Phase Transformations from Nanocrystalline to Amorphous (Zr70Ni25Al5)100-xWx (x; 0, 2, 10, 20, 35 at. %) and Subsequent Consolidation
Abstract
1. Introduction
2. Materials and Methods
2.1. Feedstock Materials
2.2. Sample Preparations
2.2.1. Zr70Ni25Al5 Ternary System
2.2.2. Multicomponent (Zr70Ni25Al5)100-xWx(x; 2, 5, 10, 20, 35 at. %) Systems
2.3. Powder Consolidation
2.4. Sample Characterizations
2.4.1. Crystal Structure
2.4.2. Morphology
2.4.3. Thermal Analysis
2.4.4. Density and Microhardness
3. Results and Discussions
3.1. Changes in Structure, Morphology, and Composition Associated with Changing the MA Time
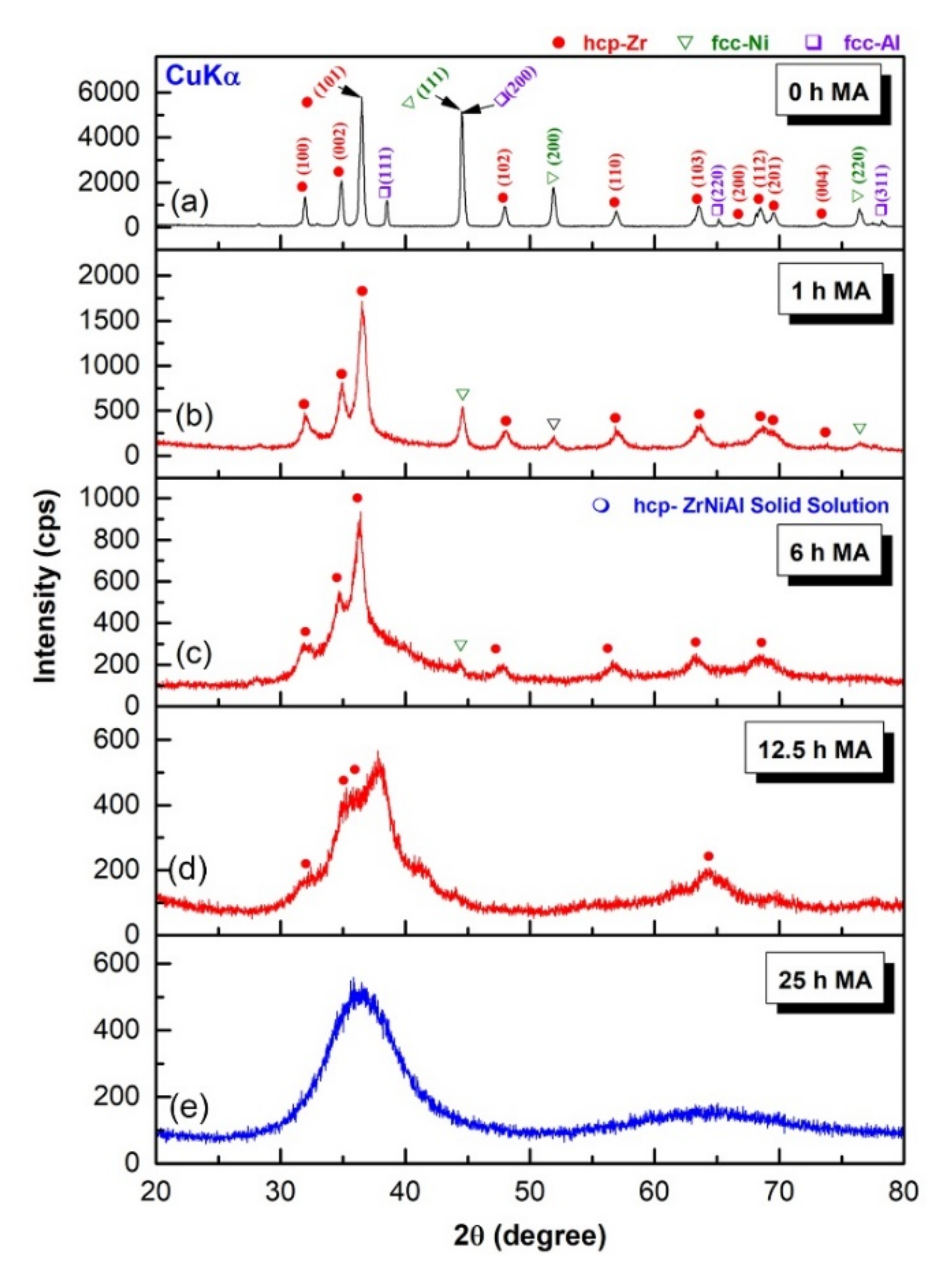
3.1.1. Metallic Glassy Zr70Ni25Al5 System
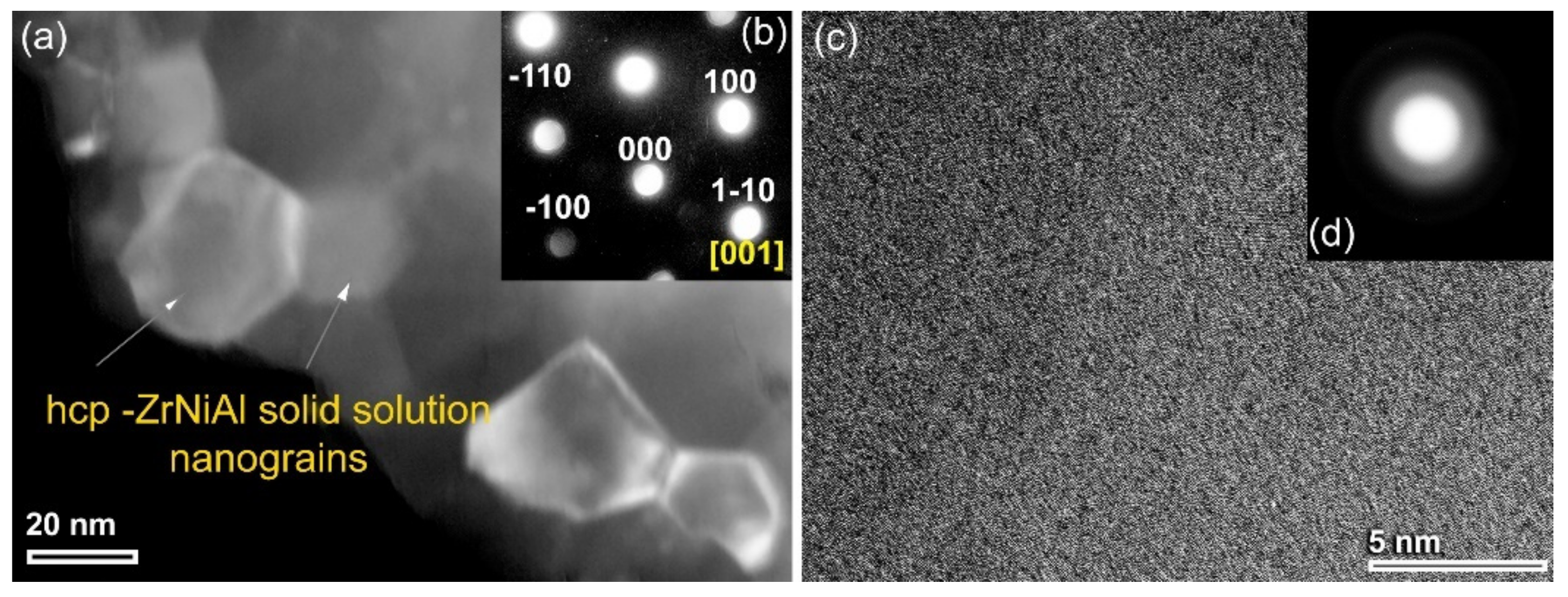
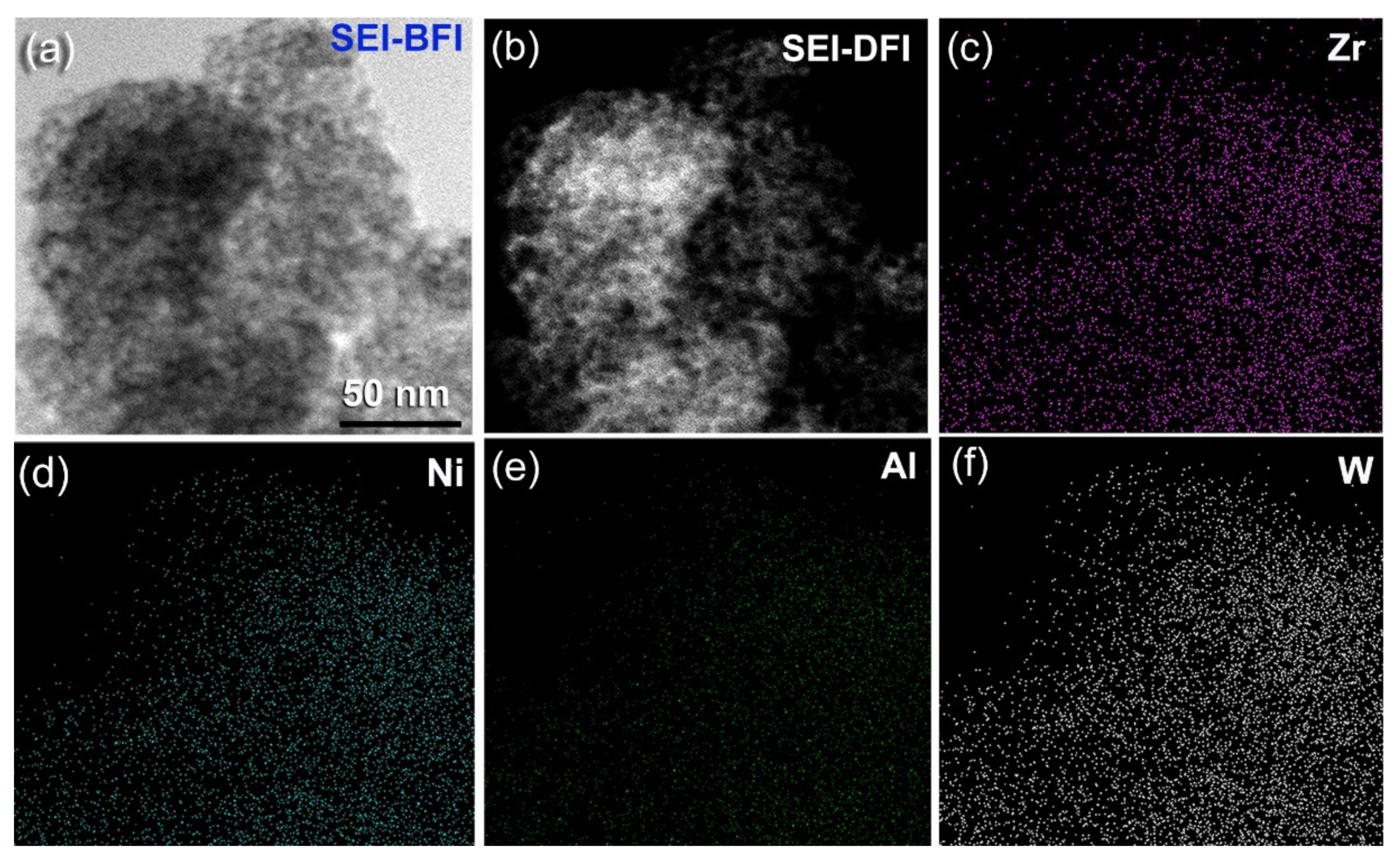
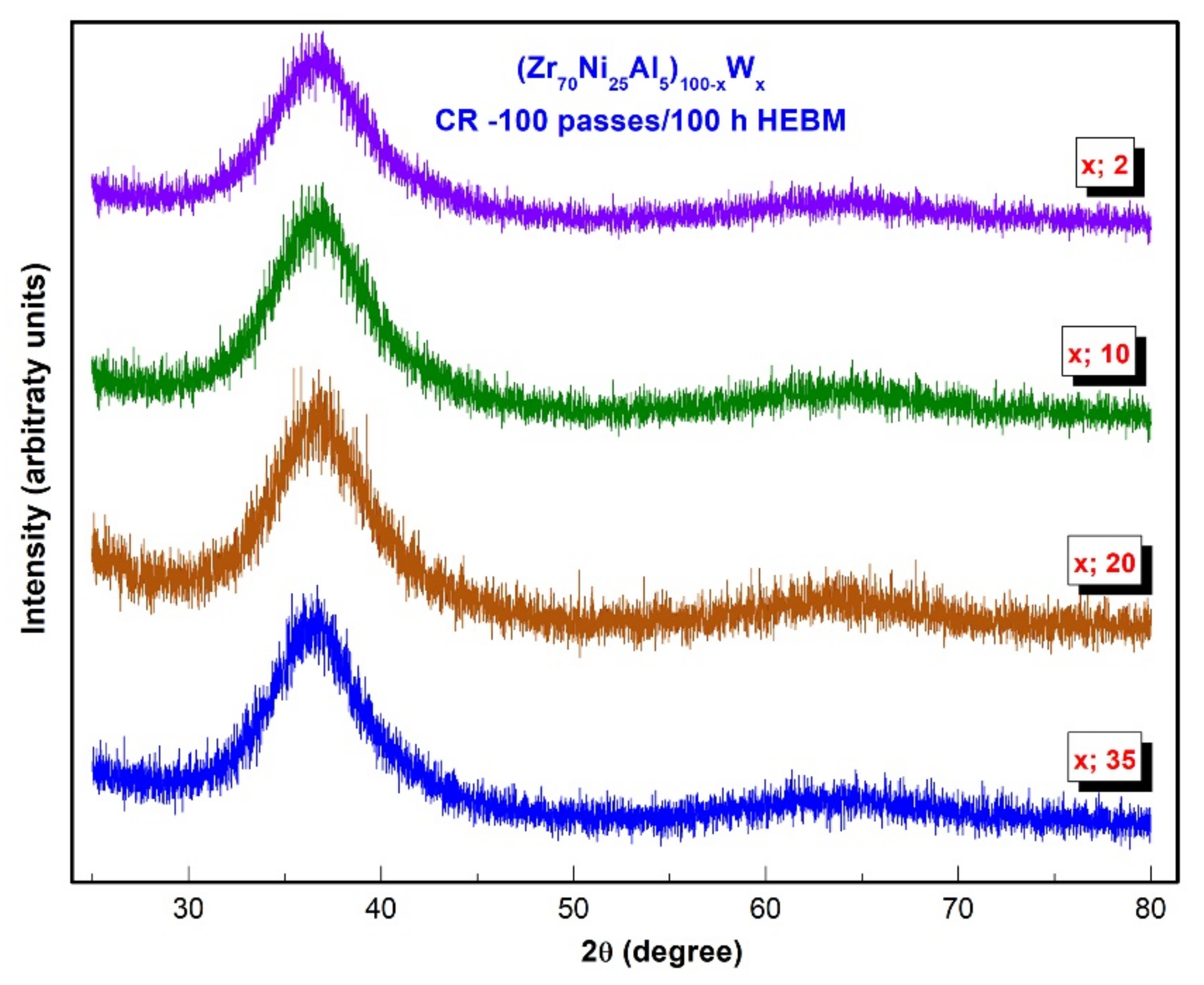
3.1.2. Metallic Glassy (Zr70Ni25Al5)100-xWx (x; 0, 2, 10, 20, 35 at. %) Systems
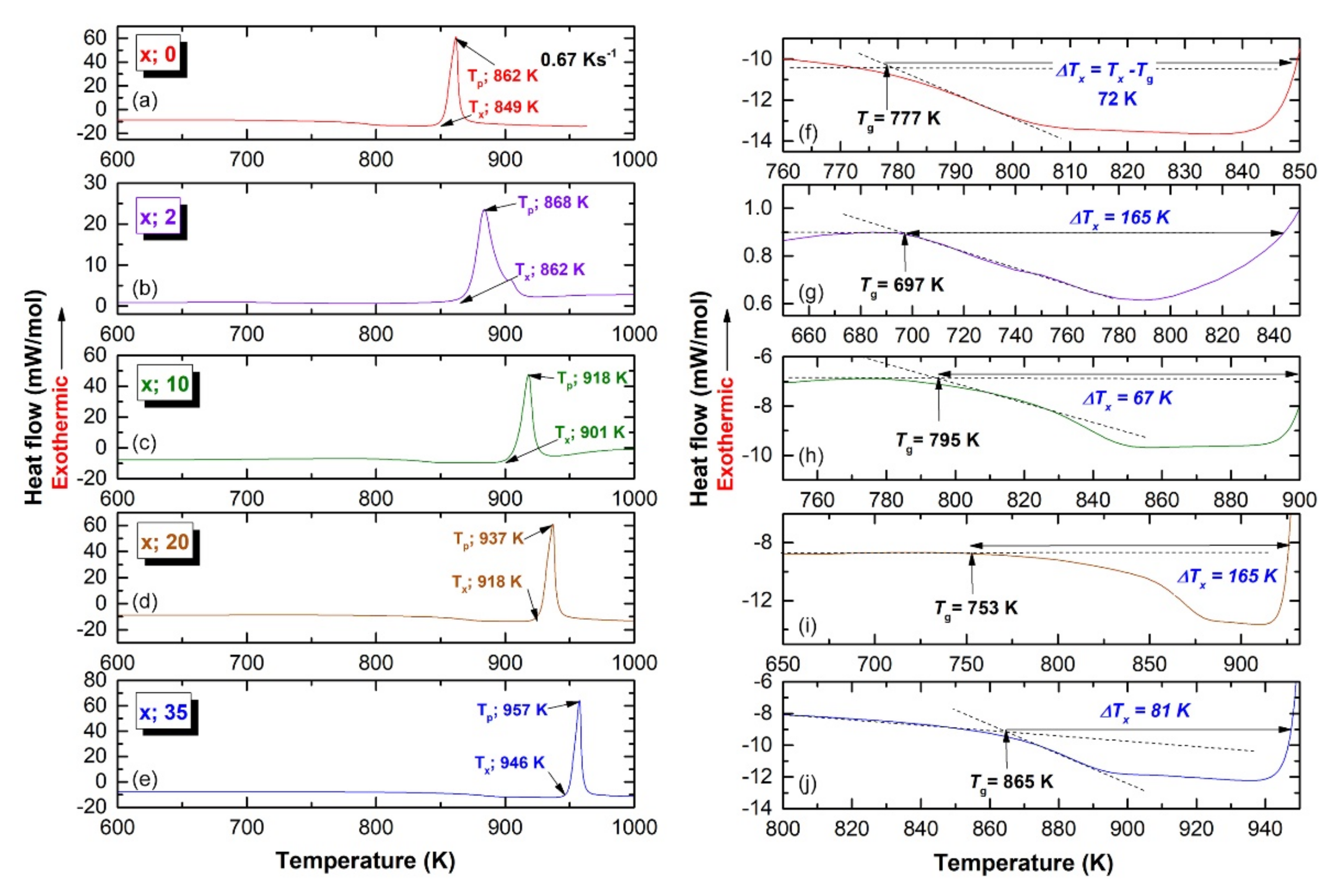
3.2. Thermal Stability
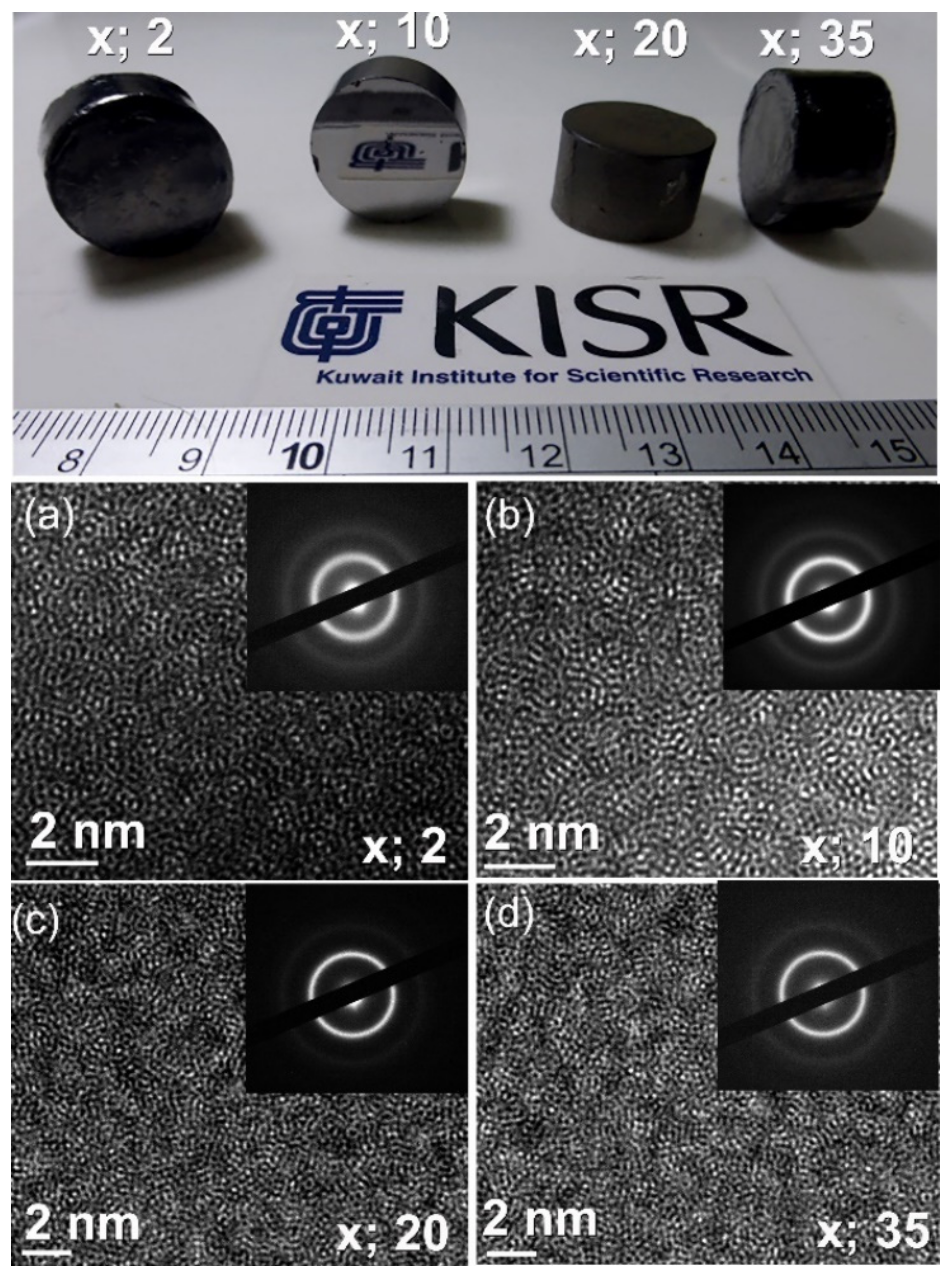
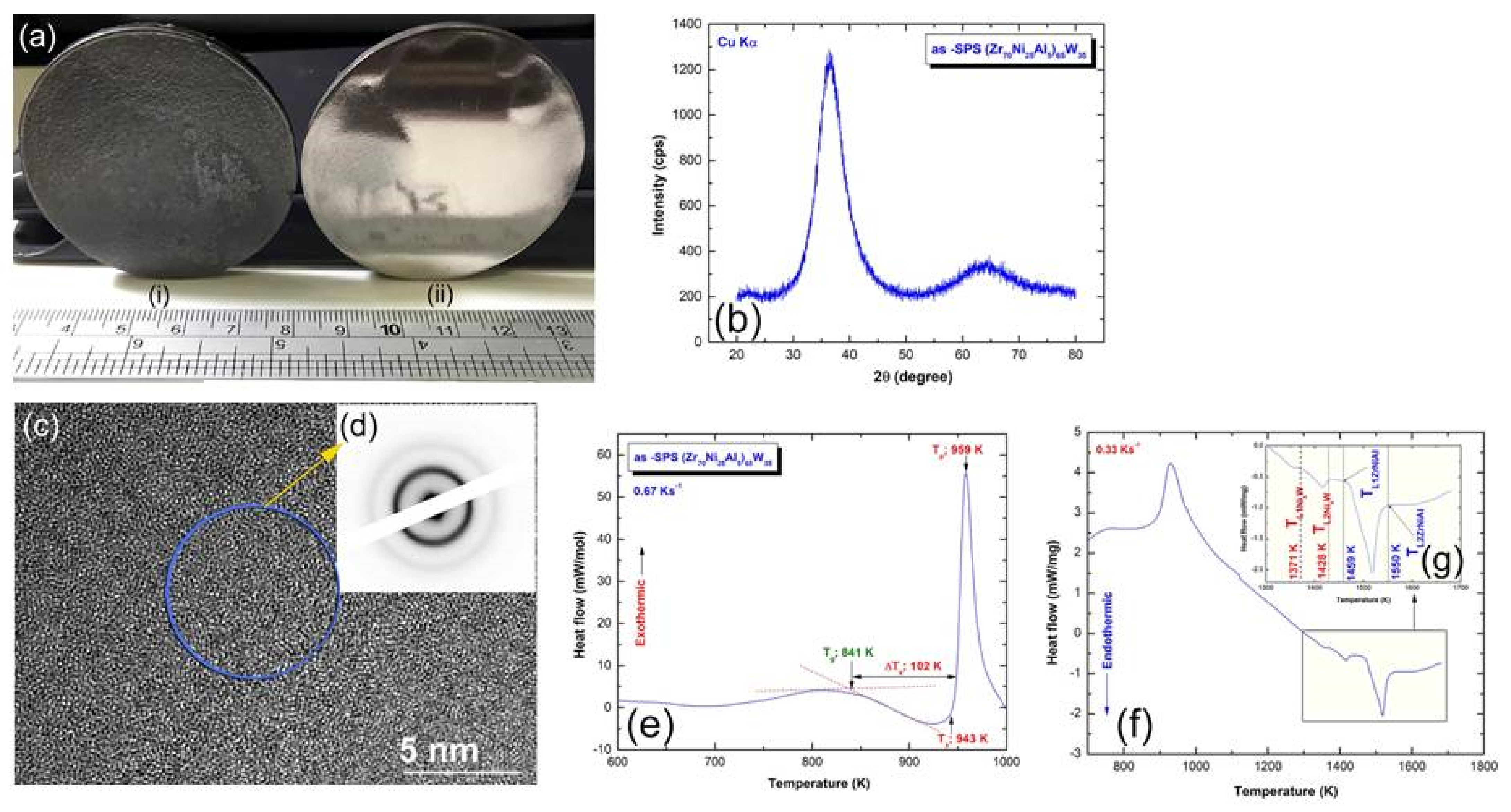
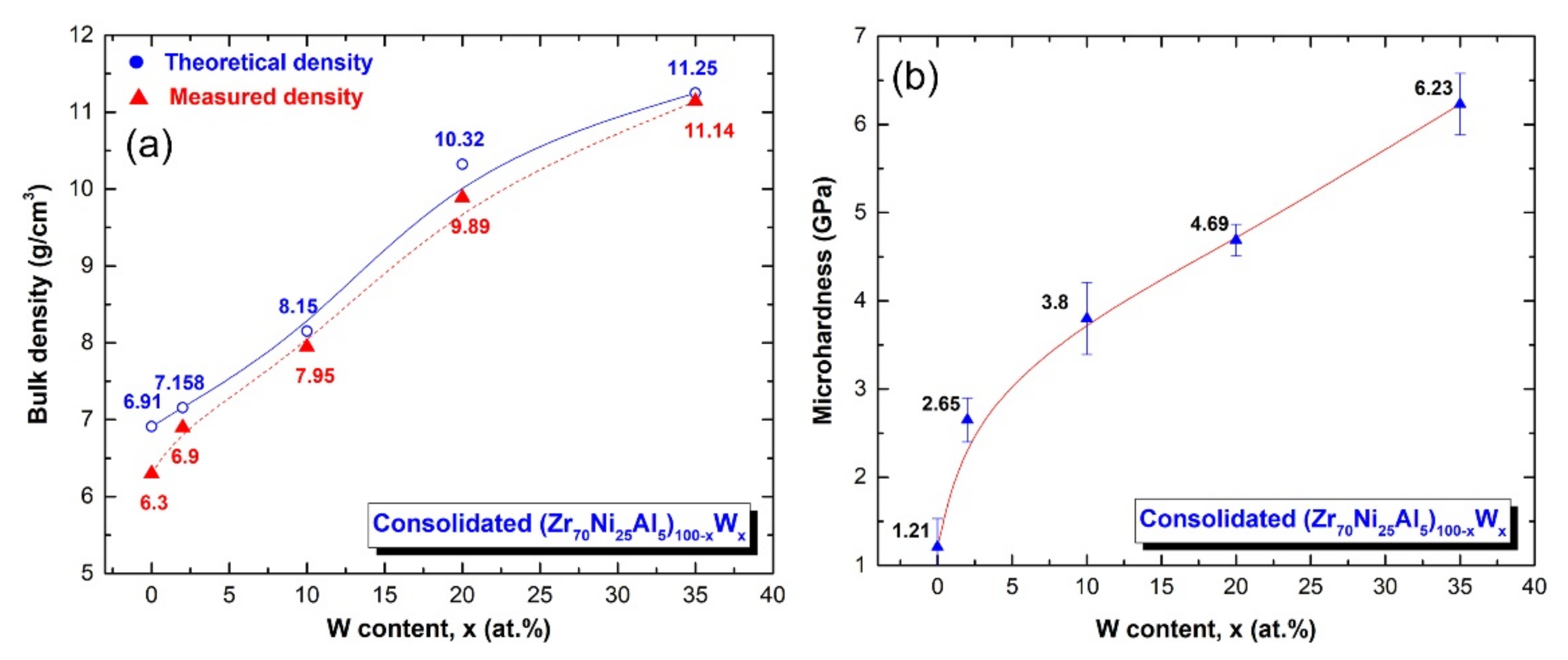
3.3. Fabrication of Bulk (Zr70Ni25Al5)100-xWx Metallic Glassy Systems with the Spark Plasma Sintering (SPS) Technique
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klement, W.; Willens, R.H.; Duwez, P. Non-crystalline structure in solidified gold–silicon alloys. Nature 1960, 187, 869–870. [Google Scholar] [CrossRef]
- Cheng, Y.T.; Johnson, W.L.; Nicolet, M.A. Crystal-to-glass transformation in metallic materials. Mater. Sci. Eng. 1988, 97, 1–13. [Google Scholar]
- Duwez, P.; Willens, R.H.; Klement, W., Jr. Continuous Series of Metastable Solid Solutions in Silver-Copper Alloys. J. Appl. Phys. 1960, 31, 1136–1138. [Google Scholar] [CrossRef]
- Lord Rayleigh, O.M.F.R.S. On convection currents in a horizontal layer of fluid, when the higher temperature is on the under side. Phil. Mag. 2009, 32, 529–546. [Google Scholar] [CrossRef]
- Herd, S.R.; Tu, K.N.; Ahn, K.Y. Formation of an amorphous Rh-Si alloy by interfacial reaction between amorphous Si and crystalline Rh thin films. Appl. Phys. Lett. 1983, 42, 597–599. [Google Scholar] [CrossRef]
- Blatter, A.; Allmen, M. Reversible amorphization in laser-quenched titanium alloys. Phys. Rev. Lett. 1985, 54, 2103–2106. [Google Scholar] [CrossRef]
