Applications of Pristine and Functionalized Carbon Nanotubes, Graphene, and Graphene Nanoribbons in Biomedicine
Abstract
:1. Introduction
2. Overview of Carbon Nanomaterials for Biomedical Applications
3. Advantages and Disadvantages of Carbon Nanomaterial
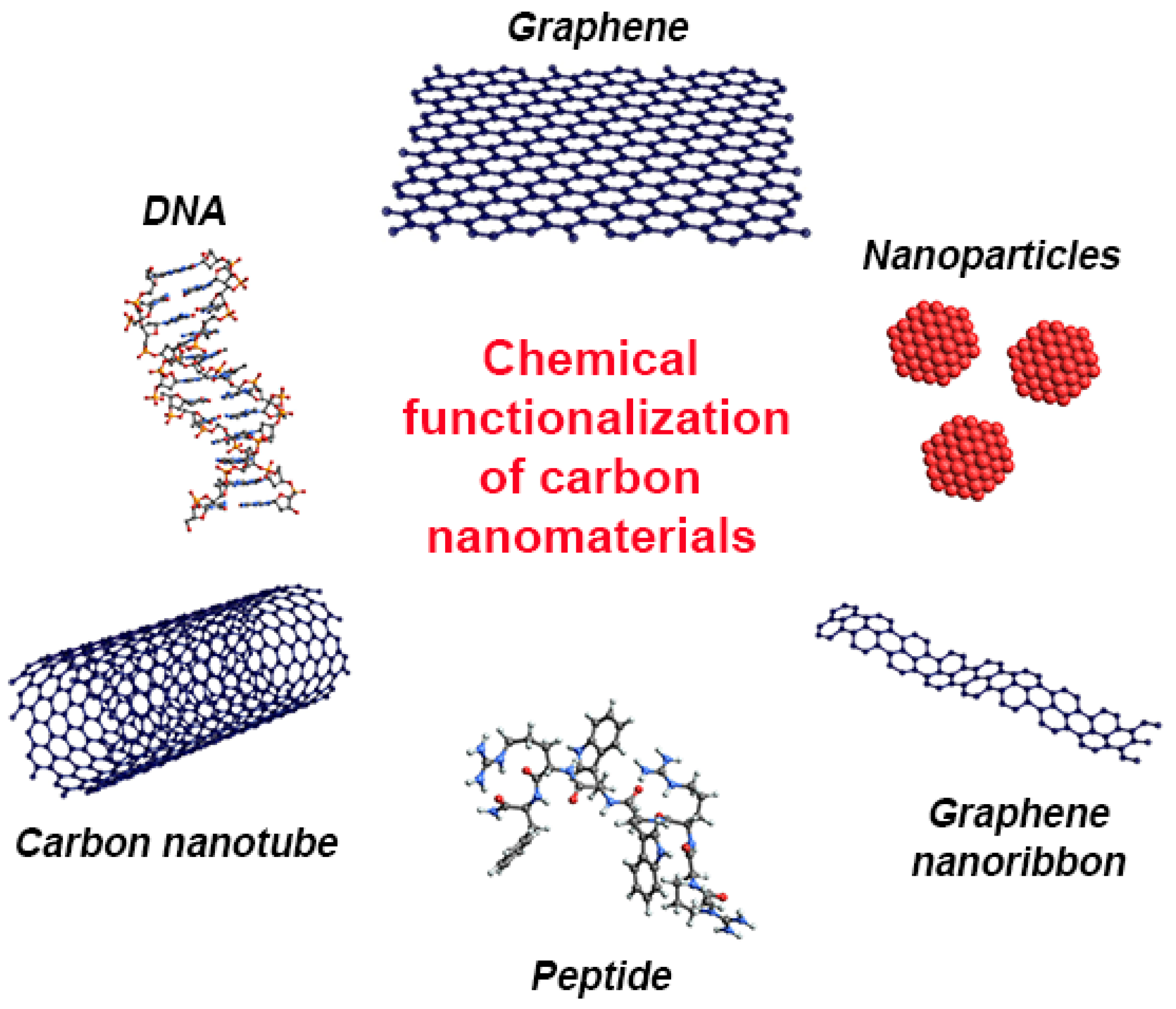
4. Types of Chemical Functionalization of Carbon Nanomaterials
4.1. Exohedral Chemical Functionalization
4.1.1. Noncovalent Chemical Functionalization
4.1.2. Covalent Chemical Functionalization
4.2. Endohedral Chemical Functionalization
5. Chemical Functionalization of Carbon Nanomaterials to Improve the Functionality in Biomedicine
5.1. Functionalization for Improving Hydrophilicity
5.2. Functionalization for Improving the Biocompatibility
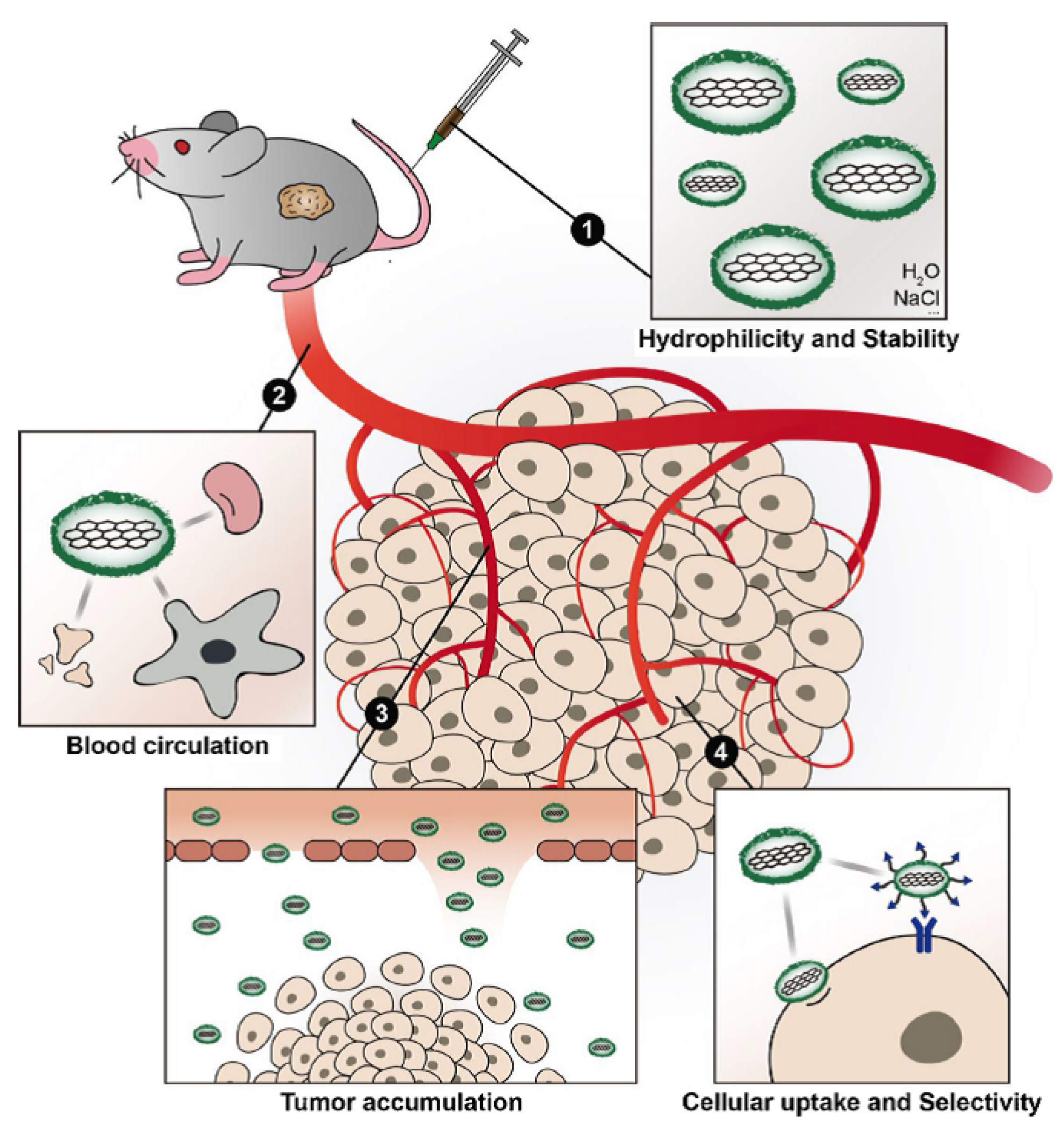
5.3. Functionalization for Improving the Blood Circulation Time and Tumor Accumulation
5.4. Functionalization for Improving the Cellular Uptake and Selectivity
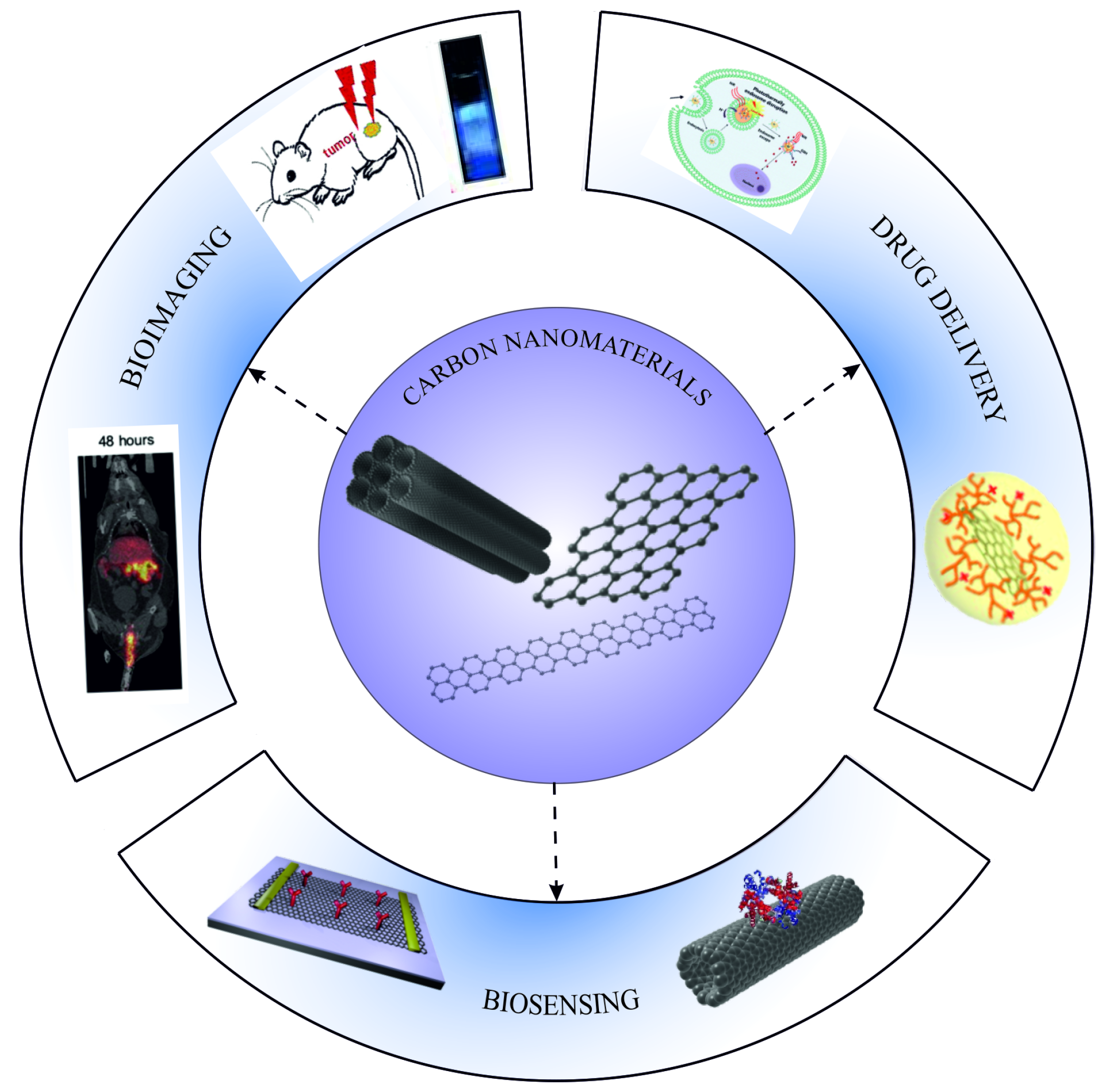
6. Application of Functionalized Carbon Nanomaterials in Biomedicine
6.1. Application of Carbon Nanotubes
6.1.1. Bioimaging
6.1.2. Drug Delivery
6.1.3. Biosensing
6.2. Applications of Graphene
6.2.1. Bioimaging
6.2.2. Drug Delivery
6.2.3. Biosensing
6.3. Application of Graphene Nanoribbons
6.3.1. Bioimaging
6.3.2. Drug Delivery
6.3.3. Biosensing
7. Toxicity Studies of Carbon Nanomaterials
8. Conclusions
9. Outlook
- The development of methods of the surface functionalization of CNMs to improve the solubility, biocompatibility, biodegradability, and targeting of functionalized CNMs and reduce their toxicity;
- The development of methods of the loading of CNMs with therapeutic drugs to increase the degree of loading and reduce the amount of nonloaded drugs;
- The comparison of the efficiency of the methods of the external and internal loading of CNTs with therapeutic drugs;
- The revealing of the correlation among the structural parameters of CNMs (i.e., diameter and length of CNTs, the size of GQDs), surface functionalization, and their accumulation in tissues and cells;
- The development of the methods of controlling the release of drugs from loaded CNMs (pH of the medium, temperature, electric stimulation);
- The development of the methods of the loading of CNMs with contrast agents for bioimaging to increase the degree of loading and reduce the amount of nonloaded contrast agents;
- The controllable modification of the physicochemical properties of CNMs loaded with contrast agents to improve the sensitivity of imaging and detection, to provide a high spatial resolution and imaging of deeper tissues;
- The development of the methods of the combined delivery of both biomedical contrast agents and therapeutic drugs using CNMs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATP | Adenosine triphosphate |
| CNMs | Carbon nanomaterials |
| CNTs | Carbon nanotubes |
| CT | Computerized tomography |
| DNA | Deoxyribonucleic acid |
| dsDNA | Double-stranded DNA |
| FET | Field effect transistor |
| FL | Fluorescence |
| GBM | Glioblastoma multiform |
| GCE | Glassy carbon electrode |
| GNRs | Graphene nanoribbons |
| GO | Graphene oxide |
| GQDs | Graphene quantum dots |
| LED | Light-emitting diode |
| Luc | Lucanthone |
| miRNA | Micro-RNA |
| MIR | Middle-infrared |
| MRI | Magnetic resonance imaging |
| MWCNT | Multiwalled carbon nanotube |
| NADH | Nicotinamide adenine dinucleotide |
| NGO | Nanoscale graphene oxide |
| NIR | Nearinfrared |
| oGNRs | Oxidized graphene nanoribbons |
| PAH | Polycyclic aromatic hydrocarbon |
| PAI | Photoacoustic imaging |
| PE | Polyethylene |
| PEG | Polyethylene glycol |
| PEI | Polyethylenimine |
| PVP | Polyvinylpyrrolidone |
| QY | Quantum yield |
| rGNRs | Reduced graphene nanoribbons |
| rGO | Reduced graphene oxide |
| RNA | Ribonucleic acid |
| SERS | Surface-enhanced Raman scattering |
| siRNA | Small interfering RNA |
| SPECT | Single-photon emission computed tomography |
| SPR | Surface plasmon resonance |
| SWCNT | Single-walled carbon nanotube |
| WL | White light |
References
- Jariwala, D.; Sangwan, V.K.; Lauhon, L.J.; Marks, T.J.; Hersam, M.C. Carbon nanomaterials for electronics, optoelectronics, photovoltaics, and sensing. Chem. Soc. Rev. 2013, 42, 2824–2860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burdanova, M.G.; Tsapenko, A.P.; Satco, D.A.; Kashtiban, R.; Mosley, C.D.W.; Monti, M.; Staniforth, M.; Sloan, J.; Gladush, Y.G.; Nasibulin, A.G.; et al. Giant Negative Terahertz Photoconductivity in Controllably Doped Carbon Nanotube Networks. ACS Photonics 2019, 6, 1058–1066. [Google Scholar] [CrossRef]
- Gladush, Y.; Mkrtchyan, A.A.; Kopylova, D.S.; Ivanenko, A.; Nyushkov, B.; Kobtsev, S.; Kokhanovskiy, A.; Khegai, A.; Melkumov, M.; Burdanova, M.; et al. Ionic Liquid Gated Carbon Nanotube Saturable Absorber for Switchable Pulse Generation. Nano Lett. 2019, 19, 5836–5843. [Google Scholar] [CrossRef] [PubMed]
- Burdanova, M.G.; Liu, M.; Staniforth, M.; Zheng, Y.; Xiang, R.; Chiashi, S.; Anisimov, A.; Kauppinen, E.I.; Maruyama, S.; Lloyd-Hughes, J. Intertube Excitonic Coupling in Nanotube Van der Waals Heterostructures. Adv. Funct. Mater. 2021, 2104969. [Google Scholar] [CrossRef]
- Deng, S.; Berry, V. Wrinkled, rippled and crumpled graphene: An overview of formation mechanism, electronic properties, and applications. Mater. Today 2016, 19, 197–212. [Google Scholar] [CrossRef]
- Dutta, S.; Pati, S.K. Novel properties of graphene nanoribbons: A review. J. Mater. Chem. 2010, 20, 8207. [Google Scholar] [CrossRef]
- Shao, D.; Yotprayoonsak, P.; Saunajoki, V.; Ahlskog, M.; Virtanen, J.; Kangas, V.; Volodin, A.; Haesendonck, C.V.; Burdanova, M.; Mosley, C.D.W.; et al. Conduction properties of thin films from a water soluble carbon nanotube/hemicellulose complex. Nanotechnology 2018, 29, 145203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burdanova, M.G.; Katyba, G.M.; Kashtiban, R.; Komandin, G.A.; Butler-Caddle, E.; Staniforth, M.; Mkrtchyan, A.A.; Krasnikov, D.V.; Gladush, Y.G.; Sloan, J.; et al. Ultrafast, high modulation depth terahertz modulators based on carbon nanotube thin films. Carbon 2021, 173, 245–252. [Google Scholar] [CrossRef]
- Burdanova, M.G.; Tsapenko, A.P.; Kharlamova, M.V.; Kauppinen, E.I.; Gorshunov, B.P.; Kono, J.; Lloyd-Hughes, J. A Review of the Terahertz Conductivity and Photoconductivity of Carbon Nanotubes and Heteronanotubes. Adv. Opt. Mater. 2021, 2101042. [Google Scholar] [CrossRef]
- Maiti, D.; Tong, X.; Mou, X.; Yang, K. Carbon-Based Nanomaterials for Biomedical Applications: A Recent Study. Front. Pharmacol. 2019, 9, 1401. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Z.; Zhang, Y. The application of carbon nanotubes in target drug delivery systems for cancer therapies. Nanoscale Res. Lett. 2011, 6, 555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCallion, C.; Burthem, J.; Rees-Unwin, K.; Golovanov, A.; Pluen, A. Graphene in therapeutics delivery: Problems, solutions and future opportunities. Eur. J. Pharm. Biopharm. 2016, 104, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Zare, H.; Ahmadi, S.; Ghasemi, A.; Ghanbari, M.; Rabiee, N.; Bagherzadeh, M.; Karimi, M.; Webster, T.J.; Hamblin, M.R.; Mostafavi, E. Carbon Nanotubes: Smart Drug/Gene Delivery Carriers. Int. J. Nanomed. 2021, 16, 1681–1706. [Google Scholar] [CrossRef]
- Rastogi, V.; Yadav, P.; Bhattacharya, S.S.; Mishra, A.K.; Verma, N.; Verma, A.; Pandit, J.K. Carbon Nanotubes: An Emerging Drug Carrier for Targeting Cancer Cells. J. Drug Deliv. 2014, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikitin, M.P.; Zelepukin, I.V.; Shipunova, V.O.; Sokolov, I.L.; Deyev, S.M.; Nikitin, P.I. Enhancement of the blood-circulation time and performance of nanomedicines via the forced clearance of erythrocytes. Nat. Biomed. Eng. 2020, 4, 717–731. [Google Scholar] [CrossRef]
- Zelepukin, I.V.; Yaremenko, A.V.; Yuryev, M.V.; Mirkasymov, A.B.; Sokolov, I.L.; Deyev, S.M.; Nikitin, P.I.; Nikitin, M.P. Fast processes of nanoparticle blood clearance: Comprehensive study. J. Control. Release 2020, 326, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Mirkasymov, A.B.; Zelepukin, I.V.; Nikitin, P.I.; Nikitin, M.P.; Deyev, S.M. In vivo blockade of mononuclear phagocyte system with solid nanoparticles: Efficiency and affecting factors. J. Control. Release 2021, 330, 111–118. [Google Scholar] [CrossRef]
- Saito, R.; Dresselhaus, G.; Dresselhaus, M.S. Physical Properties of Carbon Nanotubes; Imperial College Press: London, UK, 1998. [Google Scholar] [CrossRef]
- Merum, S.; Veluru, J.B.; Seeram, R. Functionalized carbon nanotubes in bio-world: Applications, limitations and future directions. Mater. Sci. Eng. B 2017, 223, 43–63. [Google Scholar] [CrossRef]
- Sun, P.Z.; Yang, Q.; Kuang, W.J.; Stebunov, Y.V.; Xiong, W.Q.; Yu, J.; Nair, R.R.; Katsnelson, M.I.; Yuan, S.J.; Grigorieva, I.V.; et al. Limits on gas impermeability of graphene. Nature 2020, 579, 229–232. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, Z.; Wang, J.; Li, J.; Lin, Y. Graphene and graphene oxide: Biofunctionalization and applications in biotechnology. Trends Biotechnol. 2011, 29, 205–212. [Google Scholar] [CrossRef]
