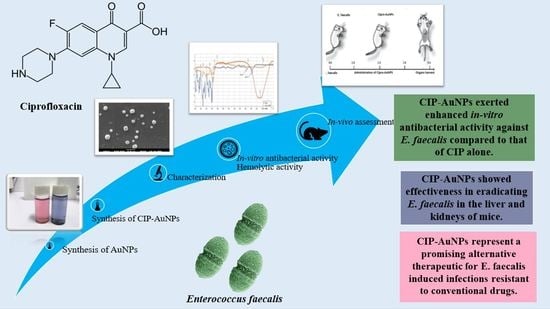
Ciprofloxacin-Loaded Gold Nanoparticles against Antimicrobial Resistance: An In Vivo Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Preparation of AuNPs and CIP-AuNPs
2.3. Characterization of AuNPs and CIP-AuNPs
2.4. Drug Loading Capacity and Encapsulation Efficiency
2.5. Drug Release Efficiency
2.6. Kinetic Analysis of the Drug Release
2.7. In Vitro Stability of CIP-AuNPs
2.8. In Vitro Antibacterial Potential of CIP-AuNPs
2.9. Hemolytic Activity of CIP-AuNPs
2.10. Colonization of E. faecalis in BALB/c Mice
2.11. In Vivo Antibacterial Activity of CIP-AuNPs
2.12. Statistical Analysis
3. Results
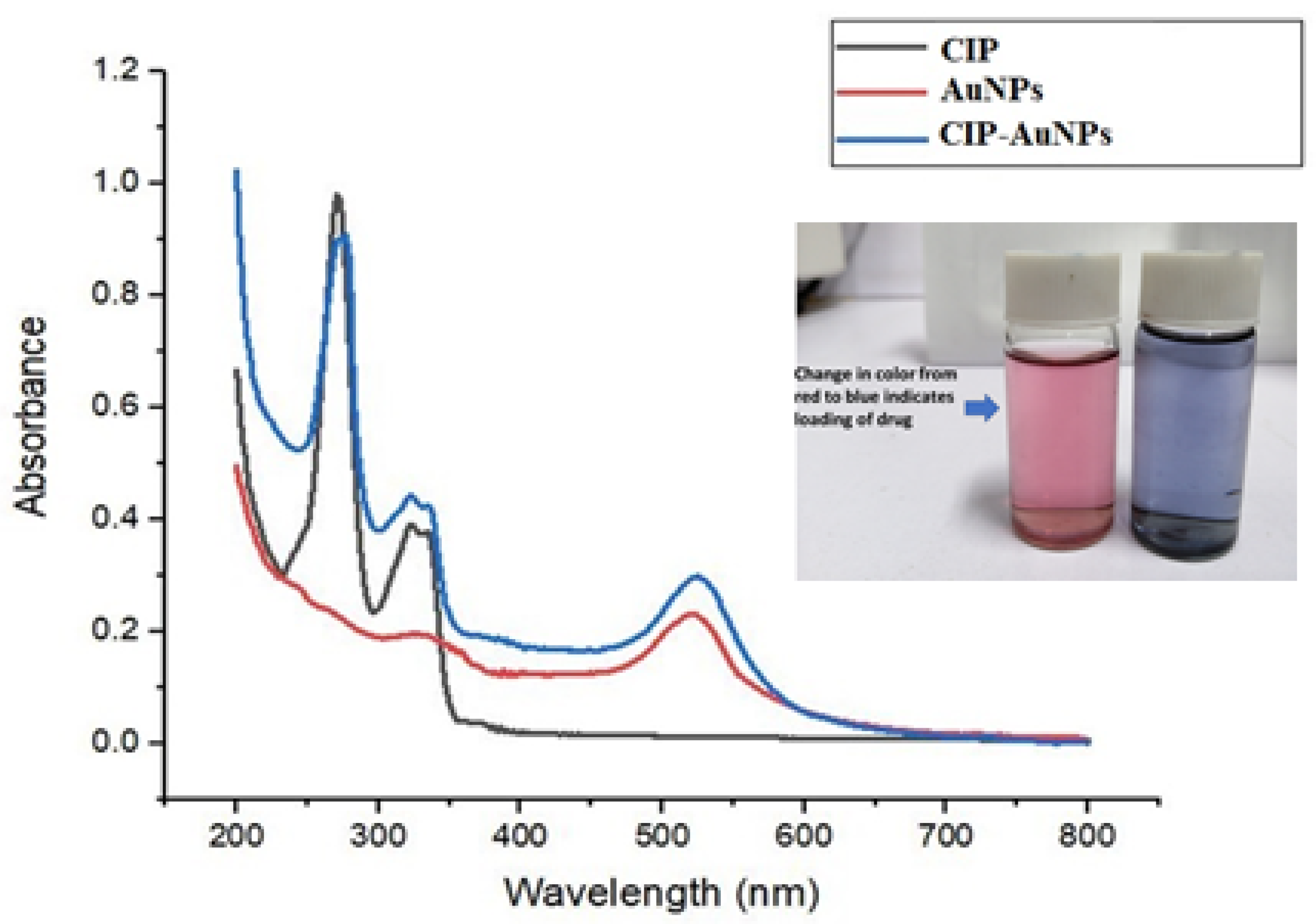
3.1. Synthesis of AuNPS and CIP-AuNPs
3.2. CIP Encapsulation Efficiency and CIP Loading Capacity
3.3. Particle Size and Zeta Potential of AuNPs and CIP-AuNPs
3.4. Surface Morphology and Elemental Chemical Composition of AuNPs by SEM–EDS
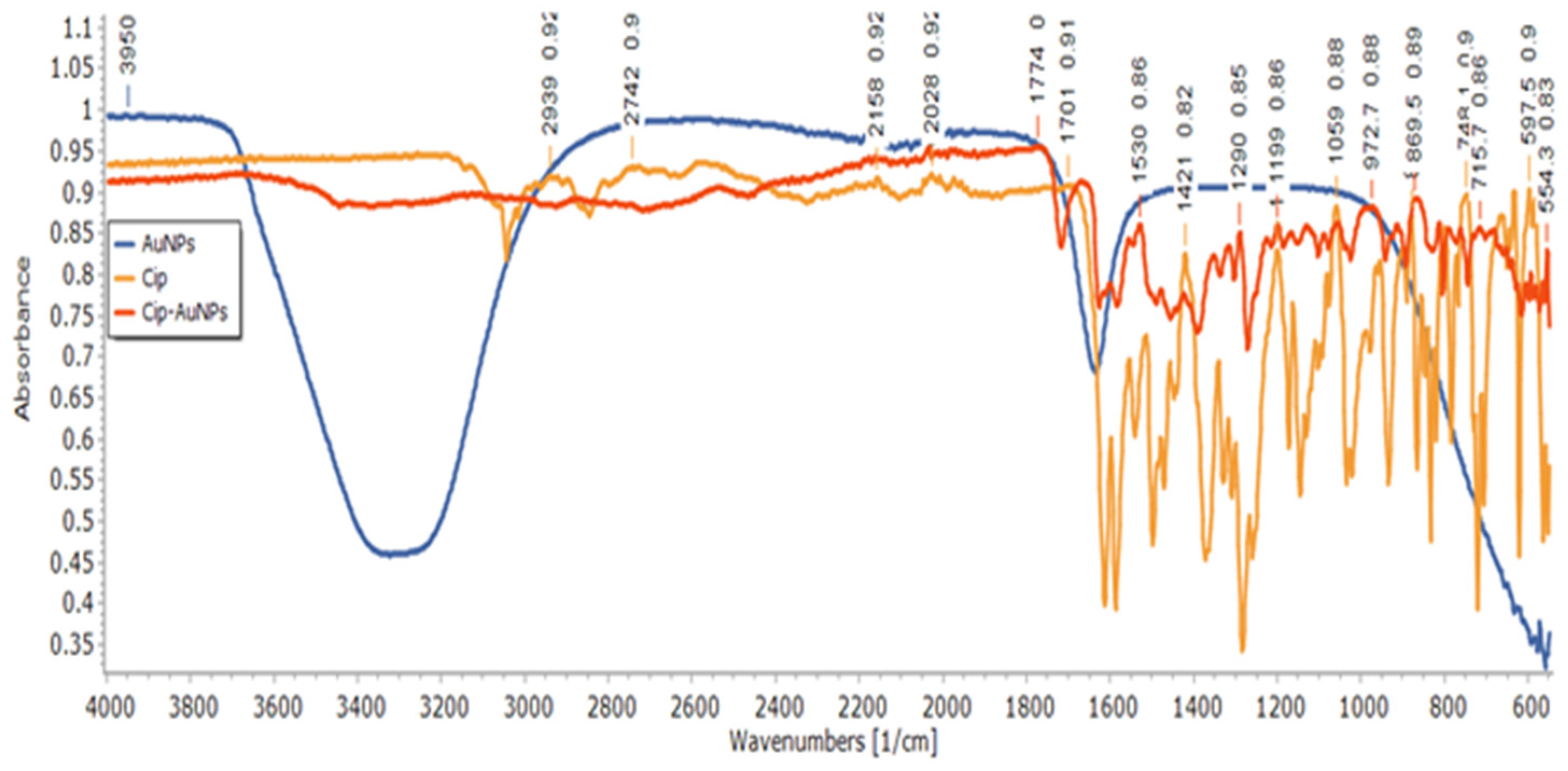
3.5. Structural Analysis of CIP-AuNPs by FTIR Spectroscopy
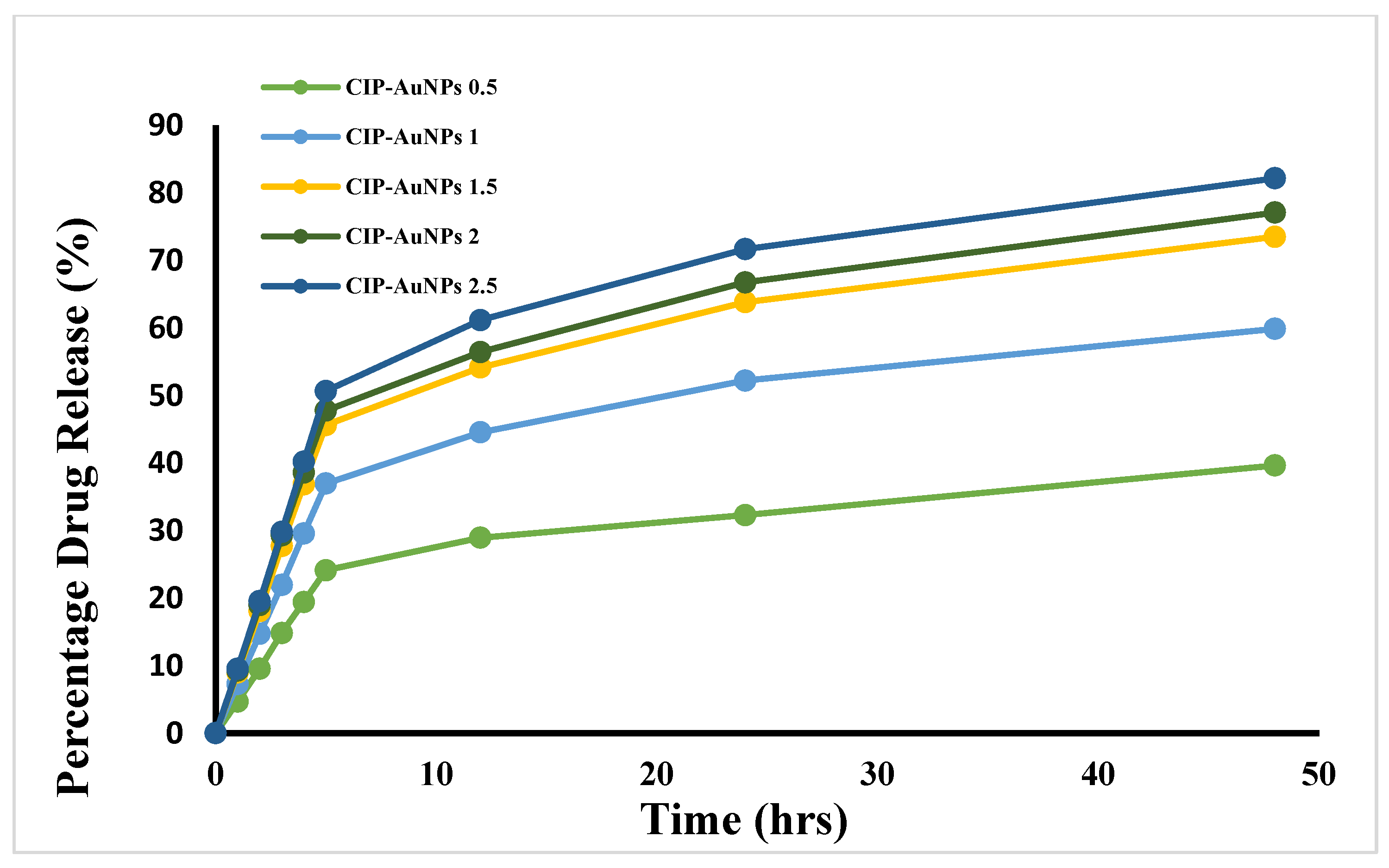
3.6. Kinetics of CIP Drug Release from AuNPs
3.7. Stability Tests on CIP-AuNPs
3.8. In Vitro Antibacterial Activity of CIP-AuNPs at the Optimized Dose
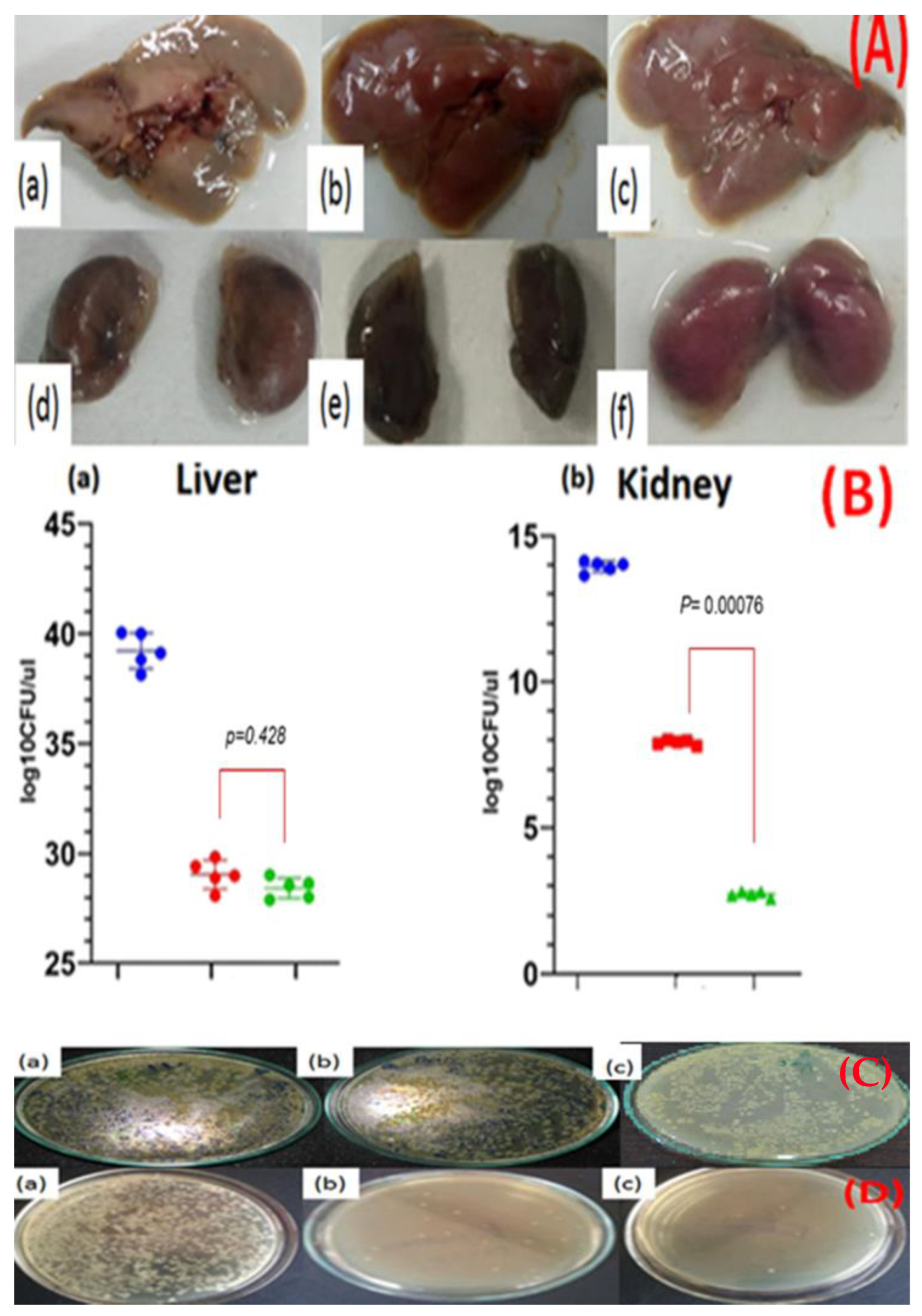
3.9. In Vivo Anticolonizing Potential of CIP-AuNPs in an Animal Model
3.10. Hemolytic Activity of CIP-AuNPs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, D.; Fan, W.; Kishen, A.; Gutmann, J.L.; Fan, B. Evaluation of the Antibacterial Efficacy of Silver Nanoparticles against Enterococcus faecalis Biofilm. J. Endod. 2014, 40, 285–290. [Google Scholar] [CrossRef]
