Graphene Transfer: A Physical Perspective
Abstract
1. Introduction
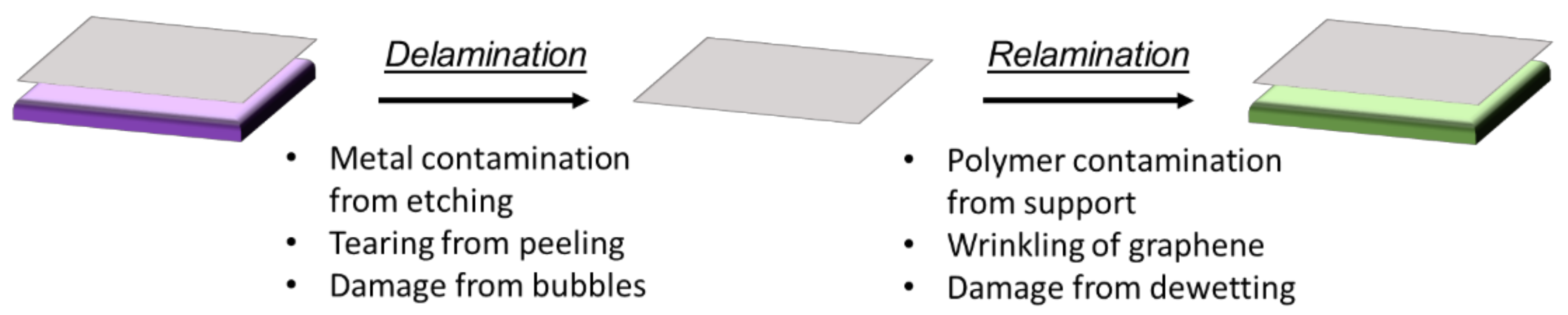
2. Issues Arising in Graphene Transfer
3. Physics of the Graphene-Substrate Interaction
3.1. General Overview
3.2. Technical Details
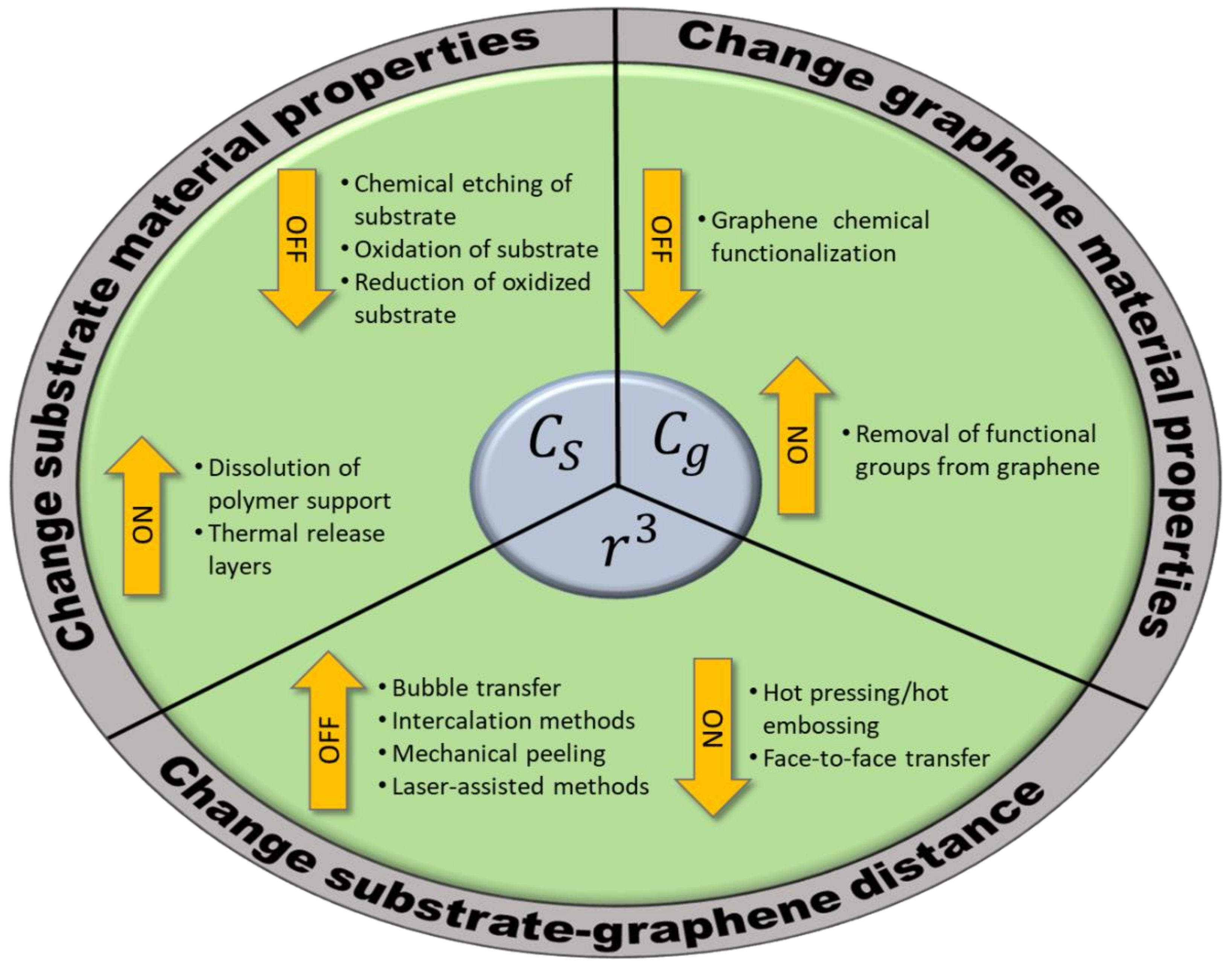
4. Transfer Strategies: Delamination of Graphene
4.1. Strategies Changing the Substrate Interaction Coefficient
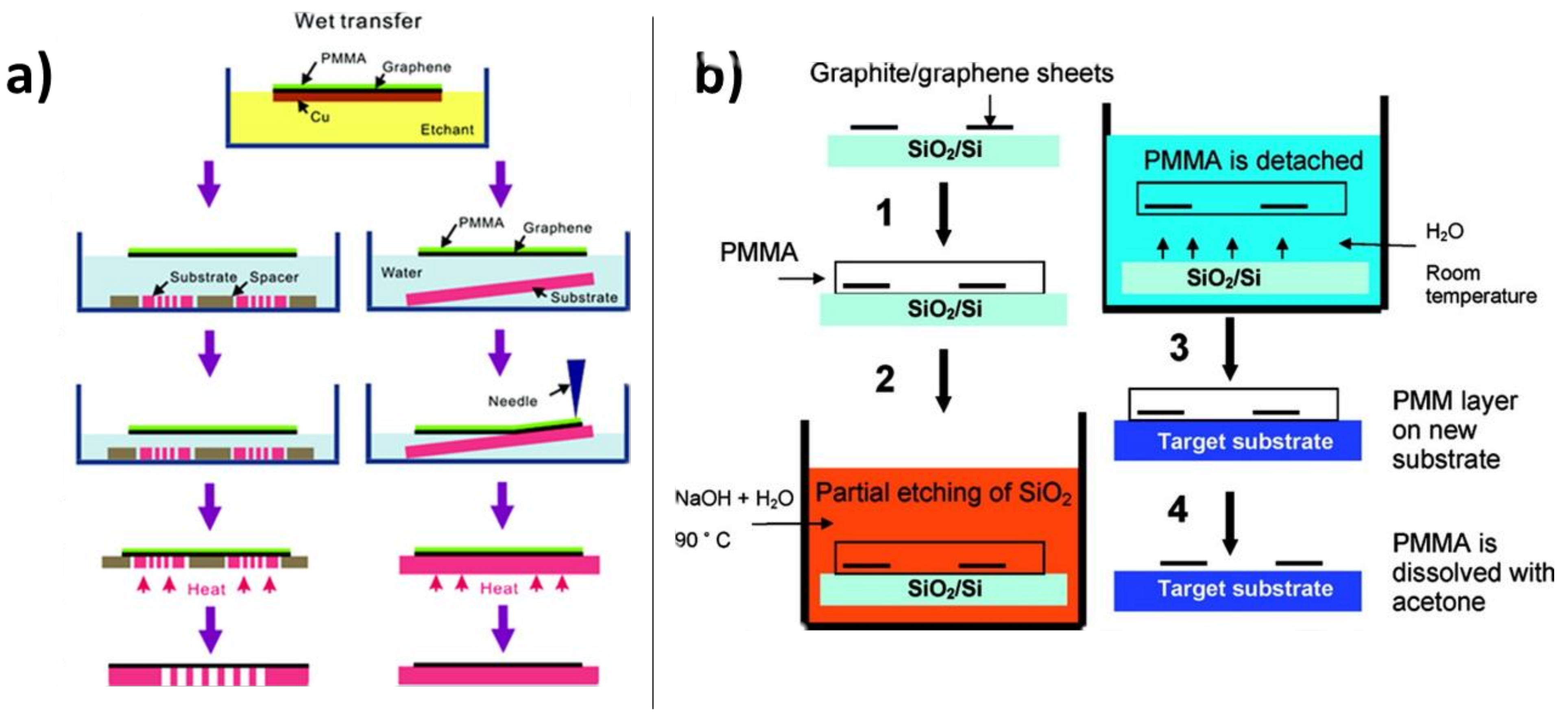
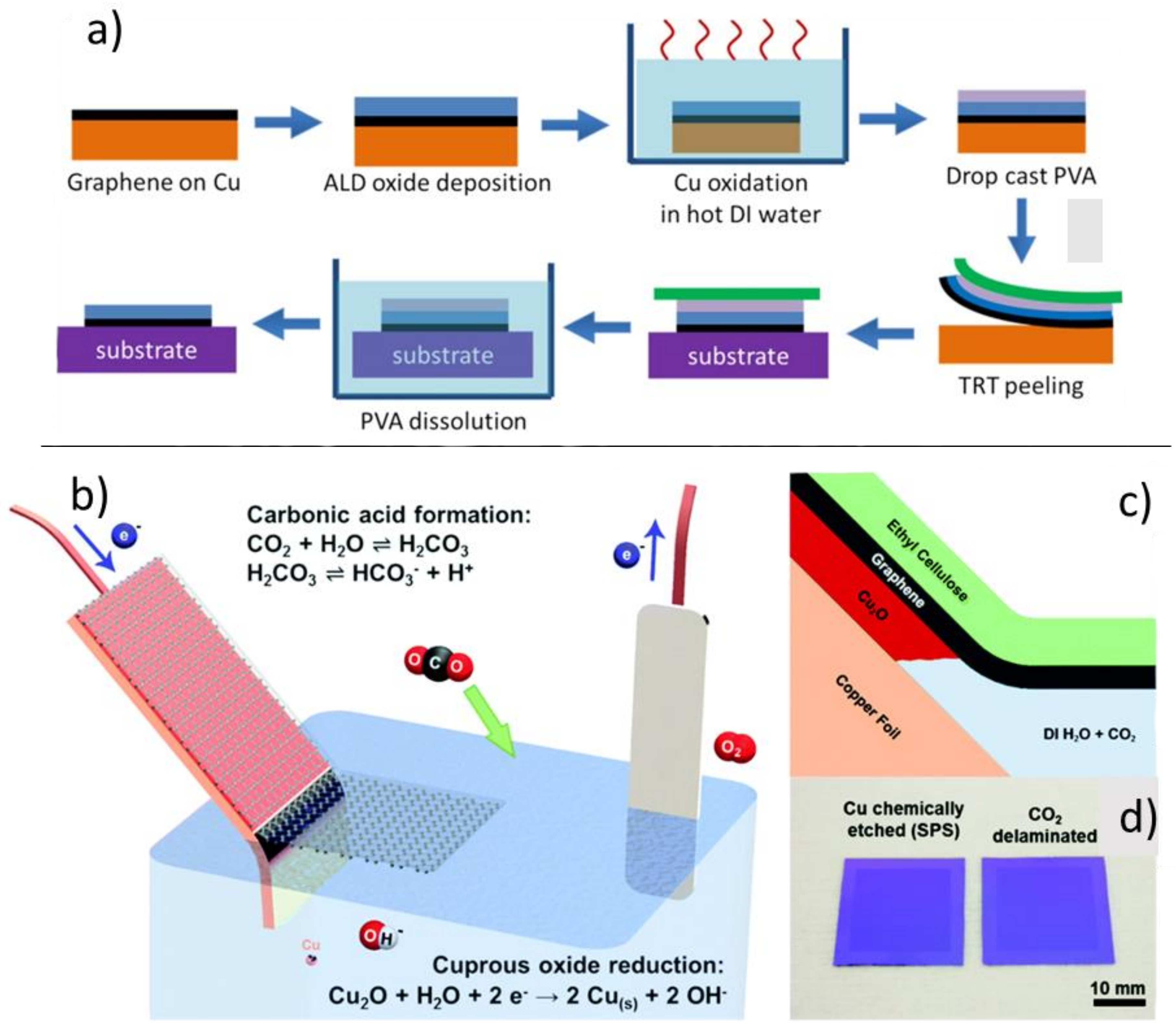
4.1.1. Chemical Etching of the Substrate
4.1.2. Non-Etching Methods to Weaken the Substrate Interaction Coefficient
4.2. Strategies Changing the Graphene Interaction Coefficient
4.3. Strategies Increasing the Graphene-Substrate Distance
4.3.1. Intercalation Methods
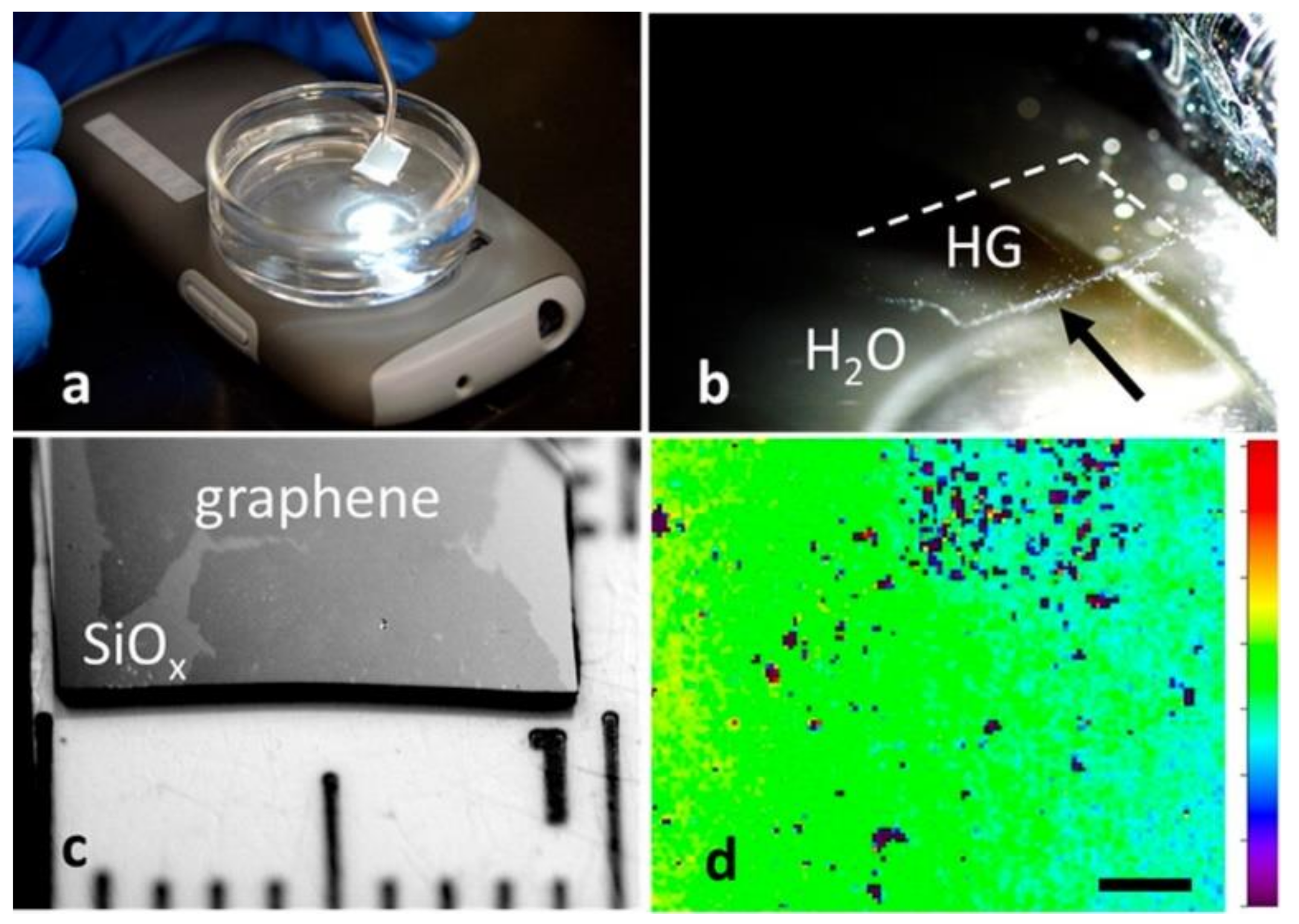
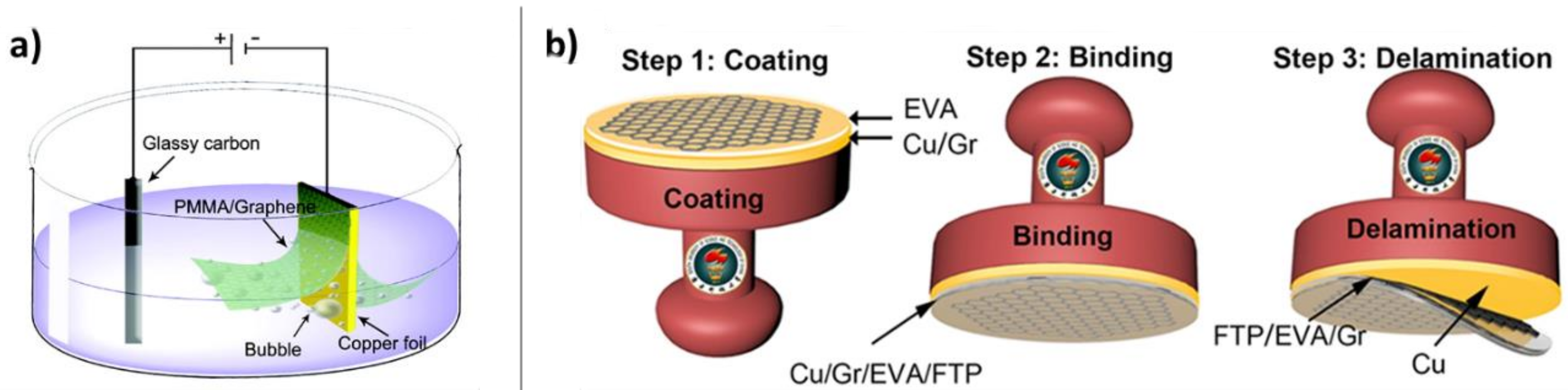
4.3.2. Electrochemical Methods, including “Bubble Transfer”
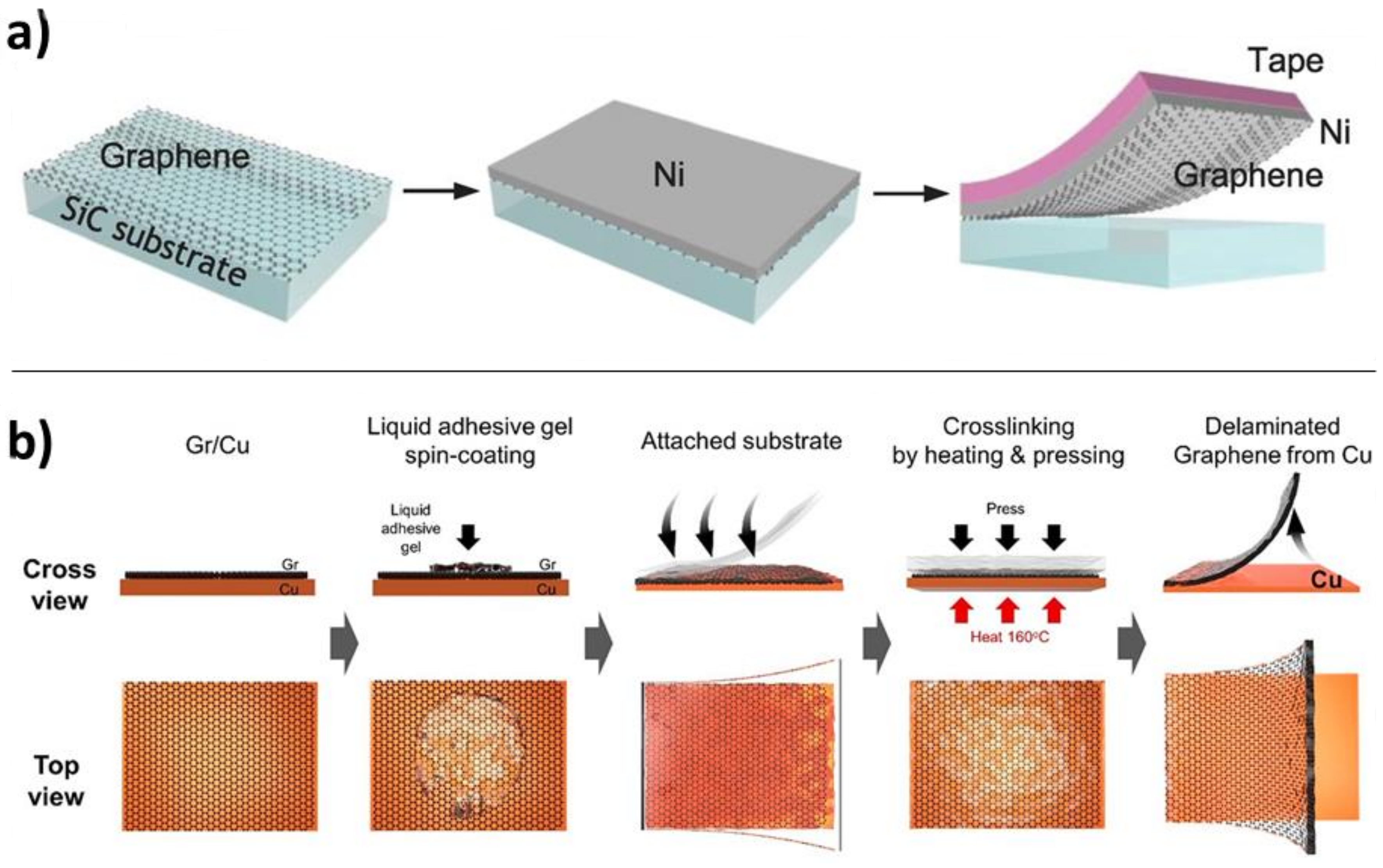
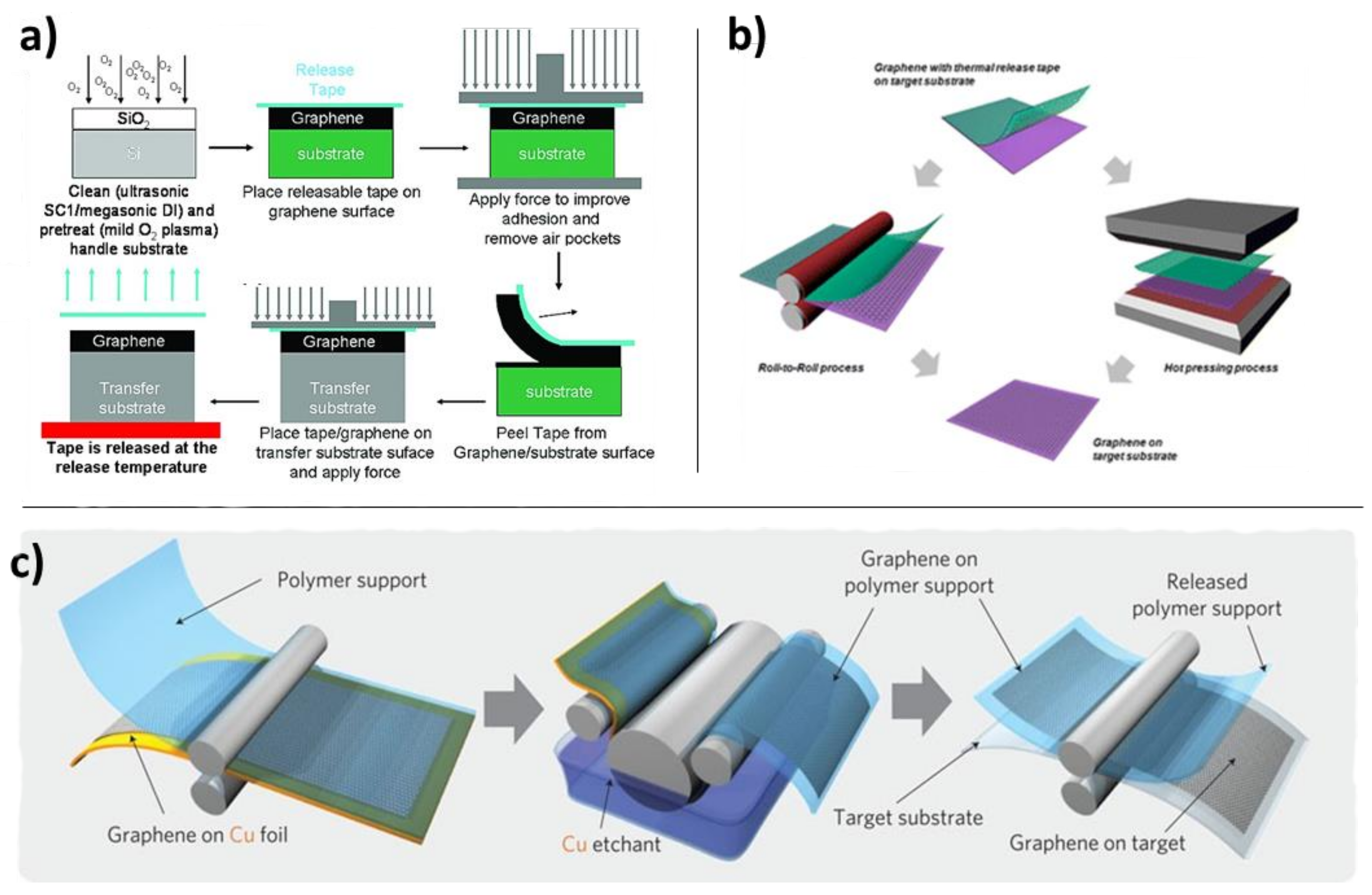
4.3.3. Mechanical Methods
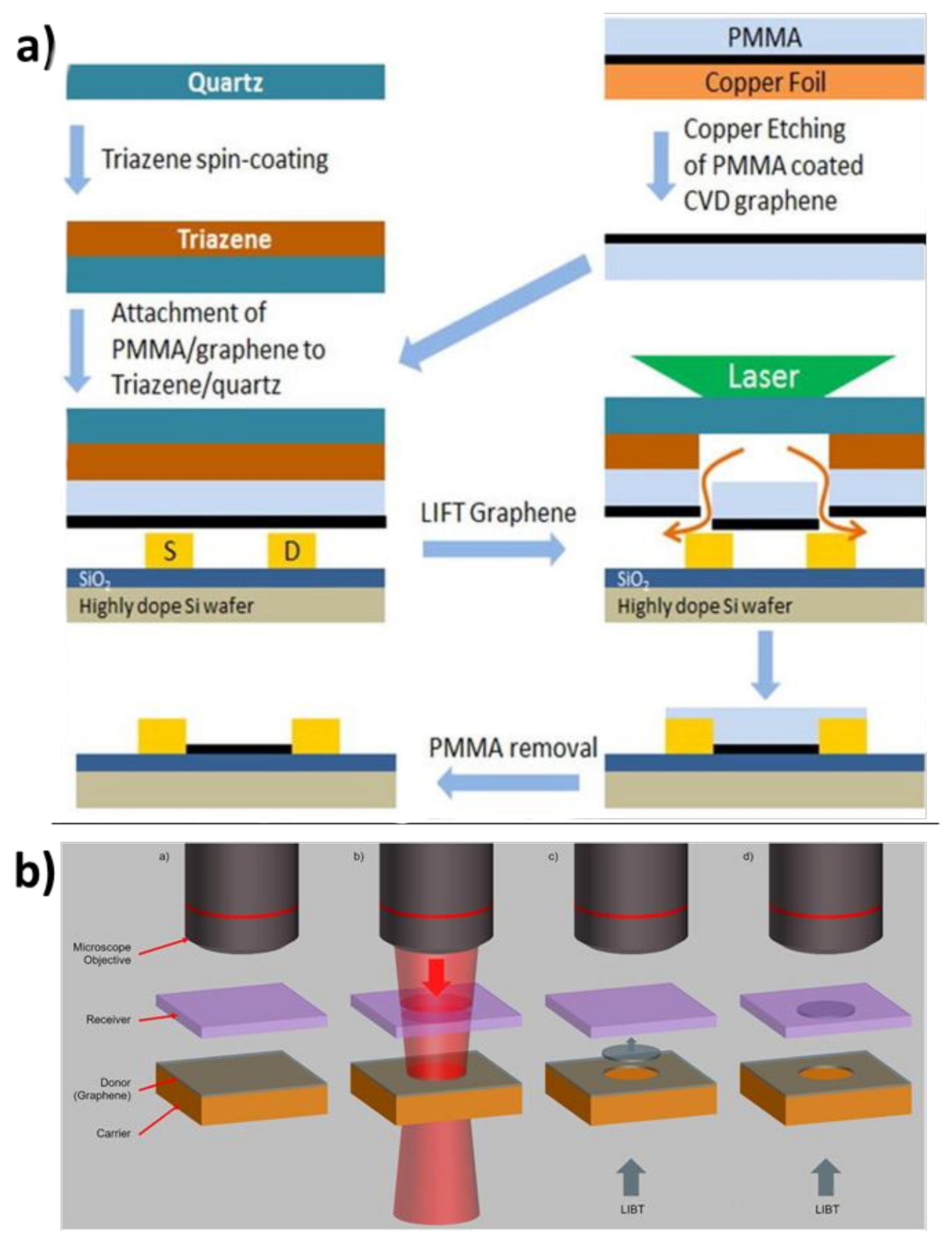
4.3.4. Laser-Assisted Methods
4.4. Other Methods and Combinations
5. Transfer Strategies: Relamination of Graphene
5.1. Strategies Changing the Substrate Interaction Coefficient
5.1.1. PMMA Removal and Cleaning
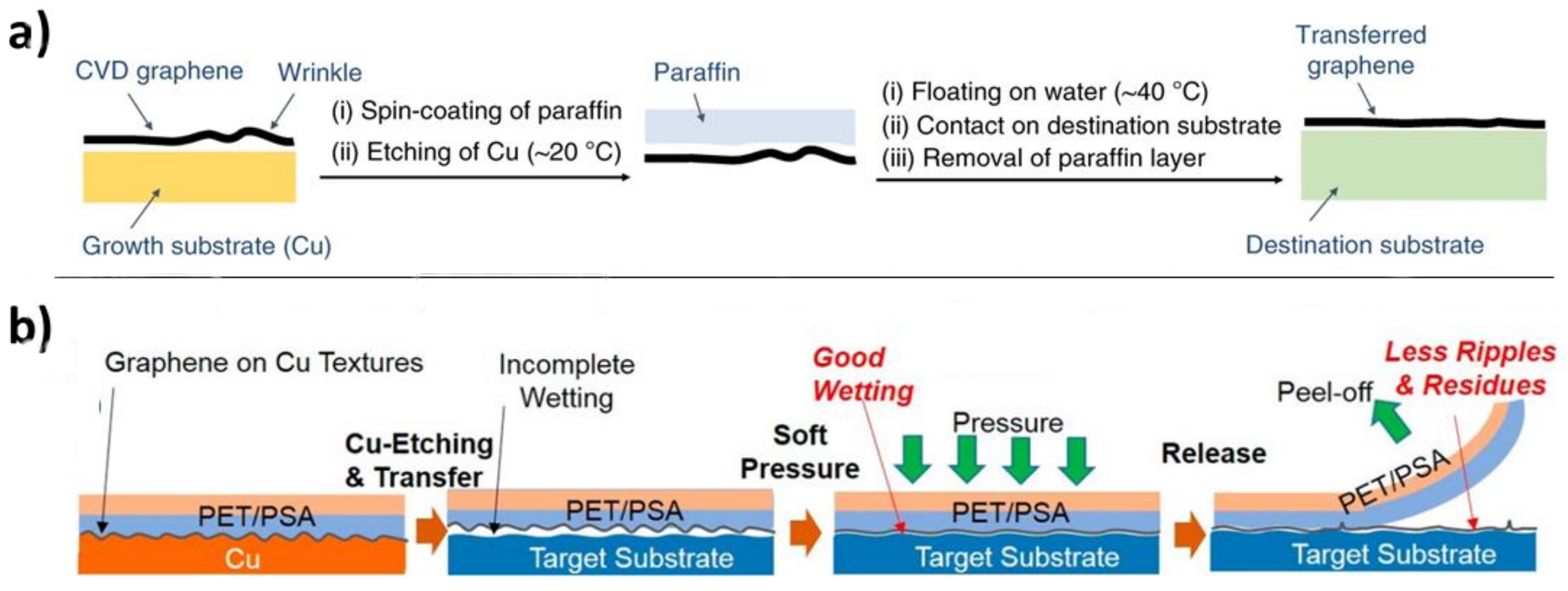
5.1.2. Releasing Layers as Polymer Support
5.1.3. Other Methods Relying on the Substrate Interaction Coefficient
5.2. Strategies Changing the Graphene Interaction Coefficient
5.3. Strategies Decreasing the Graphene-Substrate Distance
5.4. Combinations and other Strategies
6. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Castro Neto, A.H.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef]
- Schwierz, F. Graphene Transistors: Status, Prospects, and Problems. Proc. IEEE 2013, 101, 1567–1584. [Google Scholar] [CrossRef]
- Marconcini, P.; Macucci, M. Approximate calculation of the potential profile in a graphene-based device. IET Circuits Devices Syst. 2015, 9, 30–38. [Google Scholar] [CrossRef][Green Version]
- Cui, L.; Wang, J.; Sun, M. Graphene plasmon for optoelectronics. Rev. Phys. 2021, 6, 100054. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Zhou, C. Review of Chemical Vapor Deposition of Graphene and Related Applications. Accounts Chem. Res. 2013, 46, 2329–2339. [Google Scholar] [CrossRef]
- Mishra, N.; Boeckl, J.; Motta, N.; Iacopi, F. Graphene growth on silicon carbide: A review. Phys. Status Solidi A 2016, 213, 2277–2289. [Google Scholar] [CrossRef]
- Kim, J.; Park, H.; Hannon, J.B.; Bedell, S.W.; Fogel, K.; Sadana, D.K.; Dimitrakopoulos, C. Layer-Resolved Graphene Transfer via Engineered Strain Layers. Science 2013, 342, 833. [Google Scholar] [CrossRef]
- Kang, J.; Shin, D.; Bae, S.; Hong, B.H. Graphene transfer: Key for applications. Nanoscale 2012, 4, 5527–5537. [Google Scholar] [CrossRef]
- Ullah, S.; Yang, X.; Ta, H.Q.; Hasan, M.; Bachmatiuk, A.; Tokarska, K.; Trzebicka, B.; Fu, L.; Rummeli, M.H. Graphene transfer methods: A review. Nano Res. 2021, 14, 3756–3772. [Google Scholar] [CrossRef]
- Ma, L.-P.; Ren, W.; Cheng, H.-M. Transfer Methods of Graphene from Metal Substrates: A Review. Small Methods 2019, 3, 1900049. [Google Scholar] [CrossRef]
- Chen, Y.; Gong, X.-L.; Gai, J.-G. Progress and Challenges in Transfer of Large-Area Graphene Films. Adv. Sci. 2016, 3, 1500343. [Google Scholar] [CrossRef]
- Chen, M.; Haddon, R.C.; Yan, R.; Bekyarova, E. Advances in transferring chemical vapour deposition graphene: A review. Mater. Horiz. 2017, 4, 1054–1063. [Google Scholar] [CrossRef]
- Qing, F.; Zhang, Y.; Niu, Y.; Stehle, R.; Chen, Y.; Li, X. Towards large-scale graphene transfer. Nanoscale 2020, 12, 10890–10911. [Google Scholar] [CrossRef]
- Bellucci, L.; Cavallucci, T.; Tozzini, V. From the Buffer Layer to Graphene on Silicon Carbide: Exploring Morphologies by Computer Modeling. Front. Mater. 2019, 6, 198. [Google Scholar] [CrossRef]
