Aerogels Based on Reduced Graphene Oxide/Cellulose Composites: Preparation and Vapour Sensing Abilities
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Cellulose/rGO Composite Aerogels
2.3. Characterization
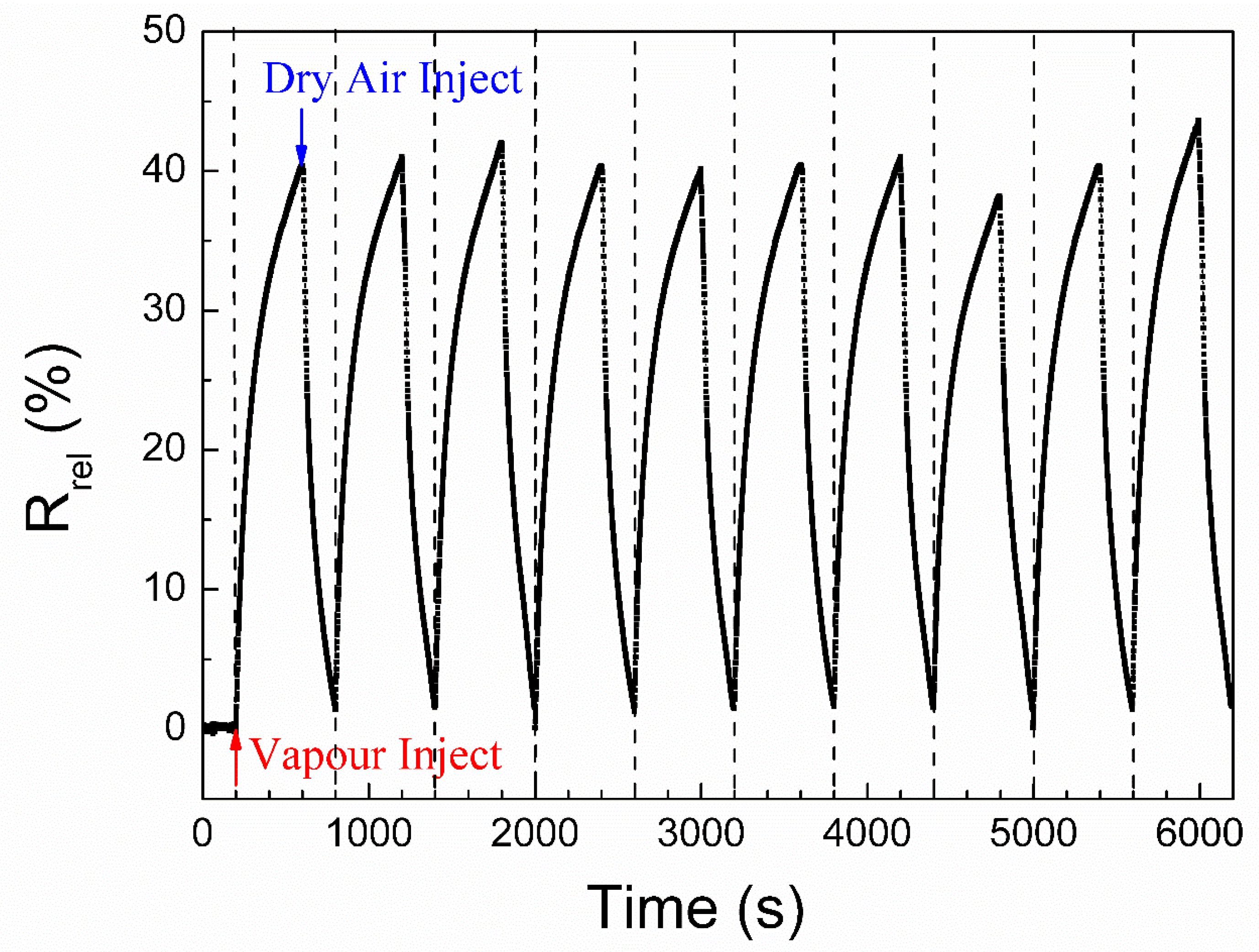
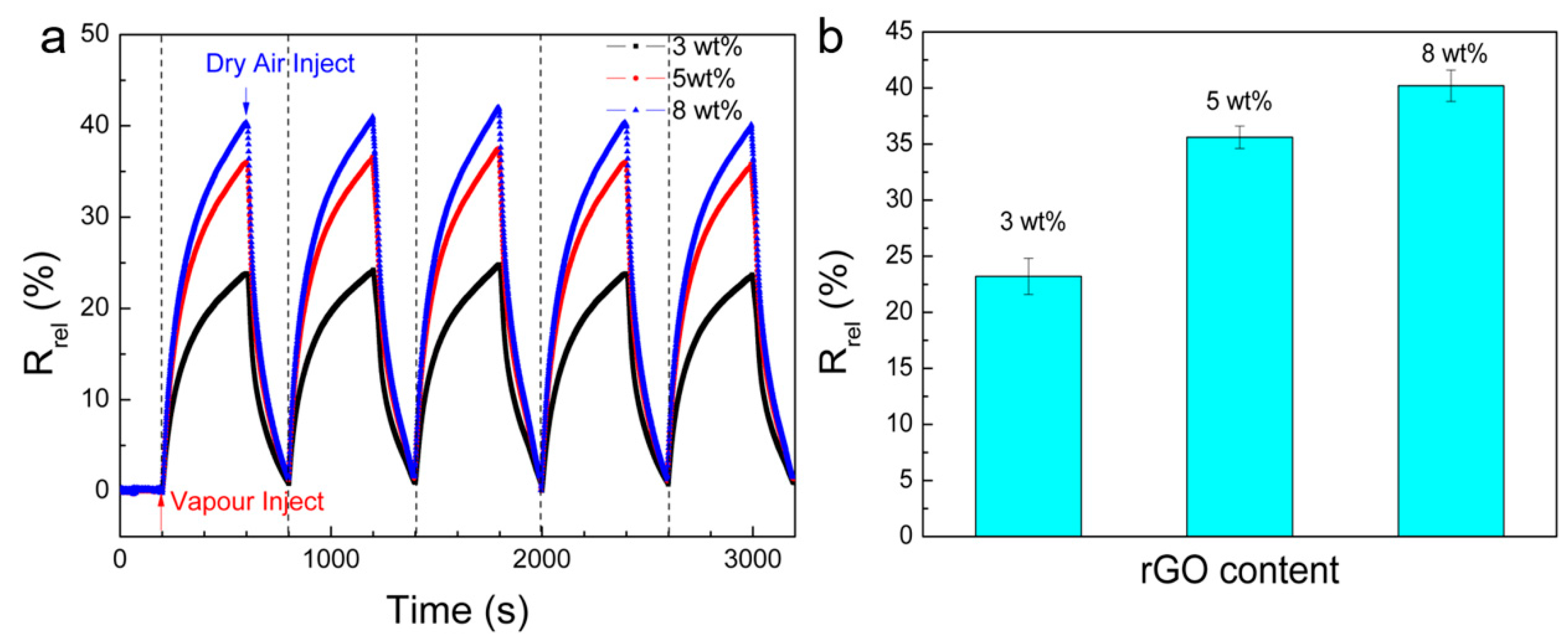
2.4. Vapour Sensing Tests
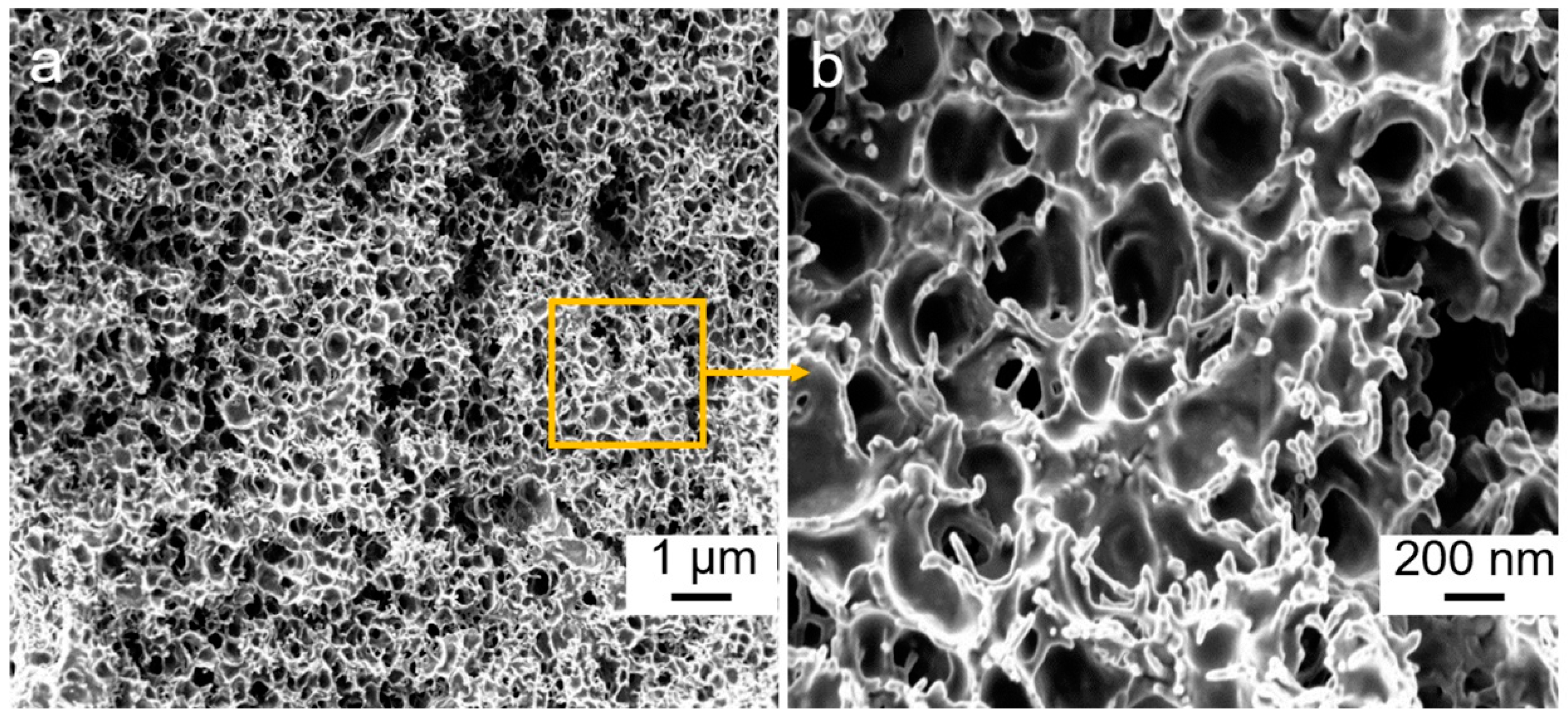
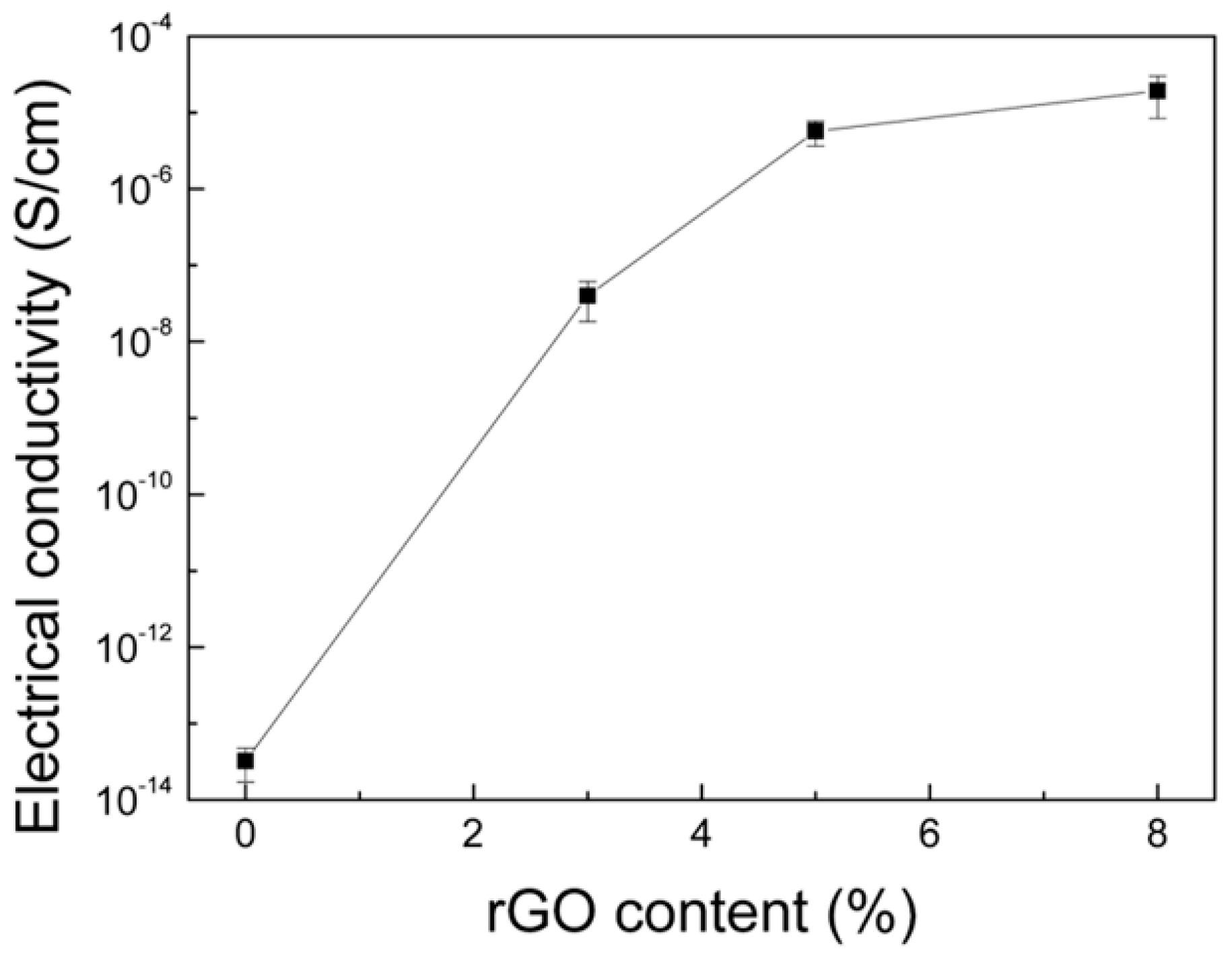
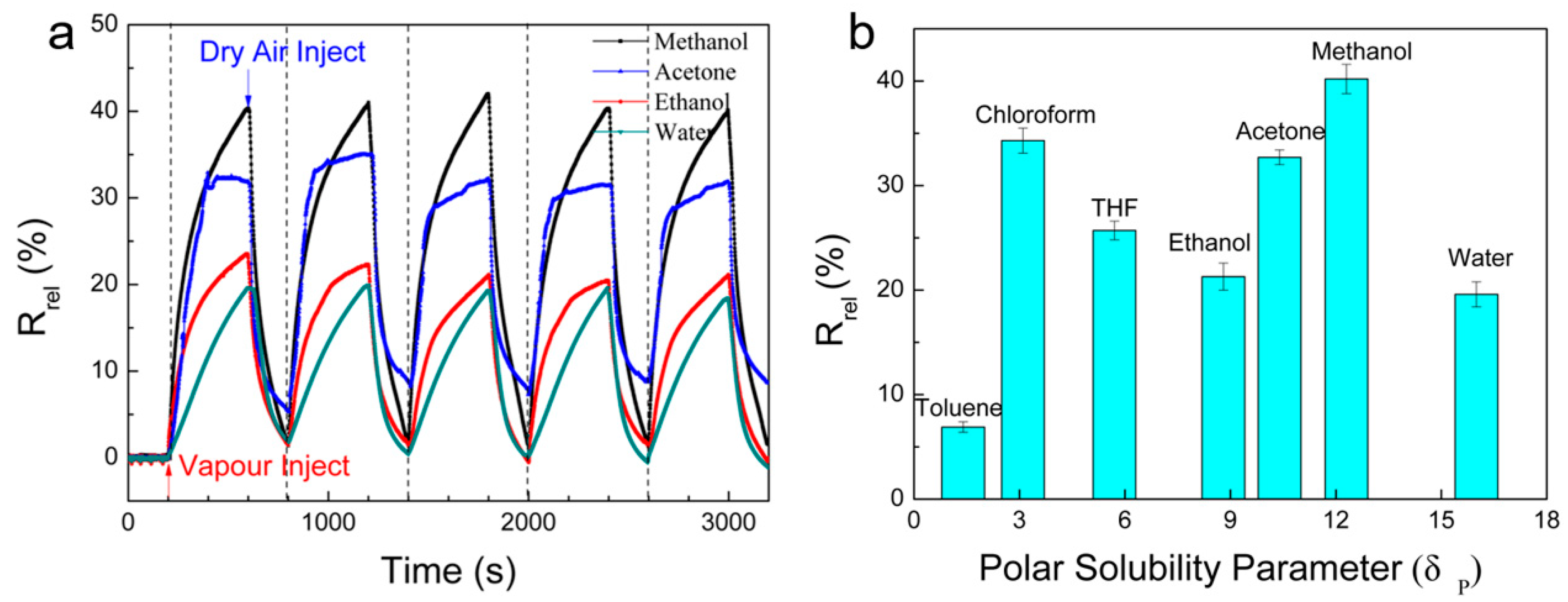
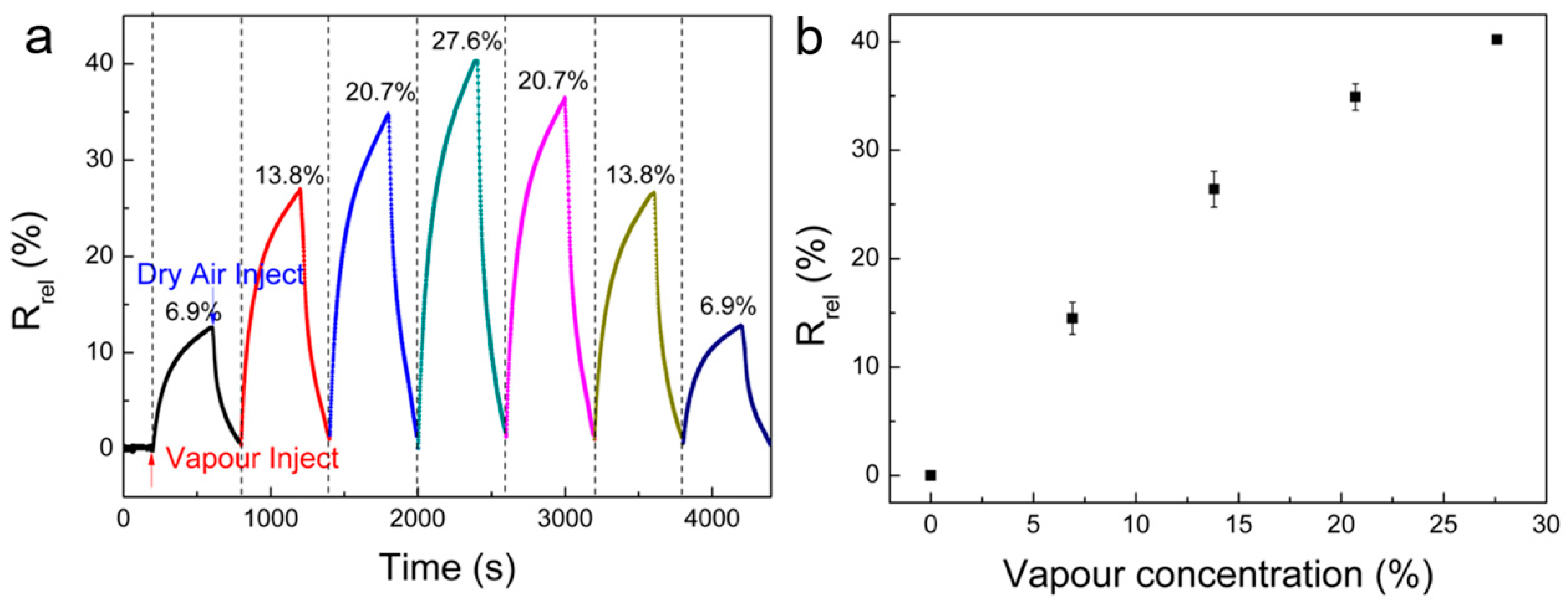
3. Results and Discussion
| Polymer/ Solvent | Rrel (%) | Pi (kPa) | Ci (%) | Vmol (cm3/mol) | δD (MPa−0.5) | δP (MPa−0.5) | δH (MPa−0.5) | DSP (MPa−0.5) |
|---|---|---|---|---|---|---|---|---|
| Cellulose | 24.4 | 14.9 | 30.9 | |||||
| Water | 19.6 | 3.2 | 3.2 | 18.0 | 15.5 | 16.0 | 42.3 | 21.2 |
| Methanol | 40.2 | 28.0 | 27.6 | 40.7 | 15.1 | 12.3 | 22.3 | 20.7 |
| Ethanol | 21.3 | 8.0 | 7.9 | 58.5 | 15.8 | 8.8 | 19.4 | 21.6 |
| Acetone | 32.7 | 30.6 | 30.2 | 77.0 | 15.5 | 10.4 | 7.0 | 30.1 |
| Toluene | 6.9 | 3.8 | 3.8 | 106.9 | 18.0 | 1.4 | 2.0 | 34.4 |
| THF | 25.7 | 23.5 | 23.2 | 81.7 | 16.8 | 5.7 | 8.0 | 29.0 |
| Chloroform | 34.3 | 80.7 | 79.6 | 80.7 | 17.3 | 3.1 | 5.7 | 31.2 |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baimpos, T.; Boutikos, P.; Nikolakis, V.; Kouzoudis, D. A polymer-Metglas sensor used to detect volatile organic compounds. Sens. Actuators A Phys. 2010, 158, 249–253. [Google Scholar] [CrossRef]
