Facile Synthesis of Peptide-Conjugated Gold Nanoclusters with Different Lengths
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Characterizations
2.3. Protein Engineering
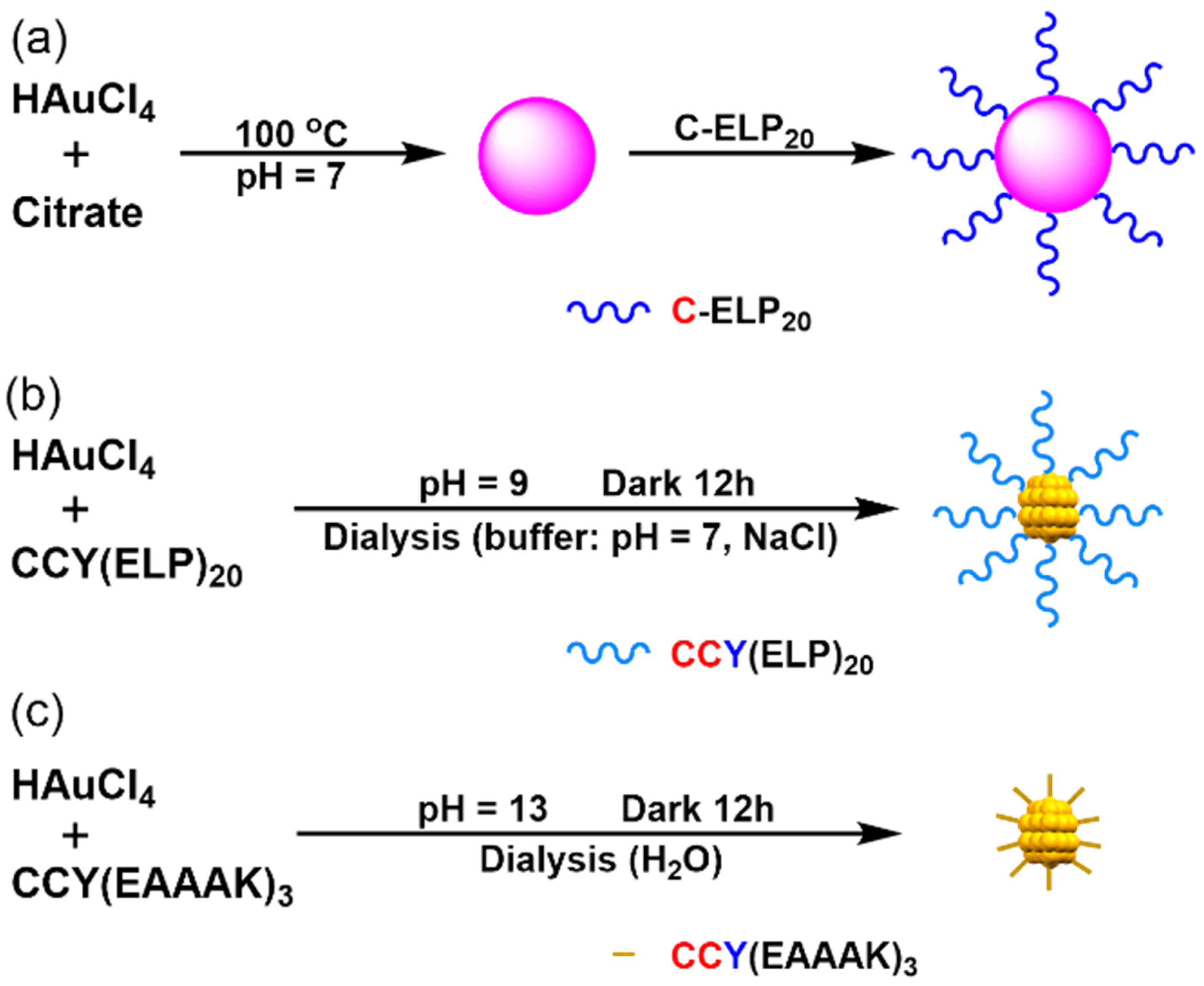
2.4. Synthesis of (ELP)20-Au Nanospheres
2.5. Synthesis of (ELP)20-Au Nanoclusters
2.6. Synthesis of (EAAAK)3-Au Nanoclusters
3. Results and Discussions
3.1. Synthesis and Characterization of Au-Cit and (ELP)20-Au NSs
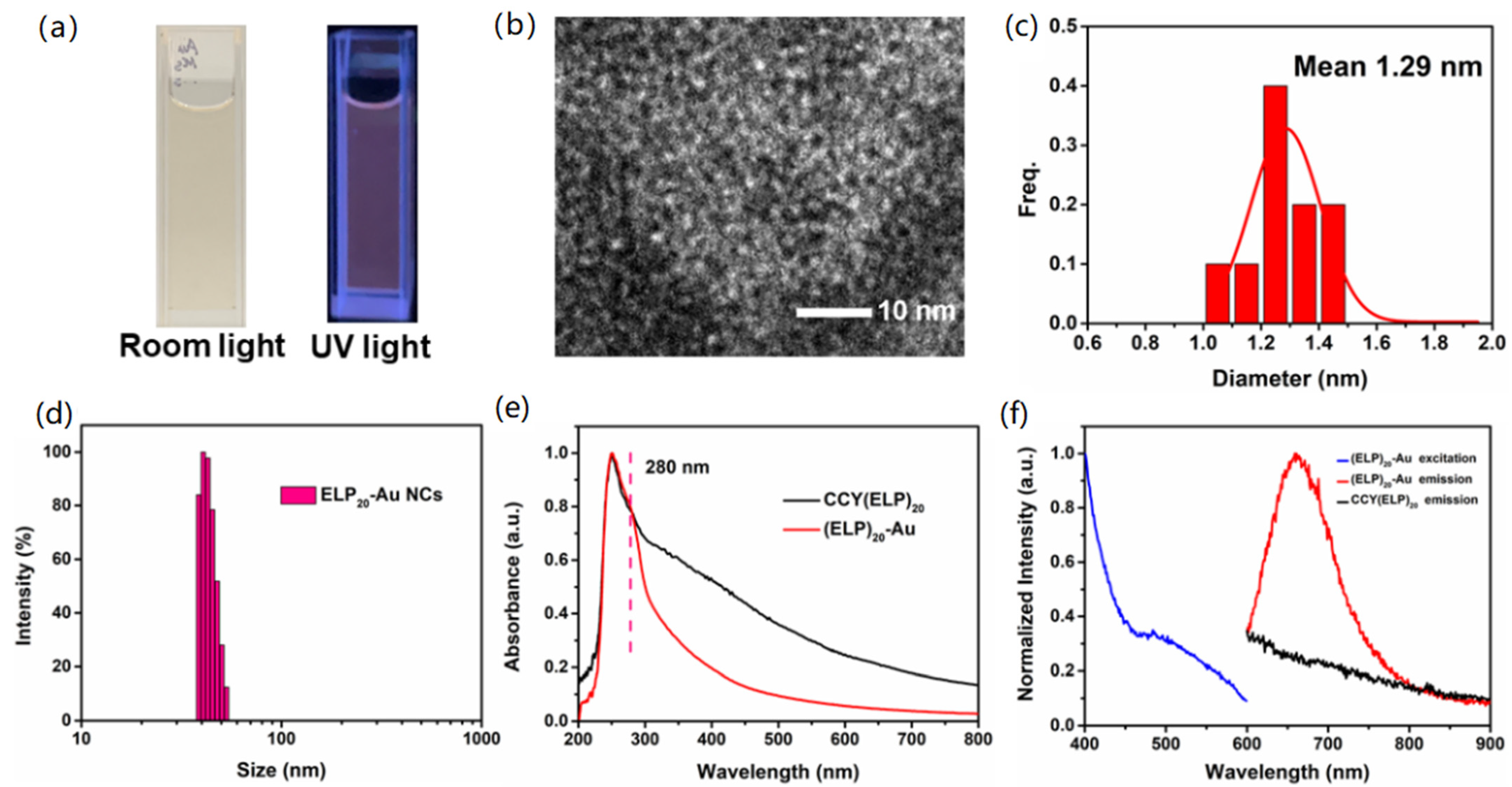
3.2. Synthesis and Characterization of (ELP)20-Au NCs
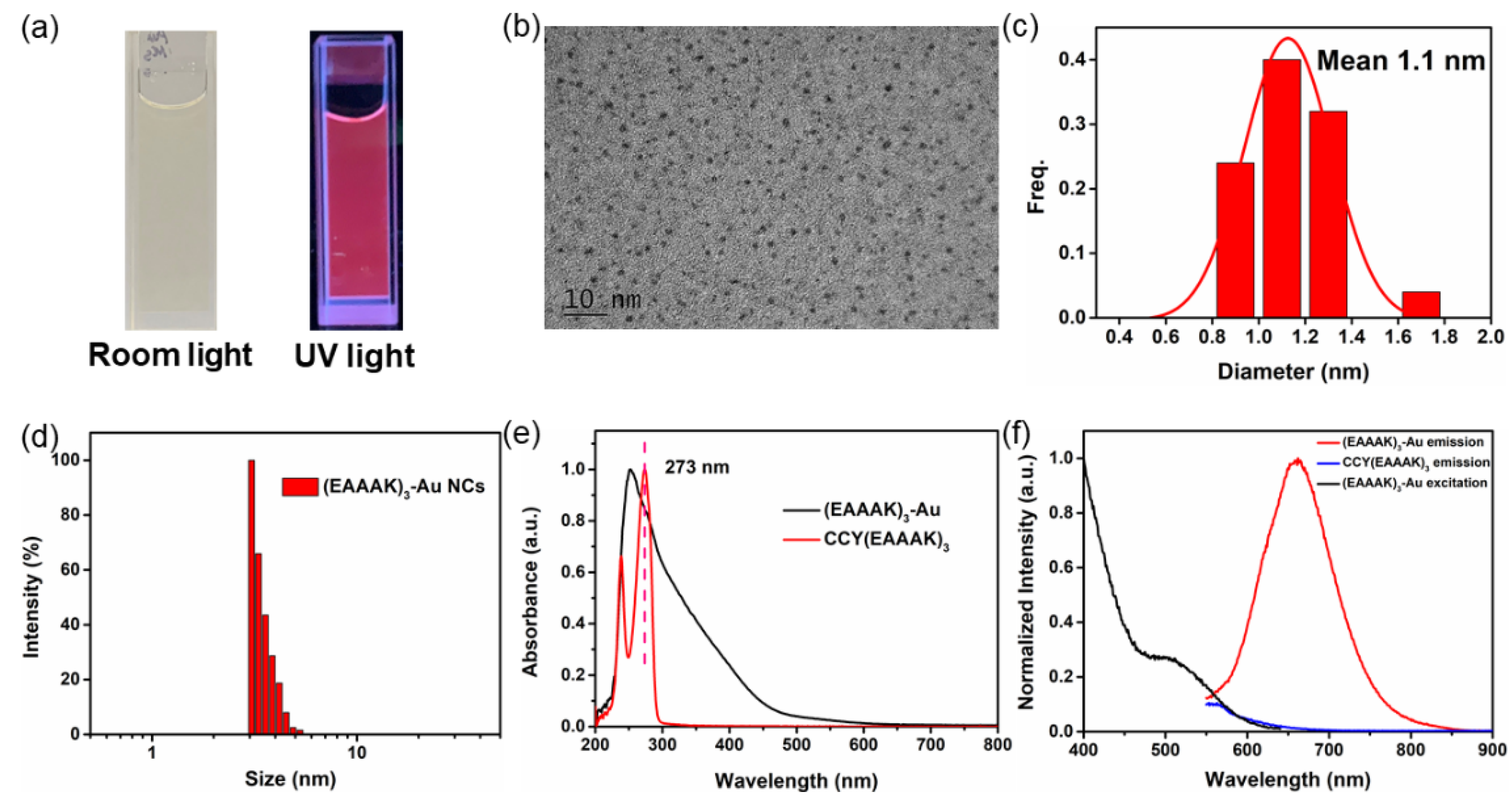
3.3. Synthesis and Characterization of (EAAAK)3-Au Nanoclusters
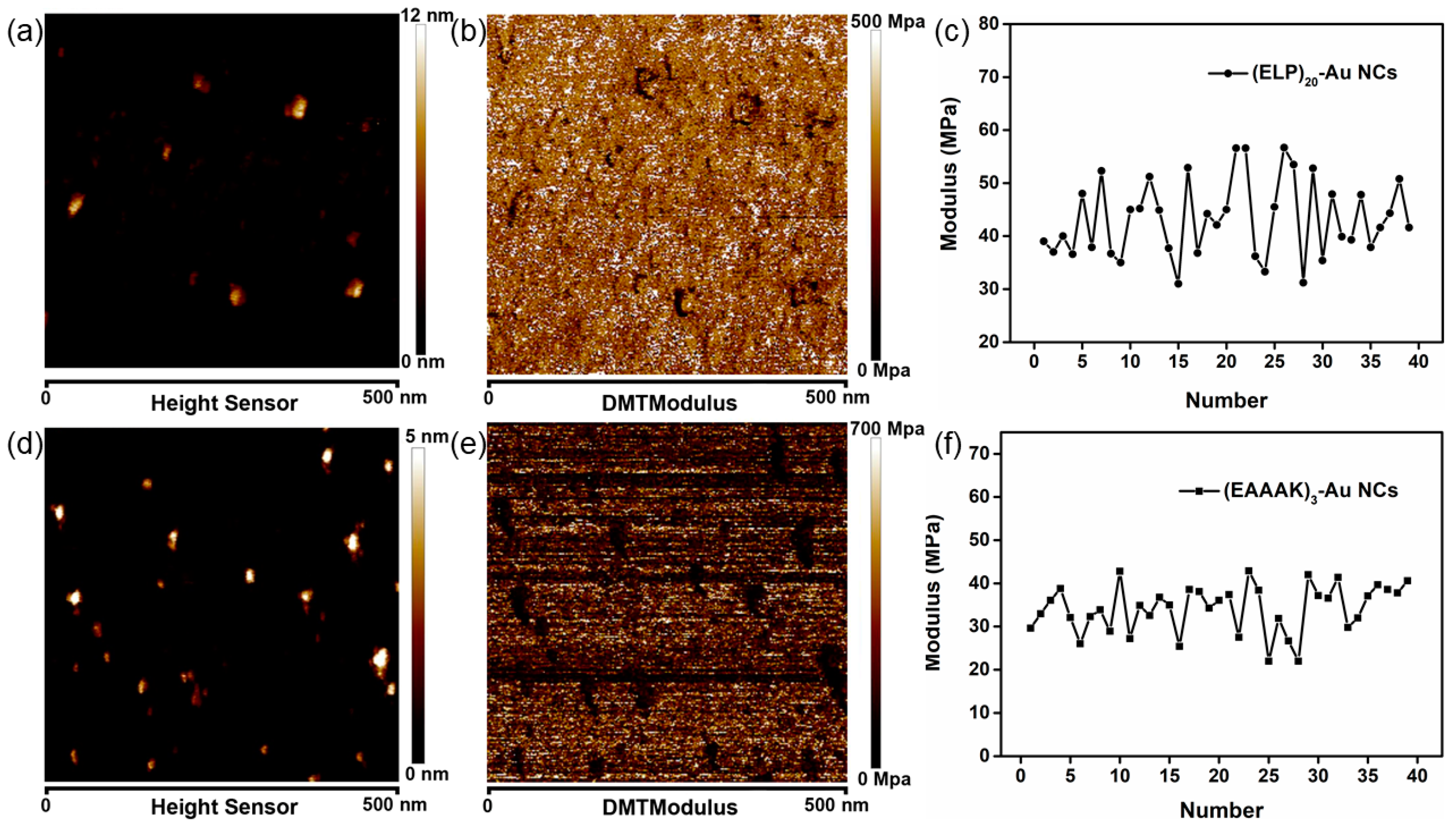
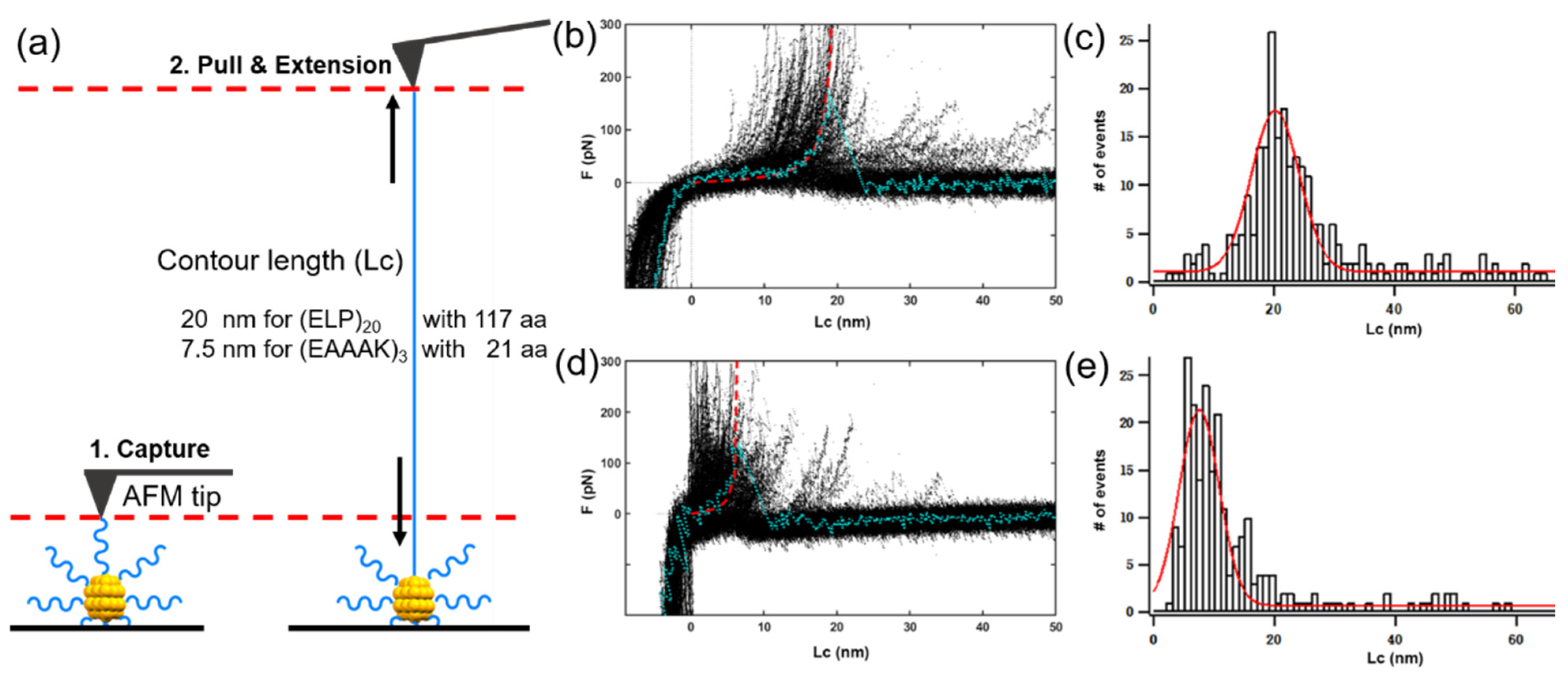
3.4. Characterization of Peptide-Modified Nanocluster by Atomic Force Microscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, Y.-H.; Jeon, J.; Hong, S.H.; Rhim, W.-K.; Lee, Y.-S.; Youn, H.; Chung, J.-K.; Lee, M.C.; Lee, D.S.; Kang, K.W.; et al. Tumor targeting and imaging using cyclic rgd-pegylated gold nanoparticle probes with directly conjugated iodine-125. Small 2011, 7, 2052–2060. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Peng, D.; Hao, H.; Hu, J.; Wang, D.; Wang, K.; Liu, J.; Guo, X.; Wei, Y.; Gao, W. Thermally triggered in situ assembly of gold nanoparticles for cancer multimodal imaging and photothermal therapy. ACS Appl. Mater. Interfaces 2017, 9, 10453–10460. [Google Scholar] [CrossRef]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold nanoparticles in chemical and biological sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [PubMed] [Green Version]
- Zhou, W.; Gao, X.; Liu, D.; Chen, X. Gold nanoparticles for in vitro diagnostics. Chem. Rev. 2015, 115, 10575–10636. [Google Scholar]
- Xiao, Y.; Wang, W.; Tian, X.; Tan, X.; Yang, T.; Gao, P.; Xiong, K.; Tu, Q.; Wang, M.; Maitz, M.F.; et al. A versatile surface bioengineering strategy based on mussel-inspired and bioclickable peptide mimic. Research 2020, 2020, 7236946. [Google Scholar] [CrossRef]
- Huang, T.; Zhao, M.; Yu, Q.; Feng, Z.; Xie, M.; Liu, S.; Zhang, K.Y.; Zhao, Q.; Huang, W. De novo design of polymeric carrier to photothermally release singlet oxygen for hypoxic tumor treatment. Research 2019, 2019, 9269081. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Zeng, C.; Li, Q.; Higaki, T.; Jin, R. Gold nanoclusters: Bridging gold complexes and plasmonic nanoparticles in photophysical properties. Nanomaterials 2019, 9, 933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visheratina, A.; Kotov, N.A. Inorganic nanostructures with strong chiroptical activity. CCS Chem. 2020, 2, 583–604. [Google Scholar] [CrossRef]
