Novel Biocompatible with Animal Cells Composite Material Based on Organosilicon Polymers and Fullerenes with Light-Induced Bacteriostatic Properties
Abstract
:1. Introduction
2. Materials and Methods
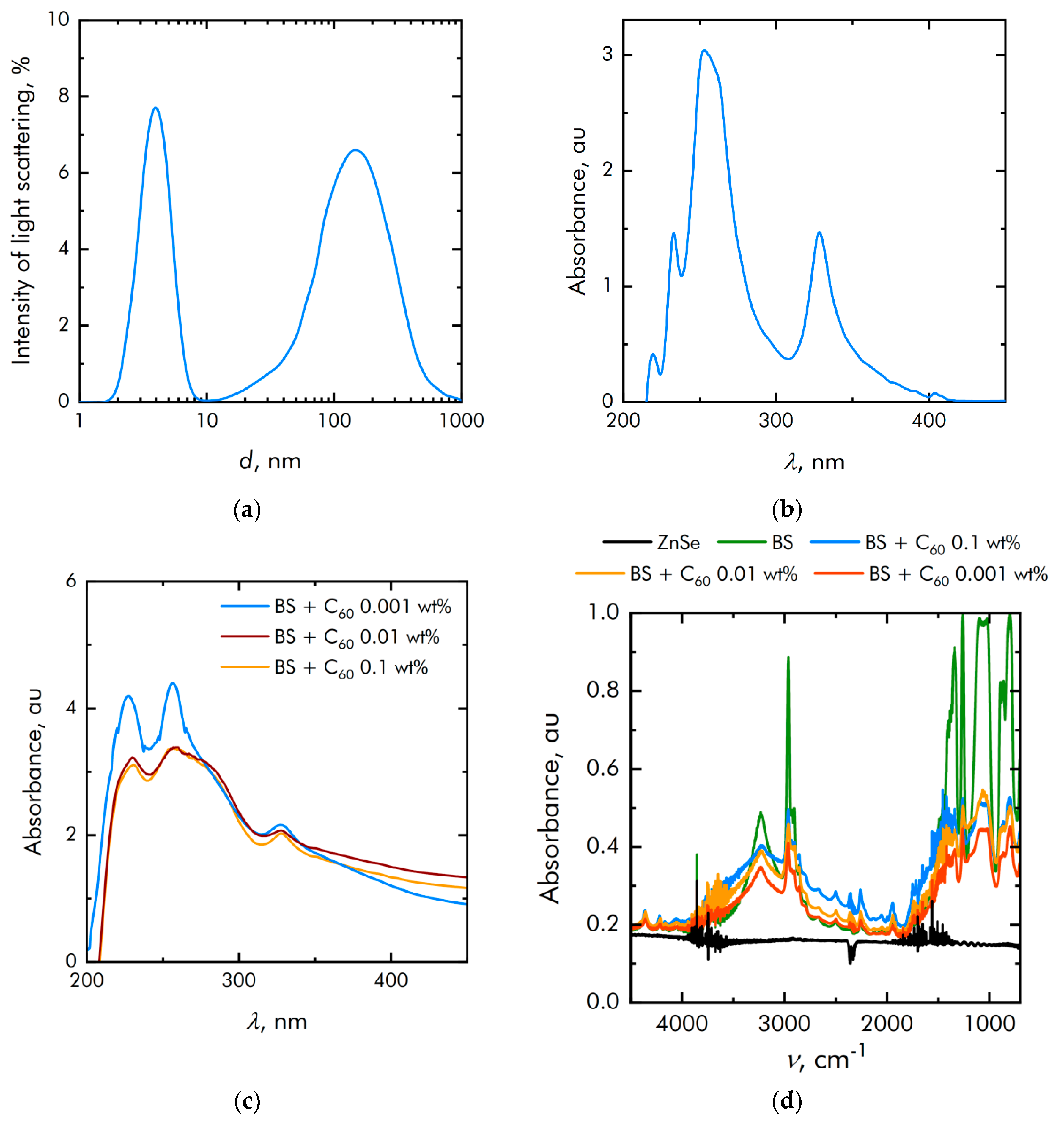
2.1. Fullerene C60 Nanoparticles Characteristics Assay
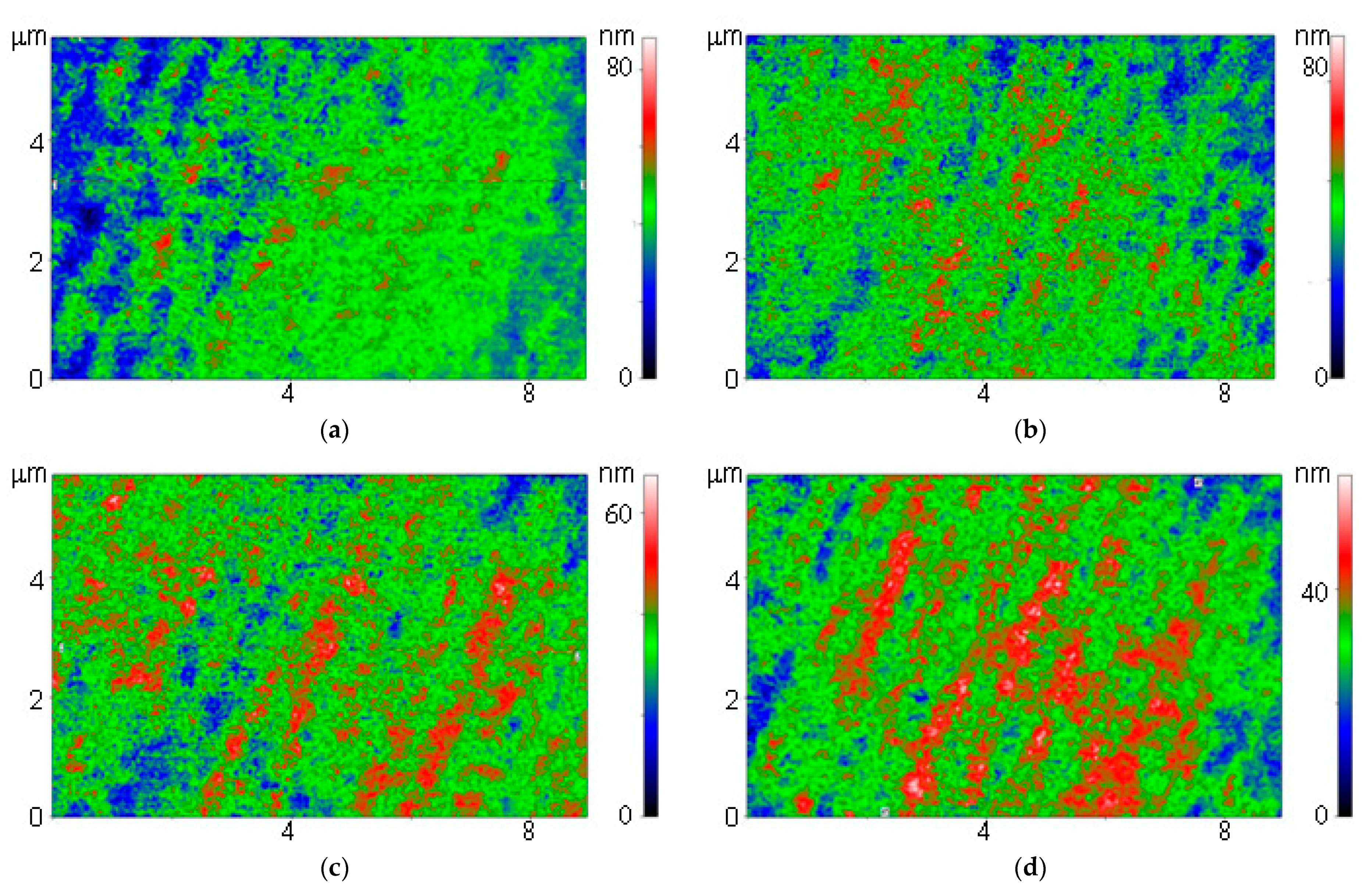
2.2. Borosiloxane Composites Synthesis and Rheological Characteristics Assay
2.3. Hydrogen Peroxide Concentration Measurement
2.4. OH-Radicals Concentration Measurement
2.5. Long-Lived Reactive Protein Species Concentration Measurement
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Bactericidal Activity Assay
2.8. Cell Culture
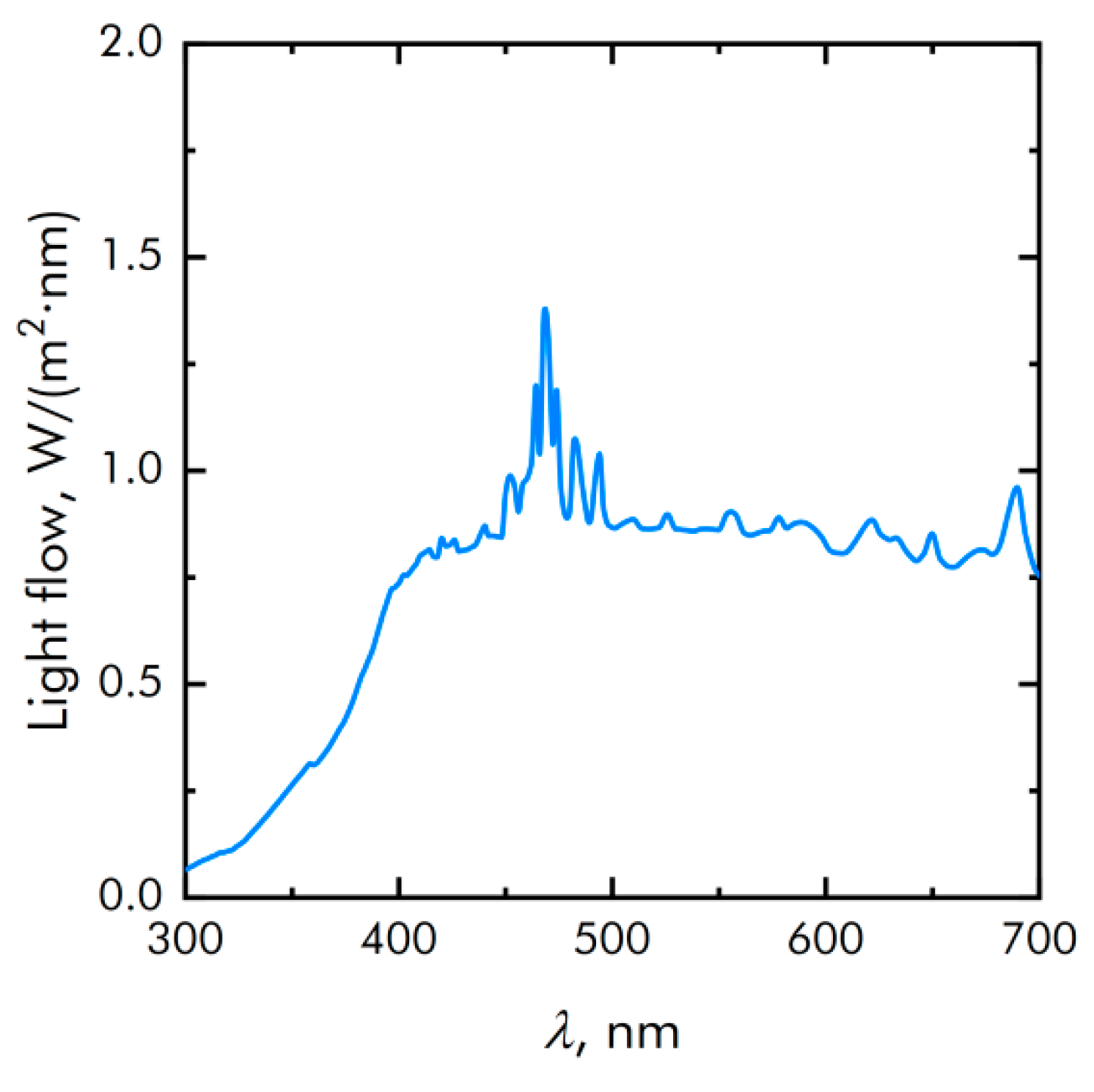
2.9. Exposure to Visible Light
2.10. Statistic
3. Results
3.1. Physicochemical Characteristics of Materials and Composite
3.2. Effect of Composite on ROS Generation and Damage to Biopolymers
3.3. Bacteriostatic Properties of the Composite
3.4. Biocompatibility with Mammalian Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. Do iron oxide nanoparticles have significant antibacterial properties? Antibiotics 2021, 10, 884. [Google Scholar] [CrossRef]
- Ye, L.; Kollie, L.; Liu, X.; Guo, W.; Ying, X.; Zhu, J.; Yang, S.; Yu, M. Antitumor Activity and Potential Mechanism of Novel Fullerene Derivative Nanoparticles. Molecules 2021, 26, 3252. [Google Scholar] [CrossRef]
- Gataullin, A.R.; Bogdanova, S.A.; Galyametdinov, Y.G. Dispersion of fullerene C60 in organized media. Liq. Cryst. Appl. 2019, 19, 6–13. [Google Scholar] [CrossRef]
- Maas, M. Carbon nanomaterials as antibacterial colloids. Materials 2016, 9, 617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Liu, X.; Han, H. Evaluation of antibacterial effects of carbon nanomaterials against copper-resistant Ralstonia solanacearum. Colloids Surf. B Biointerfaces 2013, 103, 136–142. [Google Scholar] [CrossRef]
- Elias, L.; Taengua, R.; Frígols, B.; Selesa, B.; Serrano-Aroca, A. Carbon nanomaterials and LED irradiation as antibacterial strategies against gram-positive multidrug-resistant pathogens. Int. J. Mol. Sci. 2019, 20, 3603. [Google Scholar] [CrossRef] [Green Version]
- Xin, Q.; Shah, H.; Nawaz, A.; Xie, W.; Akram, M.Z.; Batool, A.; Tian, L.; Jan, S.U.; Boddula, R.; Guo, B.; et al. Antibacterial carbon-based nanomaterials. Adv. Mater. 2019, 31, e1804838. [Google Scholar] [CrossRef]
- Hu, W.; Peng, C.; Luo, W.; Lv, M.; Li, X.; Li, D.; Huang, Q.; Fan, C. Graphene-based antibacterial paper. ACS Nano 2010, 27, 4317–4323. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Hashemi, H.; Feng, J.; Jafari, S.M. Carbon nanomaterials against pathogens; the antimicrobial activity of carbon nanotubes, graphene/graphene oxide, fullerenes, and their nanocomposites. Colloid Interface Sci. 2020, 284, 102250. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Dadashpour, M.; Hejazi, M.; Hasanzadeh, M.; Behnam, B.; de la Guardia, M.; Shadjou, N.; Mokhtarzadeh, A. Anti-bacterial activity of graphene oxide as a new weapon nanomaterial to combat multidrug-resistance bacteria. Mater. Sci. Eng. C 2017, 74, 568–581. [Google Scholar] [CrossRef] [PubMed]
