Electrochemical Oxidation of Ti15Mo Alloy—The Impact of Anodization Parameters on Surface Morphology of Nanostructured Oxide Layers
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrochemical Oxidation of Ti15Mo Alloy
2.2. Crystalline Structure of Anodized Oxide Layers
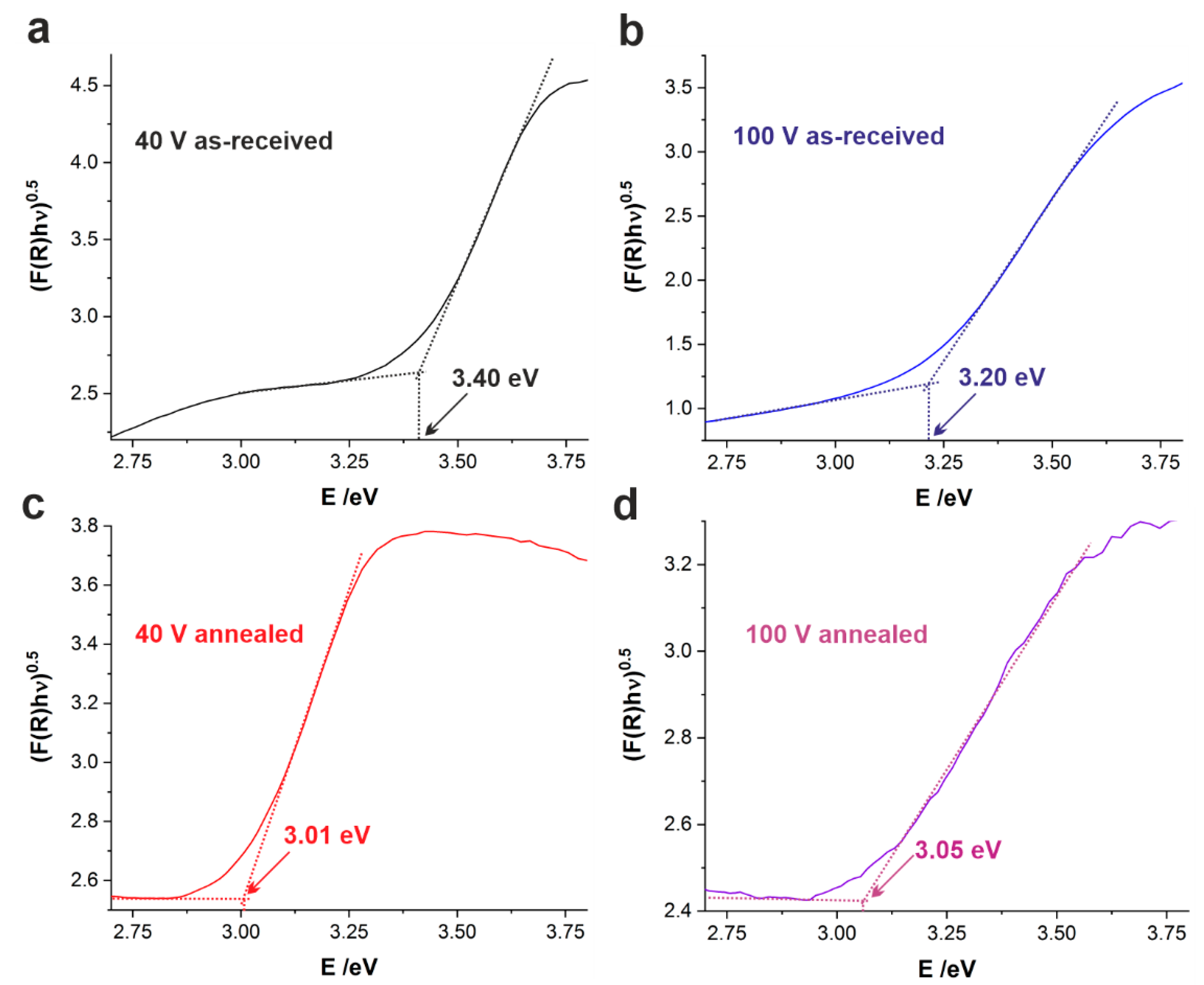
2.3. UV–Vis Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santhosh, R.; Geetha, M.; Rao, M.N. Recent Developments in Heat Treatment of Beta Titanium Alloys for Aerospace Applications. Trans. Indian Inst. Met. 2017, 70, 1681–1688. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.; Hu, J. Recent advances in the development of aerospace materials. Prog. Aerosp. Sci. 2018, 97, 22–34. [Google Scholar] [CrossRef]
- Gao, A.; Hang, R.; Bai, L.; Tang, B.; Chu, P.K. Electrochemical surface engineering of titanium-based alloys for biomedical application. Electrochim. Acta 2018, 271, 699–718. [Google Scholar] [CrossRef]
- Zhang, L.-C.; Chen, L.-Y. A Review on Biomedical Titanium Alloys: Recent Progress and Prospect. Adv. Eng. Mater. 2019, 21, 1–29. [Google Scholar] [CrossRef]
- Hang, R.; Zhao, F.; Yao, X.; Tang, B.; Chu, P.K. Self-assembled anodization of NiTi alloys for biomedical applications. Appl. Surf. Sci. 2020, 517, 146118. [Google Scholar] [CrossRef]
- İzmir, M.; Ercan, B. Anodization of titanium alloys for orthopedic applications. Front. Chem. Sci. Eng. 2019, 13, 28–45. [Google Scholar] [CrossRef]
- Ghicov, A.; Schmuki, P. Self-ordering electrochemistry: A review on growth and functionality of TiO2 nanotubes and other self-aligned MOx structures. Chem. Commun. 2009, 2009, 2791–2808. [Google Scholar] [CrossRef]
- Sulka, G.D.; Kapusta-Kołodziej, J.; Brzózka, A.; Jaskuła, M. Fabrication of nanoporous TiO2 by electrochemical anodization. Electrochim. Acta 2010, 55, 4359–4367. [Google Scholar] [CrossRef]
- Sulka, G.D. Highly Ordered Anodic Porous Alumina Formation by Self-Organized Anodizing. In Nanostructured Materials in Electrochemistry; Wiley-VCH: Weinheim, Germany, 2008; pp. 1–116. [Google Scholar]