- Loynachan, C.N.; Soleimany, A.P.; Dudani, J.S.; Lin, Y.; Najer, A.; Bekdemir, A.; Chen, Q.; Bhatia, S.N.; Stevens, M.M. Renal clearable catalytic gold nanoclusters for in vivo disease monitoring. Nat. Nanotechnol. 2019, 14, 883–890. [Google Scholar] [CrossRef] [Green Version]
- Liang, G.; Jin, X.; Zhang, S.; Xing, D. Rgd peptide-modified fluorescent gold nanoclusters as highly efficient tumor-targeted radiotherapy sensitizers. Biomaterials 2017, 144, 95–104. [Google Scholar] [CrossRef]
- Liang, G.; Ye, D.; Zhang, X.; Dong, F.; Chen, H.; Zhang, S.; Li, J.; Shen, X.; Kong, J. One-pot synthesis of gd3+-functionalized gold nanoclusters for dual model (fluorescence/magnetic resonance) imaging. J. Mater. Chem. B 2013, 1, 3545–3552. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Zeng, C.; Zhou, M.; Chen, Y. Atomically precise colloidal metal nanoclusters and nanoparticles: Fundamentals and opportunities. Chem. Rev. 2016, 116, 10346–10413. [Google Scholar] [PubMed]
- Wang, Y.; Cui, Y.; Zhao, Y.; Liu, R.; Sun, Z.; Li, W.; Gao, X. Bifunctional peptides that precisely biomineralize au clusters and specifically stain cell nuclei. Chem. Commun. 2012, 48, 871–873. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Qin, Z.; Xu, H.; Li, G. Heterogeneous cross-coupling over gold nanoclusters. Nanomaterials 2019, 9, 838. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.; Sheng, H.; Astruc, D.; Zhu, M. Atomically precise noble metal nanoclusters as efficient catalysts: A bridge between structure and properties. Chem. Rev. 2020, 120, 526–622. [Google Scholar]
- Jiang, Y.; Miao, P. DNA dumbbell and chameleon silver nanoclusters for mirna logic operations. Research 2020, 2020, 1091605. [Google Scholar] [CrossRef] [Green Version]
- Tang, H.; Gu, Z.; Ding, H.; Li, Z.; Xiao, S.; Wu, W.; Jiang, X. Nanoscale crystalline sheets and vesicles assembled from nonplanar cyclic π-conjugated molecules. Research 2019, 2019, 1953926. [Google Scholar] [PubMed] [Green Version]
- Shoshan, M.S.; Vonderach, T.; Hattendorf, B.; Wennemers, H. Peptide-coated platinum nanoparticles with selective toxicity against liver cancer cells. Angew. Chem. Int. Ed. 2019, 58, 4901–4905. [Google Scholar] [CrossRef]