- Jung, N.; Kim, N.; Jockusch, S.; Turro, N.J.; Kim, P.; Brus, L. Charge Transfer Chemical Doping of Few Layer Graphenes: Charge Distribution and Band Gap Formation. Nano Lett. 2009, 9, 4133–4137. [Google Scholar] [CrossRef]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Zhang, L.; Yoon, Y.; Weber, P.K.; Wang, H.; Guo, J.; Dai, H. N-Doping of Graphene Through Electrothermal Reactions with Ammonia. Science 2009, 324, 768. [Google Scholar] [CrossRef]
- Vicarelli, L.; Heerema, S.J.; Dekker, C.; Zandbergen, H.W. Controlling Defects in Graphene for Optimizing the Electrical Properties of Graphene Nanodevices. ACS Nano 2015, 9, 3428–3435. [Google Scholar] [CrossRef]
- Araujo, P.T.; Terrones, M.; Dresselhaus, M.S. Defects and impurities in graphene-like materials. Mater. Today 2012, 15, 98–109. [Google Scholar] [CrossRef]
- Tian, W.; Li, W.; Yu, W.; Liu, X. A Review on Lattice Defects in Graphene: Types, Generation, Effects and Regulation. Micromachines 2017, 8, 163. [Google Scholar] [CrossRef]
- Lupina, G.; Kitzmann, J.; Costina, I.; Lukosius, M.; Wenger, C.; Wolff, A.; Vaziri, S.; Östling, M.; Pasternak, I.; Krajewska, A.; et al. Residual Metallic Contamination of Transferred Chemical Vapor Deposited Graphene. ACS Nano 2015, 9, 4776–4785. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Lu, C.-C.; Yeh, C.-H.; Jin, C.; Suenaga, K.; Chiu, P.-W. Graphene Annealing: How Clean Can It Be? Nano Lett. 2011, 12, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Kim, Y.-S.; Yang, E.-H. The influence of thermal annealing to remove polymeric residue on the electronic doping and morphological characteristics of graphene. Carbon 2013, 65, 35–45. [Google Scholar] [CrossRef]
- Ishigami, M.; Chen, J.H.; Cullen, W.G.; Fuhrer, M.S.; Williams, E.D. Atomic Structure of Graphene on SiO2. Nano Lett. 2007, 7, 1643–1648. [Google Scholar] [CrossRef]
- Belyaeva, L.A.; Fu, W.; Arjmandi-Tash, H.; Schneider, G.F. Molecular Caging of Graphene with Cyclohexane: Transfer and Electrical Transport. ACS Cent. Sci. 2016, 2, 904–909. [Google Scholar] [CrossRef]
- Li, X.; Zhu, Y.; Cai, W.; Borysiak, M.; Han, B.; Chen, D.; Piner, R.D.; Colombo, L.; Ruoff, R.S. Transfer of Large-Area Graphene Films for High-Performance Transparent Conductive Electrodes. Nano Lett. 2009, 9, 4359–4363. [Google Scholar] [CrossRef]
- Brintlinger, T.H.; Bassim, N.D.; Winterstein, J.; Ng, A.; Lodge, M.S.; Ishigami, M.; Whitener, K.; Sheehan, P.; Stroud, R.M.; Robinson, J.T. Characterizing Multi-layer Pristine Graphene, Its Contaminants, and Their Origin Using Transmission Electron Microscopy. Microsc. Microanal. 2017, 23, 1740–1741. [Google Scholar] [CrossRef][Green Version]
- Miseikis, V.; Xiang, S.; Roddaro, S.; Heun, S.; Coletti, C. Perfecting the Growth and Transfer of Large Single-Crystal CVD Graphene: A Platform Material for Optoelectronic Applications. In GraphITA: Selected Papers from the Workshop on Synthesis, Characterization and Technological Exploitation of Graphene and 2D Materials Beyond Graphene; Morandi, V., Ottaviano, L., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 113–124. [Google Scholar] [CrossRef]
- Ambrosi, A.; Pumera, M. The CVD graphene transfer procedure introduces metallic impurities which alter the graphene electrochemical properties. Nanoscale 2014, 6, 472–476. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Borin Barin, G.; Song, Y.; de Fátima Gimenez, I.; Souza Filho, A.G.; Barreto, L.S.; Kong, J. Optimized graphene transfer: Influence of polymethylmethacrylate (PMMA) layer concentration and baking time on graphene final performance. Carbon 2015, 84, 82–90. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.; Xu, X.; Dubuisson, E.; Bao, Q.; Lu, J.; Loh, K.P. Electrochemical Delamination of CVD-Grown Graphene Film: Toward the Recyclable Use of Copper Catalyst. ACS Nano 2011, 5, 9927–9933. [Google Scholar] [CrossRef]
