Dendritic Polymers as Promising Additives for the Manufacturing of Hybrid Organoceramic Nanocomposites with Ameliorated Properties Suitable for an Extensive Diversity of Applications
Abstract
1. Introduction
2. Methods of Preparation
2.1. Simple Coating by Absorption of Dendritic Additives into the Pores of the Ceramic
2.2. Attachment of Polymers with Covalent Bonds to the Surface of the Ceramic Substrate
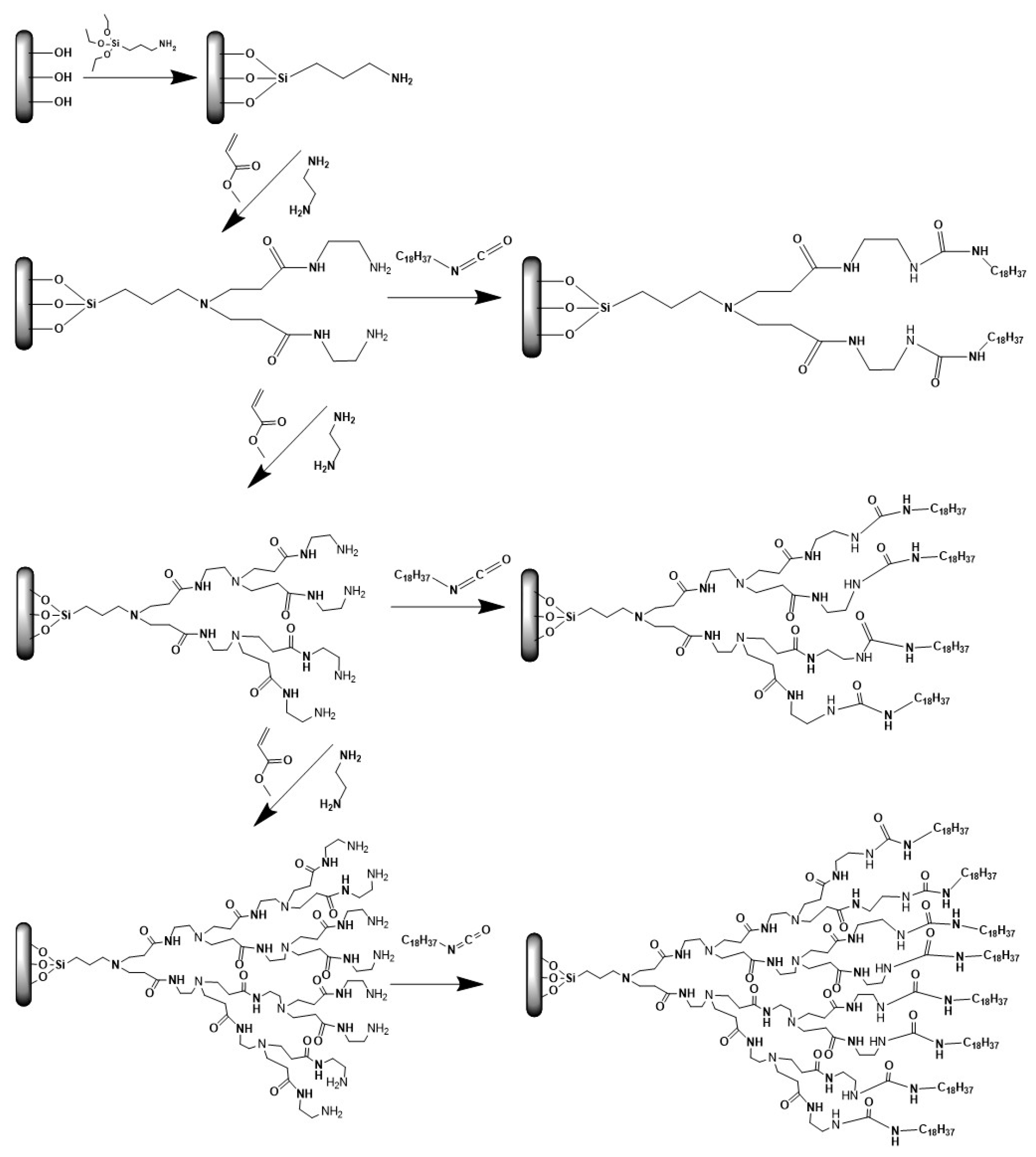
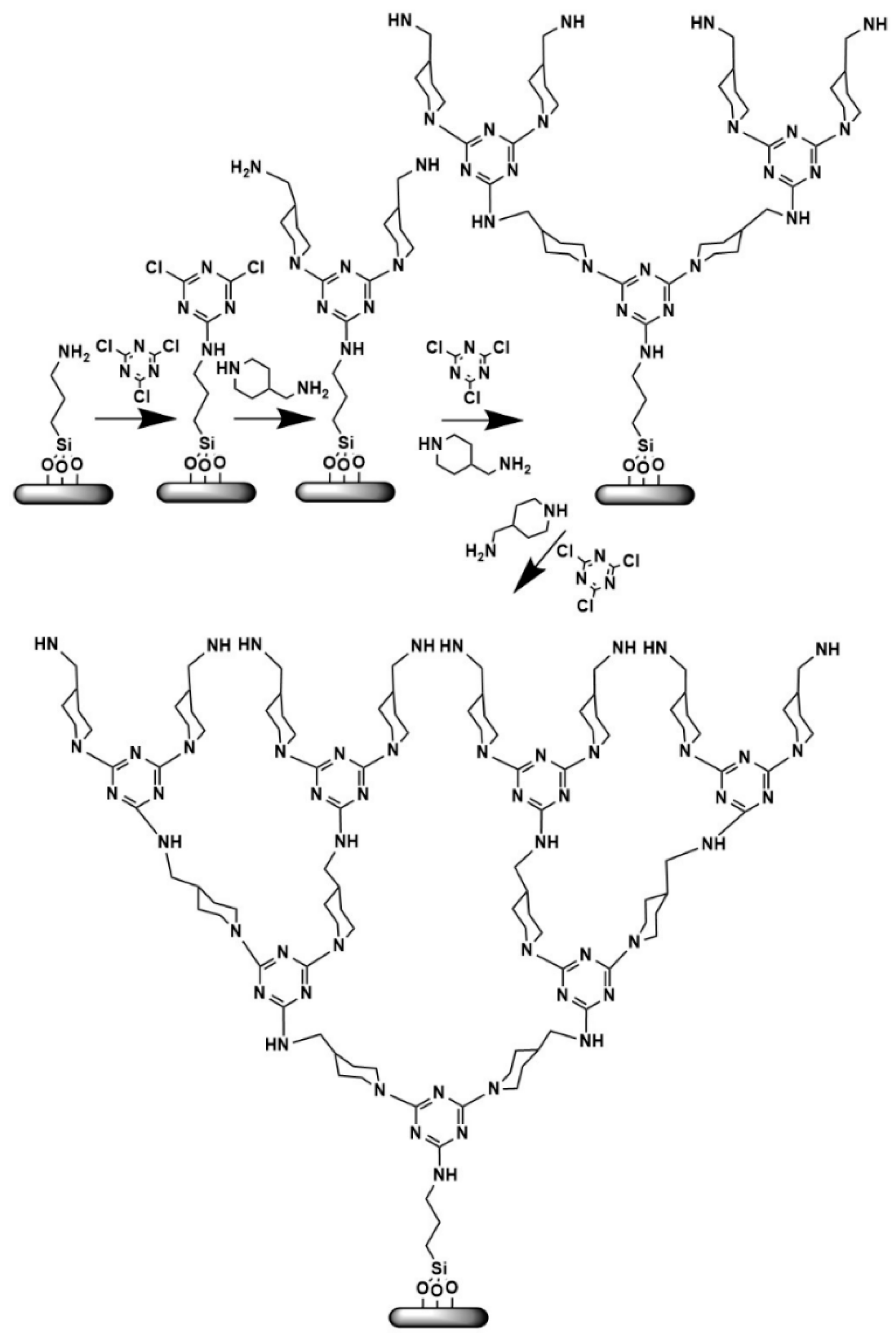
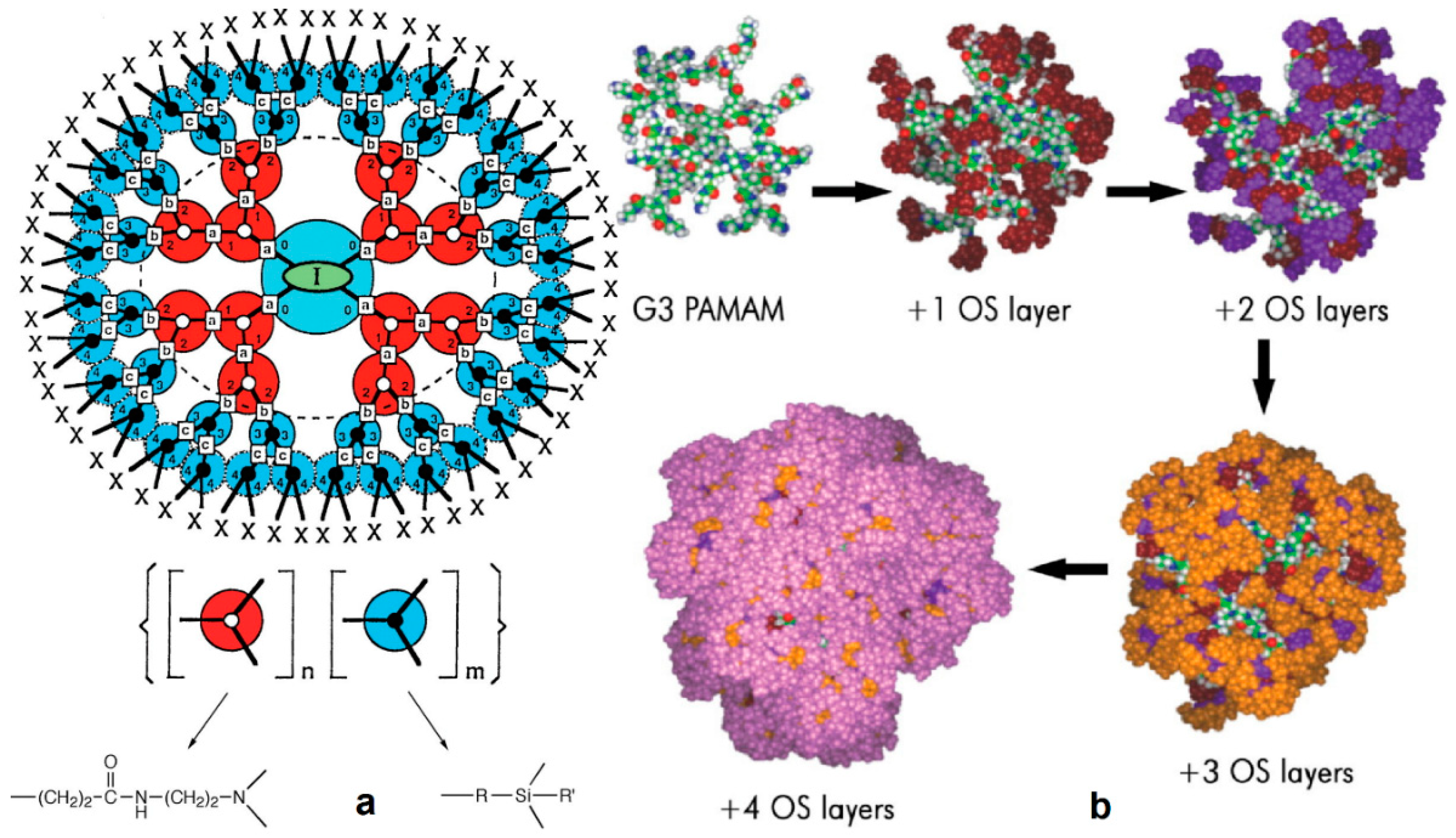
2.3. Direct Growth of Dendritic Polymers on the Surface of Ceramics
2.4. Sol-Gel Cross-Linking of Silyl Dendritic Polymers
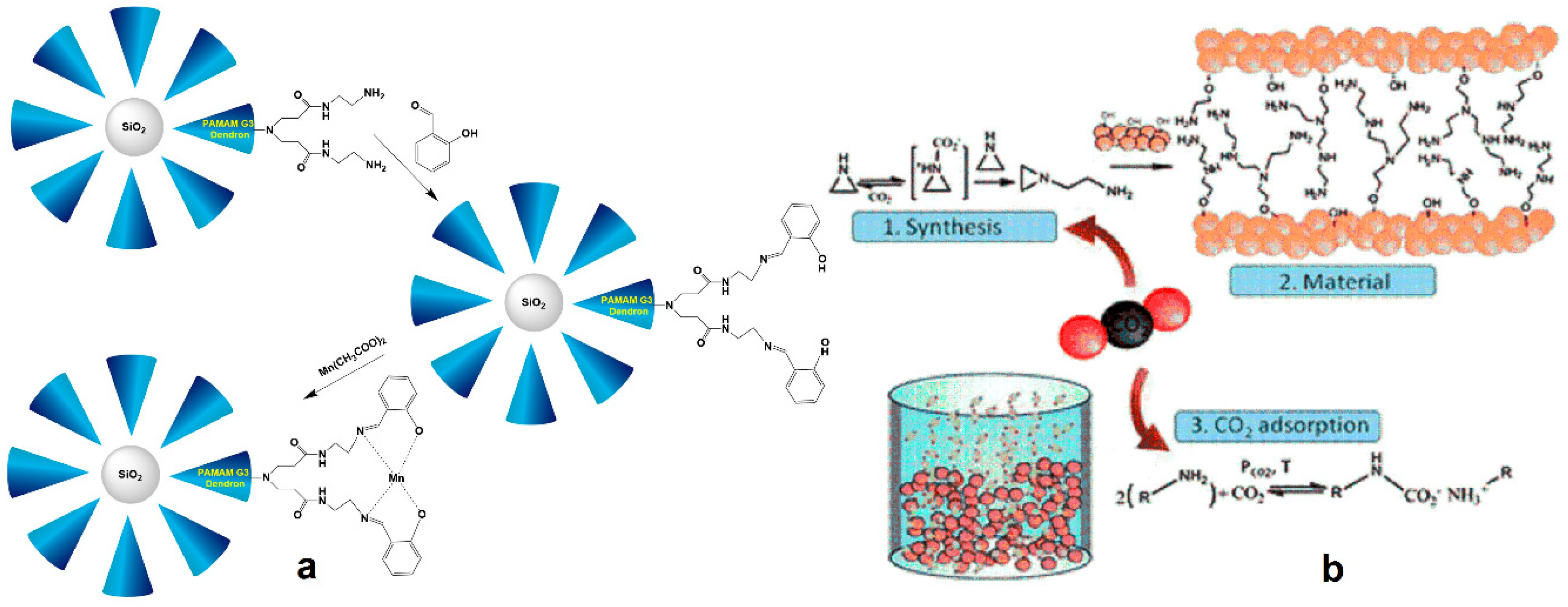
2.5. Biomimetic Reactions
3. Improvement Strategies for Performance Optimization
4. Concluding Remarks—Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Camargo, P.H.C.; Satyanarayana, K.G.; Wypych, F. Nanocomposites: Synthesis, structure, properties and new application opportunities. Mater. Res. 2009, 12, 1–39. [Google Scholar] [CrossRef]
- Parveen, S.; Misra, R.; Sahoo, S.K. Nanoparticles: A boon to drug delivery, therapeutics, diagnostics and imaging. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 147–166. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Niihara, K. New design concept of structural ceramics. Ceramic nanocomposites. J. Ceram. Soc. Jpn. Int. Ed. 1991, 99, 945–952. [Google Scholar] [CrossRef]
- Kiani, A.; Rahmani, M.; Sivakumar, M.; Tan, B. Nanoceramics: Synthesis, characterization, and applications. J. Nanomater. 2014, 2014. [Google Scholar] [CrossRef]
- Thomas, S.; Harshita, B.S.P.; Mishra, P.; Talegaonkar, S. Ceramic Nanoparticles: Fabrication Methods and Applications in Drug Delivery. Curr. Pharm. Des. 2015, 21, 6165–6188. [Google Scholar] [CrossRef]
- Meulenberg, W.A.; Schulze-Küppers, F.; Deibert, W.; Gestel, T.V.; Baumann, S. Ceramic Membranes: Materials–Components–Potential Applications. ChemBioEng Rev. 2019, 6, 198–208. [Google Scholar] [CrossRef]
- Benfer, S.; Árki, P.; Tomandl, G. Ceramic membranes for filtration applications—Preparation and characterization. Adv. Eng. Mater. 2004, 6, 495–500. [Google Scholar] [CrossRef]
- Acharya, M.; Foley, H.C. Spray-coating of nanoporous carbon membranes for air separation. J. Membr. Sci. 1999, 161, 1–5. [Google Scholar] [CrossRef]
- de Lange, R.S.A.; Keizer, K.; Burggraaf, A.J. Analysis and theory of gas transport in microporous sol-gel derived ceramic membranes. J. Membr. Sci. 1995, 104, 81–100. [Google Scholar] [CrossRef]
- Montanaro, L.; Saracco, G. Influence of some precursors on the physico-chemical characteristics of transition aluminas for the preparation of ceramic catalytic filters. Ceram. Int. 1995, 21, 43–49. [Google Scholar] [CrossRef]
- Bodzek, M.; Konieczny, K. Comparison of ceramic and capillary membranes in the treatment of natural water by means of ultrafiltration and microfiltration. Desalination 1998, 119, 191–197. [Google Scholar] [CrossRef]
- Bodzek, M.; Konieczny, K.; Rajca, M. Membranes in water and wastewater disinfection—Review. Arch. Environ. Prot. 2019, 45, 3–18. [Google Scholar] [CrossRef]
- Russo, C. A new membrane process for the selective fractionation and total recovery of polyphenols, water and organic substances from vegetation waters (VW). J. Membr. Sci. 2007, 288, 239–246. [Google Scholar] [CrossRef]
- Wang, P.; Xu, N.; Shi, J. A pilot study of the treatment of waste rolling emulsion using zirconia microfiltration membranes. J. Membr. Sci. 2000, 173, 159–166. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, G.; Xu, N.; Shi, J. Preparation and application in oil-water separation of ZrO2/α-Al2O3 MF membrane. J. Membr. Sci. 1998, 142, 235–243. [Google Scholar] [CrossRef]
- Nair, P.; Mizukami, F.; Okubo, T.; Nair, J.; Keizer, K.; Burggraaf, A.J. High-temperature catalyst supports and ceramic membranes: Metastability and particle packing. AIChE J. 1997, 43, 2710–2714. [Google Scholar] [CrossRef]
- Komolikov, Y.I.; Blaginina, L.A. Technology of ceramic ultrafiltration membranes. Ogneup. Tekhnicheskaya Keram. 2002, 43, 20–28. [Google Scholar]
- Koo, O.M.; Rubinstein, I.; Onyuksel, H. Role of nanotechnology in targeted drug delivery and imaging: A concise review. Nanomed. Nanotechnol. Biol. Med. 2005, 1, 193–212. [Google Scholar] [CrossRef]
- Moreno-Vega, A.I.; Gómez-Quintero, T.; Nuñez-Anita, R.E.; Acosta-Torres, L.S.; Castaño, V. Polymeric and ceramic nanoparticles in biomedical applications. J. Nanotechnol. 2012, 2012. [Google Scholar] [CrossRef]
- Manzano, M.; Vallet-Regí, M. Mesoporous Silica Nanoparticles for Drug Delivery. Adv. Funct. Mater. 2020, 30, 3–5. [Google Scholar] [CrossRef]
- Croissant, J.G.; Fatieiev, Y.; Almalik, A.; Khashab, N.M. Mesoporous Silica and Organosilica Nanoparticles: Physical Chemistry, Biosafety, Delivery Strategies, and Biomedical Applications. Adv. Healthc. Mater. 2018, 7, 1700831. [Google Scholar] [CrossRef] [PubMed]
- Castillo, R.R.; Vallet-Regí, M. Functional mesoporous silica nanocomposites: Biomedical applications and biosafety. Int. J. Mol. Sci. 2019, 20, 929. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Mu, Y.; Peng, C.; Lavin, M.F.; Shao, H.; Du, Z. Understanding the mechanisms of silica nanoparticles for nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 13, e1658. [Google Scholar] [CrossRef]
- Tang, F.; Li, L.; Chen, D. Mesoporous silica nanoparticles: Synthesis, biocompatibility and drug delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef]
- Croissant, J.G.; Fatieiev, Y.; Khashab, N.M. Degradability and Clearance of Silicon, Organosilica, Silsesquioxane, Silica Mixed Oxide, and Mesoporous Silica Nanoparticles. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Singh, D.; Singh, S.; Sahu, J.; Srivastava, S.; Singh, M.R. Ceramic nanoparticles: Recompense, cellular uptake and toxicity concerns. Artif. Cells Nanomed. Biotechnol. 2016, 44, 401–409. [Google Scholar] [CrossRef]