- Sedgley, C.; Buck, G.; Appelbe, O. Prevalence of Enterococcus faecalis at Multiple Oral Sites in Endodontic Patients Using Culture and PCR. J. Endod. 2006, 32, 104–109. [Google Scholar] [CrossRef]
- Saleh, I.M.; Ruyter, I.E.; Haapasalo, M.; Ørstavik, D. Survival of Enterococcus faecalis in infected dentinal tu-bules after root canal filling with different root canal sealers in vitro. Int. Endod. J. 2004, 37, 193–198. [Google Scholar] [CrossRef]
- Liu, H.; Wei, X.; Ling, J.; Wang, W.; Huang, X. Biofilm Formation Capability of Enterococcus faecalis Cells in Starvation Phase and Its Susceptibility to Sodium Hypochlorite. J. Endod. 2010, 36, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, M.S.; Clewell, D.B.; Ike, Y.; Shankar, N. Enterococci: From Commensals to Leading Causes of Drug Resistant Infection; Eye and Ear Infirmary: Boston, MA, USA, 2014. [Google Scholar]
- Livermore, D.M.; Blaser, M.; Carrs, O.; Cassell, G.; Fishman, N.; Guidos, R.; Levy, S.; Powers, J.; Norrby, R.; Tillotson, G.; et al. Discovery research: The scientific challenge of finding new antibiotics. J. Antimicrob. Chemother. 2011, 66, 1941–1944. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.; Lopes, D.; Martins, M.C.L.; Van Dijck, P.; Nunes, C.; Reis, S. Impact of nanosystems in Staphylococcus aureus biofilms treatment. FEMS Microbiol. Rev. 2019, 43, 622–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vassallo, A.; Silletti, M.F.; Faraone, I.; Milella, L. Nanoparticulate Antibiotic Systems as Antibacterial Agents and Antibiotic Delivery Platforms to Fight Infections. J. Nanomater. 2020, 2020. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Kumar, S.S.; Manikandan, M.; Saravanan, M. Photocatalytic properties and antimicrobial ef-ficacy of Fe doped CuO nanoparticles against the pathogenic bacteria and fungi. Microb. Pathog. 2018, 122, 84–89. [Google Scholar] [CrossRef]