- Wang, M.; Yang, E.H.; Vajtai, R.; Kono, J.; Ajayan, P.M. Effects of etchants in the transfer of chemical vapor deposited graphene. J. Appl. Phys. 2018, 123, 195103. [Google Scholar] [CrossRef]
- Huang, Y.; Pan, Y.-H.; Yang, R.; Bao, L.-H.; Meng, L.; Luo, H.-L.; Cai, Y.-Q.; Liu, G.-D.; Zhao, W.-J.; Zhou, Z.; et al. Universal mechanical exfoliation of large-area 2D crystals. Nat. Commun. 2020, 11, 2453. [Google Scholar] [CrossRef]
- Liang, X.; Sperling, B.A.; Calizo, I.; Cheng, G.; Hacker, C.A.; Zhang, Q.; Obeng, Y.; Yan, K.; Peng, H.; Li, Q.; et al. Toward Clean and Crackless Transfer of Graphene. ACS Nano 2011, 5, 9144–9153. [Google Scholar] [CrossRef]
- Pirkle, A.; Chan, J.; Venugopal, A.; Hinojos, D.; Magnuson, C.W.; McDonnell, S.; Colombo, L.; Vogel, E.M.; Ruoff, R.S.; Wallace, R.M. The effect of chemical residues on the physical and electrical properties of chemical vapor deposited graphene transferred to SiO2. Appl. Phys. Lett. 2011, 99, 122108. [Google Scholar] [CrossRef]
- Zhu, W.; Low, T.; Perebeinos, V.; Bol, A.A.; Zhu, Y.; Yan, H.; Tersoff, J.; Avouris, P. Structure and Electronic Transport in Graphene Wrinkles. Nano Lett. 2012, 12, 3431–3436. [Google Scholar] [CrossRef]
- Deng, S.; Berry, V. Wrinkled, rippled and crumpled graphene: An overview of formation mechanism, electronic properties, and applications. Mater. Today 2016, 19, 197–212. [Google Scholar] [CrossRef]
- Lui, C.H.; Liu, L.; Mak, K.F.; Flynn, G.W.; Heinz, T.F. Ultraflat graphene. Nature 2009, 462, 339–341. [Google Scholar] [CrossRef]
- Bunch, J.S.; Dunn, M.L. Adhesion mechanics of graphene membranes. Solid State Commun. 2012, 152, 1359–1364. [Google Scholar] [CrossRef]
- Das, S.; Lahiri, D.; Lee, D.-Y.; Agarwal, A.; Choi, W. Measurements of the adhesion energy of graphene to metallic substrates. Carbon 2013, 59, 121–129. [Google Scholar] [CrossRef]
- Koenig, S.P.; Boddeti, N.G.; Dunn, M.L.; Bunch, J.S. Ultrastrong adhesion of graphene membranes. Nat. Nanotechnol. 2011, 6, 543–546. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces, 3rd ed.; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Lifshitz, E.M. The Theory of Molecular Attractive Forces between Solids. Perspect. Theor. Phys. Pergamon 1956, 2, 73–83. [Google Scholar]
- Zhang, Y.; Brar, V.W.; Girit, C.; Zettl, A.; Crommie, M.F. Origin of spatial charge inhomogeneity in graphene. Nat. Phys. 2009, 5, 722–726. [Google Scholar] [CrossRef]
- Hamaker, H.C. The London—van der Waals attraction between spherical particles. Physica 1937, 4, 1058–1072. [Google Scholar] [CrossRef]
- McLachlan, A.D.; Longuet-Higgins, H.C. Retarded dispersion forces between molecules. Proc. R. Soc. London. Ser. A. Math. Phys. Sci. 1963, 271, 387–401. [Google Scholar] [CrossRef]
- McLachlan, A.D. Three-body dispersion forces. Mol. Phys. 1963, 6, 423–427. [Google Scholar] [CrossRef]
- Dzyaloshinskii, I.E.; Lifshitz, E.M.; Pitaevskii, L.P. The general theory of van der Waals forces. Adv. Phys. 1961, 10, 165–209. [Google Scholar] [CrossRef]
- Hwang, C.; Siegel, D.A.; Mo, S.-K.; Regan, W.; Ismach, A.; Zhang, Y.; Zettl, A.; Lanzara, A. Fermi velocity engineering in graphene by substrate modification. Sci. Rep. 2012, 2, 590. [Google Scholar] [CrossRef]
- Elias, D.C.; Gorbachev, R.V.; Mayorov, A.S.; Morozov, S.V.; Zhukov, A.A.; Blake, P.; Ponomarenko, L.A.; Grigorieva, I.V.; Novoselov, K.S.; Guinea, F.; et al. Dirac cones reshaped by interaction effects in suspended graphene. Nat. Phys. 2011, 7, 701–704. [Google Scholar] [CrossRef]
- Suk, J.W.; Kitt, A.; Magnuson, C.W.; Hao, Y.; Ahmed, S.; An, J.; Swan, A.K.; Goldberg, B.B.; Ruoff, R.S. Transfer of CVD-Grown Monolayer Graphene onto Arbitrary Substrates. ACS Nano 2011, 5, 6916–6924. [Google Scholar] [CrossRef] [PubMed]