- Vakifahmetoglu, C.; Zeydanli, D.; Colombo, P. Porous polymer derived ceramics. Mater. Sci. Eng. R 2016, 106, 1–30. [Google Scholar] [CrossRef]
- Kitsou, I.; Panagopoulos, P.; Maggos, T.; Arkas, M.; Tsetsekou, A. Development of SiO2@TiO2 core-shell nanospheres for catalytic applications. Appl. Surf. Sci. 2018, 441, 223–231. [Google Scholar] [CrossRef]
- Tsiourvas, D.; Tsetsekou, A.; Arkas, M.; Diplas, S.; Mastrogianni, E. Covalent attachment of a bioactive hyperbranched polymeric layer to titanium surface for the biomimetic growth of calcium phosphates. J. Mater. Sci. Mater. Med. 2011, 22, 85–96. [Google Scholar] [CrossRef][Green Version]
- Vallet-Regí, M.; Ruiz-Hernández, E. Bioceramics: From bone regeneration to cancer nanomedicine. Adv. Mater. 2011, 23, 5177–5218. [Google Scholar] [CrossRef] [PubMed]
- El-Ghannam, A. Bone reconstruction: From bioceramics to tissue engineering. Expert Rev. Med. Devices 2005, 2, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Habraken, W.J.E.M.; Wolke, J.G.C.; Jansen, J.A. Ceramic composites as matrices and scaffolds for drug delivery in tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Salinas, A.J. Ceramics as Bone Repair Materials, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; ISBN 9780081024515. [Google Scholar]
- Tomalia, D.A.; Fréchet, J.M.J. Discovery of dendrimers and dendritic polymers: A brief historical perspective. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 2719–2728. [Google Scholar] [CrossRef]
- Fréchet, J.M.J.; Tomalia, D.A. (Eds.) Dendrimers and Other Dendritic Polymers; John Wiley & Sons, Ltd.: Chichester, UK, 2001; ISBN 9780470845820. [Google Scholar]
- Jaymand, M.; Lotfi, M.; Lotfi, R. Functional dendritic compounds: Potential prospective candidates for dental restorative materials and: In situ re-mineralization of human tooth enamel. RSC Adv. 2016, 6, 43127–43146. [Google Scholar] [CrossRef]
- Svenson, S.; Tomalia, D.A. Dendrimers in biomedical applications-reflections on the field. Adv. Drug Deliv. Rev. 2012, 64, 102–115. [Google Scholar] [CrossRef]
- Sheikhpour, M.; Barani, L.; Kasaeian, A. Biomimetics in drug delivery systems: A critical review. J. Control. Release 2017, 253, 97–109. [Google Scholar] [CrossRef]
- Leiro, V.; Moreno, P.M.; Sarmento, B.; Durão, J.; Gales, L.; Pêgo, A.P.; Barrias, C.C. Design and Preparation of Biomimetic and Bioinspired Materials; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; ISBN 9780081007464. [Google Scholar]
- Fruchon, S.; Poupot, R. Pro-inflammatory versus anti-inflammatory effects of dendrimers: The two faces of immuno-modulatory nanoparticles. Nanomaterials 2017, 7, 251. [Google Scholar] [CrossRef]
- Fan, Y.; Sun, W.; Shi, X. Design and Biomedical Applications of Poly(amidoamine)-Dendrimer-Based Hybrid Nanoarchitectures. Small Methods 2017, 1, 1700224. [Google Scholar] [CrossRef]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef]
- Le, N.T.T.; Nguyen, T.N.Q.; Cao, V.D.; Hoang, D.T.; Ngo, V.C.; Thi, T.T.H. Recent progress and advances of multi-stimuli-responsive dendrimers in drug delivery for cancer treatment. Pharmaceutics 2019, 11, 591. [Google Scholar] [CrossRef] [PubMed]
- Devnarain, N.; Osman, N.; Fasiku, V.O.; Makhathini, S.; Salih, M.; Ibrahim, U.H.; Govender, T. Intrinsic stimuli-responsive nanocarriers for smart drug delivery of antibacterial agents—An in-depth review of the last two decades. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 13, e1664. [Google Scholar] [CrossRef] [PubMed]
- Majumder, J.; Minko, T. Multifunctional and stimuli-responsive nanocarriers for targeted therapeutic delivery. Expert Opin. Drug Deliv. 2020, 1–23. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Hu, X.; Ali, D.M.; Wang, M.-H. Emerging Strategies in Stimuli-Responsive Nanocarriers as the Drug Delivery System for Enhanced Cancer Therapy. Curr. Pharm. Des. 2019, 25, 2609–2625. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Singh, A.; Pardhi, V.; Sunil Dubey, P.K. Dendrimer (polyamidoamine, polypropylene imine, poly-L-lysine, carbosilane dendrimers, triazine dendrimers) as promising tool for anticancer therapeutics. In Polymeric Nanoparticles as a Promising Tool for Anti-Cancer Therapeutics; Wolff, A.G., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; pp. 233–255. ISBN 9780128169636. [Google Scholar]
- Wang, B.Y.; Liao, M.L.; Hong, G.C.; Chang, W.W.; Chu, C.C. Near-Infrared-Triggered photodynamic therapy toward breast cancer cells using Dendrimer-Functionalized upconversion nanoparticles. Nanomaterials 2017, 7, 269. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi-Gahrouei, D.; Khaniabadi, P.; Khaniabadi, B.; Shahbazi-Gahrouei, S. Medical imaging modalities using nanoprobes for cancer diagnosis: A literature review on recent findings. J. Res. Med. Sci. 2019, 24, 38. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wang, S.H.; Van Antwerp, M.E.; Chen, X.; Baker, J.R. Targeting and detecting cancer cells using spontaneously formed multifunctional dendrimer-stabilized gold nanoparticles. Analyst 2009, 134, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sun, J.; Zhu, H.; Wu, H.; Zhang, H.; Gu, Z.; Luo, K. Recent advances in development of dendriticpolymer-based nanomedicines for cancer diagnosis. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, e1670. [Google Scholar] [CrossRef]
- El Kadib, A.; Katir, N.; Bousmina, M.; Majoral, J.P. Dendrimer-silica hybrid mesoporous materials. New J. Chem. 2012, 36, 241–255. [Google Scholar] [CrossRef]
- Gaikwad, A.V.; Boffa, V.; ten Elshof, J.E.; Rothenberg, G. Cat-in-a-Cup: Facile Separation of Large Homogeneous Catalysts. Angew. Chem. 2008, 120, 5487–5490. [Google Scholar] [CrossRef]