- Celis, A.; Nair, M.N.; Taleb-Ibrahimi, A.; Conrad, E.H.; Berger, C.; de Heer, W.A.; Tejeda, A. Graphene nanoribbons: Fabrication, properties and devices. J. Phys. D Appl. Phys. 2016, 49, 143001. [Google Scholar] [CrossRef]
- Zhu, Y.; Higginbotham, A.L.; Tour, J.M. Covalent Functionalization of Surfactant-Wrapped Graphene Nanoribbons. Chem. Mater. 2009, 21, 5284–5291. [Google Scholar] [CrossRef]
- Johnson, A.P.; Gangadharappa, H.; Pramod, K. Graphene nanoribbons: A promising nanomaterial for biomedical applications. J. Control. Release 2020, 325, 141–162. [Google Scholar] [CrossRef]
- Zhou, Y.; Fang, Y.; Ramasamy, R. Non-Covalent Functionalization of Carbon Nanotubes for Electrochemical Biosensor Development. Sensors 2019, 19, 392. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Speranza, G. Functionalization of Carbon Nanomaterials for Biomedical Applications. C J. Carbon Res. 2019, 5, 72. [Google Scholar] [CrossRef] [Green Version]
- Bardhan, N.M. 30 years of advances in functionalization of carbon nanomaterials for biomedical applications: A practical review. J. Mater. Res. 2016, 32, 107–127. [Google Scholar] [CrossRef] [Green Version]
- Kharlamova, M.V. Nickelocene-Filled Purely Metallic Single-Walled Carbon Nanotubes: Sorting and Tuning the Electronic Properties. Nanomaterials 2021, 11, 2500. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Kramberger, C.; Saito, T.; Pichler, T. Diameter and metal-dependent growth properties of inner tubes inside metallocene-filled single-walled carbon nanotubes. Fullerenes Nanotub. Carbon Nanostruct. 2019, 28, 20–26. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Kramberger, C.; Rudatis, P.; Yanagi, K.; Eder, D. Characterization of the Electronic Properties of Single-Walled Carbon Nanotubes Filled with an Electron Donor—Rubidium Iodide: Multifrequency Raman and X-ray Photoelectron Spectroscopy Studies. Phys. Status Solidi 2019, 256, 1900209. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Kramberger, C.; Rudatis, P.; Pichler, T.; Eder, D. Revealing the doping effect of encapsulated lead halogenides on single-walled carbon nanotubes. Appl. Phys. 2019, 125, 25320. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Kramberger, C.; Domanov, O.; Mittelberger, A.; Yanagi, K.; Pichler, T.; Eder, D. Fermi level engineering of metallicity-sorted metallic single-walled carbon nanotubes by encapsulation of few-atom-thick crystals of silver chloride. J. Mater. Sci. 2018, 53, 13018–13029. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Kramberger, C.; Domanov, O.; Mittelberger, A.; Saito, T.; Yanagi, K.; Pichler, T.; Eder, D. Comparison of Doping Levels of Single-Walled Carbon Nanotubes Synthesized by Arc-Discharge and Chemical Vapor Deposition Methods by Encapsulated Silver Chloride. Phys. Status Solidi 2018, 255, 1800178. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Kramberger, C.; Mittelberger, A.; Yanagi, K.; Pichler, T.; Eder, D. Silver Chloride Encapsulation-Induced Modifications of Raman Modes of Metallicity-Sorted Semiconducting Single-Walled Carbon Nanotubes. J. Spectrosc. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Kramberger, C.; Sato, Y.; Saito, T.; Suenaga, K.; Pichler, T.; Shiozawa, H. Chiral vector and metal catalyst-dependent growth kinetics of single-wall carbon nanotubes. Carbon 2018, 133, 283–292. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Kramberger, C.; Sauer, M.; Yanagi, K.; Saito, T.; Pichler, T. Inner tube growth and electronic properties of metallicity-sorted nickelocene-filled semiconducting single-walled carbon nanotubes. Appl. Phys. A 2018, 124, 124247. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Kramberger, C.; Yanagi, K.; Sauer, M.; Saito, T.; Pichler, T. Separation of Nickelocene-Filled Single-Walled Carbon Nanotubes by Conductivity Type and Diameter. Phys. Status Solidi 2017, 254, 1700178. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Kramberger, C.; Saito, T.; Sato, Y.; Suenaga, K.; Pichler, T.; Shiozawa, H. Chirality-dependent growth of single-wall carbon nanotubes as revealed inside nano-test tubes. Nanoscale 2017, 9, 7998–8006. [Google Scholar] [CrossRef] [Green Version]
- Kharlamova, M.V. Investigation of growth dynamics of carbon nanotubes. Beilstein J. Nanotechnol. 2017, 8, 826–856. [Google Scholar] [CrossRef] [Green Version]
- Kharlamova, M.V.; Kramberger, C.; Mittelberger, A. Raman spectroscopy study of the doping effect of the encapsulated terbium halogenides on single-walled carbon nanotubes. Appl. Phys. A 2017, 123, 123239. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Kramberger, C.; Saito, T.; Shiozawa, H.; Pichler, T. Growth dynamics of inner tubes inside cobaltocene-filled single-walled carbon nanotubes. Appl. Phys. A 2016, 122, 22749. [Google Scholar] [CrossRef]
- Kharlamova, M.V. Electronic properties of single-walled carbon nanotubes filled with manganese halogenides. Appl. Phys. A 2016, 122, 22791. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Kramberger, C.; Pichler, T. Semiconducting response in single-walled carbon nanotubes filled with cadmium chloride. Phys. Status Solidi 2016, 253, 2433–2439. [Google Scholar] [CrossRef]
- Kharlamova, M.V. Advances in tailoring the electronic properties of single-walled carbon nanotubes. Prog. Mater. Sci. 2016, 77, 125–211. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Sauer, M.; Saito, T.; Sato, Y.; Suenaga, K.; Pichler, T.; Shiozawa, H. Doping of single-walled carbon nanotubes controlled via chemical transformation of encapsulated nickelocene. Nanoscale 2015, 7, 1383–1391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kharlamova, M.V. Raman Spectroscopy Study of the Doping Effect of the Encapsulated Iron, Cobalt, and Nickel Bromides on Single-Walled Carbon Nanotubes. J. Spectrosc. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Sauer, M.; Egorov, A.; Kramberger, C.; Saito, T.; Pichler, T.; Shiozawa, H. Temperature-dependent inner tube growth and electronic structure of nickelocene-filled single-walled carbon nanotubes. Phys. Status Solid 2015, 252, 2485–2490. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Kramberger, C.; Sauer, M.; Yanagi, K.; Pichler, T. Comprehensive spectroscopic characterization of high purity metallicity-sorted single-walled carbon nanotubes. Phys. Status Solidi 2015, 252, 2512–2518. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Volykhov, A.A.; Yashina, L.V.; Egorov, A.V.; Lukashin, A.V. Experimental and theoretical studies on the electronic properties of praseodymium chloride-filled single-walled carbon nanotubes. J. Mater. Sci. 2015, 50, 5419–5430. [Google Scholar] [CrossRef]
- Kashtiban, R.J.; Burdanova, M.G.; Vasylenko, A.; Wynn, J.; Medeiros, P.V.C.; Ramasse, Q.; Morris, A.J.; Quigley, D.; Lloyd-Hughes, J.; Sloan, J. Linear and Helical Cesium Iodide Atomic Chains in Ultranarrow Single-Walled Carbon Nanotubes: Impact on Optical Properties. ACS Nano 2021, 15, 13389–13398. [Google Scholar] [CrossRef]
- Kharlamova, M.V. Rare-earth metal halogenide encapsulation-induced modifications in Raman spectra of single-walled carbon nanotubes. Appl. Phys. A 2014, 118, 27–35. [Google Scholar] [CrossRef]
- Kramberger, C.; Kharlamova, M.V.; Yanagi, K. Multifrequency Raman spectroscopy on bulk (11, 10) chirality enriched semiconducting single-walled carbon nanotubes. Phys. Status Solidi 2014, 251, 2432–2436. [Google Scholar] [CrossRef]
- Kharlamova, M.V. Novel approach to tailoring the electronic properties of single-walled carbon nanotubes by the encapsulation of high-melting gallium selenide using a single-step process. JETP Lett. 2013, 98, 272–277. [Google Scholar] [CrossRef]
- Kharlamova, M.V. Electronic properties of pristine and modified single-walled carbon nanotubes. Uspekhi Fiz. Nauk. 2013, 183, 1145–1174. [Google Scholar] [CrossRef] [Green Version]
- Kharlamova, M.V.; Sauer, M.; Saito, T.; Krause, S.; Liu, X.; Yanagi, K.; Pichler, T.; Shiozawa, H. Inner tube growth properties and electronic structure of ferrocene-filled large diameter single-walled carbon nanotubes. Phys. Status Solidi 2013, 250, 2575–2580. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Yashina, L.V.; Eliseev, A.A.; Volykhov, A.A.; Neudachina, V.S.; Brzhezinskaya, M.M.; Zyubina, T.S.; Lukashin, A.V.; Tretyakov, Y.D. Single-walled carbon nanotubes filled with nickel halogenides: Atomic structure and doping effect. Phys. Status Solidi 2012, 249, 2328–2332. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Niu, J.J. Donor doping of single-walled carbon nanotubes by filling of channels with silver. J. Exp. Theor. Phys. 2012, 115, 485–491. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Niu, J.J. New method of the directional modification of the electronic structure of single-walled carbon nanotubes by filling channels with metallic copper from a liquid phase. JETP Lett. 2012, 95, 314–319. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Yashina, L.V.; Volykhov, A.A.; Niu, J.J.; Neudachina, V.S.; Brzhezinskaya, M.M.; Zyubina, T.S.; Belogorokhov, A.I.; Eliseev, A.A. Acceptor doping of single-walled carbon nanotubes by encapsulation of zinc halogenides. Eur. Phys. J. B 2012, 85, 8534. [Google Scholar] [CrossRef]