- Lyon, D.Y.; Brunet, L.; Hinkal, G.W.; Wiesner, M.R.; Alvarez, P.J. Antibacterial activity of fullerene water suspensions (nC60) is not due to ROS-mediated damage. Nano Lett. 2008, 8, 1539–1543. [Google Scholar] [CrossRef] [PubMed]
- Lyon, D.Y.; Adams, L.K.; Falkner, J.C.; Alvarez, P.J. Antibacterial activity of fullerene water suspensions: Effects of preparation method and particle size. Environ. Sci. Technol. 2006, 40, 4360–4366. [Google Scholar] [CrossRef]
- Lyon, D.Y.; Alvarez, P.J. Fullerene water suspension (nC60) exerts antibacterial effects via ROS-independent protein oxidation. Environ. Sci. Technol. 2008, 42, 8127–8132. [Google Scholar] [CrossRef] [PubMed]
- Reynoso, E.; Durantini, A.M.; Solis, C.A.; Macor, L.P.; Otero, L.A.; Gervaldo, M.A.; Durantini, E.N.; Heredia, D.A. Photoactive antimicrobial coating based on a PEDOT-fullerene C60 polymeric dyad. RSC Adv. 2021, 11, 23519–23532. [Google Scholar] [CrossRef]
- Virovska, D.; Paneva, D.; Manolova, N.; Rashkov, I.; Karashanova, D. Photocatalytic self-cleaning poly(L-lactide) materials based on a hybrid between nanosized zinc oxide and expanded graphite or fullerene. Mater. Sci. Eng. C 2016, 60, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Al-Jumaili, A.; Alancherry, S.; Bazaka, K.; Jacob, M.V. Review on the antimicrobial properties of carbon nanostructures. Materials 2017, 10, 1066. [Google Scholar] [CrossRef] [PubMed]
- Díez-Pascual, A.M. State of the Art in the Antibacterial and Antiviral Applications of Carbon-Based Polymeric Nanocomposites. Int. J. Mol. Sci. 2021, 22, 10511. [Google Scholar] [CrossRef]
- Moon, J.; Jiang, H.; Lee, E.C. Physical Surface Modification of Carbon-Nanotube/Polydimethylsiloxane Composite Electrodes for High-Sensitivity DNA Detection. Nanomaterials 2021, 11, 2661. [Google Scholar] [CrossRef]
- Wu, S.; Wang, X.; Li, Z.; Zhang, S.; Xing, F. Recent Advances in the Fabrication and Application of Graphene Microfluidic Sensors. Micromachines 2020, 11, 1059. [Google Scholar] [CrossRef]
- Chodkowski, M.; Sulym, I.Y.; Terpiłowski, K.; Sternik, D. Surface Properties of Silica—MWCNTs/PDMS Composite Coatings Deposited on Plasma Activated Glass Supports. Appl. Sci. 2021, 11, 9256. [Google Scholar] [CrossRef]
- Emelyantsev, S.; Prazdnova, E.; Chistyakov, V.; Alperovich, I. Biological Effects of C60 Fullerene Revealed with Bacterial Biosensor—Toxic or Rather Antioxidant? Biosensors 2019, 9, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarasamma, S.; Audira, G.; Juniardi, S.; Sampurna, B.P.; Lai, Y.H.; Hao, E.; Chen, J.R.; Hsiao, C.D. Evaluation of the effects of carbon 60 nanoparticle exposure to adult zebrafish: A behavioral and biochemical approach to elucidate the mechanism of toxicity. Int. J. Mol. Sci. 2018, 19, 3853. [Google Scholar] [CrossRef] [Green Version]