- Goswami, N.; Yao, Q.; Luo, Z.; Li, J.; Chen, T.; Xie, J. Luminescent metal nanoclusters with aggregation-induced emission. J. Phys. Chem. Lett. 2016, 7, 962–975. [Google Scholar] [CrossRef]
- Shang, L.; Dong, S.; Nienhaus, G.U. Ultra-small fluorescent metal nanoclusters: Synthesis and biological applications. Nano Today 2011, 6, 401–418. [Google Scholar]
- Huang, L.-y.; Yu, Y.-s.; Lu, X.; Ding, H.-m.; Ma, Y.Q. Designing a nanoparticle-containing polymeric substrate for detecting cancer cells by computer simulations. Nanoscale 2019, 11, 2170–2178. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Hung, M.J.; Chen, Y.A.; Wang, T.F.; Ou, Y.R.; Chen, S.H. Identifying reducing and capping sites of protein-encapsulated gold nanoclusters. Molecules 2019, 24, 1630. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Chu, K.; Liu, L. Zwitterionic gel coating endows gold nanoparticles with ultrastability. Langmuir 2019, 35, 1369–1378. [Google Scholar] [CrossRef]
- Su, D.; Gao, L.; Gao, F.; Zhang, X.; Gao, X. Peptide and protein modified metal clusters for cancer diagnostics. Chem. Sci. 2020, 11, 5614–5629. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Uertz, J.; Yohan, D.; Chithrani, B.D. Peptide modified gold nanoparticles for improved cellular uptake, nuclear transport, and intracellular retention. Nanoscale 2014, 6, 12026–12033. [Google Scholar] [CrossRef]
- Ni, C.; Zhou, J.; Kong, N.; Bian, T.; Zhang, Y.; Huang, X.; Xiao, Y.; Yang, W.; Yan, F. Gold nanoparticles modulate the crosstalk between macrophages and periodontal ligament cells for periodontitis treatment. Biomaterials 2019, 206, 115–132. [Google Scholar] [CrossRef]
- Yan, L.; Cai, Y.; Zheng, B.; Yuan, H.; Guo, Y.; Xiao, D.; Choi, M.M.F. Microwave-assisted synthesis of bsa-stabilized and hsa-protected gold nanoclusters with red emission. J. Mater. Chem. 2012, 22, 1000–1005. [Google Scholar] [CrossRef]
- Goswami, N.; Zheng, K.; Xie, J. Bio-ncs—The marriage of ultrasmall metal nanoclusters with biomolecules. Nanoscale 2014, 6, 13328–13347. [Google Scholar] [CrossRef]