- Altomare, M.; Pfoch, O.; Tighineanu, A.; Kirchgeorg, R.; Lee, K.; Selli, E.; Schmuki, P. Molten o-H3PO4: A New Electrolyte for the Anodic Synthesis of Self-Organized Oxide Structures—WO3 Nanochannel Layers and Others. J. Am. Chem. Soc. 2015, 137, 5646–5649. [Google Scholar] [CrossRef]
- Shin, H.-C.; Dong, J.; Liu, M. Porous Tin Oxides Prepared Using an Anodic Oxidation Process. Adv. Mater. 2004, 16, 237–240. [Google Scholar] [CrossRef]
- Mika, K.; Socha, R.P.; Nyga, P.; Wiercigroch, E.; Malek, K.; Jarosz, M.; Uchacz, T.; Sulka, G.D.; Zaraska, L. Electrochemical synthesis and characterization of dark nanoporous zinc oxide films. Electrochim. Acta 2019, 305, 349–359. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; You, S.; Hu, Y. One-step electrosynthesis of visible light responsive double-walled alloy titanium dioxide nanotube arrays for use in photocatalytic degradation of dibutyl phthalate. RSC Adv. 2020, 10, 21238–21247. [Google Scholar] [CrossRef]
- Babilas, D.; Służalska, K.; Krząkała, A.; Maciej, A.; Socha, R.P.; Dercz, G.; Tylko, G.; Michalska, J.; Osyczka, A.M.; Simka, W. Plasma electrolytic oxidation of a Ti–15Mo alloy in silicate solutions. Mater. Lett. 2013, 100, 252–256. [Google Scholar] [CrossRef]
- Bolat, G.; Mareci, D.; Chelariu, R.; Izquierdo, J.M.; González, S.; Souto, R. Investigation of the electrochemical behaviour of TiMo alloys in simulated physiological solutions. Electrochim. Acta 2013, 113, 470–480. [Google Scholar] [CrossRef]
- Bouchemel, H.; Benchettara, A. Corrosion Behavior of a New Ti–3Mo Alloy in Simulated Body Fluid for Biomedical Applications. Arab. J. Sci. Eng. 2013, 39, 139–146. [Google Scholar] [CrossRef]
- Chaves, J.; Escada, A.L.; Rodrigues, A.D.G.; Claro, A.P.R.A. Characterization of the structure, thermal stability and wettability of the TiO2 nanotubes growth on the Ti-7.5Mo alloy surface. Appl. Surf. Sci. 2016, 370, 76–82. [Google Scholar] [CrossRef]
