SDS-Stabilized CuInSe2/ZnS Multinanocomposites Prepared by Mechanochemical Synthesis for Advanced Biomedical Application
Abstract
:1. Introduction
2. Materials and Methods
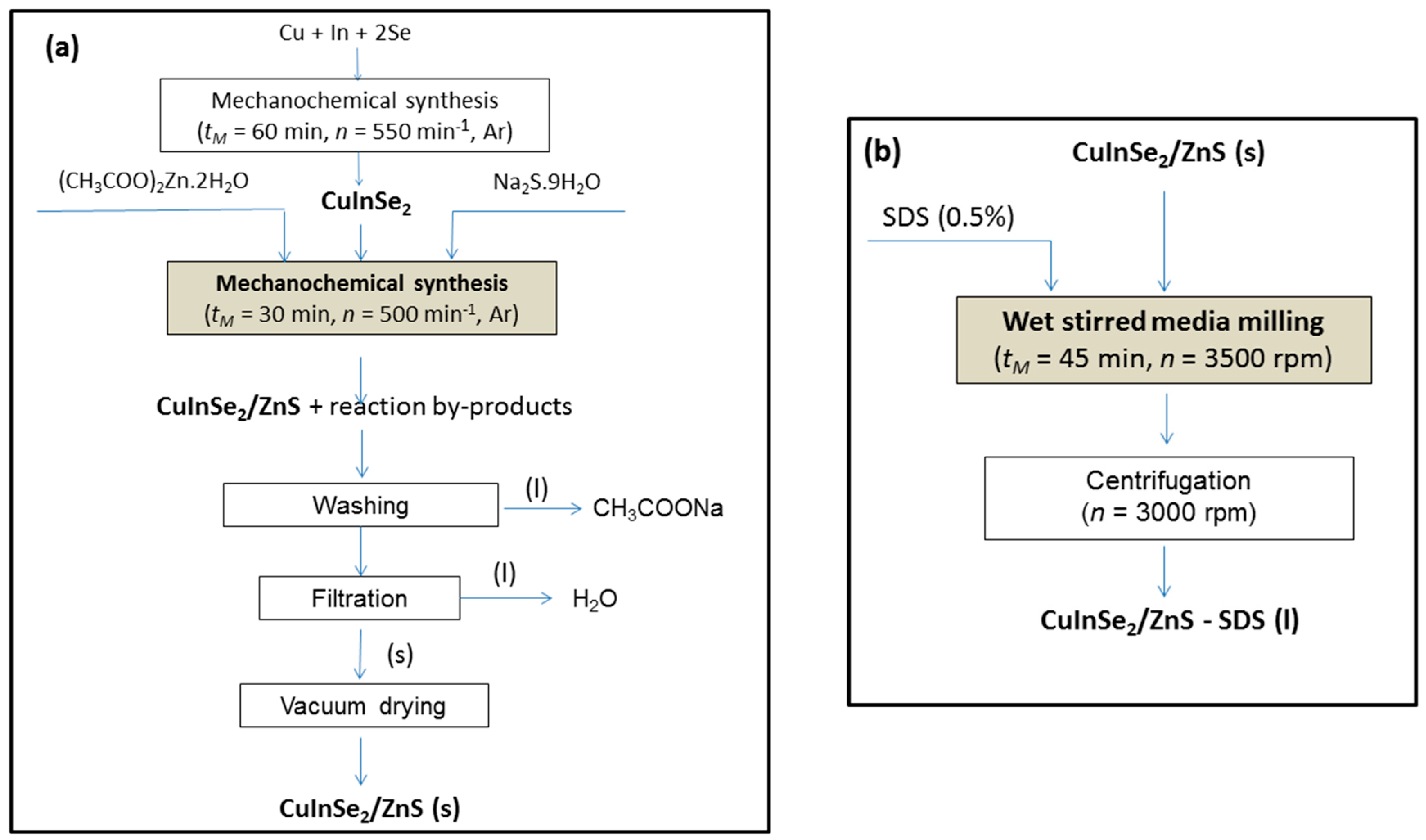
2.1. Mechanochemical Synthesis of CuInSe2/ZnS Nanocrystals and SDS Capped CuInSe2/ZnS Nanosuspensions
2.2. Characterization Methods
2.3. Biological Activity
2.3.1. Multiple Myeloma Cell Lines
2.3.2. Nanoparticles Sensitivity by Cell-Based MTT Assay
2.3.3. Nanoparticle Sensitivity by Flow Cytometry Analysis
3. Results and Discussion
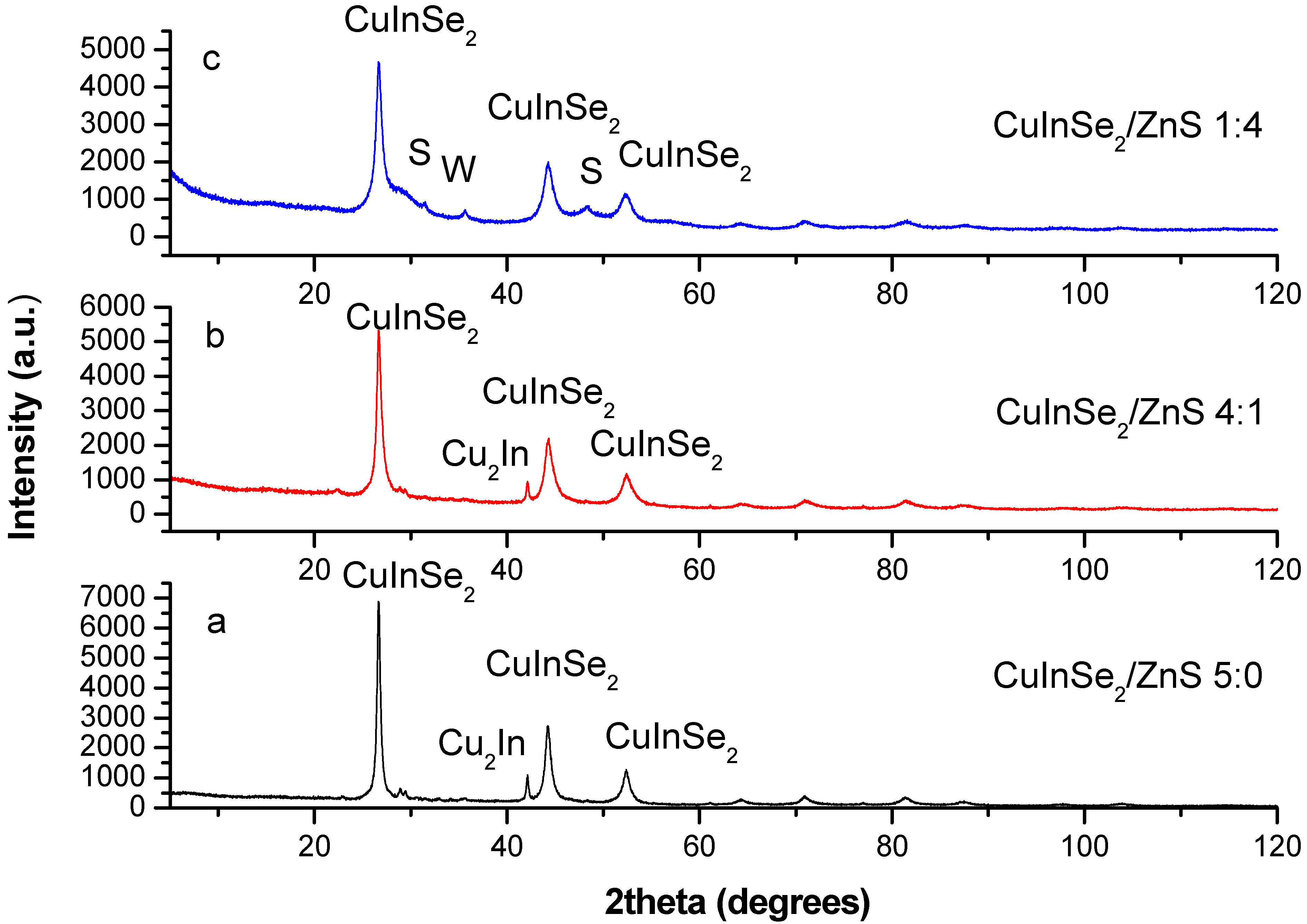
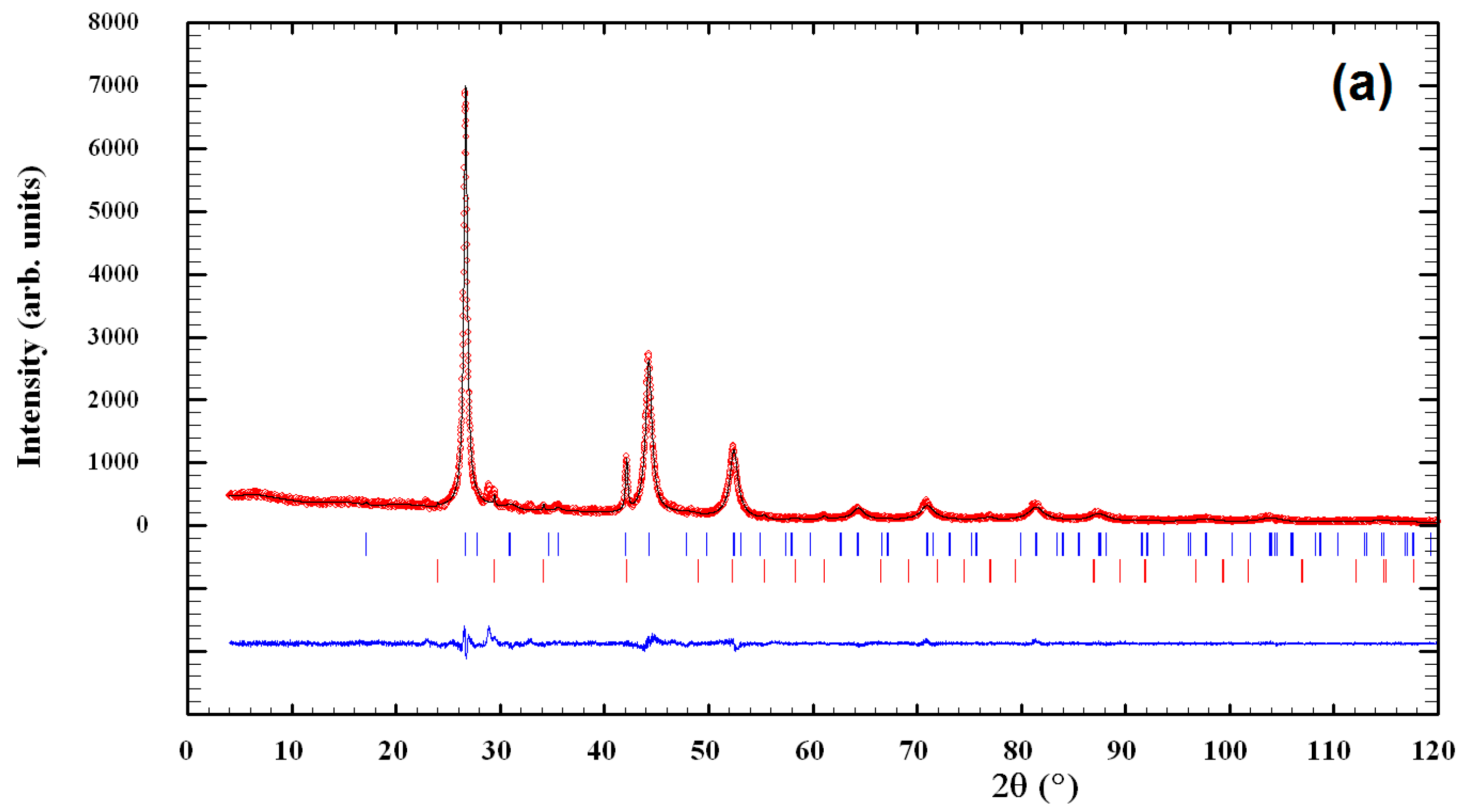
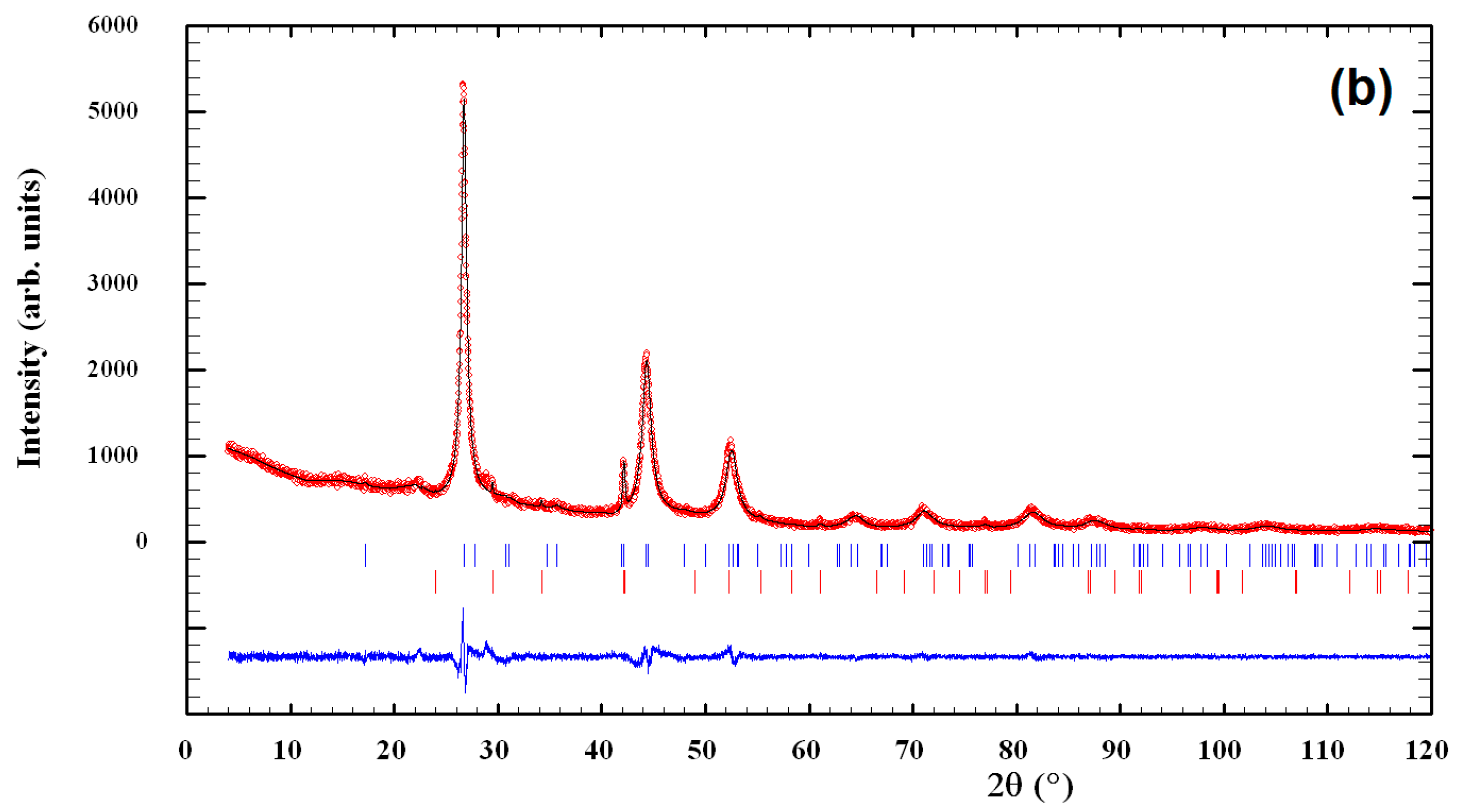
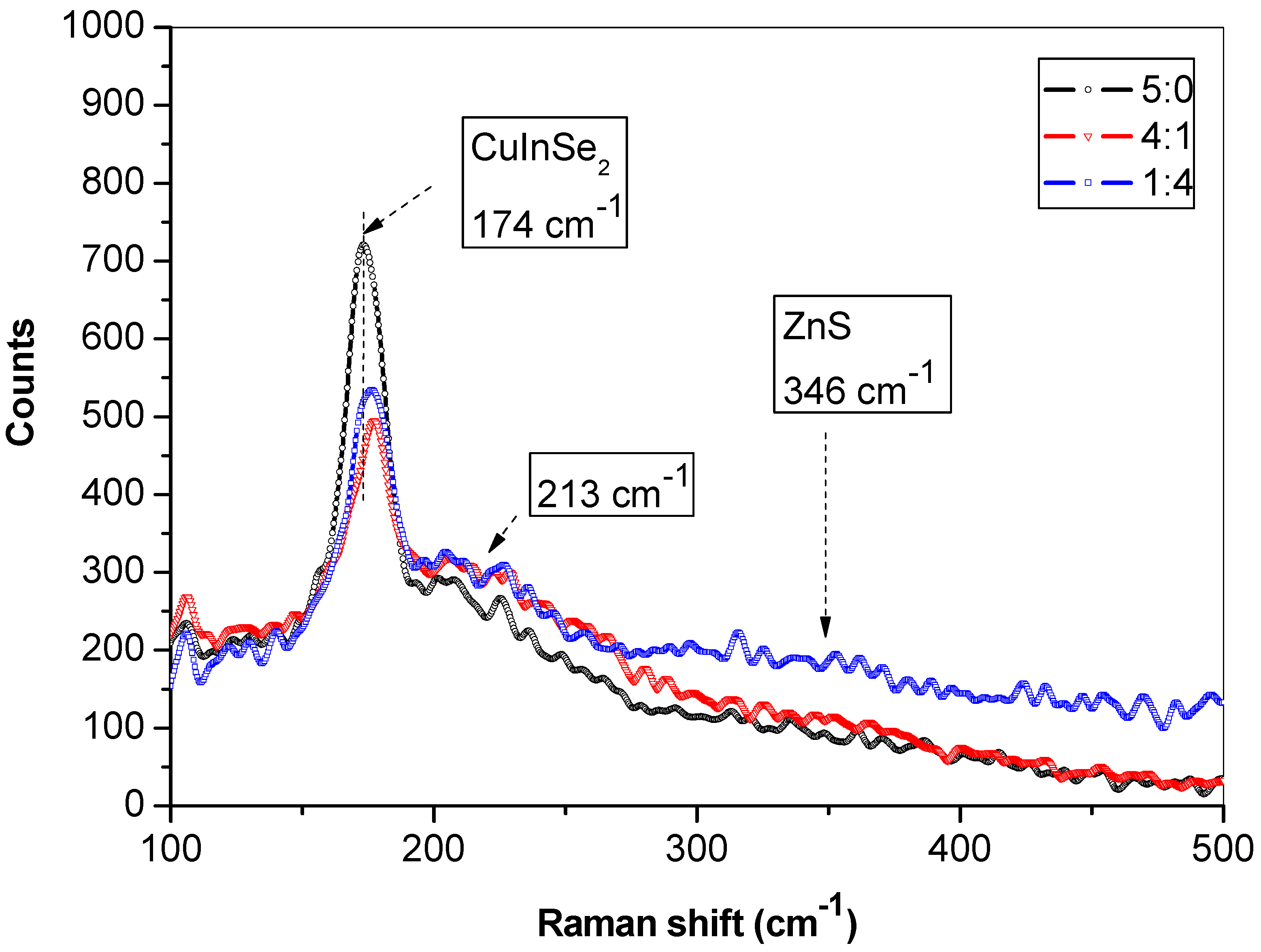
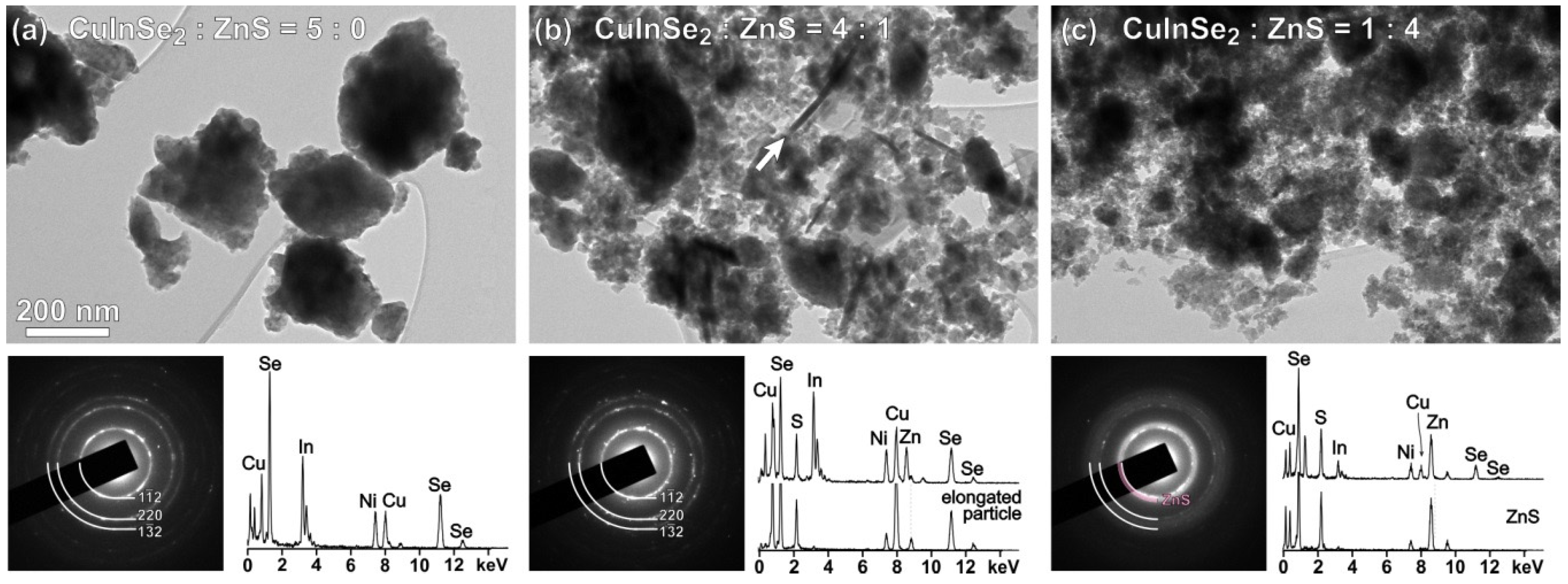
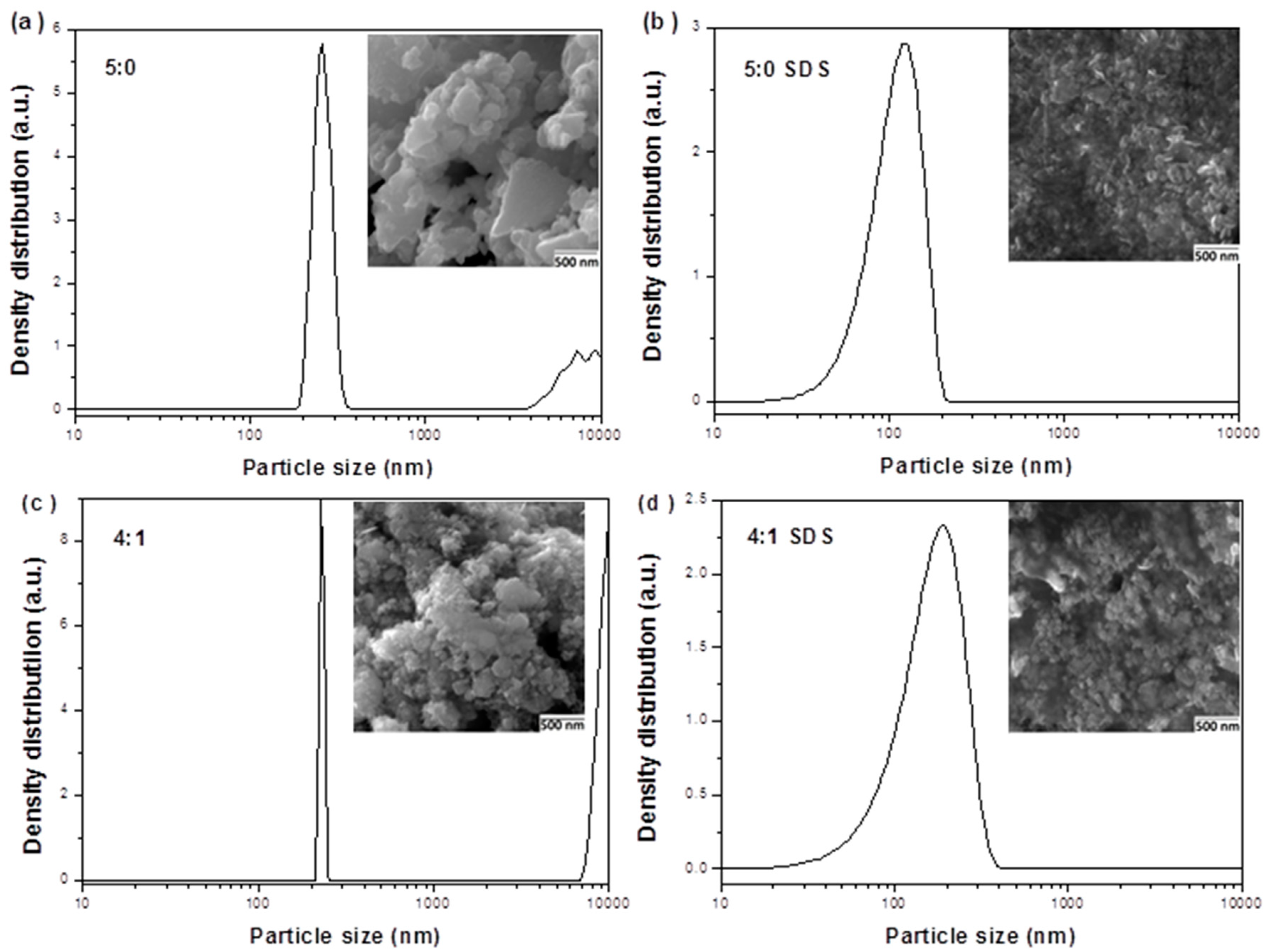
3.1. Characterization of CuInSe2/ZnS Nanocrystals
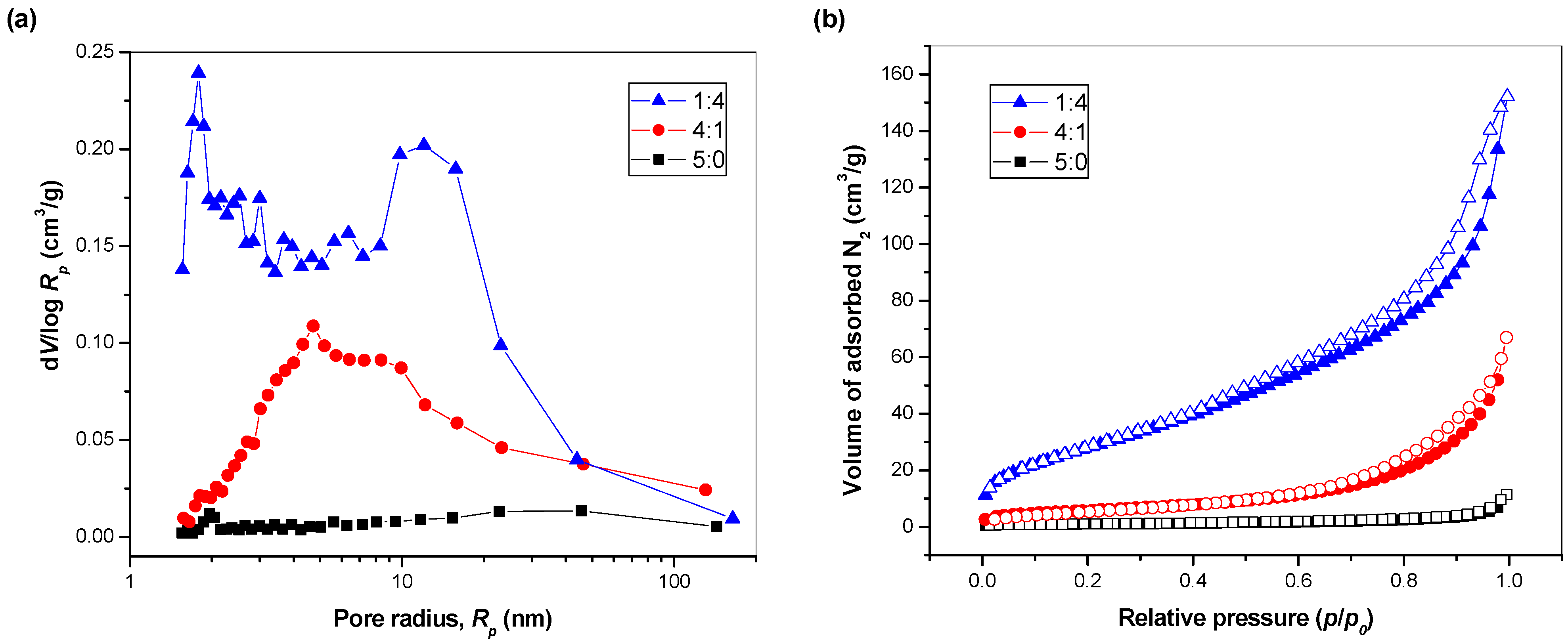
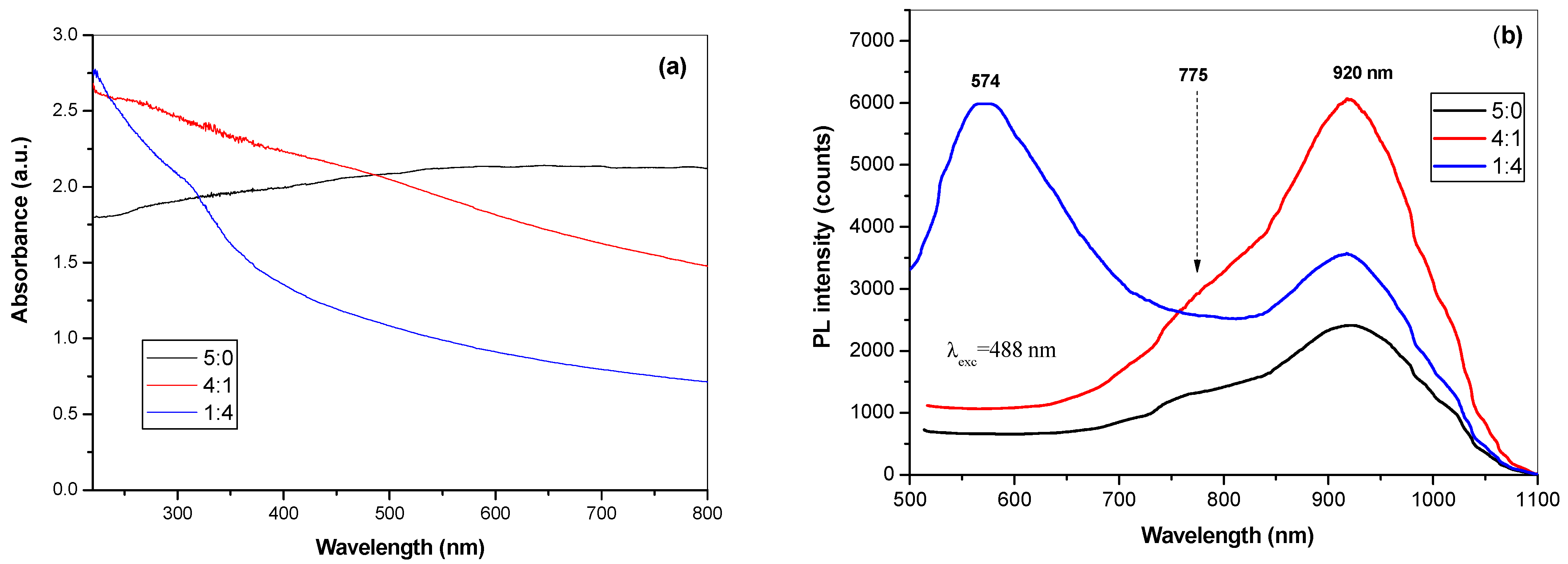
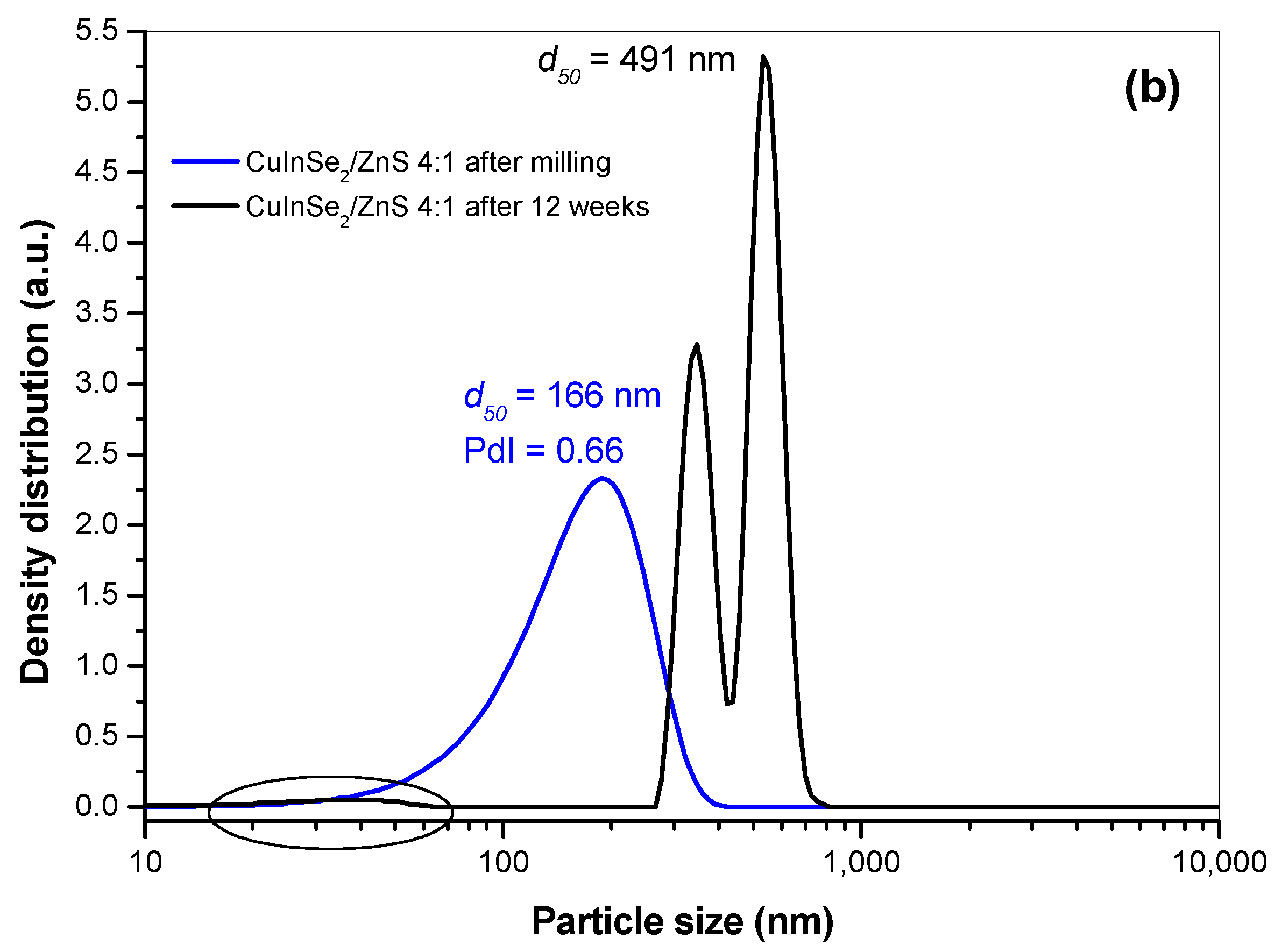
3.2. Characterization of SDS Capped CuInSe2/ZnS Nanosuspensions
3.3. Biological Activity of SDS Capped CuInSe2/ZnS 5:0 and 4:1 Nanosuspensions
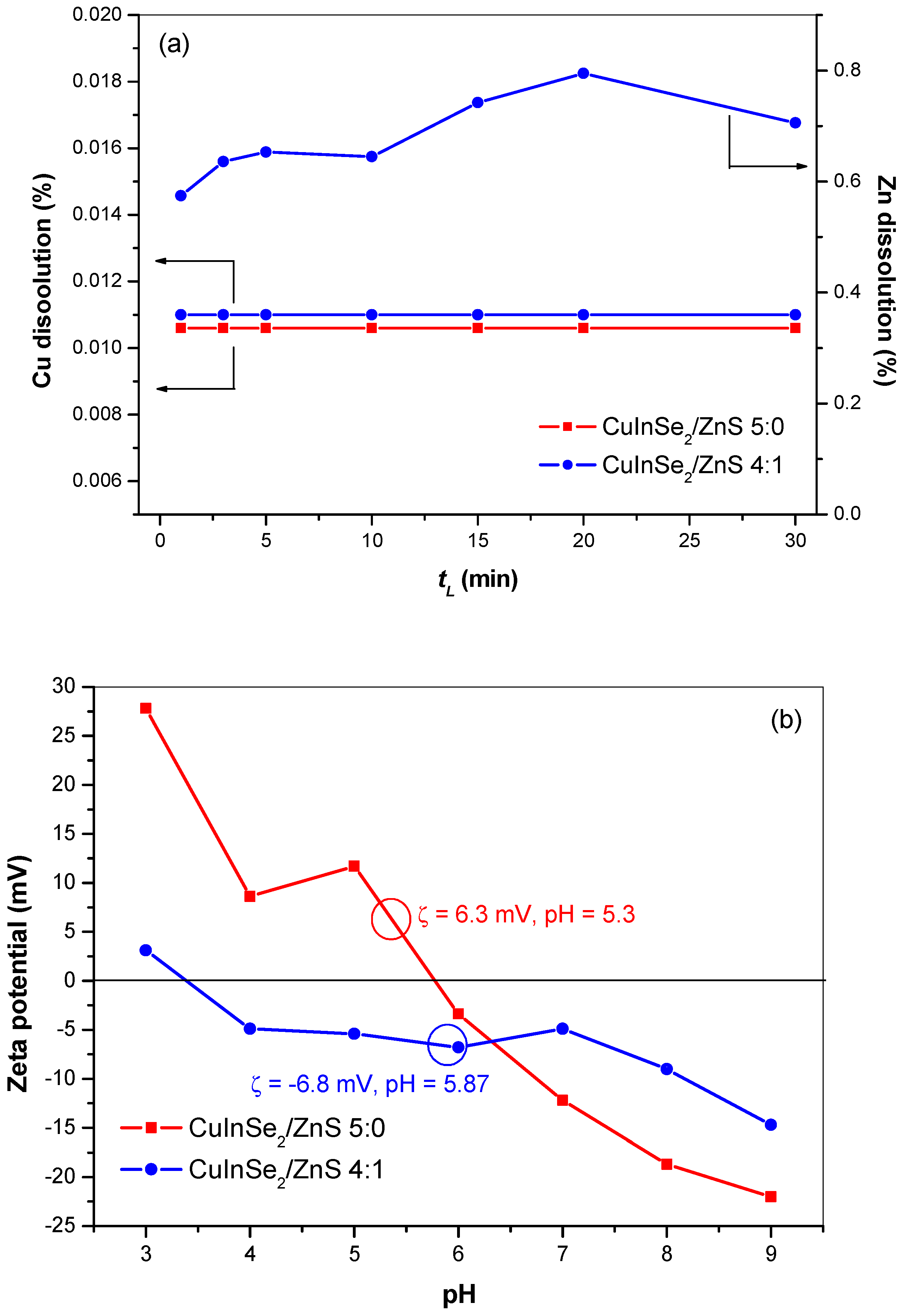
3.3.1. Dissolution of Copper and Zinc from CuInSe2/ZnS Nanocrystals
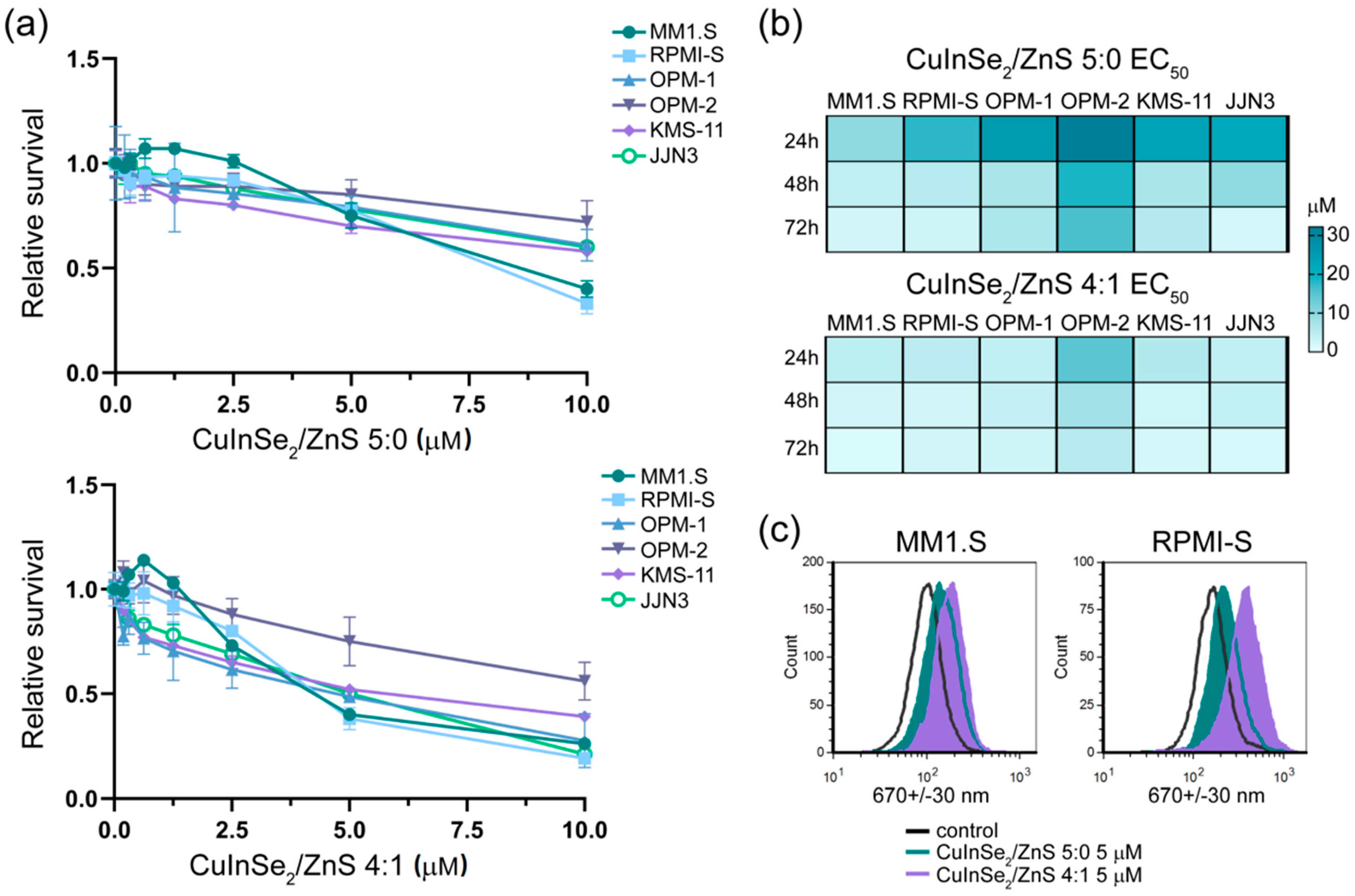