- Yeh, X.L.; Samwer, K.; Johnson, W.L. Formation of an amorphous metallic hydride by reaction of hydrogen with crystalline intermetallic compounds—A new method of synthesizing metallic glasses. Appl. Phys. Lett. 1983, 42, 242–244. [Google Scholar] [CrossRef][Green Version]
- Aoki, K.; Nagano, M.; Yanagitani, A.; Masumoto, T. Hydrogen-induced amorphization and its effect on magnetic properties of the Laves-phase GdFe2 compound. J. Appl. Phys. 1987, 62, 3314–3316. [Google Scholar] [CrossRef]
- Taguchi, K.; Shinozaki, K.; Okumura, H.; Michioka, C.; Yoshimura, K.; Ishihara, K.N. Discovery of Amorphous Iron Hydrides via Novel Quiescent Reaction in Aqueous Solution. Sci. Rep. 2020, 10, 6199. [Google Scholar] [CrossRef]
- Schwarz, R.B.; Johnson, W.L. Formation of an Amorphous Alloy by Solid-State Reaction of the Pure Polycrystalline Metals. Phys. Rev. Lett. 1983, 51, 415–418. [Google Scholar] [CrossRef]
- Barbour, J.C.; Nastasi, M.; Mayer, J.W. Mobility of Ni versus Zr in an amorphous Ni-Zr alloy. Appl. Phys. Lett. 1986, 48, 517–519. [Google Scholar] [CrossRef]
- Shi, H.; Zhao, W.; Wei, X.; Ding, Y.; Shen, X.; Liu, W. Effect of Ti addition on mechanical properties and corrosion resistance of Ni-free Zr-based bulk metallic glasses for potential biomedical applications. J. Alloy. Compd. 2019, 815, 152636. [Google Scholar] [CrossRef]
- Han, K.; Wang, Y.; Qiang, J.; Jiang, H.; Gu, L. Low-cost Zr-based bulk metallic glasses for biomedical devices applications. J. Non Cryst. Solids 2019, 520, 119442. [Google Scholar] [CrossRef]
- El-Eskandrany, M.S.; Al-Azmi, A. Potential applications of cold sprayed Cu50Ti20Ni30 metallic glassy alloy powders for antibacterial protective coating in medical and food sectors. J. Mech. Behav. Biomed. Mater. 2016, 56, 183–194. [Google Scholar] [CrossRef]
- Han, K.; Qiang, J.; Wang, Y.; Häussler, P. Zr-Al-Co-Cu bulk metallic glasses for biomedical devices applications. J. Alloy. Compd. 2017, 729, 144–149. [Google Scholar] [CrossRef]
- Rajan, T.; Arockiarajan, A. Thin film metallic glasses for bioimplants and surgical tools: A review. J. Alloy. Compd. 2021, 876, 159939. [Google Scholar] [CrossRef]
