Potential Production of Theranostic Boron Nitride Nanotubes (64Cu-BNNTs) Radiolabeled by Neutron Capture
Abstract
:1. Introduction
2. Experiment
2.1. Raw Materials
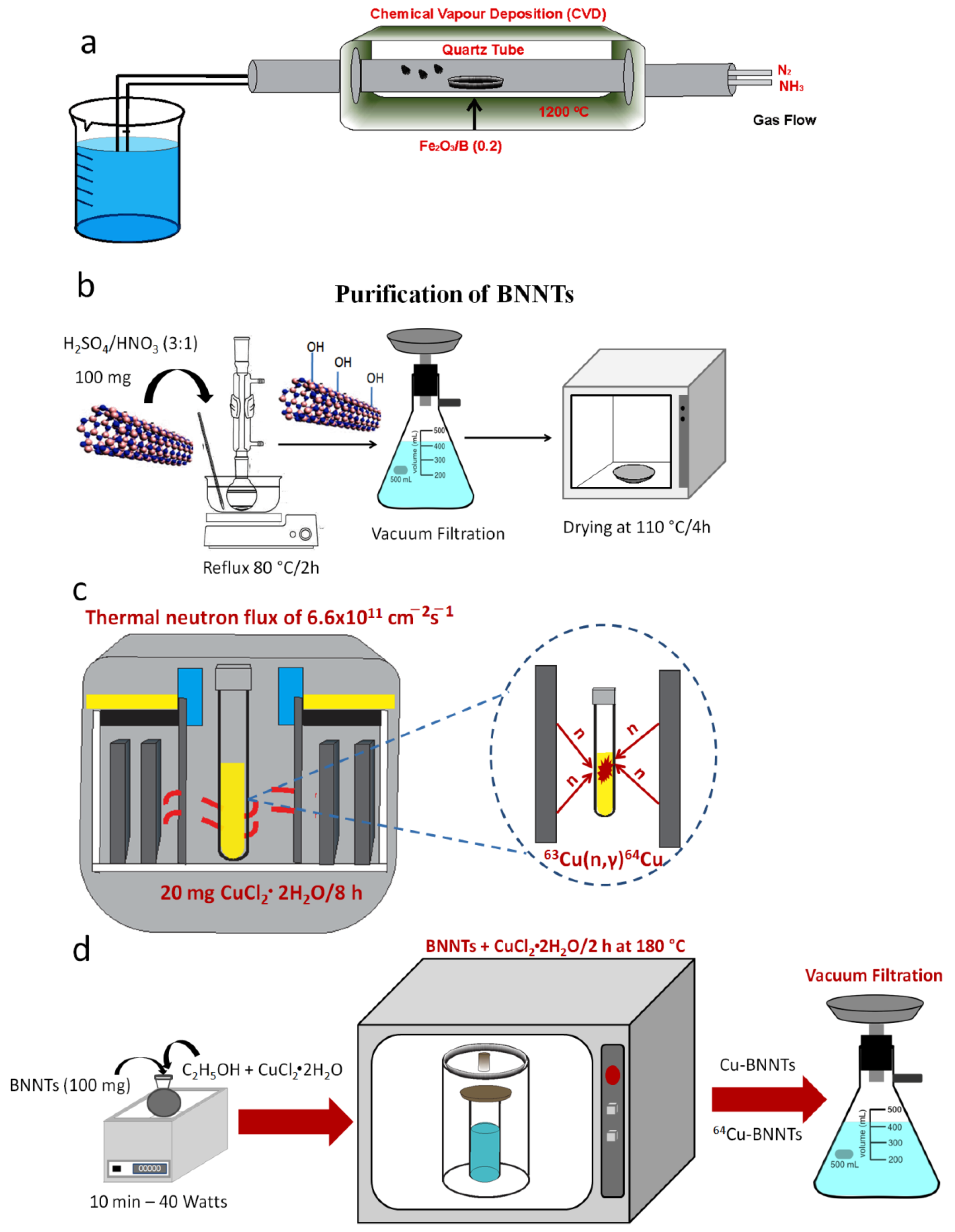
2.2. Synthesis and Purification of Boron Nitride Nanotubes
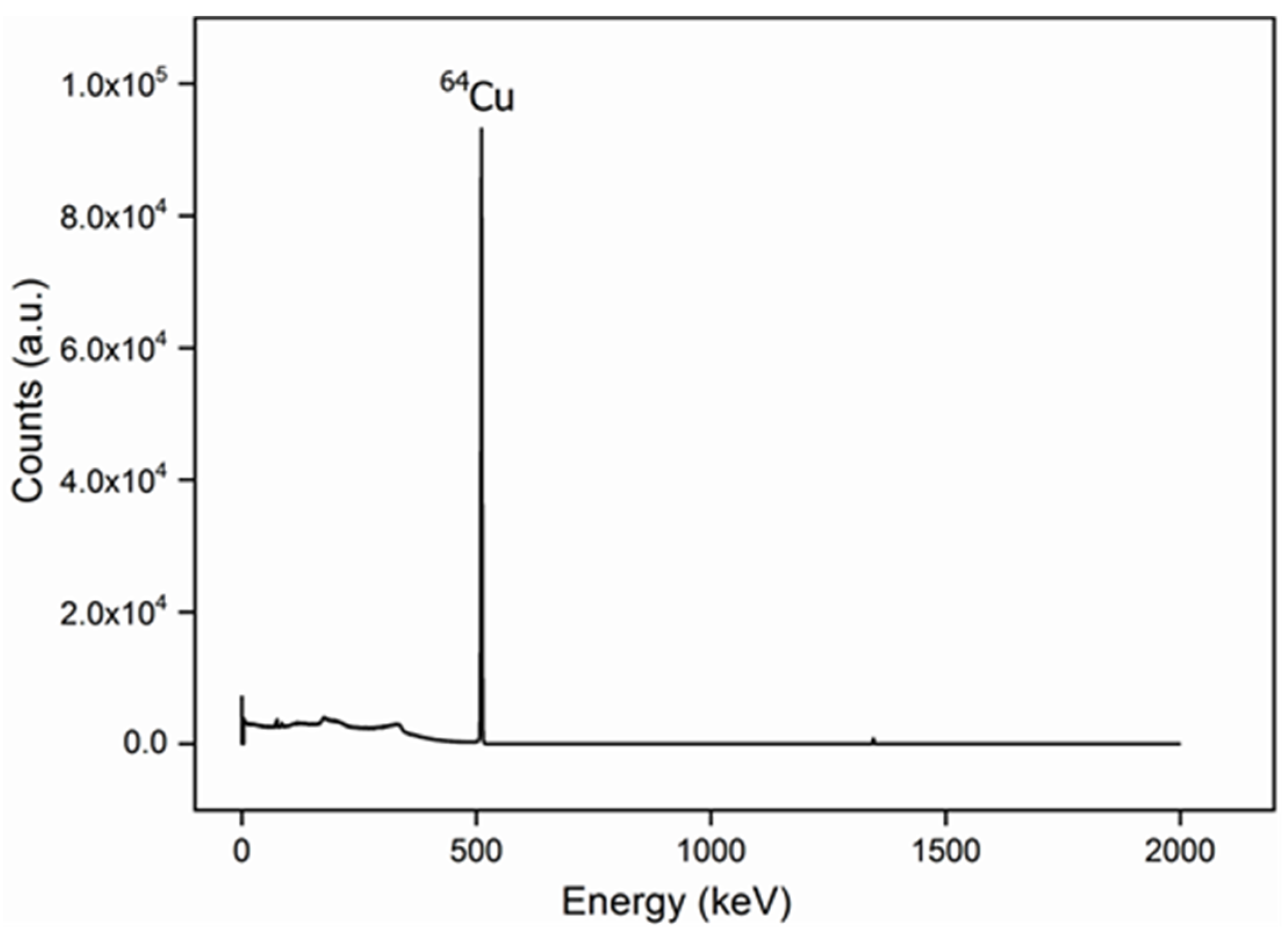
2.2.1. Activation Process of 64Cu Radioisotope
2.2.2. Incorporation of Cu and 64Cu to the BNNT Samples
3. Characterization
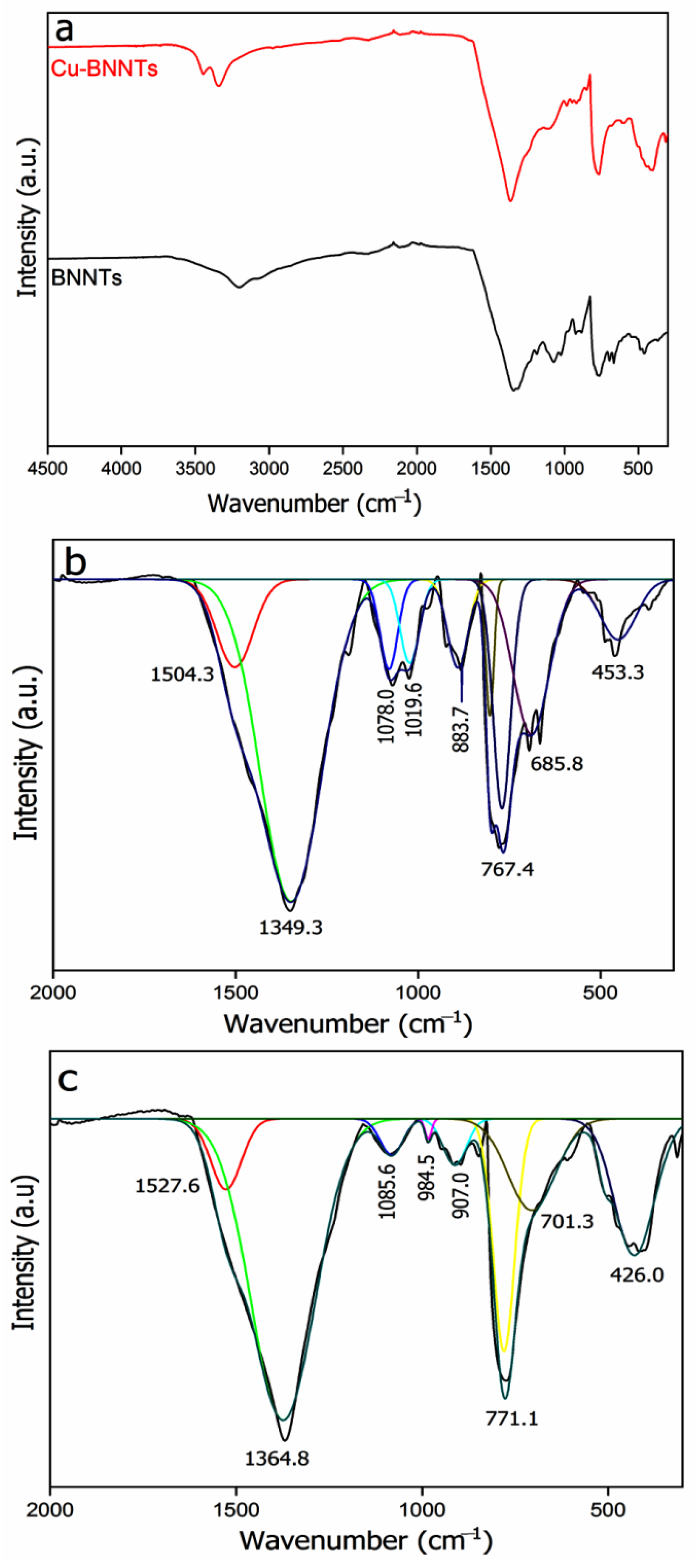
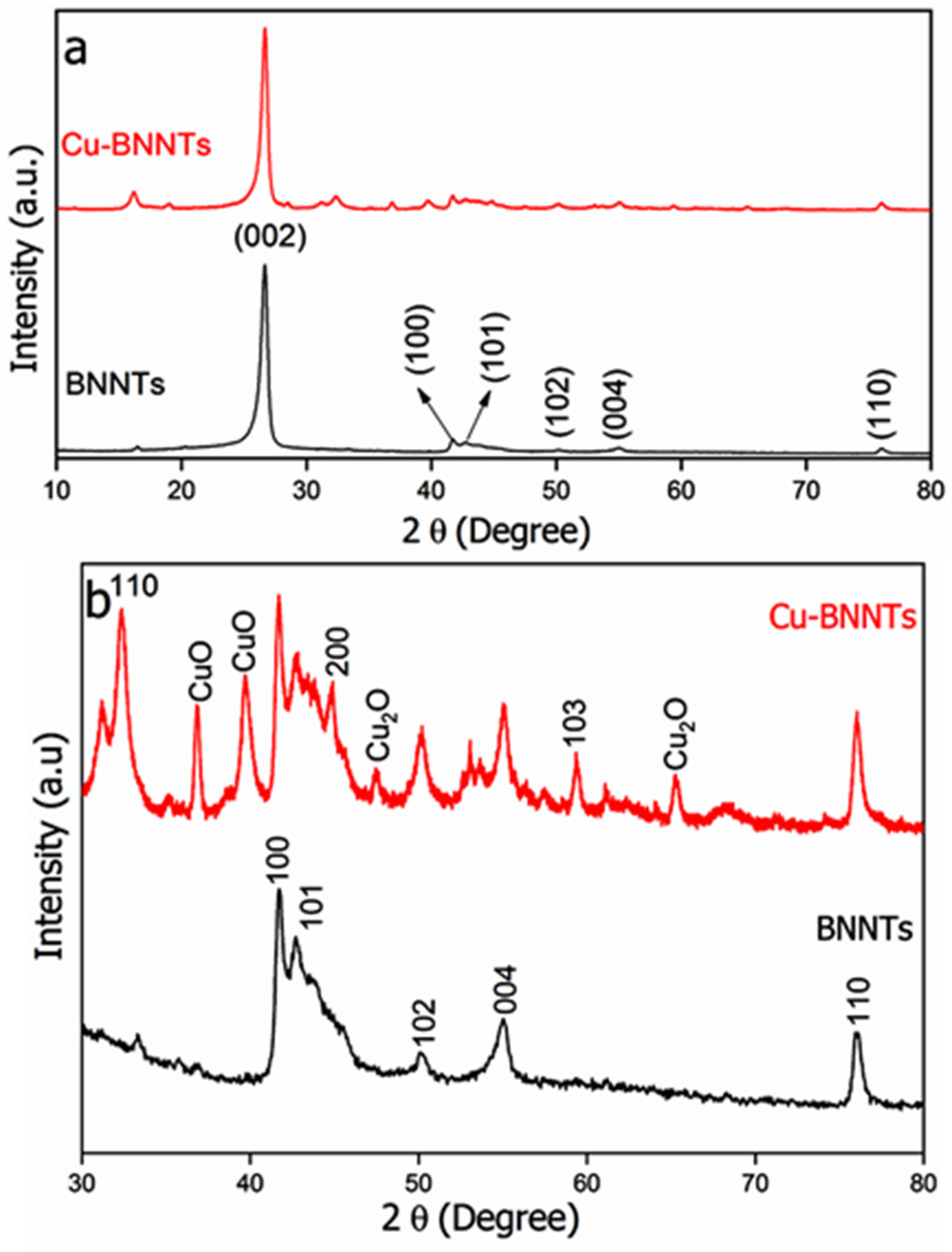
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chopra, N.G.; Luyken, R.J.; Cherrey, K.; Crespi, V.H.; Cohen, M.L.; Louie, S.G.; Zettl, A. Boron Nitride Nanotubes. Science 1995, 269, 966–967. [Google Scholar] [CrossRef]
- Terrones, M.; Romo-Herrera, J.M.; Cruz-Silva, E.; López-Urías, F.; Muñoz-Sandoval, E.; Velázquez-Salazar, J.J.; Terrones, H.; Bando, Y.; Golberg, D. Pure and dopedboronnitridenanotubes. Mater. Today 2007, 10, 30–38. [Google Scholar] [CrossRef]
- Golberg, D.; Bando, Y.; Huang, Y.; Terao, T.; Mitome, M.; Tang, C.; Zhi, C. Boron nitride nanotubes and nanosheets. ACS Nano 2010, 4, 2979–2993. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zou, J.; Campbell, S.J.; le Caer, G. Boron nitride nanotubes: Pronounced resistance to oxidation. Appl. Phys. Lett. 2004, 84, 2430–2432. [Google Scholar] [CrossRef] [Green Version]
