Enhanced Energy Density for P-Doped Hierarchically Porous Carbon-Based Symmetric Supercapacitor with High Operation Potential in Aqueous H2SO4 Electrolyte
Abstract
:1. Introduction
2. Experimental Section
2.1. Material Preparation
2.2. Material Characterization
2.3. Electrochemical Measurements
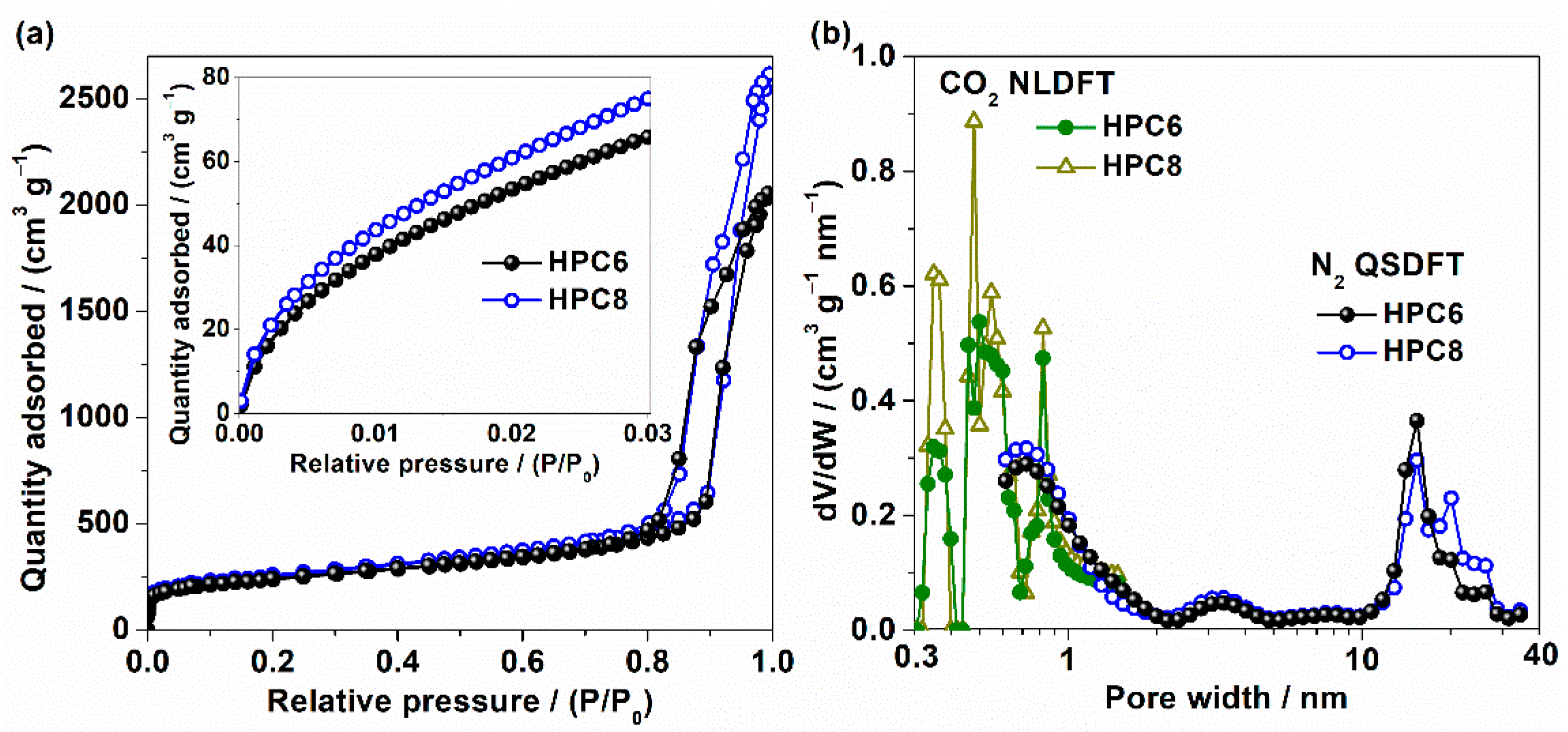
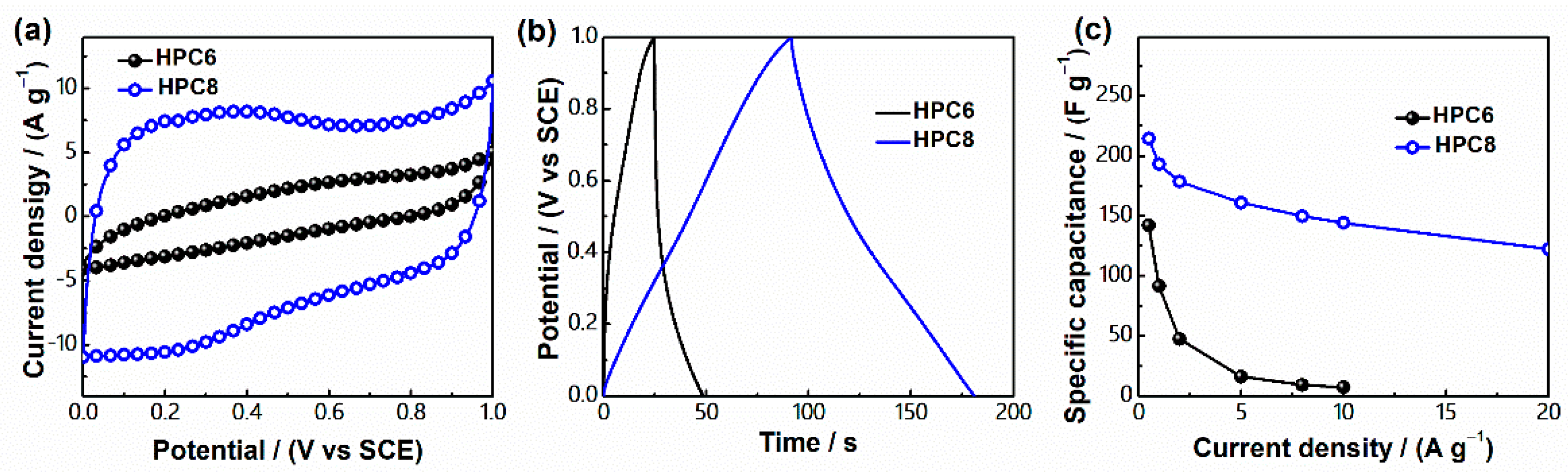
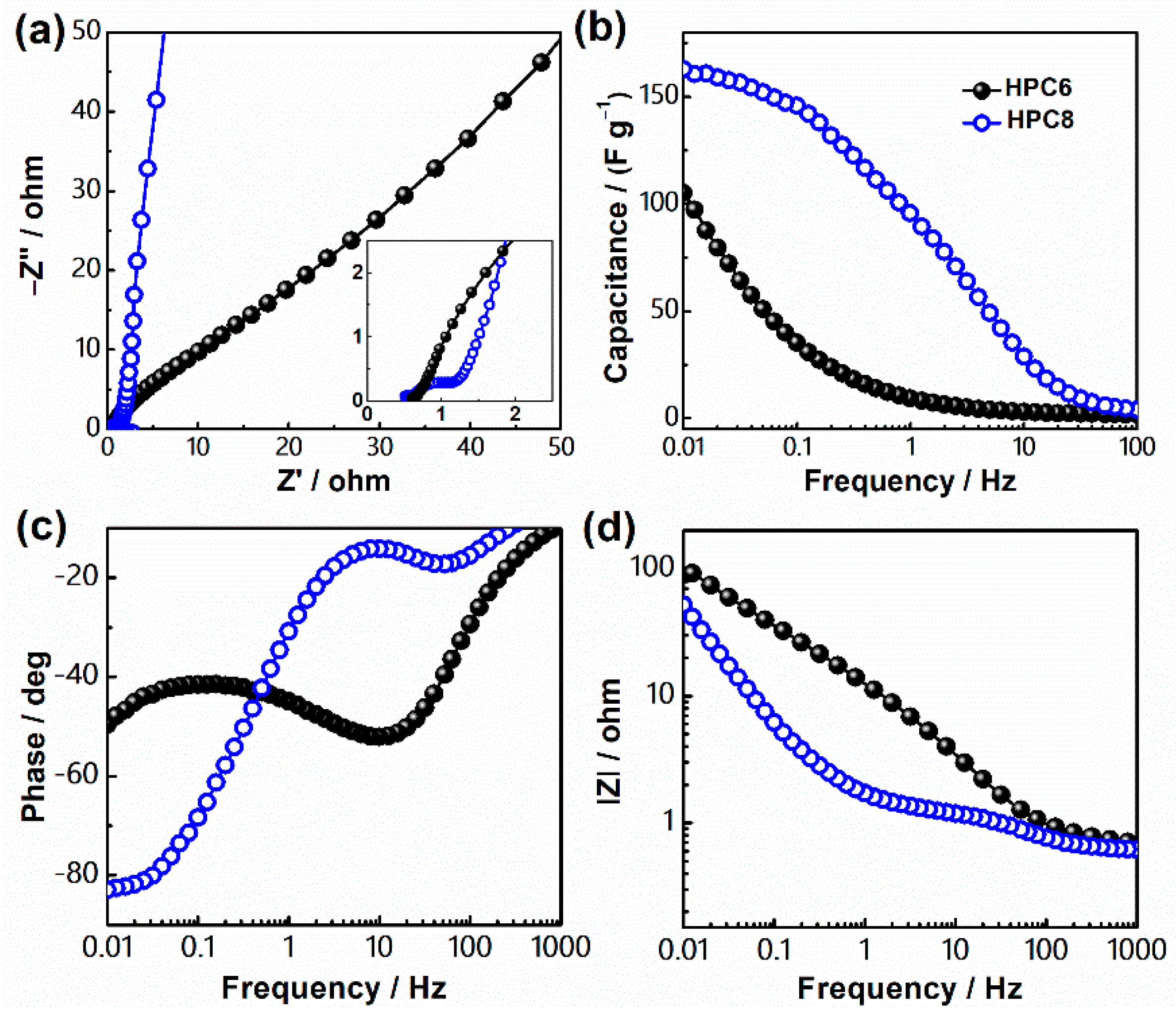
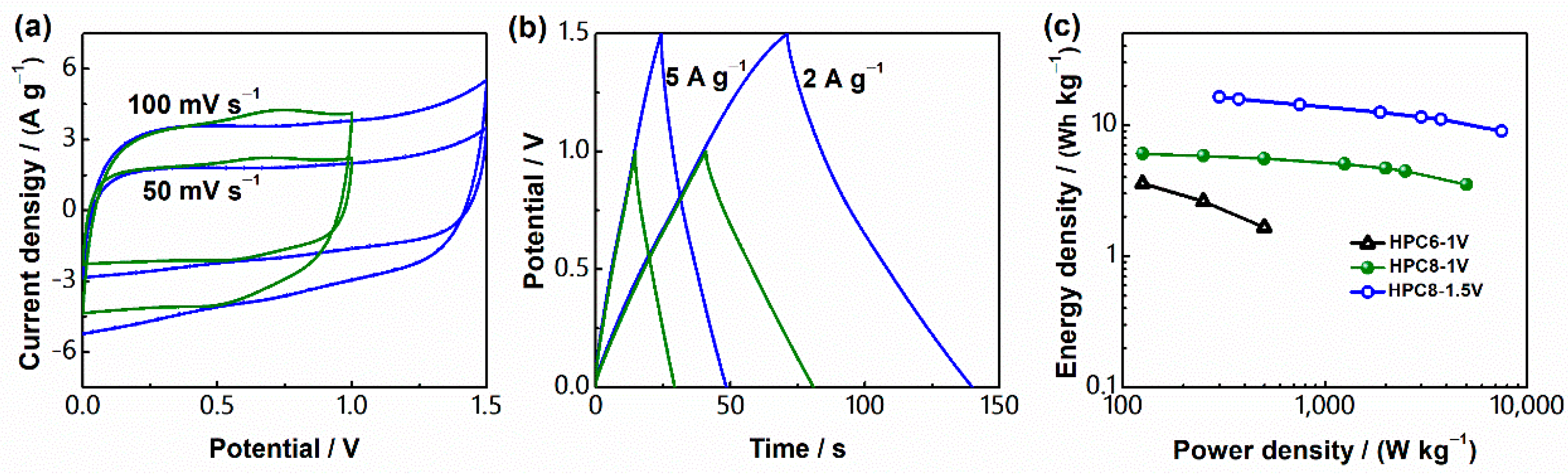
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Szewczyk, A. Measurement of noise in supercapacitors. Metrol. Meas. Syst. 2017, 24, 645–652. [Google Scholar] [CrossRef]
- Yang, X.Q.; Liu, A.R.; Zhao, Y.W.; Lu, H.J.; Zhang, Y.J.; Wei, W.; Li, Y.; Liu, S.Q. Three-dimensional macroporous polypyrrole-derived graphene electrode prepared by the hydrogen bubble dynamic template for supercapacitors and metal-free catalysts. ACS Appl. Mater. Interfaces 2015, 7, 23731–23740. [Google Scholar] [CrossRef]
- Li, K.L.; Feng, S.H.; Jing, C.; Chen, Y.X.; Liu, X.Y.; Zhang, Y.X.; Zhou, L. Assembling a double shell on a diatomite skeleton ternary complex with conductive polypyrrole for the enhancement of supercapacitors. Chem. Commun. 2019, 55, 13773–13776. [Google Scholar] [CrossRef]
- Bo, X.K.; Xiang, K.; Zhang, Y.; Shen, Y.; Chen, S.Y.; Wang, Y.Z.; Xie, M.J.; Guo, X.F. Microwave-assisted conversion of biomass wastes to pseudocapacitive mesoporous carbon for high-performance supercapacitor. J. Energy Chem. 2019, 39, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Najib, S.; Bakan, F.; Abdullayeva, N.; Bahariqushchi, R.; Kasap, S.; Franzo, G.; Sankir, M.; Sankir, N.D.; Mirabella, S.; Erdem, E. Tailoring morphology to control defect structures in ZnO electrodes for high-performance supercapacitor devices. Nanoscale 2020, 12, 16162–16172. [Google Scholar] [CrossRef]
- Beguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and electrolytes for advanced supercapacitors. Adv. Mater. 2014, 26, 2219–2251. [Google Scholar] [CrossRef]
- Fic, K.; Lota, G.; Meller, M.; Frackowiak, E. Novel insight into neutral medium as electrolyte for high-voltage supercapacitors. Energy Environ. Sci. 2012, 5, 5842–5850. [Google Scholar] [CrossRef]
- He, L.; Liu, Y.; Li, C.Y.; Yang, D.Z.; Wang, W.G.; Yan, W.Q.; Zhou, W.B.; Wu, Z.X.; Wang, L.L.; Huang, Q.H.; et al. A low-cost Zn-based aqueous supercapacitor with high energy density. ACS Appl. Energ. Mater. 2019, 2, 5835–5842. [Google Scholar] [CrossRef]
- Pham, V.H.; Nguyen-Phan, T.D.; Tong, X.; Rajagopalan, B.; Chung, J.S.; Dickerson, J.H. Hydrogenated TiO2@reduced graphene oxide sandwich-like nanosheets for high voltage supercapacitor applications. Carbon 2018, 126, 135–144. [Google Scholar] [CrossRef]
- Dou, Q.Y.; Lei, S.L.; Wang, D.W.; Zhang, Q.N.; Xiao, D.W.; Guo, H.W.; Wang, A.P.; Yang, H.; Li, Y.L.; Shi, S.Q.; et al. Safe and high-rate supercapacitors based on an “acetonitrile/water in salt” hybrid electrolyte. Energy Environ. Sci. 2018, 11, 3212–3219. [Google Scholar] [CrossRef]