- Reina, A.; Son, H.; Jiao, L.; Fan, B.; Dresselhaus, M.S.; Liu, Z.; Kong, J. Transferring and Identification of Single- and Few-Layer Graphene on Arbitrary Substrates. J. Phys. Chem. C 2008, 112, 17741–17744. [Google Scholar] [CrossRef]
- Reina, A.; Jia, X.; Ho, J.; Nezich, D.; Son, H.; Bulovic, V.; Dresselhaus, M.S.; Kong, J. Large Area, Few-Layer Graphene Films on Arbitrary Substrates by Chemical Vapor Deposition. Nano Lett. 2008, 9, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Bae, S.; Jang, H.; Jang, S.; Zhu, S.-E.; Sim, S.H.; Song, Y.I.; Hong, B.H.; Ahn, J.-H. Wafer-Scale Synthesis and Transfer of Graphene Films. Nano Lett. 2010, 10, 490–493. [Google Scholar] [CrossRef]
- Park, H.; Lim, C.; Lee, C.-J.; Kang, J.; Kim, J.; Choi, M.; Park, H. Optimized poly(methyl methacrylate)-mediated graphene-transfer process for fabrication of high-quality graphene layer. Nanotechnology 2018, 29, 415303. [Google Scholar] [CrossRef]
- Cabrero-Vilatela, A.; Alexander-Webber, J.A.; Sagade, A.A.; Aria, A.I.; Braeuninger-Weimer, P.; Martin, M.B.; Weatherup, R.S.; Hofmann, S. Atomic layer deposited oxide films as protective interface layers for integrated graphene transfer. Nanotechnology 2017, 28, 485201. [Google Scholar] [CrossRef]
- Wang, M.C.; Moestopo, W.P.; Takekuma, S.; Barna, S.F.; Haasch, R.T.; Nam, S. A sustainable approach to large area transfer of graphene and recycling of the copper substrate. J. Mater. Chem. C 2017, 5, 11226–11232. [Google Scholar] [CrossRef]
- Wang, X.; Tabakman, S.M.; Dai, H. Atomic Layer Deposition of Metal Oxides on Pristine and Functionalized Graphene. J. Am. Chem. Soc. 2008, 130, 8152–8153. [Google Scholar] [CrossRef]
- Grebel, H.; Stan, L.; Sumant, A.V.; Liu, Y.; Gosztola, D.; Ocola, L.; Fisher, B. Transfer of Graphene with Protective Oxide Layers. ChemEngineering 2018, 2, 58. [Google Scholar] [CrossRef]
- Cherian, C.T.; Giustiniano, F.; Martin-Fernandez, I.; Andersen, H.; Balakrishnan, J.; Özyilmaz, B. ‘Bubble-Free’ Electrochemical Delamination of CVD Graphene Films. Small 2015, 11, 189–194. [Google Scholar] [CrossRef]
- Whitener, K.E.; Lee, W.K.; Bassim, N.D.; Stroud, R.M.; Robinson, J.T.; Sheehan, P.E. Transfer of Chemically Modified Graphene with Retention of Functionality for Surface Engineering. Nano Lett. 2016, 16, 1455–1461. [Google Scholar] [CrossRef]
- Whitener, K.E., Jr. Review Article: Hydrogenated graphene: A user’s guide. J. Vac. Sci. Technol. A 2018, 36, 05G401. [Google Scholar] [CrossRef]
- Elias, D.C.; Nair, R.R.; Mohiuddin, T.M.G.; Morozov, S.V.; Blake, P.; Halsall, M.P.; Ferrari, A.C.; Boukhvalov, D.W.; Katsnelson, M.I.; Geim, A.K.; et al. Control of Graphene’s Properties by Reversible Hydrogenation: Evidence for Graphane. Science 2009, 323, 610–613. [Google Scholar] [CrossRef]
- Whitener, K.E., Jr.; Lee, W.K.; Campbell, P.M.; Robinson, J.T.; Sheehan, P.E. Chemical hydrogenation of single-layer graphene enables completely reversible removal of electrical conductivity. Carbon 2014, 72, 348–353. [Google Scholar] [CrossRef]
- Whitener, K.E.; Robinson, J.T.; Sheehan, P.E. Protection from Below: Stabilizing Hydrogenated Graphene Using Graphene Underlayers. Langmuir 2017, 33, 13749–13756. [Google Scholar] [CrossRef]
- Pumera, M.; Wong, C.H.A. Graphane and hydrogenated graphene. Chem. Soc. Rev. 2013, 42, 5987–5995. [Google Scholar] [CrossRef]
- Robinson, J.T.; Zalalutdinov, M.; Baldwin, J.W.; Snow, E.S.; Wei, Z.; Sheehan, P.; Houston, B.H. Wafer-scale Reduced Graphene Oxide Films for Nanomechanical Devices. Nano Lett. 2008, 8, 3441–3445. [Google Scholar] [CrossRef]
- Haridas, D.; Yoseph, S.P.; So, C.R.; Whitener, K.E., Jr. Transfer of printed electronic structures using graphene oxide and gelatin enables reversible and biocompatible interface with living cells. Mater. Sci. Eng. C 2021, 120, 111685. [Google Scholar] [CrossRef]