- Zhi, C.; Bando, Y.; Tang, C.; Golberg, D. Boron nitride nanotubes. Mater. Sci. Eng. R Rep. 2010, 70, 92–111. [Google Scholar] [CrossRef]
- Golberg, D.; Bando, Y.; Tang, C.; Zhi, C. Boron Nitride Nanotubes. Adv. Mater. 2007, 19, 2413–2432. [Google Scholar] [CrossRef]
- Cohen, M.L.; Zettl, A. The physics of boron nitride nanotubes. Phys. Today 2010, 63, 34–38. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.; Zhang, X.; Puthirath, A.B.; Meiyazhagan, A.; Bhattacharyya, S.; Rahman, M.M.; Babu, G.; Susarla, S.; Saju, S.K.; Tran, M.K.; et al. Structure, Properties and Applications of Two-Dimensional Hexagonal Boron Nitride. Adv. Mater. 2021, 2021, 2101589. [Google Scholar] [CrossRef]
- Ahmad, P.; Khandaker, M.U.; Amin, Y.M.; Khan, Z.R. Influence of growth duration on size and morphology of boron nitride nanotubes grown via chemical vapor deposition technique. J. Phys. Chem. Solids 2015, 85, 226–232. [Google Scholar] [CrossRef]
- Guo, L.; Singh, R.N. Catalytic growth of boron nitride nanotubes using gas precursors. Phys. E Low-Dimens. Syst. Nanostruct. 2009, 41, 448–453. [Google Scholar] [CrossRef]
- Ciofani, G.; Raffa, V.; Menciassi, A.; Cuschieri, A. Boron nitride nanotubes: An innovative tool for nanomedicine. In Vitro 2009, 4, 8–10. [Google Scholar] [CrossRef]
