Effect of Water and Glycerol in Deoxygenation of Coconut Oil over Bimetallic NiCo/SAPO-11 Nanocatalyst under N2 Atmosphere
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Catalyst Preparation
2.3. Catalyst Characterization
2.4. Catalytic Deoxygenation Testing in Continuous-Flow Fixed-Bed Reactor
2.5. Liquid and Gaseous Product Analysis
3. Results and Discussion
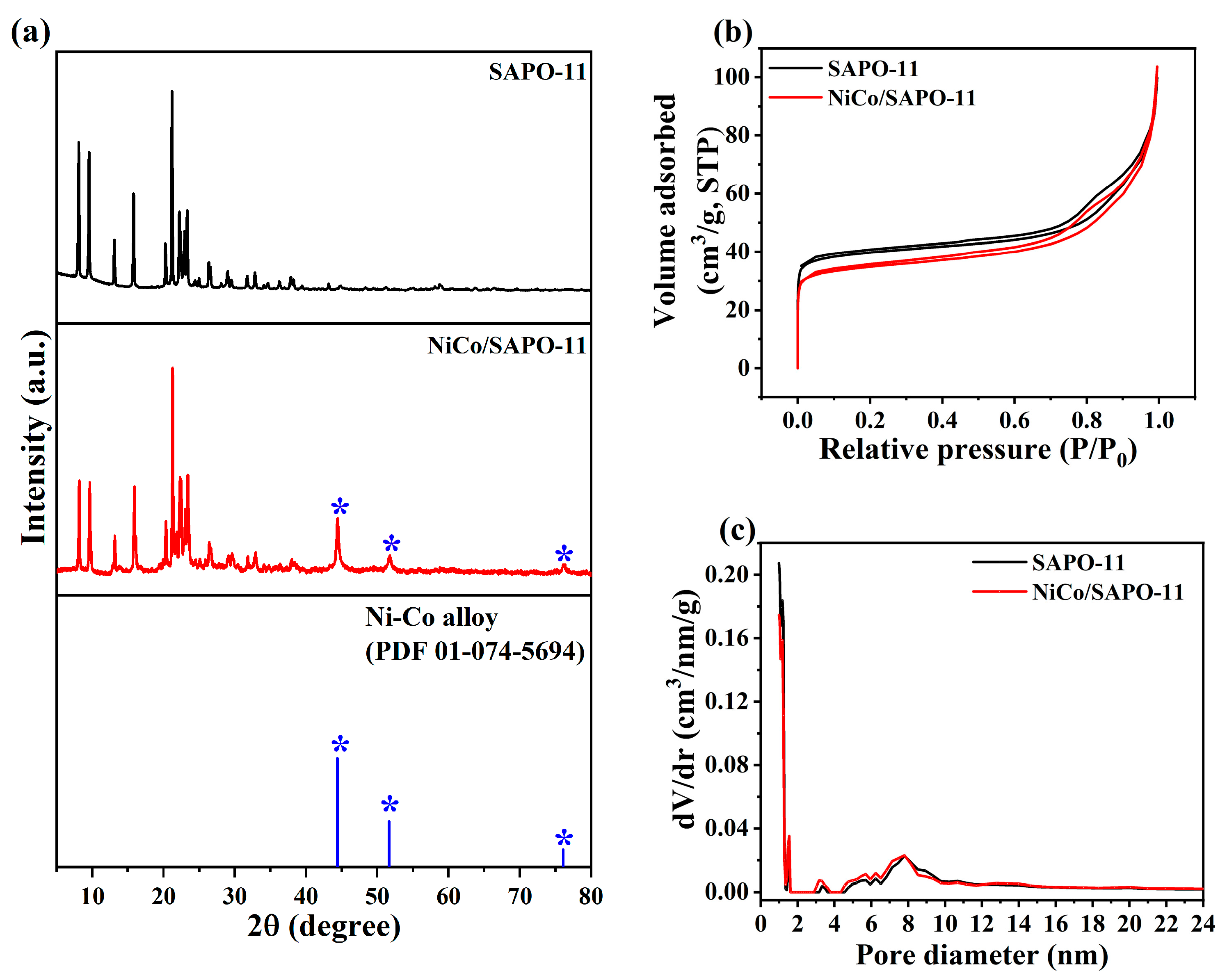
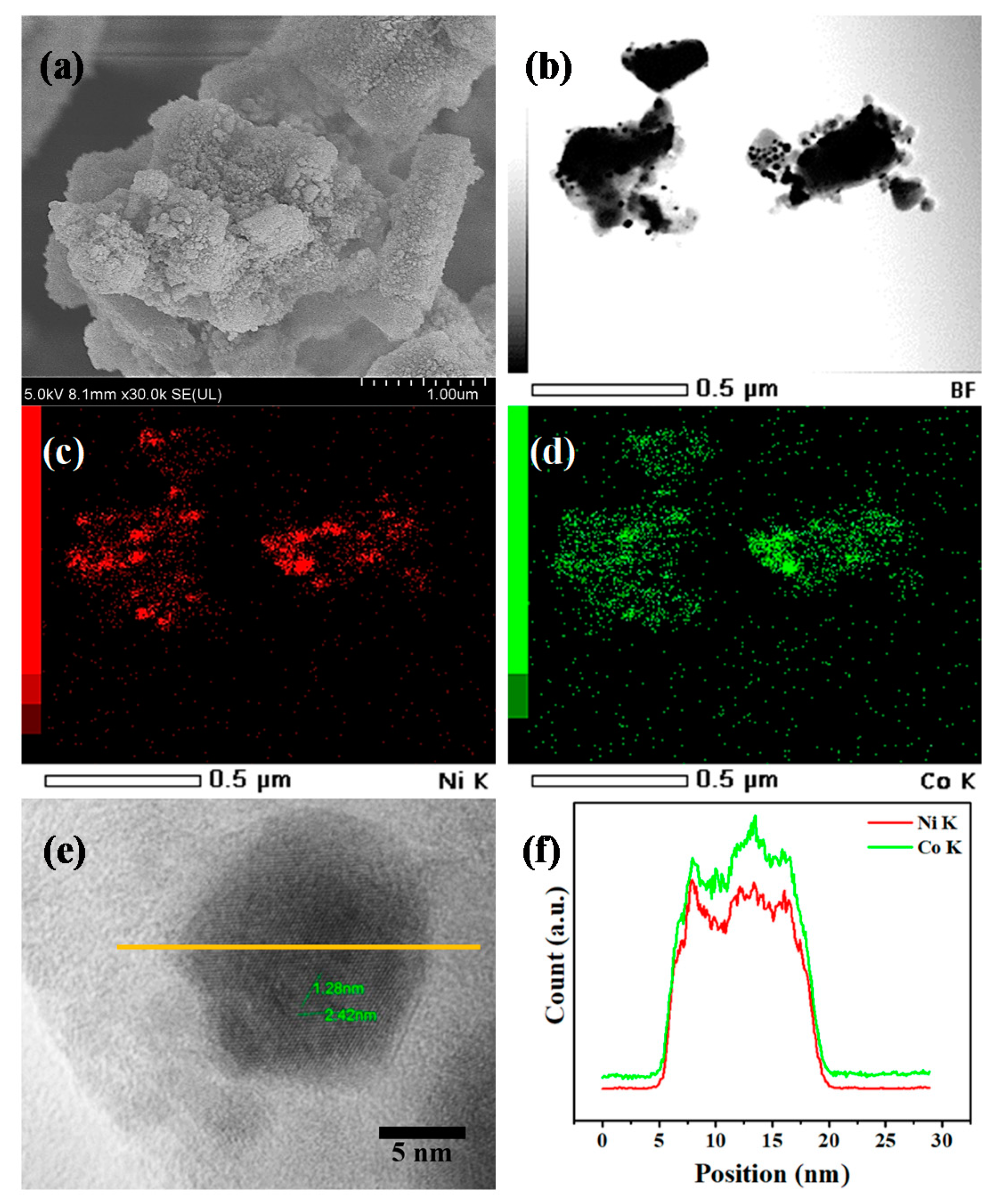
3.1. Catalyst Characterization
3.2. Feed Compositions
3.3. Hydrogen Production from Glycerol Aqueous-Phase Reforming (Gly-APR) over NiCo/SAPO-11
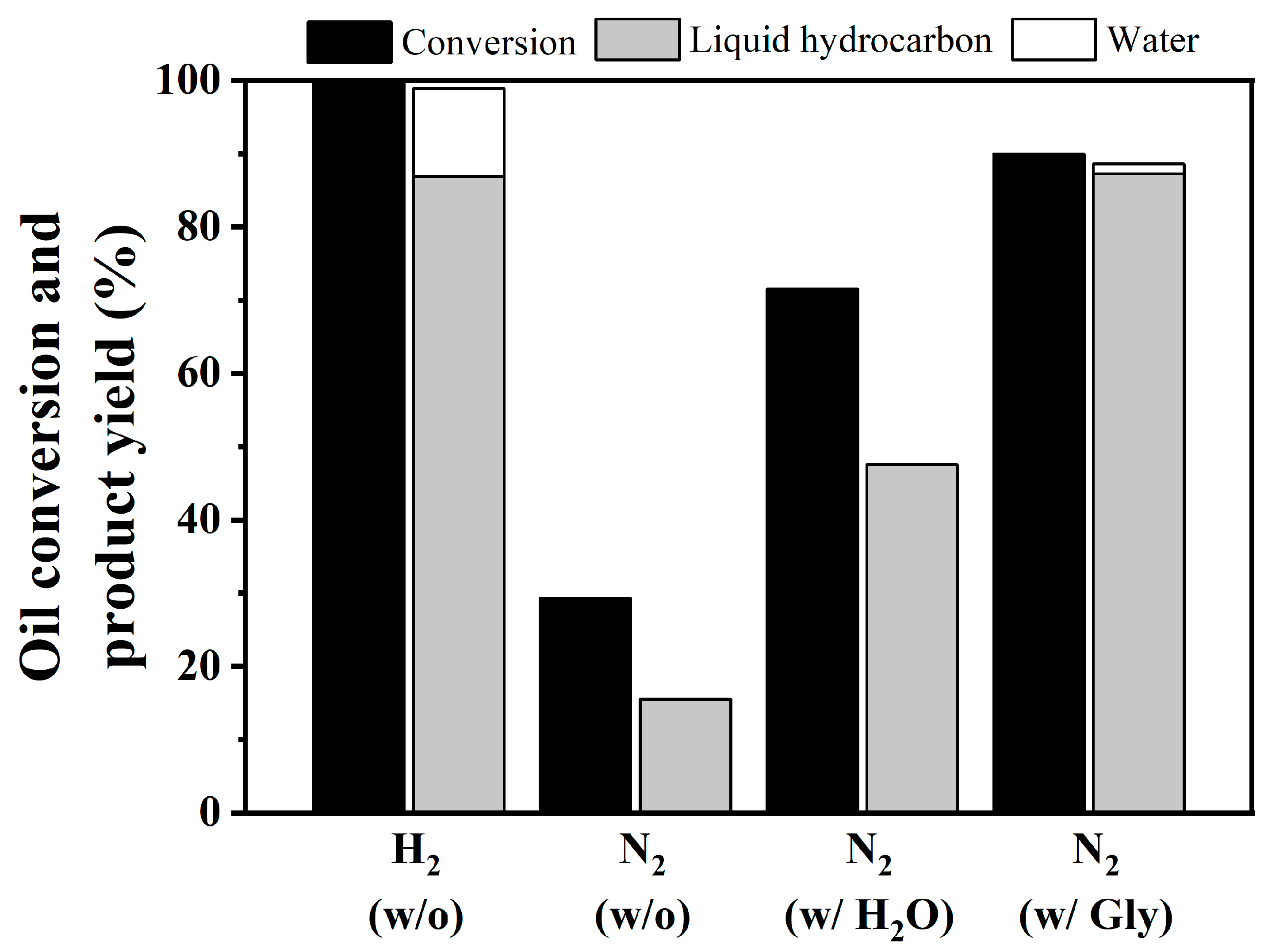
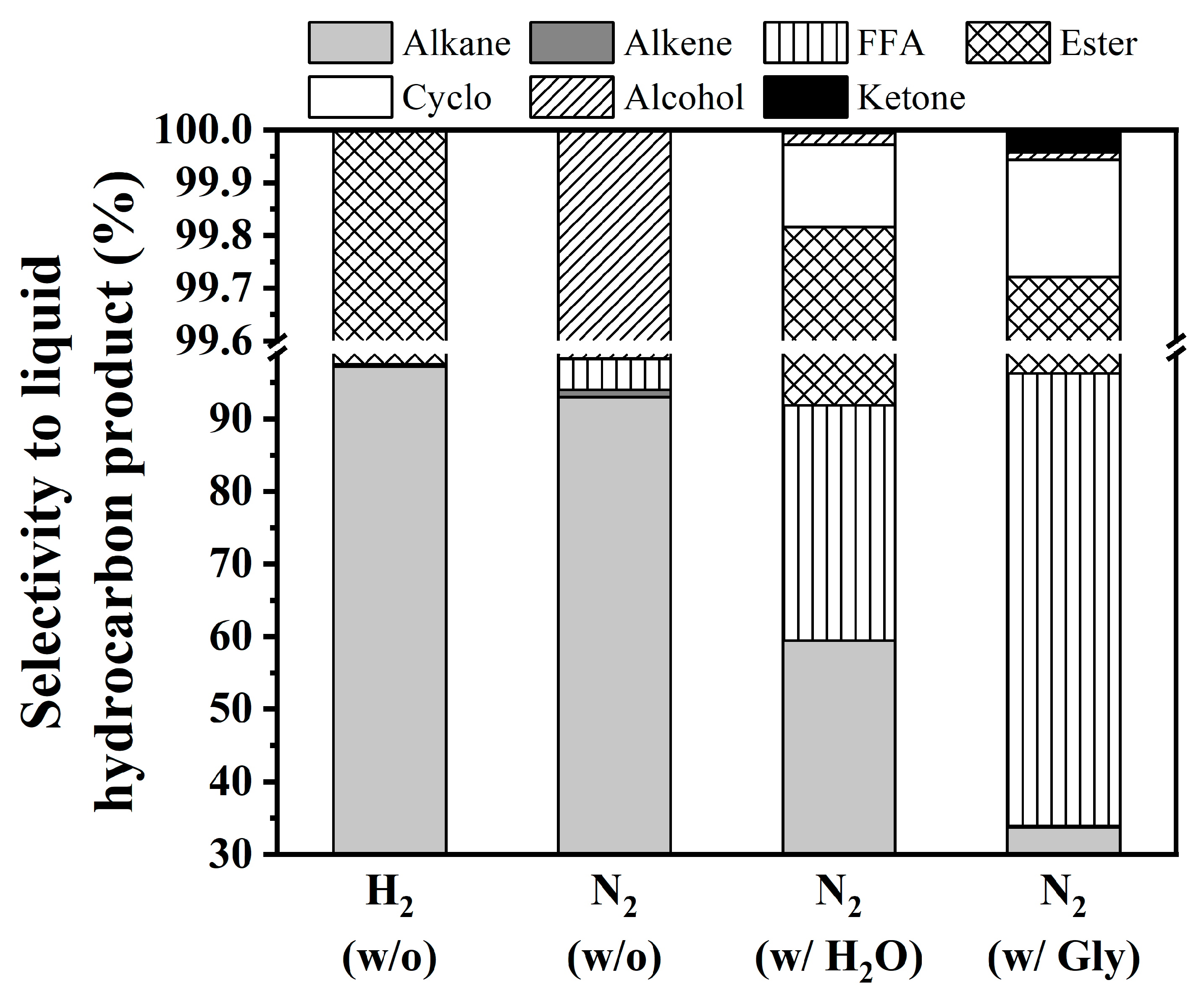
3.4. Catalytic Performance of NiCo/SAPO-11 over In-Situ HDO of Coconut Oil
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yeletsky, P.M.; Kukushkin, R.G.; Yakovlev, V.A.; Chen, B.H. Recent advances in one-stage conversion of lipid-based biomass-derived oils into fuel components—aromatics and isomerized alkanes. Fuel 2020, 278, 118255. [Google Scholar] [CrossRef]
- The European Parliament; The Council of the European Union. Directive 2009/28/EC of the European Parliament and of the Council of 23 April 2009, on the Promotion of the Use of Energy from Renewable Sources and Amending and Subsequently Repealing Directives 2001/77/EC and 2003/30/EC; The European Parliament: Brussels, Belgium, 2009. Available online: https://eur-lex.europa.eu/eli/dir/2009/30/oj (accessed on 18 November 2020).