- Uzair, B.; Liaqat, A.; Iqbal, H.; Menaa, B.; Razzaq, A.; Thiripuranathar, G.; Rana, N.F.; Menaa, F. Green and Cost-Effective Synthesis of Metallic Nanoparticles by Algae: Safe Methods for Translational Medicine. Bioengineering 2020, 7, 129. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [Green Version]
- Dykman, L.; Khlebtsov, N. Gold nanoparticles in biomedical applications: Recent advances and perspectives. Chem. Soc. Rev. 2012, 41, 2256–2282. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Raouf, N.; Al-Enazi, N.M.; Ibraheem, I.B. Green biosynthesis of gold nanoparticles using Galaxaura elongata and characterization of their antibacterial activity. Arab. J. Chem. 2017, 10, S3029–S3039. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Geng, X.; Liu, D.; Li, Z. Near-infrared light-enhanced protease-conjugated gold nanorods as a photo-thermal antimicrobial agent for elimination of exotoxin and biofilms. International journal of nanomedicine. Int. J. Nanomed. 2019, 14, 8047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhazouani, A.; Gamrani, H.; El Achaby, M.; Aziz, K.; Gebrati, L.; Uddin, S.; Aziz, F. Synthesis and Toxicity of Graphene Oxide Nanoparticles: A Literature Review of In vitro and In vivo Studies. BioMed Res. Int. 2021, 2021. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.N.; Scoffield, J.; Andukuri, A.; Alexander, G.C.; Walker, T.; Kim, S.; Choi, S.C.; Brott, B.C.; Eleazer, P.D.; Lee, J.-Y.; et al. Evaluation of ciprofloxacin and metronidazole encapsulated biomimetic nanomatrix gel on Enterococcus faecalis and Treponema denticola. Biomater. Res. 2015, 19, 9. [Google Scholar] [CrossRef] [Green Version]