- Pan, J.; Ivanov, Y.P.; Zhou, W.H.; Li, Y.; Greer, A.L. Strain-hardening and suppression of shear-banding in rejuvenated metallic glass. Nature 2020, 578, 559–562. [Google Scholar] [CrossRef] [PubMed]
- Greer, A.L. Confusion by Design. Nature 1993, 366, 303–304. [Google Scholar] [CrossRef]
- Inoue, A. High strength bulk amorphous alloys with low critical cooling rates (overview). Mater. Trans. JIM 1995, 36, 866–875. [Google Scholar] [CrossRef]
- Bajpai, S.; Nisar, A.; Sharma, R.K.; Schwarz, U.D.; Balani, K.; Datye, A. Effect of fictive temperature on tribological properties of Zr44Ti11Cu10Ni10Be25 bulk metallic glasses. Wear 2021, 486, 204075. [Google Scholar] [CrossRef]
- Yang, W.; Sun, X.; Liu, H.; Yu, C.; Li, W.; Inoue, A.; Şopu, D.; Eckert, J.; Tang, C. Structural homology of the strength for metallic glasses. J. Mater. Sci. Technol. 2020, 81, 123–130. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, Y.W.; Cheng, X.W.; Fu, Q.; Xin, Z.H.; Xu, Z.Q.; Cheng, H.W. Effects of Al replacement on glass forming ability and mechanical properties of Zr-based bulk metallic glasses. J. Non Cryst. Solids 2021, 568, 120962. [Google Scholar] [CrossRef]
- Polosan, S.; Ganea, P.; Nitescu, A. Structural, magneto-optical and dielectric properties of phosphate tellurite glasses. Mater. Res. Bull. 2021, 143, 111455. [Google Scholar] [CrossRef]
- Chen, S.H.; Yue, T.M.; Tsui, C.P.; Chan, K.C. Flaw-induced plastic-flow dynamics in bulk metallic glasses under tension. Sci. Rep. 2016, 6, 36130. [Google Scholar] [CrossRef]
- Li, W.; Gao, Y.; Bei, H. Instability analysis and free volume simulations of shear band directions and arrangements in notched metallic glasses. Sci. Rep. 2016, 6, 34878. [Google Scholar] [CrossRef]
- Herrero-Gómez, C.; Samwer, K. Stress and temperature dependence of the avalanche dynamics during creep deformation of metallic glasses. Sci. Rep. 2016, 6, 33503. [Google Scholar] [CrossRef]
- Chen, C.; Gao, M.; Wang, C.; Wang, W.-H.; Wang, T.-C. Fracture behaviors under pure shear loading in bulk metallic glasses. Sci. Rep. 2016, 6, 39522. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Liu, H.; Zhao, Y.; Inoue, A.; Jiang, K.; Huo, J.; Ling, H.; Li, Q.; Shen, B. Mechanical properties and structural features of novel Fe-based bulk metallic glasses with unprecedented plasticity. Sci. Rep. 2014, 4, 6233. [Google Scholar] [CrossRef] [PubMed]