- Ibanez, F.J.; Zamborini, F.P. Chemiresistive sensing of volatile organic compounds with films of surfactant-stabilized gold and gold-silver alloy nanoparticles. ACS Nano 2008, 2, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Consales, M.; Crescitelli, A.; Penza, M.; Aversa, P.; Veneri, P.D.; Giordano, M.; Cusano, A. SWCNT nano-composite optical sensors for VOC and gas trace detection. Sens. Actuators B Chem. 2009, 138, 351–361. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, S.; Liu, H.; Hu, S.; Zhang, D.; Ning, H. A survey on gas sensing technology. Sensors 2012, 12, 9635–9665. [Google Scholar] [CrossRef]
- Kennedy, Z.; Christ, J.; Evans, K.; Arey, B.; Sweet, L.; Warner, M.; Erikson, R.; Barrett, C. 3D-printed poly (vinylidene fluoride)/carbon nanotube composites as a tunable, low-cost chemical vapour sensing platform. Nanoscale 2017, 9, 5458–5466. [Google Scholar] [CrossRef]
- Şahin, S.; Altun, S.; Altındal, A.; Odabaş, Z. Synthesis of novel azo-bridged phthalocyanines and their toluene vapour sensing properties. Sens. Actuators B Chem. 2015, 206, 601–608. [Google Scholar] [CrossRef]
- Kumari, M.; Ding, B.; Blaikie, R. Enhanced resonant absorption in dye-doped polymer thin-film cavities for water vapour sensing. Sens. Actuators B Chem. 2016, 231, 88–94. [Google Scholar] [CrossRef]
- Sharma, S.; Madou, M. A new approach to gas sensing with nanotechnology. Philos. Trans. R. Soc. A Math. Philos. Trans. R. Soc. 2012, 370, 2448–2473. [Google Scholar] [CrossRef]
- Patra, M.; Manzoor, K.; Manoth, M.; Negi, S.; Vadera, S.; Kumar, N. Nanotechnology applications for chemical and biological sensors. Def. Sci. J. 2008, 58. [Google Scholar] [CrossRef]
- Sun, P.; Cao, Y.; Liu, J.; Sun, Y.; Ma, J.; Lu, G. Dispersive SnO2 nanosheets: Hydrothermal synthesis and gas-sensing properties. Sens. Actuators B Chem. 2011, 156, 779–783. [Google Scholar] [CrossRef]