- Safari, J.; Zarnegar, Z.; Sadeghi, M.; Enayati-Najafabadi, A. Dendritic macromolecules supported Ag nanoparticles as efficient catalyst for the reduction of 4-nitrophenol. J. Mol. Struct. 2016, 1125, 772–776. [Google Scholar] [CrossRef]
- Arkas, M.; Tsiourvas, D.; Paleos, C.M. Organosilicon dendritic networks in porous ceramics for water purification. Chem. Mater. 2005, 17, 3439–3444. [Google Scholar] [CrossRef]
- Arkas, M.; Tsiourvas, D.; Paleos, C.M. Functional dendritic polymers for the development of hybrid materials for water purification. Macromol. Mater. Eng. 2010, 295, 883–898. [Google Scholar] [CrossRef]
- Arkas, M.; Allabashi, R.; Tsiourvas, D.; Mattausch, E.M.; Perfler, R. Organic/inorganic hybrid filters based on dendritic and cyclodextrin “nanosponges” for the removal of organic pollutants from water. Environ. Sci. Technol. 2006, 40, 2771–2777. [Google Scholar] [CrossRef]
- Allabashi, R.; Arkas, M.; Hörmann, G.; Tsiourvas, D. Removal of some organic pollutants in water employing ceramic membranes impregnated with cross-linked silylated dendritic and cyclodextrin polymers. Water Res. 2007, 41, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Qin, Y.; Zhang, H.; Shang, Y.; Sun, M.; Liu, B.; Li, Z. Electronic structure-transport property relationships of polyferrocenylene, polyferrocenylacetylene, and polyferrocenylsilane. J. Phys. Chem. C 2010, 114, 9469–9477. [Google Scholar] [CrossRef]
- Tsetsekou, A.; Arkas, M.; Kritikaki, A.; Simonetis, S.; Tsiourvas, D. Optimization of hybrid hyperbranched polymer/ceramic filters for the efficient absorption of polyaromatic hydrocarbons from water. J. Membr. Sci. 2008, 311, 128–135. [Google Scholar] [CrossRef]
- Zamponi, S.; Kijak, A.M.; Sommer, A.J.; Marassi, R.; Kulesza, P.J.; Cox, J.A. Electrochemistry of Prussian Blue in silica sol-gel electrolytes doped with polyamidoamine dendrimers. J. Solid State Electrochem. 2002, 6, 528–533. [Google Scholar] [CrossRef]
- Ainalem, M.L.; Campbell, R.A.; Nylander, T. Interactions between DNA and poly(amido amine) dendrimers on Silica surfaces. Langmuir 2010, 26, 8625–8635. [Google Scholar] [CrossRef]
- Xu, X.; Lü, S.; Gao, C.; Bai, X.; Feng, C.; Gao, N.; Liu, M. Multifunctional drug carriers comprised of mesoporous silica nanoparticles and polyamidoamine dendrimers based on layer-by-layer assembly. Mater. Des. 2015, 88, 1127–1133. [Google Scholar] [CrossRef]
- Díaz, I.; García, B.; Alonso, B.; Casado, C.M.; Morán, M.; Losada, J.; Pérez-Pariente, J. Ferrocenyl dendrimers incorporated into mesoporous silica: New hybrid redox-active materials. Chem. Mater. 2003, 15, 1073–1079. [Google Scholar] [CrossRef]
- Arkas, M.; Tsiourvas, D.; Paleos, C.M. Functional dendrimeric “Nanosponges” for the removal of polycyclic aromatic hydrocarbons from water. Chem. Mater. 2003, 15, 2844–2847. [Google Scholar] [CrossRef]
- Barakat, M.A.; Ramadan, M.H.; Alghamdi, M.A.; Algarny, S.S.; Woodcock, H.L.; Kuhn, J.N. Remediation of Cu(II), Ni(II), and Cr(III) ions from simulated wastewater by dendrimer/titania composites. J. Environ. Manag. 2013, 117, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Kang, D.J.; Blamire, M.G.; Huck, W.T.S. High-Resolution Contact Printing with Dendrimers. Nano Lett. 2002, 2, 347–349. [Google Scholar] [CrossRef]
- Esumi, K.; Goino, M. Adsorption of poly(amidoamine) dendrimers on alumina/water and silica/water interfaces. Langmuir 1998, 14, 4466–4470. [Google Scholar] [CrossRef]
- Tokarczyk, K.; Jachimska, B. Quantitative interpretation of PAMAM dendrimers adsorption on silica surface. J. Colloid Interface Sci. 2017, 503, 86–94. [Google Scholar] [CrossRef]
- Ottaviani, M.F.; Turro, N.J.; Jockusch, S.; Tomalia, D.A. EPR investigation of the adsorption of dendrimers on porous surfaces. J. Phys. Chem. B 2003, 107, 2046–2053. [Google Scholar] [CrossRef]
- Fu, S.; Zhu, M.; Zhu, Y. Organosilicon polymer-derived ceramics: An overview. J. Adv. Ceram. 2019, 8, 457–478. [Google Scholar] [CrossRef]
- Ottenbrite, R.M.; Yin, R.; Zengin, H.; Suzuki, K.; Siddiqui, J.A. Surface Modification of Silica Particles and Silica Glass Beads Polymer Surface Modification of Particles. Spec. Monomers Polym. 2000, 13, 170–183. [Google Scholar]
- González, B.; Colilla, M.; De Laorden, C.L.; Vallet-Regí, M. A novel synthetic strategy for covalently bonding dendrimers to ordered mesoporous silica: Potential drug delivery applications. J. Mater. Chem. 2009, 19, 9012–9024. [Google Scholar] [CrossRef]
- González, B.; Colilla, M.; Díez, J.; Pedraza, D.; Guembe, M.; Izquierdo-Barba, I.; Vallet-Regí, M. Mesoporous silica nanoparticles decorated with polycationic dendrimers for infection treatment. Acta Biomater. 2018, 68, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Nadrah, P.; Porta, F.; Planinšek, O.; Kros, A.; Gaberšček, M. Poly(propylene imine) dendrimer caps on mesoporous silica nanoparticles for redox-responsive release: Smaller is better. Phys. Chem. Chem. Phys. 2013, 15, 10740–10748. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Milla, M.; Gómez, R.; Pérez-Serrano, J.; Sánchez-Nieves, J.; de la Mata, F.J. Functionalization of silica with amine and ammonium alkyl chains, dendrons and dendrimers: Synthesis and antibacterial properties. Mater. Sci. Eng. C 2020, 109, 110526. [Google Scholar] [CrossRef] [PubMed]
- Martínez, Á.; Fuentes-Paniagua, E.; Baeza, A.; Sánchez-Nieves, J.; Cicuéndez, M.; Gõmez, R.; De La Mata, F.J.; González, B.; Vallet-Regí, M. Mesoporous Silica Nanoparticles Decorated with Carbosilane Dendrons as New Non-viral Oligonucleotide Delivery Carriers. Chem.-A Eur. J. 2015, 21, 15651–15666. [Google Scholar] [CrossRef]
- Andrés, R.; Jesús, E.D.; Fierro, J.L.G.; Terreros, P. Bifunctional carbosilane dendrons for the immobilization of zirconocene catalysts on silica. New, J. Chem. 2011, 35, 2203–2211. [Google Scholar] [CrossRef]