- Xiao, D.W.; Dou, Q.Y.; Zhang, L.; Ma, Y.L.; Shi, S.Q.; Lei, S.L.; Yu, H.Y.; Yan, X.B. Optimization of organic/water hybrid electrolytes for high-rate carbon-based supercapacitor. Adv. Funct. Mater. 2019, 29, 8. [Google Scholar] [CrossRef]
- Thareja, S.; Kumar, A. “Water-in-salt” electrolyte-based high-voltage (2.7 V) sustainable symmetric supercapacitor with superb electrochemical performance-an analysis of the role of electrolytic ions in extending the cell voltage. ACS Sustain. Chem. Eng. 2021, 9, 2338–2347. [Google Scholar] [CrossRef]
- Tatlisu, A.; Huang, Z.F.; Chen, R.Y. High-voltage and low-temperature aqueous supercapacitor enabled by “Water-in-Imidazolium chloride” electrolytes. ChemSusChem 2018, 11, 3899–3904. [Google Scholar] [CrossRef]
- Suo, L.M.; Borodin, O.; Gao, T.; Olguin, M.; Ho, J.; Fan, X.L.; Luo, C.; Wang, C.S.; Xu, K. “Water-in-salt” electrolyte enables high-voltage aqueous lithium-ion chemistries. Science 2015, 350, 938–943. [Google Scholar] [CrossRef]
- Qian, X.Y.; Miao, L.; Jiang, J.X.; Ping, G.C.; Xiong, W.; Lv, Y.K.; Liu, Y.F.; Gan, L.H.; Zhua, D.Z.; Liu, M.X. Hydrangea-like N/O codoped porous carbons for high-energy supercapacitors. Chem. Eng. J. 2020, 388, 124208. [Google Scholar] [CrossRef]
- Li, C.Y.; Wu, W.Z.; Wang, P.; Zhou, W.B.; Wang, J.; Chen, Y.H.; Fu, L.J.; Zhu, Y.S.; Wu, Y.P.; Huang, W. Fabricating an aqueous symmetric supercapacitor with a stable high working voltage of 2 V by using an alkaline-acidic electrolyte. Adv. Sci. 2019, 6, 1801665. [Google Scholar] [CrossRef] [Green Version]