- Yashina, L.V.; Eliseev, A.A.; Kharlamova, M.V.; Volykhov, A.A.; Egorov, A.V.; Savilov, S.V.; Lukashin, A.V.; Püttner, R.; Belogorokhov, A.I. Growth and Characterization of One-Dimensional SnTe Crystals within the Single-Walled Carbon Nanotube Channels. J. Phys. Chem. C 2011, 115, 3578–3586. [Google Scholar] [CrossRef]
- Eliseev, A.; Yashina, L.; Brzhezinskaya, M.; Chernysheva, M.; Kharlamova, M.; Verbitsky, N.; Lukashin, A.; Kiselev, N.; Kumskov, A.; Zakalyuhin, R.; et al. Structure and electronic properties of AgX (X = Cl, Br, I)-intercalated single-walled carbon nanotubes. Carbon 2010, 48, 2708–2721. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Eliseev, A.A.; Yashina, L.V.; Petukhov, D.I.; Liu, C.P.; Wang, C.Y.; Semenenko, D.A.; Belogorokhov, A.I. Study of the electronic structure of single-walled carbon nanotubes filled with cobalt bromide. JETP Lett. 2010, 91, 196–200. [Google Scholar] [CrossRef]
- Eliseev, A.A.; Kharlamova, M.V.; Chernysheva, M.V.; Lukashin, A.V.; Tretyakov, Y.D.; Kumskov, A.S.; Kiselev, N.A. Preparation and properties of single-walled nanotubes filled with inorganic compounds. Russ. Chem. Rev. 2009, 78, 833–854. [Google Scholar] [CrossRef]
- Marega, R.; Bonifazi, D. Filling carbon nanotubes for nanobiotechnological applications. New J. Chem. 2014, 38, 22–27. [Google Scholar] [CrossRef]
- de Melo-Diogo, D.; Lima-Sousa, R.; Alves, C.G.; Costa, E.C.; Louro, R.O.; Correia, I.J. Functionalization of graphene family nanomaterials for application in cancer therapy. Colloids Surfaces B Biointerfaces 2018, 171, 260–275. [Google Scholar] [CrossRef]
- Feng, L.; Li, K.; Shi, X.; Gao, M.; Liu, J.; Liu, Z. Smart pH-Responsive Nanocarriers Based on Nano-Graphene Oxide for Combined Chemo- and Photothermal Therapy Overcoming Drug Resistance. Adv. Healthc. Mater. 2014, 3, 1261–1271. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, L.; Li, X.; Zeng, J.; Jia, X.; Liu, P. Biocompatible Graphene Oxide Nanoparticle-Based Drug Delivery Platform for Tumor Microenvironment-Responsive Triggered Release of Doxorubicin. Langmuir 2014, 30, 10419–10429. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, L.; Li, X.; Jia, X.; Liu, L.; Zeng, J.; Guo, J.; Liu, P. Functionalized Graphene Oxide Nanoparticles for Cancer Cell-Specific Delivery of Antitumor Drug. Bioconjug. Chem. 2015, 26, 128–136. [Google Scholar] [CrossRef]
- Alibolandi, M.; Mohammadi, M.; Taghdisi, S.M.; Ramezani, M.; Abnous, K. Fabrication of aptamer decorated dextran coated nano-graphene oxide for targeted drug delivery. Carbohydr. Polym. 2017, 155, 218–229. [Google Scholar] [CrossRef]
- Bao, H.; Pan, Y.; Ping, Y.; Sahoo, N.G.; Wu, T.; Li, L.; Li, J.; Gan, L.H. Chitosan-Functionalized Graphene Oxide as a Nanocarrier for Drug and Gene Delivery. Small 2011, 7, 1569–1578. [Google Scholar] [CrossRef]
- Qin, C.; Fei, J.; Cai, P.; Zhao, J.; Li, J. Biomimetic membrane-conjugated graphene nanoarchitecture for light-manipulating combined cancer treatment in vitro. J. Colloid Interface Sci. 2016, 482, 121–130. [Google Scholar] [CrossRef]
- Sheng, Z.; Song, L.; Zheng, J.; Hu, D.; He, M.; Zheng, M.; Gao, G.; Gong, P.; Zhang, P.; Ma, Y.; et al. Protein-assisted fabrication of nano-reduced graphene oxide for combined in vivo photoacoustic imaging and photothermal therapy. Biomaterials 2013, 34, 5236–5243. [Google Scholar] [CrossRef] [PubMed]
- Imani, R.; Emami, S.H.; Faghihi, S. Synthesis and characterization of an octaarginine functionalized graphene oxide nano-carrier for gene delivery applications. Phys. Chem. Chem. Phys. 2015, 17, 6328–6339. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, M. Research Progress on PI3K/AKT/mTOR Signaling Pathway in Burkitt Lymphoma. Cancer Res. Prev. Treat. 2019, 46, 169. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, J.J.; Cheng, F.F.; Zheng, T.T.; Wang, C.; Zhu, J.J. Green and facile synthesis of highly biocompatible graphene nanosheets and its application for cellular imaging and drug delivery. J. Mater. Chem. 2011, 21, 12034. [Google Scholar] [CrossRef]
- Martín, C.; Kostarelos, K.; Prato, M.; Bianco, A. Biocompatibility and biodegradability of 2D materials: Graphene and beyond. Chem. Commun. 2019, 55, 5540–5546. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kim, J.H. Synthesis, toxicity, biocompatibility, and biomedical applications of graphene and graphene-related materials. Int. J. Nanomed. 2016, 11, 1927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, K.; Gong, H.; Shi, X.; Wan, J.; Zhang, Y.; Liu, Z. In vivo biodistribution and toxicology of functionalized nano-graphene oxide in mice after oral and intraperitoneal administration. Biomaterials 2013, 34, 2787–2795. [Google Scholar] [CrossRef]
- Luo, N.; Weber, J.K.; Wang, S.; Luan, B.; Yue, H.; Xi, X.; Du, J.; Yang, Z.; Wei, W.; Zhou, R.; et al. PEGylated graphene oxide elicits strong immunological responses despite surface passivation. Nat. Commun. 2017, 8, 14537. [Google Scholar] [CrossRef] [Green Version]
- Chong, Y.; Ge, C.; Yang, Z.; Garate, J.A.; Gu, Z.; Weber, J.K.; Liu, J.; Zhou, R. Reduced Cytotoxicity of Graphene Nanosheets Mediated by Blood-Protein Coating. ACS Nano 2015, 9, 5713–5724. [Google Scholar] [CrossRef]
- Mahanta, S.; Paul, S. Bovine α-lactalbumin functionalized graphene oxide nano-sheet exhibits enhanced biocompatibility: A rational strategy for graphene-based targeted cancer therapy. Colloids Surfaces B Biointerfaces 2015, 134, 178–187. [Google Scholar] [CrossRef]
- Mu, L.; Gao, Y.; Hu, X. l-Cysteine: A biocompatible, breathable and beneficial coating for graphene oxide. Biomaterials 2015, 52, 301–311. [Google Scholar] [CrossRef]
- Jin, R.; Ji, X.; Yang, Y.; Wang, H.; Cao, A. Self-Assembled Graphene–Dextran Nanohybrid for Killing Drug-Resistant Cancer Cells. ACS Appl. Mater. Interfaces 2013, 5, 7181–7189. [Google Scholar] [CrossRef]
- Cheng, C.; Nie, S.; Li, S.; Peng, H.; Yang, H.; Ma, L.; Sun, S.; Zhao, C. Biopolymer functionalized reduced graphene oxide with enhanced biocompatibility via mussel inspired coatings/anchors. J. Mater. Chem. B 2013, 1, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Sitharaman, B.; Kanakia, S.; Toussaint, J.; Chowdhury, S.M.; Lalwani, G.; Tembulkar, T.; Button, T.; Shroyer, K.; Moore, W. Physicochemical characterization of a novel graphene-based magnetic resonance imaging contrast agent. Int. J. Nanomed. 2013, 2013, 2821–2833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rana, V.K.; Choi, M.C.; Kong, J.Y.; Kim, G.Y.; Kim, M.J.; Kim, S.H.; Mishra, S.; Singh, R.P.; Ha, C.S. Synthesis and Drug-Delivery Behavior of Chitosan-Functionalized Graphene Oxide Hybrid Nanosheets. Macromol. Mater. Eng. 2010, 296, 131–140. [Google Scholar] [CrossRef]
- Cheng, C.; Li, S.; Nie, S.; Zhao, W.; Yang, H.; Sun, S.; Zhao, C. General and Biomimetic Approach to Biopolymer-Functionalized Graphene Oxide Nanosheet through Adhesive Dopamine. Biomacromolecules 2012, 13, 4236–4246. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhang, S.; Zhang, G.; Sun, X.; Lee, S.T.; Liu, Z. Graphene in Mice: Ultrahigh In Vivo Tumor Uptake and Efficient Photothermal Therapy. Nano Lett. 2010, 10, 3318–3323. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wan, J.; Zhang, S.; Zhang, Y.; Lee, S.T.; Liu, Z. In Vivo Pharmacokinetics, Long-Term Biodistribution, and Toxicology of PEGylated Graphene in Mice. ACS Nano 2010, 5, 516–522. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S. Graphene Nanomaterials: Synthesis, Biocompatibility, and Cytotoxicity. Int. J. Mol. Sci. 2018, 19, 3564. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.; Wan, J.; Zhang, S.; Tian, B.; Zhang, Y.; Liu, Z. The influence of surface chemistry and size of nanoscale graphene oxide on photothermal therapy of cancer using ultra-low laser power. Biomaterials 2012, 33, 2206–2214. [Google Scholar] [CrossRef]
- Xu, C.; Shi, S.; Feng, L.; Chen, F.; Graves, S.A.; Ehlerding, E.B.; Goel, S.; Sun, H.; England, C.G.; Nickles, R.J.; et al. Long circulating reduced graphene oxide–iron oxide nanoparticles for efficient tumor targeting and multimodality imaging. Nanoscale 2016, 8, 12683–12692. [Google Scholar] [CrossRef]
- Li, J.; Lyv, Z.; Li, Y.; Liu, H.; Wang, J.; Zhan, W.; Chen, H.; Chen, H.; Li, X. A theranostic prodrug delivery system based on Pt(IV) conjugated nano-graphene oxide with synergistic effect to enhance the therapeutic efficacy of Pt drug. Biomaterials 2015, 51, 12–21. [Google Scholar] [CrossRef]
- Yang, H.; Bremner, D.H.; Tao, L.; Li, H.; Hu, J.; Zhu, L. Carboxymethyl chitosan-mediated synthesis of hyaluronic acid-targeted graphene oxide for cancer drug delivery. Carbohydr. Polym. 2016, 135, 72–78. [Google Scholar] [CrossRef]
