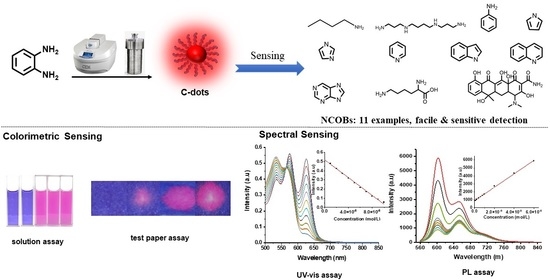
Facile and Sensitive Detection of Nitrogen-Containing Organic Bases with Near Infrared C-Dots Derived Assays
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Apparatus
2.2. Synthesis of C-Dots
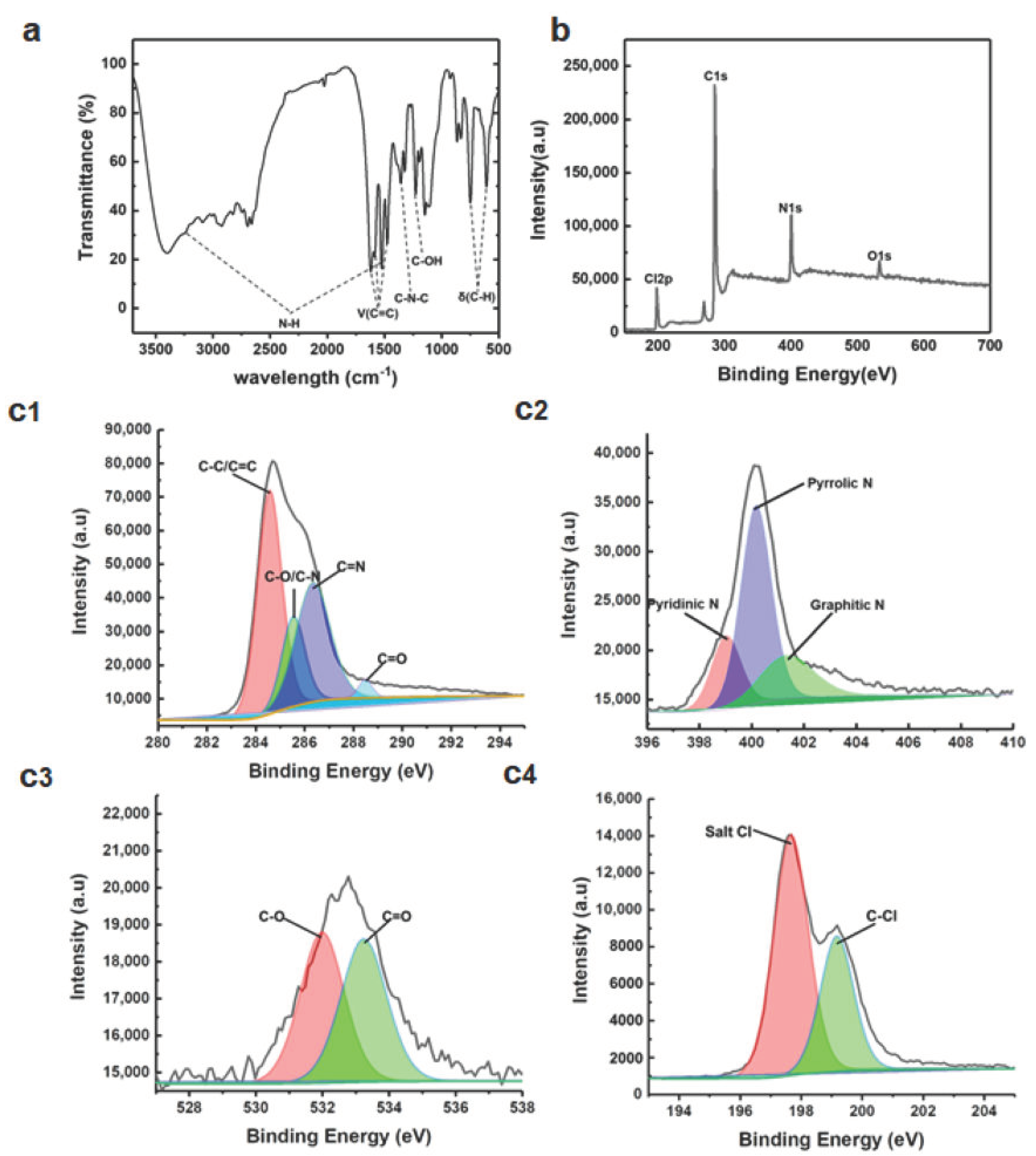
2.3. Characterizations of C-Dots
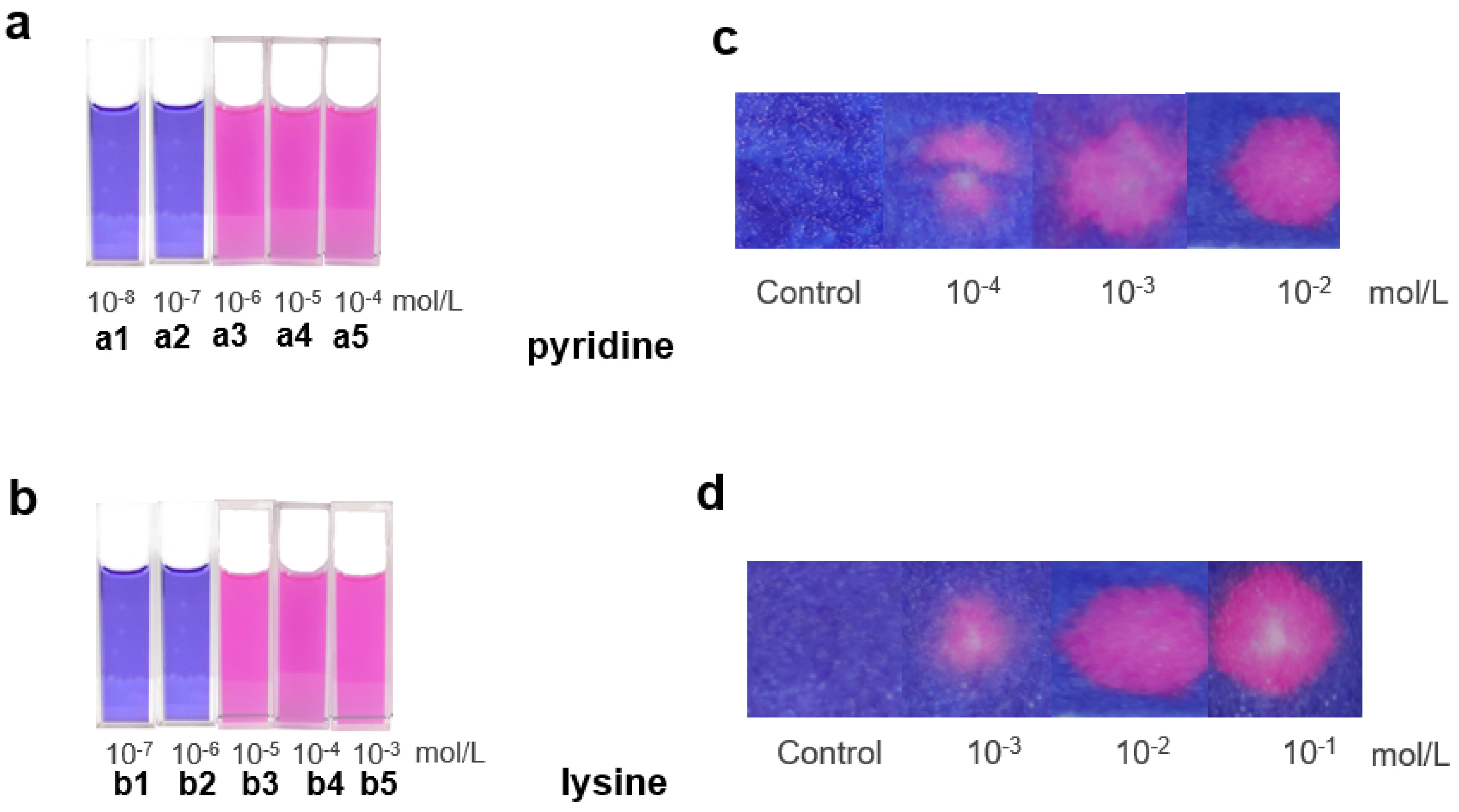
2.4. Detection of Pyridine
2.5. Solution Assays
2.6. Test Paper Assays
2.7. Spectral Sensing Assays Based on UV–vis Absorption
2.8. Spectral Sensing Assays Based on Fluorescence Spectroscopy
2.9. Difference in Percentage (Diff. %) between Calculated Values and Actual Values
3. Experimental Results and Discussion
3.1. Synthesis and Characterization of C-Dots
3.2. Colorimetric Sensing Assay Development
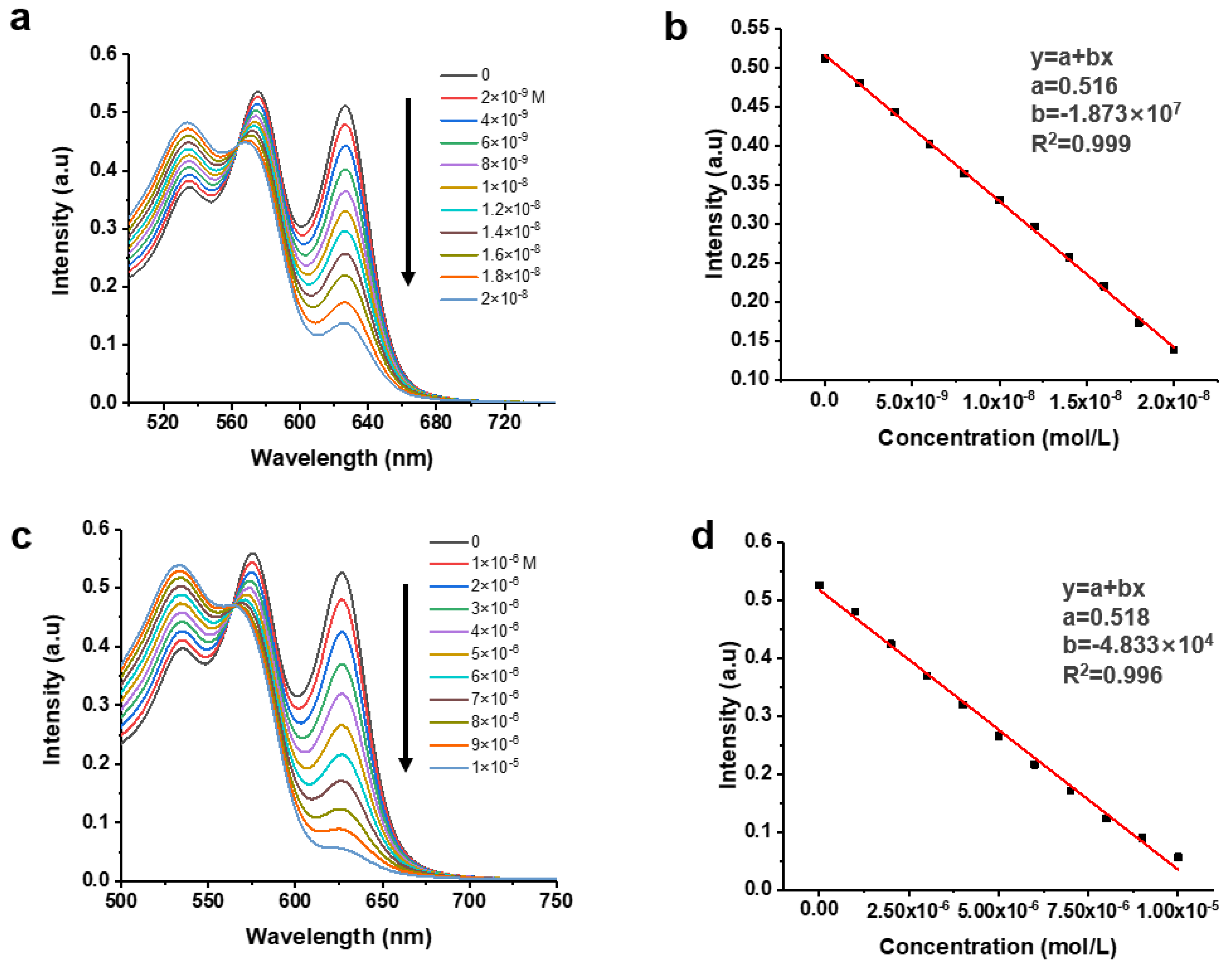
3.3. Spectral Sensing Assay (UV-vis and PL) Development
3.4. Reliability and Selectivity of the Assay
3.5. Advantages of the Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Pavlova, N.N.; Thompson, C.B. Cancer cell metabolism: The essential role of the nonessential amino acid, glutamine. EMBO J. 2017, 36, 1302–1315. [Google Scholar] [CrossRef] [Green Version]
- Anjalin, M.; Kanagathara, N.; Suganthi, A.B. A brief review on aniline and its derivatives. Mater. Today Proc. 2020, 33, 4751–4755. [Google Scholar] [CrossRef]
- Zablotskaya, A.; Segal, I.; Geronikaki, A.; Shestakova, I.; Nikolajeva, V.; Makarenkova, G. N-Heterocyclic choline analogues based on 1, 2, 3, 4-tetrahydro (iso) quinoline scaffold with anticancer and anti-infective dual action. Pharmacol. Rep. 2017, 69, 575–581. [Google Scholar] [CrossRef]