- Liang, N.; Ji, Y.S.; Xu, J.; Zuo, D.Y.; Chen, D.Z.; Zhang, H.W. An asymmetric electric double-layer capacitor with a janus membrane and two different aqueous electrolytes. J. Power Sources 2019, 423, 68–71. [Google Scholar] [CrossRef]
- Xia, H.F.; Zhang, B.; Wang, C.H.; Cao, L.; Luo, B.; Fan, X.M.; Zhang, J.F.; Ou, X. Surface engineered carbon-cloth with broadening voltage window for boosted energy density aqueous supercapacitors. Carbon 2020, 162, 136–146. [Google Scholar] [CrossRef]
- Qin, T.F.; Chen, H.D.; Zhang, Y.A.; Chen, X.T.; Liu, L.; Yan, D.; Ma, S.; Hou, J.; Yu, F.; Peng, S.L. Modulating surface chemistry of heteroatom-rich micropore carbon cloth electrode for aqueous 2.1 V high-voltage window all-carbon supercapacitor. J. Power Sources 2019, 431, 232–238. [Google Scholar] [CrossRef]
- Deng, Y.L.; Ji, Y.J.; Wu, H.M.; Chen, F. Enhanced electrochemical performance and high voltage window for supercapacitor based on multi-heteroatom modified porous carbon materials. Chem. Commun. 2019, 55, 1486–1489. [Google Scholar] [CrossRef]
- Hulicova-Jurcakova, D.; Puziy, A.M.; Poddubnaya, O.I.; Suarez-Garcia, F.; Tascon, J.M.D.; Lu, G.Q. Highly stable performance of supercapacitors from phosphorus-enriched carbons. J. Am. Chem. Soc. 2009, 131, 5026–5027. [Google Scholar] [CrossRef]
- Wen, Y.Y.; Wang, B.; Huang, C.C.; Wang, L.Z.; Hulicova-Jurcakova, D. Synthesis of phosphorus-doped graphene and its wide potential window in aqueous supercapacitors. Chem. Eur. J. 2015, 21, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Sun, T.; Hulicova-Jurcakova, D. Wide electrochemical window of supercapacitors from coffee bean-derived phosphorus-rich carbons. ChemSusChem 2013, 6, 2330–2339. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.Y.; Rufford, T.E.; Hulicova-Jurcakova, D.; Wang, L.Z. Nitrogen and phosphorous co-doped graphene monolith for supercapacitors. ChemSusChem 2016, 9, 513–520. [Google Scholar] [CrossRef]
- Yu, M.H.; Lin, D.; Feng, H.B.; Zeng, Y.X.; Tong, Y.X.; Lu, X.H. Boosting the energy density of carbon-based aqueous supercapacitors by optimizing the surface charge. Angew. Chem. Int. Ed. 2017, 56, 5454–5459. [Google Scholar] [CrossRef]
- Park, S.H.; Bak, S.M.; Kim, K.H.; Jegal, J.P.; Lee, S.I.; Lee, J.; Kim, K.B. Solid-state microwave irradiation synthesis of high quality graphene nanosheets under hydrogen containing atmosphere. J. Mater. Chem. 2011, 21, 680–686. [Google Scholar] [CrossRef]
- Hasegawa, G.; Deguchi, T.; Kanamori, K.; Kobayashi, Y.; Kageyama, H.; Abe, T.; Nakanishi, K. High-level doping of nitrogen, phosphorus, and sulfur into activated carbon monoliths and their electrochemical capacitances. Chem. Mater. 2015, 27, 4703–4712. [Google Scholar] [CrossRef]
- Eliad, L.; Salitra, G.; Soffer, A.; Aurbach, D. Ion sieving effects in the electrical double layer of porous carbon electrodes: Estimating effective ion size in electrolytic solutions. J. Phys. Chem. B 2001, 105, 6880–6887. [Google Scholar] [CrossRef]
- Wu, X.Z.; Xing, W.; Florek, J.; Zhou, J.; Wang, G.Q.; Zhuo, S.P.; Xue, Q.Z.; Yan, Z.F.; Kleitz, F. On the origin of the high capacitance of carbon derived from seaweed with an apparently low surface area. J. Mater. Chem. A 2014, 2, 18998–19004. [Google Scholar] [CrossRef]