- Zhu, H.; Zhou, B.; Chan, L.; Du, Y.; Chen, T. Transferrin-functionalized nanographene oxide for delivery of platinum complexes to enhance cancer-cell selectivity and apoptosis-inducing efficacy. Int. J. Nanomed. 2017, 12, 5023–5038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Curran, E.C.; Hinds, T.R.; Wang, E.H.; Zheng, N. Crystal structure of a TAF1-TAF7 complex in human transcription factor IID reveals a promoter binding module. Cell Res. 2014, 24, 1433–1444. [Google Scholar] [CrossRef] [Green Version]
- Qin, X.; Guo, Z.; Liu, Z.; Zhang, W.; Wan, M.; Yang, B. Folic acid-conjugated graphene oxide for cancer targeted chemo-photothermal therapy. J. Photochem. Photobiol. B Biol. 2013, 120, 156–162. [Google Scholar] [CrossRef]
- Miao, W.; Shim, G.; Kang, C.M.; Lee, S.; Choe, Y.S.; Choi, H.G.; Oh, Y.K. Cholesteryl hyaluronic acid-coated, reduced graphene oxide nanosheets for anti-cancer drug delivery. Biomaterials 2013, 34, 9638–9647. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.H.; Fang, R.H.; Chen, Y.W.; Liao, B.J.; Chen, I.W.; Chen, S.Y. Photoresponsive Protein–Graphene–Protein Hybrid Capsules with Dual Targeted Heat-Triggered Drug Delivery Approach for Enhanced Tumor Therapy. Adv. Funct. Mater. 2014, 24, 4144–4155. [Google Scholar] [CrossRef]
- Cherkasov, V.R.; Mochalova, E.N.; Babenyshev, A.V.; Rozenberg, J.M.; Sokolov, I.L.; Nikitin, M.P. Antibody-directed metal-organic framework nanoparticles for targeted drug delivery. Acta Biomater. 2020, 103, 223–236. [Google Scholar] [CrossRef]
- Shipunova, V.O.; Kotelnikova, P.A.; Aghayeva, U.F.; Stremovskiy, O.A.; Novikov, I.A.; Schulga, A.A.; Nikitin, M.P.; Deyev, S.M. Self-assembling nanoparticles biofunctionalized with magnetite-binding protein for the targeted delivery to HER2/neu overexpressing cancer cells. J. Magn. Magn. Mater. 2019, 469, 450–455. [Google Scholar] [CrossRef]
- Burenin, A.G.; Urusov, A.E.; Betin, A.V.; Orlov, A.V.; Nikitin, M.P.; Ksenevich, T.I.; Gorshkov, B.G.; Zherdev, A.V.; Dzantiev, B.B.; Nikitin, P.I. Direct immunosensing by spectral correlation interferometry: Assay characteristics versus antibody immobilization chemistry. Anal. Bioanal. Chem. 2015, 407, 3955–3964. [Google Scholar] [CrossRef]
- Shipunova, V.O.; Sogomonyan, A.S.; Zelepukin, I.V.; Nikitin, M.P.; Deyev, S.M. PLGA Nanoparticles Decorated with Anti-HER2 Affibody for Targeted Delivery and Photoinduced Cell Death. Molecules 2021, 26, 3955. [Google Scholar] [CrossRef]
- Kim, H.; Lee, D.; Kim, J.; il Kim, T.; Kim, W.J. Photothermally Triggered Cytosolic Drug Delivery via Endosome Disruption Using a Functionalized Reduced Graphene Oxide. ACS Nano 2013, 7, 6735–6746. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Feng, L.; Liu, Z. Stimuli responsive drug delivery systems based on nano-graphene for cancer therapy. Adv. Drug Deliv. Rev. 2016, 105, 228–241. [Google Scholar] [CrossRef]
- Rivera, E.J.; Sethi, R.; Qu, F.; Krishnamurthy, R.; Muthupillai, R.; Alford, M.; Swanson, M.A.; Eaton, S.S.; Eaton, G.R.; Wilson, L.J. Nitroxide Radicals@US-Tubes: New Spin Labels for Biomedical Applications. Adv. Funct. Mater. 2012, 22, 3691–3698. [Google Scholar] [CrossRef]
- Rivera, E.J.; Tran, L.A.; Hernández-Rivera, M.; Yoon, D.; Mikos, A.G.; Rusakova, I.A.; Cheong, B.Y.; da Graça Cabreira-Hansen, M.; Willerson, J.T.; Perin, E.C.; et al. Bismuth@US-tubes as a potential contrast agent for X-ray imaging applications. J. Mater. Chem. B 2013, 1, 4792. [Google Scholar] [CrossRef] [Green Version]
- Ashcroft, J.; Hartman, K.; Kissell, K.; Mackeyev, Y.; Pheasant, S.; Young, S.; Van der Heide, P.; Mikos, A.; Wilson, L. Single-Molecule I2@US-Tube Nanocapsules: A New X-ray Contrast-Agent Design. Adv. Mater. 2007, 19, 573–576. [Google Scholar] [CrossRef]
- Munari, S.D.; Sandoval, S.; Pach, E.; Ballesteros, B.; Tobias, G.; Anthony, D.C.; Davis, B.G. In vivo behaviour of glyco-NaI@SWCNT ‘nanobottles’. Inorg. Chim. Acta 2019, 495, 118933. [Google Scholar] [CrossRef]
- D’Accolti, L.; Gajewska, A.; Kierkowicz, M.; Martincic, M.; Nacci, A.; Sandoval, S.; Ballesteros, B.; Tobias, G.; Ros, T.D.; Fusco, C. Epoxidation of Carbon Nanocapsules: Decoration of Single-Walled Carbon Nanotubes Filled with Metal Halides. Nanomaterials 2018, 8, 137. [Google Scholar] [CrossRef] [Green Version]
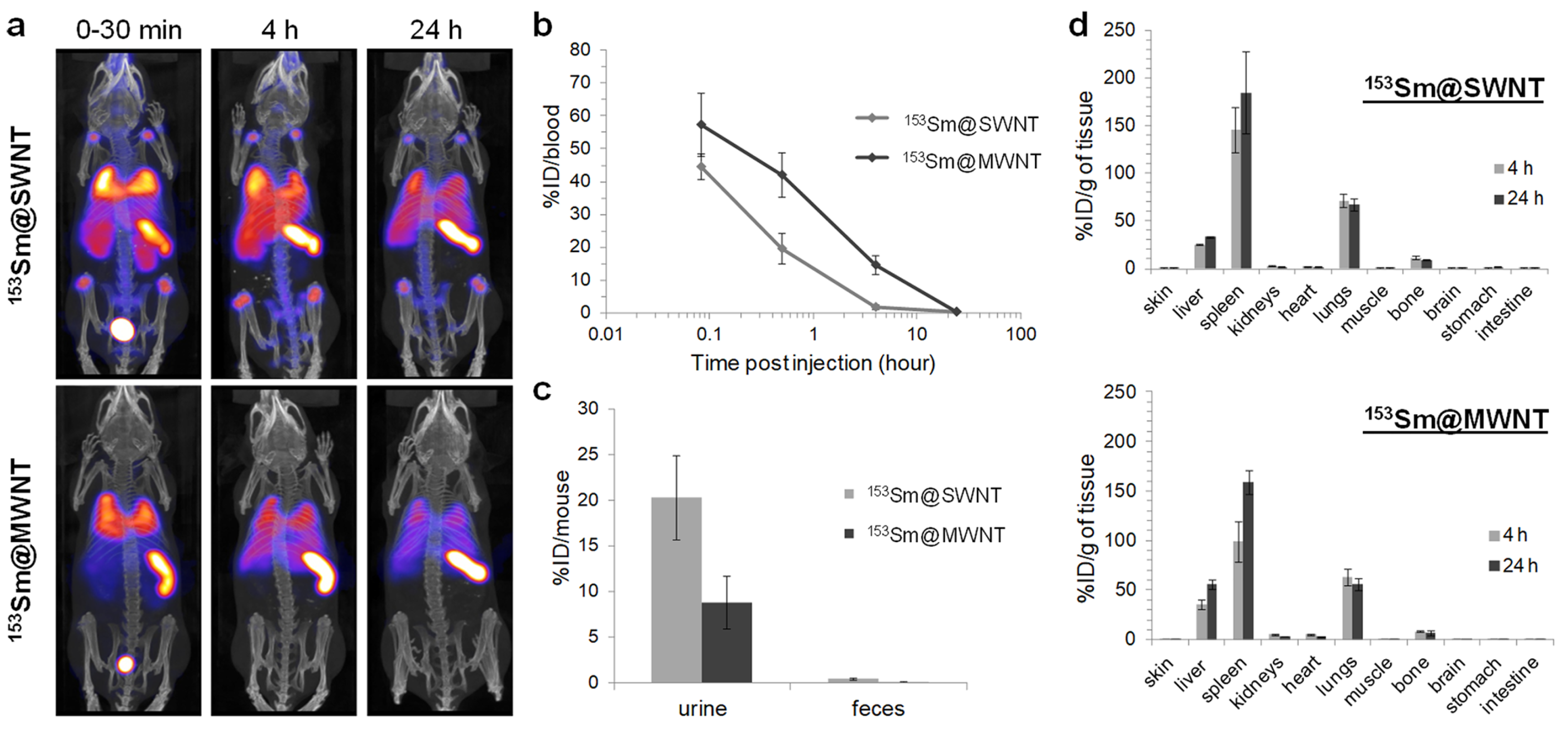
- Wang, J.T.W.; Klippstein, R.; Martincic, M.; Pach, E.; Feldman, R.; Šefl, M.; Michel, Y.; Asker, D.; Sosabowski, J.K.; Kalbac, M.; et al. Neutron Activated 153Sm Sealed in Carbon Nanocapsules for in Vivo Imaging and Tumor Radiotherapy. ACS Nano 2020, 14, 129–141. [Google Scholar] [CrossRef]
- Yang, K.; Feng, L.; Hong, H.; Cai, W.; Liu, Z. Preparation and functionalization of graphene nanocomposites for biomedical applications. Nat. Protoc. 2013, 8, 2392–2403. [Google Scholar] [CrossRef] [Green Version]
- de Garibay, A.P.R.; Spinato, C.; Klippstein, R.; Bourgognon, M.; Martincic, M.; Pach, E.; Ballesteros, B.; Ménard-Moyon, C.; Al-Jamal, K.T.; Tobias, G.; et al. Evaluation of the immunological profile of antibody-functionalized metal-filled single-walled carbon nanocapsules for targeted radiotherapy. Sci. Rep. 2017, 7, 42605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spinato, C.; de Garibay, A.P.R.; Kierkowicz, M.; Pach, E.; Martincic, M.; Klippstein, R.; Bourgognon, M.; Wang, J.T.W.; Ménard-Moyon, C.; Al-Jamal, K.T.; et al. Design of antibody-functionalized carbon nanotubes filled with radioactivable metals towards a targeted anticancer therapy. Nanoscale 2016, 8, 12626–12638. [Google Scholar] [CrossRef] [Green Version]
- Serpell, C.J.; Rutte, R.N.; Geraki, K.; Pach, E.; Martincic, M.; Kierkowicz, M.; Munari, S.D.; Wals, K.; Raj, R.; Ballesteros, B.; et al. Carbon nanotubes allow capture of krypton, barium and lead for multichannel biological X-ray fluorescence imaging. Nat. Commun. 2016, 7, 13118. [Google Scholar] [CrossRef] [Green Version]
- Tregubov, A.; Sokolov, I.; Babenyshev, A.; Nikitin, P.; Cherkasov, V.; Nikitin, M. Magnetic hybrid magnetite/metal organic framework nanoparticles: Facile preparation, post-synthetic biofunctionalization and tracking in vivo with magnetic methods. J. Magn. Magn. Mater. 2018, 449, 590–596. [Google Scholar] [CrossRef]
- Ringaci, A.; Yaremenko, A.; Shevchenko, K.; Zvereva, S.; Nikitin, M. Metal-organic frameworks for simultaneous gene and small molecule delivery in vitro and in vivo. Chem. Eng. J. 2021, 418, 129386. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Orlov, A.V.; Pushkarev, A.V.; Mochalova, E.N.; Nikitin, P.I.; Nikitin, M.P. Development and label-free investigation of logic-gating biolayers for smart biosensing. Sens. Actuators B Chem. 2018, 257, 971–979. [Google Scholar] [CrossRef]
- Vashist, S.K.; Zheng, D.; Pastorin, G.; Al-Rubeaan, K.; Luong, J.H.; Sheu, F.S. Delivery of drugs and biomolecules using carbon nanotubes. Carbon 2011, 49, 4077–4097. [Google Scholar] [CrossRef]
- Su, Z.; Zhu, S.; Donkor, A.D.; Tzoganakis, C.; Honek, J.F. Controllable Delivery of Small-Molecule Compounds to Targeted Cells Utilizing Carbon Nanotubes. J. Am. Chem. Soc. 2011, 133, 6874–6877. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Mataraza, J.M.; Qin, Z.H.; Huang, Z.; Huang, J.; Chiles, T.C.; Carnahan, D.; Kempa, K.; Ren, Z. Highly efficient molecular delivery into mammalian cells using carbon nanotube spearing. Nat. Methods 2005, 2, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Lipert, K.; Krämer, K.; Hampel, S.; Füssel, S.; Meye, A.; Klingeler, R.; Ritschel, M.; Leonhardt, A.; Büchner, B.; et al. Biocompatibility of Iron Filled Carbon Nanotubes In Vitro. J. Nanosci. Nanotechnol. 2009, 9, 5709–5716. [Google Scholar] [CrossRef] [PubMed]
- Marega, R.; Leo, F.D.; Pineux, F.; Sgrignani, J.; Magistrato, A.; Naik, A.D.; Garcia, Y.; Flamant, L.; Michiels, C.; Bonifazi, D. Functionalized Fe-Filled Multiwalled Carbon Nanotubes as Multifunctional Scaffolds for Magnetization of Cancer Cells. Adv. Funct. Mater. 2013, 23, 3173–3184. [Google Scholar] [CrossRef]
- Volder, M.F.L.D.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon Nanotubes: Present and Future Commercial Applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef] [Green Version]
- Cherkasov, V.R.; Mochalova, E.N.; Babenyshev, A.V.; Vasilyeva, A.V.; Nikitin, P.I.; Nikitin, M.P. Nanoparticle Beacons: Supersensitive Smart Materials with On/Off-Switchable Affinity to Biomedical Targets. ACS Nano 2020, 14, 1792–1803. [Google Scholar] [CrossRef]
- Znoyko, S.L.; Orlov, A.V.; Bragina, V.A.; Nikitin, M.P.; Nikitin, P.I. Nanomagnetic lateral flow assay for high-precision quantification of diagnostically relevant concentrations of serum TSH. Talanta 2020, 216, 120961. [Google Scholar] [CrossRef] [PubMed]
- Guteneva, N.V.; Znoyko, S.L.; Orlov, A.V.; Nikitin, M.P.; Nikitin, P.I. Rapid lateral flow assays based on the quantification of magnetic nanoparticle labels for multiplexed immunodetection of small molecules: Application to the determination of drugs of abuse. Microchim. Acta 2019, 186, 621. [Google Scholar] [CrossRef] [PubMed]