3.3.2. Cytotoxicity of CuInSe2/ZnS 5:0-SDS and CuInSe2/ZnS 4:1-SDS Nanosuspensions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vollath, D. Nanomaterials: An Introduction to Synthesis, Properties and Applications, 2nd ed.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2013. [Google Scholar]
- Chuang, P.H.; Lin, C.C.; Liu, R.S. Emission-Tunable CuInS2/Zns Quantum Dots: Structure, Optical Properties, and Application in White Light-Emitting Diodes with High Color Rendering Index. ACS Appl. Mater. Interfaces 2014, 6, 15379–15387. [Google Scholar] [CrossRef] [PubMed]
- Kang, F.; Ao, J.P.; Sun, G.Z.; He, Q.; Sun, Y. Structure and Photovoltaic Characteristics of CuInSe2 Thin Films Prepared by Pulse-Reverse Electrodeposition and Selenization Process. J. Alloys Compd. 2009, 478, L25–L27. [Google Scholar] [CrossRef]
- Jiang, D.X.; Cao, L.X.; Su, G.; Liu, W.; Qu, H.; Sun, Y.G.; Dong, B.H. Shell Thickness Dependence of Luminescence Intensity in Core/Shell ZnS:Mn/ZnS Nanoparticles. Mater. Chem. Phys. 2009, 115, 795–798. [Google Scholar] [CrossRef]
- Fang, X.S.; Zhai, T.Y.; Gautam, U.K.; Li, L.; Wu, L.M.; Yoshio, B.; Golberg, D. ZnS Nanostructures: From Synthesis to Applications. Prog. Mater Sci. 2011, 56, 175–287. [Google Scholar] [CrossRef]