- Chen, Y.; Phipps, M.L.; Werner, J.H.; Chakraborty, S.; Martinez, J.S. DNA templated metal nanoclusters: From emergent properties to unique applications. Acc. Chem. Res. 2018, 51, 2756–2763. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.-D.; Cong, V.T.; Baek, C.; Min, J. Fabrication of peptide stabilized fluorescent gold nanocluster/graphene oxide nanocomplex and its application in turn-on detection of metalloproteinase-9. Biosens. Bioelectron. 2017, 89, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Nonappa, N. Luminescent gold nanoclusters for bioimaging applications. Beilstein J. Nanotechnol. 2020, 11, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Wu, S.; Chang, X.; Liu, F.; Zhang, T.; Wang, B.; Zhang, K.-Q. Aprotinin encapsulated gold nanoclusters: A fluorescent bioprobe with dynamic nuclear targeting and selective detection of trypsin and heavy metal. Bioconjug. Chem. 2018, 29, 4140–4148. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, S.; Khamari, L.; Nandi, S.; Mukherjee, S. Discriminating single base pair mismatches in DNA using glutathione-templated copper nanoclusters. J. Phys. Chem. C 2019, 123, 29047–29056. [Google Scholar] [CrossRef]
- Yarramala, D.S.; Baksi, A.; Pradeep, T.; Rao, C.P. Green synthesis of protein-protected fluorescent gold nanoclusters (auncs): Reducing the size of auncs by partially occupying the ca2+ site by la3+ in apo-α-lactalbumin. ACS Sustain. Chem. Eng. 2017, 5, 6064–6069. [Google Scholar] [CrossRef]
- Neupane, S.; Pan, Y.; Li, H.; Patnode, K.; Farmakes, J.; Liu, G.; Yang, Z. Engineering protein–gold nanoparticle/nanorod complexation via surface modification for protein immobilization and potential therapeutic applications. ACS Appl. Nano Mater. 2018, 1, 4053–4063. [Google Scholar] [CrossRef]
- Rival, J.V.; Mymoona, P.; Lakshmi, K.M.; Pradeep, T.; Shibu, E.S. Self-assembly of precision noble metal nanoclusters: Hierarchical structural complexity, colloidal superstructures, and applications. Small 2021, 17, 2005718. [Google Scholar] [CrossRef] [PubMed]
- Azubel, M.; Carter, S.D.; Weiszmann, J.; Zhang, J.; Jensen, G.J.; Li, Y.; Kornberg, R.D. Fgf21 trafficking in intact human cells revealed by cryo-electron tomography with gold nanoparticles. Elife 2019, 8, e43146. [Google Scholar] [CrossRef]
- Lundahl, M.L.E.; Fogli, S.; Colavita, P.E.; Scanlan, E.M. Aggregation of protein therapeutics enhances their immunogenicity: Causes and mitigation strategies. RSC Chem. Biol. 2021, 2, 1004–1020. [Google Scholar] [CrossRef]
- Xie, J.; Zheng, Y.; Ying, J.Y. Protein-directed synthesis of highly fluorescent gold nanoclusters. J. Am. Chem. Soc. 2009, 131, 888–889. [Google Scholar] [CrossRef]