- Escada, A.L.; Nakazato, R.Z.; Claro, A.P.R.A. Influence of Anodization Parameters in the TiO2 Nanotubes Formation on Ti-7.5Mo Alloy Surface for Biomedical Application. Mater. Res. 2017, 20, 1282–1290. [Google Scholar] [CrossRef]
- Mareci, D.; Dragoi, E.-N.; Bolat, G.; Chelariu, R.; Gordin, D.; Curteanu, S. Modelling the influence of pH, fluoride, and caffeine on the corrosion resistance of TiMo alloys by artificial neural networks developed with differential evolution algorithm. Mater. Corros. 2015, 66, 982–994. [Google Scholar] [CrossRef]
- Oliveira, N.; Guastaldi, A.; Piazza, S.; Sunseri, C. Photo-electrochemical investigation of anodic oxide films on cast Ti–Mo alloys. I. Anodic behaviour and effect of alloy composition. Electrochim. Acta 2009, 54, 1395–1402. [Google Scholar] [CrossRef]
- Simka, W.; Krząkała, A.; Korotin, D.M.; Zhidkov, I.S.; Kurmaev, E.; Cholakh, S.O.; Kuna, K.; Dercz, G.; Michalska, J.; Suchanek, K.; et al. Modification of a Ti–Mo alloy surface via plasma electrolytic oxidation in a solution containing calcium and phosphorus. Electrochim. Acta 2013, 96, 180–190. [Google Scholar] [CrossRef]
- Park, J.; Bauer, S.; Von Der Mark, K.; Schmuki, P. Nanosize and Vitality: TiO2 Nanotube Diameter Directs Cell Fate. Nano Lett. 2007, 7, 1686–1691. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Liu, Y.; Zhang, P.; Zhang, X.; Liu, J.; Chen, T.; Su, P.; Li, H.; Zhou, Y. The nanoscale geometry of TiO2 nanotubes influences the osteogenic differentiation of human adipose-derived stem cells by modulating H3K4 trimethylation. Biomaterials 2015, 39, 193–205. [Google Scholar] [CrossRef]
- Zollfrank, C.; Gutbrod, K.; Wechsler, P.; Guggenbichler, J.P. Antimicrobial activity of transition metal acid MoO3 prevents microbial growth on material surfaces. Mater. Sci. Eng. C 2012, 32, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Piçarra, S.; Lopes, E.; Almeida, P.L.; De Lencastre, H.; Aires-De-Sousa, M. Novel coating containing molybdenum oxide nanoparticles to reduce Staphylococcus aureus contamination on inanimate surfaces. PLoS ONE 2019, 14, e0213151. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Luo, J.; Li, Z.; Yin, Y.; Jie, S. Enhanced Photoelectrochemical Properties from Mo-Doped TiO2 Nanotube Arrays Film. Coatings 2020, 10, 75. [Google Scholar] [CrossRef]
