High Selectivity and Reusability of Biomass-Based Adsorbent for Chloramphenicol Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of Adsorbent
2.3. Adsorption Properties
2.4. Reuse Studies
2.5. Characterization
2.6. Adsorption Isotherm
2.7. Adsorption Kinetics
3. Results and Discussion
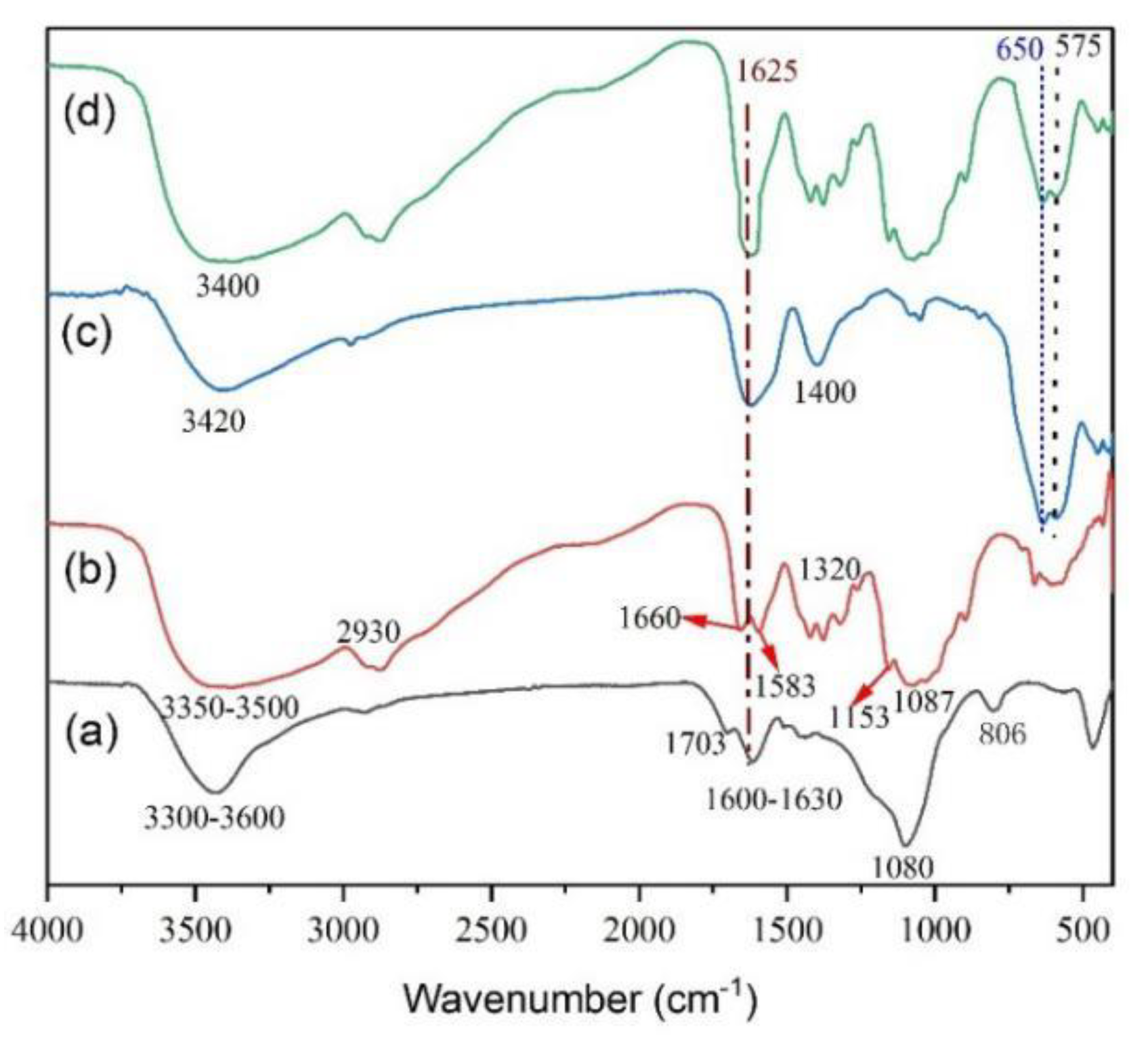
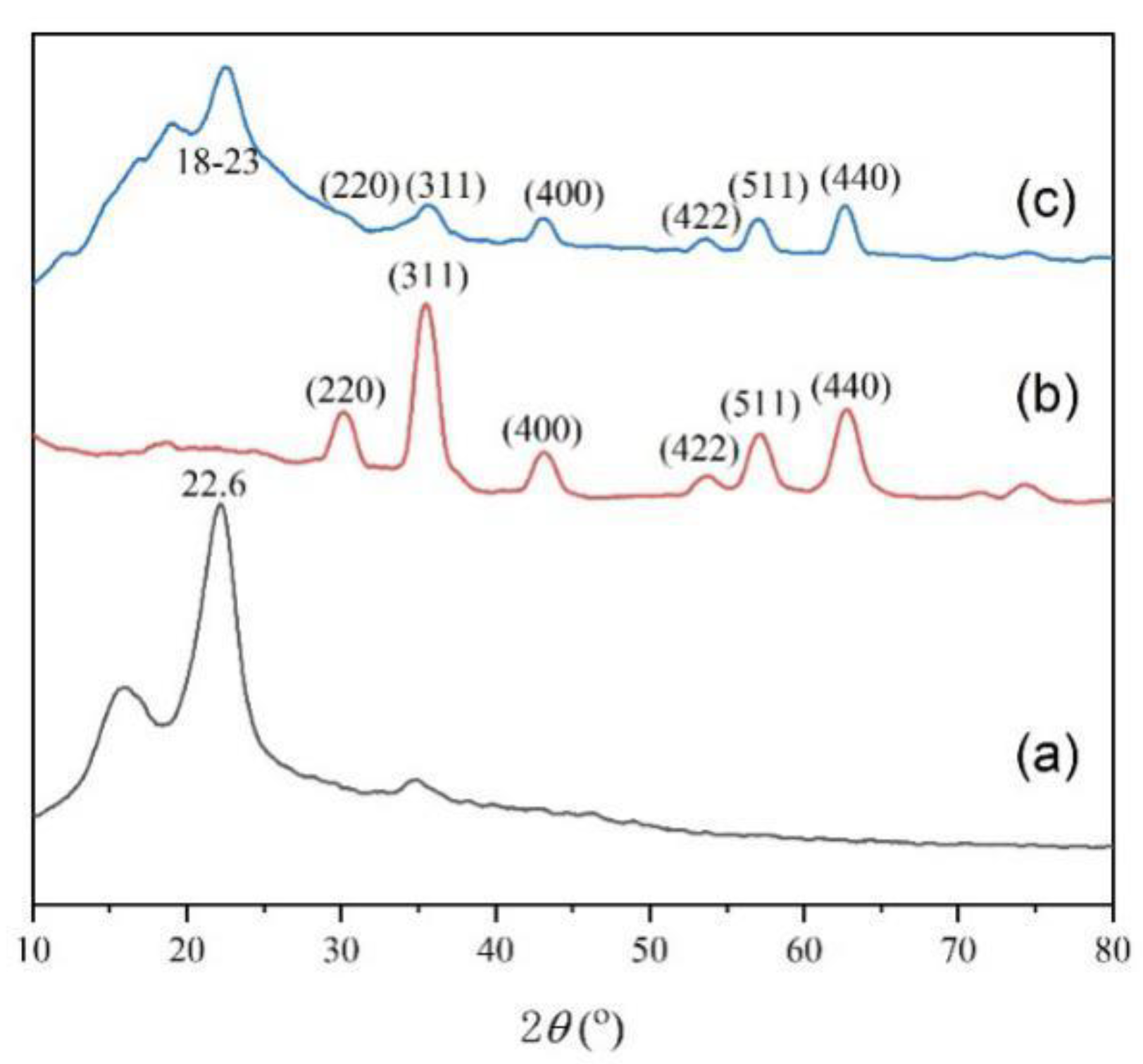
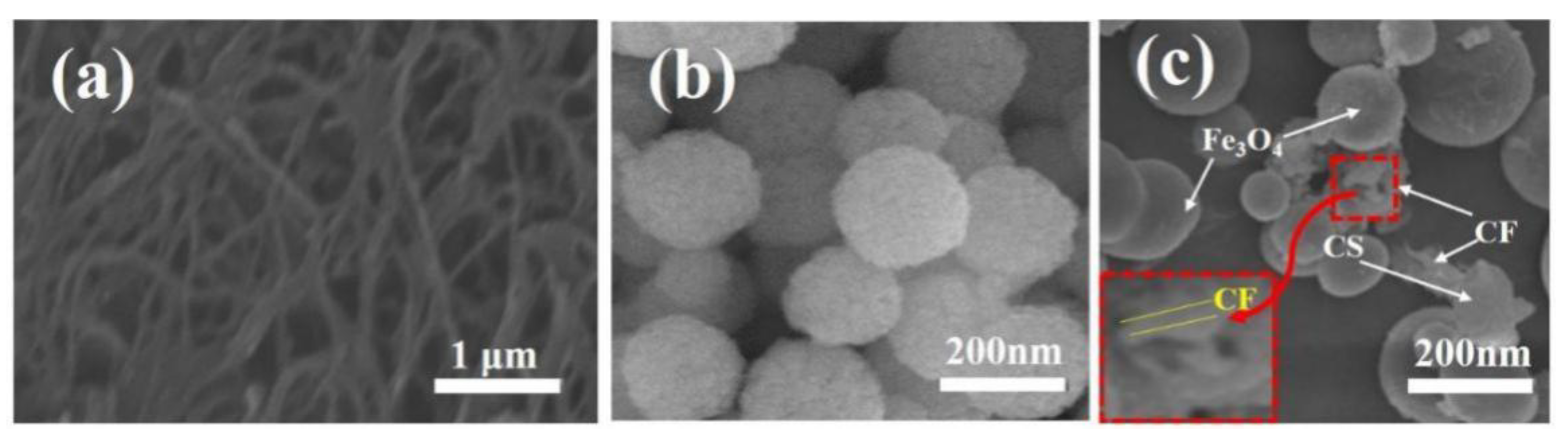
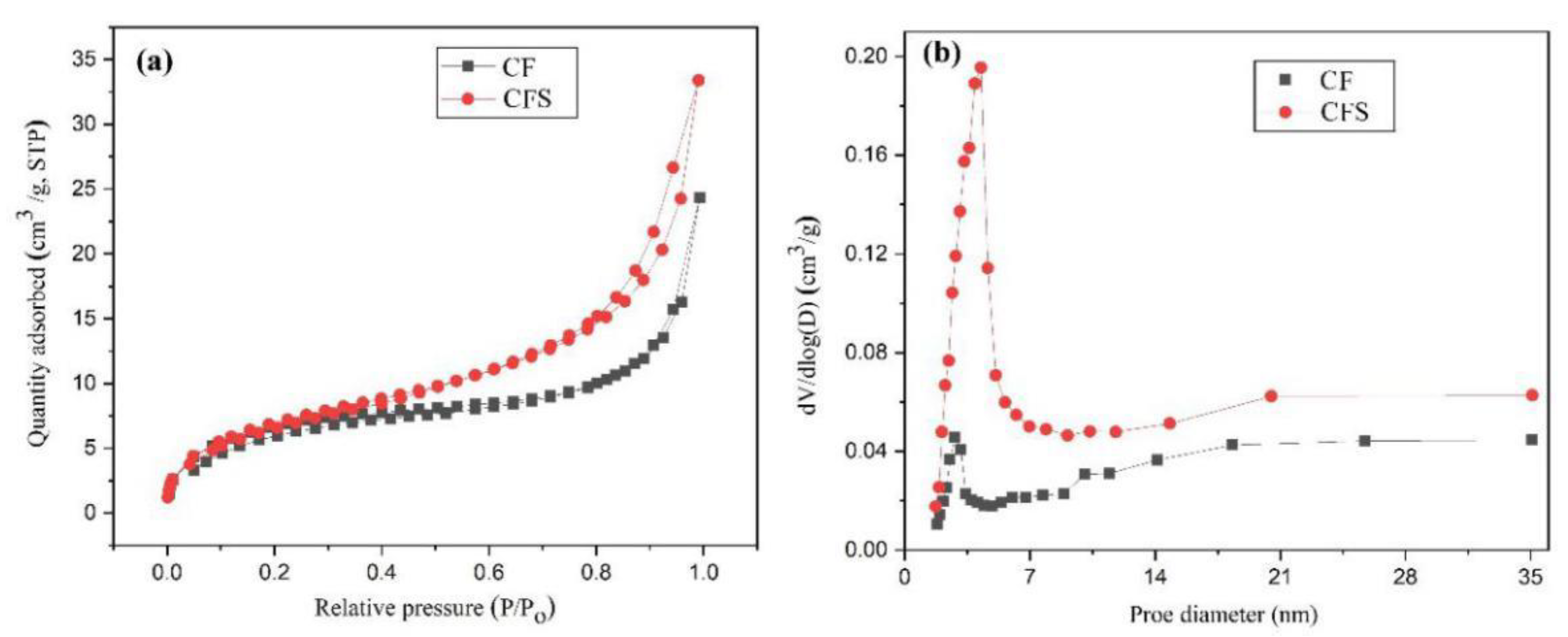
3.1. Composition and Structure of the CFS Adsorbent
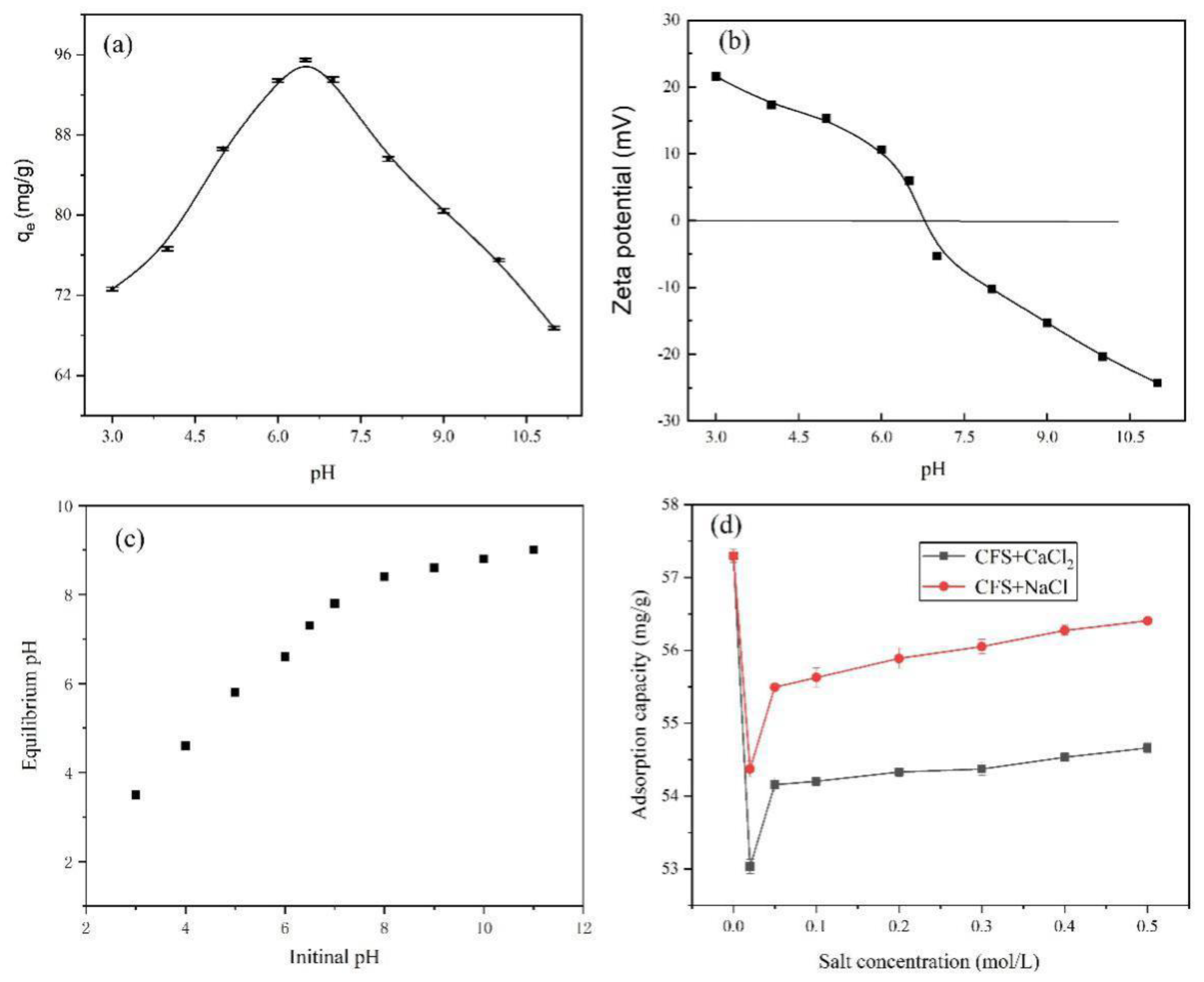
3.2. Effects of pH and Ionic Strength
3.3. Adsorption Isotherm Model
3.4. Kinetics Models
3.5. Thermodynami
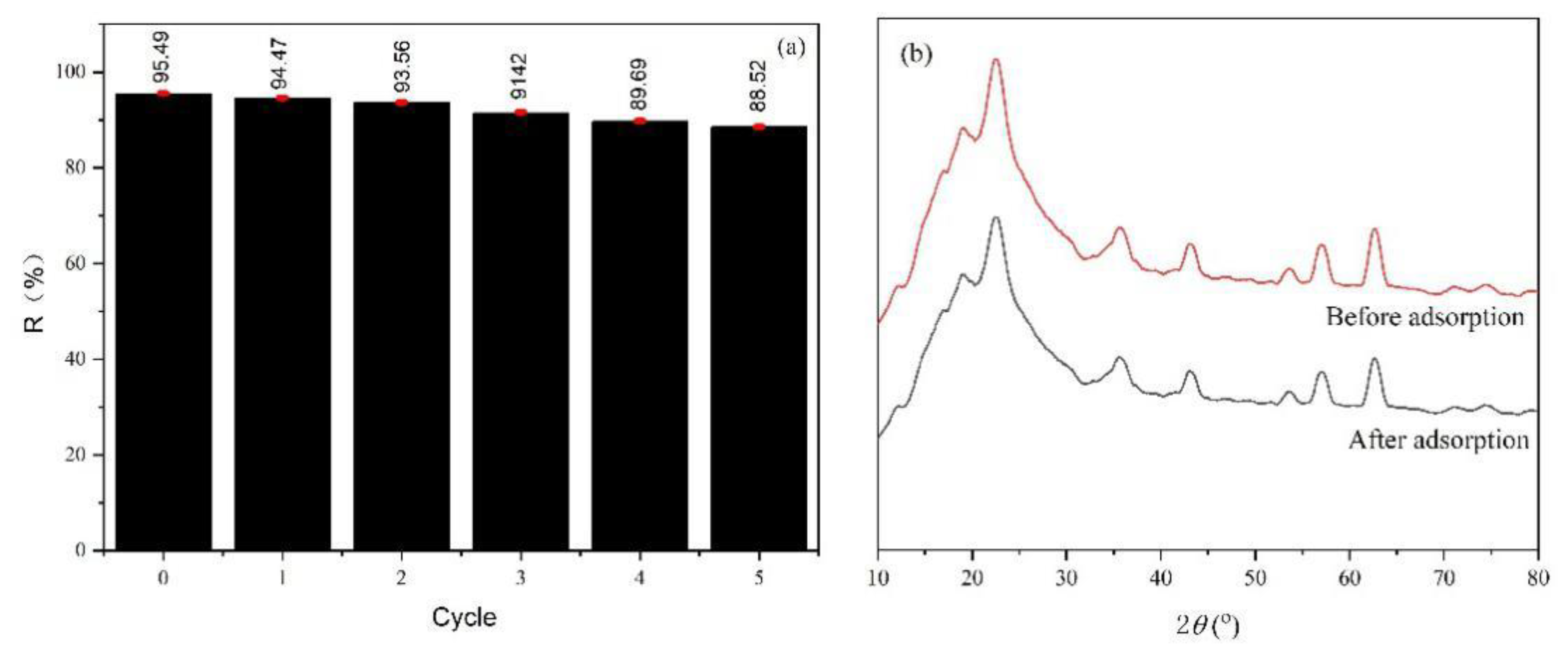
3.6. Recycling Ability and Adsorbent Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahammad, N.A.; Zulkifli, M.A.; Ahmad, M.A.; Hameed, B.H.; Mohd Din, A.T. Desorption of chloramphenicol from ordered mesoporous carbon-alginate beads: Effects of operating parameters, and isotherm, kinetics, and regeneration studies. J. Environ. Chem. Eng. 2021, 9, 105015. [Google Scholar] [CrossRef]
- Rahimi, Z.; Shahbazi, Y.; Ahmadi, F. Polypyrrole as an efficient solid-phase extraction sorbent for determination of chloramphenicol residue in chicken liver, kidney, and meat. Food Anal. Methods 2016, 10, 955–963. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, Q.; Jian, H.X.; Wang, C.P.; Xing, B.S.; Sun, H.W.; Hao, Y.L. Effect of biochar-derived dissolved organic matter on adsorption of sulfamethoxazole and chloramphenicol. J. Hazard. Mater. 2020, 396, 122598. [Google Scholar] [CrossRef]