- Sun, X.; Qu, R.; Sun, C.; Zhang, Y.; Sun, S.; Ji, C.; Yin, P. Sol-gel preparation and Hg(II) adsorption properties of silica-gel supported low generation polyamidoamine dendrimers polymer adsorbents. Ind. Eng. Chem. Res. 2014, 53, 2878–2888. [Google Scholar] [CrossRef]
- Xu, X.; Lü, S.; Gao, C.; Wang, X.; Bai, X.; Gao, N.; Liu, M. Facile preparation of pH-sensitive and self-fluorescent mesoporous silica nanoparticles modified with PAMAM dendrimers for label-free imaging and drug delivery. Chem. Eng. J. 2015, 266, 171–178. [Google Scholar] [CrossRef]
- Newkome, G.R.; Yoo, K.S.; Kabir, A.; Malik, A. Synthesis of benzyl-terminated dendrons for use in high-resolution capillary gas chromatography. Tetrahedron Lett. 2001, 42, 7537–7541. [Google Scholar] [CrossRef]
- Kabir, A.; Hamlet, C.; Yoo, K.S.; Newkome, G.R.; Malik, A. Capillary microextraction on sol-gel dendrimer coatings. J. Chromatogr. A 2004, 1034, 1–11. [Google Scholar] [CrossRef]
- Riegert, D.; Bareille, L.; Laurent, R.; Majoral, J.P.; Caminade, A.M.; Chaumonnot, A. Silica Functionalized by Bifunctional Dendrimers: Hybrid Nanomaterials for Trapping CO2. Eur. J. Inorg. Chem. 2016, 2016, 3103–3110. [Google Scholar] [CrossRef]
- Pang, Y.; Zeng, G.; Tang, L.; Zhang, Y.; Liu, Y.; Lei, X.; Li, Z.; Zhang, J.; Xie, G. PEI-grafted magnetic porous powder for highly effective adsorption of heavy metal ions. Desalination 2011, 281, 278–284. [Google Scholar] [CrossRef]
- Sorokina, S.A.; Kuchkina, N.V.; Lawson, B.P.; Krasnova, I.Y.; Nemygina, N.A.; Nikoshvili, L.Z.; Talanova, V.N.; Stein, B.D.; Pink, M.; Morgan, D.G.; et al. Pyridylphenylene dendrons immobilized on the surface of chemically modified magnetic silica as efficient stabilizing molecules of Pd species. Appl. Surf. Sci. 2019, 488, 865–873. [Google Scholar] [CrossRef]
- Chung, Y.M.; Rhee, H.K. Silica-supported dendritic chiral auxiliaries for enantioselective addition of diethylzinc to benzaldehyde. Comptes Rendus Chim. 2003, 6, 695–705. [Google Scholar] [CrossRef]
- Karakhanov, E.; Maximov, A.; Kardasheva, Y.; Semernina, V.; Zolotukhina, A.; Ivanov, A.; Abbott, G.; Rosenberg, E.; Vinokurov, V. Pd nanoparticles in dendrimers immobilized on silica-polyamine composites as catalysts for selective hydrogenation. ACS Appl. Mater. Interfaces 2014, 6, 8807–8816. [Google Scholar] [CrossRef]
- Hameau, A.; Collière, V.; Grimoud, J.; Fau, P.; Roques, C.; Caminade, A.-M.; Turrin, C.-O. PPH dendrimers grafted on silica nanoparticles: Surface chemistry, characterization, silver colloids hosting and antibacterial activity. RSC Adv. 2013, 3, 19015. [Google Scholar] [CrossRef]
- Mathews, B.T.; Beezer, A.E.; Snowden, M.J.; Hardy, M.J.; Mitchell, J.C. The synthesis of immobilised chiral dendrimers. New J. Chem. 2001, 25, 807–818. [Google Scholar] [CrossRef]
- Gerrans, K.; Luhrs, A.; Feider, C.; Margerum, L.D. Silica nanoparticles functionalized with polyamidoamine (PAMAM) dendrimers as platforms for photoluminescence (PL) sensing of copper and cyanide ions. J. Colloid Interface Sci. 2016, 470, 276–283. [Google Scholar] [CrossRef]
- Nakanishi, Y.; Imae, T. Preparation of siloxy focal dendron-protected TiO2 nanoparticles and their photocatalysis. J. Colloid Interface Sci. 2006, 297, 122–129. [Google Scholar] [CrossRef]
- Pawlaczyk, M.; Pasieczna-Patkowska, S.; Ryczkowski, J.; Schroeder, G. Photoacoustic infrared spectroscopic studies of silica surface functionalized by dendrimers. Vib. Spectrosc. 2019, 103, 102943. [Google Scholar] [CrossRef]
- Pawlaczyk, M.; Schroeder, G. Adsorption studies of Cu(II) ions on dendrimer-grafted silica-based materials. J. Mol. Liq. 2019, 281, 176–185. [Google Scholar] [CrossRef]
- Acosta, E.J.; Gonzalez, S.O.; Simanek, E.E. Synthesis, characterization, and application of melamine-based dendrimers supported on silica gel. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 168–177. [Google Scholar] [CrossRef]
- Bu, J.; Li, R.; Quah, C.W.; Carpenter, K.J. Propagation of PAMAM dendrons on silica gel: A study on the reaction kinetics. Macromolecules 2004, 37, 6687–6694. [Google Scholar] [CrossRef]
- Chu, C.C.; Ueno, N.; Imae, T. Solid-phase synthesis of amphiphilic dendron-surface-modified silica particles and their application toward water purification. Chem. Mater. 2008, 20, 2669–2676. [Google Scholar] [CrossRef]
- Wu, K.; Luan, L.; Xing, J.X.; Ma, S.; Xue, Z.; Xu, W.; Niu, Y. Removal of Zn(II) and Co(II) from N,N-dimethylformamide by polyamidoamine dendrimers decorated silica: Performance and mechanism. J. Mol. Liq. 2020, 308, 113073. [Google Scholar] [CrossRef]
- Fu, T.; Niu, Y.; Zhou, Y.; Wang, K.; Mu, Q.; Qu, R.; Chen, H.; Yuan, B.; Yang, H. Adsorption of Mn(II) from aqueous solution by silica-gel supported polyamidoamine dendrimers: Experimental and DFT study. J. Taiwan Inst. Chem. Eng. 2019, 97, 189–199. [Google Scholar] [CrossRef]
- Qiu, Z.; Niu, Y.; Fu, T.; Wang, K.; Mu, Q.; Wang, F. Removal of Ni(II) from fuel ethanol by PAMAM dendrimers/silica hybrid materials: Combined experimental and theoretical study. Chem. Eng. Res. Des. 2019, 144, 174–184. [Google Scholar] [CrossRef]
- Song, X.; Niu, Y.; Zhang, P.; Zhang, C.; Zhang, Z.; Zhu, Y.; Qu, R. Removal of Co(II) from fuel ethanol by silica-gel supported PAMAM dendrimers: Combined experimental and theoretical study. Fuel 2017, 199, 91–101. [Google Scholar] [CrossRef]
- Ren, B.; Wang, K.; Zhang, B.; Li, H.; Niu, Y.; Chen, H.; Yang, Z.; Li, X.; Zhang, H. Adsorption behavior of PAMAM dendrimers functionalized silica for Cd(II) from aqueous solution: Experimental and theoretical calculation. J. Taiwan Inst. Chem. Eng. 2019, 101, 80–91. [Google Scholar] [CrossRef]
- Zhu, Y.; Niu, Y.; Li, H.; Ren, B.; Qu, R.; Chen, H.; Zhang, Y. Removal of Cd(II) and Fe(III) from DMSO by silica gel supported PAMAM dendrimers: Equilibrium, thermodynamics, kinetics and mechanism. Ecotoxicol. Environ. Saf. 2018, 162, 253–260. [Google Scholar] [CrossRef]