- Ueda, G.; Antanasijevic, A.; Fallas, J.A.; Sheffler, W.; Copps, J.; Ellis, D.; Hutchinson, G.B.; Moyer, A.; Yasmeen, A.; Tsybovsky, Y.; et al. Tailored design of protein nanoparticle scaffolds for multivalent presentation of viral glycoprotein antigens. Elife 2020, 9, e57659. [Google Scholar] [CrossRef]
- Fauser, J.; Savitskiy, S.; Fottner, M.; Trauschke, V.; Gulen, B. Sortase-mediated quantifiable enzyme immobilization on magnetic nanoparticles. Bioconjug. Chem. 2020, 31, 1883–1892. [Google Scholar] [CrossRef]
- Tian, F.; Li, G.; Zheng, B.; Liu, Y.; Shi, S.; Deng, Y.; Zheng, P. Verification of sortase for protein conjugation by single-molecule force spectroscopy and molecular dynamics simulations. Chem. Commun. 2020, 56, 3943–3946. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zheng, B.; Liu, Y.; Shi, S.; Nie, J.; Wu, T.; Zheng, P. Oaaep1-mediated enzymatic synthesis and immobilization of polymerized protein for single-molecule force spectroscopy. JoVE 2020, 156, e60774. [Google Scholar] [CrossRef]
- Higashi, N.; Ochiai, T.; Kanazawa, C.; Koga, T. Site-specific adsorption of gold nanoparticles coated with thermo-responsive peptides. Polym. J. 2013, 45, 523–528. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.P.; Ta, D.T.; Nash, M.A. Mechanical polyprotein assembly using sfp and sortase-mediated domain oligomerization for single-molecule studies. Small Methods 2018, 2, 1800039. [Google Scholar] [CrossRef]
- Ott, W.; Jobst, M.A.; Bauer, M.S.; Durner, E.; Milles, L.F.; Nash, M.A.; Gaub, H.E. Elastin-like polypeptide linkers for single-molecule force spectroscopy. Acs Nano 2017, 11, 6346–6354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ta, D.T.; Vanella, R.; Nash, M.A. Bioorthogonal elastin-like polypeptide scaffolds for immunoassay enhancement. ACS Appl. Mater. Interfaces 2018, 10, 30147–30154. [Google Scholar] [CrossRef] [Green Version]
- Yuan, G.; Liu, H.; Ma, Q.; Li, X.; Nie, J.; Zuo, J.; Zheng, P. Single-molecule force spectroscopy reveals that iron–ligand bonds modulate proteins in different modes. J. Phys. Chem. Lett. 2019, 10, 5428–5433. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Liu, Z.; Liu, H.; Nash, M.A. Next generation methods for single-molecule force spectroscopy on polyproteins and receptor-ligand complexes. Front. Mol. Biosci. 2020, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Ding, X.; Liu, H.; Yuan, G.; Tian, F.; Shi, S.; Yang, Y.; Li, G.; Zheng, P. Single-molecule force spectroscopy reveals that the fe–n bond enables multiple rupture pathways of the 2fe2s cluster in a mitoneet monomer. Anal. Chem. 2020, 92, 14783–14789. [Google Scholar] [CrossRef]