- Zhou, J.; Feng, B.; Lu, X.; Duan, K. Novel one-step fabrication of highly ordered Mo-doped TiO2 nanotubes arrays with enhanced visible light catalytic activity. J. Mater. Sci. Mater. Electron. 2018, 29, 18388–18396. [Google Scholar] [CrossRef]
- Shrestha, N.K.; Nah, Y.-C.; Tsuchiya, H.; Schmuki, P. Self-organized nano-tubes of TiO2–MoO3 with enhanced electrochromic properties. Chem. Commun. 2009, 2009, 2008–2010. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, L.; Jin, B.; Huang, L.; Gan, Y. Enhanced photoelectrochemical properties and water splitting activity of self-ordered MoO3-TiO2 nanotubes. Appl. Surf. Sci. 2016, 364, 410–415. [Google Scholar] [CrossRef]
- Hsu, H.-C.; Hsu, S.-K.; Wu, S.-C.; Hung, Y.-H.; Ho, W.-F. Surface modification of nanotubular anodized Ti–7.5Mo alloy using NaOH treatment for biomedical application. Thin Solid Films 2020, 710, 138273. [Google Scholar] [CrossRef]
- Chen, P.C.; Hsieh, S.-J.; Chen, C.C.; Zou, J. The Microstructure and Capacitance Characterizations of Anodic Titanium Based Alloy Oxide Nanotube. J. Nanomater. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Oliveira, N.T.; Verdério, J.F.; Bolfarini, C. Obtaining self-organized nanotubes on biomedical Ti–Mo alloys. Electrochem. Commun. 2013, 35, 139–141. [Google Scholar] [CrossRef]
- Rangel, A.L.; Chaves, J.A.M.; Escada, A.L.; Konatu, R.T.; Popat, K.C.; Claro, A.P.R.A. Modification of the Ti15Mo alloy surface through TiO2 nanotube growth—An in vitro study. J. Appl. Biomater. Funct. Mater. 2018, 16, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Paramasivam, I.; Shrestha, N.K.; Schmuki, P. MoO3 in Self-Organized TiO2 Nanotubes for Enhanced Photocatalytic Activity. Chem. Asian J. 2010, 5, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Macak, J.M.; Schmuki, P. Flexible self-organization of two size-scales oxide nanotubes on Ti45Nb alloy. Electrochem. Commun. 2007, 9, 2403–2407. [Google Scholar] [CrossRef]