- Wang, T.; Huang, D.; Yang, Z.; Xu, S.; He, G.; Li, X.; Hu, N.; Yin, G.; He, D.; Zhang, L. A review on graphene-based gas/vapor sensors with unique properties and potential applications. Nano-Micro Lett. 2016, 8, 95–119. [Google Scholar] [CrossRef] [PubMed]
- Potyrailo, R.A. Polymeric sensor materials: Toward an alliance of combinatorial and rational design tools? Angew. Chem. Int. Ed. 2006, 45, 702–723. [Google Scholar] [CrossRef]
- Wang, X.; Meng, S.; Ma, W.; Pionteck, J.; Gnanaseelan, M.; Zhou, Z.; Sun, B.; Qin, Z.; Zhu, M. Fabrication and gas sensing behavior of poly (3,4-ethylenedioxythiophene) coated polypropylene fiber with engineered interface. React. Funct. Polym. 2017, 112, 74–80. [Google Scholar] [CrossRef]
- Wang, X.; Meng, S.; Tebyetekerwa, M.; Weng, W.; Pionteck, J.; Sun, B.; Qin, Z.; Zhu, M. Nanostructured polyaniline/poly(styrene-butadiene-styrene) composite fiber for use as highly sensitive and flexible ammonia sensor. Synth. Met. 2017, 233, 86–93. [Google Scholar] [CrossRef]
- Konwer, S.; Guha, A.K.; Dolui, S.K. Graphene oxide-filled conducting polyaniline composites as methanol-sensing materials. J. Mater. Sci. 2013, 48, 1729–1739. [Google Scholar] [CrossRef]
- Bhandari, S. Polymer/carbon composites for sensor application. In Carbon-Containing Polymer Composites; Springer: Singapore, 2019; pp. 503–531. [Google Scholar]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183. [Google Scholar] [CrossRef] [PubMed]
- Brownson, D.A.; Kampouris, D.K.; Banks, C.E. An overview of graphene in energy production and storage applications. J. Power Sources 2011, 196, 4873–4885. [Google Scholar] [CrossRef]