- Ciofani, G.; Danti, S.; Genchi, G.G.; Mazzolai, B.; Mattoli, V. Boron nitride nanotubes: Biocompatibility and potential spill-over in nanomedicine. Small 2013, 9, 1672–1685. [Google Scholar] [CrossRef] [PubMed]
- Soares, D.C.F.; Ferreira, T.H.; Ferreira, C.D.A.; Cardoso, V.N.; de Sousa, E.M.B. Boron nitride nanotubes radiolabeled with 99mTc: Preparation, physicochemical characterization, biodistribution study, and scintigraphic imaging in Swiss mice. Int. J. Pharm. 2012, 423, 489–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Silva, W.M.; Silva, R.H.d.A.e.; Cipreste, M.F.; Andrade, G.F.; Gastelois, P.L.; Macedo, W.A.d.; de Sousa, E.M.B. Boron nitride nanotubes radiolabeled with 153Sm and 159Gd: Potential application in nanomedicine. Appl. Radiat. Isot 2020, 2020, 157. [Google Scholar] [CrossRef] [PubMed]
- Zangirolami, D.M.; Oliveira, A.H.; Ferreira, A.V. Thermal and Epithermal Neutron Fluence Rates in the Irradiation Facilities of the TRIGA IPR-R1 Nuclear Reactor. Brazilian J. Phys. 2010, 2021, 40. [Google Scholar] [CrossRef] [Green Version]
- Mughabghab, S.F. Resonance Integrals and G-Factors. Int. Nucl. Data Commun. 2003, 11376, 7. [Google Scholar]
- Hossain, I.; Sharip, N.; Viswanathan, K.K. Efficiency and resolution of HPGe and NaI(Tl) detectors using gamma-ray spectroscopy. Sci. Res. Essays 2012, 7, 86–89. [Google Scholar] [CrossRef]