- Lun-Fu, A.V.; Bubenchikov, A.M.; Bubenchikov, M.A.; Ovchinnikov, V.A. Numerical Simulation of Interaction Between Kr+ Ion and Rotating C60 Fullerene Towards for Nanoarchitectonics of Fullerene Materials. Crystals 2021, 11, 1204. [Google Scholar] [CrossRef]
- Tselukin, V.; Dzhumieva, A.; Yakovlev, A.; Mostovoy, A.; Zakirova, S.; Strilets, A.; Lopukhova, M. Electrodeposition and Corrosion Properties of Nickel—Graphene Oxide Composite Coatings. Materials 2021, 14, 5624. [Google Scholar] [CrossRef]
- Armentano, I.; Dottori, M.; Fortunati, E.; Mattioli, S.; Kenny, J.M. Biodegradable polymer matrix nanocomposites for tissue engineering: A review. Polym. Degrad. Stab. 2010, 95, 2126–2146. [Google Scholar] [CrossRef]
- Zhang, N.; Xu, C.; Azer, A.; Liu, H. Dispersibility and characterization of polyvinyl alcohol–coated magnetic nanoparticles in poly (glycerol sebacate) for biomedical applications. J. Nanopart. Res. 2019, 21, 1–11. [Google Scholar] [CrossRef]
- Jayalekshmi, A.C.; Victor, S.P.; Sharma, C.P. Magnetic and degradable polymer/bioactive glass composite nanoparticles for biomedical applications. Colloids Surf. B Biointerfaces 2013, 101, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Boval’dinova, K.A.; Sherstneva, N.E.; Fel’dshtein, M.M.; Moskalets, A.P.; Khokhlov, A.R. Pressure-sensitive adhesives with tunable tackiness. Polym. Sci. 2019, 61, 458–470. [Google Scholar] [CrossRef]
- Aleshina, A.L.; Shibaeva, A.V.; Philippova, O.E.; Khokhlova, A.R. Self-healing double polymer networks with dynamic cross-links. Dokl. Akad. Nauk 2020, 491, 64–68. (In Russian) [Google Scholar]
- Xu, C.; Wang, Y.; Wu, J.; Song, S.; Cao, S.; Xuan, S.; Gong, X. Anti-impact response of Kevlar sandwich structure with silly putty core. Compos. Sci. Technol. 2017, 153, 168–177. [Google Scholar] [CrossRef]
- Solomatin, A.S.; Tsareva, Y.V.; Mashchenko, V.I.; Savin, A.V.; Chigrinov, V.G.; Chausov, D.N. New principles of organizing an interactive multi-channel visual information flow by display and projective means on the basis of ordered crystalline 4-cyano-4-octyloxybiphenyl microstructures in borosiloxane gels. Liq. Cryst. Appl. 2020, 20, 57–64. [Google Scholar] [CrossRef]
- Talreja, K.; Chauhan, I.; Ghosh, A.; Majumdar, A.; Butola, B.S. Functionalization of silica particles to tune the impact resistance of shear thickening fluid treated aramid fabrics. RSC Adv. 2017, 78, 49787–49794. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Wang, R.; Liu, X.; Fang, S.; Sun, Y. Shear-thickening fluid using oxygen-plasma-modified multi-walled carbon nanotubes to improve the quasi-static stab resistance of Kevlar fabrics. Polymers 2018, 12, 1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ermakova, M.V.; Mashchenko, V.I.; Chausova, O.V.; Solomatin, A.S.; Volosnikova, N.I.; Chausov, D.N. Formation of ordered crystalline microstructures of 4-cyano-4-octyloxydiphenyl in borosiloxane gels. Liq. Cryst. Appl. 2019, 19, 61–66. [Google Scholar] [CrossRef]
- Liu, Z.; Picken, S.J.; Besseling, N.A. Polyborosiloxanes (PBSs), synthetic kinetics, and characterization. Macromolecules 2014, 47, 4531–4537. [Google Scholar] [CrossRef]