- Liu, X.Y.; Braun, G.B.; Zhong, H.Z.; Hall, D.J.; Han, W.L.; Qin, M.D.; Zhao, C.Z.; Wang, M.N.; She, Z.G.; Cao, C.B.; et al. Tumor-Targeted Multimodal Optical Imaging with Versatile Cadmium-Free Quantum Dots. Adv. Funct. Mater. 2016, 26, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Dvoracek, C.; Lee, K.H.; Galloway, J.F.; Bhang, H.E.C.; Pomper, M.G.; Searson, P.C. CuInSe2/ZnS Core/Shell NIR Quantum Dots For Biomedical Imaging. Small 2011, 7, 3148–3152. [Google Scholar] [CrossRef] [Green Version]
- Yarema, O.; Bozyigit, D.; Rousseau, I.; Nowack, L.; Yarema, M.; Heiss, W.; Wood, V. Highly Luminescent, Size- and Shape-Tunable Copper Indium Selenide Based Colloidal Nanocrystals. Chem. Mater. 2013, 25, 3753–3757. [Google Scholar] [CrossRef]
- Lian, W.; Tu, D.; Hu, P.; Song, X.; Gong, Z.; Chen, T.; Song, J.; Chen, Z.; Chen., X. Broadband Excitable NIR-II Luminescent Nano-Bioprobes Based on CuInSe2 Quantum Dots for the Detection of Circulating Tumor Cells. NanoToday 2020, 35, 100943. [Google Scholar] [CrossRef]
- Abdullah, H.; Habibi, S. Effect Of Annealing Temperature on CuInSe2/Zns Thin-Film Solar Cells Fabricated by using Electron Beam Evaporation. Int. J. Photoenergy 2013, 2013, 1–5. [Google Scholar] [CrossRef]