- Végh, J. The Shirley background revised. J. Electron Spectros. Relat. Phenomena 2006, 151, 159–164. [Google Scholar] [CrossRef]
- Végh, J. The analytical form of the Shirley-type background. J. Electron Spectros. Relat. Phenomena 1988, 46, 411–417. [Google Scholar] [CrossRef]
- Nazar, A.M.D.; Silva, W.M.; de Oliveira, E.F.; de Sousa, E.M.B. Boron nitride nanotubes decorated with magnetite nanoparticles for application as a multifunctional system in cancer treatment. Nano-Struct. Nano-Objects 2020, 24, 100616. [Google Scholar] [CrossRef]
- da Silva, W.M.; Monteiro, G.A.A.; Gastelois, P.L.; de Sousa, R.G.; Macedo, W.A.d.; Sousa, E.M.B. Efficient sensitive polymer-grafted boron nitride nanotubes by microwave-assisted process. Nano-Struct. Nano-Objects 2018, 15, 186–196. [Google Scholar] [CrossRef]
- Harrison, H.; Lamb, J.T.; Nowlin, K.S.; Guenthner, A.J.; Ghiassi, K.B.; Kelkar, A.D.; Alston, J.R. Quantification of hexagonal boron nitride impurities in boron nitride nanotubes: Via FTIR spectroscopy. Nanoscale Adv. 2019, 1, 1693–1701. [Google Scholar] [CrossRef] [Green Version]
- da Silva, W.M.; Ribeiro, H.; Ferreira, T.H.; Ladeira, L.O.; Sousa, E.M.B. Synthesis of boron nitride nanostructures from catalyst of iron compounds via thermal chemical vapor deposition technique. Phys. E Low-Dimens. Syst. Nanostruct. 2017, 89, 177–182. [Google Scholar] [CrossRef]