- Hitit, A.; Şahin, H. The effect of iron content on glass forming ability and thermal stability of Co–Fe–Ni–Ta–Nb–B–Si bulk metallic glass. Metals 2017, 7, 7. [Google Scholar] [CrossRef]
- Shi, Z.; Li, R.; Li, X.; Wang, C.; Zhang, T. Controllable brittleness in soft-magnetic Fe-P-C-B metallic glasses through composition design. Mater. Sci. Eng. A 2019, 766, 138385. [Google Scholar] [CrossRef]
- Xu, K.; Ling, H.; Li, Q.; Li, J.; Yao, K.; Guo, S. Effects of Co substitution for Fe on the glass forming ability and properties of Fe80P13C7 bulk metallic glasses. Intermetallics 2014, 51, 53–58. [Google Scholar] [CrossRef]
- Xu, S.; Wang, J.; Wang, N.; Han, Z.T.; Wan, Y. Soft magnetic properties and corrosion resistance of the annealed (Fe0.7Co0.15Ni0.15)75B21Nb4 metallic glasses. Mater. Today Commun. 2021, 26, 101906. [Google Scholar] [CrossRef]
- Cui, G.; Li, X.; Shan, G.; Gao, H.; Wong, K.W.; Zhang, J. Depression of direct exchange couplings in metallic glasses: A comparative study of critical and electronic behavior in Gd6Co4.85 intermetallic compound and metallic glass. Intermetallics 2020, 124, 106878. [Google Scholar] [CrossRef]
- Chen, H.; Dong, B.; Zhou, S.; Li, X.; Qin, J. Structural, magnetic, and electronic properties of Fe82Si4B10P4 metallic glass. Sci. Rep. 2018, 8, 5680. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shan, G.; Li, J.; Wang, Y.; Shek, C.H. Structures and physical properties of two magnetic Fe-based metallic glasses. J. Alloy. Compd. 2021, 747, 636–639. [Google Scholar] [CrossRef]
- Ayyagari, A.; Hasannaeimi, V.; Arora, H.; Mukherjee, S. Electrochemical and Friction Characteristics of Metallic Glass Composites at the Microstructural Length-scales. Sci. Rep. 2018, 8, 906. [Google Scholar] [CrossRef]
- Burkov, A.; Chigrin, P. Effect of tungsten, molybdenum, nickel and cobalt on the corrosion and wear performance of Fe-based metallic glass coatings. Surf. Coat. Technol. 2018, 351, 68–77. [Google Scholar] [CrossRef]
- Si, J.J.; Chen, X.H.; Cai, Y.H.; Wu, Y.D.; Wang, T.; Hui, X.H. Corrosion behavior of Cr-based bulk metallic glasses in hydrochloric acid solutions. Corros. Sci. 2016, 107, 123–132. [Google Scholar] [CrossRef]