- Dong, H.; Qiang, Z.; Hu, J.; Qu, J. Degradation of chloramphenicol by UV/chlorine treatment: Kinetics, mechanism and enhanced formation of halonitromethanes. Water Res. 2017, 121, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhang, Y.; Li, J.; Hao, Q.; Li, X.; Liu, F. Heterogeneous activation of peroxymonosulfate by a biochar-supported Co3O4 composite for efficient degradation of chloramphenicols. Environ. Pollut. 2020, 257, 113610. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Li, P.; Zhang, M.; Wang, J.; Gao, Y.; Zhang, T.; Zhou, G.; Li, F. Designing three-dimensional half-embedded ES-PAN/AHCNs adsorption membrane for removal of Pb(II), Cu(II) and Cr(III). Colloids Surf. A 2021, 627, 127177. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Adeniyi, A.G.; Adelodun, A.A. Recent advances on the adsorption of herbicides and pesticides from polluted waters: Performance evaluation via physical attributes. J. Ind. Eng. Chem. 2021, 93, 117–137. [Google Scholar] [CrossRef]
- Wu, G.Y.; Liu, Q.; Wang, J.Y.; Xia, S.Y.; Wu, H.L.; Zong, J.X.; Han, J.G.; Xing, W.N. Facile fabrication of rape straw biomass fiber/β-CD/Fe3O4 as adsorbent for effective removal of ibuprofen. Ind. Crop. Prod. 2021, 173, 114150. [Google Scholar] [CrossRef]
- Mukwevho, N.; Gusain, R.; Fosso-Kankeu, E.; Kumar, N.; Waanders, F.; Ray, S.S. Removal of naphthalene from simulated wastewater through adsorption-photodegradation by ZnO/Ag/GO nanocomposite. J. Ind. Eng. Chem. 2020, 81, 393. [Google Scholar] [CrossRef]
- Yuan, J.; Zhu, Y.; Wang, J.; Gan, L.; He, M.; Zhang, T.; Li, P.; Qiu, F. Preparation and application of Mg-Al composite oxide/coconut shell carbon fiber for effective removal of phosphorus from domestic sewage. Food Bioprod. Process 2021, 126, 293–304. [Google Scholar] [CrossRef]