- Bragina, V.A.; Znoyko, S.L.; Orlov, A.V.; Pushkarev, A.V.; Nikitin, M.P.; Nikitin, P.I. Analytical Platform with Selectable Assay Parameters Based on Three Functions of Magnetic Nanoparticles: Demonstration of Highly Sensitive Rapid Quantitation of Staphylococcal Enterotoxin B in Food. Anal. Chem. 2019, 91, 9852–9857. [Google Scholar] [CrossRef]
- Shevchenko, K.G.; Cherkasov, V.R.; Tregubov, A.A.; Nikitin, P.I.; Nikitin, M.P. Surface plasmon resonance as a tool for investigation of noncovalent nanoparticle interactions in heterogeneous self-assembly & disassembly systems. Biosens. Bioelectron. 2017, 88, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sireesha, M.; Babu, V.J.; Kiran, A.S.K.; Ramakrishna, S. A review on carbon nanotubes in biosensor devices and their applications in medicine. Nanocomposites 2018, 4, 36–57. [Google Scholar] [CrossRef]
- Lin, Y.; Lu, F.; Tu, Y.; Ren, Z. Glucose Biosensors Based on Carbon Nanotube Nanoelectrode Ensembles. Nano Lett. 2004, 4, 191–195. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, Z.; He, S.; Zhang, B.; Li, X.; Yao, M. Direct electron transfer of glucose oxidase and biosensing for glucose based on PDDA-capped gold nanoparticle modified graphene/multi-walled carbon nanotubes electrode. Biosens. Bioelectron. 2014, 52, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Vilian, A.T.E.; Chen, S.M. Direct electrochemistry and electrocatalysis of glucose oxidase based poly(l-arginine)-multi-walled carbon nanotubes. RSC Adv. 2014, 4, 50771–50781. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, Y.; Zhu, Y.; Wu, J.; Li, K.; Lin, G.; Li, X.; Liu, R.; Liu, X.; Wong, C.P. Facile one-step fabrication of glucose oxidase loaded polymeric nanoparticles decorating MWCNTs for constructing glucose biosensing platform: Structure matters. Biosens. Bioelectron. 2019, 135, 153–159. [Google Scholar] [CrossRef]
- Gallay, P.; Eguílaz, M.; Rivas, G. Designing electrochemical interfaces based on nanohybrids of avidin functionalized-carbon nanotubes and ruthenium nanoparticles as peroxidase-like nanozyme with supramolecular recognition properties for site-specific anchoring of biotinylated residues. Biosens. Bioelectron. 2020, 148, 111764. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Q.; Wang, R.; Yoon, S.; Ahn, J.; Yang, D.; Tian, J.; Li, J.; Zhou, Q. Multi-walled carbon nanotubes for the immobilization of enzyme in glucose biosensors. Electrochem. Commun. 2003, 5, 800–803. [Google Scholar] [CrossRef]
- Yang, N.; Chen, X.; Ren, T.; Zhang, P.; Yang, D. Carbon nanotube based biosensors. Sens. Actuators B Chem. 2015, 207, 690–715. [Google Scholar] [CrossRef]
- Tang, X.; Bansaruntip, S.; Nakayama, N.; Yenilmez, E.; lan Chang, Y.; Wang, Q. Carbon Nanotube DNA Sensor and Sensing Mechanism. Nano Lett. 2006, 6, 1632–1636. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Shi, M.; Zhao, L.; Zhao, S.; Hu, K.; Chen, Z.F.; Chen, J.; Liang, H. Carbon nanotube signal amplification for ultrasensitive fluorescence polarization detection of DNA methyltransferase activity and inhibition. Biosens. Bioelectron. 2014, 54, 285–291. [Google Scholar] [CrossRef]
- Dong, X.; Lu, X.; Zhang, K.; Zhang, Y. Chronocoulometric DNA biosensor based on a glassy carbon electrode modified with gold nanoparticles, poly(dopamine) and carbon nanotubes. Microchim. Acta 2012, 180, 101–108. [Google Scholar] [CrossRef]
- Baj-Rossi, C.; Micheli, G.D.; Carrara, S. Electrochemical Detection of Anti-Breast-Cancer Agents in Human Serum by Cytochrome P450-Coated Carbon Nanotubes. Sensors 2012, 12, 6520–6537. [Google Scholar] [CrossRef]
- Park, Y.K.; Bold, B.; Lee, W.K.; Jeon, M.H.; An, K.H.; Jeong, S.Y.; Shim, Y.K. d±-Galactose-Conjugated Single-Walled Carbon Nanotubes as New Chemical Probes for Electrochemical Biosensors for the Cancer Marker Galectin-3. Int. J. Mol. Sci. 2011, 12, 2946–2957. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.T.; Zhang, R.; Zou, L.; Zhu, J.J. A label-free cytosensor for the enhanced electrochemical detection of cancer cells using polydopamine-coated carbon nanotubes. Analyst 2012, 137, 1316–1318. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-Area Synthesis of High-Quality and Uniform Graphene Films on Copper Foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Si, Y.; Samulski, E.T. Synthesis of Water Soluble Graphene. Nano Lett. 2008, 8, 1679–1682. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Sun, X.; Liu, Z.; Welsher, K.; Robinson, J.T.; Goodwin, A.; Zaric, S.; Dai, H. Nano-graphene oxide for cellular imaging and drug delivery. Nano Res. 2008, 1, 203–212. [Google Scholar] [CrossRef] [Green Version]
- Yoo, J.M.; Kang, J.H.; Hong, B.H. Graphene-based nanomaterials for versatile imaging studies. Chem. Soc. Rev. 2015, 44, 4835–4852. [Google Scholar] [CrossRef]
- Yang, K.; Feng, L.; Shi, X.; Liu, Z. Nano-graphene in biomedicine: Theranostic applications. Chem. Soc. Rev. 2013, 42, 530–547. [Google Scholar] [CrossRef]
- Chen, M.L.; Liu, J.W.; Hu, B.; Chen, M.L.; Wang, J.H. Conjugation of quantum dots with graphene for fluorescence imaging of live cells. Analyst 2011, 136, 4277. [Google Scholar] [CrossRef]
- Yan, X.; Niu, G.; Lin, J.; Jin, A.J.; Hu, H.; Tang, Y.; Zhang, Y.; Wu, A.; Lu, J.; Zhang, S.; et al. Enhanced fluorescence imaging guided photodynamic therapy of sinoporphyrin sodium loaded graphene oxide. Biomaterials 2015, 42, 94–102. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Bai, L.; Shang, W.; Xie, W.; Ma, H.; Fu, Y.; Fang, D.; Sun, H.; Fan, L.; Han, M.; et al. Facile synthesis of water-soluble, highly fluorescent graphene quantum dots as a robust biological label for stem cells. J. Mater. Chem. 2012, 22, 7461. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, J.; Qiao, C.; Tang, S.; Li, Y.; Yuan, W.; Li, B.; Tian, L.; Liu, F.; Hu, R.; et al. Strongly green-photoluminescent graphene quantum dots for bioimaging applications. Chem. Commun. 2011, 47, 6858. [Google Scholar] [CrossRef]
- Pan, D.; Guo, L.; Zhang, J.; Xi, C.; Xue, Q.; Huang, H.; Li, J.; Zhang, Z.; Yu, W.; Chen, Z.; et al. Cutting sp2 clusters in graphene sheets into colloidal graphene quantum dots with strong green fluorescence. J. Mater. Chem. 2012, 22, 3314. [Google Scholar] [CrossRef]
- Peng, J.; Gao, W.; Gupta, B.K.; Liu, Z.; Romero-Aburto, R.; Ge, L.; Song, L.; Alemany, L.B.; Zhan, X.; Gao, G.; et al. Graphene Quantum Dots Derived from Carbon Fibers. Nano Lett. 2012, 12, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Chen, C.; Zheng, X.; Gao, L.; Cui, Z.; Yang, H.; Guo, C.; Chi, Y.; Li, C.M. One-step and high yield simultaneous preparation of single- and multi-layer graphene quantum dots from CX-72 carbon black. J. Mater. Chem. 2012, 22, 8764. [Google Scholar] [CrossRef]
- Cheng, Z.; Zaki, A.A.; Hui, J.Z.; Muzykantov, V.R.; Tsourkas, A. Multifunctional Nanoparticles: Cost Versus Benefit of Adding Targeting and Imaging Capabilities. Science 2012, 338, 903–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Wei, L.; Wang, J.; Peng, F.; Luo, D.; Cui, R.; Niu, Y.; Qin, X.; Liu, Y.; Sun, H.; et al. Cell imaging by graphene oxide based on surface enhanced Raman scattering. Nanoscale 2012, 4, 7084. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, Z.; Zhong, H.; Qin, X.; Wan, M.; Yang, B. Graphene oxide based surface-enhanced Raman scattering probes for cancer cell imaging. Phys. Chem. Chem. Phys. 2013, 15, 2961. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xue, L.; Zhu, Z.; Zhao, X.; Qian, J. Graphene oxide nanoparticles for two-photon fluorescence imaging of zebrafish. Opt. Quantum Electron. 2016, 48, 519. [Google Scholar] [CrossRef]
- Liu, Q.; Guo, B.; Rao, Z.; Zhang, B.; Gong, J.R. Strong Two-Photon-Induced Fluorescence from Photostable, Biocompatible Nitrogen-Doped Graphene Quantum Dots for Cellular and Deep-Tissue Imaging. Nano Lett. 2013, 13, 2436–2441. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, A.; Chavva, S.R.; Fan, Z.; Sinha, S.S.; Nellore, B.P.V.; Ray, P.C. Extremely High Two-Photon Absorbing Graphene Oxide for Imaging of Tumor Cells in the Second Biological Window. J. Phys. Chem. Lett. 2014, 5, 2150–2154. [Google Scholar] [CrossRef] [PubMed]
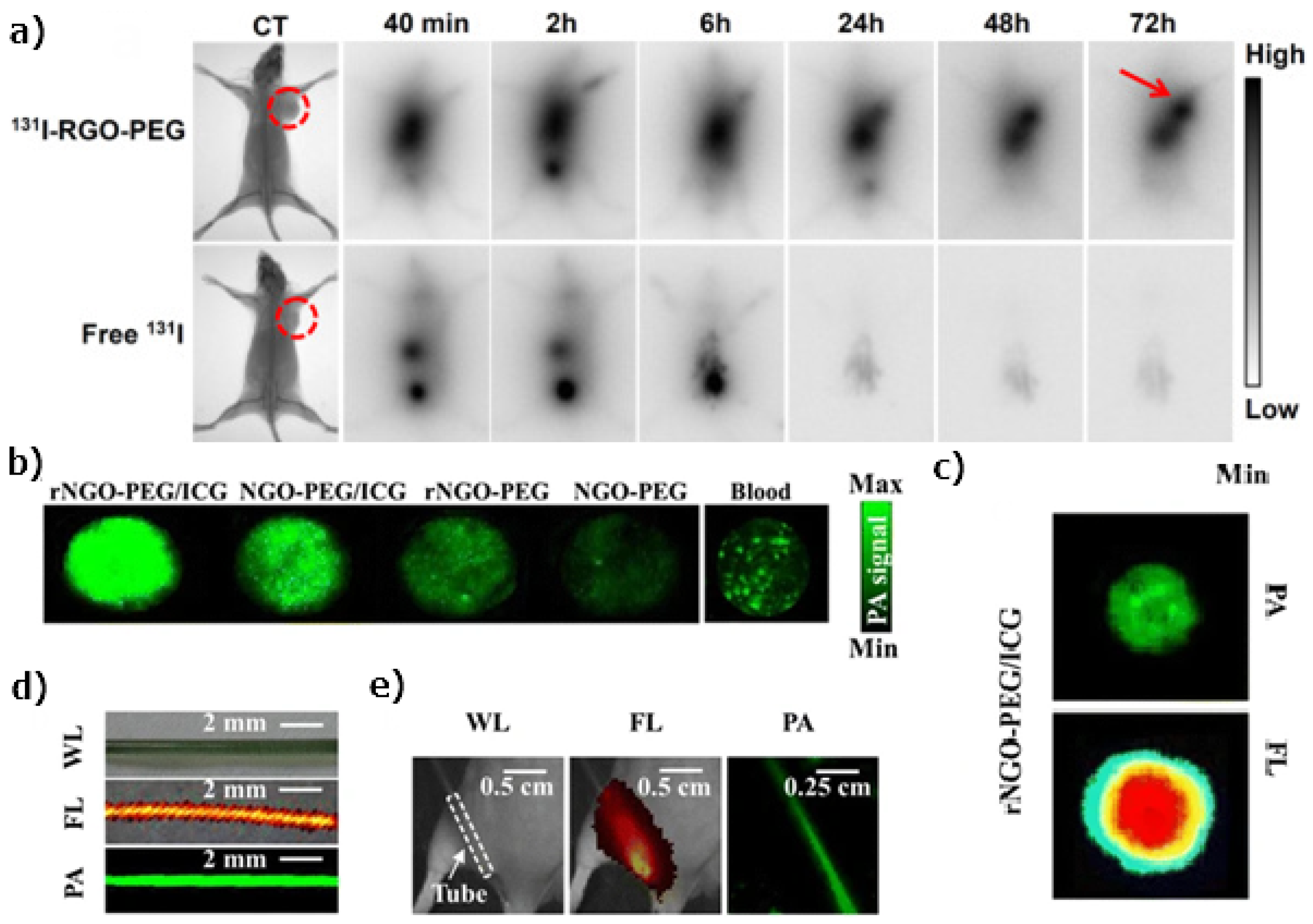
- Chen, L.; Zhong, X.; Yi, X.; Huang, M.; Ning, P.; Liu, T.; Ge, C.; Chai, Z.; Liu, Z.; Yang, K. Radionuclide 131I labeled reduced graphene oxide for nuclear imaging guided combined radio- and photothermal therapy of cancer. Biomaterials 2015, 66, 21–28. [Google Scholar] [CrossRef]
- Hong, H.; Zhang, Y.; Engle, J.W.; Nayak, T.R.; Theuer, C.P.; Nickles, R.J.; Barnhart, T.E.; Cai, W. In vivo targeting and positron emission tomography imaging of tumor vasculature with 66Ga-labeled nano-graphene. Biomaterials 2012, 33, 4147–4156. [Google Scholar] [CrossRef] [Green Version]
