Photostability of Contrast Agents for Photoacoustics: The Case of Gold Nanorods
Abstract
1. Introduction
2. Photoacoustic Imaging in a Nutshell
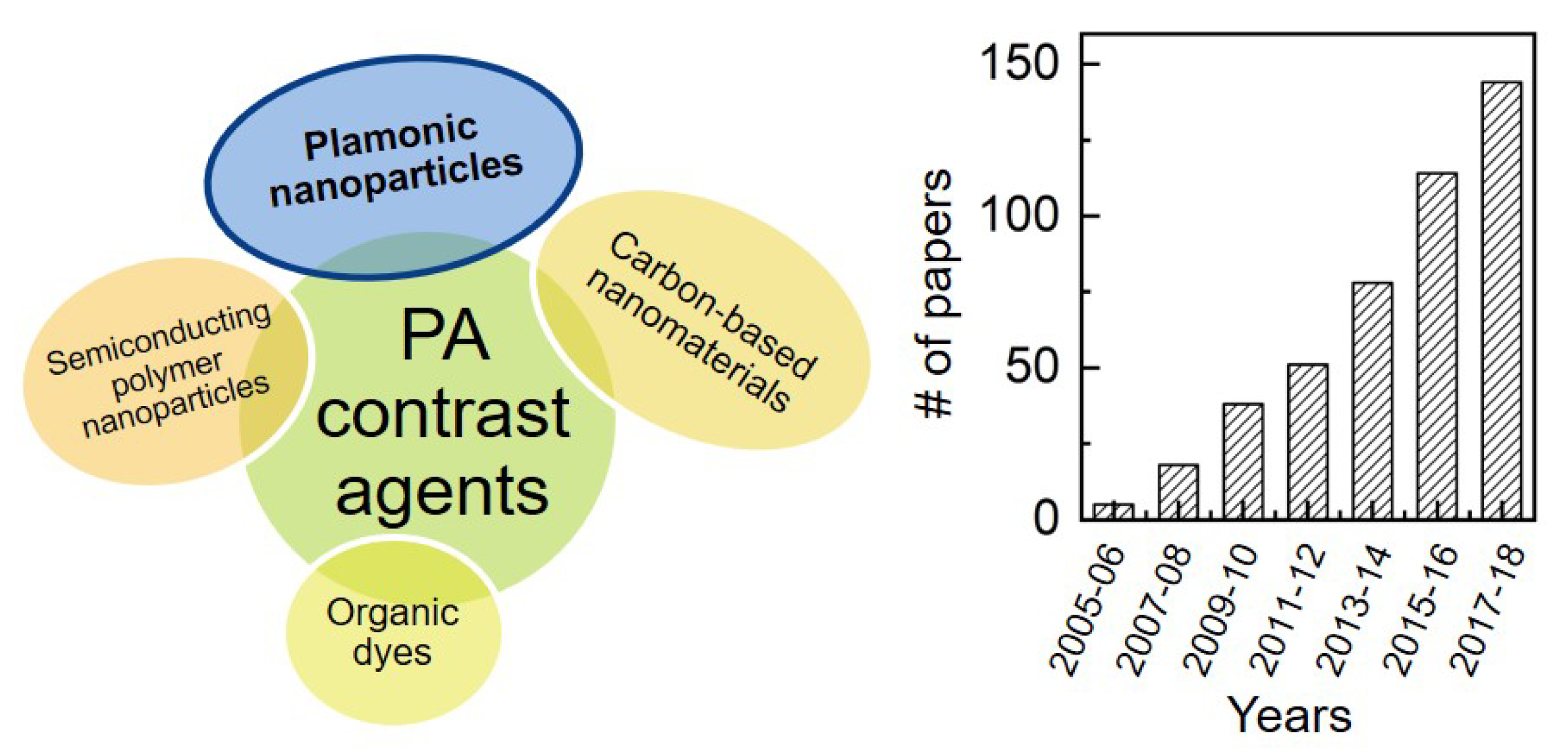
3. Contrast Agents for Photoacoustic Imaging
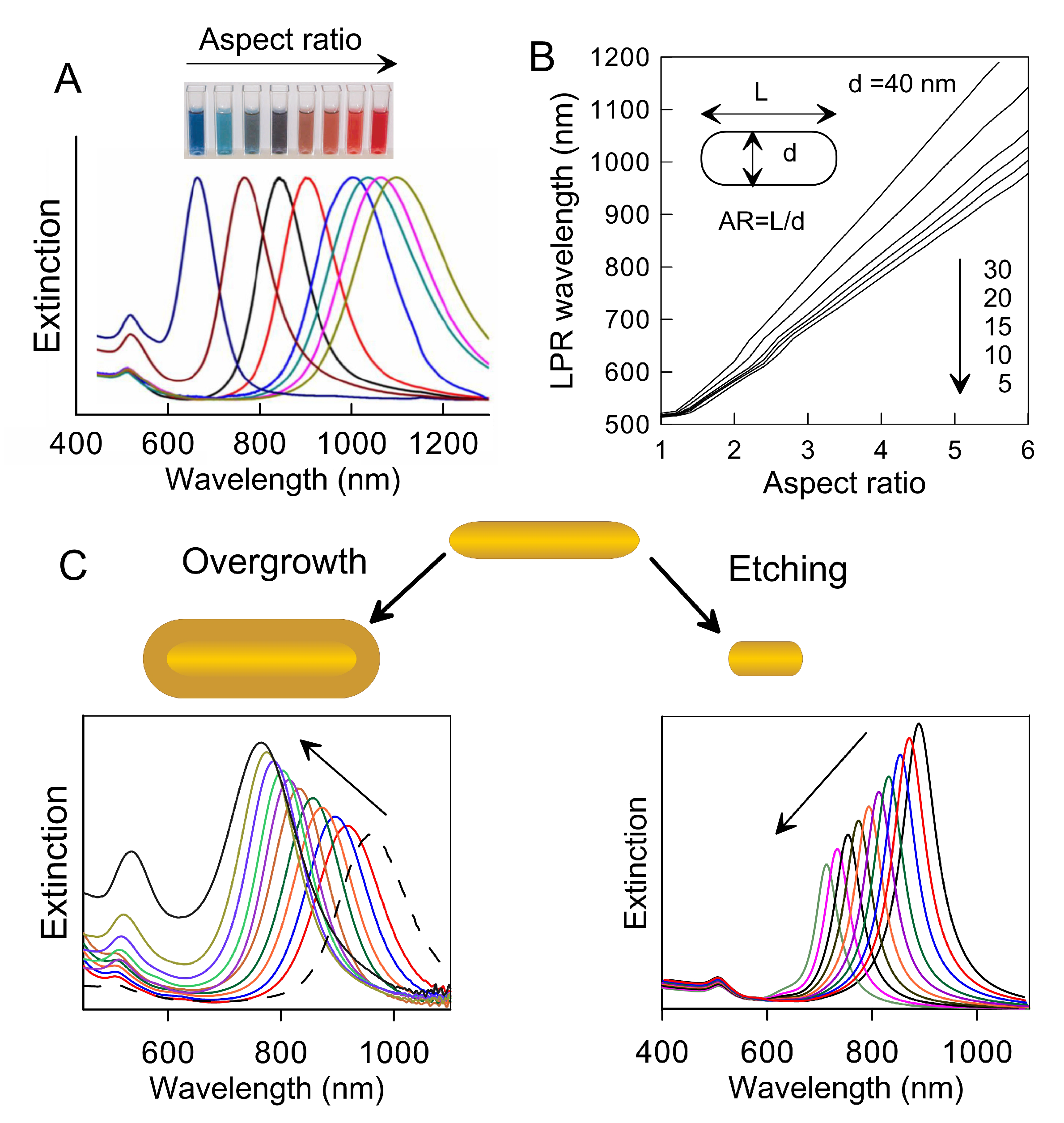
3.1. Gold Nanorods: Many Pros and Few Critical Issues
3.2. Photoinstability of Gold Nanorods: Theoretical Notions
3.2.1. Pre-Melting of Gold Nanorods
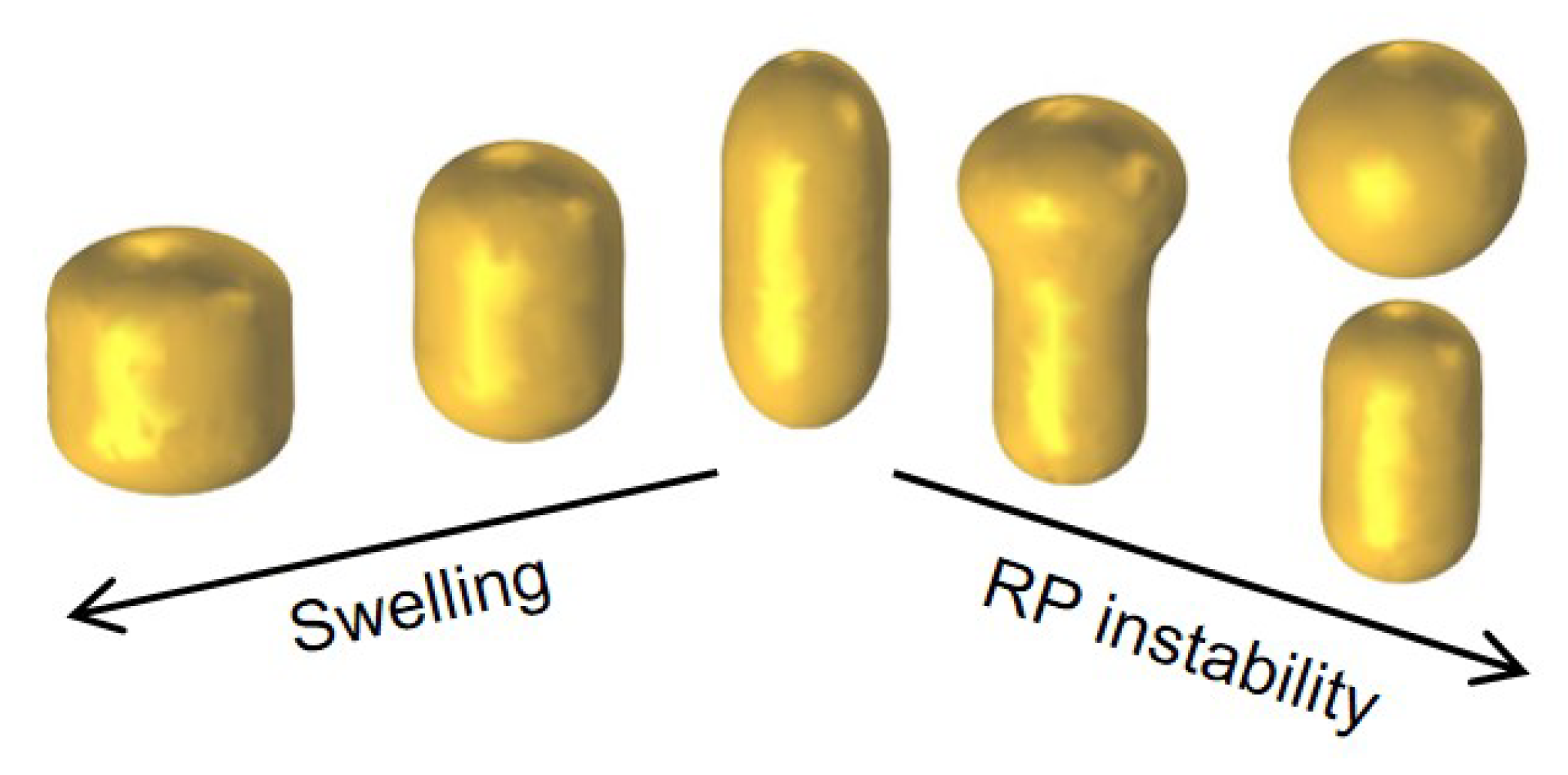
3.2.2. Pathway to the Spheroidization of Gold Nanorods
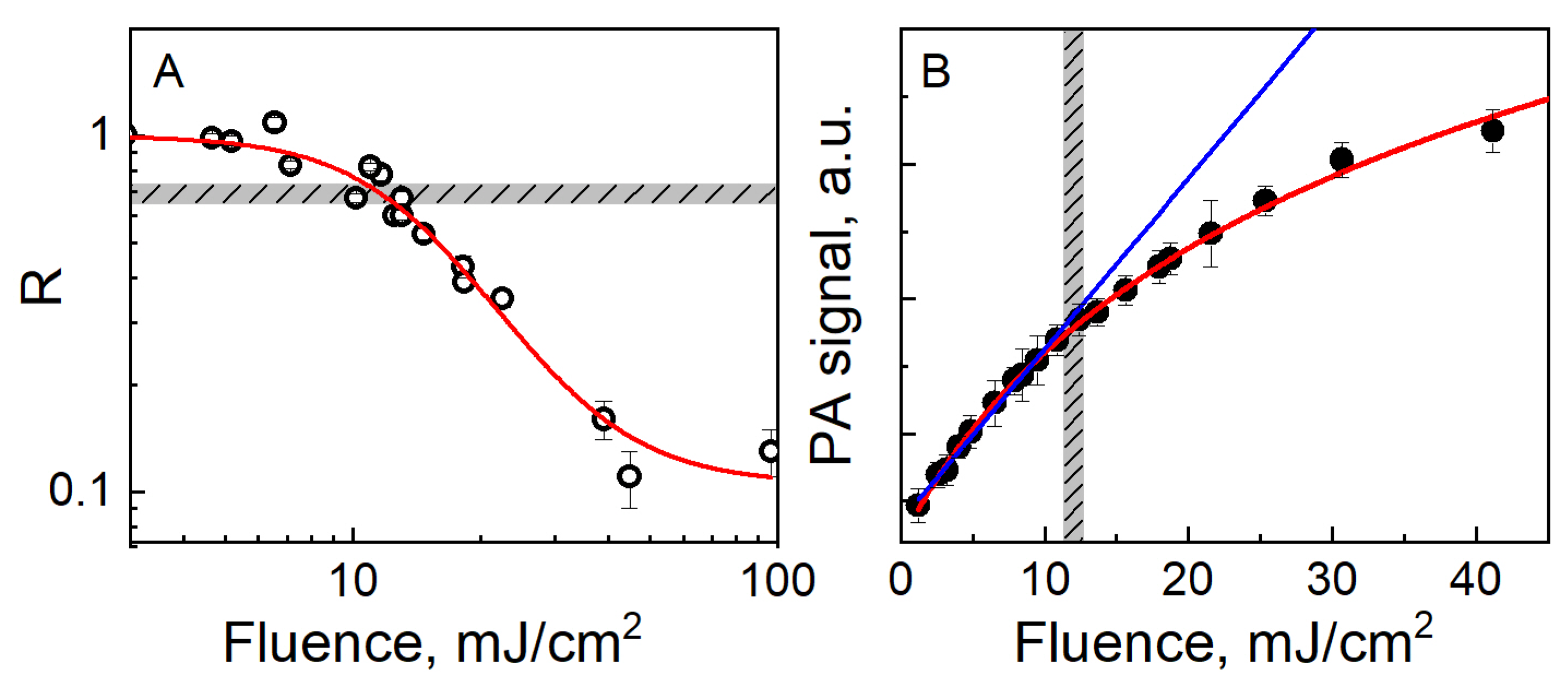
3.3. Methods to Characterize the Instability
4. Strategies to Improve the Photostability of Gold Nanorods
4.1. Improving the Thermal Stability of Gold Nanorods: The Effect of Coating