- Cai, W.; Zhu, Y.; Li, X.; Piner, R.D.; Ruoff, R.S. Large area few-layer graphene/graphite films as transparent thin conducting electrodes. Appl. Phys. Lett. 2009, 95, 123115. [Google Scholar] [CrossRef]
- Leenaerts, O.; Partoens, B.; Peeters, F. Adsorption of H2O, NH3, CO, NO2, and NO on graphene: A first-principles study. Phys. Rev. B 2008, 77, 125416. [Google Scholar] [CrossRef]
- Liu, C.; Yu, Z.; Neff, D.; Zhamu, A.; Jang, B.Z. Graphene-based supercapacitor with an ultrahigh energy density. Nano Lett. 2010, 10, 4863–4868. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Ren, W.; Wen, L.; Gao, L.; Zhao, J.; Chen, Z.; Zhou, G.; Li, F.; Cheng, H.-M. Graphene anchored with Co3O4 nanoparticles as anode of lithium ion batteries with enhanced reversible capacity and cyclic performance. ACS Nano 2010, 4, 3187–3194. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Bi, H.; Wan, D.; Huang, F. Novel Cu nanowires/graphene as the back contact for CdTe solar cells. Adv. Funct. Mater. 2012, 22, 1267–1271. [Google Scholar] [CrossRef]
- Schedin, F.; Geim, A.; Morozov, S.; Hill, E.; Blake, P.; Katsnelson, M.; Novoselov, K. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Ocola, L.E.; Chen, J. Reduced graphene oxide for room-temperature gas sensors. Nanotechnology 2009, 20, 445502. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.-H.; Kim, P.; Choi, J.-Y.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706. [Google Scholar] [CrossRef]
- Sutter, P.W.; Flege, J.-I.; Sutter, E.A. Epitaxial graphene on ruthenium. Nat. Mater. 2008, 7, 406. [Google Scholar] [CrossRef]