- Azad, A.; Rostamifar, S.; Modaresi, F.; Bazrafkan, A.; Rezaie, Z. Assessment of the Antibacterial Effects of Bismuth Nanoparticles against Enterococcus faecalis. BioMed Res. Int. 2020, 2020. [Google Scholar] [CrossRef]
- Parolia, A.; Kumar, H.; Ramamurthy, S.; Davamani, F.; Pau, A. Effectiveness of chitosan-propolis nanoparticle against Enterococcus faecalis biofilms in the root canal. BMC Oral Heal. 2020, 20, 339. [Google Scholar] [CrossRef]
- Arias-Moliz, M.T.; Baca, P.; Solana, C.; Toledano, M.; Medina-Castillo, A.L.; Toledano-Osorio, M.; Osorio, R. Doxycycline-functionalized polymeric nanoparticles inhibit Enterococcus faecalis biofilm formation on dentine. Int. Endod. J. 2021, 54, 413–426. [Google Scholar] [CrossRef]
- Maliszewska, I.; Wróbel, J.; Wanarska, E.; Podhorodecki, A.; Matczyszyn, K. Synergistic effect of methylene blue and biogenic gold nanoparticles against Enterococcus faecalis. Photodiagnosis Photodyn. Ther. 2019, 27, 218–226. [Google Scholar] [CrossRef]
- Balto, H.; Bukhary, S.; Al-Omran, O.; BaHammam, A.; Al-Mutairi, B. Combined Effect of a Mixture of Silver Nanoparticles and Calcium Hydroxide against Enterococcus faecalis Biofilm. J. Endod. 2020, 46, 1689–1694. [Google Scholar] [CrossRef]