- Gurarslan, A.; Yu, Y.; Su, L.; Yu, Y.; Suarez, F.; Yao, S.; Zhu, Y.; Ozturk, M.; Zhang, Y.; Cao, L. Surface-Energy-Assisted Perfect Transfer of Centimeter-Scale Monolayer and Few-Layer MoS2 Films onto Arbitrary Substrates. ACS Nano 2014, 8, 11522–11528. [Google Scholar] [CrossRef]
- Li, H.; Wu, J.; Huang, X.; Yin, Z.; Liu, J.; Zhang, H. A Universal, Rapid Method for Clean Transfer of Nanostructures onto Various Substrates. ACS Nano 2014, 8, 6563–6570. [Google Scholar] [CrossRef]
- Calado, V.E.; Schneider, G.F.; Theulings, A.M.M.G.; Dekker, C.; Vandersypen, L.M.K. Formation and control of wrinkles in graphene by the wedging transfer method. Appl. Phys. Lett. 2012, 101, 103116. [Google Scholar] [CrossRef]
- Prydatko, A.V.; Belyaeva, L.A.; Jiang, L.; Lima, L.M.C.; Schneider, G.F. Contact angle measurement of free-standing square-millimeter single-layer graphene. Nat. Commun. 2018, 9, 4185. [Google Scholar] [CrossRef]
- Taqieddin, A.; Heiranian, M.; Aluru, N.R. Interfacial Properties of Water on Hydrogenated and Fluorinated Graphene Surfaces: Parametrization of Nonbonded Interactions. J. Phys. Chem. C 2020, 124, 21467–21475. [Google Scholar] [CrossRef]
- Son, J.; Buzov, N.; Chen, S.; Sung, D.; Ryu, H.; Kwon, J.; Kim, S.; Namiki, S.; Xu, J.; Hong, S.; et al. Tailoring Surface Properties via Functionalized Hydrofluorinated Graphene Compounds. Adv. Mater. 2019, 31, 1903424. [Google Scholar] [CrossRef]
- Zheng, L.; Li, Z.; Bourdo, S.; Watanabe, F.; Ryerson, C.C.; Biris, A.S. Catalytic hydrogenation of graphene films. Chem. Commun. 2011, 47, 1213–1215. [Google Scholar] [CrossRef]
- Lotya, M.; Hernandez, Y.; King, P.J.; Smith, R.J.; Nicolosi, V.; Karlsson, L.S.; Blighe, F.M.; De, S.; Wang, Z.; McGovern, I.T.; et al. Liquid Phase Production of Graphene by Exfoliation of Graphite in Surfactant/Water Solutions. J. Am. Chem. Soc. 2009, 131, 3611–3620. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Z.; Zheng, H.; Ren, Q.; Zou, Z.; Mei, L.; Zhang, Z.; Xia, Y.; Lin, C.-T.; Zhao, P.; et al. High-quality graphene transfer via directional etching of metal substrates. Nanoscale 2019, 11, 16001–16006. [Google Scholar] [CrossRef]
- Seo, Y.-M.; Jang, W.; Gu, T.; Seok, H.-J.; Han, S.; Choi, B.L.; Kim, H.-K.; Chae, H.; Kang, J.; Whang, D. Defect-Free Mechanical Graphene Transfer Using n-Doping Adhesive Gel Buffer. ACS Nano 2021, 15, 11276–11284. [Google Scholar] [CrossRef]
- Fujita, J.-I.; Hiyama, T.; Hirukawa, A.; Kondo, T.; Nakamura, J.; Ito, S.-I.; Araki, R.; Ito, Y.; Takeguchi, M.; Pai, W.W. Near room temperature chemical vapor deposition of graphene with diluted methane and molten gallium catalyst. Sci. Rep. 2017, 7, 12371. [Google Scholar] [CrossRef]
- Enoki, T.; Suzuki, M.; Endo, M. Graphite Intercalation Compounds and Applications; Oxford University Press: New York, NY, USA, 2003. [Google Scholar]
- Verguts, K.; Schouteden, K.; Wu, C.-H.; Peters, L.; Vrancken, N.; Wu, X.; Li, Z.; Erkens, M.; Porret, C.; Huyghebaert, C.; et al. Controlling Water Intercalation Is Key to a Direct Graphene Transfer. ACS Appl. Mater. Interfaces 2017, 9, 37484–37492. [Google Scholar] [CrossRef]
- Guo, H.; Wang, X.; Huang, L.; Jin, X.; Yang, Z.; Zhou, Z.; Hu, H.; Zhang, Y.-Y.; Lu, H.; Zhang, Q.; et al. Insulating SiO2 under Centimeter-Scale, Single-Crystal Graphene Enables Electronic-Device Fabrication. Nano Lett. 2020, 20, 8584–8591. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Zhang, Y.; Liu, M.; Ji, Q.; Gao, T.; Zhang, Y.; Liu, Z. Clean transfer of graphene on Pt foils mediated by a carbon monoxide intercalation process. Nano Res. 2013, 6, 671–678. [Google Scholar] [CrossRef]