- Reina, A.; Jia, X.; Ho, J.; Nezich, D.; Son, H.; Bulovic, V.; Dresselhaus, M.S.; Kong, J. Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition. Nano Lett. 2008, 9, 30–35. [Google Scholar] [CrossRef]
- Mao, S.; Pu, H.; Chen, J. Graphene oxide and its reduction: Modeling and experimental progress. RSC Adv. 2012, 2, 2643–2662. [Google Scholar] [CrossRef]
- Wang, Y.; Yeow, J.T. A review of carbon nanotubes-based gas sensors. J. Sens. 2009, 2009. [Google Scholar] [CrossRef]
- Hüsing, N.; Schubert, U. Aerogels—Airy materials: Chemistry, structure, and properties. Angew. Chem. Int. Ed. 1998, 37, 22–45. [Google Scholar] [CrossRef]
- Pierre, A.C.; Pajonk, G.M. Chemistry of aerogels and their applications. Chem. Rev. 2002, 102, 4243–4266. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Parale, V.G.; Jung, H.-N.-R.; Kim, Y.; Driss, Z.; Driss, D.; Bouabidi, A.; Euchy, S.; Park, H.-H. Facile synthesis of SnO2 aerogel/reduced graphene oxide nanocomposites via in situ annealing for the photocatalytic degradation of methyl orange. Nanomaterials 2019, 9, 358. [Google Scholar] [CrossRef] [PubMed]
- Zu, G.; Kanamori, K.; Nakanishi, K.; Huang, J. Superhydrophobic ultraflexible triple-network graphene/polyorganosiloxane aerogels for a high-performance multifunctional temperature/strain/pressure sensing array. Chem. Mater. 2019, 31, 6276–6285. [Google Scholar] [CrossRef]
- Thubsuang, U.; Sukanan, D.; Sahasithiwat, S.; Wongkasemjit, S.; Chaisuwan, T. Highly sensitive room temperature organic vapor sensor based on polybenzoxazine-derived carbon aerogel thin film composite. Mater. Sci. Eng. B 2015, 200, 67–77. [Google Scholar] [CrossRef]