- El-Eskandarany, M. Metallic glassy Zr70Ni20Pd10 powders for improving the hydrogenation/dehydrogenation behavior of MgH2. Sci. Rep. 2016, 6, 26936. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Al-Salem, S.M.; Ali, N.; Banyan, M.; Al-Ajmi, F.; Al-Duweesh, A. From gangue to the fuel-cells application. Sci. Rep. 2020, 10, 20022. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S. Recent developments in the fabrication, characterization and implementation of MgH2-based solid-hydrogen materials in the Kuwait Institute for Scientific Research. RSC Adv. 2019, 9, 9907–9930. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S. Metallic glassy Ti2Ni grain-growth inhibitor powder for enhancing the hydrogenation/dehydrogenation kinetics of MgH2. RSC Adv. 2019, 9, 1036–1046. [Google Scholar] [CrossRef]
- Zhao, B.; Zeng, S.; Qin, X.; Li, Z.; Zhang, S.; Zhang, H.; Zhu, Z. Cu-Ag nanocomposites in-situ formed on the surface of CuZr-based metallic glasses as highly efficient catalysts for improving catalytic behavior and reusability towards wastewater treatment. Appl. Surf. Sci. 2021, 568, 150920. [Google Scholar] [CrossRef]
- Hattori, M.; Katsuragawa, N.; Yamaura, S.; Ozawa, M. Three-way catalytic properties and microstructures of metallic glass driven composite catalysts. Catal. Today 2021, 375, 273–281. [Google Scholar] [CrossRef]
- Zhao, B.; Zhu, Z.; Dong, X.Q.; Li, Z.; Zhang, H. Highly efficient and stable CuZr-based metallic glassy catalysts for azo dye degradation. J. Mater. Sci. Technol. 2020, 46, 88–97. [Google Scholar] [CrossRef]
- Hasannaeimi, V.; Mukherjee, S. Noble-metal based metallic glasses as highly catalytic materials for hydrogen oxidation reaction in fuel cells. Sci. Rep. 2019, 9, 12136. [Google Scholar] [CrossRef]
- Chen, Y.; Dai, Z.W.; Jiang, J.Z. High entropy metallic glasses: Glass formation, crystallization and properties. J. Alloy. Compd. 2021, 866, 158852. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Inoue, A. Bulk Metallic Glasses, 2nd ed.; CRC Press UK Limited: London, UK, 2018. [Google Scholar]
- Sun, Y.; Concustell, A.; Greer, A. Thermomechanical processing of metallic glasses: Extending the range of the glassy state. Nat Rev. Mater. 2016, 1, 16039. [Google Scholar] [CrossRef]
- Axinte, E. Metallic glasses from “alchemy” to pure science: Present and future of design, processing and applications of glassy metals. Mater. Des. 2012, 35, 518–556. [Google Scholar] [CrossRef]