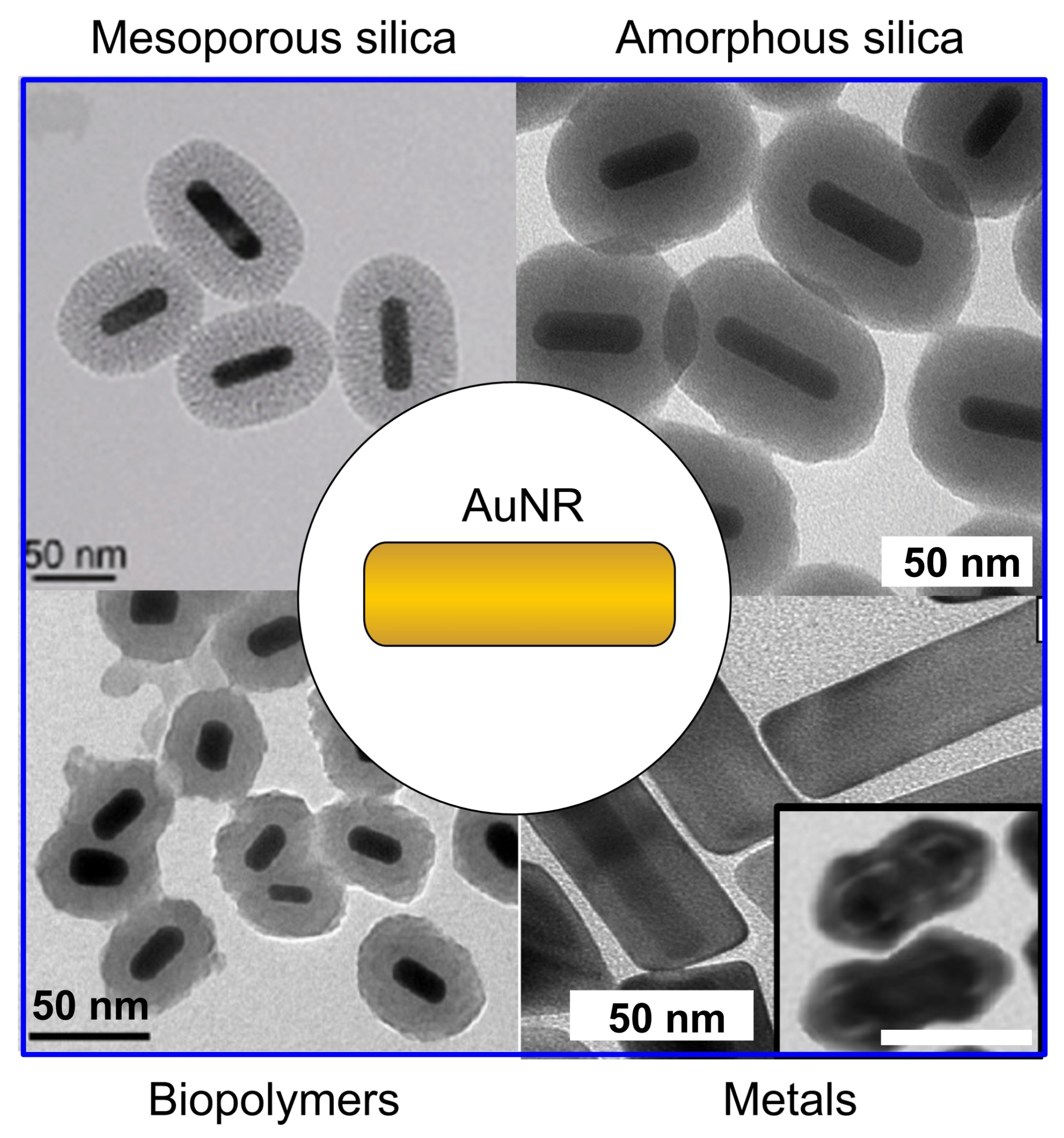
4.1.1. Coating of Gold Nanorods with Silica and Organic Polymers: Useful Protocols
4.1.2. Coating of Gold Nanorods with Silica and Organic Polymers: Pros and Cons
4.2. Lowering the Thermal Resistance at the Interface between Gold and Water: The Effect of Miniaturization and Roughness
4.2.1. Miniaturization of Gold Nanorods: Useful Protocols
4.2.2. Miniaturization of Gold Nanorods: Pros and Cons
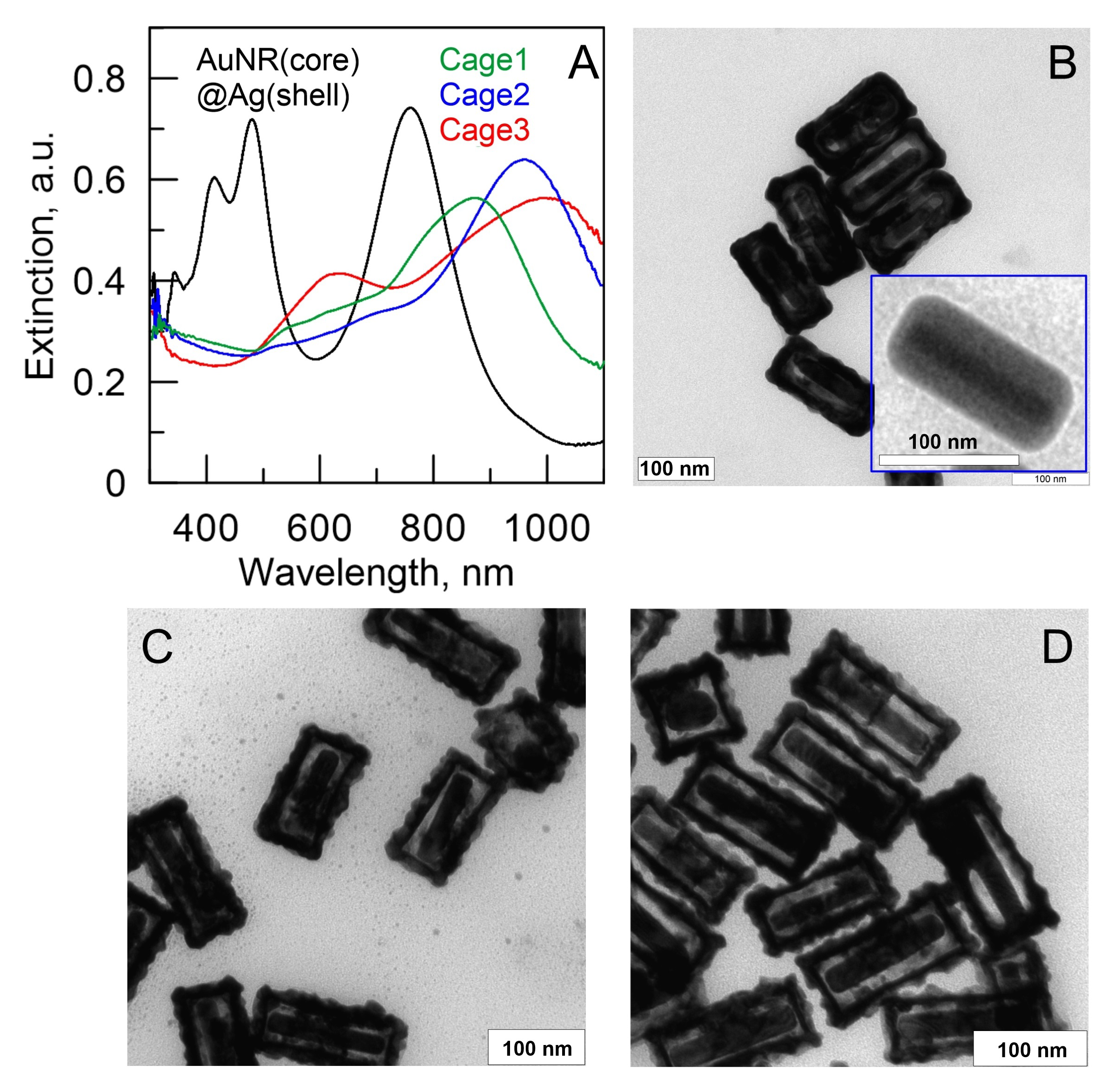
4.3. Alternative Strategies
5. Conclusions and Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef] [PubMed]
- Dykman, L.; Khlebtsov, N. Gold nanoparticles in biomedical applications: Recent advances and perspectives. Chem. Soc. Rev. 2012, 41, 2256–2282. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhao, P.; Astruc, D. Anisotropic Gold Nanoparticles: Synthesis, Properties, Applications, and Toxicity. Angew. Chem. 2014, 53, 1756–1789. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Wei, Q.; Wei, A.; Cheng, J.X. Gold Nanorods as Contrast Agents for Biological Imaging: Optical Properties, Surface Conjugation and Photothermal Effects. Photochem. Photobiol. 2009, 85, 21–32. [Google Scholar] [CrossRef]
- Chen, H.; Shao, L.; Li, Q.; Wang, J. Gold nanorods and their plasmonic properties. Chem. Soc. Rev. 2013, 42, 2679–2724. [Google Scholar] [CrossRef]
- Riley, R.S.; Day, E.S. Gold nanoparticle-mediated photothermal therapy: Applications and opportunities for multimodal cancer treatment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1449. [Google Scholar] [CrossRef]
- Wang, L.V.; Hu, S. Photoacoustic Tomography: In Vivo Imaging from Organelles to Organs. Science 2012, 335, 1458–1462. [Google Scholar] [CrossRef]
- Beard, P. Biomedical photoacoustic imaging. Interface Focus 2011, 1, 602–631. [Google Scholar] [CrossRef]
- Steinberg, I.; Huland, D.M.; Vermesh, O.; Frostig, H.E.; Tummers, W.S.; Gambhir, S.S. Photoacoustic clinical imaging. Photoacoustics 2019, 14, 77–98. [Google Scholar] [CrossRef]
- Kim, C.; Erpelding, T.N.; Jankovic, L.; Pashley, M.D.; Wang, L.V. Deeply penetrating in vivo photoacoustic imaging using a clinical ultrasound array system. Biomed. Opt. Express 2010, 1, 278–284. [Google Scholar] [CrossRef]