- Li, X.; Zhang, D.; Xiang, K.; Huang, G. Synthesis of polyborosiloxane and its reversible physical crosslinks. RSC Adv. 2014, 62, 32894–32901. [Google Scholar] [CrossRef]
- Palmer, R.M.; Green, P.C. Energy Absorbing Material. U.S. Patent US7381460B2, 3 June 2008. [Google Scholar]
- Speck, O.; Speck, T. An overview of bioinspired and biomimetic self-repairing materials. Biomimetics 2019, 4, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, C.D.; Green, P.A. Method and Device for Detecting Fascia Damage and Repairing the Same. U.S. Patent US20170228094A1, 10 August 2017. [Google Scholar]
- Tee, B.C.K.; Wang, C.; Allen, R.; Bao, Z. An electrically and mechanically self-healing composite with pressure- and flexion-sensitive properties for electronic skin applications. Nat. Nanotechnol. 2012, 7, 825–832. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, B.; Jiang, W.; Yang, Y.; Leow, W.R.; Wang, H.; Chen, X. A mechanically and electrically self-healing supercapacitor. Adv. Mater. 2014, 26, 3638–3643. [Google Scholar] [CrossRef]
- Belyaev, V.V.; Mashchenko, V.I.; Chausov, D.N.; Solomatin, A.S. A Method of Obtaining of a Mixture of Liquid Crystal with a Polymer for Display Technology and Optoelectronics. RF Patent 2607454, 3 October 2015. [Google Scholar]
- Mashchenko, V.I.; Sitnikov, N.N.; Khabibullina, I.A.; Chausov, D.N.; Shelyakov, A.V.; Spiridonov, V.V. Effect of boric acid on the structure and properties of borosiloxanes. Polym. Sci. 2021, 63, 91–99. [Google Scholar]
- Gudkov, S.V.; Simakin, A.V.; Bunkin, N.F.; Shafeev, G.A.; Astashev, M.E.; Glinushkin, A.P.; Grinberg, M.A.; Vodeneev, V.A. Development and application of photoconversion fluoropolymer films for greenhouses located at high or polar latitudes. J. Photochem. Photobiol. 2020, 213, 112056. [Google Scholar] [CrossRef] [PubMed]
- Andrievsky, G.V.; Klochkov, V.K.; Bordyuh, A.B.; Dovbeshko, G.I. Comparative analysis of two aqueous-colloidal solutions of C60 fullerene with help of FTIR reflectance and UV-Vis spectroscopy. Chem. Phys. Lett. 2002, 364, 8–17. [Google Scholar] [CrossRef]
- Kirsanov, E.A.; Timoshin, Y.N. Non-Newtonian flow of structured systems. II. Analysis of flow curve. Liq. Cryst. Appl. 2012, 4, 71–80. (In Russian) [Google Scholar]
- Shcherbakov, I.A.; Baimler, I.V.; Gudkov, S.V.; Lyakhov, G.A.; Mikhailova, G.N.; Pustovoy, V.I.; Sarimov, R.M.; Simakin, A.V.; Troitsky, A.V. Influence of a Constant Magnetic Field on Some Properties of Water Solutions. Dokl. Phys. 2020, 65, 9–11. [Google Scholar] [CrossRef]
- Belov, S.V.; Lobachevsky, Y.P.; Danilejko, Y.K.; Egorov, A.B.; Simakin, A.V.; Maleki, A.; Temnov, A.A.; Dubinin, M.V.; Gudkov, S.V. The role of mitochondria in the dual effect of low-temperature plasma on human bone marrow stem cells: From apoptosis to activation of cell proliferation. Appl. Sci. 2020, 10, 8971. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Lyakhov, G.A.; Pustovoy, V.I.; Shcherbakov, I.A. Influence of Mechanical Effects on the hydrogen peroxide concentration in aqueous solutions. Phys. Wave Phenom. 2019, 27, 141–144. [Google Scholar] [CrossRef]