- Chopra, S.; Singh, A.; Venugopalan, P.; Singh, N.; Kaur, N. Organic nanoparticles for visual detection of spermidine and spermine in vapors and aqueous phase. ACS Sustain. Chem. Eng. 2017, 5, 1287–1296. [Google Scholar] [CrossRef]
- Ullrich, T.; Weirich, S.; Jeltsch, A. Development of an epigenetic tetracycline sensor system based on DNA methylation. PLoS ONE 2020, 15, e0232701. [Google Scholar] [CrossRef]
- Cho, Y.B.; Jeong, S.H.; Chun, H.; Kim, Y.S. Selective colorimetric detection of dissolved ammonia in water via modified Berthelot’s reaction on porous paper. Sens. Actuators B 2018, 256, 167–175. [Google Scholar] [CrossRef]
- Peng, Z.L.; Han, X.; Li, S.H.; Al-Youbi, A.O.; Bashammakh, A.S.; El-Shahawi, M.S.; Leblanc, R.M. Carbon dots: Biomacromolecule interaction, bioimaging and nanomedicine. Coord. Chem. Rev. 2017, 343, 256–277. [Google Scholar] [CrossRef]
- Ji, C.; Zhou, Y.; Leblanc, R.M.; Peng, Z. Recent developments of carbon dots in biosensing: A review. ACS Sens. 2020, 5, 2724–2741. [Google Scholar] [CrossRef]
- Mintz, K.; Waidely, E.; Zhou, Y.; Peng, Z.; Al-Youbi, A.O.; Bashammakh, A.S.; El-Shahawi, M.S.; Leblanc, R.M. Carbon dots and gold nanoparticles based immunoassay for detection of alpha-L-fucosidase. Anal. Chim. Acta 2018, 1041, 114–121. [Google Scholar] [CrossRef]
- Sun, X.C.; Lei, Y. Fluorescent carbon dots and their sensing applications. TrAC Trends Anal. Chem. 2017, 89, 163–180. [Google Scholar] [CrossRef]
- Li, M.; Chen, T.; Gooding, J.J.; Liu, J. Review of carbon and graphene quantum dots for sensing. ACS Sens. 2019, 4, 1732–1748. [Google Scholar] [CrossRef]
- Xu, D.; Lin, Q.; Chang, H.T.J.S.M. Recent advances and sensing applications of carbon dots. Small Methods 2020, 4, 1900387. [Google Scholar] [CrossRef]