- Hong, H.; Yang, K.; Zhang, Y.; Engle, J.W.; Feng, L.; Yang, Y.; Nayak, T.R.; Goel, S.; Bean, J.; Theuer, C.P.; et al. In Vivo Targeting and Imaging of Tumor Vasculature with Radiolabeled, Antibody-Conjugated Nanographene. ACS Nano 2012, 6, 2361–2370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, S.; Yang, K.; Hong, H.; Chen, F.; Valdovinos, H.F.; Goel, S.; Barnhart, T.E.; Liu, Z.; Cai, W. VEGFR targeting leads to significantly enhanced tumor uptake of nanographene oxide in vivo. Biomaterials 2015, 39, 39–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fazaeli, Y.; Akhavan, O.; Rahighi, R.; Aboudzadeh, M.R.; Karimi, E.; Afarideh, H. In vivo SPECT imaging of tumors by 198, 199Au-labeled graphene oxide nanostructures. Mater. Sci. Eng. C 2014, 45, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, C.; Zeng, G.; You, Y.; Wang, H.; Gong, X.; Zheng, R.; Kim, J.; Kim, C.; Song, L. Indocyanine Green Loaded Reduced Graphene Oxide for In Vivo Photoacoustic/Fluorescence Dual-Modality Tumor Imaging. Nanoscale Res. Lett. 2016, 11, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Z.; Ma, T.; Zhao, E.; Docter, D.; Yang, W.; Stauber, R.H.; Gao, M. Small is Smarter: Nano MRI Contrast Agents—Advantages and Recent Achievements. Small 2015, 12, 556–576. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Sun, D.; Dai, Y.; Shen, J.; You, J.; Han, C.; Xu, K. Aptamer-Directed Specific Drug Delivery and Magnetic Resonance Imaging of Renal Carcinoma Cells In Vitro and In Vivo. J. Biomed. Nanotechnol. 2016, 12, 1604–1616. [Google Scholar] [CrossRef]
- Yang, K.; Hu, L.; Ma, X.; Ye, S.; Cheng, L.; Shi, X.; Li, C.; Li, Y.; Liu, Z. Multimodal Imaging Guided Photothermal Therapy using Functionalized Graphene Nanosheets Anchored with Magnetic Nanoparticles. Adv. Mater. 2012, 24, 1868–1872. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Huo, P.; Zhang, R.; Liu, B. Antibacterial Properties of Graphene-Based Nanomaterials. Nanomaterials 2019, 9, 737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salaheldin, T.A.; Loutfy, S.A.; Ramadan, M.A.; Youssef, T.; Mousa, S.A. IR-enhanced photothermal therapeutic effect of graphene magnetite nanocomposite on human liver cancer HepG2 cell model. Int. J. Nanomed. 2019, 14, 4397–4412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Y.; Bao, H.; Sahoo, N.G.; Wu, T.; Li, L. Water-Soluble Poly(N-isopropylacrylamide)–Graphene Sheets Synthesized via Click Chemistry for Drug Delivery. Adv. Funct. Mater. 2011, 21, 2754–2763. [Google Scholar] [CrossRef]
- Liu, Z.; Robinson, J.T.; Sun, X.; Dai, H. PEGylated Nanographene Oxide for Delivery of Water-Insoluble Cancer Drugs. J. Am. Chem. Soc. 2008, 130, 10876–10877. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Xia, J.; Zhao, Q.; Liu, L.; Zhang, Z. Functional Graphene Oxide as a Nanocarrier for Controlled Loading and Targeted Delivery of Mixed Anticancer Drugs. Small 2010, 6, 537–544. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, X.; Liu, Z.; Ma, Y.; Huang, Y.; Chen, Y. High-Efficiency Loading and Controlled Release of Doxorubicin Hydrochloride on Graphene Oxide. J. Phys. Chem. C 2008, 112, 17554–17558. [Google Scholar] [CrossRef]
- Fan, X.; Jiao, G.; Gao, L.; Jin, P.; Li, X. The preparation and drug delivery of a graphene–carbon nanotube–Fe3O4 nanoparticle hybrid. J. Mater. Chem. B 2013, 1, 2658. [Google Scholar] [CrossRef]
- Chen, M.L.; He, Y.J.; Chen, X.W.; Wang, J.H. Quantum-Dot-Conjugated Graphene as a Probe for Simultaneous Cancer-Targeted Fluorescent Imaging, Tracking, and Monitoring Drug Delivery. Bioconjug. Chem. 2013, 24, 387–397. [Google Scholar] [CrossRef]
- Mauri, E.; Salvati, A.; Cataldo, A.; Mozetic, P.; Basoli, F.; Abbruzzese, F.; Trombetta, M.; Bellucci, S.; Rainer, A. Graphene-laden hydrogels: A strategy for thermally triggered drug delivery. Mater. Sci. Eng. C 2021, 118, 111353. [Google Scholar] [CrossRef]
- Miao, W.; Shim, G.; Lee, S.; Lee, S.; Choe, Y.S.; Oh, Y.K. Safety and tumor tissue accumulation of pegylated graphene oxide nanosheets for co-delivery of anticancer drug and photosensitizer. Biomaterials 2013, 34, 3402–3410. [Google Scholar] [CrossRef]
- Volarevic, V.; Paunovic, V.; Markovic, Z.; Markovic, B.S.; Misirkic-Marjanovic, M.; Todorovic-Markovic, B.; Bojic, S.; Vucicevic, L.; Jovanovic, S.; Arsenijevic, N.; et al. Large Graphene Quantum Dots Alleviate Immune-Mediated Liver Damage. ACS Nano 2014, 8, 12098–12109. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, F.; Han, H.; Yang, L.; Zhang, G.; Fan, Z. Functionalized graphene oxide as a drug carrier for loading pirfenidone in treatment of subarachnoid hemorrhage. Colloids Surfaces B Biointerfaces 2015, 129, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Iannazzo, D.; Pistone, A.; Ferro, S.; Luca, L.D.; Monforte, A.M.; Romeo, R.; Buemi, M.R.; Pannecouque, C. Graphene Quantum Dots Based Systems As HIV Inhibitors. Bioconjug. Chem. 2018, 29, 3084–3093. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Veerapandian, M.; Zhang, L.H.; Yun, K.; Kim, S.J. Antibacterial Efficiency of Graphene Nanosheets against Pathogenic Bacteria via Lipid Peroxidation. J. Phys. Chem. C 2012, 116, 17280–17287. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Daye, A.A.; Eppakayala, V.; hoi Kim, J. Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa. Int. J. Nanomed. 2012, 7, 5901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hui, L.; Piao, J.G.; Auletta, J.; Hu, K.; Zhu, Y.; Meyer, T.; Liu, H.; Yang, L. Availability of the Basal Planes of Graphene Oxide Determines Whether It Is Antibacterial. ACS Appl. Mater. Interfaces 2014, 6, 13183–13190. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Ma, R.; Gao, M.; Tian, X.; Li, Y.Q.; Zeng, L.; Li, R. Antibacterial applications of graphene oxides: Structure-activity relationships, molecular initiating events and biosafety. Sci. Bull. 2018, 63, 133–142. [Google Scholar] [CrossRef]
- Scaffaro, R.; Botta, L.; Maio, A.; Gallo, G. PLA graphene nanoplatelets nanocomposites: Physical properties and release kinetics of an antimicrobial agent. Compos. Part B Eng. 2017, 109, 138–146. [Google Scholar] [CrossRef]
- Xu, L.Q.; Liao, Y.B.; Li, N.N.; Li, Y.J.; Zhang, J.Y.; Wang, Y.B.; Hu, X.F.; Li, C.M. Vancomycin-assisted green synthesis of reduced graphene oxide for antimicrobial applications. J. Colloid Interface Sci. 2018, 514, 733–739. [Google Scholar] [CrossRef]
- Huang, Y.P.; Hung, C.M.; Hsu, Y.C.; Zhong, C.Y.; Wang, W.R.; Chang, C.C.; Lee, M.J. Suppression of Breast Cancer Cell Migration by Small Interfering RNA Delivered by Polyethylenimine-Functionalized Graphene Oxide. Nanoscale Res. Lett. 2016, 11, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Zhang, B.; Zhou, L.; Shi, Y.; Li, Z.; Xia, Y.; Tian, J. Imaging Dendrimer-Grafted Graphene Oxide Mediated Anti-miR-21 Delivery With an Activatable Luciferase Reporter. ACS Appl. Mater. Interfaces 2016, 8, 9014–9021. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Liu, M.; Zhang, L.; Huang, J.; Yao, J.; Zhang, Z. Polyethylenimine-functionalized graphene oxide as an efficient gene delivery vector. J. Mater. Chem. 2011, 21, 7736. [Google Scholar] [CrossRef]
- Mo, R.; Jiang, T.; Sun, W.; Gu, Z. ATP-responsive DNA-graphene hybrid nanoaggregates for anticancer drug delivery. Biomaterials 2015, 50, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Lai, W.F.; Wong, W.T. Use of graphene-based materials as carriers of bioactive agents. Asian J. Pharm. Sci. 2020, 13. [Google Scholar] [CrossRef]
- Song, Y.; Qu, K.; Zhao, C.; Ren, J.; Qu, X. Graphene Oxide: Intrinsic Peroxidase Catalytic Activity and Its Application to Glucose Detection. Adv. Mater. 2010, 22, 2206–2210. [Google Scholar] [CrossRef]
- Wang, K.; Liu, Q.; Guan, Q.M.; Wu, J.; Li, H.N.; Yan, J.J. Enhanced direct electrochemistry of glucose oxidase and biosensing for glucose via synergy effect of graphene and CdS nanocrystals. Biosens. Bioelectron. 2011, 26, 2252–2257. [Google Scholar] [CrossRef]
- Peña-Bahamonde, J.; Nguyen, H.N.; Fanourakis, S.K.; Rodrigues, D.F. Recent advances in graphene-based biosensor technology with applications in life sciences. J. Nanobiotechnol. 2018, 16, 75. [Google Scholar] [CrossRef] [Green Version]
- Shan, C.; Yang, H.; Han, D.; Zhang, Q.; Ivaska, A.; Niu, L. Electrochemical determination of NADH and ethanol based on ionic liquid-functionalized graphene. Biosens. Bioelectron. 2010, 25, 1504–1508. [Google Scholar] [CrossRef]
- Zhou, M.; Zhai, Y.; Dong, S. Electrochemical Sensing and Biosensing Platform Based on Chemically Reduced Graphene Oxide. Anal. Chem. 2009, 81, 5603–5613. [Google Scholar] [CrossRef]
- Lim, C.X.; Hoh, H.Y.; Ang, P.K.; Loh, K.P. Direct Voltammetric Detection of DNA and pH Sensing on Epitaxial Graphene: An Insight into the Role of Oxygenated Defects. Anal. Chem. 2010, 82, 7387–7393. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, G.; Zhu, L.; Li, G. Graphene quantum dots-based platform for the fabrication of electrochemical biosensors. Electrochem. Commun. 2011, 13, 31–33. [Google Scholar] [CrossRef]
- Xu, H.; Dai, H.; Chen, G. Direct electrochemistry and electrocatalysis of hemoglobin protein entrapped in graphene and chitosan composite film. Talanta 2010, 81, 334–338. [Google Scholar] [CrossRef]