- Ohtomo, M.; Sekine, Y.; Wang, S.; Hibino, H.; Yamamoto, H. Etchant-free graphene transfer using facile intercalation of alkanethiol self-assembled molecules at graphene/metal interfaces. Nanoscale 2016, 8, 11503–11510. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, B.N.; Smitha, A.S.; Wu, Y.; Cai, N.; Li, Y.; Huang, Z.; Wang, W.; Shi, R.; Wang, J.; Liu, S.; et al. A Universal Stamping Method of Graphene Transfer for Conducting Flexible and Transparent Polymers. Sci. Rep. 2019, 9, 3999. [Google Scholar] [CrossRef]
- Gao, L.; Ren, W.; Xu, H.; Jin, L.; Wang, Z.; Ma, T.; Ma, L.-P.; Zhang, Z.; Fu, Q.; Peng, L.-M.; et al. Repeated growth and bubbling transfer of graphene with millimetre-size single-crystal grains using platinum. Nat. Commun. 2012, 3, 699. [Google Scholar] [CrossRef]
- Kang, S.J.; Kim, B.; Kim, K.S.; Zhao, Y.; Chen, Z.; Lee, G.H.; Hone, J.; Kim, P.; Nuckolls, C. Inking Elastomeric Stamps with Micro-Patterned, Single Layer Graphene to Create High-Performance OFETs. Adv. Mater. 2011, 23, 3531–3535. [Google Scholar] [CrossRef]
- Ko, P.J.; Takahashi, H.; Koide, S.; Sakai, H.; Thu, T.V.; Okada, H.; Sandhu, A. Simple method to transfer graphene from metallic catalytic substrates to flexible surfaces without chemical etching. J. Phys. Conf. Ser. 2013, 433, 012002. [Google Scholar] [CrossRef]
- Lock, E.H.; Baraket, M.; Laskoski, M.; Mulvaney, S.P.; Lee, W.K.; Sheehan, P.E.; Hines, D.R.; Robinson, J.T.; Tosado, J.; Fuhrer, M.S.; et al. High-Quality Uniform Dry Transfer of Graphene to Polymers. Nano Lett. 2011, 12, 102–107. [Google Scholar] [CrossRef]
- Lock, E.H.; Hernández, S.C.; Anderson, T.J.; Schmucker, S.W.; Laskoski, M.; Mulvaney, S.P.; Bezares, F.J.; Caldwell, J.D.; Sheehan, P.E.; Robinson, J.T.; et al. Etch free graphene transfer to polymers. Surf. Coat. Technol. 2014, 241, 118–122. [Google Scholar] [CrossRef]
- Fechine, G.J.M.; Martin-Fernandez, I.; Yiapanis, G.; Bentini, R.; Kulkarni, E.S.; Bof de Oliveira, R.V.; Hu, X.; Yarovsky, I.; Castro Neto, A.H.; Özyilmaz, B. Direct dry transfer of chemical vapor deposition graphene to polymeric substrates. Carbon 2015, 83, 224–231. [Google Scholar] [CrossRef]
- Smits, E.C.P.; Walter, A.; de Leeuw, D.M.; Asadi, K. Laser induced forward transfer of graphene. Appl. Phys. Lett. 2017, 111, 173101. [Google Scholar] [CrossRef]
- Praeger, M.; Papazoglou, S.; Pesquera, A.; Zurutuza, A.; Levi, A.; Naveh, D.; Zergioti, I.; Eason, R.W.; Mills, B. Laser-induced backward transfer of monolayer graphene. Appl. Surf. Sci. 2020, 533, 147488. [Google Scholar] [CrossRef]
- Yoo, J.-H.; Park, J.B.; Ahn, S.; Grigoropoulos, C.P. Laser-Induced Direct Graphene Patterning and Simultaneous Transferring Method for Graphene Sensor Platform. Small 2013, 9, 4269–4275. [Google Scholar] [CrossRef]
- Papazoglou, S.; Petridis, C.; Kymakis, E.; Kennou, S.; Raptis, Y.S.; Chatzandroulis, S.; Zergioti, I. In-situ sequential laser transfer and laser reduction of graphene oxide films. Appl. Phys. Lett. 2018, 112, 183301. [Google Scholar] [CrossRef]
- Goodfriend, N.T.; Heng, S.Y.; Nerushev, O.A.; Gromov, A.V.; Bulgakov, A.V.; Okada, M.; Xu, W.; Kitaura, R.; Warner, J.; Shinohara, H.; et al. Blister-based-laser-induced-forward-transfer: A non-contact, dry laser-based transfer method for nanomaterials. Nanotechnology 2018, 29, 385301. [Google Scholar] [CrossRef]
- Chandrashekar, B.N.; Deng, B.; Smitha, A.S.; Chen, Y.; Tan, C.; Zhang, H.; Peng, H.; Liu, Z. Roll-to-Roll Green Transfer of CVD Graphene onto Plastic for a Transparent and Flexible Triboelectric Nanogenerator. Adv. Mater. 2015, 27, 5210–5216. [Google Scholar] [CrossRef]
- Shivayogimath, A.; Whelan, P.R.; Mackenzie, D.M.A.; Luo, B.; Huang, D.; Luo, D.; Wang, M.; Gammelgaard, L.; Shi, H.; Ruoff, R.S.; et al. Do-It-Yourself Transfer of Large-Area Graphene Using an Office Laminator and Water. Chem. Mater. 2019, 31, 2328–2336. [Google Scholar] [CrossRef]