- Halkai, K.; Mudda, J.A.; Shivanna, V.; Rathod, V.; Halkai, R. Antibacterial efficacy of biosynthesized silver nanoparticles against Enterococcus faecalis Biofilm: An in vitro study. Contemp. Clin. Dent. 2018, 9, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, Y.; Zhang, H.; Lei, L. Nano-graphene oxide with antisense walR RNA inhibits the pathogenicity of Enterococcus faecalis in periapical periodontitis. J. Dent. Sci. 2019, 15, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Martini, C.; Longo, F.; Castagnola, R.; Marigo, L.; Grande, N.M.; Cordaro, M.; Cacaci, M.; Papi, M.; Palmieri, V.; Bugli, F.; et al. Antimicrobial and Antibiofilm Properties of Graphene Oxide on Enterococcus faecalis. Antibiotics 2020, 9, 692. [Google Scholar] [CrossRef] [PubMed]
- Herizchi, R.; Abbasi, E.; Milani, M.; Akbarzadeh, A. Current methods for synthesis of gold nanoparticles. Artif. Cells Nanomed. Biotechnol. 2016, 44, 596–602. [Google Scholar] [CrossRef]
- Tom, R.T.; Suryanarayanan, V.; Reddy, P.G.; Baskaran, S.; Pradeep, T. Ciprofloxacin-protected gold nanopar-ticles. Langmuir 2004, 20, 1909–1914. [Google Scholar] [CrossRef]
- Topal, G.R.; Devrim, B.; Eryilmaz, M.; Bozkir, A. Design of ciprofloxacin-loaded nano-and micro-composite particles for dry powder inhaler formulations: Preparation, in vitro characterisation, and antimicro-bial efficacy. J. Microencapsul. 2018, 35, 533–547. [Google Scholar] [CrossRef]
- Arafa, M.G.; Mousa, H.A.; Afifi, N.N. Preparation of PLGA-chitosan based nanocarriers for enhancing anti-bacterial effect of ciprofloxacin in root canal infection. Drug Deliv. 2020, 27, 26–39. [Google Scholar] [CrossRef] [Green Version]
- Albuquerque, M.T.; Valera, M.C.; Moreira, C.S.; Bresciani, E.; de Melo, R.M.; Bottino, M.C. Effects of ciprof-loxacin-containing scaffolds on Enterococcus faecalis biofilms. J. Endod. 2015, 41, 710–714. [Google Scholar] [CrossRef]