- Luo, J.; Jiang, S.; Liu, X. Electrochemical sensor for bovine hemoglobin based on a novel graphene-molecular imprinted polymers composite as recognition element. Sens. Actuators B Chem. 2014, 203, 782–789. [Google Scholar] [CrossRef]
- Dey, R.S.; Raj, C.R. Development of an Amperometric Cholesterol Biosensor Based on Graphene-Pt Nanoparticle Hybrid Material. J. Phys. Chem. C 2010, 114, 21427–21433. [Google Scholar] [CrossRef]
- Lu, C.H.; Yang, H.H.; Zhu, C.L.; Chen, X.; Chen, G.N. A Graphene Platform for Sensing Biomolecules. Angew. Chem. Int. Ed. 2009, 48, 4785–4787. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Y.; Zhao, D.; Zeng, D.; Xia, J.; Aldalbahi, A.; Wang, C.; San, L.; Fan, C.; Zuo, X.; et al. Universal Fluorescence Biosensor Platform Based on Graphene Quantum Dots and Pyrene-Functionalized Molecular Beacons for Detection of MicroRNAs. ACS Appl. Mater. Interfaces 2015, 7, 16152–16156. [Google Scholar] [CrossRef]
- Lu, C.H.; Zhu, C.L.; Li, J.; Liu, J.J.; Chen, X.; Yang, H.H. Using graphene to protect DNA from cleavage during cellular delivery. Chem. Commun. 2010, 46, 3116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.L.; Yan, X.P.; Meng, K.; Wang, S.F. Graphene Oxide Based Photoinduced Charge Transfer Label-Free Near-Infrared Fluorescent Biosensor for Dopamine. Anal. Chem. 2011, 83, 8787–8793. [Google Scholar] [CrossRef]
- Nguyen, P.D.; Cong, V.T.; Baek, C.; Min, J. Fabrication of peptide stabilized fluorescent gold nanocluster/graphene oxide nanocomplex and its application in turn-on detection of metalloproteinase-9. Biosens. Bioelectron. 2017, 89, 666–672. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, C.; Han, L.; Jin, L.; Zhou, M.; Dong, S. Label-free, regenerative and sensitive surface plasmon resonance and electrochemical aptasensors based on graphene. Chem. Commun. 2011, 47, 7794. [Google Scholar] [CrossRef]
- Vasilescu, A.; Gáspár, S.; Gheorghiu, M.; David, S.; Dinca, V.; Peteu, S.; Wang, Q.; Li, M.; Boukherroub, R.; Szunerits, S. Surface Plasmon Resonance based sensing of lysozyme in serum on Micrococcus lysodeikticus-modified graphene oxide surfaces. Biosens. Bioelectron. 2017, 89, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, Q.; Yang, X.; Wang, K.; Zhang, H.; Nie, W. High sensitivity surface plasmon resonance biosensor for detection of microRNA and small molecule based on graphene oxide-gold nanoparticles composites. Talanta 2017, 174, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Hwang, E.Y.; Choo, J.; Lim, D.W. PEGylated nanographene-mediated metallic nanoparticle clusters for surface enhanced Raman scattering-based biosensing. Analyst 2018, 143, 2604–2615. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Wang, Y.; Liu, Y.; Liu, H.; Fu, L.; Wen, J.; Li, J.; Wei, P.; Chen, L. A graphene oxide/gold nanoparticle-based amplification method for SERS immunoassay of cardiac troponin I. Analyst 2019, 144, 1582–1589. [Google Scholar] [CrossRef] [PubMed]
- Demeritte, T.; Nellore, B.P.V.; Kanchanapally, R.; Sinha, S.S.; Pramanik, A.; Chavva, S.R.; Ray, P.C. Hybrid Graphene Oxide Based Plasmonic-Magnetic Multifunctional Nanoplatform for Selective Separation and Label-Free Identification of Alzheimer’s Disease Biomarkers. ACS Appl. Mater. Interfaces 2015, 7, 13693–13700. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Liang, R.P.; Bai, J.M.; Qiu, J.D. Using Graphene Quantum Dots as Photoluminescent Probes for Protein Kinase Sensing. Anal. Chem. 2013, 85, 9148–9155. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, X.; Sun, J.; Jiao, S.; Chen, H.; Gao, F.; Wang, L. Fluorescent blood glucose monitor by hemin-functionalized graphene quantum dots based sensing system. Anal. Chim. Acta 2014, 810, 71–78. [Google Scholar] [CrossRef]
- Zor, E.; Morales-Narváez, E.; Zamora-Gálvez, A.; Bingol, H.; Ersoz, M.; Merkoçi, A. Graphene Quantum Dots-based Photoluminescent Sensor: A Multifunctional Composite for Pesticide Detection. ACS Appl. Mater. Interfaces 2015, 7, 20272–20279. [Google Scholar] [CrossRef]
- Dong, X.; Shi, Y.; Huang, W.; Chen, P.; Li, L.J. Electrical Detection of DNA Hybridization with Single-Base Specificity Using Transistors Based on CVD-Grown Graphene Sheets. Adv. Mater. 2010, 22, 1649–1653. [Google Scholar] [CrossRef]
- Stine, R.; Robinson, J.T.; Sheehan, P.E.; Tamanaha, C.R. Real-Time DNA Detection Using Reduced Graphene Oxide Field Effect Transistors. Adv. Mater. 2010, 22, 5297–5300. [Google Scholar] [CrossRef]
- Danielson, E.; Sontakke, V.A.; Porkovich, A.J.; Wang, Z.; Kumar, P.; Ziadi, Z.; Yokobayashi, Y.; Sowwan, M. Graphene based field-effect transistor biosensors functionalized using gas-phase synthesized gold nanoparticles. Sens. Actuators B Chem. 2020, 320, 128432. [Google Scholar] [CrossRef]
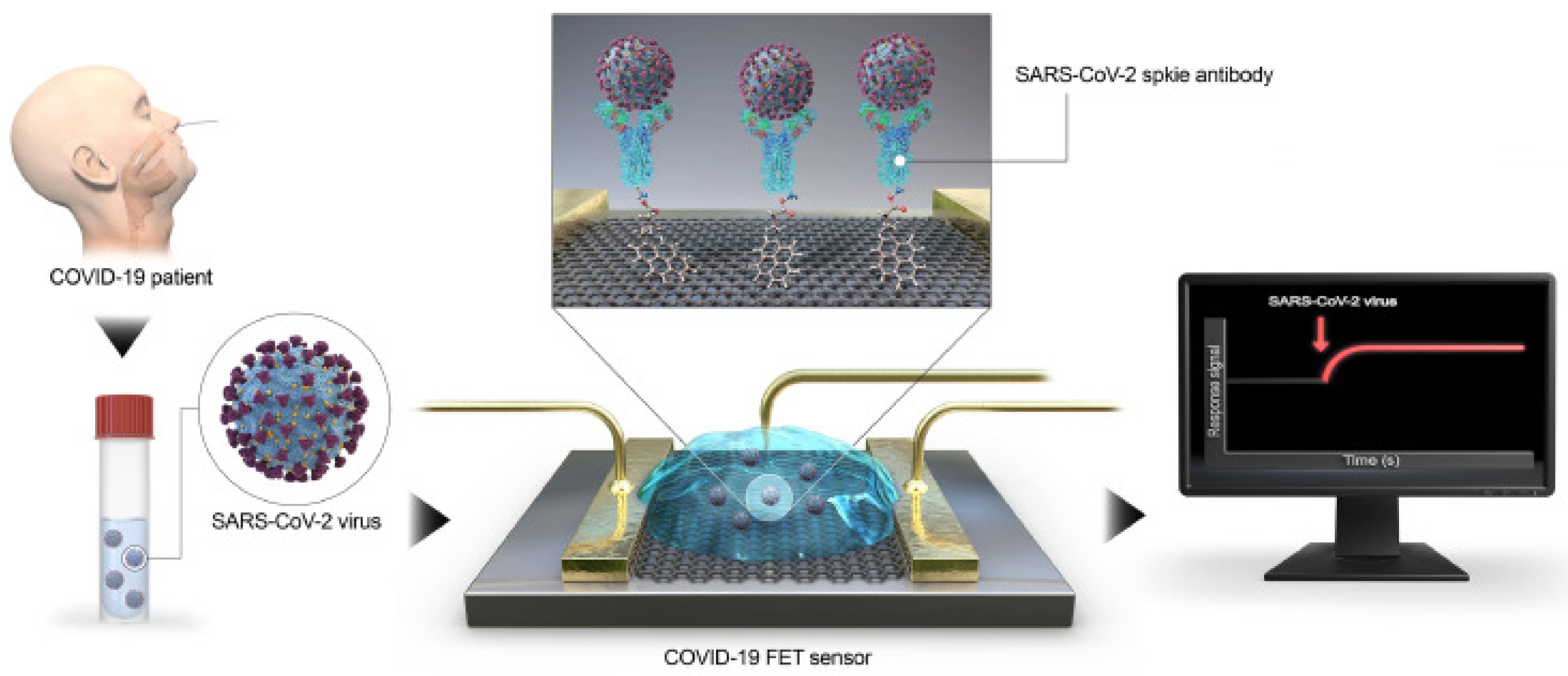
- Seo, G.; Lee, G.; Kim, M.J.; Baek, S.H.; Choi, M.; Ku, K.B.; Lee, C.S.; Jun, S.; Park, D.; Kim, H.G.; et al. Rapid Detection of COVID-19 Causative Virus (SARS-CoV-2) in Human Nasopharyngeal Swab Specimens Using Field-Effect Transistor-Based Biosensor. ACS Nano 2020, 14, 5135–5142. [Google Scholar] [CrossRef] [Green Version]
- Sengupta, J.; Hussain, C.M. Graphene-based field-effect transistor biosensors for the rapid detection and analysis of viruses: A perspective in view of COVID-19. Carbon Trends 2021, 2, 100011. [Google Scholar] [CrossRef]
- Gizzatov, A.; Keshishian, V.; Guven, A.; Dimiev, A.M.; Qu, F.; Muthupillai, R.; Decuzzi, P.; Bryant, R.G.; Tour, J.M.; Wilson, L.J. Enhanced MRI relaxivity of aquated Gd3+ ions by carboxyphenylated water-dispersed graphene nanoribbons. Nanoscale 2014, 6, 3059–3063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.; Tao, H.; Yang, K.; Feng, L.; Cheng, L.; Shi, X.; Li, Y.; Guo, L.; Liu, Z. A functionalized graphene oxide-iron oxide nanocomposite for magnetically targeted drug delivery, photothermal therapy, and magnetic resonance imaging. Nano Res. 2012, 5, 199–212. [Google Scholar] [CrossRef]
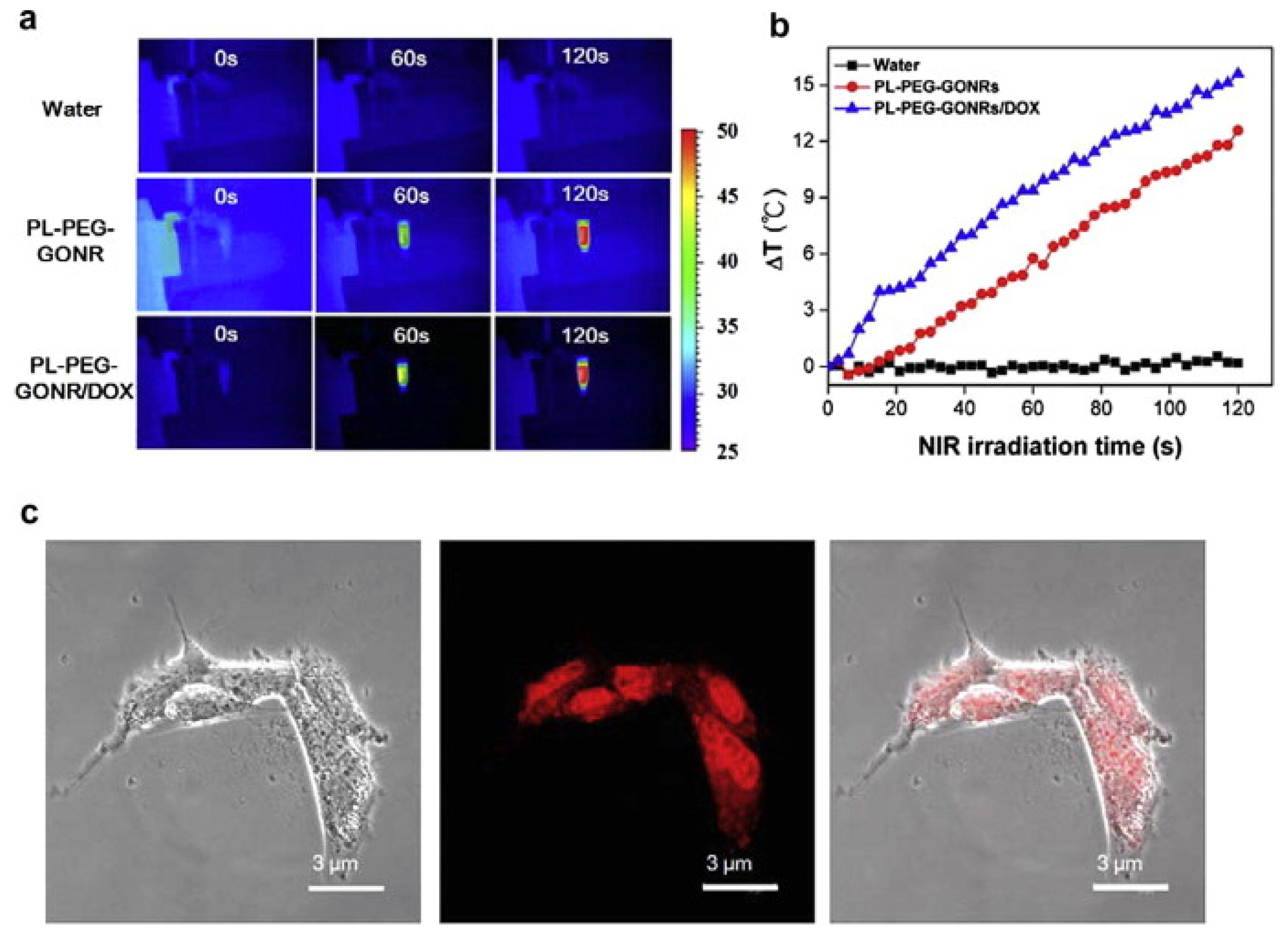
- Akhavan, O.; Ghaderi, E.; Emamy, H. Nontoxic concentrations of PEGylated graphene nanoribbons for selective cancer cell imaging and photothermal therapy. J. Mater. Chem. 2012, 22, 20626. [Google Scholar] [CrossRef]
- Zhao, H.; Ding, R.; Zhao, X.; Li, Y.; Qu, L.; Pei, H.; Yildirimer, L.; Wu, Z.; Zhang, W. Graphene-based nanomaterials for drug and/or gene delivery, bioimaging, and tissue engineering. Drug Discov. Today 2017, 22, 1302–1317. [Google Scholar] [CrossRef] [PubMed]
- Lalwani, G.; Cai, X.; Nie, L.; Wang, L.V.; Sitharaman, B. Graphene-based contrast agents for photoacoustic and thermoacoustic tomography. Photoacoustics 2013, 1, 62–67. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, S.M.; Surhland, C.; Sanchez, Z.; Chaudhary, P.; Kumar, M.S.; Lee, S.; Peña, L.A.; Waring, M.; Sitharaman, B.; Naidu, M. Graphene nanoribbons as a drug delivery agent for lucanthone mediated therapy of glioblastoma multiforme. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.J.; Lin, C.W.; Yang, H.W.; Lin, K.J.; Wey, S.P.; Sun, C.L.; Wei, K.C.; Yen, T.C.; Lin, C.I.; Ma, C.C.M.; et al. Biodistribution of PEGylated graphene oxide nanoribbons and their application in cancer chemo-photothermal therapy. Carbon 2014, 74, 83–95. [Google Scholar] [CrossRef]
- Foreman, H.C.C.; Lalwani, G.; Kalra, J.; Krug, L.T.; Sitharaman, B. Gene delivery to mammalian cells using a graphene nanoribbon platform. J. Mater. Chem. B 2017, 5, 2347–2354. [Google Scholar] [CrossRef]
- Youn, H.C.; Bak, S.M.; Kim, M.S.; Jaye, C.; Fischer, D.A.; Lee, C.W.; Yang, X.Q.; Roh, K.C.; Kim, K.B. High-Surface-Area Nitrogen-Doped Reduced Graphene Oxide for Electric Double-Layer Capacitors. ChemSusChem 2015, 8, 1875–1884. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Soroshnia, S.; Hashemi, S.A.; Babapoor, A.; Ghasemi, Y.; Savardashtaki, A.; Amani, A.M. Graphene nano-ribbon based high potential and efficiency for DNA, cancer therapy and drug delivery applications. Drug Metab. Rev. 2019, 51, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Valentini, F.; Calcaterra, A.; Ruggiero, V.; Pichichero, E.; Martino, A.; Iosi, F.; Bertuccini, L.; Antonaroli, S.; Mardente, S.; Zicari, A.; et al. Functionalized Graphene Derivatives: Antibacterial Properties and Cytotoxicity. J. Nanomater. 2019, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Peng, C.; Luo, W.; Lv, M.; Li, X.; Li, D.; Huang, Q.; Fan, C. Graphene-Based Antibacterial Paper. ACS Nano 2010, 4, 4317–4323. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Li, Y.; Lin, H.; Guo, B.; Du, Y.; Li, X.; Jia, H.; Zhao, X.; Tang, J.; Zhang, L. Functional graphene oxide as a plasmid-based Stat3 siRNA carrier inhibits mouse malignant melanoma growth in vivo. Nanotechnology 2013, 24, 105102. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, A.B.M.; Leszczynska, D. Electrochemically Prepared Unzipped Single Walled Carbon Nanotubes-MnO2 Nanostructure Composites for Hydrogen Peroxide and Glucose Sensing. Chemosensors 2019, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Jothi, L.; Jayakumar, N.; Jaganathan, S.; Nageswaran, G. Ultrasensitive and selective non-enzymatic electrochemical glucose sensor based on hybrid material of graphene nanosheets/graphene nanoribbons/nickel nanoparticle. Mater. Res. Bull. 2018, 98, 300–307. [Google Scholar] [CrossRef]