- Ren, Y.; Zhu, C.; Cai, W.; Li, H.; Hao, Y.; Wu, Y.; Chen, S.; Wu, Q.; Piner, R.D.; Ruoff, R.S. An improved method for transferring graphene grown by chemical vapor deposition. Nano 2012, 7, 1150001. [Google Scholar] [CrossRef]
- Yang, X.; Yan, M. Removing contaminants from transferred CVD graphene. Nano Res. 2020, 13, 599–610. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, L.; Jia, K.; Liu, X.; Cheng, T.; Peng, H.; Lin, L.; Liu, Z. New Growth Frontier: Superclean Graphene. ACS Nano 2020, 14, 10796–10803. [Google Scholar] [CrossRef]
- Wang, X.; Dolocan, A.; Chou, H.; Tao, L.; Dick, A.; Akinwande, D.; Willson, C.G. Direct Observation of Poly(Methyl Methacrylate) Removal from a Graphene Surface. Chem. Mater. 2017, 29, 2033–2039. [Google Scholar] [CrossRef]
- Kern, W. The Evolution of Silicon Wafer Cleaning Technology. J. Electrochem. Soc. 1990, 137, 1887–1892. [Google Scholar] [CrossRef]
- Sun, J.; Finklea, H.O.; Liu, Y. Characterization and electrolytic cleaning of poly(methyl methacrylate) residues on transferred chemical vapor deposited graphene. Nanotechnology 2017, 28, 125703. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Huh, J.N.; Lee, W.S.; Bae, I.-G. Simple and rapid cleaning of graphenes with a ‘bubble-free’ electrochemical treatment. J. Mater. Chem. C 2018, 6, 2234–2244. [Google Scholar] [CrossRef]
- Song, J.; Kam, F.-Y.; Png, R.-Q.; Seah, W.-L.; Zhuo, J.-M.; Lim, G.-K.; Ho, P.K.H.; Chua, L.-L. A general method for transferring graphene onto soft surfaces. Nat. Nanotechnol. 2013, 8, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, J.D.; Anderson, T.J.; Culbertson, J.C.; Jernigan, G.G.; Hobart, K.D.; Kub, F.J.; Tadjer, M.J.; Tedesco, J.L.; Hite, J.K.; Mastro, M.A.; et al. Technique for the Dry Transfer of Epitaxial Graphene onto Arbitrary Substrates. ACS Nano 2010, 4, 1108–1114. [Google Scholar] [CrossRef]
- Kang, J.; Hwang, S.; Kim, J.H.; Kim, M.H.; Ryu, J.; Seo, S.J.; Hong, B.H.; Kim, M.K.; Choi, J.-B. Efficient Transfer of Large-Area Graphene Films onto Rigid Substrates by Hot Pressing. ACS Nano 2012, 6, 5360–5365. [Google Scholar] [CrossRef]
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.; Park, J.-S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Ri Kim, H.; Song, Y.I.; et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nano 2010, 5, 574–578. [Google Scholar] [CrossRef]
- Martins, L.G.P.; Song, Y.; Zeng, T.; Dresselhaus, M.S.; Kong, J.; Araujo, P.T. Direct transfer of graphene onto flexible substrates. Proc. Natl. Acad. Sci. USA 2013, 110, 17762–17767. [Google Scholar] [CrossRef]
- Ballesio, A.; Parmeggiani, M.; Verna, A.; Frascella, F.; Cocuzza, M.; Pirri, C.F.; Marasso, S.L. A novel hot embossing Graphene transfer process for flexible electronics. Microelectron. Eng. 2019, 209, 16–19. [Google Scholar] [CrossRef]
- Kim, H.H.; Lee, S.K.; Lee, S.G.; Lee, E.; Cho, K. Wetting-Assisted Crack- and Wrinkle-Free Transfer of Wafer-Scale Graphene onto Arbitrary Substrates over a Wide Range of Surface Energies. Adv. Funct. Mater. 2016, 26, 2070–2077. [Google Scholar] [CrossRef]
- Kafiah, F.; Laoui, T.; Abdelsalam, E.; Atieh, M.A.; Khan, Z.; Alkasrawi, M. Monolayer Graphene Transfer onto Hydrophilic Substrates: A New Protocol Using Electrostatic Charging. Membranes 2020, 10, 358. [Google Scholar] [CrossRef]
- Leong, W.S.; Wang, H.; Yeo, J.; Martin-Martinez, F.J.; Zubair, A.; Shen, P.-C.; Mao, Y.; Palacios, T.; Buehler, M.J.; Hong, J.-Y.; et al. Paraffin-enabled graphene transfer. Nat. Commun. 2019, 10, 867. [Google Scholar] [CrossRef]
- Kim, S.J.; Choi, T.; Lee, B.; Lee, S.; Choi, K.; Park, J.B.; Yoo, J.M.; Choi, Y.S.; Ryu, J.; Kim, P.; et al. Ultraclean Patterned Transfer of Single-Layer Graphene by Recyclable Pressure Sensitive Adhesive Films. Nano Lett. 2015, 15, 3236–3240. [Google Scholar] [CrossRef]