- Petrov, T.; Markova-Deneva, I.; Chauvet, O.; Nikolov, R.; Denev, I. Sem and ft-ir spectroscopy study of Cu, Sn and Cu-Sn nanoparticles. J. Univ. Chem. Technol. Metall. 2012, 47, 197–206. [Google Scholar]
- Amelkovich, Y.A.; Nazarenko, O.B.; Sechin, A.I.; Visakh, P.M. Characterization of copper nanopowders after natural aging. IOP Conf. Ser. Mater. Sci. Eng. 2015, 81, 012072. [Google Scholar] [CrossRef] [Green Version]
- da Silva, W.M.; Ferreira, T.H.; de Morais, C.A.; Leal, A.S.; Sousa, E.M.B. Samarium doped boron nitride nanotubes. Appl. Radiat. Isot 2018, 131, 30–35. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Shrivastava, V.; Tomar, R.S. Biofabrication of copper nanoparticles: A next-generation antibacterial agent against wound-associated pathogens. Turkish J. Pharm. Sci. 2018, 15, 238–247. [Google Scholar]
- Betancourt-Galindo, R.; Reyes-Rodriguez, P.Y.; Puente-Urbina, B.A.; Avila-Orta, C.A.; Rodríguez-Fernández, O.S.; Cadenas-Pliego, G.; Lira-Saldivar, R.H.; García-Cerda, L.A. Synthesis of copper nanoparticles by thermal decomposition and their antimicrobial properties. J. Nanomater. 2014, 2014, 10. [Google Scholar] [CrossRef] [Green Version]