- Liu, H.; Li, P.; Yu, H.; Zhang, T.; Qiu, F. Controlled fabrication of functionalized nanoscale zero-valent iron/celluloses composite with silicon as protective layer for arsenic removal. Chem. Eng. Res. Des. 2019, 151, 242–251. [Google Scholar] [CrossRef]
- Zhu, W.; Jiang, X.; Jiang, K.; Liu, F.; You, F.; Yao, C. Fabrication of reusable carboxymethyl cellulose/graphene oxide composite aerogel with large surface area for adsorption of methylene blue. Nanomaterials 2021, 11, 1609. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Johir, M.A.H.; Sornalingam, K.; Belhaj, D.; Kallel, M. Nano-Fe0 immobilized onto functionalized biochar gaining excellent stability during sorption and reduction of chloramphenicol via transforming to reusable magnetic composite. Chem. Eng. J. 2017, 322, 571–581. [Google Scholar] [CrossRef]
- Zhao, H.; Lang, Y. Adsorption behaviors and mechanisms of florfenicol by magnetic functionalized biochar and reed biochar. J. Taiwan Inst. Chem. Eng. 2018, 88, 152–160. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; Elgarahy, A.M.; Khan, Z.A.; Almughamisi, M.S.; Al-Bogami, A.S. Perspectives regarding metal/mineral-incorporating materials for water purification: With special focus on Cr(VI) removal. Mater. Adv. 2020, 1, 1546–1574. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; Al-Bogami, A.S.; Elgarahy, A.M. Efficient Retention of chromate from industrial wastewater onto a green magnetic polymer based on shrimp peels. J. Polym. Environ. 2018, 26, 2018–2029. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; El-Bindary, A.A.; Ismail, A.; Morshidy, A.M. Magnetic chitosan grafted with polymerized thiourea for remazol brilliant blue R recovery: Effects of uptake conditions. J. Dispers. Sci. Technol. 2017, 38, 943–952. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; Atia, A.A.; Guibal, E. Fast removal of uranium from aqueous solutions using tetraethylenepentamine modified magnetic chitosan resin. Bioresour. Technol. 2014, 160, 107–114. [Google Scholar] [CrossRef]
- Zhu, Y.; Rong, J.; Mao, K.; Yang, D.; Zhang, T.; Qiu, F.; Pan, J. Fe3O4@chitosan-bound boric acid composite as pH-responsive reusable adsorbent for selective recognition and capture of cis-diol-containing shikimic acid. Appl. Organomet. Chem. 2020, 34, e5415. [Google Scholar] [CrossRef]
- Wang, J.; Yang, F. Preparation of 2-hydroxypropyl-b-cyclodextrin polymers crosslinked by poly(acrylic acid) for efficient removal of chloramphenicol. Mater. Lett. 2021, 284, 128882. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; Qiu, F.; Li, P. Synthesis and characterization of β-cyclodextrin-carboxymethyl cellulose-graphene oxide composite materials and its application for removal of basic fuchsin. J. Iran. Chem. Soc. 2017, 14, 1827–1837. [Google Scholar] [CrossRef]
- Weber, T.W.; Chakravorti, R.K. Pore and solid diffusion models for fixed-bed adsorbers. J. Am. Inst. Chem. Eng. 1974, 20, 228–238. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, N.; Feng, C.; Li, M.; Gao, Y. Chromium removal using a magnetic corncob biochar/polypyrrole composite by adsorption combined with reduction: Reaction pathway and contribution degree. Colloids Surf. A 2018, 556, 201–209. [Google Scholar] [CrossRef]