- Wu, X.Z.; Zhou, J.; Xing, W.; Zhang, Y.; Bai, P.; Xu, B.J.; Zhuo, S.P.; Xue, Q.Z.; Yan, Z.F. Insight into high areal capacitances of low apparent surface area carbons derived from nitrogen-rich polymers. Carbon 2015, 94, 560–567. [Google Scholar] [CrossRef]
- Saha, D.; Li, Y.C.; Bi, Z.H.; Chen, J.H.; Keum, J.K.; Hensley, D.K.; Grappe, H.A.; Meyer, H.M.; Dai, S.; Paranthaman, M.P.; et al. Studies on supercapacitor electrode material from activated lignin-derived mesoporous carbon. Langmuir 2014, 30, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Hulicova-Jurcakova, D.; Kodama, M.; Shiraishi, S.; Hatori, H.; Zhu, Z.H.; Lu, G.Q. Nitrogen-enriched nonporous carbon electrodes with extraordinary supercapacitance. Adv. Funct. Mater. 2009, 19, 1800–1809. [Google Scholar] [CrossRef]


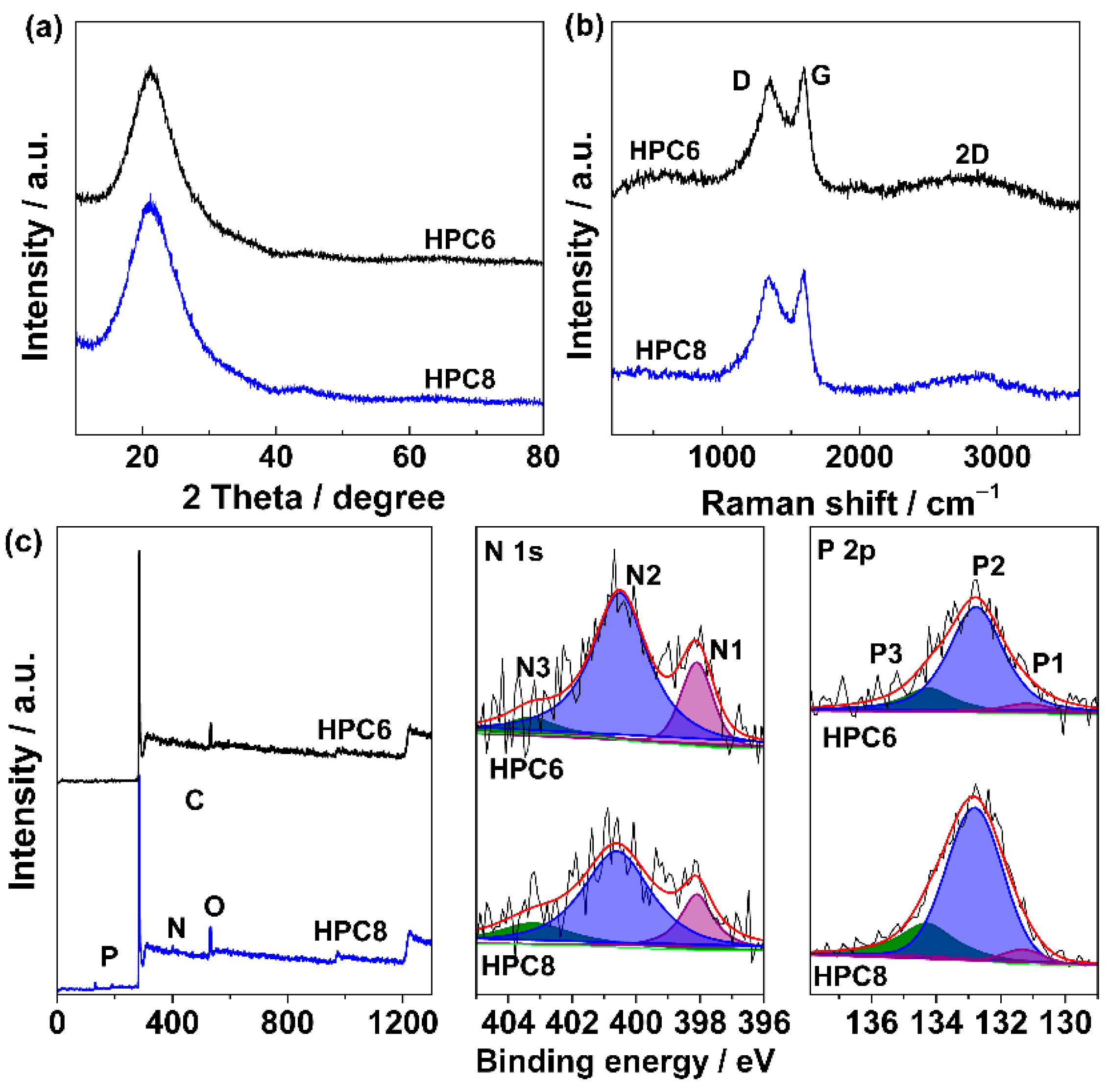
| Sample | C a | O a | N a | P a | C b | O b | N b | P b |
|---|---|---|---|---|---|---|---|---|
| HPC6 | 90.25 | 4.23 | 5.19 | 0.33 | 91.01 | 5.97 | 2.45 | 0.58 |
| HPC8 | 87.11 | 5.56 | 4.55 | 2.78 | 87.88 | 8.67 | 0.96 | 2.49 |
| Sample | Vt a | SBET b | SQSDFT c | Smic c | Smeso c | SNLDFT d | S<0.6 nm d | S0.6–1.5 nm d |
|---|---|---|---|---|---|---|---|---|
| (cm3 g−1) | (m2 g−1) | |||||||
| HPC6 | 3.18 | 843 | 828 | 480 | 348 | 691 | 437 | 254 |
| HPC8 | 4.05 | 892 | 904 | 518 | 386 | 787 | 492 | 295 |
| C8 | 2.18 | 458 | 445 | 227 | 218 | - | - | - |