- Chen, X.; Zaro, J.L.; Shen, W.-C. Fusion protein linkers: Property, design and functionality. Adv. Drug Deliv. Rev. 2013, 65, 1357–1369. [Google Scholar] [CrossRef] [Green Version]
- Bastús, N.G.; Comenge, J.; Puntes, V. Kinetically controlled seeded growth synthesis of citrate-stabilized gold nanoparticles of up to 200 nm: Size focusing versus ostwald ripening. Langmuir 2011, 27, 11098–11105. [Google Scholar] [CrossRef]
- Liu, Y.; Shipton, M.K.; Ryan, J.; Kaufman, E.D.; Franzen, S.; Feldheim, D.L. Synthesis, stability, and cellular internalization of gold nanoparticles containing mixed peptide−poly(ethylene glycol) monolayers. Anal. Chem. 2007, 79, 2221–2229. [Google Scholar] [CrossRef]
- Yuan, Q.; Wang, Y.; Zhao, L.; Liu, R.; Gao, F.; Gao, L.; Gao, X. Peptide protected gold clusters: Chemical synthesis and biomedical applications. Nanoscale 2016, 8, 12095–12104. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Wu, T.; Wang, M.; Shi, S.; Yuan, G.; Li, X.; Chong, H.; Wu, B.; Zheng, P. Enzymatic biosynthesis and immobilization of polyprotein verified at the single-molecule level. Nat. Commun. 2019, 10, 2775–2785. [Google Scholar] [CrossRef]
- Garg, S.; Singaraju, G.S.; Yengkhom, S.; Rakshit, S. Tailored polyproteins using sequential staple and cut. Bioconj. Chem. 2018, 29, 1714–1719. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, C.; Li, J.; Zhang, J.; Fan, C.; Willner, I.; Tian, H. Functional DNA structures and their biomedical applications. CCS Chem. 2020, 2, 707–728. [Google Scholar] [CrossRef]
- Yang, B.; Liu, H.; Liu, Z.; Doenen, R.; Nash, M.A. Influence of fluorination on single-molecule unfolding and rupture pathways of a mechanostable protein adhesion complex. Nano Lett. 2020, 20, 8940–8950. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Ma, Q.; Wu, T.; Wang, M.; Li, X.; Zuo, J.; Zheng, P. Multistep protein unfolding scenarios from the rupture of a complex metal cluster cd3s9. Sci. Rep. 2019, 9, 10518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, F.; Tong, B.; Sun, L.; Shi, S.; Zheng, B.; Wang, Z.; Dong, X.; Zheng, P. N501Y mutation of spike protein in sars-cov-2 strengthens its binding to receptor ace2. Elife 2021, 10, e69091. [Google Scholar] [CrossRef]