- Abdullah, A.Z.; Razali, N.; Mootabadi, H.; Salamatinia, B. Critical technical areas for future improvement in biodiesel technologies. Environ. Res. Lett. 2007, 2, 34001. [Google Scholar] [CrossRef]
- Ogunkunle, O.; Ahmed, N.A. A review of global current scenario of biodiesel adoption and combustion in vehicular diesel engines. Energy Rep. 2019, 5, 1560–1579. [Google Scholar] [CrossRef]
- Huang, D.; Zhou, H.; Lin, L. Biodiesel: An alternative to conventional fuel. Energy Procedia 2012, 16, 1874–1885. [Google Scholar] [CrossRef]
- Phumpradit, S.; Reubroycharoen, P.; Kuchonthara, P.; Ngamcharussrivichai, C.; Hinchiranan, N. Partial hydrogenation of palm oil-derived biodiesel over Ni/Electrospun silica fiber catalysts. Catalysts 2020, 10, 993. [Google Scholar] [CrossRef]
- Li, X.; Luo, X.; Jin, Y.; OchoaLi, J.; Zhang, H.; Zhang, A.; Xie, J. Heterogeneous sulfur-free hydrodeoxygenation catalysts for selectively upgrading the renewable bio-oils to second generation biofuels. Renew. Sustain. Energy Rev. 2018, 82, 3762–3797. [Google Scholar] [CrossRef]
- Kordulis, C.; Bourikas, K.; Gousi, M.; Kordouli, E.; Lycourghiotis, A. Development of nickel based catalysts for the transformation of natural triglycerides and related compounds into green diesel: A critical review. Appl. Catal. B Environ. 2016, 181, 156–196. [Google Scholar] [CrossRef]
- Jin, W.; Pastor-Pérez, L.; Villora-Picó, J.J.; Sepúlveda-Escribano, A.; Gu, S.; Reina, T.R. Investigating new routes for biomass upgrading: “H2-free” hydrodeoxygenation using Ni-based catalysts. ACS Sustain. Chem. Eng. 2019, 7, 16041–16049. [Google Scholar] [CrossRef]
- Choo, M.-Y.; Juan, J.C.; Oi, L.E.; Ling, T.C.; Ng, E.-P.; Noorsaadah, A.R.; Centi, G.; Lee, K.T. The role of nanosized zeolite Y in the H2-free catalytic deoxygenation of triolein. Catal. Sci. Technol. 2019, 9, 772–782. [Google Scholar] [CrossRef]
- Hollak, S. Catalytic Deoxygenation of Fatty Acids and Triglycerides for Production of Fuels and Chemicals. Ph.D. Thesis, Utrecht University, Utrecht, The Netherlands, 2014. [Google Scholar]
- Chiappero, M.; Do, P.T.M.; Crossley, S.; Lobban, L.L.; Resasco, D.E. Direct conversion of triglycerides to olefins and paraffins over noble metal supported catalysts. Fuel 2011, 90, 1155–1165. [Google Scholar] [CrossRef]
- Morgan, T.; Grubb, D.; Santillan-Jimenez, E.; Crocker, M. Conversion of triglycerides to hydrocarbons over supported metal catalysts. Top. Catal. 2010, 53, 820–829. [Google Scholar] [CrossRef]
- Miao, C.; Marin-Flores, O.; Davidson, S.D.; Li, T.; Dong, T.; Gao, D.; Wang, Y.; Garcia-Pérez, M.; Chen, S. Hydrothermal catalytic deoxygenation of palmitic acid over nickel catalyst. Fuel 2016, 166, 302–308. [Google Scholar] [CrossRef]
- Asikin-Mijan, N.; Lee, H.V.; Juan, J.C.; Noorsaadah, A.R.; Ong, H.C.; Razali, S.M.; Taufiq-Yap, Y.H. Promoting deoxygenation of triglycerides via Co-Ca loaded SiO2-Al2O3 catalyst. Appl. Catal. Gen. 2018, 552, 38–48. [Google Scholar] [CrossRef]
- Cortright, R.D.; Davda, R.R.; Dumesic, J.A. Hydrogen from catalytic reforming of biomass-derived hydrocarbons in liquid water. Nature 2002, 418, 964–967. [Google Scholar] [CrossRef]