- Regan, W.; Alem, N.; Alemán, B.; Geng, B.; Girit, Ç.; Maserati, L.; Wang, F.; Crommie, M.; Zettl, A. A direct transfer of layer-area graphene. Appl. Phys. Lett. 2010, 96, 113102. [Google Scholar] [CrossRef]
- Qi, P.; Huang, Y.; Yao, Y.; Li, Q.; Lian, Y.; Lin, L.; Wang, X.; Gu, Y.; Li, L.; Deng, Z.; et al. Wax-assisted crack-free transfer of monolayer CVD graphene: Extending from standalone to supported copper substrates. Appl. Surf. Sci. 2019, 493, 81–86. [Google Scholar] [CrossRef]
- Nasir, T.; Kim, B.J.; Kim, K.-W.; Lee, S.H.; Lim, H.K.; Lee, D.K.; Jeong, B.J.; Kim, H.C.; Yu, H.K.; Choi, J.-Y. Design of softened polystyrene for crack- and contamination-free large-area graphene transfer. Nanoscale 2018, 10, 21865–21870. [Google Scholar] [CrossRef]
- Qu, J.; Li, B.-W.; Shen, Y.; Huo, S.; Xu, Y.; Liu, S.; Song, B.; Wang, H.; Hu, C.; Feng, W. Evaporable Glass-State Molecule-Assisted Transfer of Clean and Intact Graphene onto Arbitrary Substrates. ACS Appl. Mater. Interfaces 2019, 11, 16272–16279. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, C.; Zou, Z.; Wu, Z.; Yin, S.; Zhang, Z.; Liu, J.; Xia, Y.; Lin, C.-T.; Zhao, P.; et al. A scalable polymer-free method for transferring graphene onto arbitrary surfaces. Carbon 2020, 161, 479–485. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, Q.; Zhang, X.; Chen, Z.; Zheng, H.; Liu, J.; Jin, Y.; Lin, C.-T.; Zhao, Y.; Zhao, P. Facile Graphene Transfer Using Commercially Available Liquid Bandage. ACS Appl. Nano Mater. 2021, 4, 7272–7279. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Ni, G.-X.; Liu, Y.; Liu, B.; Castro Neto, A.H.; Loh, K.P. Face-to-face transfer of wafer-scale graphene films. Nature 2014, 505, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, S.; Dev, P.; Friedman, A.L.; Stine, R.; Robinson, J.T.; Reinecke, T.L.; Sheehan, P.E. van der Waals Screening by Single-Layer Graphene and Molybdenum Disulfide. ACS Nano 2014, 8, 12410–12417. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, Y.; Guo, W. Screening effect of monolayer van der Waals crystals on surface deicing: A molecular simulation study. Phys. Chem. Chem. Phys. 2020, 22, 27873–27881. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Z.; Guo, W. Van der Waals screening by graphenelike monolayers. Phys. Rev. B 2018, 97, 241411. [Google Scholar] [CrossRef]
- Gaire, B.; Singla, S.; Dhinojwala, A. Screening of hydrogen bonding interactions by a single layer graphene. Nanoscale 2021, 13, 8098–8106. [Google Scholar] [CrossRef]
- Cao, Y.; Fatemi, V.; Fang, S.; Watanabe, K.; Taniguchi, T.; Kaxiras, E.; Jarillo-Herrero, P. Unconventional superconductivity in magic-angle graphene superlattices. Nature 2018, 556, 43–50. [Google Scholar] [CrossRef]
- Hanbicki, A.T.; Chuang, H.-J.; Rosenberger, M.R.; Hellberg, C.S.; Sivaram, S.V.; McCreary, K.M.; Mazin, I.I.; Jonker, B.T. Double Indirect Interlayer Exciton in a MoSe2/WSe2 van der Waals Heterostructure. ACS Nano 2018, 12, 4719–4726. [Google Scholar] [CrossRef]
- Yasuda, K.; Wang, X.; Watanabe, K.; Taniguchi, T.; Jarillo-Herrero, P. Stacking-engineered ferroelectricity in bilayer boron nitride. Science 2021, 372, 1458–1462. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Langston, X.; Whitener, K.E., Jr. Graphene Transfer: A Physical Perspective. Nanomaterials 2021, 11, 2837. https://doi.org/10.3390/nano11112837
Langston X, Whitener KE Jr. Graphene Transfer: A Physical Perspective. Nanomaterials. 2021; 11(11):2837. https://doi.org/10.3390/nano11112837
Chicago/Turabian StyleLangston, Xavier, and Keith E. Whitener, Jr. 2021. "Graphene Transfer: A Physical Perspective" Nanomaterials 11, no. 11: 2837. https://doi.org/10.3390/nano11112837
APA StyleLangston, X., & Whitener, K. E., Jr. (2021). Graphene Transfer: A Physical Perspective. Nanomaterials, 11(11), 2837. https://doi.org/10.3390/nano11112837