| Sample | H2SO4 | KOH | ||
|---|---|---|---|---|
| Cg (F g−1) a | Cs (μF cm−2) b | Cg (F g−1) a | Cs (μF cm−2) b | |
| HPC6 | 142 | 17 | 132 | 16 |
| HPC8 | 215 | 24 | 181 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Yang, X.; Feng, W.; Wang, X.; Miao, Z.; Zhou, P.; Zhao, J.; Zhou, J.; Zhuo, S. Enhanced Energy Density for P-Doped Hierarchically Porous Carbon-Based Symmetric Supercapacitor with High Operation Potential in Aqueous H2SO4 Electrolyte. Nanomaterials 2021, 11, 2838. https://doi.org/10.3390/nano11112838
Wu X, Yang X, Feng W, Wang X, Miao Z, Zhou P, Zhao J, Zhou J, Zhuo S. Enhanced Energy Density for P-Doped Hierarchically Porous Carbon-Based Symmetric Supercapacitor with High Operation Potential in Aqueous H2SO4 Electrolyte. Nanomaterials. 2021; 11(11):2838. https://doi.org/10.3390/nano11112838
Chicago/Turabian StyleWu, Xiaozhong, Xinping Yang, Wei Feng, Xin Wang, Zhichao Miao, Pengfei Zhou, Jinping Zhao, Jin Zhou, and Shuping Zhuo. 2021. "Enhanced Energy Density for P-Doped Hierarchically Porous Carbon-Based Symmetric Supercapacitor with High Operation Potential in Aqueous H2SO4 Electrolyte" Nanomaterials 11, no. 11: 2838. https://doi.org/10.3390/nano11112838
APA StyleWu, X., Yang, X., Feng, W., Wang, X., Miao, Z., Zhou, P., Zhao, J., Zhou, J., & Zhuo, S. (2021). Enhanced Energy Density for P-Doped Hierarchically Porous Carbon-Based Symmetric Supercapacitor with High Operation Potential in Aqueous H2SO4 Electrolyte. Nanomaterials, 11(11), 2838. https://doi.org/10.3390/nano11112838