- Byeon, I.-S.; Hwang, I.-J.; Choe, H.-C.; Brantley, W.A. Electrochemically-coated hydroxyapatite films on nanotubular Ti Nb alloys prepared in solutions containing Ca, P, and Zn ions. Thin Solid Films 2016, 620, 132–138. [Google Scholar] [CrossRef]
- Kim, W.-G.; Choe, H.-C. Nanostructure and corrosion behaviors of nanotube formed Ti-Zr alloy. Trans. Nonferrous Met. Soc. China 2009, 19, 1005–1008. [Google Scholar] [CrossRef]
- Minagar, S.; Li, Y.; Berndt, C.C.; Wen, C. The influence of titania–zirconia–zirconium titanate nanotube characteristics on osteoblast cell adhesion. Acta Biomater. 2015, 12, 281–289. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Akaki, T.; Nakata, J.; Terada, D.; Tsuji, N.; Koizumi, Y.; Minamino, Y.; Schmuki, P.; Fujimoto, S. Anodic oxide nanotube layers on Ti–Ta alloys: Substrate composition, microstructure and self-organization on two-size scales. Corros. Sci. 2009, 51, 1528–1533. [Google Scholar] [CrossRef]
- Kapusta-Kołodziej, J.; Syrek, K.; Pawlik, A.; Jarosz, M.; Tynkevych, O.; Sulka, G.D. Effects of anodizing potential and temperature on the growth of anodic TiO2 and its photoelectrochemical properties. Appl. Surf. Sci. 2017, 396, 1119–1129. [Google Scholar] [CrossRef]
- Suhadolnik, L.; Marinko, Ž.; Ponikvar-Svet, M.; Tavčar, G.; Kovač, J.; Čeh, M. Influence of Anodization-Electrolyte Aging on the Photocatalytic Activity of TiO2 Nanotube Arrays. J. Phys. Chem. C 2020, 124, 4073–4080. [Google Scholar] [CrossRef]
- Babilas, D.; Urbańczyk, E.; Sowa, M.; Maciej, A.; Korotin, D.M.; Zhidkov, I.; Basiaga, M.; Krok-Borkowicz, M.; Szyk-Warszyńska, L.; Pamuła, E.; et al. On the electropolishing and anodic oxidation of Ti-15Mo alloy. Electrochim. Acta 2016, 205, 256–265. [Google Scholar] [CrossRef]