- Shirui, Z.; Yabin, D.; Mingyan, Y.; Xiaolong, G.; Xinwei, X.; Yupeng, J.; Yang, X. Microwave annealing effects on ZnO films deposited by atomic layer deposition. J. Semicond. 2014, 35, 1–7. [Google Scholar]
- Li, J.; Li, J.; Yin, Y.; Chen, Y.; Bi, X. Water-assisted chemical vapor deposition synthesis of boron nitride nanotubes and their photoluminescence property. Nanotechnology 2013, 24, 365605. [Google Scholar] [CrossRef]
- Hubácek, M.; Sato, T.; Ishii, T. A Coexistence of Boron Nitride and Boric Oxide. J. Sol. 1994, 109, 384–390. [Google Scholar] [CrossRef]
- Ferreira, T.H.; Marino, A.; Rocca, A.; Liakos, I.; Nitti, S.; Athanassiou, A.; Mattoli, V.; Mazzolai, B.; de Sousa, E.M.B.; Ciofani, G. Folate-grafted boron nitride nanotubes: Possible exploitation in cancer therapy. Int. J. Pharm. 2015, 481, 56–63. [Google Scholar] [CrossRef]
- Monteiro, G.A.A.; de Sousa, R.G.; da Silva, W.M.; Gastelois, P.L.; Macedo, W.A.d.; de Sousa, E.M.B. Microwave radiation-assisted covalent functionalization of boron nitride nanotubes and their grafting with cationic thermo and pH-sensitive hydrogel. Appl. Nanosci. 2021, 11, 505–520. [Google Scholar] [CrossRef]
- Jin, Z.; Liu, C.; Qi, K.; Cui, X. Photo-reduced Cu / CuO nanoclusters on TiO2 nanotube arrays as highly efficient and reusable catalyst. Sci. Rep. 2017, 7, 39695. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Das, R.; Chowdhury, I.H.; Bhanja, P.; Naskar, M.K. RSC Advances. RSC Adv. 2015, 5, 101519–101524. [Google Scholar] [CrossRef]