- Polychronopoulou, K.; Charisiou, N.; Papageridis, K.; Sebastian, V.; Hinder, S.; Dabbawala, A.; AlKhoori, A.; Baker, M.; Goula, M. The effect of Ni addition onto a Cu-based ternary support on the H2 production over glycerol steam reforming reaction. Nanomaterials 2018, 8, 931. [Google Scholar] [CrossRef]
- Chen, Z.; Kukushkin, R.G.; Yeletsky, P.M.; Saraev, A.A.; Bulavchenko, O.A.; Millan, M. Coupling hydrogenation of guaiacol with in-situ hydrogen production by glycerol aqueous reforming over Ni/Al2O3 and Ni-X/Al2O3 (X = Cu, Mo, P) Catalysts. Nanomaterials 2020, 10, 1420. [Google Scholar] [CrossRef]
- Aman, D.; Radwan, D.; Ebaid, M.; Mikhail, S.; van Steen, E. Comparing nickel and cobalt perovskites for steam reforming of glycerol. Mol. Catal. 2018, 452, 60–67. [Google Scholar] [CrossRef]
- Li, K.T.; Yen, R.H. Aqueous-phase hydrogenolysis of glycerol over Re promoted Ru catalysts encapuslated in porous silica nanoparticles. Nanomaterials 2018, 8, 153. [Google Scholar] [CrossRef]
- Zhong, H.; Yao, G.; Cui, X.; Yan, P.; Wang, X.; Jin, F. Selective conversion of carbon dioxide into methane with a 98% yield on an in-situ formed Ni nanoparticle catalyst in water. Chem. Eng. J. 2019, 357, 421–427. [Google Scholar] [CrossRef]
- Hu, X.; Li, P.; Zhang, X.; Yu, B.; Lv, C.; Zeng, N.; Luo, J.; Zhang, Z.; Song, J.; Liu, Y. Ni-based catalyst derived from NiAl layered double hydroxide for vapor phase catalytic exchange between hydrogen and water. Nanomaterials 2019, 9, 1688. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, Z.; Song, H.; Bai, P.; Xing, W.; Yan, Z.; Zhao, L.; Zhang, Z.; Gao, X. Synthesis of hierarchical SAPO-11 for hydroisomerization reaction in refinery processes. Appl. Petrochem. Res. 2014, 4, 351–358. [Google Scholar] [CrossRef][Green Version]
- Zuo, H.; Liu, Q.; Wang, T.; Ma, L.; Zhang, Q.; Zhang, Q. Hydrodeoxygenation of methyl palmitate over supported Ni catalysts for diesel-like fuel production. Energy Fuels 2012, 26, 3747–3755. [Google Scholar] [CrossRef]
- Srifa, A.; Faungnawakij, K.; Itthibenchapong, V.; Assabumrungrat, S. Roles of monometallic catalysts in hydrodeoxygenation of palm oil to green diesel. Chem. Eng. J. 2015, 278, 249–258. [Google Scholar] [CrossRef]
- Kaewmeesri, R.; Srifa, A.; Itthibenchapong, V.; Faungnawakij, K. Deoxygenation of waste chicken fats to green diesel over Ni/Al2O3: Effect of water and free fatty acid content. Energy Fuels 2015, 29, 833–840. [Google Scholar] [CrossRef]
- Huynh, T.M.; Armbruster, U.; Phan, B.M.Q.; Nguyen, D.A.; Martin, A. The influence of cobalt in bimetallic Ni-Co catalyst supported on H-Beta for phenol hydrodeoxygenation. Chim. Oggi. Chem. Today 2014, 32, 40–44. [Google Scholar]
- Sotomayor, F.J.; Cychosz, K.A.; Thommes, M. Characterization of micro/mesoporous materials by physisorption: Concepts and case studies. Acc. Mater. Surf. Res. 2018, 3, 36–37. [Google Scholar]
- Said, S.; Zaky, M.T. Pt/SAPO-11 catalysts: Effect of platinum loading method on the hydroisomerization of n-hexadecane. Catal. Lett. 2019, 149, 2119–2131. [Google Scholar] [CrossRef]
- San-José-Alonso, D.; Juan-Juan, J.; Illán-Gómez, M.J.; Román-Martínez, M.C. Ni, Co and bimetallic Ni–Co catalysts for the dry reforming of methane. Appl. Catal. A Gen. 2009, 371, 54–59. [Google Scholar] [CrossRef]