- Zhang, S.; Niu, Y.; Chen, Z.; Chen, H.; Yang, Z.; Bai, L.; Yuan, B. Removal of Fe(III) from ethanol by silica-gel supported ester-terminated pamam dendrimers: Experimental and dft calculation. Desalin. Water Treat. 2019, 164, 310–318. [Google Scholar] [CrossRef]
- Niu, Y.; Qu, R.; Sun, C.; Wang, C.; Chen, H.; Ji, C.; Zhang, Y.; Shao, X.; Bu, F. Adsorption of Pb(II) from aqueous solution by silica-gel supported hyperbranched polyamidoamine dendrimers. J. Hazard. Mater. 2013, 244–245, 276–286. [Google Scholar] [CrossRef]
- Shaaban, A.F.; Khalil, A.A.; Lasheen, T.A.; Nouh, E.S.A.; Ammar, H. Polyamidoamine dendrimers modified silica gel for uranium(VI) removal from aqueous solution using batch and fixed-bed column methods. Desalin. Water Treat. 2018, 102, 197–210. [Google Scholar] [CrossRef]
- Reynhardt, J.P.K.; Yang, Y.; Sayari, A.; Alper, H. Polyamidoamine dendrimers prepared Inside the channels of pore-expanded periodic mesoporous silica. Adv. Funct. Mater. 2005, 15, 1641–1646. [Google Scholar] [CrossRef]
- Sakai, K.; Kunitake, M.; Teng, T.C.; Katada, A.; Harada, T.; Yoshida, K.; Yamanaka, K.; Asami, Y.; Sakata, M.; Hirayama, C. Designable Size Exclusion Chromatography Columns Based on Dendritic Polymer-Modified Porous Silica Particles. Chem. Mater. 2003, 15, 4091–4097. [Google Scholar] [CrossRef]
- Niu, Y.; Qu, R.; Chen, H.; Mu, L.; Liu, X.; Wang, T.; Zhang, Y.; Sun, C. Synthesis of silica gel supported salicylaldehyde modified PAMAM dendrimers for the effective removal of Hg(II) from aqueous solution. J. Hazard. Mater. 2014, 278, 267–278. [Google Scholar] [CrossRef]
- Niu, Y.; Yang, J.; Qu, R.; Gao, Y.; Du, N.; Chen, H.; Sun, C.; Wang, W. Synthesis of Silica-Gel-Supported Sulfur-Capped PAMAM Dendrimers for Efficient Hg(II) Adsorption: Experimental and DFT Study. Ind. Eng. Chem. Res. 2016, 55, 3679–3688. [Google Scholar] [CrossRef]
- Zhang, P.; Niu, Y.; Qiao, W.; Xue, Z.; Bai, L.; Chen, H. Experimental and DFT investigation on the adsorption mechanism of silica gel supported sulfur-capped PAMAM dendrimers for Ag(I). J. Mol. Liq. 2018, 263, 390–398. [Google Scholar] [CrossRef]
- Song, X.; Niu, Y.; Qiu, Z.; Zhang, Z.; Zhou, Y.; Zhao, J.; Chen, H. Adsorption of Hg(II) and Ag(I) from fuel ethanol by silica gel supported sulfur-containing PAMAM dendrimers: Kinetics, equilibrium and thermodynamics. Fuel 2017, 206, 80–88. [Google Scholar] [CrossRef]
- Li, H.; Zheng, Z.; Cao, M.; Cao, R. Stable gold nanoparticle encapsulated in silica-dendrimers organic-inorganic hybrid composite as recyclable catalyst for oxidation of alcohol. Microporous Mesoporous Mater. 2010, 136, 42–49. [Google Scholar] [CrossRef]
- Reynhardt, J.P.K.; Yang, Y.; Sayari, A.; Alper, H. Rhodium complexed C2-PAMAM dendrimers supported on large pore Davisil silica as catalysts for the hydroformylation of olefins. Adv. Synth. Catal. 2005, 347, 1379–1388. [Google Scholar] [CrossRef]
- Bourque, S.C.; Alper, H.; Manzer, L.E.; Arya, P. Hydroformylation reactions using recyclable rhodium-complexed dendrimers on silica [4]. J. Am. Chem. Soc. 2000, 122, 956–957. [Google Scholar] [CrossRef]
- Reynhardt, J.P.K.; Yang, Y.; Sayari, A.; Alper, H. Periodic mesoporous silica-supported recyclable rhodium-complexed dendrimer catalysts. Chem. Mater. 2004, 16, 4095–4102. [Google Scholar] [CrossRef]
- Lu, S.M.; Alper, H. Intramolecular carbonylation reactions with recyclable palladium-complexed dendrimers on silica: Synthesis of oxygen, nitrogen, or sulfur-containing medium ring fused heterocycles. J. Am. Chem. Soc. 2005, 127, 14776–14784. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.M.; Alper, H. Synthesis of large ring macrocycles (12-18) by recyclable palladiumComplexed dendrimers on silica gel catalyzed intramolecular cyclocarbonylation reactions. Chem.-A Eur. J. 2007, 13, 5908–5916. [Google Scholar] [CrossRef] [PubMed]
- Antebi, S.; Arya, P.; Manzer, L.E.; Alper, H. Carbonylation reactions of iodoarenes with PAMAM dendrimer-palladium catalysts immobilized on silica. J. Org. Chem. 2002, 67, 6623–6631. [Google Scholar] [CrossRef]
- Reynhardt, J.P.K.; Alper, H. Hydroesterification Reactions with Palladium-Complexed PAMAM Dendrimers Immobilized on Silica. J. Org. Chem. 2003, 68, 8353–8360. [Google Scholar] [CrossRef]
- Alper, H.; Arya, P.; Bourque, S.C.; Jefferson, G.R.; Manzer, L.E. Heck reaction using palladium complexed to dendrimers on silica. Can. J. Chem. 2000, 78, 920–924. [Google Scholar] [CrossRef]
- Bu, J.; Judeh, Z.M.A.; Ching, C.B.; Kawi, S. Epoxidation of olefins catalyzed by Mn(II) salen complex anchored on PAMAM-SiO2 dendrimer. Catal. Lett. 2003, 85, 183–187. [Google Scholar] [CrossRef]
- Wang, S.; Su, P.; Ding, F.; Yang, Y. Immobilization of cellulase on polyamidoamine dendrimer-grafted silica. J. Mol. Catal. B Enzym. 2013, 89, 35–40. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, L.; Jiang, Y.; Gao, W.; Wang, Y.; Yang, X.; Yang, X.; Liu, Z. Gold Nanorods with Silica Shell and PAMAM Dendrimers for Efficient Photothermal Therapy and Low Toxic Codelivery of Anticancer Drug and siRNA. Adv. Mater. Interfaces 2017, 4, 1–9. [Google Scholar] [CrossRef]
- Hicks, J.C.; Drese, J.H.; Fauth, D.J.; Gray, M.L.; Qi, G.; Jones, C.W. Designing adsorbents for CO2 capture from flue gas-hyperbranched aminosilicas capable of capturing CO2 reversibly. J. Am. Chem. Soc. 2008, 130, 2902–2903. [Google Scholar] [CrossRef] [PubMed]
- Drese, J.H.; Choi, S.; Lively, R.P.; Koros, W.J.; Fauth, D.J.; Gray, M.L.; Jones, C.W. Synthesis-structure-property relationships for Hyperbranched aminosilica CO2 adsorbents. Adv. Funct. Mater. 2009, 19, 3821–3832. [Google Scholar] [CrossRef]
- López-Aranguren, P.; Vega, L.F.; Domingo, C. A new method using compressed CO2 for the in situ functionalization of mesoporous silica with hyperbranched polymers. Chem. Commun. 2013, 49, 11776–11778. [Google Scholar] [CrossRef]