- Mondal, P.; Sinha, A.; Salam, N.; Roy, A.S.; Jana, N.R.; Islam, S.M. Enhanced catalytic performance by copper nanoparticle-graphene based composite. RSC Adv. 2013, 3, 5615–5623. [Google Scholar] [CrossRef]
- Fernandez, V.; Kiani, D.; Fairley, N.; Felpin, F.X.; Baltrusaitis, J. Curve fitting complex X-ray photoelectron spectra of graphite-supported copper nanoparticles using informed line shapes. Appl. Surf. Sci. 2020, 505, 143841. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, H.; Lee, J.C.; Kim, K.M.; Lee, K.C.; Ha, H.J.; Choi, T.H.; An, G.I.; Cheon, G.J. A simple Cu-64 production and its application of Cu-64 ATSM. Appl. Radiat. Isot 2009, 67, 1190–1194. [Google Scholar] [CrossRef]
- Pérot, B.; Jallu, F.; Passard, C.; Gueton, O.; Allinei, P.-G.; Loubet, L.; Estre, N.; Simon, E.; Carasco, C.; Roure, C.; et al. The characterization of radioactive waste: A critical review of techniques implemented or under development at CEA. EPJ Nucl. Sci. Technol. 2018, 4, 3. [Google Scholar] [CrossRef]
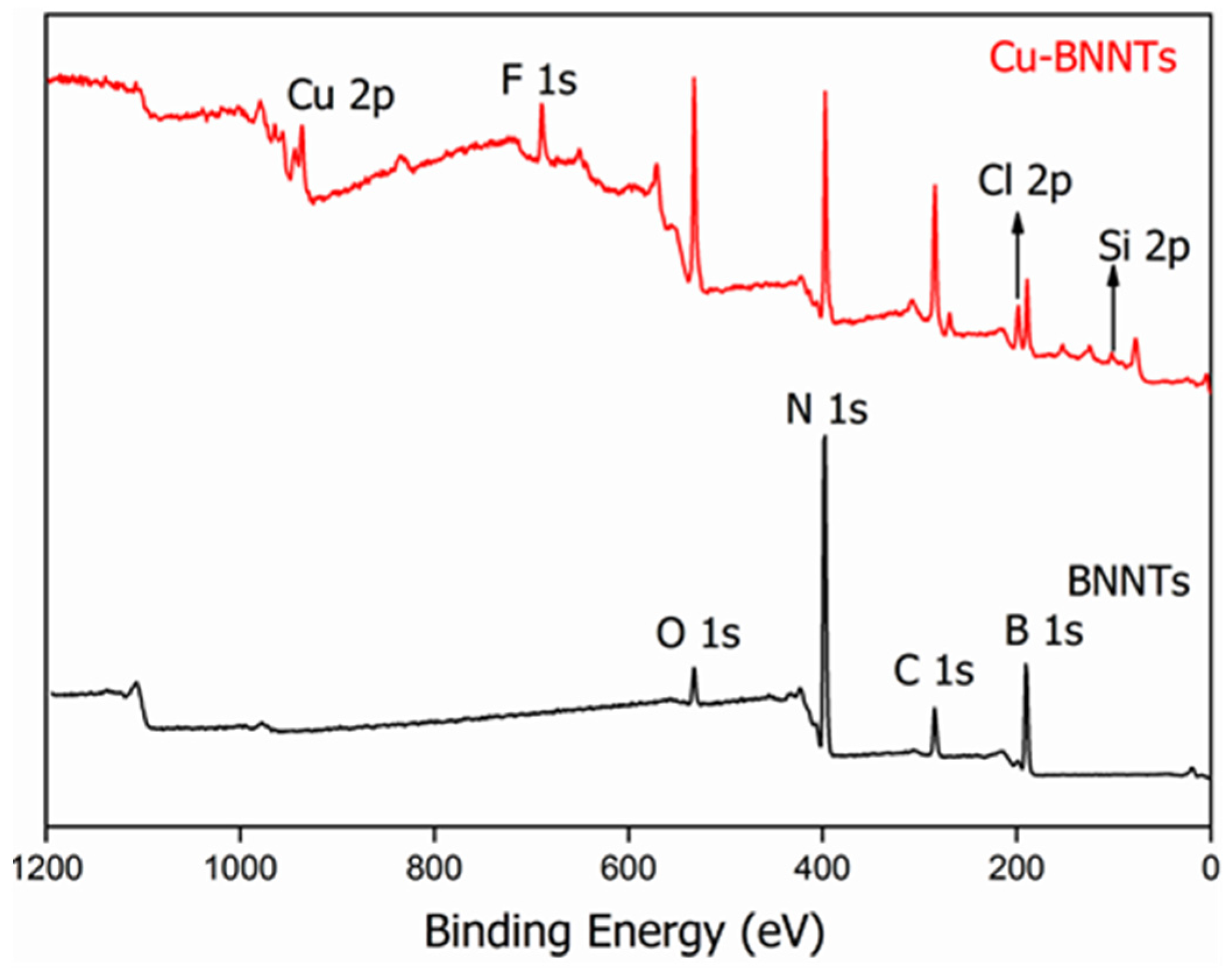
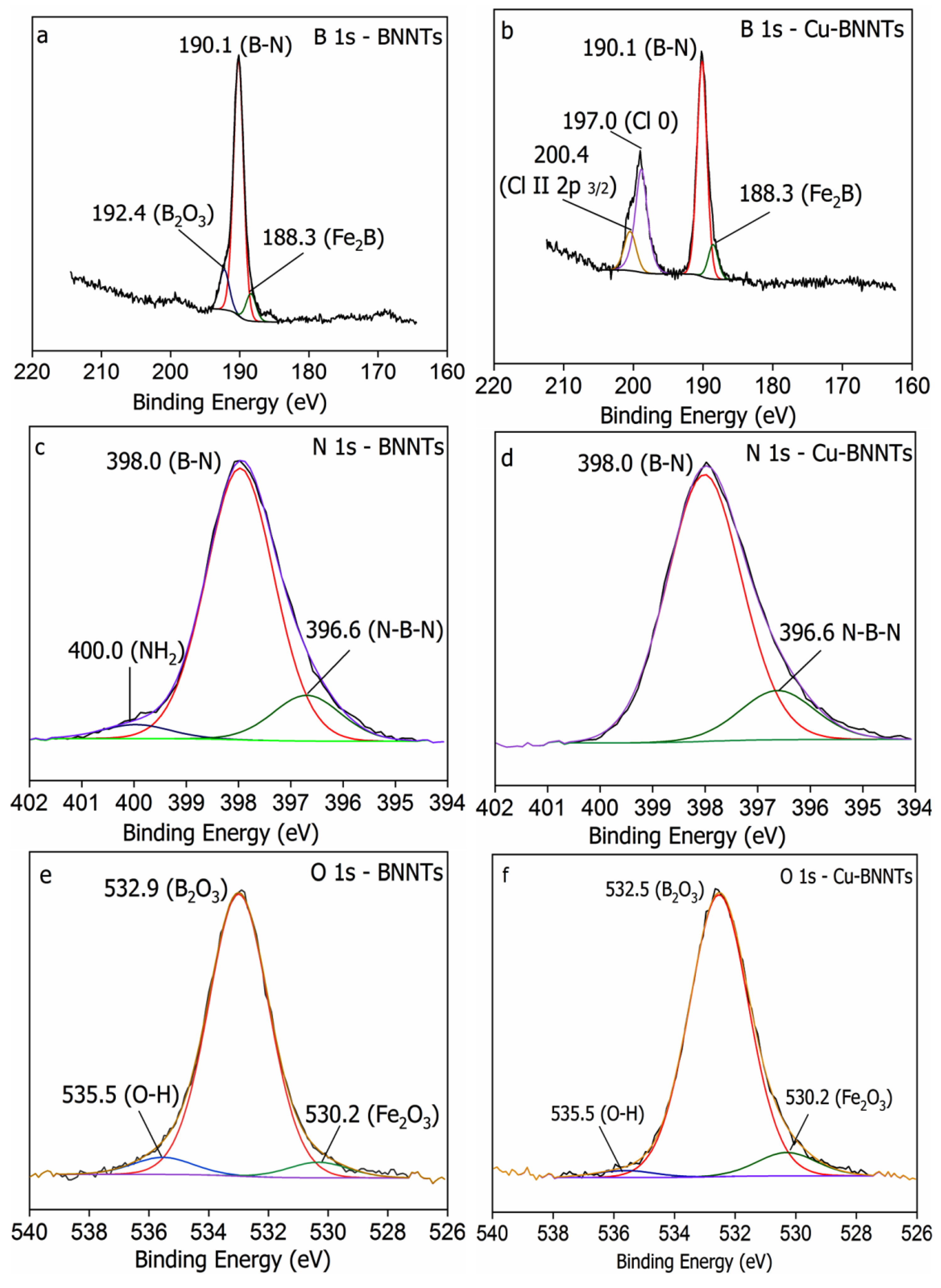
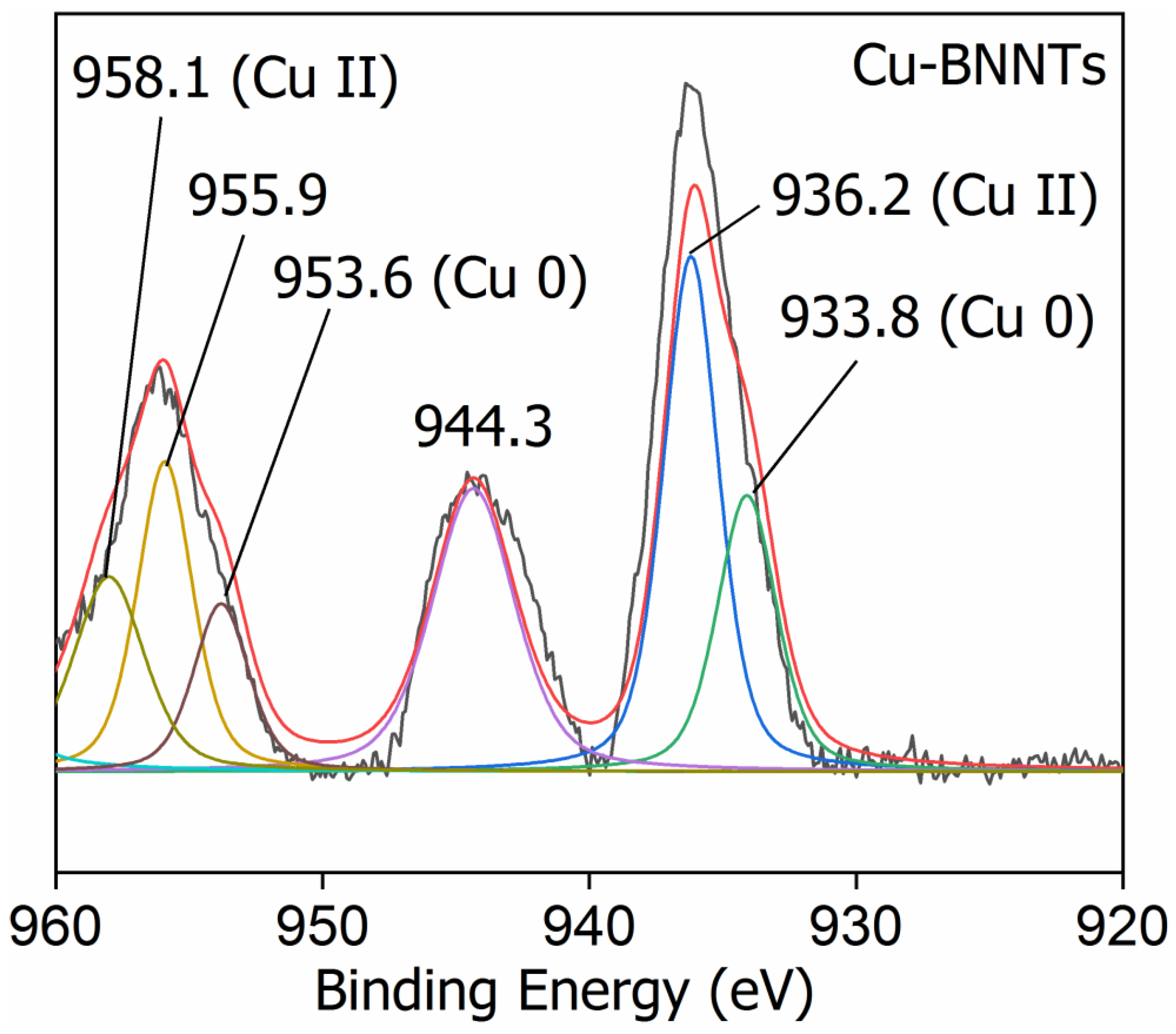

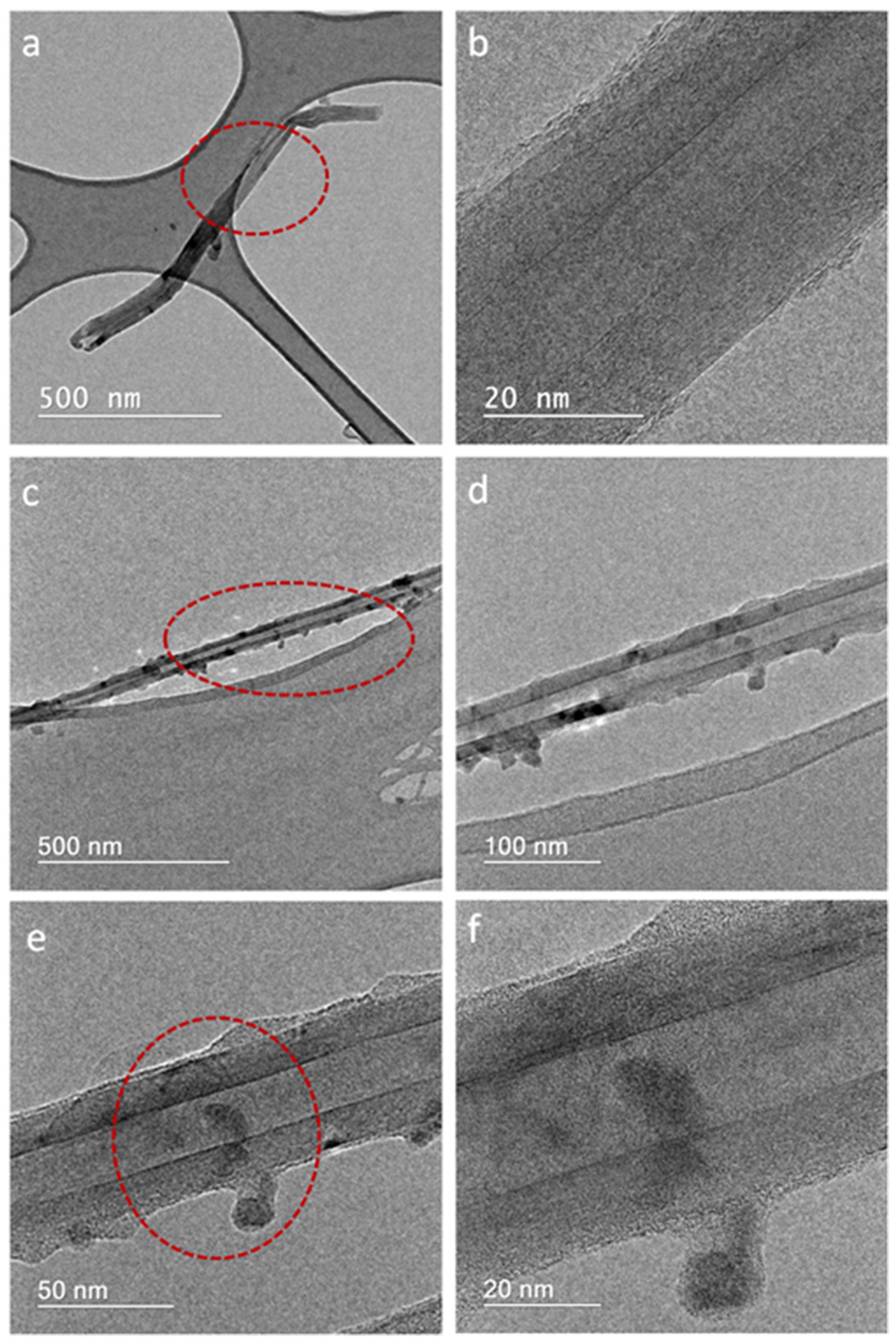