- Feng, Q.; Zhao, X.; Guo, Y.; Liu, M.; Wang, P. Stochastic DNA walker for electrochemical biosensing sensitized with gold nanocages@graphene nanoribbons. Biosens. Bioelectron. 2018, 108, 97–102. [Google Scholar] [CrossRef]
- Rastgoo, M.; Fathipour, M. Interaction of DNA nucleobases with boron, nitrogen, and sulfur doped graphene nano-ribbon for sequencing: An Ab initio study. Appl. Surf. Sci. 2019, 492, 634–643. [Google Scholar] [CrossRef]
- Lavanya, J.; Gomathi, N. High-sensitivity ascorbic acid sensor using graphene sheet/graphene nanoribbon hybrid material as an enhanced electrochemical sensing platform. Talanta 2015, 144, 655–661. [Google Scholar] [CrossRef]
- Vlăsceanu, G.M.; Amărandi, R.M.; Ioniță, M.; Tite, T.; Iovu, H.; Pilan, L.; Burns, J.S. Versatile graphene biosensors for enhancing human cell therapy. Biosens. Bioelectron. 2018, 117, 283–302. [Google Scholar] [CrossRef] [PubMed]
- Rostami, S.; Mehdinia, A.; Niroumand, R.; Jabbari, A. Enhanced LSPR performance of graphene nanoribbons-silver nanoparticles hybrid as a colorimetric sensor for sequential detection of dopamine and glutathione. Anal. Chim. Acta 2020, 1120, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Wen, G.; Jing, Q.; Liang, A.; Jiang, Z. A new SERS strategy for quantitative analysis of trace microalbuminuria based on immunorecognition and graphene oxide nanoribbon catalysis. Int. J. Nanomed. 2018, 13, 6099–6107. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Zhu, M.; Liu, Q.; Guo, Y.; Wang, H.; Wang, K. Fabricating photoelectrochemical aptasensor for sensitive detection of aflatoxin B1 with visible-light-driven BiOBr/nitrogen-doped graphene nanoribbons. J. Electroanal. Chem. 2019, 840, 67–73. [Google Scholar] [CrossRef]
- Jothi, L.; Jaganathan, S.K.; Nageswaran, G. An electrodeposited Au nanoparticle/porous graphene nanoribbon composite for electrochemical detection of alpha-fetoprotein. Mater. Chem. Phys. 2020, 242, 122514. [Google Scholar] [CrossRef]
- Huan, J.; Liu, Q.; Fei, A.; Qian, J.; Dong, X.; Qiu, B.; Mao, H.; Wang, K. Amplified solid-state electrochemiluminescence detection of cholesterol in near-infrared range based on CdTe quantum dots decorated multiwalled carbon nanotubes@reduced graphene oxide nanoribbons. Biosens. Bioelectron. 2015, 73, 221–227. [Google Scholar] [CrossRef]
- Dong, X.; Long, Q.; Wang, J.; Chan-Park, M.B.; Huang, Y.; Huang, W.; Chen, P. A graphene nanoribbon network and its biosensing application. Nanoscale 2011, 3, 5156. [Google Scholar] [CrossRef]
- Nikitin, M.; Gabbasov, R.; Cherepanov, V.; Chuev, M.; Polikarpov, M.; Panchenko, V.; Deyev, S. Magnetic Nanoparticle Degradation in vivo Studied by Mossbauer Spectroscopy. AIP Conf. Proc. 2010, 401–408. [Google Scholar] [CrossRef]
- Zelepukin, I.V.; Yaremenko, A.V.; Ivanov, I.N.; Yuryev, M.V.; Cherkasov, V.R.; Deyev, S.M.; Nikitin, P.I.; Nikitin, M.P. Long-Term Fate of Magnetic Particles in Mice: A Comprehensive Study. ACS Nano 2021, 15, 11341–11357. [Google Scholar] [CrossRef] [PubMed]
- Chuev, M.A.; Cherepanov, V.M.; Deyev, S.M.; Mischenko, I.N.; Nikitin, M.P.; Polikarpov, M.A.; Panchenko, V.Y. Interpretation of the Mossbauer Spectra of the Magnetic Nanoparticles in Mouse Spleen. AIP Conf. Proc. 2010, 1311, 322. [Google Scholar] [CrossRef]
- Aldieri, E.; Fenoglio, I.; Cesano, F.; Gazzano, E.; Gulino, G.; Scarano, D.; Attanasio, A.; Mazzucco, G.; Ghigo, D.; Fubini, B. The Role of Iron Impurities in the Toxic Effects Exerted by Short Multiwalled Carbon Nanotubes (MWCNT) in Murine Alveolar Macrophages. J. Toxicol. Environ. Health Part A 2013, 76, 1056–1071. [Google Scholar] [CrossRef] [PubMed]
- Pulskamp, K.; Diabate, S.; Krug, H. Carbon nanotubes show no sign of acute toxicity but induce intracellular reactive oxygen species in dependence on contaminants. Toxicol. Lett. 2007, 168, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Smart, S.; Cassady, A.; Lu, G.; Martin, D. The biocompatibility of carbon nanotubes. Carbon 2006, 44, 1034–1047. [Google Scholar] [CrossRef]
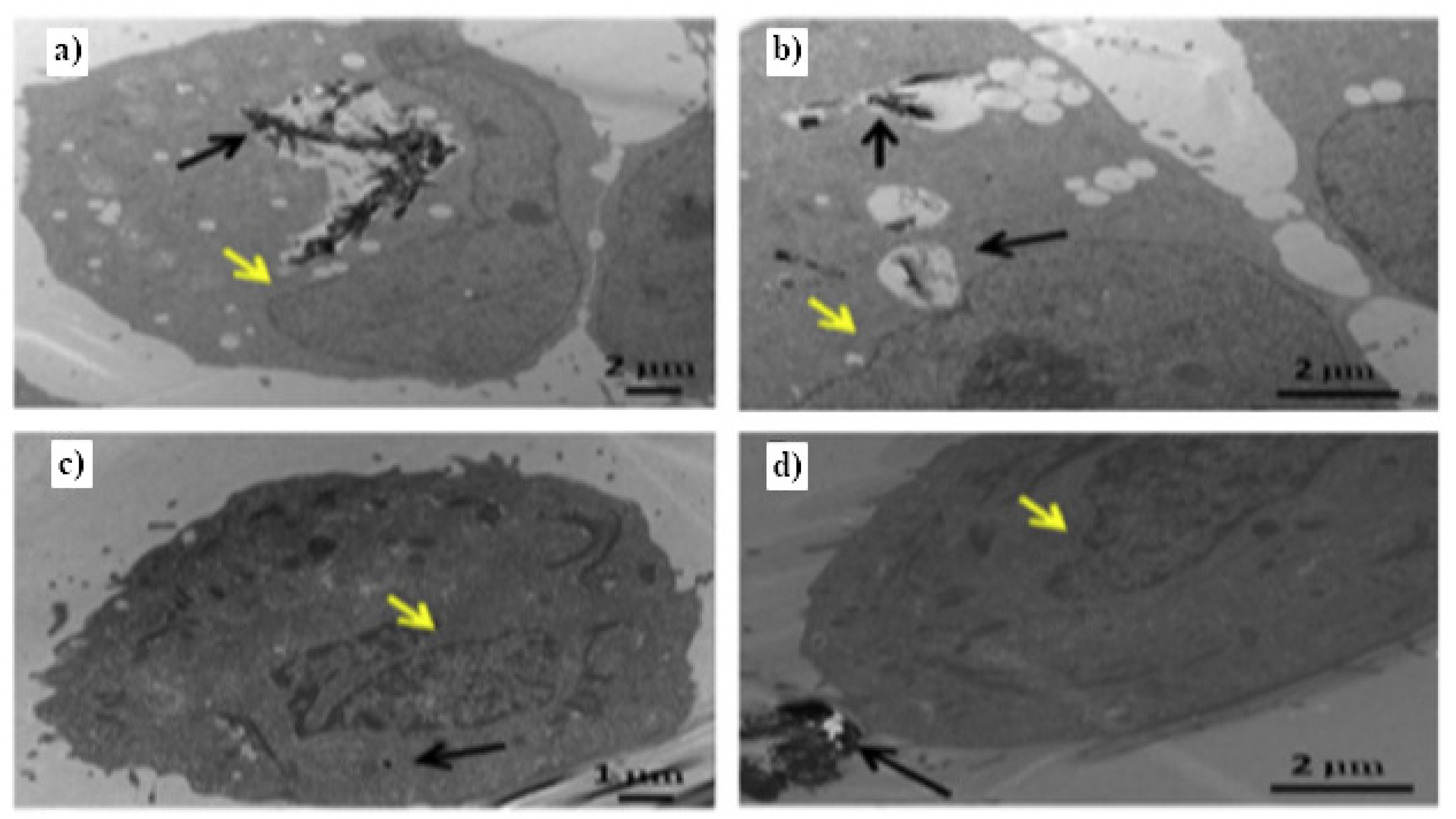
- Kolosnjaj-Tabi, J.; Hartman, K.B.; Boudjemaa, S.; Ananta, J.S.; Morgant, G.; Szwarc, H.; Wilson, L.J.; Moussa, F. In Vivo Behavior of Large Doses of Ultrashort and Full-Length Single-Walled Carbon Nanotubes after Oral and Intraperitoneal Administration to Swiss Mice. ACS Nano 2010, 4, 1481–1492. [Google Scholar] [CrossRef]
- Poland, C.A.; Duffin, R.; Kinloch, I.; Maynard, A.; Wallace, W.A.H.; Seaton, A.; Stone, V.; Brown, S.; MacNee, W.; Donaldson, K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat. Nanotechnol. 2008, 3, 423–428. [Google Scholar] [CrossRef]
- Monteiro-Riviere, N.A.; Inman, A.O.; Wang, Y.Y.; Nemanich, R.J. Surfactant effects on carbon nanotube interactions with human keratinocytes. Nanomed. Nanotechnol. Biol. Med. 2005, 1, 293–299. [Google Scholar] [CrossRef]
- Shvedova, A.; Castranova, V.; Kisin, E.; Schwegler-Berry, D.; Murray, A.; Gandelsman, V.; Maynard, A.; Baron, P. Exposure to Carbon Nanotube Material: Assessment of Nanotube Cytotoxicity using Human Keratinocyte Cells. J. Toxicol. Environ. Health Part A 2003, 66, 1909–1926. [Google Scholar] [CrossRef]
- Reddy, A.R.N.; Krishna, D.; Himabindu, V.; Reddy, Y.N. Single walled carbon nanotubes induce cytotoxicity and oxidative stress in HEK293 cells. Toxicol. Environ. Chem. 2014, 96, 931–940. [Google Scholar] [CrossRef]
- Tahara, Y.; Nakamura, M.; Yang, M.; Zhang, M.; Iijima, S.; Yudasaka, M. Lysosomal membrane destabilization induced by high accumulation of single-walled carbon nanohorns in murine macrophage RAW 264.7. Biomaterials 2012, 33, 2762–2769. [Google Scholar] [CrossRef]
- Zhang, Y.; Petibone, D.; Xu, Y.; Mahmood, M.; Karmakar, A.; Casciano, D.; Ali, S.; Biris, A.S. Toxicity and efficacy of carbon nanotubes and graphene: The utility of carbon-based nanoparticles in nanomedicine. Drug Metab. Rev. 2014, 46, 232–246. [Google Scholar] [CrossRef]
- An, S.S.; Wu, S.Y.; Hulme, J. Current applications of graphene oxide in nanomedicine. Int. J. Nanomed. 2015, 10, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.; Yang, S.T.; Liu, J.H.; Dong, E.; Wang, Y.; Cao, A.; Liu, Y.; Wang, H. In vitro toxicity evaluation of graphene oxide on A549 cells. Toxicol. Lett. 2011, 200, 201–210. [Google Scholar] [CrossRef]
- Chong, Y.; Ma, Y.; Shen, H.; Tu, X.; Zhou, X.; Xu, J.; Dai, J.; Fan, S.; Zhang, Z. The in vitro and in vivo toxicity of graphene quantum dots. Biomaterials 2014, 35, 5041–5048. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, A.; Panchakarla, L.S.; Chandran, P.; Menon, D.; Nair, S.; Rao, C.N.R.; Koyakutty, M. Differential nano-bio interactions and toxicity effects of pristine versus functionalized graphene. Nanoscale 2011, 3, 2461. [Google Scholar] [CrossRef]
- Liao, K.H.; Lin, Y.S.; Macosko, C.W.; Haynes, C.L. Cytotoxicity of Graphene Oxide and Graphene in Human Erythrocytes and Skin Fibroblasts. ACS Appl. Mater. Interfaces 2011, 3, 2607–2615. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ruan, J.; Song, H.; Zhang, J.; Wo, Y.; Guo, S.; Cui, D. Biocompatibility of Graphene Oxide. Nanoscale Res. Lett. 2010, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Marzi, L.D.; Ottaviano, L.; Perrozzi, F.; Nardone, M.; Santucci, S.; de Lapuente, J.; Borrás, M.; Treossi, E.; Palermo, V.; Poma, A.M.G. Flake size-dependent cyto and genotoxic evaluation of graphene oxide on in vitro A549, CaCo2 and vero cell lines. J. Biol. Regul. Homeost. Agents 2014, 28 2, 281–289. [Google Scholar]
- Chatterjee, N.; Yang, J.; Choi, J. Differential genotoxic and epigenotoxic effects of graphene family nanomaterials (GFNs) in human bronchial epithelial cells. Mutat. Res. Toxicol. Environ. Mutagen. 2016, 798–799, 1–10. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burdanova, M.G.; Kharlamova, M.V.; Kramberger, C.; Nikitin, M.P. Applications of Pristine and Functionalized Carbon Nanotubes, Graphene, and Graphene Nanoribbons in Biomedicine. Nanomaterials 2021, 11, 3020. https://doi.org/10.3390/nano11113020
Burdanova MG, Kharlamova MV, Kramberger C, Nikitin MP. Applications of Pristine and Functionalized Carbon Nanotubes, Graphene, and Graphene Nanoribbons in Biomedicine. Nanomaterials. 2021; 11(11):3020. https://doi.org/10.3390/nano11113020
Chicago/Turabian StyleBurdanova, Maria G., Marianna V. Kharlamova, Christian Kramberger, and Maxim P. Nikitin. 2021. "Applications of Pristine and Functionalized Carbon Nanotubes, Graphene, and Graphene Nanoribbons in Biomedicine" Nanomaterials 11, no. 11: 3020. https://doi.org/10.3390/nano11113020
APA StyleBurdanova, M. G., Kharlamova, M. V., Kramberger, C., & Nikitin, M. P. (2021). Applications of Pristine and Functionalized Carbon Nanotubes, Graphene, and Graphene Nanoribbons in Biomedicine. Nanomaterials, 11(11), 3020. https://doi.org/10.3390/nano11113020