- Acosta, E.J.; Carr, C.S.; Simanek, E.E.; Shantz, D.F. Engineering nanospaces: Iterative synthesis of melamine-based dendrimers on amine-functionalized SBA-15 leading to complex hybrids with controllable chemistry and porosity. Adv. Mater. 2004, 16, 985–989. [Google Scholar] [CrossRef]
- Bhagiyalakshmi, M.; Park, S.D.; Cha, W.S.; Jang, H.T. Development of TREN dendrimers over mesoporous SBA-15 for CO2 adsorption. Appl. Surf. Sci. 2010, 256, 6660–6666. [Google Scholar] [CrossRef]
- Yoo, S.; Yeu, S.; Sherman, R.L.; Simanek, E.E.; Shantz, D.F.; Ford, D.M. Reverse-selective membranes formed by dendrimers on mesoporous ceramic supports. J. Membr. Sci. 2009, 334, 16–22. [Google Scholar] [CrossRef]
- Tsubokawa, N.; Hayashi, S.; Nishimura, J. Grafting of hyperbranched polymers onto ultrafine silica: Postgraft polymerization of vinyl monomers initiated by pendant azo groups of grafted polymer chains on the surface. Prog. Org. Coat. 2002, 44, 69–74. [Google Scholar] [CrossRef]
- Hayashi, S.; Fujiki, K.; Tsubokawa, N. Grafting of hyperbranched polymers onto ultrafine silica: Postgraft polymerization of vinyl monomers initiated by pendant initiating groups of polymer chains grafted onto the surface. React. Funct. Polym. 2000, 46, 193–201. [Google Scholar] [CrossRef]
- Dvornic, P.R. PAMAMOS: The first commercial silicon-containing dendrimers and their applications. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 2755–2773. [Google Scholar] [CrossRef]
- Dvornic, P.R.; Li, J.; De Leuze-Jallouli, A.M.; Reeves, S.D.; Owen, M.J. Nanostructured dendrimer-based networks with hydrophilic polyamidoamine and hydrophobic organosilicon domains. Macromolecules 2002, 35, 9323–9333. [Google Scholar] [CrossRef]
- Chujo, Y.; Matsuki, H.; Kure, S.; Saegusa, T.; Yazawa, T. Control of pore size of porous silica by means of pyrolysis of an organic-inorganic polymer hybrid. J. Chem. Soc. Chem. Commun. 1994, 635–636. [Google Scholar] [CrossRef]
- Tsiourvas, D.; Tsetsekou, A.; Papavasiliou, A.; Arkas, M.; Boukos, N. A novel hybrid sol-gel method for the synthesis of highly porous silica employing hyperbranched poly(ethyleneimine) as a reactive template. Microporous Mesoporous Mater. 2013, 175, 59–66. [Google Scholar] [CrossRef]
- Turrin, C.O.; Maraval, V.; Caminade, A.M.; Majoral, J.P.; Mehdi, A.; Reyé, C. Organic-Inorganic hybrid materials incorporating phosphorus-containing dendrimers. Chem. Mater. 2000, 12, 3848–3856. [Google Scholar] [CrossRef]
- Vardavoulias, M.; Gkomoza, E.; Tsetsekou, A.; Kitsou, I.; Arkas, M.; Papageorgiou, M.; González, S.M.S.; Cubillos, Y.L.; Cepas, V.; Jensen, H.E.; et al. Hydrogel and Xerogel Active Ingredient Carriers Made from Dendritic Polymers and Silica for Solid Substrate Coating Applications. Greek Patent Application Number 2019010000585, 30 December 2019. [Google Scholar]
- Talham, D.R. Biomineralization: Principles and Concepts in Bioinorganic Materials Chemistry Stephen Mann. Oxford University Press, New York, 2001. Cryst. Growth Des. 2002, 2, 675. [Google Scholar] [CrossRef]
- Sumper, M.; Brunner, E. Learning from diatoms: Nature’s tools for the production of nanostructured silica. Adv. Funct. Mater. 2006, 16, 17–26. [Google Scholar] [CrossRef]
- Lechner, C.C.; Becker, C.F.W. Silaffins in silica biomineralization and biomimetic silica precipitation. Mar. Drugs 2015, 13, 5297–5333. [Google Scholar] [CrossRef] [PubMed]
- Knecht, M.R.; Wright, D.W. Amine-terminated dendrimers as biomimetic templates for silica nanosphere formation. Langmuir 2004, 20, 4728–4732. [Google Scholar] [CrossRef] [PubMed]
- Knecht, M.R.; Sewell, S.L.; Wright, D.W. Size control of dendrimer-templated silica. Langmuir 2005, 21, 2058–2061. [Google Scholar] [CrossRef]
- Coradin, T.; Durupthy, O.; Livage, J. Interactions of amino-containing peptides with sodium silicate and colloidal silica: A biomimetic approach of silicification. Langmuir 2002, 18, 2331–2336. [Google Scholar] [CrossRef]
- Kobler, J.; Möller, K.; Bein, T. Colloidal Suspensions of Functionalized Mesoporous Silica Nanoparticles. ACS Nano 2008, 2, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Petrakli, F.; Arkas, M.; Tsetsekou, A. α-Alumina nanospheres from nano-dispersed boehmite synthesized by a wet chemical route. J. Am. Ceram. Soc. 2018, 101, 3508–3519. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, S.P.; Wang, W.M.; Chen, H.M.; Wang, Z.H.; Yu, X. Bin Preparation of nanocrystalline ceramic oxide powders in the presence of anionic starburst dendrimer. Mater. Lett. 2004, 58, 3285–3289. [Google Scholar] [CrossRef]
- Arkas, M.; Tsiourvas, D. Organic/inorganic hybrid nanospheres based on hyperbranched poly(ethylene imine) encapsulated into silica for the sorption of toxic metal ions and polycyclic aromatic hydrocarbons from water. J. Hazard. Mater. 2009, 170, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Nemanashi-Maumela, M.; Nongwe, I.; Motene, R.C.; Davids, B.L.; Meijboom, R. Au and Ag nanoparticles encapsulated within silica nanospheres using dendrimers as dual templating agent and their catalytic activity. Mol. Catal. 2017, 438, 184–196. [Google Scholar] [CrossRef]
- Arkas, M.; Kithreoti, G.; Boukos, N.; Kitsou, I.; Petrakli, F.; Panagiotaki, K. Two completely different biomimetic reactions mediated by the same matrix producing inorganic/organic/inorganic hybrid nanoparticles. Nano-Struct. Nano-Objects 2018, 14, 138–148. [Google Scholar] [CrossRef]
- Kröger, N.; Deutzmann, R.; Bergsdorf, C.; Sumper, M. Species-specific polyamines from diatoms control silica morphology. Proc. Natl. Acad. Sci. USA 2000, 97, 14133–14138. [Google Scholar] [CrossRef]
- Mizutani, T.; Nagase, H.; Fujiwara, N.; Ogoshi, H. Silicic Acid Polymerization Catalyzed by Amines and Polyamines. Bull. Chem. Soc. Jpn. 1998, 71, 2017–2022. [Google Scholar] [CrossRef]