- Budavari, S.; O’Neil, M.J.; Smith, A.; Heckelman, P.E. The Merck Index; Merck: Rahway, NJ, USA, 1989; Volume 11. [Google Scholar]
- Wang, B.; Yu, J.; Sui, L.; Zhu, S.; Tang, Z.; Yang, B.; Lu, S. Rational design of multi-color-emissive carbon dots in a single reaction system by hydrothermal. Adv. Sci. 2021, 8, 2001453. [Google Scholar] [CrossRef]
- Soni, N.; Singh, S.; Sharma, S.; Batra, G.; Kaushik, K.; Rao, C.; Verma, N.C.; Mondal, B.; Yadav, A.; Nandi, C.K. Absorption and emission of light in red emissive carbon nanodots. Chem. Sci. 2021, 12, 3615–3626. [Google Scholar] [CrossRef]
- Liu, J.; Li, D.; Zhang, K.; Yang, M.; Sun, H.; Yang, B. One-step hydrothermal synthesis of nitrogen-doped conjugated carbonized polymer dots with 31% efficient red emission for in vivo imaging. Small 2018, 14, 1703919. [Google Scholar] [CrossRef]
- Jia, H.; Wang, Z.; Yuan, T.; Yuan, F.; Li, X.; Li, Y.; Tan, Z.a.; Fan, L.; Yang, S. Electroluminescent warm white light-emitting diodes based on passivation enabled bright red bandgap emission carbon quantum dots. Adv. Sci. 2019, 6, 1900397. [Google Scholar] [CrossRef] [Green Version]
- Shrivastava, A.; Gupta, V.B. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21. [Google Scholar] [CrossRef]
- Thomsen, V.; Schatzlein, D.; Mercuro, D. Limits of detection in spectroscopy. Spectroscopy 2003, 18, 112–114. [Google Scholar]
- Bakier, Y.; Ghali, M.; Zahra, W. Highly sensitive fluorescent detection of pyridine using small size carbon quantum dots derived from folic acid. J. Phys. D Appl. Phys. 2020, 53, 405103. [Google Scholar] [CrossRef]