- Qi, H.; Liu, J.; Pionteck, J.; Pötschke, P.; Mäder, E. Carbon nanotube–cellulose composite aerogels for vapour sensing. Sens. Actuators B Chem. 2015, 213, 20–26. [Google Scholar] [CrossRef]
- Chen, Y.; Pötschke, P.; Pionteck, J.; Voit, B.; Qi, H. Smart cellulose/graphene composites fabricated by in situ chemical reduction of graphene oxide for multiple sensing applications. J. Mater. Chem. A 2018, 6, 7777–7785. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, H.; Shen, G.; Cheng, P.; Zhang, J.; Guo, S. Reduction of graphene oxide via L-ascorbic acid. Chem. Commun. 2010, 46, 1112–1114. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, J.; Cui, X.; Wang, Y.; Gao, Y.; Sun, P.; Liu, F.; Shimanoe, K.; Yamazoe, N.; Lu, G. Enhanced gas sensing properties to acetone vapor achieved by α-Fe2O3 particles ameliorated with reduced graphene oxide sheets. Sens. Actuators B Chem. 2017, 241, 904–914. [Google Scholar] [CrossRef]
- Varghese, S.S.; Lonkar, S.; Singh, K.; Swaminathan, S.; Abdala, A. Recent advances in graphene based gas sensors. Sens. Actuators B Chem. 2015, 218, 160–183. [Google Scholar] [CrossRef]
- Zhang, B.; Fu, R.W.; Zhang, M.Q.; Dong, X.M.; Lan, P.L.; Qiu, J.S. Preparation and characterization of gas-sensitive composites from multi-walled carbon nanotubes/polystyrene. Sens. Actuators B Chem. 2005, 109, 323–328. [Google Scholar] [CrossRef]
- Chen, S.G.; Hu, J.W.; Zhang, M.Q.; Li, M.W.; Rong, M.Z. Gas sensitivity of carbon black/waterborne polyurethane composites. Carbon 2004, 42, 645–651. [Google Scholar] [CrossRef]
- Fan, Q.; Qin, Z.; Villmow, T.; Pionteck, J.; Pötschke, P.; Wu, Y.; Voit, B.; Zhu, M. Vapor sensing properties of thermoplastic polyurethane multifilament covered with carbon nanotube networks. Sens. Actuators B Chem. 2011, 156, 63–70. [Google Scholar] [CrossRef]