- Manevich, Y.; Held, K.D.; Biaglow, J.E. Coumarin-3-Carboxylic Acid as a Detector for Hydroxyl Radicals Generated Chemically and by Gamma Radiation. Radiat. Res. 1997, 148, 580–591. [Google Scholar] [CrossRef]
- Baimler, I.; Simakin, A.; Uvarov, O.; Volkov, M.; Gudkov, S. Generation of hydroxyl radicals during laser breakdown of aqueous solutions in the presence of Fe and Cu nanoparticles of different sizes. Phys. Wave Phenom. 2020, 28, 107–110. [Google Scholar] [CrossRef]
- Holley, A.E.; Cheeseman, K.H. Measuring free radical reactions in vivo. Br. Med. Bull. 1993, 49, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, S.V.; Garmash, S.A.; Shtarkman, I.N.; Chernikov, A.V.; Karp, O.E.; Bruskov, V.I. Long-lived protein radicals induced by X-ray irradiation are the source of reactive oxygen species in aqueous medium. Dokl. Biochem. Biophys. 2010, 430, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Sharapov, M.G.; Novoselov, V.I.; Penkov, N.V.; Fesenko, E.E.; Vedunova, M.V.; Bruskov, V.I.; Gudkov, S.V. Protective and adaptogenic role of peroxiredoxin 2 (Prx2) in neutralization of oxidative stress induced by ionizing radiation. Free Radic. Biol. Med. 2019, 134, 76–86. [Google Scholar] [CrossRef]
- Bruskov, V.I.; Gaziev, A.I.; Malakhova, L.V.; Mantsygin, I.; Morenkov, O.S. Monoclonal antibodies to 8-oxo-2′-deoxyguanosine (8-hydroxyguanosine). Characteristics and use for determining DNA damage by active forms of oxygen. Biochemistry 1996, 61, 737–744. [Google Scholar]
- Garmash, S.A.; Smirnova, V.S.; Karp, O.E.; Usacheva, A.M.; Berezhnov, A.V.; Ivanov, V.E.; Chernikov, A.V.; Bruskov, V.I.; Gudkov, S.V. Pro-oxidative, genotoxic and cytotoxic properties of uranyl ions. J. Environ. Radioact. 2014, 127, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, A.R.; Trahan, N.D.; Son, M.S. Growth and maintenance of Escherichia coli laboratory strains. Curr. Protoc. 2021, 1, e20. [Google Scholar] [CrossRef]
- Barkhudarov, E.M.; Kossyi, I.A.; Anpilov, A.M.; Ivashkin, P.I.; Artem’ev, K.V.; Moryakov, I.V.; Misakyan, M.A.; Christofi, N.; Burmistrov, D.E.; Smirnova, V.V.; et al. New nanostructured carbon coating inhibits bacterial growth, but does not influence on animal cells. Nanomaterials 2020, 10, 2130. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Simakin, A.V.; Konushkin, S.V.; Ivannikov, A.Y.; Nasakina, E.O.; Shatova, L.A.; Kolmakov, A.G.; Sevostyanov, M.A. Preparation, structural and microstructural characterization of Ti–30Nb–10Ta–5Zr alloy for biomedical applications. J. Mater. Res. Technol. 2020, 9, 16018–16028. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Simakin, A.V.; Sevostyanov, M.A.; Konushkin, S.V.; Losertová, M.; Ivannikov, A.Y.; Kolmakov, A.G.; Izmailov, A.Y. Manufacturing and study of mechanical properties, structure and compatibility with biological objects of plates and wire from new Ti-25Nb-13Ta-5Zr alloy. Metals 2020, 10, 1584. [Google Scholar] [CrossRef]