- Gong, Y.; Du, C.; Wang, X.; Guo, H.; Yang, F. First stable (Z)-configuration of cyanostilbene derivative: An effective “turn-on “fluorescent sensor for lysine in aqueous media. Microchem. J. 2021, 162, 105866. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Fu, H.; Wang, W.; Han, D.; An, X. Experimental and theoretical study on the excellent amine-sensing performance of Au decorated WO3 needle-like nanocomposites. Mater. Chem. Phys. 2019, 234, 122–132. [Google Scholar] [CrossRef]
- Bhamore, J.R.; Murthy, Z.; Kailasa, S.K. Fluorescence turn-off detection of spermine in biofluids using pepsin mediated synthesis of gold nanoclusters as a probe. J. Mol. Liq. 2019, 280, 18–24. [Google Scholar] [CrossRef]
- Feng, H.-J.; Xu, L.; Liu, B.; Jiao, H. Europium metal–organic frameworks as recyclable and selective turn-off fluorescent sensors for aniline detection. Dalton Trans. 2016, 45, 17392–17400. [Google Scholar] [CrossRef]
- Ahmed, H.M.; Ghali, M.; Zahra, W.K.; Ayad, M. Optical sensing of pyridine based on green synthesis of passivated carbon dots. Mater. Today Proc. 2020, 33, 1845–1848. [Google Scholar] [CrossRef]
- Pandey, S.P.; Singh, P.K. A polyelectrolyte based ratiometric optical sensor for arginine and lysine. Sens. Actuators B 2020, 303, 127182. [Google Scholar] [CrossRef]
- Park, S.; Lee, S.-Y. Photoluminescent microrods from the self-assembly of a biomimetic molecule: Application for the optical detection of pyrrole. Sens. Actuators B 2014, 202, 690–698. [Google Scholar] [CrossRef]
- Manshaei, F.; Bagheri, H.; Eshaghi, A. Turn-off chelation-enhanced fluorescence sensing of carbon dot-metallic deep eutectic solvent by imidazole-based small molecules. Sens. Actuators B 2021, 344, 130228. [Google Scholar] [CrossRef]