- Kang, X.J.; Yang, Y.C.; Huang, L.J.; Tao, Y.; Wang, L.; Pan, D.C. Large-Scale Synthesis of Water-Soluble CuInSe2/ZnS and AgInSe2/ZnS Core/Shell Quantum Dots. Green. Chem. 2015, 17, 4482–4488. [Google Scholar] [CrossRef]
- Li, W.J.; Pan, Z.X.; Zhong, X.H. CuInSe2 and CuInSe2-ZnS Based High Efficiency “Green” Quantum Dot Sensitized Solar Cells. J. Mater. Chem. A 2015, 3, 1649–1655. [Google Scholar] [CrossRef]
- Zhong, H.Z.; Wang, Z.B.; Bovero, E.; Lu, Z.H.; Van Veggel, F.C.J.M.; Scholes, G.D. Colloidal CuInSe2 Nanocrystals in the Quantum Confinement Regime: Synthesis, Optical Properties, and Electroluminescence. J. Mater. Chem. C 2011, 115, 12396–12402. [Google Scholar] [CrossRef]
- Mazing, D.S.; Karmanov, A.A.; Matyushkin, L.B.; Aleksandrova, O.A.; Pronin, I.A.; Moshnikov, V.A. Synthesis and Characterization of CuInSe2 Core–Shell Quantum Dots. Glass Phys. Chem 2016, 42, 497–504. [Google Scholar] [CrossRef]
- Bujňáková, Z.; Baláž, M.; Dutková, E.; Baláž, P.; Kello, M.; Mojžišová, G.; Mojžiš, J.; Vilková, M.; Imrich, J.; Psotka, M. Mechanochemical Approach for the Capping of Mixed Core CdS/ZnS Nanocrystals: Elimination of Cadmium Toxicity. J. Colloid Interface Sci. 2017, 486, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Bujňáková, Z.; Baláž, M.; Zdurienčiková, M.; Sedlák, J.; Čaplovičová, M.; Čaplovič, L.; Dutková, E.; Zorkovská, A.; Turianicová, E.; Baláž, P.; et al. Preparation, Properties and Anticancer Effects of Mixed As4S4/ZnS Nanoparticles Capped by Poloxamer 407. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 541–551. [Google Scholar] [CrossRef]
- Bujňáková, Z.; Dutková, E.; Zorkovská, A.; Baláž, M.; Kováč, J.; Kello, M.; Mojžiš, J.; Briančin, J.; Baláž, P. Mechanochemical Synthesis and in Vitro Studies of Chitosan-Coated InAs/Zns Mixed Nanocrystals. J. Mater. Sci. 2017, 52, 721–735. [Google Scholar] [CrossRef]
- Kelly, M.A.; Altria, K.D.; Clark, B.J. Quantitative Analysis of Sodium Dodecyl Sulphate by Capillary Electrophoresis. J. Chromatogr. A 1997, 781, 67–71. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, R. Interaction Between Casein and Sodium Dodecyl Sulfate. J. Colloid Interface Sci. 2007, 315, 685–692. [Google Scholar] [CrossRef]
- Raymond, C.; Rowe, P.J.S.; Marian, E. Quinn Handbook of Pharmaceutical Excipients, 6th ed.; Pharmaceutical Press and American Pharmacists Association: Washington, DC, USA, 2009. [Google Scholar]
- Niraula, T.P.; Bhattarai, A.; Chatterjee, S.K. Sodium Dodecylsulphate: A Very Useful Surfactant for Scientific Investigations. J. Knowl. Innov. 2014, 2, 111–113. [Google Scholar]