- Sevostyanov, M.A.; Kolmakov, A.G.; Sergiyenko, K.V.; Kaplan, M.A.; Baikin, A.S.; Gudkov, S.V. Mechanical, physical-chemical and biological properties of the new Ti-30Nb-13Ta-5Zr alloy. J. Mater. Sci. 2020, 55, 14516–14529. [Google Scholar] [CrossRef]
- Borisov, S.N.; Voronkov, M.G.; Lukewitz, E.Y. Organoelement Compounds. Derivatives of Inorganogens; Publishing House “Chemistry”: Leningrad, Russia, 1966; 544p. [Google Scholar]
- Canaparo, R.; Foglietta, F.; Limongi, T.; Serpe, L. Biomedical applications of reactive oxygen species generation by metal nanoparticles. Materials 2021, 14, 53. [Google Scholar] [CrossRef]
- Abdal Dayem, A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.M.; Choi, H.Y.; Cho, S.G. The role of reactive oxygen species (ROS) in the biological activities of metallic nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henry, T.B.; Petersen, E.J.; Compton, R.N. Aqueous fullerene aggregates (nC60) generate minimal reactive oxygen species and are of low toxicity in fish: A revision of previous reports. Curr. Opin. Biotechnol. 2011, 22, 533–537. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H.A.; Alshamsan, A. Mechanism of ROS scavenging and antioxidant signalling by redox metallic and fullerene nanomaterials: Potential implications in ROS associated degenerative disorders. Biochim. Biophys. Acta 2017, 1861, 802–813. [Google Scholar] [CrossRef]
- Dugan, L.L.; Lovett, E.G.; Quick, K.L.; Lotharius, J.; Lin, T.T.; O’malley, K.L. Fullerene-based antioxidants and neurodegenerative disorders. Parkinsonism Relat. Disord. 2001, 7, 243–246. [Google Scholar] [CrossRef]
- Andrievsky, G.V.; Bruskov, V.I.; Tykhomyrov, A.A.; Gudkov, S.V. Peculiarities of the antioxidant and radioprotective effects of hydrated C60 fullerene nanostuctures in vitro and in vivo. Free Radic. Biol. Med. 2009, 47, 786–793. [Google Scholar] [CrossRef]
- Yin, J.J.; Fu, P.P.; Lutterodt, H.; Zhou, Y.T.; Antholine, W.E.; Wamer, W. Dual role of selected antioxidants found in dietary supplements: Crossover between anti- and pro-oxidant activities in the presence of copper. J. Agric. Food. Chem. 2012, 60, 2554–2561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gudkov, S.V.; Guryev, E.L.; Gapeyev, A.B.; Sharapov, M.G.; Bunkin, N.F.; Shkirin, A.V.; Zabelina, T.S.; Glinushkin, A.P.; Sevost’yanov, M.A.; Belosludtsev, K.N.; et al. Unmodified hydrated C60 fullerene molecules exhibit antioxidant properties, prevent damage to DNA and proteins induced by reactive oxygen species and protect mice against injuries caused by radiation-induced oxidative stress. Nanomedicine 2019, 15, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Matsui, S.; Shimizu, Y.; Matsuda, T. Genotoxicity of colloidal fullerene C60. Environ. Sci. Technol. 2011, 45, 4133–4138. [Google Scholar] [CrossRef]