- Kong, J.; Zheng, Y.; Xiao, L.; Dai, B.; Meng, Y.; Ma, Z.; Wang, J.; Huang, X. Synthesis and comparison studies of activated carbons based folium cycas for ciprofloxacin adsorption. Colloids Surf. A 2020, 606, 125519. [Google Scholar] [CrossRef]
- Shi, Z.; Xu, C.; Guan, H.; Li, L.; Fan, L.; Wang, Y.; Liu, L.; Meng, Q.; Zhang, R. Magnetic metal organic frameworks (MOFs) composite for removal of lead and malachite green in wastewater. Colloids Surf. A 2018, 539, 382–390. [Google Scholar] [CrossRef] [Green Version]
- Ogungbenro, A.E.; Quang, D.V.; Al-Ali, K.A.; Vega, L.F.; Abu-Zahr, M.R.M. Synthesis and characterization of activated carbon from biomass date seeds for carbon dioxide adsorption. J. Environ. Chem. Eng. 2020, 8, 104257. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Z.; Li, Z.; Wu, H. Removal of nitrogen and phosphorus pollutants from water by FeCl3-impregnated biochar. Ecol. Eng. 2020, 149, 105792. [Google Scholar]
- Zhao, H.; Liu, X.; Cao, Z.; Zhan, Y.; Shi, X.; Yang, Y.; Zhou, J.; Xu, J. Adsorption behavior and mechanism of chloramphenicols, sulfonamides, and non-antibiotic pharmaceuticals on multi-walled carbon nanotubes. J. Hazard. Mater. 2016, 310, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, A.C.; dos Reis, G.S.; Pavan, F.A.; Lima, É.C.; Foletto, E.L.; Dotto, G.L. Improvement of activated carbon characteristics by sonication and its application for pharmaceutical contaminant adsorption. Environ. Sci. Pollut. Res. 2018, 25, 24713–24725. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Hu, F.; Lam, F.; Wang, Y.; Liu, Z.; Dai, H. Adsorption behavior and mechanisms of ciprofloxacin from aqueous solution by ordered mesoporous carbon and bamboo-based carbon. J. Colloid Interface Sci. 2015, 460, 349–360. [Google Scholar] [CrossRef]