| Elements | Bending Energy (eV) | Atomic Percentage (at.%) |
|---|---|---|
| O 1s | 532.51 | 16.047 |
| C 1s | 284.49 | 26.010 |
| N 1s | 398.12 | 21.914 |
| F 1s | 689.51 | 4.179 |
| Cu 2p | 936.50 | 2.771 |
| Cl 2p | 198.53 | 3.004 |
| B 1s | 190.10 | 23.936 |
| Si 2p | 102.53 | 2.138 |
| B:N | - | 1.090 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, W.M.; Ribeiro, H.; Taha-Tijerina, J.J. Potential Production of Theranostic Boron Nitride Nanotubes (64Cu-BNNTs) Radiolabeled by Neutron Capture. Nanomaterials 2021, 11, 2907. https://doi.org/10.3390/nano11112907
Silva WM, Ribeiro H, Taha-Tijerina JJ. Potential Production of Theranostic Boron Nitride Nanotubes (64Cu-BNNTs) Radiolabeled by Neutron Capture. Nanomaterials. 2021; 11(11):2907. https://doi.org/10.3390/nano11112907
Chicago/Turabian StyleSilva, Wellington Marcos, Helio Ribeiro, and Jose Jaime Taha-Tijerina. 2021. "Potential Production of Theranostic Boron Nitride Nanotubes (64Cu-BNNTs) Radiolabeled by Neutron Capture" Nanomaterials 11, no. 11: 2907. https://doi.org/10.3390/nano11112907
APA StyleSilva, W. M., Ribeiro, H., & Taha-Tijerina, J. J. (2021). Potential Production of Theranostic Boron Nitride Nanotubes (64Cu-BNNTs) Radiolabeled by Neutron Capture. Nanomaterials, 11(11), 2907. https://doi.org/10.3390/nano11112907