- Totsuka, Y.; Kato, T.; Masuda, S.I.; Ishino, K.; Matsumoto, Y.; Goto, S.; Kawanish, M.; Yagi, T.; Wakabayashi, K. In vitro and in vivo genotoxicity induced by fullerene (C60) and kaolin. Genes Environ. 2011, 33, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Bernstein, R.; Prat, F.; Foote, C.S. On the mechanism of DNA cleavage by fullerenes investigated in model systems: Electron transfer from guanosine and 8-oxo-guanosine derivatives to C60. J. Am. Chem. Soc. 1999, 121, 464–465. [Google Scholar] [CrossRef]
- Gao, J.; Wang, H.L.; Shreve, A.; Iyer, R. Fullerene derivatives induce premature senescence: A new toxicity paradigm or novel biomedical applications. Toxicol. Appl. Pharmacol. 2010, 244, 130–143. [Google Scholar] [CrossRef]
- Takahashi, M.; Kato, H.; Doi, Y.; Hagiwara, A.; Hirata-Koizumi, M.; Ono, A.; Kubota, R.; Nishimura, T.; Hirose, A. Sub-acute oral toxicity study with fullerene C60 in rats. J. Toxicol. Sci. 2012, 37, 353–361. [Google Scholar] [CrossRef] [Green Version]
- Sumner, S.C.; Snyder, R.W.; Wingard, C.; Mortensen, N.P.; Holland, N.A.; Shannahan, J.H.; Dhungana, S.; Pathmasiri, W.; Han, L.; Lewin, A.H.; et al. Distribution and biomarkers of carbon-14-labeled fullerene C60 ([14C(U)]C60) in female rats and mice for up to 30 days after intravenous exposure. J. Appl. Toxicol. 2015, 35, 1452–1464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Ma, Z.; Liu, G.; Wei, H.; Xie, X. Antibacterial activity of cationic cyclen-functionalized fullerene derivatives: Membrane stress. Dig. J. Nanomater. Biostruct. 2016, 11, 753–761. [Google Scholar]
- Zhang, Y.; Zhang, H.; Zou, Q.; Xing, R.; Jiao, T.; Yan, X. An injectable dipeptide-fullerene supramolecular hydrogel for photodynamic antibacterial therapy. J. Mater. Chem. B 2016, 6, 7335–7342. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Wu, X.; Ouyang, P.; Shi, M.; Li, Q.; Maimaiti, T.; Lan, S.; Yang, S.; Chang, X. Surface modification mediates the interaction between fullerene and lysozyme: Protein structure and antibacterial activity. Environ. Sci. Nano 2021, 8, 76–85. [Google Scholar] [CrossRef]
- Aquino, A.; Chan, J.; Giolma, K.; Loh, M. The effect of a fullerene water suspension on the growth, cell viability, and membrane integrity of Escherichia coli B23. Mater. Sci. 2010, 14, 13–20. [Google Scholar]
- Mashino, T.; Nishikawa, D.; Takahashi, K.; Usui, N.; Yamori, T.; Seki, M.; Endo, T.; Mochizuki, M. Antibacterial and antiproliferative activity of cationic fullerene derivatives. Bioorg. Med. Chem. Lett. 2003, 13, 4395–4397. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Lyon, D.Y.; Li, Q.; Alvarez, P.J. Effect of soil sorption and aquatic natural organic matter on the antibacterial activity of a fullerene water suspension. Environ. Toxicol. Chem. 2008, 27, 1888–1894. [Google Scholar] [CrossRef]
- Skariyachan, S.; Parveen, A.; Garka, S. Nanoparticle Fullerene (C60) demonstrated stable binding with antibacterial potential towards probable targets of drug resistant Salmonella typhi—A computational perspective and in vitro investigation. J. Biomol. Struct. Dyn. 2017, 35, 3449–3468. [Google Scholar] [CrossRef]