- Chen, M. A brief overview of bulk metallic glasses. NPG Asia Mater. 2011, 3, 82–90. [Google Scholar] [CrossRef]
- Greer, A.L. Metallic glasses on the threshold. Mater. Today 2009, 12, 14–22. [Google Scholar] [CrossRef]
- Inoue, A.; Takeuchi, A. Recent development and application products of bulk glassy alloys. Acta Mater. 2011, 59, 2243–2267. [Google Scholar] [CrossRef]
- Johnson, W.L. Bulk glass-forming metallic alloys: Science and technology. MRS Bull. 1999, 24, 42–56. [Google Scholar] [CrossRef]
- Koch, C.C.; Cavin, O.B.; McKamey, C.G.; Scarbrough, J.O. Preparation of “amorphous” Ni60Nb40 by mechanical alloying. Appl. Phys. Lett. 1983, 43, 1017–1019. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S. Mechanical Alloying: Energy, Surface Protective Coating and Medical Applications, 3rd ed.; Elsevier: Oxford, UK, 2020. [Google Scholar]
- Battezzati, L.; Enzo, S.; Schiffini, L.; Cocco, G. Formation and crystallization of amorphous Ni2Ti powders prepared by mechanical alloying. J. Less Common Met. 1988, 145, 301–308. [Google Scholar] [CrossRef]
- Weeber, A.W.; Loeff, P.I.; Barker, H. Glass-forming range of transition metal-transition metal alloys prepared by mechanical alloying. J. Less Common Met. 1988, 145, 293–299. [Google Scholar] [CrossRef]
- Schultz, L. Glass-forming ranges in transition metal-Zr alloys prepared by mechanical alloying. J. Less Common Met. 1988, 145, 233–249. [Google Scholar] [CrossRef]
- Hellstern, E.; Schultz, L. Progress of the amorphization reaction during mechanical alloying in Fe-Zr. J. Appl. Phys. 1988, 63, 1408. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Itoh, F.; Aoki, K.; Suzuki, K. Preparation of AlxTa1-x amorphous alloy powder by mechanical alloying. J. Non Cryst. Solids 1990, 118, 729–732. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Aoki, K.; Suzuki, K. Calorimetric characterization of the amorphization process for milled Al50Nb50 alloy powders. Scr. Metall. Mater. 1991, 25, 1695–1700. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S. Solid-state amorphization reaction in mechanically deformed AlxHf100−x multilayered composite powders and the effect of annealing. J. Alloy. Compd. 1999, 284, 295–307. [Google Scholar] [CrossRef]