- Cao, W.; Dong, C.; Kim, S.; Hou, D.; Tai, W.; Du, L.; Im, W.; Zhang, X.F. Biomechanical characterization of sars-cov-2 spike rbd and human ace2 protein-protein interaction. Biophys. J. 2021, 120, 1011–1019. [Google Scholar] [CrossRef]
- Khoury, L.R.; Popa, I. Chemical unfolding of protein domains induces shape change in programmed protein hydrogels. Nat. Commun. 2019, 10, 5439. [Google Scholar] [CrossRef] [PubMed]
- Rico, F.; Russek, A.; Gonzalez, L.; Grubmuller, H.; Scheuring, S. Heterogeneous and rate-dependent streptavidin-biotin unbinding revealed by high-speed force spectroscopy and atomistic simulations. Proc. Natl. Acad. Sci. USA 2019, 116, 6594–6601. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Qian, H.-j.; Liu, Z.; Ju, H.; Lu, Z.-y.; Zhang, H.; Chi, L.; Cui, S. Towards unveiling the exact molecular structure of amorphous red phosphorus by single-molecule studies. Angew. Chem. Int. Ed. 2019, 58, 1659–1663. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Li, X.; Li, H.; Zhang, W. Quantifying thiol–gold interactions towards the efficient strength control. Nat. Commun. 2014, 5, 4348–4356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.; Zhu, Z.; Wen, J.; Wang, X.; Qin, M.; Cao, Y.; Ma, H.; Wang, W. Single molecule study of force-induced rotation of carbon–carbon double bonds in polymers. ACS Nano 2017, 11, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, H. Single molecule force spectroscopy reveals that a two-coordinate ferric site is critical for the folding of holo-rubredoxin. Nanoscale 2020, 12, 22564–22573. [Google Scholar] [CrossRef]
- Le, S.; Yu, M.; Yan, J. Direct single-molecule quantification reveals unexpectedly high mechanical stability of vinculin-talin/alpha-catenin linkages. Sci. Adv. 2019, 5, eaav2720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.; Wu, X.; Gao, X.; Yu, Y.; Lei, H.; Zhu, Z.; Shi, Y.; Chen, Y.; Qin, M.; Wang, W.; et al. Maleimide–thiol adducts stabilized through stretching. Nat. Chem. 2019, 11, 310–319. [Google Scholar] [CrossRef]
- Li, Y.R.; Wen, J.; Qin, M.; Cao, Y.; Ma, H.B.; Wane, W. Single-molecule mechanics of catechol-iron coordination bonds. ACS Biomater. Sci. Eng. 2017, 3, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Brückner, S.; Schubert, R.; Kraushaar, T.; Hartmann, R.; Hoffmann, D.; Jelli, E.; Drescher, K.; Müller, D.J.; Oliver Essen, L.; Mösch, H.-U. Kin discrimination in social yeast is mediated by cell surface receptors of the flo11 adhesin family. Elife 2020, 9, e55587. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Heenan, P.R.; Edwards, D.T.; Uyetake, L.; Perkins, T.T. Quantifying the initial unfolding of bacteriorhodopsin reveals retinal stabilization. Angew. Chem. Int. Ed. 2019, 58, 1710–1713. [Google Scholar] [CrossRef]