- Marriam, I.; Wang, X.; Tebyetekerwa, M.; Chen, G.; Zabihi, F.; Pionteck, J.; Peng, S.; Ramakrishna, S.; Yang, S.; Zhu, M. A bottom-up approach to design wearable and stretchable smart fibers with organic vapor sensing behaviors and energy storage properties. J. Mater. Chem. 2018, 6, 13633–13643. [Google Scholar] [CrossRef]
- Wang, X.; Meng, S.; Tebyetekerwa, M.; Li, Y.; Pionteck, J.; Sun, B.; Qin, Z.; Zhu, M. Highly sensitive and stretchable piezoresistive strain sensor based on conductive poly(styrene-butadiene-styrene)/few layer graphene composite fiber. Compos. Part A 2018, 105, 291–299. [Google Scholar] [CrossRef]
- Li, Y.; Pötschke, P.; Pionteck, J.; Voit, B. Electrical and vapor sensing behaviors of polycarbonate composites containing hybrid carbon fillers. Eur. Polym. J. 2018, 108, 461–471. [Google Scholar] [CrossRef]
- Tabačiarová, J.; Krajči, J.; Pionteck, J.; Reuter, U.; Omastová, M.; Mičušík, M. Styrene Butadiene Rubber/Carbon Filler-Based Vapor Sensors. Macromol. Chem. Phys. 2016, 217, 1149–1160. [Google Scholar] [CrossRef]
- Elidrissi, A.; El Barkany, S.; Amhamdi, H.; Maaroufi, A.; Hammouti, B. New approach to predict the solubility of polymers. Application: Cellulose acetate at various DS, prepared from alfa “stipa-tenassicima” of eastern morocco. J. Mater. Environ. Sci 2012, 3, 270–285. [Google Scholar]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook; CRC press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Bouvree, A.; Feller, J.-F.; Castro, M.; Grohens, Y.; Rinaudo, M. Conductive polymer nano-biocomposites (CPC): Chitosan-carbon nanoparticle a good candidate to design polar vapour sensors. Sens. Actuators B Chem. 2009, 138, 138–147. [Google Scholar] [CrossRef]