- Assali, M.; Zaid, A.N.; Abdallah, F.; Almasri, M.; Khayyat, R. Single-walled carbon nanotubes-ciprofloxacin nanoantibiotic: Strategy to improve ciprofloxacin antibacterial activity. Int. J. Nanomed. 2017, 12, 6647–6659. [Google Scholar] [CrossRef] [Green Version]
- Rana, N.F.; Sauvageot, N.; Laplace, J.-M.; Bao, Y.; Nes, I.; Rincé, A.; Posteraro, B.; Sanguinetti, M.; Hartke, A. Redox Balance via Lactate Dehydrogenase Is Important for Multiple Stress Resistance and Virulence in Enterococcus faecalis. Infect. Immun. 2013, 81, 2662–2668. [Google Scholar] [CrossRef] [Green Version]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. Drug Res. 2010, 67, 217–223. [Google Scholar]
- Bettini, R.; Catellani, P.L.; Santi, P.; Massimo, G.; Peppas, N.A.; Colombo, P. Translocation of drug particles in HPMC matrix gel layer: Effect of drug solubility and influence on release rate. J. Control. Release 2001, 70, 383–391. [Google Scholar] [CrossRef]
- Naz, S.S.; Islam, N.U.; Shah, M.R.; Alam, S.S.; Iqbal, Z.; Bertino, M.; Franzel, L.; Ahmed, A. Enhanced biocidal activity of Au nanoparticles synthesized in one pot using 2, 4-dihydroxybenzene carbodithioic acid as a reducing and stabilizing agent. J. Nanobiotechnol. 2013, 11, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhammad, Z.; Raza, A.; Ghafoor, S.; Naeem, A.; Naz, S.S.; Riaz, S.; Ahmed, W.; Rana, N.F. PEG capped methotrexate silver nanoparticles for efficient anticancer activity and biocompatibility. Eur. J. Pharm. Sci. 2016, 91, 251–255. [Google Scholar] [CrossRef]