- Martins, A.C.; Pezoti, O.; Cazetta, A.L.; Bedin, K.C.; Yamazaki, D.A.S.; Bandoch, G.F.G.; Asefa, T.; Visentainer, J.V.; Almeida, V.C. Removal of tetracycline by NaOH-activated carbon produced from macadamia nut shells: Kinetic and equilibrium studies. Chem. Eng. J. 2015, 260, 291–299. [Google Scholar] [CrossRef]
- Liao, P.; Zhan, Z.; Dai, J.; Wu, X.; Zhang, W.; Wang, K.; Yuan, S. Adsorption of tetracycline and chloramphenicol in aqueous solutions by bamboo charcoal: A batch andfixed-bed column study. Chem. Eng. J. 2013, 228, 496–505. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Johir, M.A.H.; Belhaj, D. Competitive sorption affinity of sulfonamides and chloramphenicol antibiotics toward functionalized biochar for water and wastewater treatment. Bioresour. Technol. 2017, 238, 306–312. [Google Scholar] [CrossRef]
- Wei, S.; Li, J.; Liu, Y.; Ma, J. Development of magnetic molecularly imprinted polymers with double templates for the rapid and selective determination of amphenicol antibiotics in water, blood, and egg samples. J. Chromatogr. A 2016, 1473, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Bedin, K.C.; Martins, A.C.; Cazetta, A.L.; Pezoti, O.; Almeida, V.C. KOH-activated carbon prepared from sucrose spherical carbon: Adsorption equilibrium, kinetic and thermodynamic studies for methylene blue removal. Chem. Eng. J. 2016, 286, 476–484. [Google Scholar] [CrossRef]