- Knecht, M.R.; Wright, D.W. Functional analysis of the biomimetic silica precipitating activity of the R5 peptide from Cylindrotheca fusiformis. Chem. Commun. 2003, 3, 3038–3039. [Google Scholar] [CrossRef]
- Kossovsky, N.; Gelman, A.; Sponsler, E.E.; Hnatyszyn, H.J.; Rajguru, S.; Torres, M.; Pham, M.; Crowder, J.; Zemanovich, J.; Chung, A.; et al. Surface-modified nanocrystalline ceramics for drug delivery applications. Biomaterials 1994, 15, 1201–1207. [Google Scholar] [CrossRef]
- Kossovsky, N.; Gelman, A.; Rajguru, S.; Nguyen, R.; Sponsler, E.; Hnatyszyn, H.J.; Chow, K.; Chung, A.; Torres, M.; Zemanovich, J.; et al. Control of molecular polymorphisms by a structured carbohydrate/ceramic delivery vehicle—Aquasomes. J. Control. Release 1996, 39, 383–388. [Google Scholar] [CrossRef]
- Khopade, A.J.; Khopade, S.; Jain, N.K. Development of hemoglobin aquasomes from spherical hydroxyapatite cores precipitated in the presence of half-generation poly(amidoamine) dendrimer. Int. J. Pharm. 2002, 241, 145–154. [Google Scholar] [CrossRef]
- Zhang, F.; Zhou, Z.H.; Yang, S.P.; Mao, L.H.; Chen, H.M.; Yu, X. Bin Hydrothermal synthesis of hydroxyapatite nanorods in the presence of anionic starburst dendrimer. Mater. Lett. 2005, 59, 1422–1425. [Google Scholar] [CrossRef]
- Yan, S.J.; Zhou, Z.H.; Zhang, F.; Yang, S.P.; Yang, L.Z.; Yu, X. Bin Effect of anionic PAMAM with amido groups starburst dendrimers on the crystallization of Ca10(PO4)6(OH)2 by hydrothermal method. Mater. Chem. Phys. 2006, 99, 164–169. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Zhou, P.L.; Yang, S.P.; Yu, X.B.; Yang, L.Z. Controllable synthesis of hydroxyapatite nanocrystals via a dendrimer-assisted hydrothermal process. Mater. Res. Bull. 2007, 42, 1611–1618. [Google Scholar] [CrossRef]
- Tsiourvas, D.; Tsetsekou, A.; Kammenou, M.I.; Boukos, N. Controlling the formation of hydroxyapatite nanorods with dendrimers. J. Am. Ceram. Soc. 2011, 94, 2023–2029. [Google Scholar] [CrossRef]
- Donners, J.J.J.M.; Nolte, R.J.M.; Sommerdijk, N.A.J.M. Dendrimer-based hydroxyapatite composites with remarkable materials properties. Adv. Mater. 2003, 15, 313–316. [Google Scholar] [CrossRef]
- Cahill, B.P.; Papastavrou, G.; Koper, G.J.M.; Borkovec, M. Adsorption of poly(amido amine) (PAMAM) dendrimers on silica: Importance of electrostatic three-body attraction. Langmuir 2008, 24, 465–473. [Google Scholar] [CrossRef]
- Longtin, R.; Maroni, P.; Borkovec, M. Transition from completely reversible to irreversible adsorption of poly(amido amine) dendrimers on silica. Langmuir 2009, 25, 2928–2934. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M. Relations between pore structure parameters and their implications for characterization of MCM-41 using gas adsorption and x-ray diffraction. Chem. Mater. 1999, 11, 492–500. [Google Scholar] [CrossRef]
- Arkas, M. Hybrid organoceramics deriving from dendritic polymers, methods of preparation, optimization techniques and prospected applications. In Recent Advances in Ceramic Materials Research (Materials Science and Technologies); Nova Science Publishers: Hauppauge, NY, USA, 2013; pp. 1–30. ISBN 9781624177293. [Google Scholar]
- Arkas, M. Hybrid Organic/Inorganic Materials Based on Functionalized Dendritic Polymers: Methods of Preparation, Applications and Future Prospects. J. Mater. Sci. Eng. 2012, 1, 1000e103. [Google Scholar] [CrossRef]
- Sooklal, K.; Hanus, L.H.; Ploehn, H.J.; Murphy, C.J. A Blue-Emitting CdS/Dendrimer Nanocomposite. Adv. Mater. 1998, 10, 1083–1087. [Google Scholar] [CrossRef]
- Garcia, M.E.; Baker, L.A.; Crooks, R.M. Preparation and characterization of dendrimer-gold colloid nanocomposites. Anal. Chem. 1999, 71, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Manoth, M.; Manzoor, K.; Patra, M.K.; Pandey, P.; Vadera, S.R.; Kumar, N. Dendrigraft polymer-based synthesis of silver nanoparticles showing bright blue fluorescence. Mater. Res. Bull. 2009, 44, 714–717. [Google Scholar] [CrossRef]
- Karakhanov, E.; Maximov, A.; Zolotukhina, A.; Mamadli, A.; Vutolkina, A.; Ivanov, A. Dendrimer-stabilized Ru nanoparticles immobilized in organo-silica materials for hydrogenation of phenols. Catalysts 2017, 7, 86. [Google Scholar] [CrossRef]
- Tsiourvas, D.; Papavasiliou, A.; Deze, E.G.; Papageorgiou, S.K.; Katsaros, F.K.; Romanos, G.E.; Poulakis, E.; Philippopoulos, C.J.; Xin, Q.; Cool, P. A green route to copper loaded silica nanoparticles using hyperbranched poly(Ethylene imine) as a biomimetic template: Application in heterogeneous catalysis. Catalysts 2017, 7, 390. [Google Scholar] [CrossRef]
- Kitsou, I.; Arkas, M.; Tsetsekou, A. Synthesis and characterization of ceria-coated silica nanospheres: Their application in heterogeneous catalysis of organic pollutants. SN Appl. Sci. 2019, 1. [Google Scholar] [CrossRef]
- Gemeinhart, R.A.; Luo, D.; Saltzman, W.M. Cellular fate of a modular DNA delivery system mediated by silica nanoparticles. Biotechnol. Prog. 2005, 21, 532–537. [Google Scholar] [CrossRef]
- Radu, D.R.; Lai, C.Y.; Jeftinija, K.; Rowe, E.W.; Jeftinija, S.; Lin, V.S.Y. A polyamidoamine dendrimer-capped mesoporous silica nanosphere-based gene transfection reagent. J. Am. Chem. Soc. 2004, 126, 13216–13217. [Google Scholar] [CrossRef]
- Zang, S.; Chang, S.; Shahzad, M.B.; Sun, X.; Jiang, X.; Yang, H. Ceramics-based Drug Delivery System: A Review and Outlook. Rev. Adv. Mater. Sci. 2019, 58, 82–97. [Google Scholar] [CrossRef]
- Janaszewska, A.; Lazniewska, J.; Trzepiński, P.; Marcinkowska, M.; Klajnert-Maculewicz, B. Cytotoxicity of dendrimers. Biomolecules 2019, 9, 330. [Google Scholar] [CrossRef] [PubMed]
- Patri, A.K.; Majoros, I.J.; Baker, J.R. Dendritic polymer macromolecular carriers for drug delivery. Curr. Opin. Chem. Biol. 2002, 6, 466–471. [Google Scholar] [CrossRef]