- Ghabdian, Y.; Taheri, A.; Jahanian-Najafabadi, A. Development of novel topical formulation from fullerene with antibacterial activity against Propionibacterium acnes. Fuller. Nanotub. Carbon Nanostruct. 2021, 29, 163–173. [Google Scholar] [CrossRef]
- Tollas, S.; Bereczki, I.; Sipos, A.; Rőth, E.; Batta, G.; Daróczi, L.; Kéki, S.; Ostorházi, E.; Rozgonyi, F.; Herczegh, P. Nano-sized clusters of a teicoplanin ψ-aglycon-fullerene conjugate. Synthesis, antibacterial activity and aggregation studies. Eur. J. Med. Chem. 2012, 54, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Prylutska, S.V.; Matyshevska, O.P.; Golub, A.A.; Prylutskyy, Y.I.; Potebnya, G.P.; Ritter, U.; Scharff, P. Study of C60 fullerenes and C60-containing composites cytotoxicity in vitro. Mater. Sci. Eng. C 2007, 27, 1121–1124. [Google Scholar] [CrossRef]
- Misra, C.; Thotakura, N.; Kumar, R.; Singh, B.; Sharma, G.; Katare, O.P.; Raza, K. Improved cellular uptake, enhanced efficacy and promising pharmacokinetic profile of docetaxel employing glycine-tethered C60-fullerenes. Mater. Sci. Eng. C 2017, 76, 501–508. [Google Scholar] [CrossRef] [PubMed]
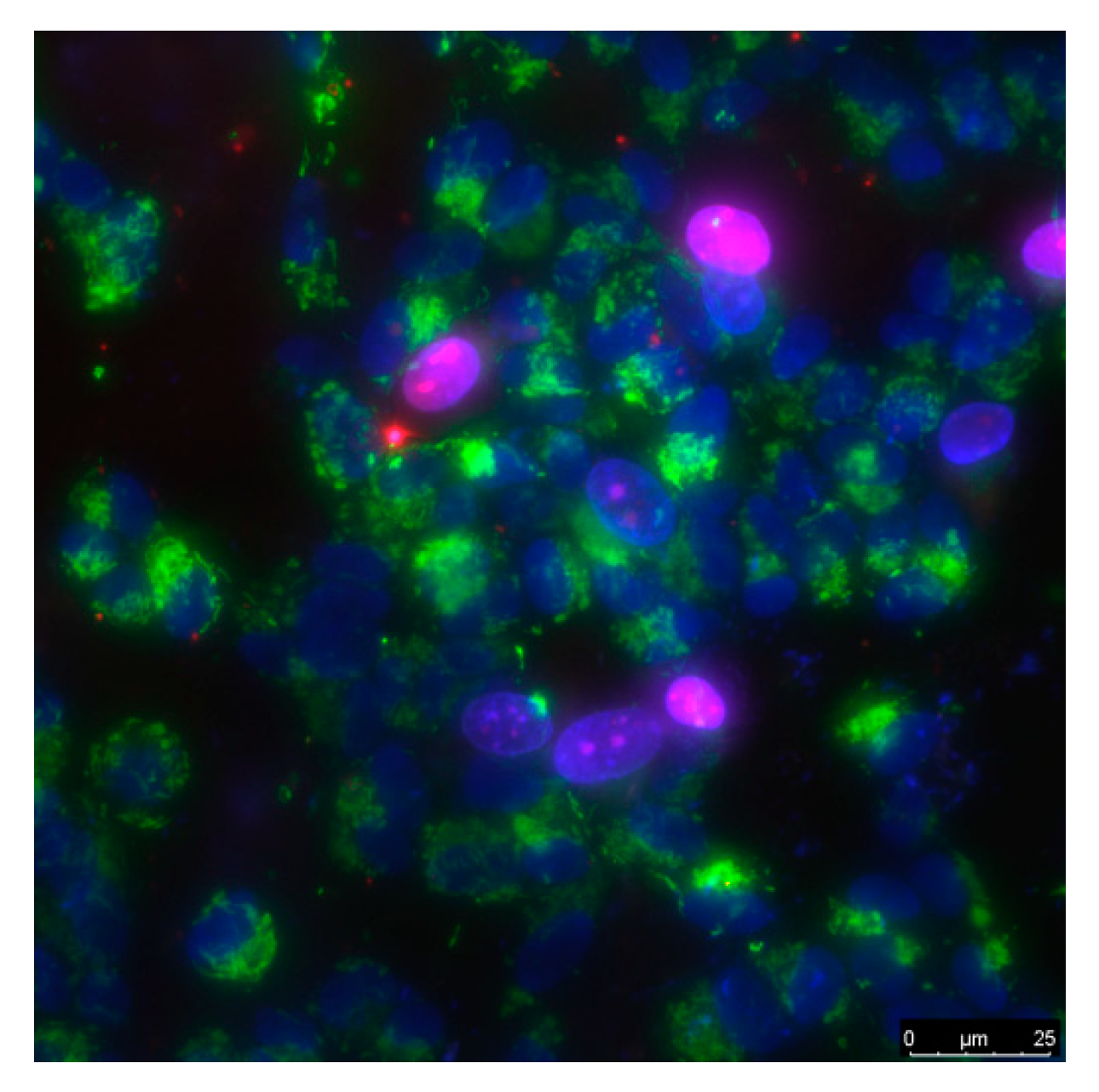
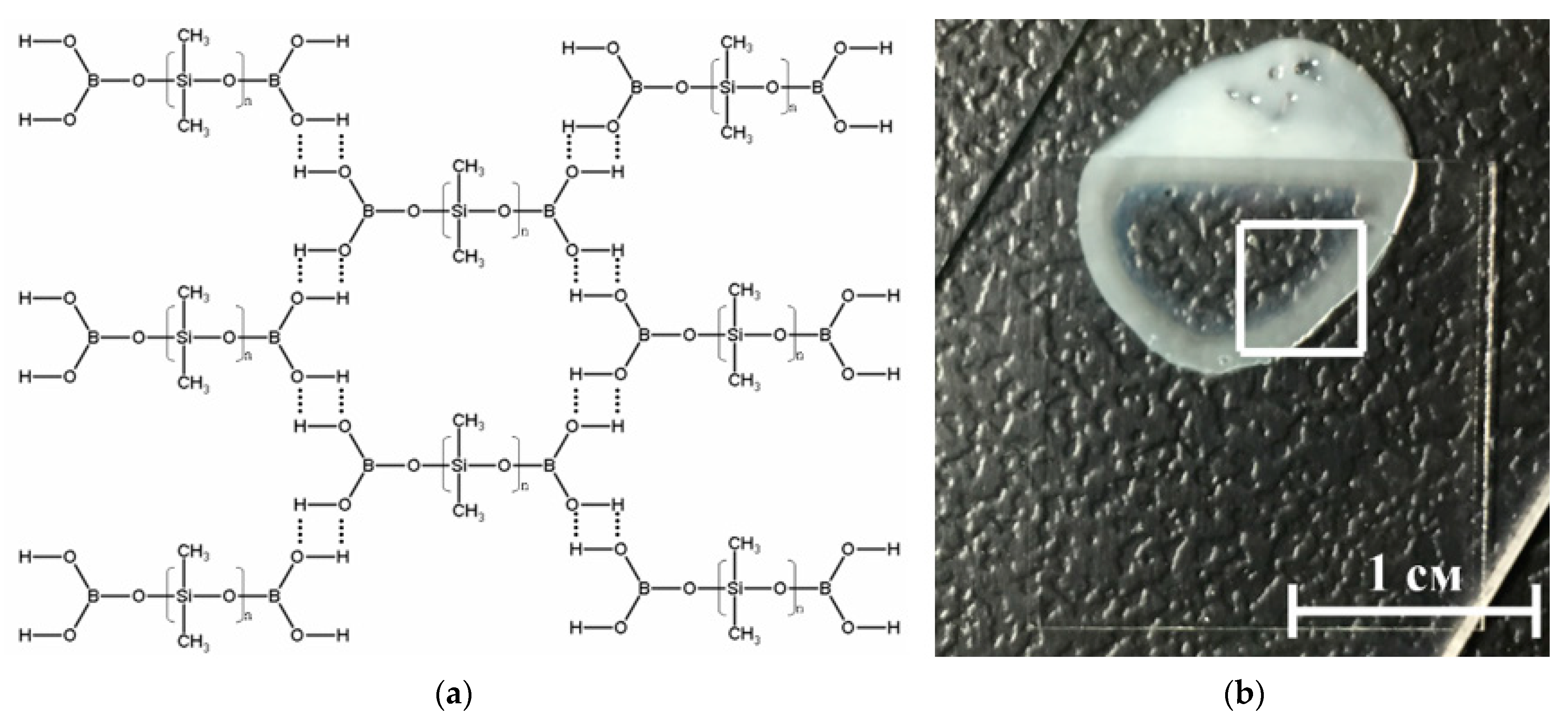


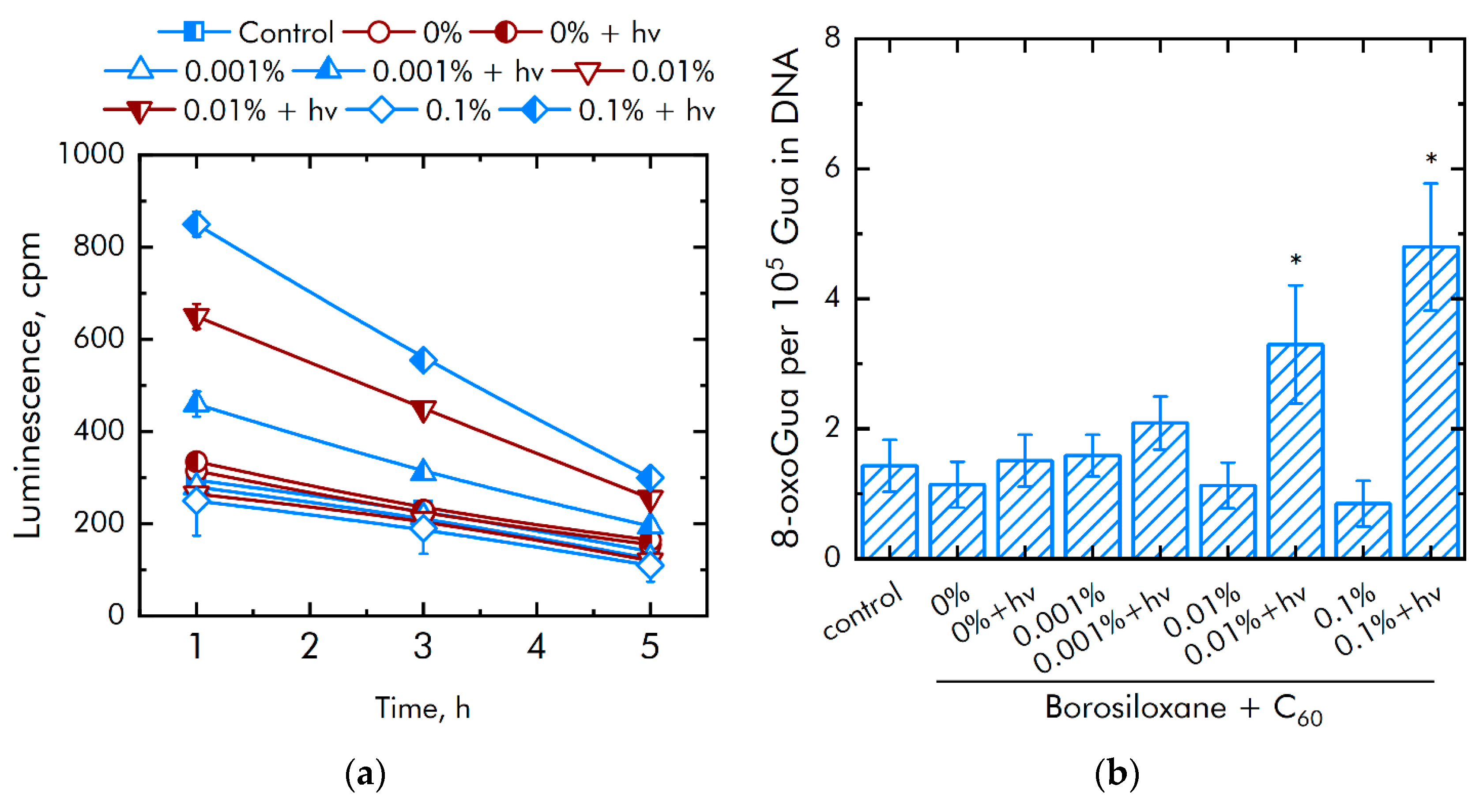

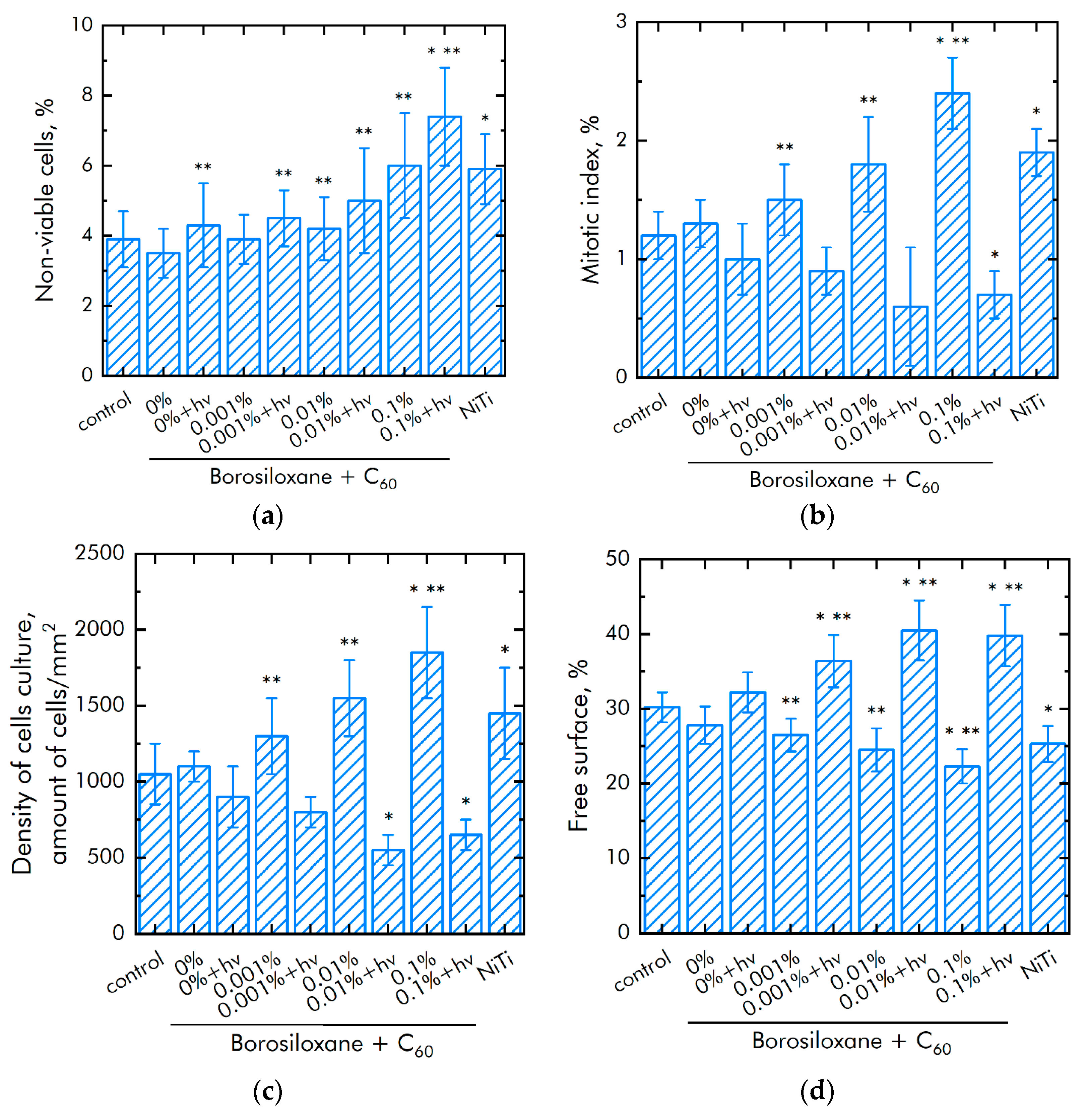
| Concentration of C60, % | Cell Culture Parameter | |
|---|---|---|
| Viable Cells, % | Mitotic Index, % | |
| Control | 95.9 ± 1.5 | 1.2 ± 0.4 |
| Control + hv | 94.3 ± 1.9 | 1.3 ± 0.2 |
| 0.0001% | 95.6 ± 2.1 | 1.4 ± 0.3 |
| 0.0001% + hv | 95.3 ± 2.3 | 1.1 ± 0.4 |
| 0.001% | 95.7 ± 1.7 | 1.5 ± 0.3 |
| 0.001% + hv | 93.2 ± 1.8 | 0.9 ± 0.4 |
| 0.01% | 94.2 ± 2.5 | 1.9 ± 0.2 * |
| 0.01% + hv | 92.1 ± 2.1 * | 0.7 ± 0.2 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gudkov, S.V.; Simakin, A.V.; Sarimov, R.M.; Kurilov, A.D.; Chausov, D.N. Novel Biocompatible with Animal Cells Composite Material Based on Organosilicon Polymers and Fullerenes with Light-Induced Bacteriostatic Properties. Nanomaterials 2021, 11, 2804. https://doi.org/10.3390/nano11112804
Gudkov SV, Simakin AV, Sarimov RM, Kurilov AD, Chausov DN. Novel Biocompatible with Animal Cells Composite Material Based on Organosilicon Polymers and Fullerenes with Light-Induced Bacteriostatic Properties. Nanomaterials. 2021; 11(11):2804. https://doi.org/10.3390/nano11112804
Chicago/Turabian StyleGudkov, Sergey V., Alexander V. Simakin, Ruslan M. Sarimov, Alexander D. Kurilov, and Denis N. Chausov. 2021. "Novel Biocompatible with Animal Cells Composite Material Based on Organosilicon Polymers and Fullerenes with Light-Induced Bacteriostatic Properties" Nanomaterials 11, no. 11: 2804. https://doi.org/10.3390/nano11112804
APA StyleGudkov, S. V., Simakin, A. V., Sarimov, R. M., Kurilov, A. D., & Chausov, D. N. (2021). Novel Biocompatible with Animal Cells Composite Material Based on Organosilicon Polymers and Fullerenes with Light-Induced Bacteriostatic Properties. Nanomaterials, 11(11), 2804. https://doi.org/10.3390/nano11112804