- Bujňáková, Z.; Dutková, E.; Kello, M.; Mojžiš, J.; Baláž, M.; Baláž, P.; Shpotyuk, O. Mechanochemistry of Chitosan-Coated Zinc Sulfide (ZnS) Nanocrystals for Bio-Imaging Applications. Nanoscale Res. Lett. 2017, 12, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Bujňáková, Z.; Dutková, E.; Baláž, M.; Turianicová, E.; Baláž, P. Stability Studies of As4S4 Nanosuspension Prepared by Wet Milling in Poloxamer 407. Int. J. Pharm. 2015, 478, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Rong, X.Y.; Laru, J.; Van Veen, B.; Kiesvaara, J.; Hirvonen, J.; Laaksonen, T.; Peltonen, L. Nanosuspensions of Poorly Soluble Drugs: Preparation and Development by Wet Milling. Int. J. Pharm. 2011, 411, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, L.; Dave, R.N.; Bilgili, E. An Intensified Vibratory Milling Process for Enhancing the Breakage Kinetics During the Preparation of Drug Nanosuspensions. AAPS PharmsSciTech 2016, 17, 389–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukáčová Bujňáková, Z.; Shpotyuk, O.; Syvorotka, I., Jr.; Demchenko, P.; Dutková, E.; Tóthová, E.; Bártová, Z. Preparation and Characterization of Stable Fluorescent As4S4/ZnS/Fe3O4 Nanosuspension Capped by Poloxamer 407 and Folic Acid. Appl. Nanosci. 2020, 10, 4651–4660. [Google Scholar] [CrossRef]