- Gao, X.; Tan, Z.; Hidajat, K.; Kawi, S. Highly reactive Ni-Co/SiO2 bimetallic catalyst via complexation with oleylamine/oleic acid organic pair for dry reforming of methane. Catal. Today 2017, 281, 250–258. [Google Scholar] [CrossRef]
- Reynoso, A.J.; Iriarte-Velasco, U.; Gutiérrez-Ortiz, M.A.; Ayastuy, J.L. Highly stable Pt/CoAl2O4 catalysts in aqueous-phase reforming of glycerol. Catal. Today 2020, in press. [Google Scholar] [CrossRef]
- Reynoso, A.J.; Ayastuy, J.L.; Iriarte-Velasco, U.; Gutiérrez-Ortiz, M.A. Cobalt aluminate spinel-derived catalysts for glycerol aqueous phase reforming. Appl. Catal. B Environ. 2018, 239, 86–101. [Google Scholar] [CrossRef]
- Wei, Y.; Li, S.; Jing, J. Synthesis of Cu–Co catalysts for methanol decomposition to hydrogen production via deposition–precipitation with urea method. Catal. Lett. 2019, 149, 2671–2682. [Google Scholar] [CrossRef]
- Wang, L.; Guan, E.; Wang, Y. Silica accelerates the selective hydrogenation of CO2 to methanol on cobalt catalysts. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Xu, J.; Gong, X.; Hu, R. Performance of a Ni-Cu-Co/Al2O3 catalyst on in-situ hydrodeoxygenation of bio-derived phenol. Catalysts 2019, 9, 952. [Google Scholar] [CrossRef]
- Papadopoulou, E.; Ioannides, T. Methanol reforming over cobalt catalysts prepared from fumarate precursors: TPD investigation. Catalysts 2016, 6, 33. [Google Scholar] [CrossRef]
- Krobkrong, N.; Itthibenchapong, V.; Khongpracha, P.; Faungnawakij, K. Deoxygenation of oleic acid under an inert atmosphere using molybdenum oxide-based catalysts. Energy Convers. Manag. 2018, 167, 1–8. [Google Scholar] [CrossRef]
- Authayanun, S.; Arpornwichanop, A.; Paengjuntuek, W.; Assabumrungrat, S. Thermodynamic study of hydrogen production from crude glycerol autothermal reforming for fuel cell applications. Int. J. Hydrogen Energy 2010, 35, 6617–6623. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, C.; Liu, Y.; Tang, S.; Chen, G.; Zhang, R.; Tang, X. Coke formation on the surface of Ni/HZSM-5 and Ni-Cu/HZSM-5 catalysts during bio-oil hydrodeoxygenation. Fuel 2017, 189, 23–31. [Google Scholar] [CrossRef]
- Gosselink, R.W.; Hollak, S.A.; Chang, S.W.; van Haveren, J.; de Jong, K.P.; Bitter, J.H.; van Es, D.S. Reaction pathways for the deoxygenation of vegetable oils and related model compounds. ChemSusChem 2013, 6, 1576–1594. [Google Scholar] [CrossRef]
- Avasthi, K.S.; Reddy, R.N.; Patel, S. Challenges in the production of hydrogen from glycerol—A biodiesel byproduct via steam reforming process. Procedia Eng. 2013, 51, 423–429. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Gong, X.; Guo, Z. The intensification technologies to water electrolysis for hydrogen production—A review. Renew. Sustain. Energy Rev. 2014, 29, 573–588. [Google Scholar] [CrossRef]
- Hollak, S.A.; Ariëns, M.A.; de Jong, K.P.; van Es, D.S. Hydrothermal deoxygenation of triglycerides over Pd/C aided by in-situ hydrogen production from glycerol reforming. ChemSusChem 2014, 7, 1057–1062. [Google Scholar] [CrossRef]