- Horcas, I.; Fernández, R.; Gómez-Rodríguez, J.M.; Colchero, J.; Gómez-Herrero, J.; Baro, A.M. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, M.; Syrek, K.; Kapusta-Kołodziej, J.; Mech, J.; Małek, K.; Hnida, K.; Łojewski, T.; Jaskuła, M.; Sulka, G.D. Heat Treatment Effect on Crystalline Structure and Photoelectrochemical Properties of Anodic TiO2 Nanotube Arrays Formed in Ethylene Glycol and Glycerol Based Electrolytes. J. Phys. Chem. C 2015, 119, 24182–24191. [Google Scholar] [CrossRef]
- Gates-Rector, S.; Blanton, T.N. The Powder Diffraction File: A quality materials characterization database. Powder Diffr. 2019, 34, 352–360. [Google Scholar] [CrossRef]
- Degen, T.; Sadki, M.; Bron, E.; König, U.; Nénert, G. The HighScore suite. Powder Diffr. 2014, 29, S13–S18. [Google Scholar] [CrossRef]
- Oliveira, N.T.; Aleixo, G.; Caram, R.; Guastaldi, A.C. Development of Ti–Mo alloys for biomedical applications: Microstructure and electrochemical characterization. Mater. Sci. Eng. A 2007, 452, 727–731. [Google Scholar] [CrossRef]
- Sulka, G.D.; Kapusta-Kołodziej, J.; Brzózka, A.; Jaskuła, M. Anodic growth of TiO2 nanopore arrays at various temperatures. Electrochim. Acta 2013, 104, 526–535. [Google Scholar] [CrossRef]
- Zaraska, L.; Bobruk, M.; Jaskuła, M.; Sulka, G.D. Growth and complex characterization of nanoporous oxide layers on metallic tin during one-step anodic oxidation in oxalic acid at room temperature. Appl. Surf. Sci. 2015, 351, 1034–1042. [Google Scholar] [CrossRef]
- Zaraska, L.; Czopik, N.; Bobruk, M.; Sulka, G.D.; Mech, J.; Jaskuła, M. Synthesis of nanoporous tin oxide layers by electrochemical anodization. Electrochim. Acta 2013, 104, 549–557. [Google Scholar] [CrossRef]
- Yahia, S.A.A.; Hamadou, L.; Kadri, A.; Benbrahim, N.; Sutter, E.M.M. Effect of Anodizing Potential on the Formation and EIS Characteristics of TiO2 Nanotube Arrays. J. Electrochem. Soc. 2012, 159, K83–K92. [Google Scholar] [CrossRef]
- Xing, J.; Lu, S.; Zhang, C.; Yin, M.; Li, D.; Song, Y. Determination of the field strength and realization of the high-field anodization of aluminum. Phys. Chem. Chem. Phys. 2017, 19, 21696–21706. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Li, C.; Yang, Y.; Xu, S.; Zhang, K.; Cui, H.; Zhu, X. Cavities between the double walls of nanotubes: Evidence of oxygen evolution beneath an anion-contaminated layer. Electrochem. Commun. 2018, 90, 34–38. [Google Scholar] [CrossRef]