- Salve, M.; Amreen, K.; Rajurkar, P.; Pattnaik, P.K.; Goel, S. Miniaturized Disposable Buckypaper-Polymer Substrate Based Electrochemical Purine Sensing Platform. ECS J. Solid State Sci. Technol. 2020, 9, 101009. [Google Scholar] [CrossRef]
- Velmurugan, S.; Zhi-Xiang, L.; Yang, T.C.; Juan, J.C. Rational design of built-in stannic oxide-copper manganate microrods p-n heterojunction for photoelectrochemical sensing of tetracycline. Chemosphere 2021, 271, 129788. [Google Scholar] [CrossRef]





| NCOBs | Aliphatic Amines | Aromatic | Five-H a | Six-H b | Fused Ring | Amino Acid | Antibiotic | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Assays | Butylamine | Spermine | Aniline | Pyrrole | Imidazole | Pyridine | Purine | Quinoline | Indole | Lysine | Tetracycline | |
| Solution (M) | 10−5 | 10−6 | 10−6 | 10−2 | 10−5 | 10−6 | 10−3 | 10−3 | 10−2 | 10−5 | 10−5 | |
| Paper (M) | 10−4 | 10−6 | 10−5 | 10−1 | 10−4 | 10−4 | 10−1 | 10−2 | 10−1 | 10−3 | 10−4 | |
| NCOBs | LOD a | Linear Range | Diff. % b | Figure c |
|---|---|---|---|---|
| butylamine | 5.50 × 10−2 µM | 0.17–3 µM | 1.00 | S3 |
| spermine | 0.70 nM | 2.12–20 nM | 0.90 | S4 |
| aniline | 0.69 nM | 2.06–20 nM | 2.77 | S5 |
| pyrrole | 0.12 mM | 0.36–2 mM | 1.87 | S6 |
| imidazole | 7.40 × 10−2 µM | 0.22–1.60 µM | 1.73 | S7 |
| pyridine | 0.75 nM | 2.27–20 nM | 2.15 | 6a,b |
| purine | 0.042 mM | 0.13–0.30 mM | 7.67 | S8 |
| quinoline | 0.015 mM | 0.045–0.20 mM | 5.43 | S9 |
| indole | 0.025 mM | 0.076–0.20 mM | 5.07 | S10 |
| lysine | 0.29 µM | 0.88–10 µM | 1.41 | 6c,d |
| tetracycline | 0.85 µM | 2.58–20 µM | 3.90 | S11 |
| NCOBs | LOD a | Linear Range | Diff. % b | Figure c |
|---|---|---|---|---|