- Hu, W.; Wang, H.; Lin, H.; Zheng, Y.; Ng, S.; Shi, M.; Zhao, Y.; Xu, R. Catalytic decomposition of oleic acid to fuels and chemicals: Roles of catalyst acidity and basicity on product distribution and reaction pathways. Catalysts 2019, 9, 1063. [Google Scholar] [CrossRef]
- Sousa, F.P.; Silva, L.N.; de Rezende, D.B.; de Oliveira, L.C.A.; Pasa, V.M.D. Simultaneous deoxygenation, cracking and isomerization of palm kernel oil and palm olein over beta zeolite to produce biogasoline, green diesel and biojet-fuel. Fuel 2018, 223, 149–156. [Google Scholar] [CrossRef]


| Fatty Acid | Content (wt%) |
|---|---|
| Octanoic acid (C8:0) | 0.9 |
| Decanoic acid (C10:0) | 3.3 |
| Dodecanoic acid (C12:0) | 30.1 |
| Myristic acid (C14:0) | 21.5 |
| Palmitic acid (C16:0) | 17.9 |
| Stearic acid (C18:0) | 5.2 |
| Oleic acid (C18:1) | 15.9 |
| Linoleic acid (C18:2) | 4.8 |
| Eicosanoic acid (C20:0) | 0.2 |
| Eicosenoic acid (C20:1) | 0.2 |
| Saturated FFAs | 79.1 |
| Unsaturated FFAs | 20.9 |
| Oxygen | 12.9 |
| Liquid Product | Retention Time (min) | Selectivity (%) | |
|---|---|---|---|
| Acetaldehyde | 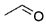 | 1.050 | 1.13 |
| Ethanol | 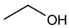 | 1.259 | 1.97 |
| 1-propanol, 2-methyl- | 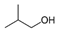 | 4.843 | 30.47 |
| 2-propanone, 1-hydroxy- |  | 10.585 | 56.75 |
| Propylene glycol | 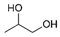 | 11.035 | 7.89 |
| Ethylene glycol | 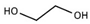 | 17.858 | 1.80 |
| Gas Product | Retention Time (min) | Yield (×10−9 mole) | Yield (×10−5 mol/mol Glycerol) |
|---|---|---|---|
| H2 | 1.78 | 0.0000084 | 0.00000024 |
| CO | 3.919 | 5.58528 | 1.5926 |
| CH4 | 4.393 | 6.85314 | 1.95412 |
| CO2 | 8.862 | 2.8679 | 0.81775 |
| Feed | Co-Reactant | Selectivity (%) | DCOx/HDO 3 | CO2/CO | |
|---|---|---|---|---|---|
| Jet Fuel 1 | Diesel Fuel 2 | ||||
| Coconut oil | H2 | 64.09 | 35.91 | 1.08 | 0.30 |
| N2 | 28.73 | 71.27 | 2.90 | 0.21 | |
| H2O | 62.58 | 37.42 | 1.05 | 0.59 | |
| Glycerol | 58.74 | 41.26 | 1.18 | 1.75 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaewmeesri, R.; Nonkumwong, J.; Witoon, T.; Laosiripojana, N.; Faungnawakij, K. Effect of Water and Glycerol in Deoxygenation of Coconut Oil over Bimetallic NiCo/SAPO-11 Nanocatalyst under N2 Atmosphere. Nanomaterials 2020, 10, 2548. https://doi.org/10.3390/nano10122548
Kaewmeesri R, Nonkumwong J, Witoon T, Laosiripojana N, Faungnawakij K. Effect of Water and Glycerol in Deoxygenation of Coconut Oil over Bimetallic NiCo/SAPO-11 Nanocatalyst under N2 Atmosphere. Nanomaterials. 2020; 10(12):2548. https://doi.org/10.3390/nano10122548
Chicago/Turabian StyleKaewmeesri, Rungnapa, Jeeranan Nonkumwong, Thongthai Witoon, Navadol Laosiripojana, and Kajornsak Faungnawakij. 2020. "Effect of Water and Glycerol in Deoxygenation of Coconut Oil over Bimetallic NiCo/SAPO-11 Nanocatalyst under N2 Atmosphere" Nanomaterials 10, no. 12: 2548. https://doi.org/10.3390/nano10122548
APA StyleKaewmeesri, R., Nonkumwong, J., Witoon, T., Laosiripojana, N., & Faungnawakij, K. (2020). Effect of Water and Glycerol in Deoxygenation of Coconut Oil over Bimetallic NiCo/SAPO-11 Nanocatalyst under N2 Atmosphere. Nanomaterials, 10(12), 2548. https://doi.org/10.3390/nano10122548