- Yang, F.; Feng, X.; Ge, F.; Zhang, T.; Qi, J.; Li, D.; Zhu, X. Rapid growth of titanium oxide nanotubes under the critical breakdown voltage: Evidence against the dissolution reaction of fluoride ions. Electrochem. Commun. 2019, 103, 17–21. [Google Scholar] [CrossRef]
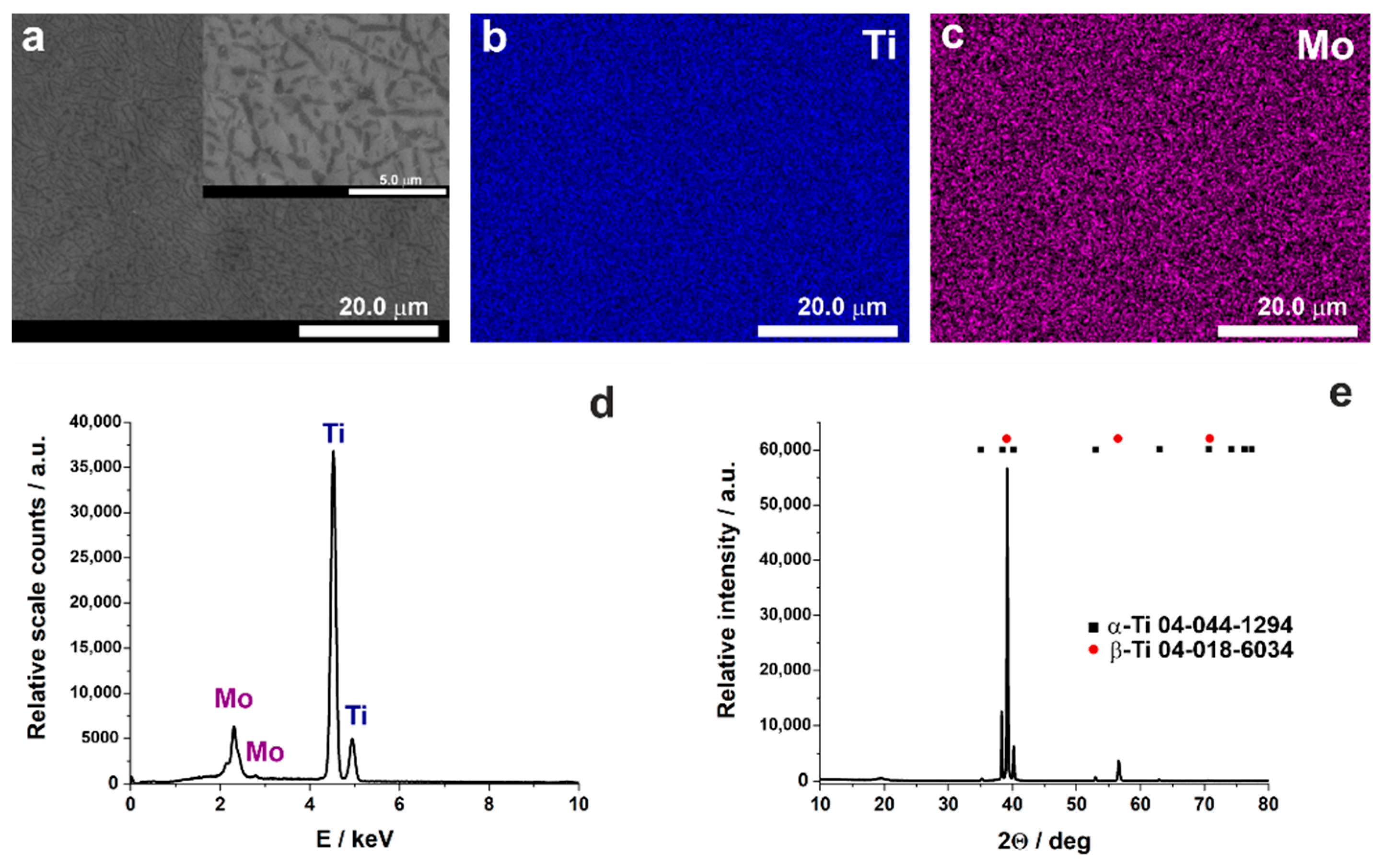
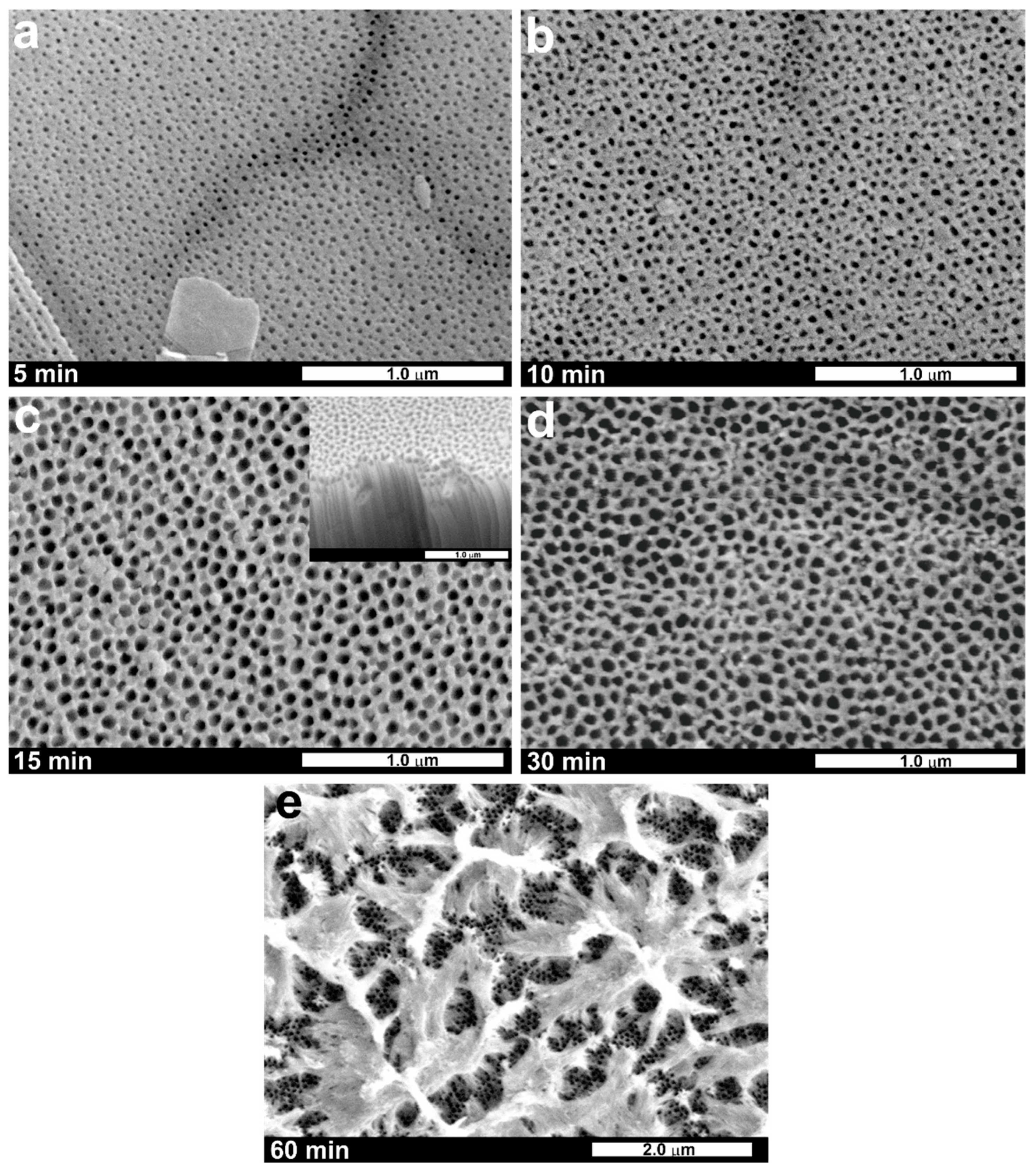

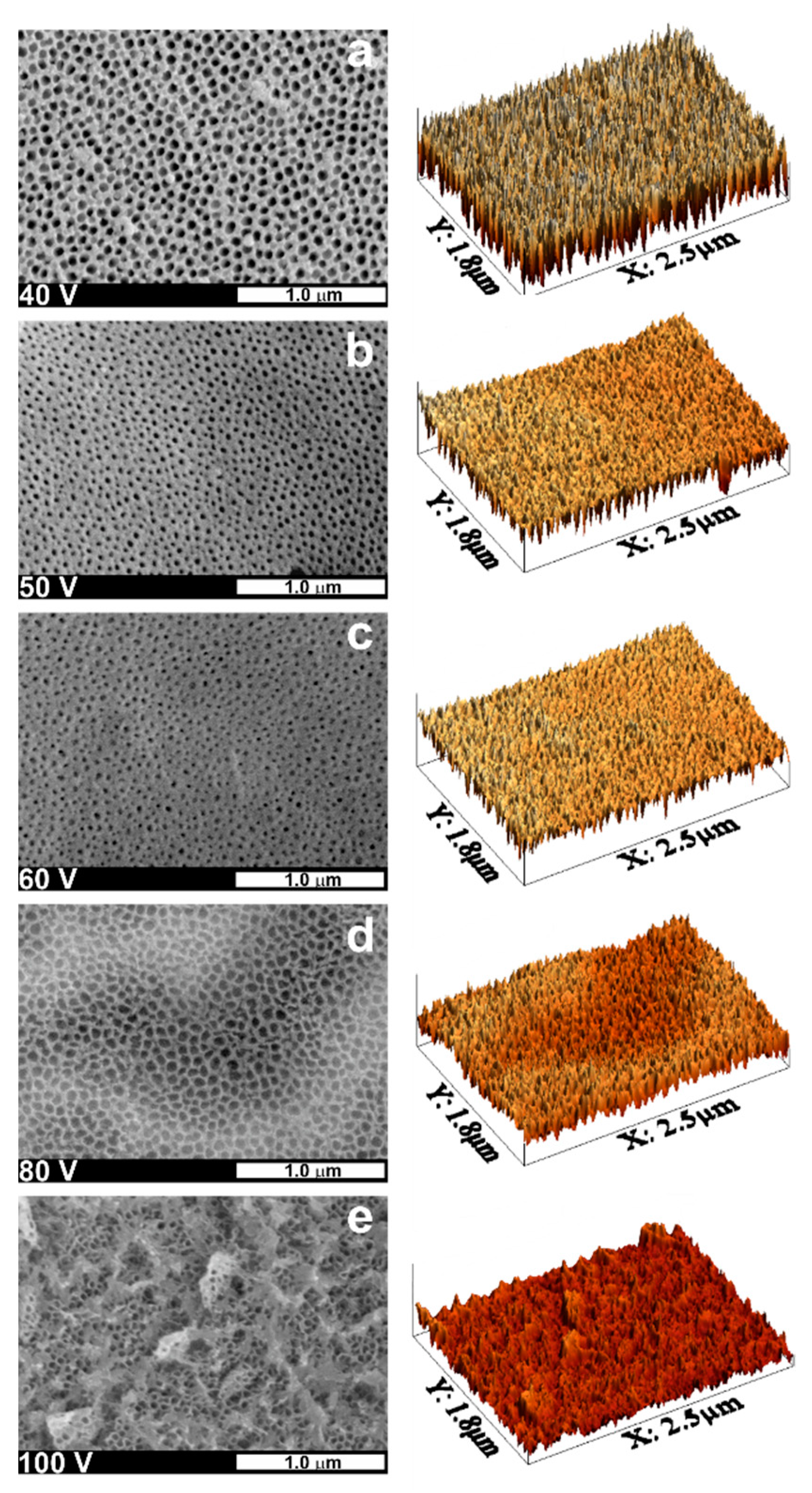
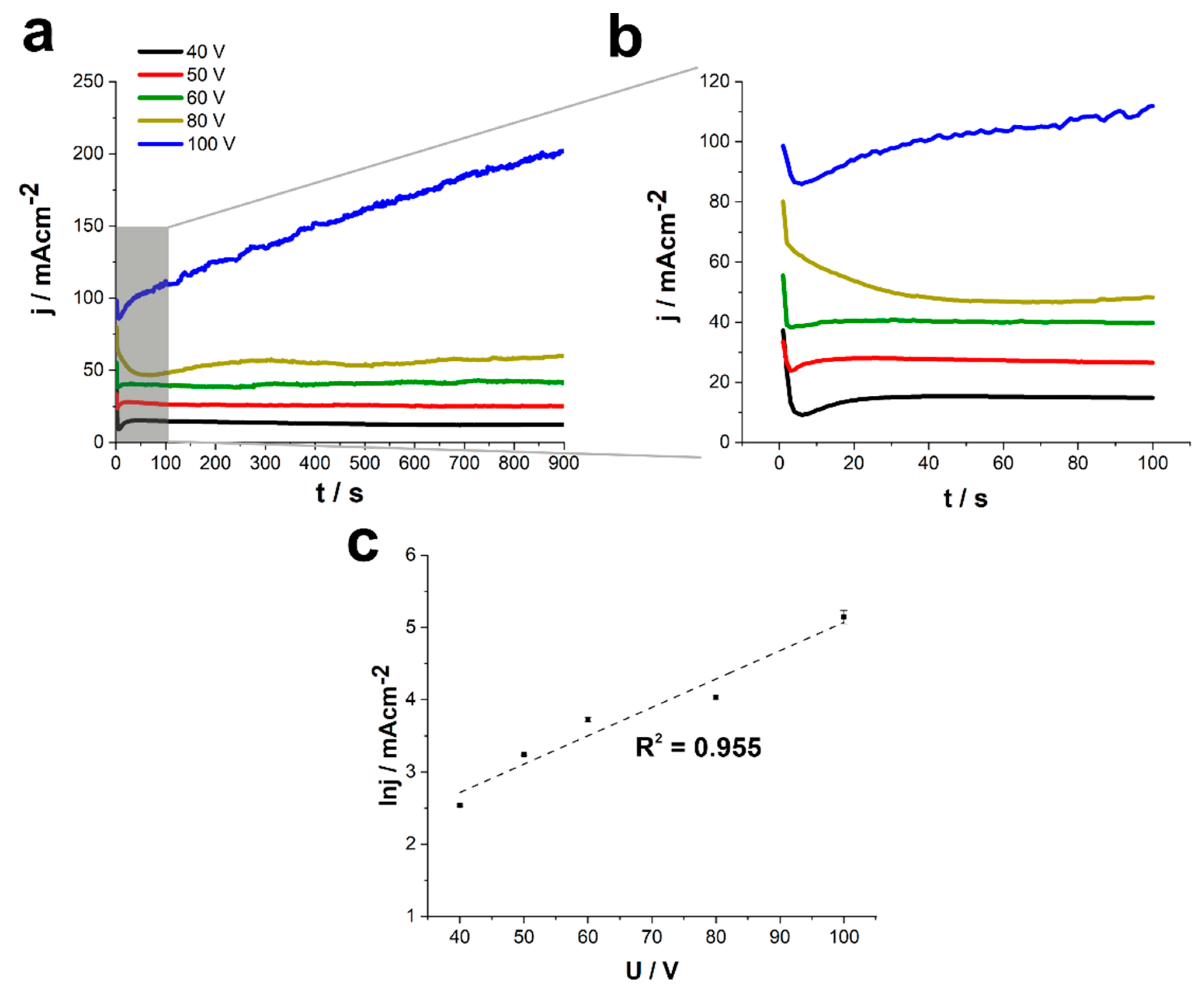
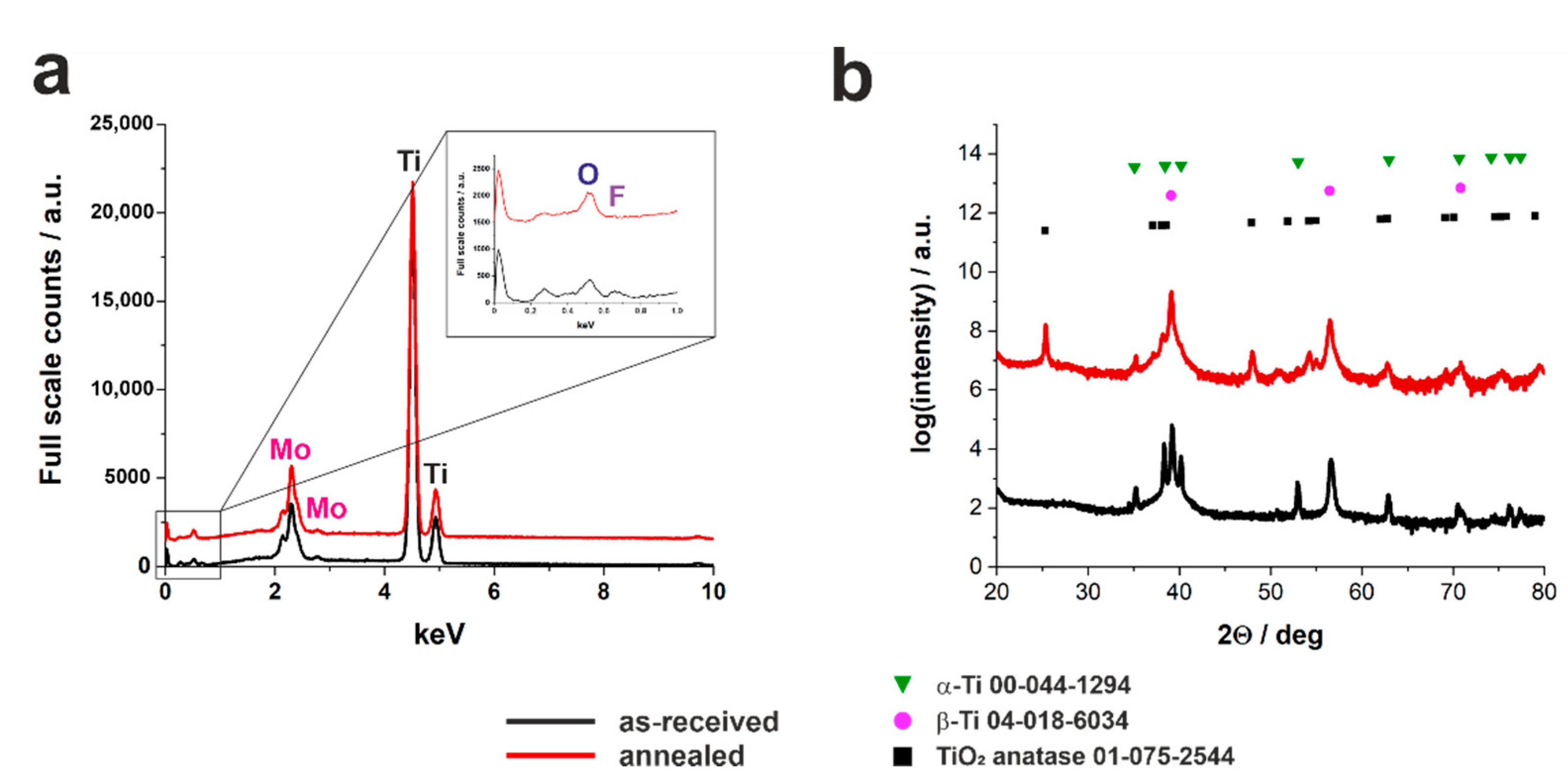
| Alloy | Electrolyte | Type of the Process | Applied Potential and Time | Oxide Layer Morphology | Ref. |
|---|---|---|---|---|---|
| Ti7Mo | 0.05 M HF in ethylene glycol (‘aged’ at 150 V for 20 h) | anodization in the three-electrode system (Pt and Ag/AgCl as counter and reference electrodes, respectively) | 20–60 V 3 h | nanotubes with a nanoporous layer on the top Dp = 15–45 nm | [28] |
| Ti7Mo | 0.18 M NH4F + 2 M H2O in ethylene glycol | anodization | 50 V 1 h | nanotubes with a nanoporous layer on the top Dp = 100 nm | [29] |
| Ti7.5Mo | 0.075 M NH4F in glycerol | anodization | 20 V and 30 V 48 h | nanotubes Dp = 80 nm (20 V) Dp = 100 nm (30 V) | [18] |
| Ti7.5Mo | 0.070 M NH4F + 0.55 M H2O in glycerol | anodization | 20 V 24 h | nanotubes Dp = 65 nm | [17] |
| Ti7.5Mo | 0.3 M NaCl with 0.14 M NH4F | anodization | 10 V 60 min | nanotubes Dp = 31–44 nm | [30] |
| Ti10Mo | 0.15 M NH4F + 1.25 M H2O in ethylene glycol | anodization | 60 V 2 h | nanotubes with a porous and net film on the top of NT | [31] |
| Ti15Mo | 0.5 M H2SO4; 1 M Na2SO4; 0.1 M NH4B5O8 | potentiodynamic anodization | 10–200 mV s−1 from the OCP value to 1–8 V vs. MSE | barrier-type anodic oxide with different oxide thickness | [20] |
| Ti6Mo Ti15Mo | 0.25 M NH4F in ethylene glycol | anodization | 20 or 60 V 6 h 40 V (2–6 h) | nanotubes Dp = 90 nm (for 40 V, 6 h) Dp = 100–120 nm (for 30 V) | [32] |