| butylamine | 0.17 µM | 0.50–20 µM | 5.03 | S12 |
| spermine | 0.50 nM | 1.52–100 nM | 3.47 | S13 |
| aniline | 0.53 nM | 1.61–10 nM | 6.20 | S14 |
| pyrrole | 1.55 mM | 4.70–100 mM | 6.10 | S15 |
| imidazole | 0.17 µM | 0.52–100 µM | 5.10 | S16 |
| pyridine | 0.68 nM | 2.06–60 nM | 6.77 | 7a,b |
| purine | 12.40 µM | 37.60–100 µM | 6.00 | S17 |
| quinoline | 17.40 µM | 52.70–1000 µM | 5.13 | S18 |
| indole | 87.10 uM | 0.26–1 mM | 4.80 | S19 |
| lysine | 0.20 µM | 0.61–20 µM | 6.00 | 7c,d |
| tetracycline | 0.20 µM | 0.60–10 µM | 4.17 | S20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, C.; Zhou, Y.; Shi, W.; Wu, J.; Han, Q.; Zhao, T.; Leblanc, R.M.; Peng, Z. Facile and Sensitive Detection of Nitrogen-Containing Organic Bases with Near Infrared C-Dots Derived Assays. Nanomaterials 2021, 11, 2607. https://doi.org/10.3390/nano11102607
Ji C, Zhou Y, Shi W, Wu J, Han Q, Zhao T, Leblanc RM, Peng Z. Facile and Sensitive Detection of Nitrogen-Containing Organic Bases with Near Infrared C-Dots Derived Assays. Nanomaterials. 2021; 11(10):2607. https://doi.org/10.3390/nano11102607
Chicago/Turabian StyleJi, Chunyu, Yiqun Zhou, Wenquan Shi, Jiajia Wu, Qiurui Han, Tianshu Zhao, Roger M. Leblanc, and Zhili Peng. 2021. "Facile and Sensitive Detection of Nitrogen-Containing Organic Bases with Near Infrared C-Dots Derived Assays" Nanomaterials 11, no. 10: 2607. https://doi.org/10.3390/nano11102607
APA StyleJi, C., Zhou, Y., Shi, W., Wu, J., Han, Q., Zhao, T., Leblanc, R. M., & Peng, Z. (2021). Facile and Sensitive Detection of Nitrogen-Containing Organic Bases with Near Infrared C-Dots Derived Assays. Nanomaterials, 11(10), 2607. https://doi.org/10.3390/nano11102607