- Drweesh, S.A.; Fathy, N.A.; Wahba, M.A.; Hanna, A.A.; Akarish, A.I.M.; Elzahany, E.A.M.; El-Sherif, I.Y.; Abou-El-Sherbini, K.S. Equilibrium, kinetic and thermodynamic studies of Pb(II) adsorption from aqueous solutions on HCl-treated Egyptian kaolin. J. Environ. Chem. Eng. 2016, 4, 1674–1684. [Google Scholar] [CrossRef]
| Model | Parameter | 298 K | 308 K | 318 K |
|---|---|---|---|---|
| LIM | qe, cal (mg/g) | 59.3600 | 62.7933 | 63.1200 |
| qm (mg/g) | 53.996 | 58.7544 | 50.1756 | |
| KL (L/mg) | 0.0504 | 0.0909 | 0.1084 | |
| R2 | 0.9916 | 0.9990 | 0.9958 | |
| SSRE (×10−5) | 3.9191 | 0.3743 | 1.5981 | |
| FIM | Kf (mg1−(1/n) L1/n g−1) | 1.4007 | 4.1343 | 4.2137 |
| 1/n | 1.61553 | 1.5744 | 1.6628 | |
| R2 | 0.9369 | 0.9837 | 0.9677 | |
| SSRE | 0.2042 | 0.0499 | 0.0994 | |
| TIM | bT (J.g/mol.mg) | 44.6432 | 43.1616 | 42.4106 |
| KT (L/mg) | 0.3015 | 0.53889 | 0.56649 | |
| R2 | 0.7273 | 0.8338 | 0.8029 | |
| SSRE (×103) | 1.3410 | 0.8555 | 1.0274 | |
| DRIM | qm (mg/g) | 71.9111 | 81.6123 | 85.8429 |
| KDR (molJ) | 4.8 × 10−6 | 1.8 × 10−6 | 1.7 × 10−6 | |
| R2 | 0.7988 | 0.8618 | 0.8612 | |
| SSRE | 0.6508 | 0.4246 | 0.4270 |
| Adsorbents | Temperature (K) | qe (mg/g) | Ref. |
|---|---|---|---|
| Si@MIPs-CAP | 298 | 32.258 | [3] |
| BC (Bamboo charcoal) | - | 8.1 | [33] |
| FBC | 298 | 21.35 | [34] |
| MRBC | 298 | 9.29 | [14] |
| RBC | 298 | 6.14 | [14] |
| Fe3O4@mSiO2@NIP | 298 | 44 | [35] |
| Aligned-MWCNT | 298 | 14.58 | [29] |
| CFS | 298–308 | 50.18–58.75 | This work |
| Model | Parameter | 298 K | 308 K | 318 K |
|---|---|---|---|---|
| PFO | qe (mg/g) | 38.5837 | 38.7306 | 38.9391 |
| K1 (/min) | 0.0013 | 0.0011 | 0.0011 | |
| R2 | 0.5687 | 0.5220 | 0.5236 | |