| Ti15Mo | 0.075 M NH4F in glycerol | anodization | 20 V 24 h | nanotubes Dp = ~65 nm | [33] |
| Ti7Mo, Ti20Mo, Ti35Mo | 0.05 M HF in ethylene glycol (electrolyte was ‘aged’ at 150 V for 20 h) | anodization | 50 V 35 min, 2 h or 3 h | Ti7Mo–nanotubes Ti20Mo and Ti35Mo–porous oxide layers | [34] |
| Ti15Mo | 0.11 M NH4F + 1.11 M H2O in ethylene glycol | anodization | 40–100 V 5–60 min | nanoporous oxide layers | this work |
| Potential (U)/V Time (t)/min | 40 | 50 | 60 | 80 | 100 |
|---|---|---|---|---|---|
| 5 | √ | ||||
| 10 | √ | ||||
| 15 | √ | √ | √ | √ | √ |
| 30 | √ | ||||
| 60 | √ |
| U [V] | Eg [eV] | |
|---|---|---|
| As-Received | Annealed | |
| 40 | 3.45 ± 0.05 | 3.05 ± 0.10 |
| 50 | 3.28 ± 0.06 | 3.09 ± 0.10 |
| 60 | 3.21 ± 0.03 | 3.08 ± 0.10 |
| 80 | 3.19 ± 0.04 | 2.95 ± 0.10 |
| 100 | 3.19 ± 0.02 | 3.04 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarosz, M.; Zaraska, L.; Kozieł, M.; Simka, W.; Sulka, G.D. Electrochemical Oxidation of Ti15Mo Alloy—The Impact of Anodization Parameters on Surface Morphology of Nanostructured Oxide Layers. Nanomaterials 2021, 11, 68. https://doi.org/10.3390/nano11010068
Jarosz M, Zaraska L, Kozieł M, Simka W, Sulka GD. Electrochemical Oxidation of Ti15Mo Alloy—The Impact of Anodization Parameters on Surface Morphology of Nanostructured Oxide Layers. Nanomaterials. 2021; 11(1):68. https://doi.org/10.3390/nano11010068
Chicago/Turabian StyleJarosz, Magdalena, Leszek Zaraska, Marcin Kozieł, Wojciech Simka, and Grzegorz D. Sulka. 2021. "Electrochemical Oxidation of Ti15Mo Alloy—The Impact of Anodization Parameters on Surface Morphology of Nanostructured Oxide Layers" Nanomaterials 11, no. 1: 68. https://doi.org/10.3390/nano11010068
APA StyleJarosz, M., Zaraska, L., Kozieł, M., Simka, W., & Sulka, G. D. (2021). Electrochemical Oxidation of Ti15Mo Alloy—The Impact of Anodization Parameters on Surface Morphology of Nanostructured Oxide Layers. Nanomaterials, 11(1), 68. https://doi.org/10.3390/nano11010068