- Riaz, S.; Rana, N.F.; Hussain, I.; Tanweer, T.; Nawaz, A.; Menaa, F.; Janjua, H.A.; Alam, T.; Batool, A.; Naeem, A.; et al. Effect of Flavonoid-Coated Gold Nanoparticles on Bacterial Colonization in Mice Organs. Nanomaterials 2020, 10, 1769. [Google Scholar] [CrossRef]
- Bhalerao, S.R.; Rote, A.R. Application of UV spectrophotometric methods for estimation of Ciprofloxacin and tinidazole in combined tablet dosage form. Int. J. Pharmacol. Pharm. Sci. 2012, 4, 4–7. [Google Scholar]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Canepa, E.; Salassi, S.; Simonelli, F.; Ferrando, R.; Rolandi, R.; Lambruschini, C.; Canepa, F.; Dante, S.; Relini, A.; Rossi, G. Non-disruptive uptake of anionic and cationic gold nanoparticles in neutral zwitterionic membranes. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Okoampah, E.; Mao, Y.; Yang, S.; Sun, S.; Zhou, C. Gold nanoparticles-biomembrane interactions: From fundamental to simulation. Colloids Surf. B Biointerfaces 2020, 196, 111312. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Austin, L.; Mackey, M.A.; El-Sayed, M.A. Size matters: Gold nanoparticles in targeted cancer drug delivery. Ther. Deliv. 2012, 3, 457–478. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.; Alam, M.G. Ciprofloxacin-Induced Acute Interstitial Nephritis and Autoimmune Hemolytic Anemia. Ren. Fail. 2003, 25, 647–651. [Google Scholar] [CrossRef] [PubMed]
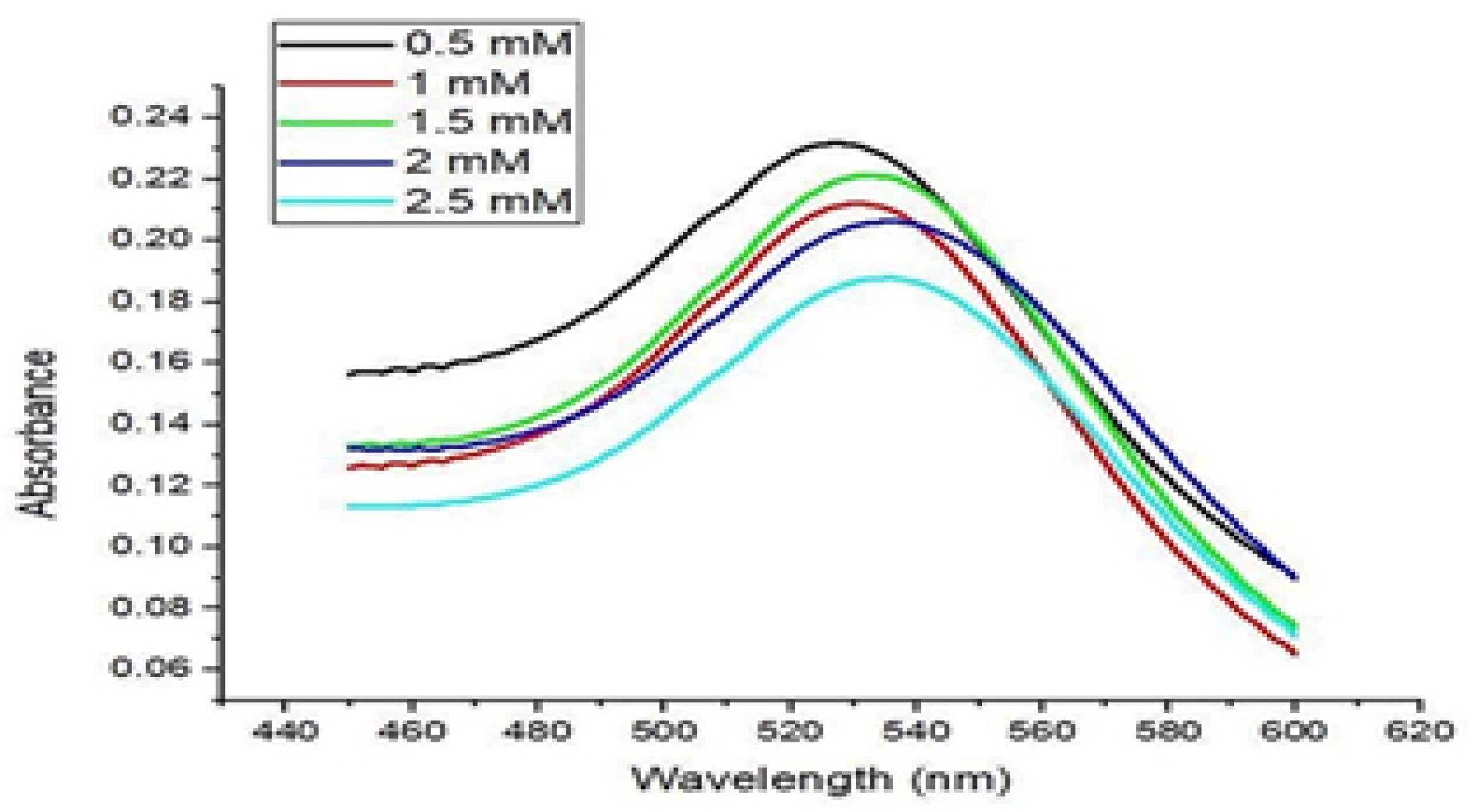
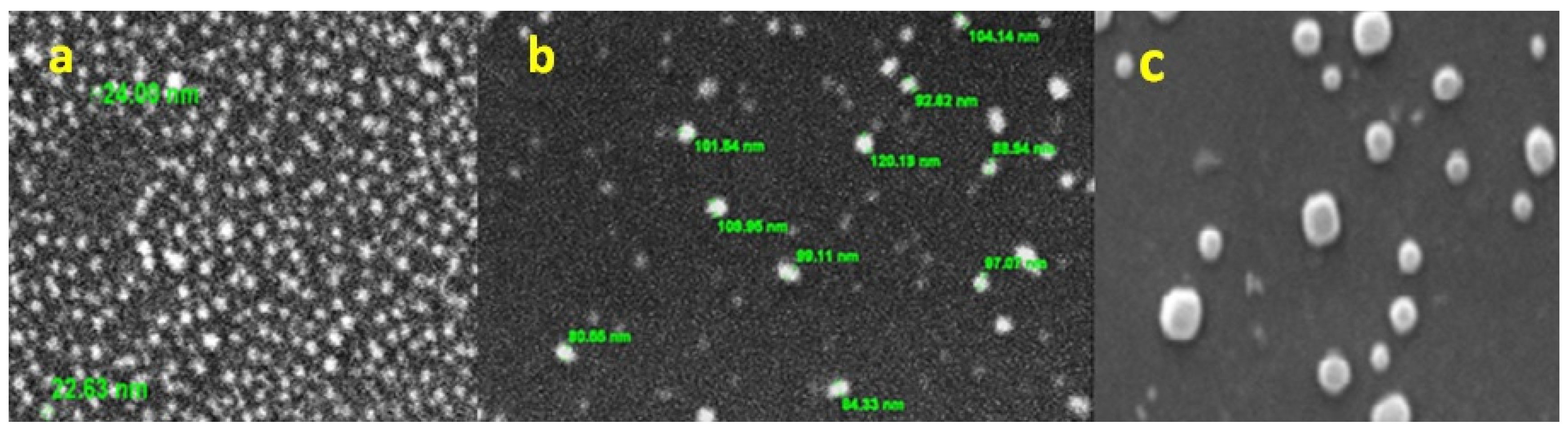


| CIP Concentration AuNPs | Encapsulation Efficiency (%) | Loading Capacity (%) |
|---|---|---|
| 0.5 mM | 24.43 | 8.85 |
| 1.0 mM | 29.30 | 15.60 |
| 1.5 mM | 30.65 | 28.85 |
| 2.0 mM | 48.92 | 33.81 |
| 2.5 mM | 60.83 | 34.54 |