- Fukunaga, T.; Nakamura, K.; Suzuki, K.; Mizutani, U. Amorphization of immiscible CuTa system by mechanical alloying and its structure observation. J. Non Cryst. Solids 1990, 118, 700–703. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Matsushita, M.; Inoue, A. Phase transformations of ball-milled Nb50Zr10Al10Ni10Cu20 powders and the effect of annealing. J. Alloy. Comp. 2001, 329, 239–252. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Inoue, A. New V45Zr20Ni20Cu10Al2.5Pd2.5 glassy alloy powder with wide supercooled liquid region. Mater. Trans. JIM 2002, 43, 770–772. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Zhang, W.; Inoue, A. Mechanically induced crystalline-glassy phase transformations of mechanically alloyed Ta55Zr10Ni10Al10Cu15 multicomponent alloy powders. J. Alloy. Compd. 2003, 350, 222–231. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Saida, J.; Inoue, A. Mechanically induced solid state devitrifications of glassy Zr65Al7.5Ni10Cu12.5Pd5 alloy powders. Acta Mater. 2003, 51, 4519–4532. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Ishihara, S.; Inoue, A. Mechanism of solid-state reaction for fabrication of new glassy V45Zr22Ni22Cu11 alloy powders and subsequent consolidation. J. Mater. Res. 2003, 18, 2435–2445. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Inoue, A. Synthesis of new bulk metallic glassy Ti60Al15Cu10W10Ni5 alloy by hot-pressing the mechanically alloyed powders at the supercooled liquid region. Met. Trans. A 2006, 37, 2231–2238. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Ishihara, S.; Inoue, A. Fabrication and characterizations of new glassy Co71Ti24B5 alloy powders and subsequent hot pressing into a fully dense bulk glass. Met. Trans. A 2005, 36, 141–147. [Google Scholar] [CrossRef]
- Madge, S.V.; Caron, A.; Gralla, R.; Wilde, G.; Mishra, S.K. Novel W-based metallic glass with high hardness and wear resistance. Intermetallics 2014, 47, 6–10. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Sumiyama, K.; Suzuki, K. Crystalline-to-amorphous phase transformation in mechanically alloyed Fe50W50 powders. Acta Metall. 1997, 45, 1175–1187. [Google Scholar] [CrossRef]