- Yin, Z.; Song, G.; Jiao, Y.; Zheng, P.; Xu, J.-F.; Zhang, X. Dissipative supramolecular polymerization powered by light. CCS Chemistry 2019, 1, 335–342. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Zhao, X.; He, B.; Zhao, Z.; Zheng, Z.; Zhang, P.; Shi, X.; Kwok, R.T.K.; Lam, J.W.Y.; Qin, A.; et al. A simple approach to bioconjugation at diverse levels: Metal-free click reactions of activated alkynes with native groups of biotargets without prefunctionalization. Research 2018, 2018, 3152870. [Google Scholar] [CrossRef] [Green Version]
- Shi, S.; Wang, Z.; Deng, Y.; Tian, F.; Wu, Q.; Zheng, P. Combination of click chemistry and enzymatic ligation for stable and efficient protein immobilization for single-molecule force spectroscopy. CCS Chem. 2021, 3, 841–847. [Google Scholar] [CrossRef]
- Fu, L.; Wang, H.; Li, H. Harvesting mechanical work from folding-based protein engines: From single-molecule mechanochemical cycles to macroscopic devices. CCS Chem. 2019, 1, 138–147. [Google Scholar] [CrossRef]
- Xing, H.; Li, Z.; Wang, W.; Liu, P.; Liu, J.; Song, Y.; Wu, Z.L.; Zhang, W.; Huang, F. Mechanochemistry of an interlocked poly [2]catenane: From single molecule to bulk gel. CCS Chem. 2020, 2, 513–523. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Q.; Liu, L.; Yang, Z.; Zheng, P. Facile Synthesis of Peptide-Conjugated Gold Nanoclusters with Different Lengths. Nanomaterials 2021, 11, 2932. https://doi.org/10.3390/nano11112932
Ma Q, Liu L, Yang Z, Zheng P. Facile Synthesis of Peptide-Conjugated Gold Nanoclusters with Different Lengths. Nanomaterials. 2021; 11(11):2932. https://doi.org/10.3390/nano11112932
Chicago/Turabian StyleMa, Qun, Lichao Liu, Zeyue Yang, and Peng Zheng. 2021. "Facile Synthesis of Peptide-Conjugated Gold Nanoclusters with Different Lengths" Nanomaterials 11, no. 11: 2932. https://doi.org/10.3390/nano11112932
APA StyleMa, Q., Liu, L., Yang, Z., & Zheng, P. (2021). Facile Synthesis of Peptide-Conjugated Gold Nanoclusters with Different Lengths. Nanomaterials, 11(11), 2932. https://doi.org/10.3390/nano11112932