- Dutková, E.; Sayagues, M.J.; Kováč, J.; Kováč, J.; Bujňáková, Z.; Briančin, J.; Zorkovská, A.; Baláž, P.; Ficeriová, J. Mechanochemically Synthesized Nanocrystalline Ternary CuInSe2 Chalcogenide Semiconductor. Mater. Lett. 2016, 173, 182–186. [Google Scholar] [CrossRef]
- Baláž, P.; Boldižárová, E.; Godočíková, E.; Briančin, J. Mechanochemical Route for Sulphide Nanoparticles Preparation. Mater. Lett. 2003, 57, 1585–1589. [Google Scholar] [CrossRef]
- Dutková, E.; Baláž, P.; Pourghahramani, P.; Velumani, S.; Ascencio, J.A.; Kostová, N.G. Properties of Mechanochemically Synthesized ZnS Nanoparticles. J. Nanosci. Nanotechnol. 2009, 9, 6600–6605. [Google Scholar] [CrossRef] [Green Version]
- STOE WinXPOW. Software Manual, Version 3.03; Stoe & Cie GmbH: Darmstadt, Germany, 2010.
- Kraus, W.; Nolze, G. Powder Cell—A Program for the Representation and Manipulation of Crystal Structures and Calculation of the Resulting X-Ray Powder Patterns. J. Appl. Crystallogr. 1996, 29, 301–303. [Google Scholar] [CrossRef]
- Kraus, W.; Nolze, G. PowderCell for Windows, Version 2.4; Federal Institute for Materials Research and Testing: Berlin, Germany, 2000.