- Yousefi, M.; Narmani, A.; Jafari, S.M. Dendrimers as efficient nanocarriers for the protection and delivery of bioactive phytochemicals. Adv. Colloid Interface Sci. 2020, 278. [Google Scholar] [CrossRef] [PubMed]
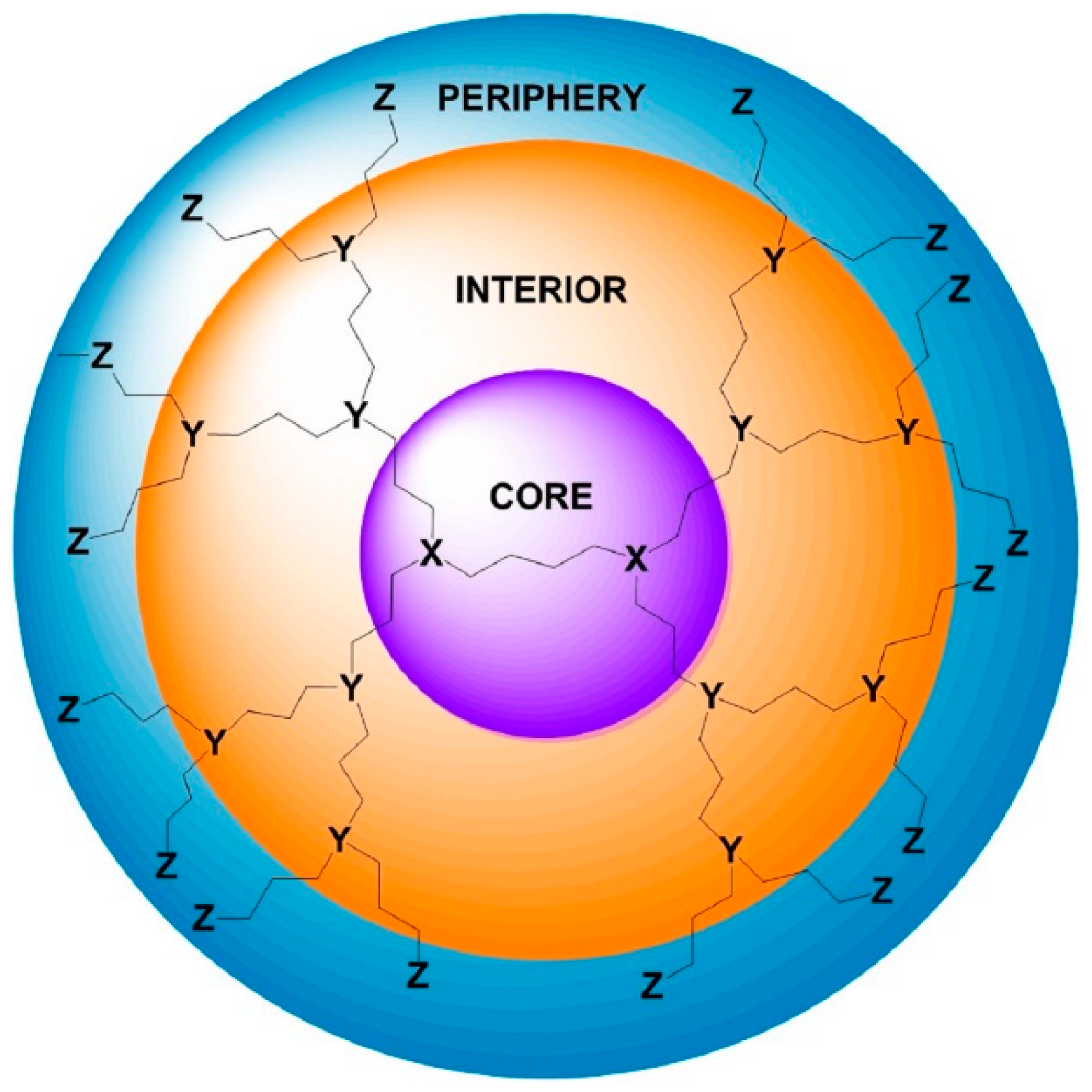
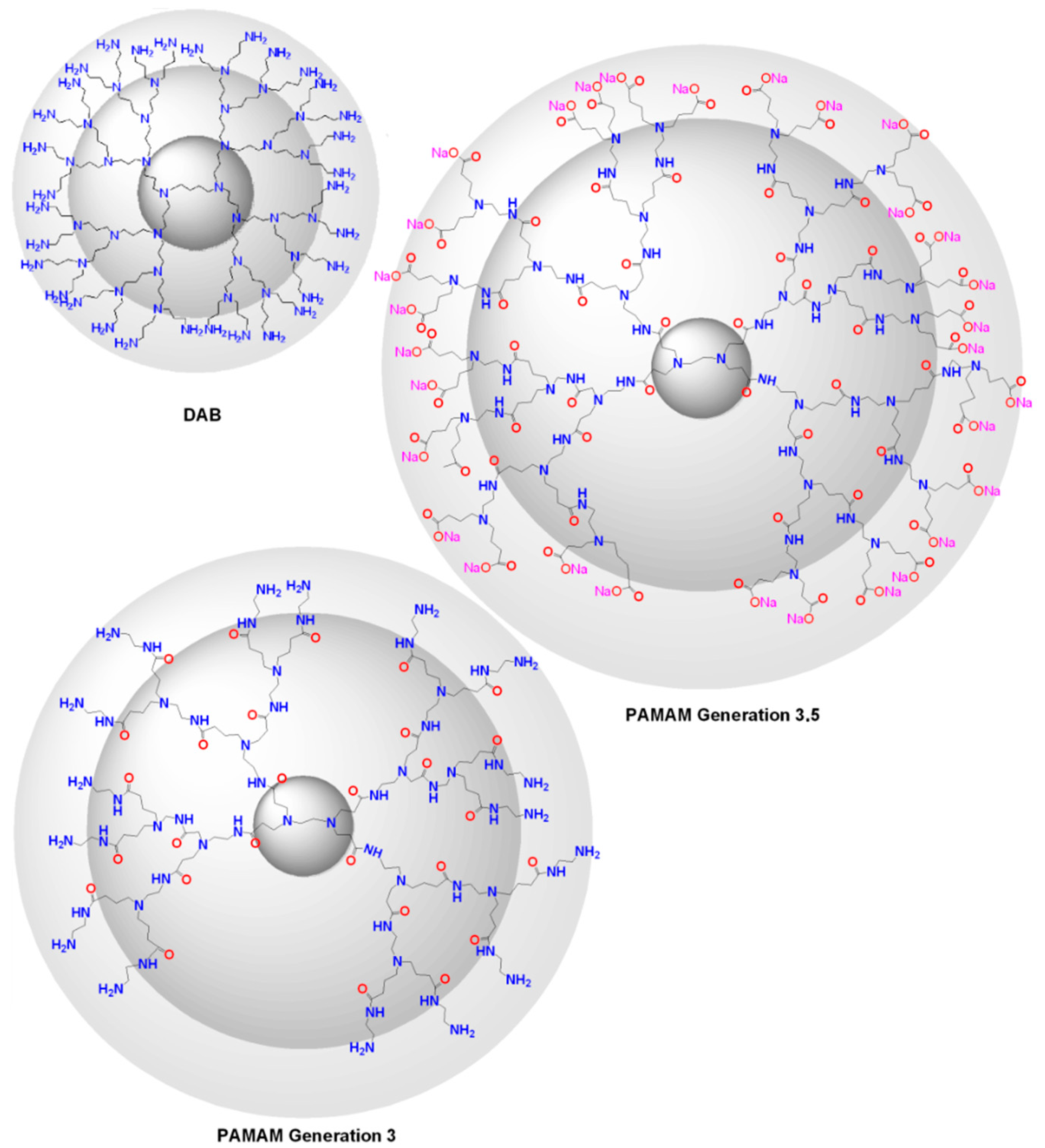
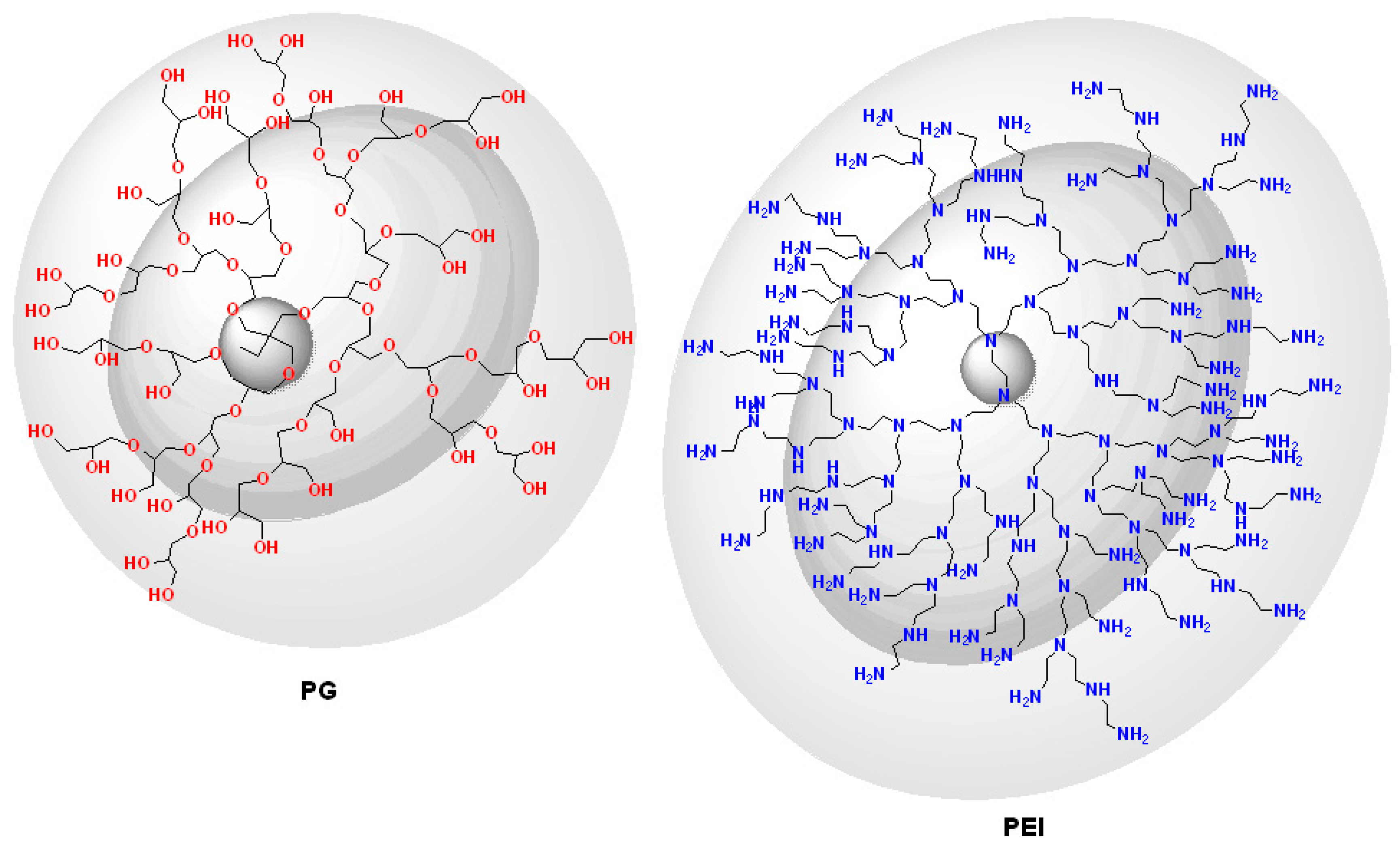
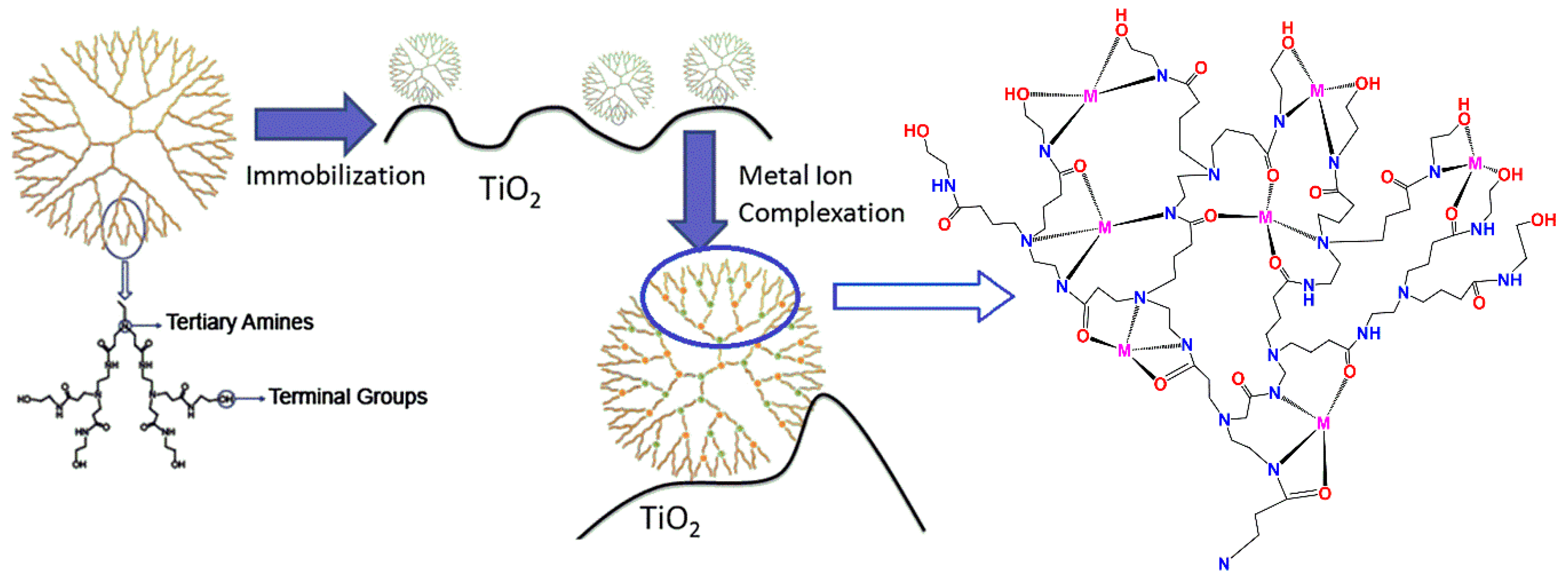
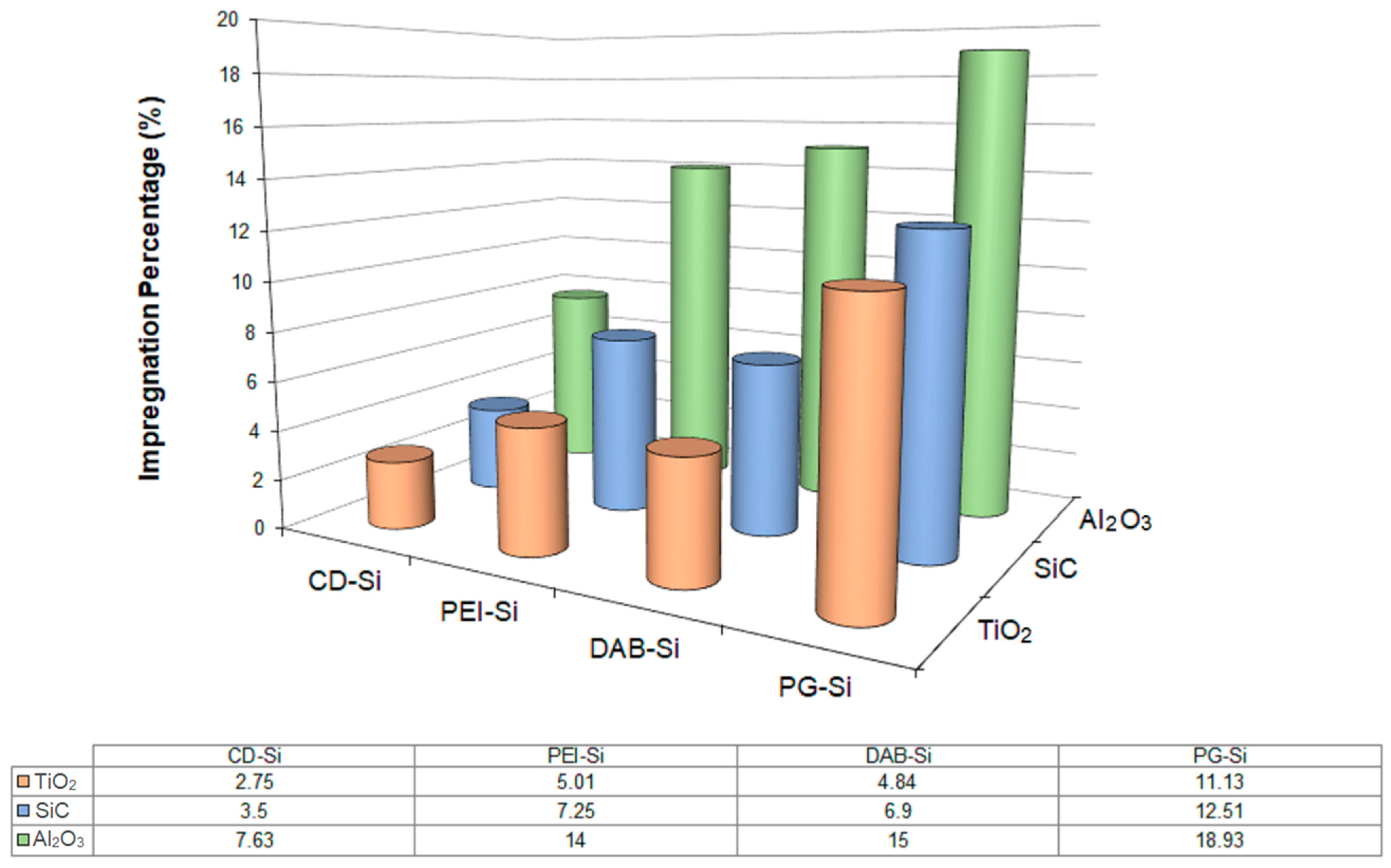
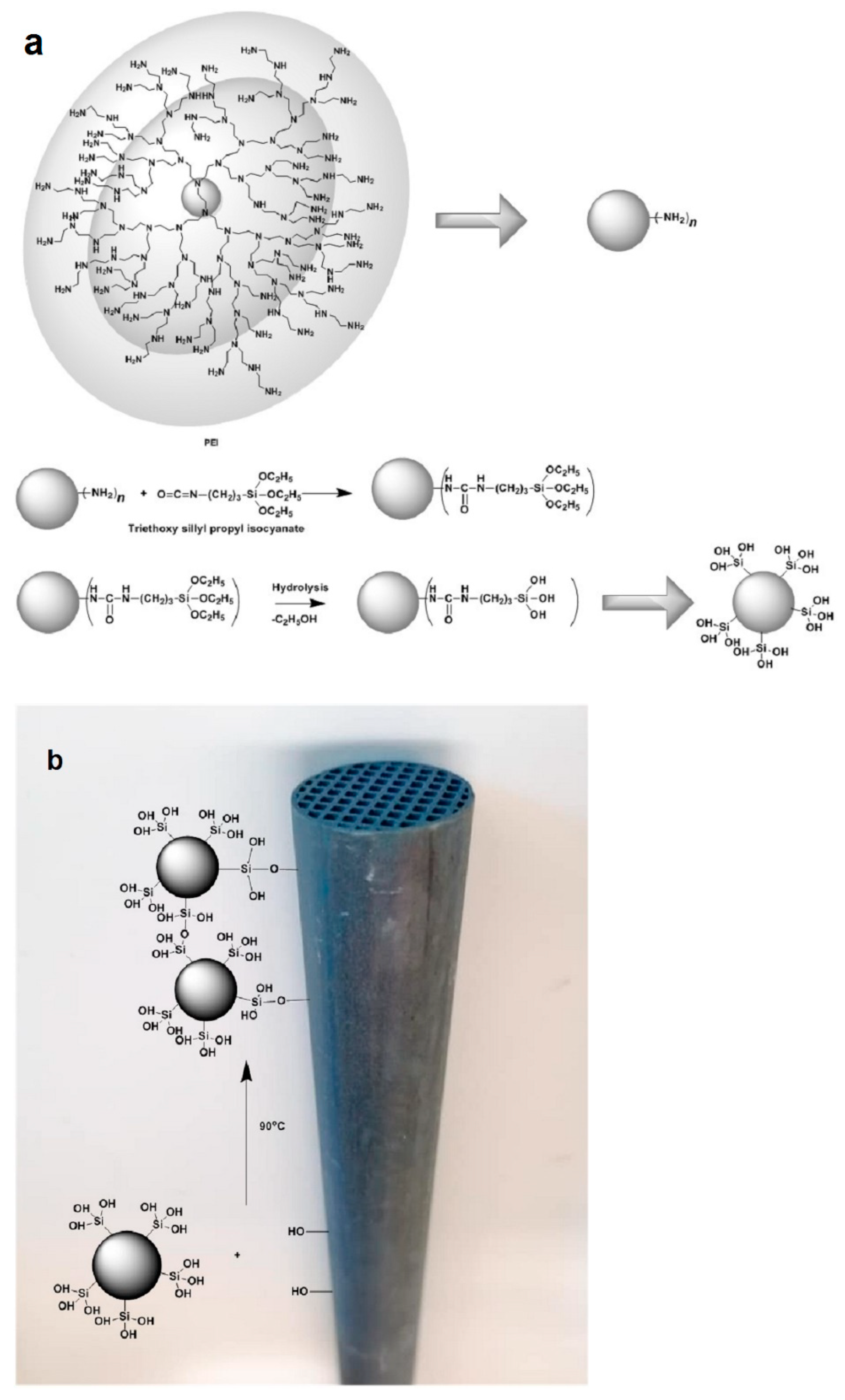
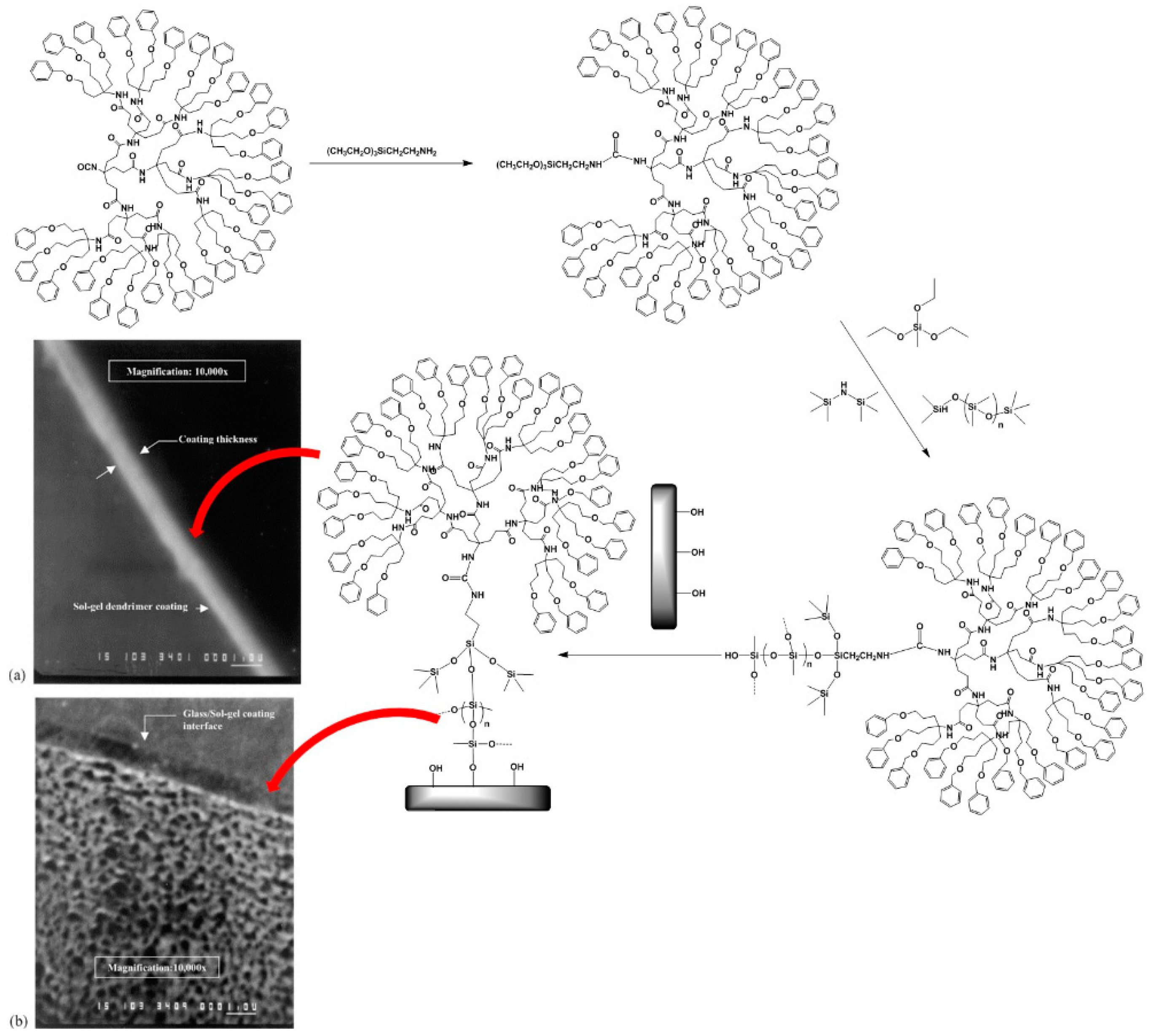
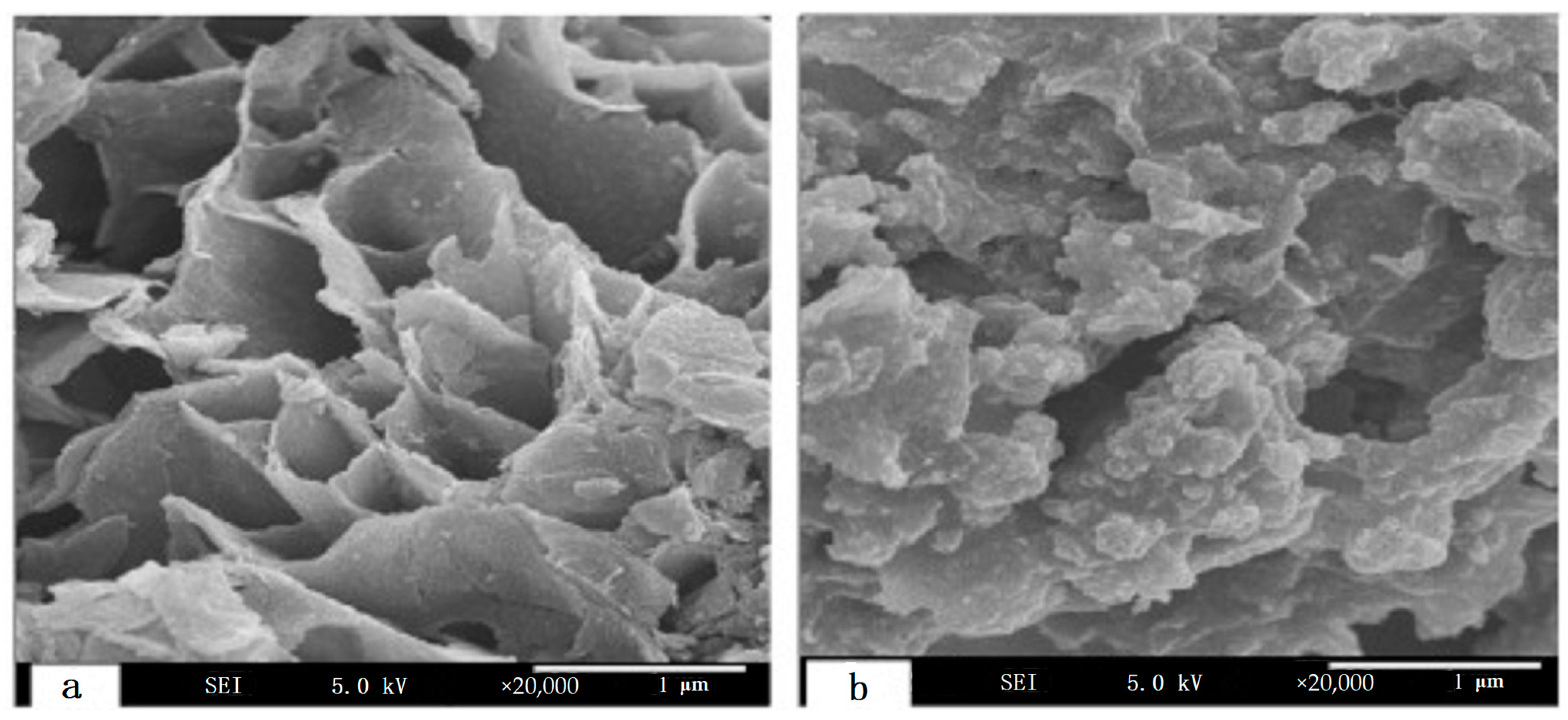
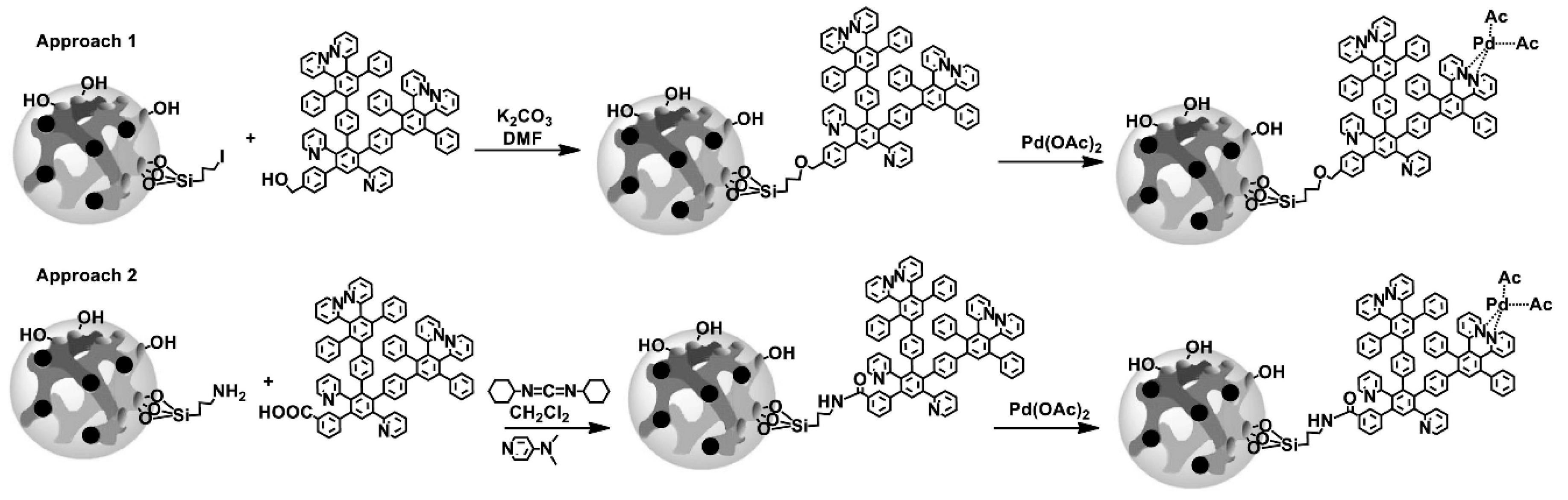
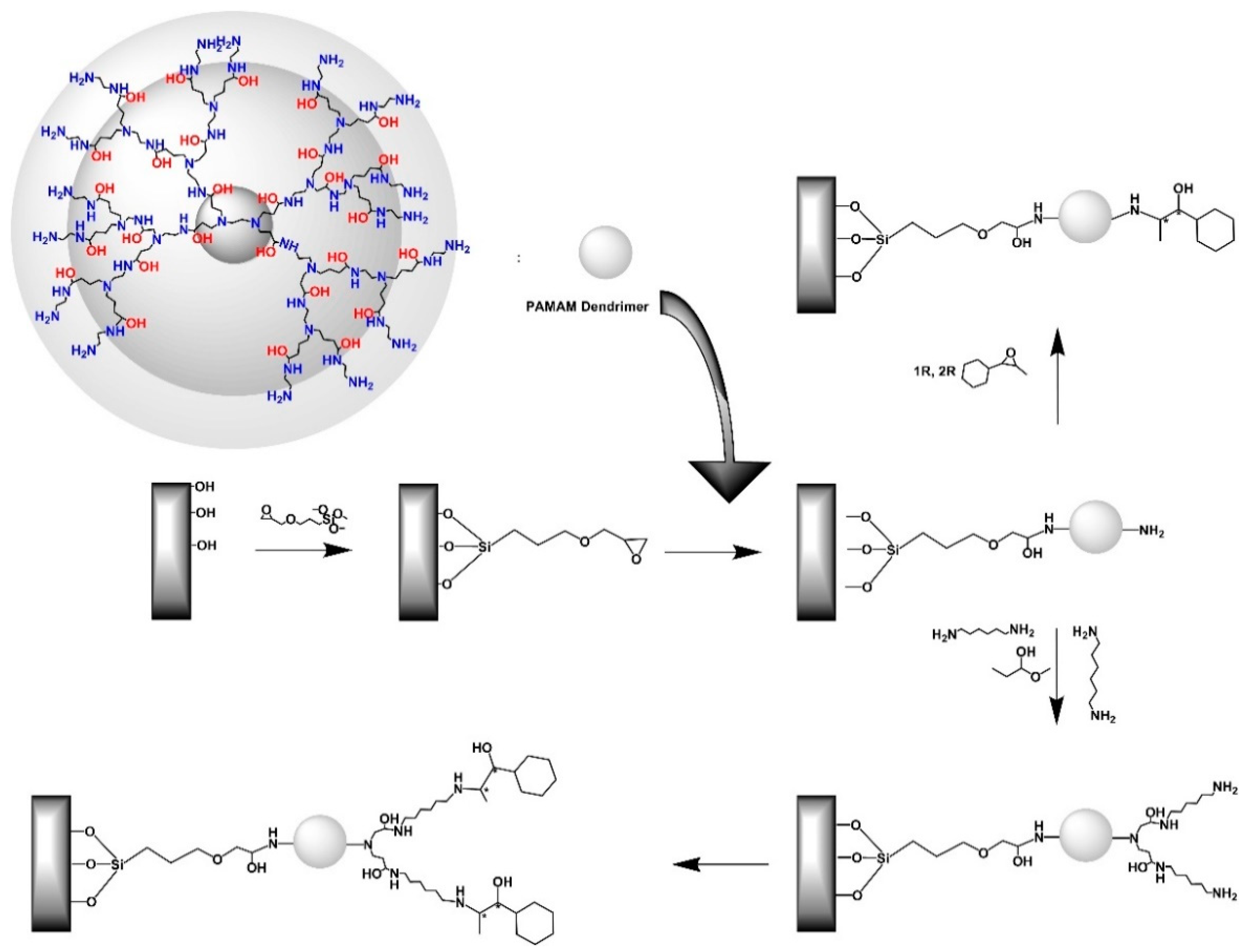
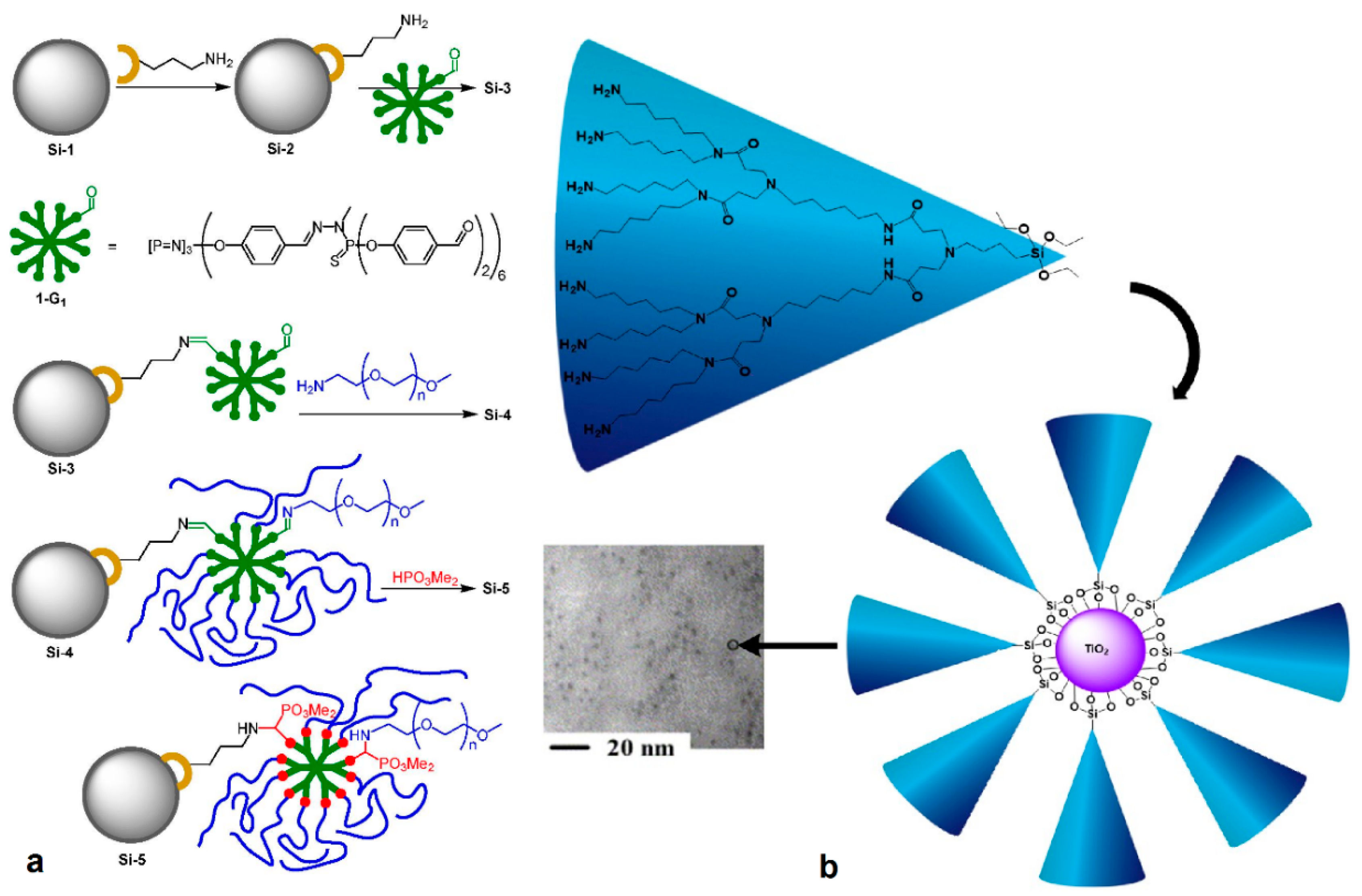
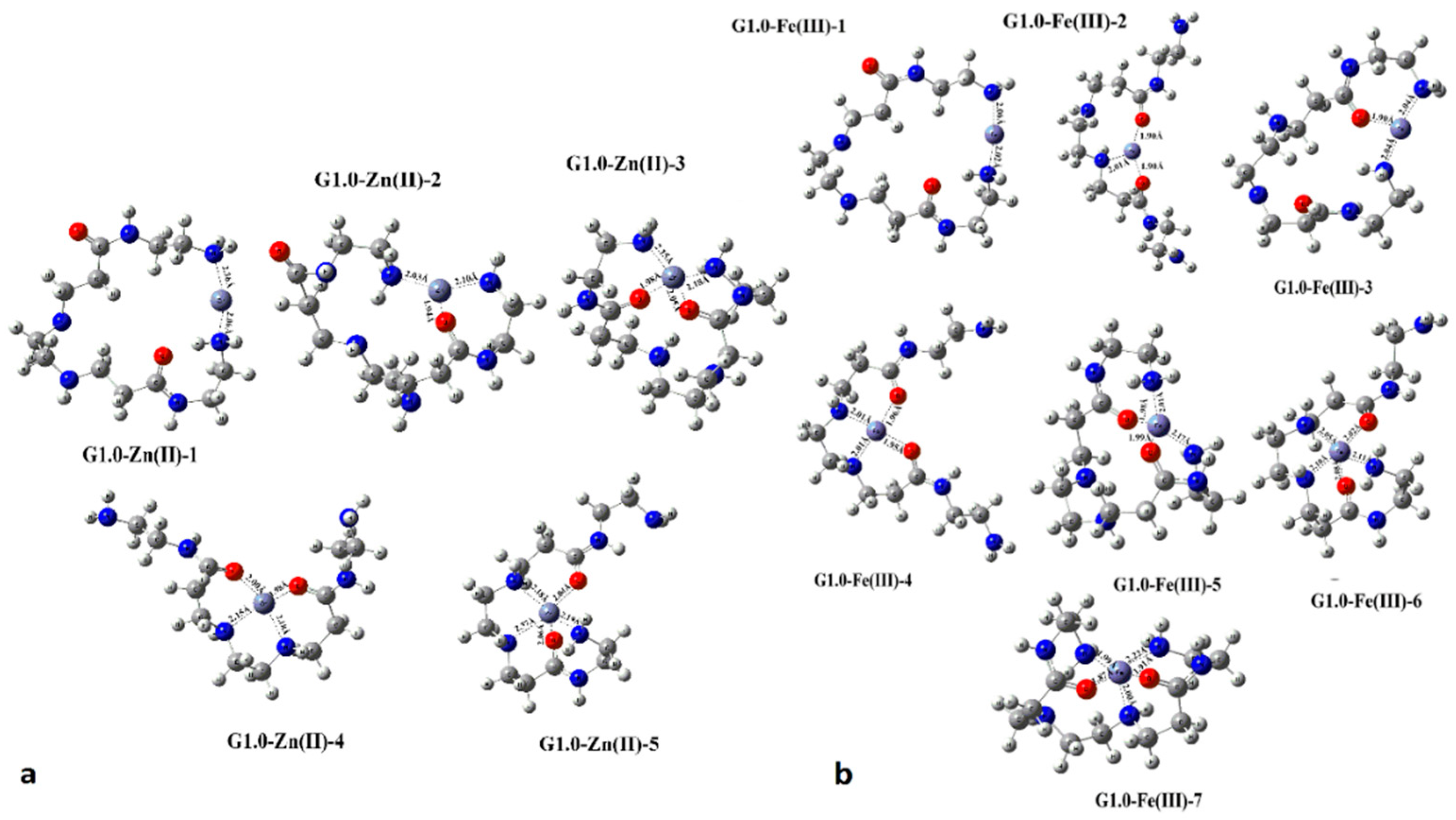


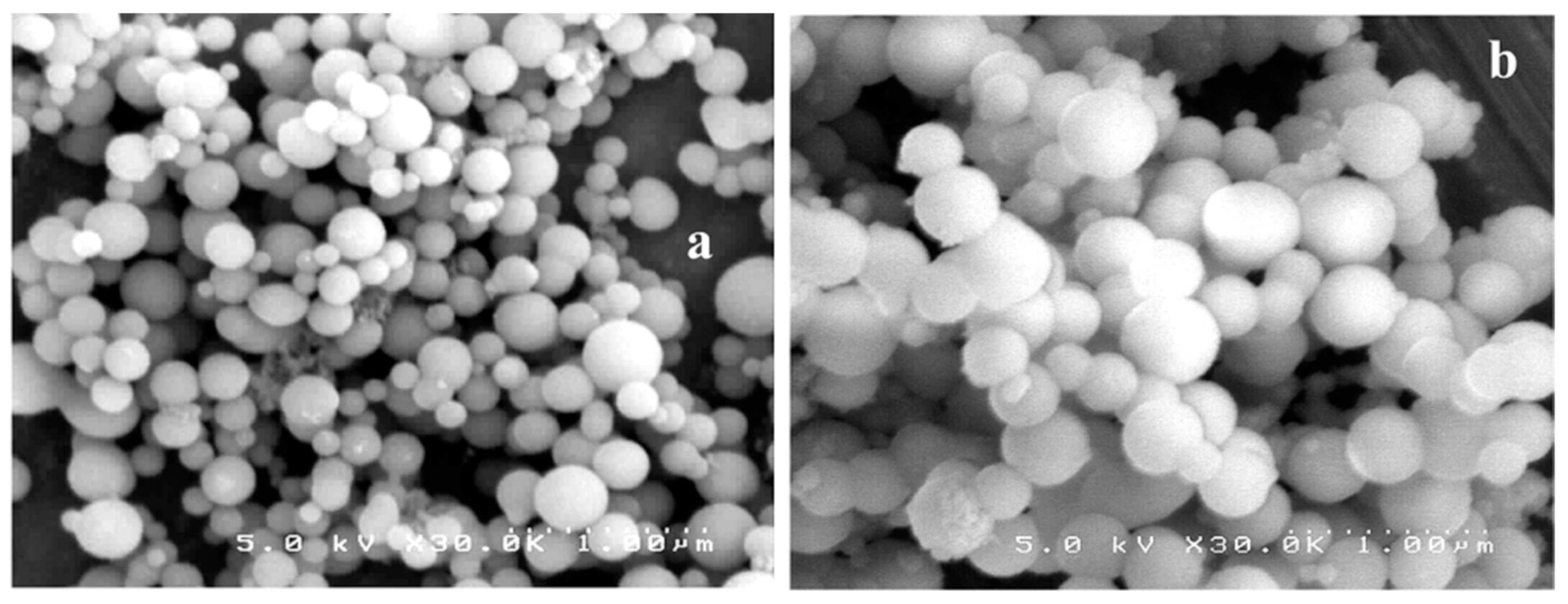
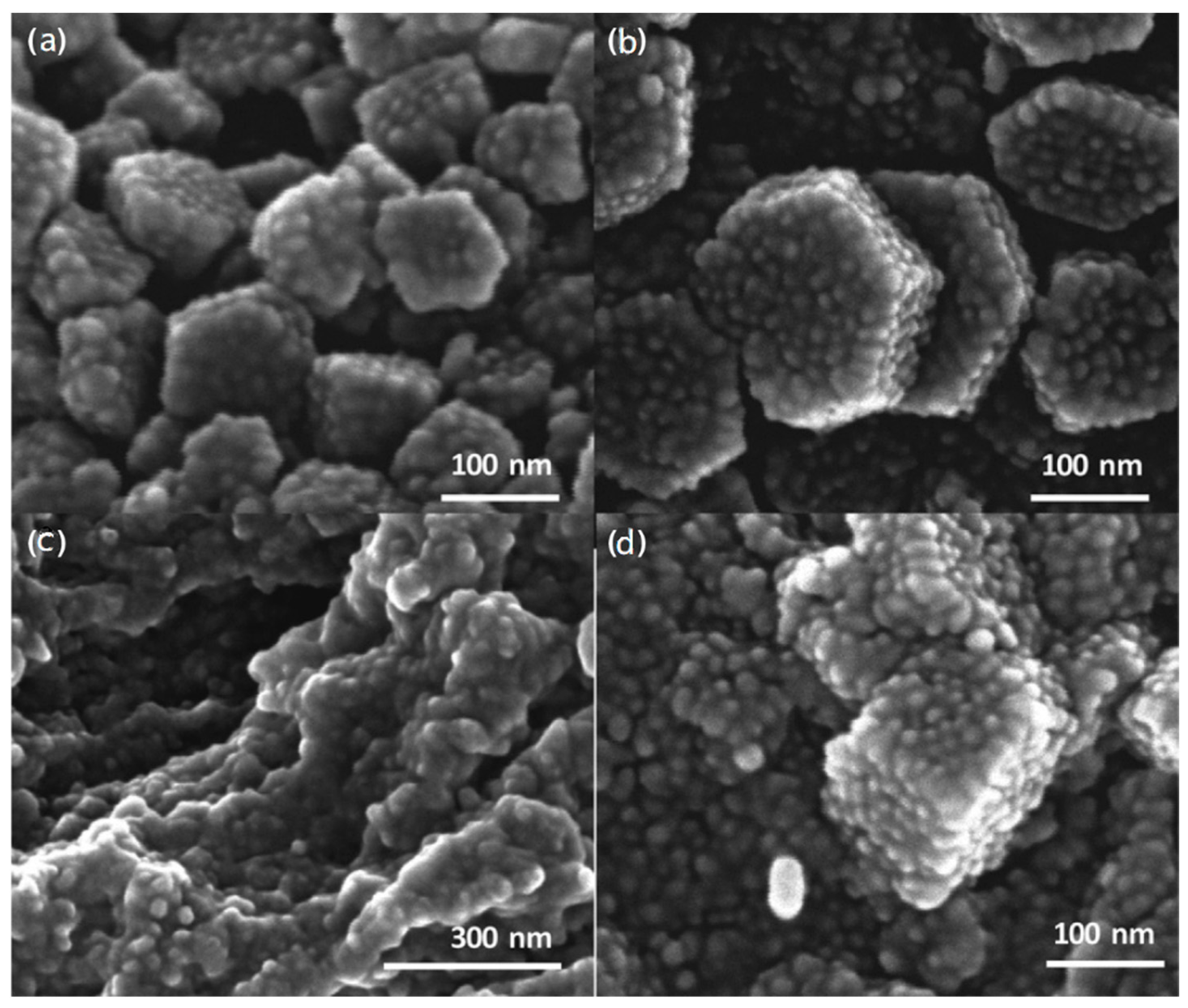
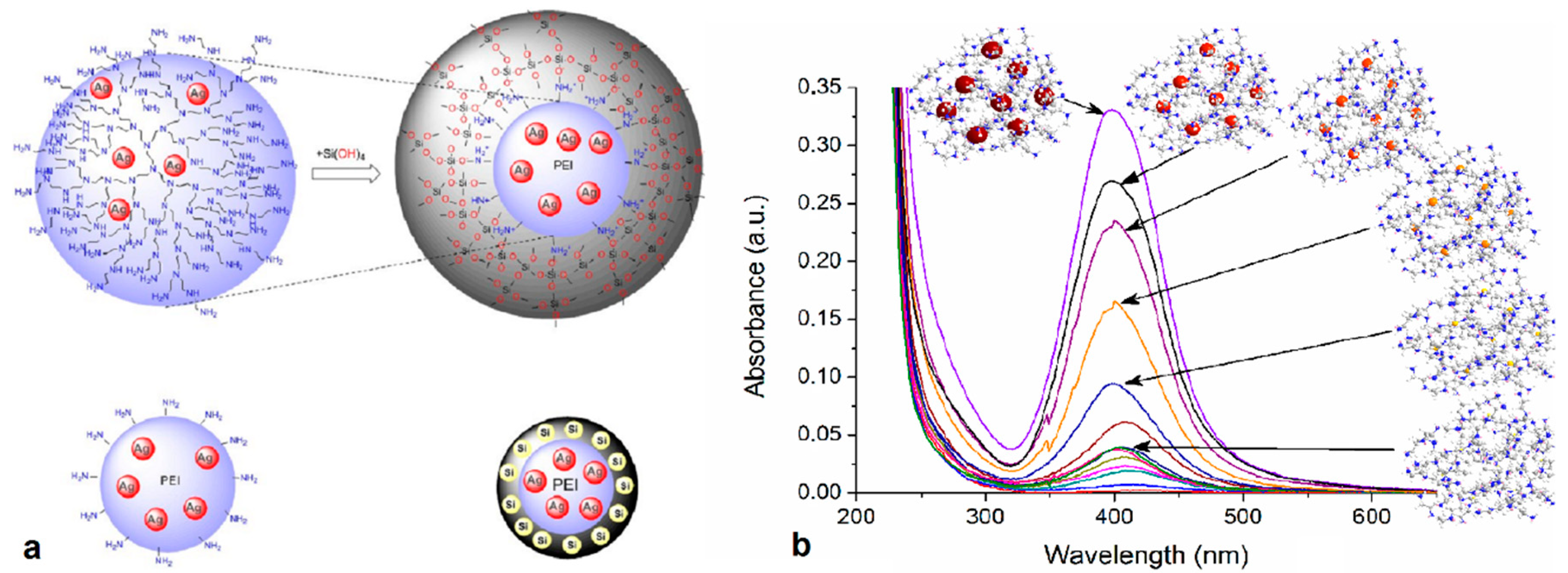
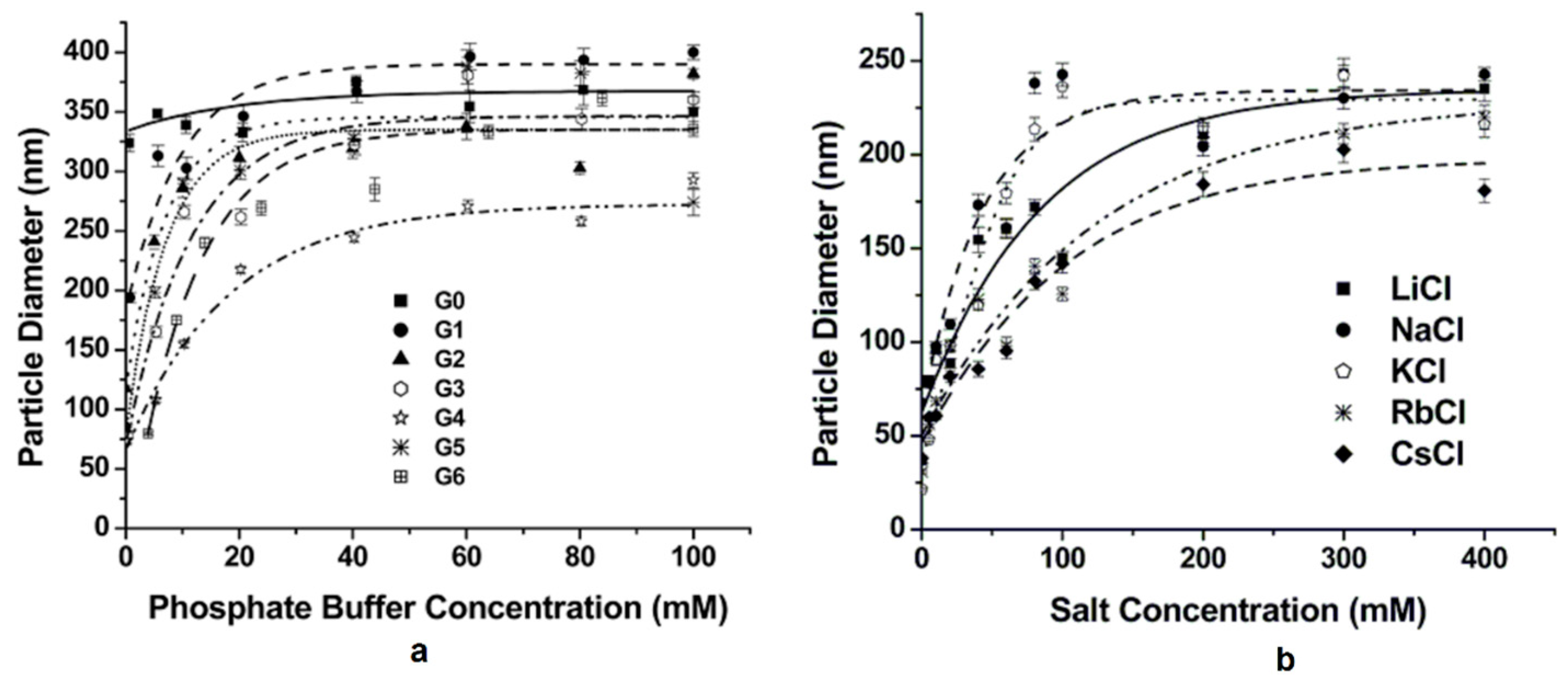
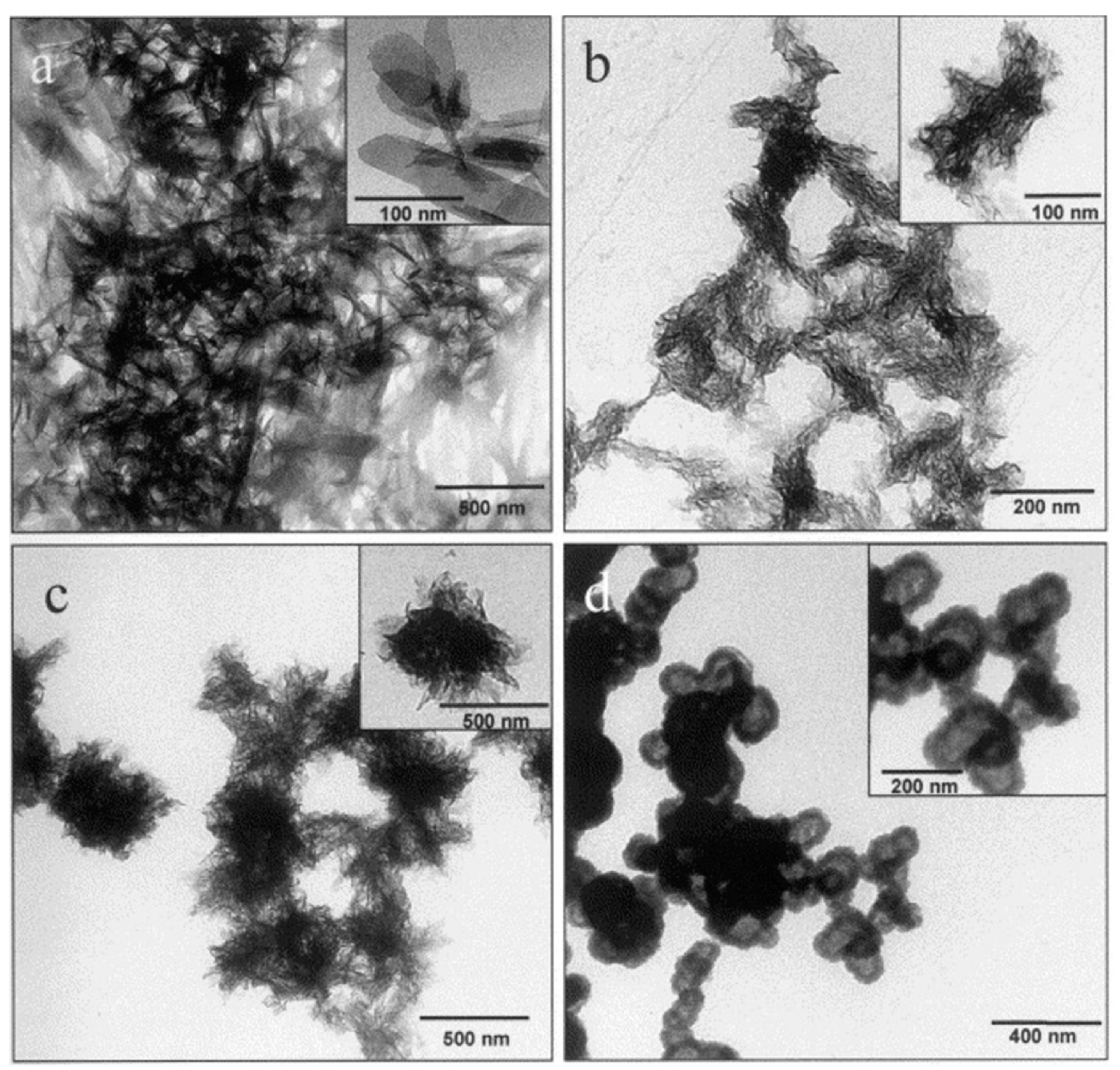
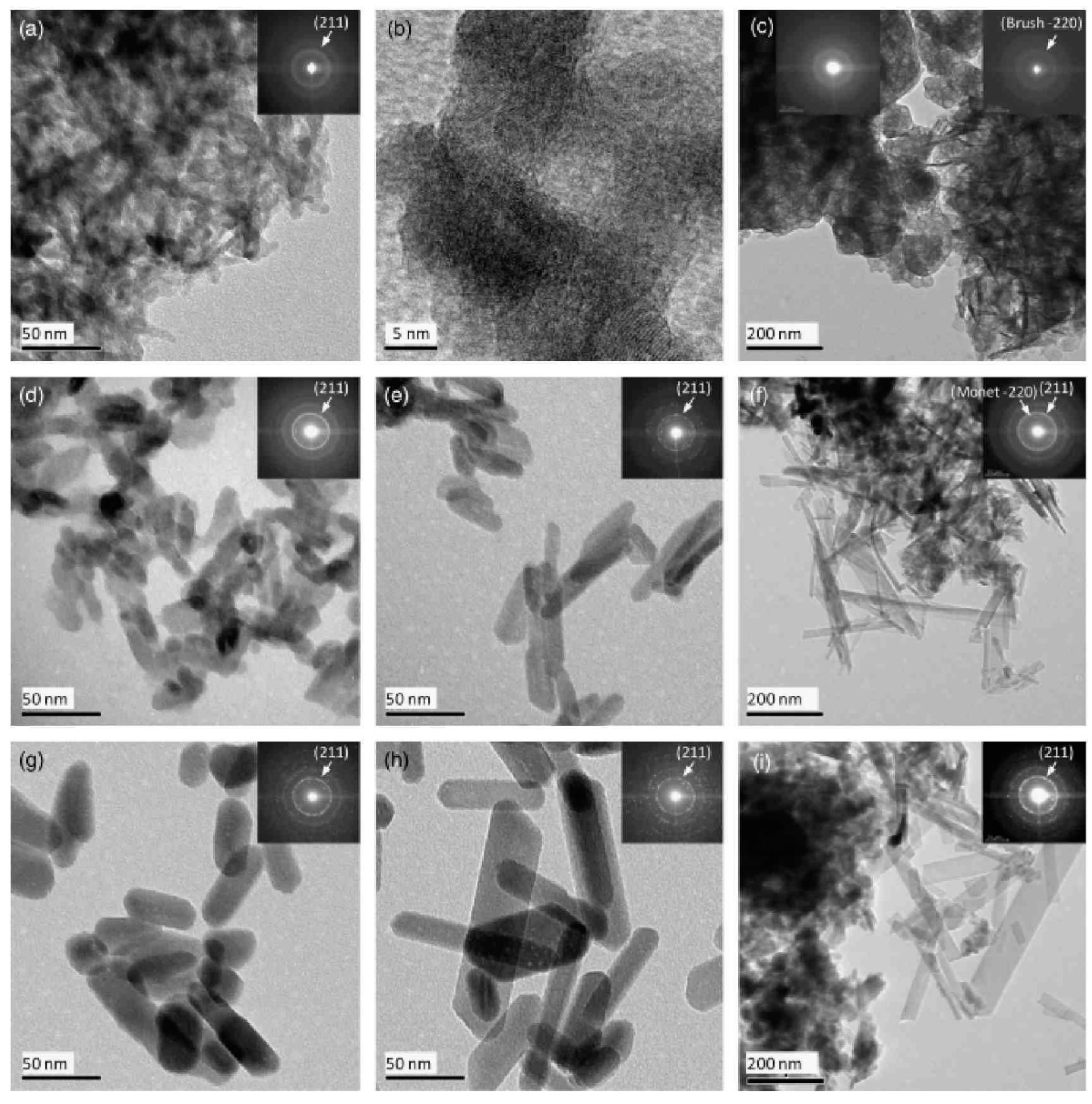
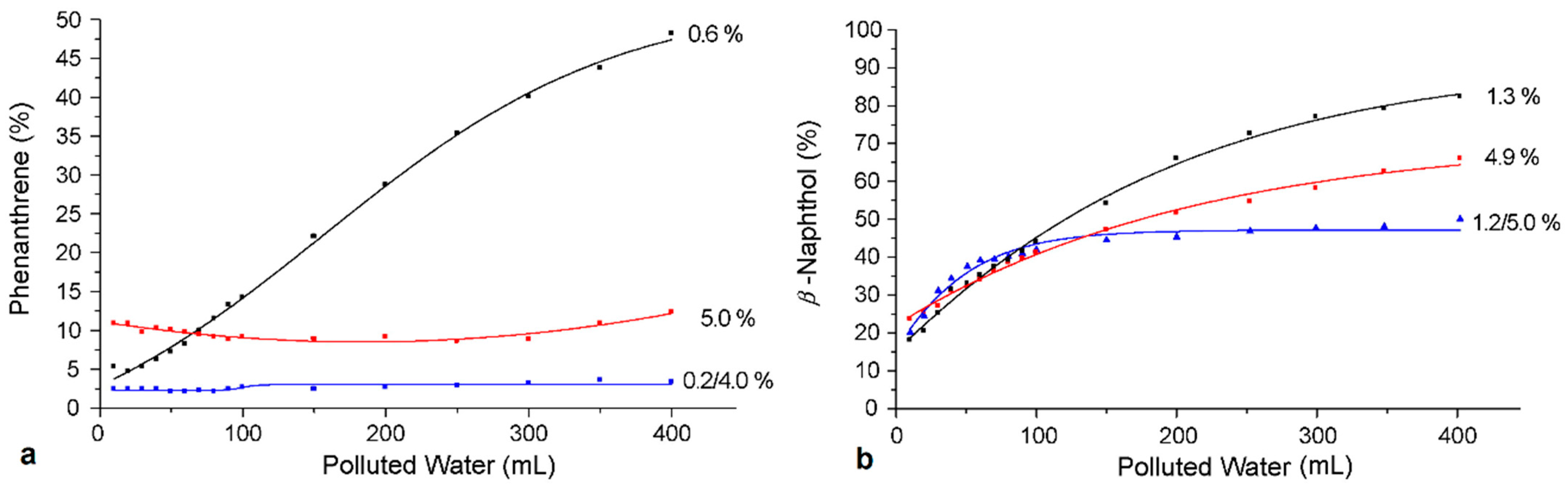
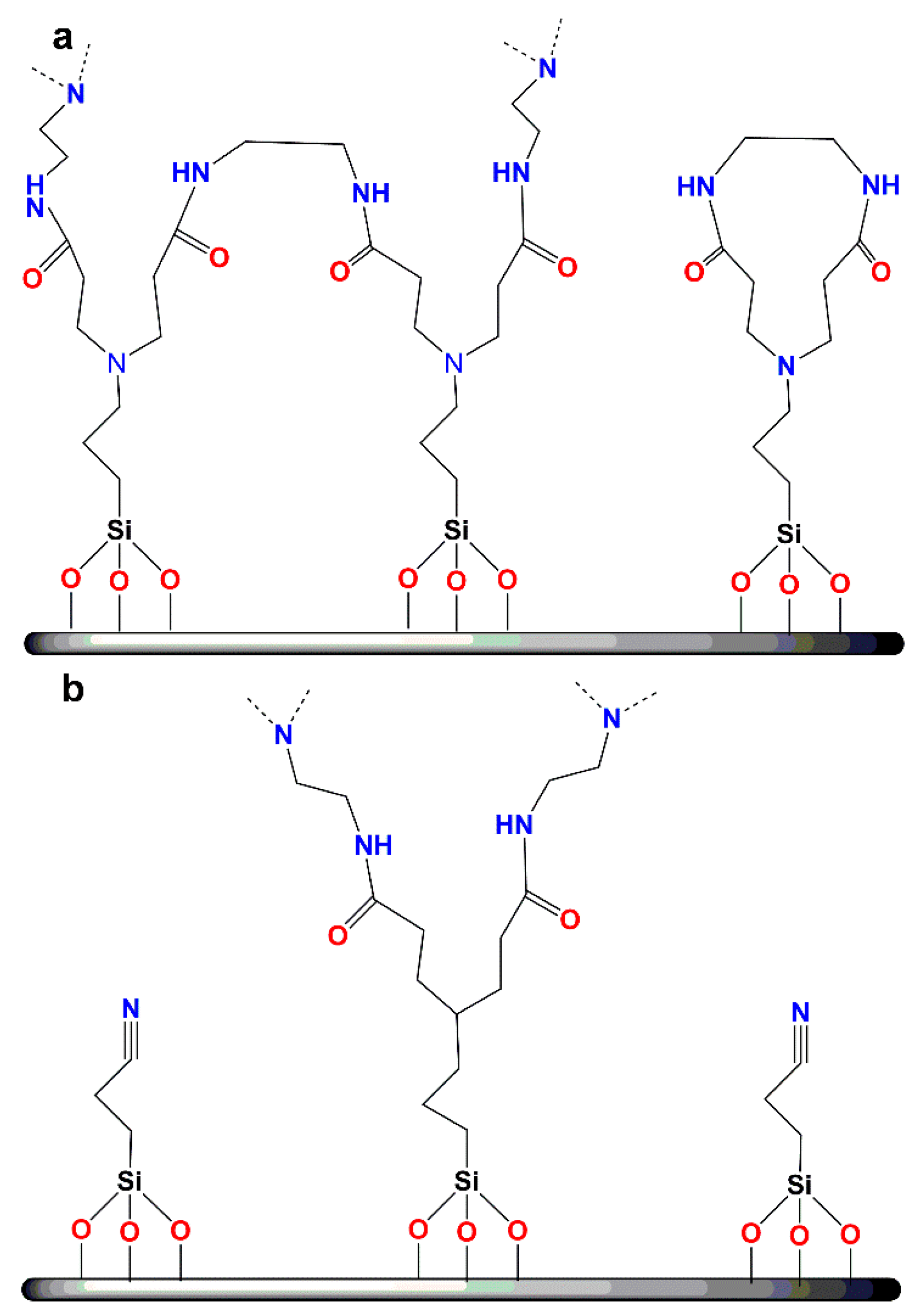
| Dendritic Polymer-Ceramic | Application | Reference |
|---|---|---|
| PPI-SiO2 | Electrochemical (Redox-Active Materials) | [65] |
| PAPAM-PDMS | High-Resolution Contact Printing | [68] |
| Benzyl-Terminated Dendrons-SiO2 | High-Resolution Capillary Gas Chromatography | [82] |
| Benzyl-Terminated Dendrons-SiO2 | Capillary microextraction | [83] |
| L-glutamic acid-Chiral-Dendrimers-SiO2 | HPLC | [90] |
| PAMAM-SiO2 | Photoluminescence Sensors | [91] |
| PAMAM-TiO2 | TiO2 Nanoparticle Stabilization | [92] |
| PAMAM-SiO2 | Size Exclusion Chromatography | [108] |
| Melamine Dendrons-Al2O3 | Polarity-Based Separation | [130] |
| PAMAMOS | Electronics, Photonics, Magnetics, Sensors, Coatings | [133,134,135] |
| Dendritic Polymer-Ceramic | Application | Reference |
|---|---|---|
| PEI-SiO2-TiO2 | Nitro-compounds Reduction | [29] |
| CBS-SiO2 | Ethylene Polymerization | [79] |
| Pyridylphenylene dendrons-SiO2 | Suzuki cross-coupling | [86] |
| Chiral-PAMAM-SiO2 | Enantiomeric Reactions | [87] |
| DAB-SiO2 | Selective Hydrogenation | [88] |
| PAMAM-SiO2 | Alcohol Oxidation | [113] |
| PAMAM-SiO2 | Olefin Hydroformylation | [114,115,116] |
| PAMAM-SiO2 | Carbonylation | [117,118,119] |
| PAMAM-SiO2 | Hydroesterification | [120] |
| PAMAM-SiO2 | Heck reaction | [121] |
| PAMAM-SiO2 | Olefin Epoxidation | [122] |
| PAMAM-SiO2 | Enzymolysis | [123] |
| PAMAM-SiO2 | Benzyl Alcohol, Methylene Blue Oxidation, Nitrophenol Reduction | [149] |
| DAB-SiO2 | Phenols Hydrogenation | [171] |
| PEI-SiO2 | CO, NO, CH4, C3H6, C3H8 Oxidation | [172] |
| PEI-SiO2-CeO2 | Nitrophenol Reduction | [173] |
| Dendritic Polymer-Ceramic | Application | Reference |
|---|---|---|
| DAB, PEI, PG-Al2O3, TiO2 SiC | PAH, BTX, THM, MTBE, Pesticides | [58,59] |
| PEI-Al2O3 | PAH | [61] |
| PAMAM-SiO2 | Fe4[Fe(CN)6]3, Co2[Fe(CN)6] | [62] |
| PAMAM-TiO2 | Cu(II), Ni(II), Cr(III) | [67] |
| PAMAM-SiO2 | Hg(II) | [80,109,110,112] |
| PPH-SiO2 | CO2 | [84] |
| PEI-SiO2 | Cu(II), Zn(II), Cd(II) | [85] |
| PAMAM-SiO2 | Cu(II) | [94] |
| Melamine Dendrons-SiO2 | Atrazine | [95] |
| PAMAM-Dendrons-SiO2 | Organic Dyes, Amphiphilic Surfactants | [97] |
| PAMAM-SiO2 | Zn(II), Co(II) | [98] |
| PAMAM-SiO2 | Mn(II) | [99] |
| PAMAM-SiO2 | Ni(II) | [100] |
| PAMAM-SiO2 | Co(II) | [101] |
| PAMAM-SiO2 | Cd(II) | [102,103] |
| PAMAM-SiO2 | Fe(III) | [103,104] |
| PAMAM-SiO2 | Pb(II) | [105] |
| PAMAM-SiO2 | U(IV) | [106] |
| PAMAM-SiO2 | Ag(I) | [111,112] |
| PEI-SiO2 | CO2 | [125,126] |
| TREN-SiO2 | CO2 | [129] |
| PEI-SiO2 | PAH, Pb(II), Hg(II), Cd(II), Cr(VI) | [148] |
| Dendritic Polymer-Ceramic | Application | Reference |
|---|---|---|
| PEI-HA | Hydroxy apatite formation | [30] |
| PAMAM-SiO2 | Gene therapy | [63] |
| PAMAM, CBS-SiO2 | Antibacterials | [77] |
| CBS-SiO2 | Oligonucleotide Delivery Carriers | [78] |
| PAMAM-SiO2 | Fluorescence Imaging | [81] |
| PPH-SiO2 | Antibacterials | [89] |
| PAMAM-SiO2 | Anticancer Photothermal Therapy | [124] |
| PEI-SiO2 | Orthopedic Implants | [138] |
| PEI-SiO2 | Antibacterials | [150] |
| PAMAM-HA | Hemoglobin Aquasomes | [156] |
| PAMAM-SiO2 | Gene transfection | [175] |
| Dendritic Polymer-Ceramic | Application | Reference |
|---|---|---|
| PAMAM-SiO2 | Doxorubicin, Curcumin | [64] |
| DAB-SiO2 | Ibuprofen | [74] |
| DAB-SiO2 | Levofloxacin | [75] |
| DAB-SiO2 | Redox-responsive release | [76] |
| PAMAM-SiO2 | Curcumin | [81] |
| PAMAM-SiO2 | Doxorubicin and Bcl-2 targeted siRNA | [124] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Douloudi, M.; Nikoli, E.; Katsika, T.; Vardavoulias, M.; Arkas, M. Dendritic Polymers as Promising Additives for the Manufacturing of Hybrid Organoceramic Nanocomposites with Ameliorated Properties Suitable for an Extensive Diversity of Applications. Nanomaterials 2021, 11, 19. https://doi.org/10.3390/nano11010019
Douloudi M, Nikoli E, Katsika T, Vardavoulias M, Arkas M. Dendritic Polymers as Promising Additives for the Manufacturing of Hybrid Organoceramic Nanocomposites with Ameliorated Properties Suitable for an Extensive Diversity of Applications. Nanomaterials. 2021; 11(1):19. https://doi.org/10.3390/nano11010019
Chicago/Turabian StyleDouloudi, Marilina, Eleni Nikoli, Theodora Katsika, Michalis Vardavoulias, and Michael Arkas. 2021. "Dendritic Polymers as Promising Additives for the Manufacturing of Hybrid Organoceramic Nanocomposites with Ameliorated Properties Suitable for an Extensive Diversity of Applications" Nanomaterials 11, no. 1: 19. https://doi.org/10.3390/nano11010019
APA StyleDouloudi, M., Nikoli, E., Katsika, T., Vardavoulias, M., & Arkas, M. (2021). Dendritic Polymers as Promising Additives for the Manufacturing of Hybrid Organoceramic Nanocomposites with Ameliorated Properties Suitable for an Extensive Diversity of Applications. Nanomaterials, 11(1), 19. https://doi.org/10.3390/nano11010019