| CIP-AuNPs | Z-Average (d. nm) | PDI | St Dev (d. nm) | Zeta Potential (mV) |
|---|---|---|---|---|
| 0.5 mM | 24.43 | 0.26 | 6.21 | −32.1 |
| 1.0 mM | 24.09 | 0.301 | 6.044 | −33.3 |
| 1.5 mM | 41 | 0.68 | 10.21 | −19.7 |
| 2.0 mM | 88.2 | 1.000 | 57.4 | −13.4 |
| 2.5 mM | 128.2 | 0.48 | 79.18 | −2.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nawaz, A.; Ali, S.M.; Rana, N.F.; Tanweer, T.; Batool, A.; Webster, T.J.; Menaa, F.; Riaz, S.; Rehman, Z.; Batool, F.; et al. Ciprofloxacin-Loaded Gold Nanoparticles against Antimicrobial Resistance: An In Vivo Assessment. Nanomaterials 2021, 11, 3152. https://doi.org/10.3390/nano11113152
Nawaz A, Ali SM, Rana NF, Tanweer T, Batool A, Webster TJ, Menaa F, Riaz S, Rehman Z, Batool F, et al. Ciprofloxacin-Loaded Gold Nanoparticles against Antimicrobial Resistance: An In Vivo Assessment. Nanomaterials. 2021; 11(11):3152. https://doi.org/10.3390/nano11113152
Chicago/Turabian StyleNawaz, Afrah, Syed Mohsin Ali, Nosheen Fatima Rana, Tahreem Tanweer, Amna Batool, Thomas J. Webster, Farid Menaa, Sundus Riaz, Zahra Rehman, Farhat Batool, and et al. 2021. "Ciprofloxacin-Loaded Gold Nanoparticles against Antimicrobial Resistance: An In Vivo Assessment" Nanomaterials 11, no. 11: 3152. https://doi.org/10.3390/nano11113152
APA StyleNawaz, A., Ali, S. M., Rana, N. F., Tanweer, T., Batool, A., Webster, T. J., Menaa, F., Riaz, S., Rehman, Z., Batool, F., Fatima, M., Maryam, T., Shafique, I., Saleem, A., & Iqbal, A. (2021). Ciprofloxacin-Loaded Gold Nanoparticles against Antimicrobial Resistance: An In Vivo Assessment. Nanomaterials, 11(11), 3152. https://doi.org/10.3390/nano11113152