- Downs, R.T.; Hall-Wallace, M. The American Mineralogist Crystal Structure Database. Am. Mineral. 2003, 88, 247–250. [Google Scholar]
- Rodriguez-Carvajal, J. Recent Developments of the Program Fullprof. Commission on Powder Diffraction (iucr). Newsletter 2001, 26, 12–19. [Google Scholar]
- Rodriguez-Carvajal, J. An Introduction to the Program Fullprof 2000 (Version 5.60); Laboratoire Léon Brillouin (Cea-Cnrs): Saclay, France, 2001. [Google Scholar]
- Rodriguez-Carvajal, J.; Roisnel, T. Line Broadening Analysis Using Fullprof*: Determination of Microstructural Properties. Mater. Sci. Forum. 2004, 443–444, 123–126. [Google Scholar] [CrossRef]
- Alvarez-Garcia, J.; Barcones, B.; Perez-Rodriguez, A.; Romano-Rodriguez, A.; Morante, J.R.; Janotti, A.; Wei, S.H.; Scheer, R. Vibrational and Crystalline Properties of Polymorphic CuInC2 (C=Se,S) Chalcogenides. Phys. Rev. B 2005, 71. [Google Scholar] [CrossRef] [Green Version]
- Zaretskaya, E.P.; Gremenok, V.F.; Riede, V.; Schmitz, W.; Bente, K.; Zalesski, V.B.; Ermakov, O. Raman Spectroscopy of CuInSe2 Thin Films Prepared by Selenization. J. Phys. Chem. Solids 2003, 64, 1989–1993. [Google Scholar] [CrossRef]
- Rincon, C.; Ramirez, F.J. Lattice-Vibrations of CuInSe2 and CuGaSe2 by Raman Microspectrometry. J. Appl. Phys. 1992, 72, 4321–4324. [Google Scholar] [CrossRef]
- Xiong, Q.H.; Wang, J.G.; Reese, O.; Voon, L.C.L.Y.; Eklund, P.C. Raman Scattering from Surface Phonons in Rectangular Cross-Sectional W-ZnS Nanowires. Nano Lett. 2004, 4, 1991–1996. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Jin, C.Q.; Gao, F.; Wu, X.L.; Zhong, W.; Li, S.H.; Chu, P.K. Raman Scattering Study of Zinc Blende and Wurtzite ZnS. J. Appl. Phys. 2009, 106, 123505. [Google Scholar] [CrossRef]
- Kim, J.H.; Rho, H.; Kim, J.; Choi, Y.J.; Park, J.G. Raman Spectroscopy of ZnS Nanostructures. J. Raman Spectrosc. 2012, 43, 906–910. [Google Scholar] [CrossRef]
- Dutková, E.; Daneu, N.; Lukáčová Bujňáková, Z.; Baláž, M.; Kováč, J.; Kováč, J., Jr.; Baláž, P. Mechanochemical Synthesis and Characterization of CuInS2/ZnS Nanocrystals. Molecules 2019, 24, 1031. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.W.; Yin, Z.Y.; Sim, D.H.; Zhang, H.; Ma, J.; Hng, H.H.; Yan, Q.Y. Growth of Dandelion-Shaped CuInSe2 Nanostructures by a Two-Step Solvothermal Process. Nanotechnology 2011, 22, 195607. [Google Scholar] [CrossRef]
- Dong, J.; Zeng, X.H.; Xia, W.W.; Zhang, X.Y.; Zhou, M.; Wang, C.X. Ferromagnetic Behavior of Non-Stoichiometric ZnS Microspheres with a Nanoplate-Netted Surface. RSC Adv. 2017, 7, 20874–20881. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.-J. Surface Chemistry and Colloids, Advanced Pharmaceutics: Physico-Chemical Principles; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- De, S.; Mandal, S. Surfactant-Assisted Shape Control of Copper Nanostructures. Colloids Surf. A Physicochem. Eng. Asp. 2013, 421, 72–83. [Google Scholar] [CrossRef]
- Viana, R.B.; Da Silva, A.B.F.; Pimentel, A.S. Infrared Spectroscopy of Anionic, Cationic, and Zwitterionic Surfactants. Adv. Phys. Chem. 2012, 2012, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Chinmay, H.; Kundu, D.; Chaudharia, A.; Jana, T. Biogenic Synthesis, Characterization, Toxicity and Photocatalysis of Zinc Sulfide Nanoparticles Using Rhamnolipids from Pseudomonas Aeruginosa BS01 as Capping and Stabilizing Agent. J. Chem. Technol. Biotechnol. 2013, 88, 1039–1048. [Google Scholar]
- Xiaodong Gao, J.C. Adsorption of Sodium Dodecyl Sulfate (SDS) at ZnSe and α-Fe2O3 Surfaces: Combining Infrared Spectroscopy and Batch Uptake Studies. J. Colloid Interface Sci. 2010, 348, 167–176. [Google Scholar]
- Soomro, R.A.; Sirajuddin, S.; Memon, N.; Shah, M.R.; Kalwar, N.H.; Hallam, K.R.; Shah, A. Synthesis of Air Stable Copper Nanoparticles and their Use in Catalysis. Adv. Mat. Lett. 2014, 5, 191–198. [Google Scholar] [CrossRef]
- Lukáčová Bujňáková, Z.; Melnyk, I.; Dutková, E.; Varhač, R.; Jakubíková, J.; Cholujová, D.; Tóthová, E.; Storozhuk, L.; Briančin, J. Nano-Bio Interface Between As4S4 Nanoparticles and Albumin Influenced by Wet Stirred Media Milling. Small 2020. under review. [Google Scholar]



| Sample | ξ (mV) | pH |
|---|---|---|
| CuInSe2/ZnS 5:0-H2O | −19 | 7.00 |
| CuInSe2/ZnS 5:0-SDS | −41 | 7.96 |
| CuInSe2/ZnS 4:1-H2O | −6.2 | 6.43 |
| CuInSe2/ZnS 4:1-SDS | −39 | 7.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutková, E.; Bujňáková, Z.L.; Sphotyuk, O.; Jakubíková, J.; Cholujová, D.; Šišková, V.; Daneu, N.; Baláž, M.; Kováč, J.; Kováč, J., Jr.; et al. SDS-Stabilized CuInSe2/ZnS Multinanocomposites Prepared by Mechanochemical Synthesis for Advanced Biomedical Application. Nanomaterials 2021, 11, 69. https://doi.org/10.3390/nano11010069
Dutková E, Bujňáková ZL, Sphotyuk O, Jakubíková J, Cholujová D, Šišková V, Daneu N, Baláž M, Kováč J, Kováč J Jr., et al. SDS-Stabilized CuInSe2/ZnS Multinanocomposites Prepared by Mechanochemical Synthesis for Advanced Biomedical Application. Nanomaterials. 2021; 11(1):69. https://doi.org/10.3390/nano11010069
Chicago/Turabian StyleDutková, Erika, Zdenka Lukáčová Bujňáková, Oleh Sphotyuk, Jana Jakubíková, Danka Cholujová, Viera Šišková, Nina Daneu, Matej Baláž, Jaroslav Kováč, Jaroslav Kováč, Jr., and et al. 2021. "SDS-Stabilized CuInSe2/ZnS Multinanocomposites Prepared by Mechanochemical Synthesis for Advanced Biomedical Application" Nanomaterials 11, no. 1: 69. https://doi.org/10.3390/nano11010069
APA StyleDutková, E., Bujňáková, Z. L., Sphotyuk, O., Jakubíková, J., Cholujová, D., Šišková, V., Daneu, N., Baláž, M., Kováč, J., Kováč, J., Jr., Briančin, J., & Demchenko, P. (2021). SDS-Stabilized CuInSe2/ZnS Multinanocomposites Prepared by Mechanochemical Synthesis for Advanced Biomedical Application. Nanomaterials, 11(1), 69. https://doi.org/10.3390/nano11010069