- Lu, J.; Kumar, B.; Castro, M.; Feller, J.-F. Vapour sensing with conductive polymer nanocomposites (CPC): Polycarbonate-carbon nanotubes transducers with hierarchical structure processed by spray layer by layer. Sens. Actuators B Chem. 2009, 140, 451–460. [Google Scholar] [CrossRef]
- Castro, M.; Lu, J.; Bruzaud, S.; Kumar, B.; Feller, J.-F. Carbon nanotubes/poly (ε-caprolactone) composite vapour sensors. Carbon 2009, 47, 1930–1942. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Pötschke, P.; Pionteck, J.; Voit, B.; Qi, H. Aerogels Based on Reduced Graphene Oxide/Cellulose Composites: Preparation and Vapour Sensing Abilities. Nanomaterials 2020, 10, 1729. https://doi.org/10.3390/nano10091729
Chen Y, Pötschke P, Pionteck J, Voit B, Qi H. Aerogels Based on Reduced Graphene Oxide/Cellulose Composites: Preparation and Vapour Sensing Abilities. Nanomaterials. 2020; 10(9):1729. https://doi.org/10.3390/nano10091729
Chicago/Turabian StyleChen, Yian, Petra Pötschke, Jürgen Pionteck, Brigitte Voit, and Haisong Qi. 2020. "Aerogels Based on Reduced Graphene Oxide/Cellulose Composites: Preparation and Vapour Sensing Abilities" Nanomaterials 10, no. 9: 1729. https://doi.org/10.3390/nano10091729
APA StyleChen, Y., Pötschke, P., Pionteck, J., Voit, B., & Qi, H. (2020). Aerogels Based on Reduced Graphene Oxide/Cellulose Composites: Preparation and Vapour Sensing Abilities. Nanomaterials, 10(9), 1729. https://doi.org/10.3390/nano10091729