- Kotynia, K.; Pawlik, P.; Filipecka, K.; Filipecki, J. Calorimetric and structural analysis of the Zr-;Fe-Co-B-Mo-W amorphous alloys doped with gadolinium. J. Alloy. Compd. 2020, 842, 155940. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Al-Hazza, A.; Al-Hajji, L.A.; Ali, N.; Al-Duweesh, A.A.; Banyan, M.; Al-Ajmi, F. Mechanical Milling: A Superior Nanotechnological Tool for Fabrication of Nanocrystalline and Nanocomposite Materials. Nanomaterials 2021, 11, 2484. [Google Scholar] [CrossRef] [PubMed]
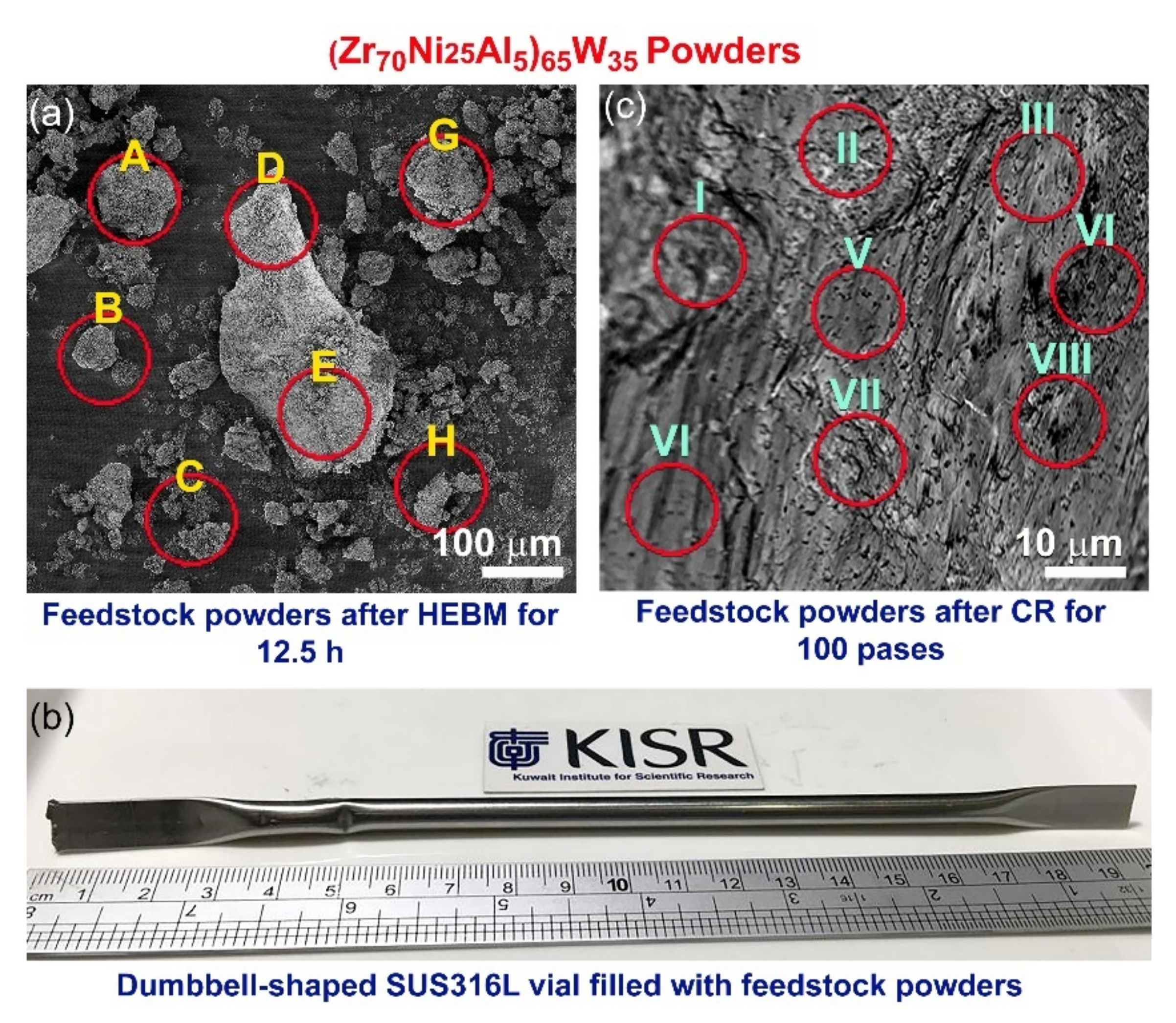




| 12.5 h | 100 Passes CR | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Element | A | B | C | D | E | H | G | I | II | III | IV | V | VI | VII | VIII |
| Zr | 2.5 | 3.7 | 8.7 | 2.1 | 4.2 | 1.8 | 36.4 | 44.8 | 46.1 | 42.5 | 41.6 | 42.8 | 48.3 | 45.2 | 44.2 |
| Ni | 1.0 | 92.8 | 83.0 | 1.9 | 2.1 | 3.6 | 24.7 | 17.2 | 15.3 | 18.7 | 14.3 | 15.1 | 14.8 | 16.1 | 18.3 |
| Al | 96.1 | 1.6 | 2.1 | 95.2 | 92.6 | 1.2 | 32.8 | 2.9 | 3.4 | 2.8 | 3.2 | 2.9 | 3.6 | 3.1 | 2.8 |
| W | 0.3 | 1.8 | 6.1 | 0.7 | 1.0 | 93.3 | 6.0 | 35.1 | 35.2 | 36.0 | 40.9 | 39.2 | 33.3 | 35.6 | 37.7 |
| Fe | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | - | - | - | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Eskandarany, M.S.; Ali, N.; Al-Ajmi, F.; Banyan, M. Phase Transformations from Nanocrystalline to Amorphous (Zr70Ni25Al5)100-xWx (x; 0, 2, 10, 20, 35 at. %) and Subsequent Consolidation. Nanomaterials 2021, 11, 2952. https://doi.org/10.3390/nano11112952
El-Eskandarany MS, Ali N, Al-Ajmi F, Banyan M. Phase Transformations from Nanocrystalline to Amorphous (Zr70Ni25Al5)100-xWx (x; 0, 2, 10, 20, 35 at. %) and Subsequent Consolidation. Nanomaterials. 2021; 11(11):2952. https://doi.org/10.3390/nano11112952
Chicago/Turabian StyleEl-Eskandarany, M. Sherif, Naser Ali, Fahad Al-Ajmi, and Mohammad Banyan. 2021. "Phase Transformations from Nanocrystalline to Amorphous (Zr70Ni25Al5)100-xWx (x; 0, 2, 10, 20, 35 at. %) and Subsequent Consolidation" Nanomaterials 11, no. 11: 2952. https://doi.org/10.3390/nano11112952
APA StyleEl-Eskandarany, M. S., Ali, N., Al-Ajmi, F., & Banyan, M. (2021). Phase Transformations from Nanocrystalline to Amorphous (Zr70Ni25Al5)100-xWx (x; 0, 2, 10, 20, 35 at. %) and Subsequent Consolidation. Nanomaterials, 11(11), 2952. https://doi.org/10.3390/nano11112952