| SSRE | 0.0699 | 0.0664 | 0.0638 | |
| PSO | qe (mg/g) | 33.0142 | 42.3012 | 41.0846 |
| K2×10−3 (g/(mg min)) | 8.5363 | 10.2812 | 9.9088 | |
| R2 | 0.9999 | 0.9999 | 0.9999 | |
| SSRE (×10−4) | 3.3791 | 0.7734 | 1.7798 | |
| IDK | Kp1 (mg/g/min1/2) | 2.4282 | 2.3006 | 2.2739 |
| C1 (mg/g) | 43.3067 | 46.6520 | 47.0841 | |
| R2 | 0.9305 | 0. 8621 | 0.8519 | |
| SSRE | 8.9674 | 0.0469 | 17.2486 | |
| Kp2 (mg/g/min1/2) | 0.1209 | 0.0921 | 0.1197 | |
| C2 (mg/g) | 59.8534 | 62.3396 | 62.1838 | |
| R2 | 0.9125 | 0.9519 | 0.8625 | |
| SSRE | 0.0121 | 18.2991 | 0.0765 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, W.; Liu, Q.; Wang, J.; Xia, S.; Ma, L.; Lu, R.; Zhang, Y.; Huang, Y.; Wu, G. High Selectivity and Reusability of Biomass-Based Adsorbent for Chloramphenicol Removal. Nanomaterials 2021, 11, 2950. https://doi.org/10.3390/nano11112950
Xing W, Liu Q, Wang J, Xia S, Ma L, Lu R, Zhang Y, Huang Y, Wu G. High Selectivity and Reusability of Biomass-Based Adsorbent for Chloramphenicol Removal. Nanomaterials. 2021; 11(11):2950. https://doi.org/10.3390/nano11112950
Chicago/Turabian StyleXing, Weinan, Qi Liu, Jingyi Wang, Siye Xia, Li Ma, Ran Lu, Yujing Zhang, Yudong Huang, and Guangyu Wu. 2021. "High Selectivity and Reusability of Biomass-Based Adsorbent for Chloramphenicol Removal" Nanomaterials 11, no. 11: 2950. https://doi.org/10.3390/nano11112950
APA StyleXing, W., Liu, Q., Wang, J., Xia, S., Ma, L., Lu, R., Zhang, Y., Huang, Y., & Wu, G. (2021). High Selectivity and Reusability of Biomass-Based Adsorbent for Chloramphenicol Removal. Nanomaterials, 11(11), 2950. https://doi.org/10.3390/nano11112950