- Kim, J.; Park, S.; Jung, Y.; Chang, S.; Park, J.; Zhang, Y.; Lovell, J.F.; Kim, C. Programmable Real-time Clinical Photoacoustic and Ultrasound Imaging System. Sci. Rep. 2016, 6, 35137. [Google Scholar] [CrossRef]
- Lin, L.; Hu, P.; Shi, J.; Appleton, C.M.; Maslov, K.; Li, L.; Zhang, R.; Wang, L.V. Single-breath-hold photoacoustic computed tomography of the breast. Nat. Commun. 2018, 9, 2352. [Google Scholar] [CrossRef]
- Chitgupi, U.; Nyayapathi, N.; Kim, J.; Wang, D.; Sun, B.; Li, C.; Carter, K.; Huang, W.C.; Kim, C.; Xia, J.; et al. Surfactant-Stripped Micelles for NIR-II Photoacoustic Imaging through 12 cm of Breast Tissue and Whole Human Breasts. Adv. Mater. 2019, 31, 1902279. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.V. Photoacoustic Imaging and Spectroscopy; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Cavigli, L.; Milanesi, A.; Khlebtsov, B.N.; Centi, S.; Ratto, F.; Khlebtsov, N.G.; Pini, R. Impact of Kapitza resistance on the stability and efficiency of photoacoustic conversion from gold nanorods. J. Colloid Interface Sci. 2020, 578, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Pang, G.A.; Poisson, F.; Laufer, J.; Haisch, C.; Bossy, E. Theoretical and Experimental Study of Photoacoustic Excitation of Silica-Coated Gold Nanospheres in Water. J. Phys. Chem. C 2020, 124, 1088–1098. [Google Scholar] [CrossRef]
- Cavigli, L.; de Angelis, M.; Ratto, F.; Matteini, P.; Rossi, F.; Centi, S.; Fusi, F.; Pini, R. Size Affects the Stability of the Photoacoustic Conversion of Gold Nanorods. J. Phys. Chem. C 2014, 118, 16140–16146. [Google Scholar] [CrossRef]
- Attia, A.B.E.; Balasundaram, G.; Moothanchery, M.; Dinish, U.; Bi, R.; Ntziachristos, V.; Olivo, M. A review of clinical photoacoustic imaging: Current and future trends. Photoacoustics 2019, 16, 100144. [Google Scholar] [CrossRef]
- Neuschmelting, V.; Burton, N.C.; Lockau, H.; Urich, A.; Harmsen, S.; Ntziachristos, V.; Kircher, M.F. Performance of a Multispectral Optoacoustic Tomography (MSOT) System equipped with 2D vs. 3D Handheld Probes for Potential Clinical Translation. Photoacoustics 2016, 4, 1–10. [Google Scholar] [CrossRef]
- Arthuis, C.J.; Novell, A.; Raes, F.; Escoffre, J.M.; Lerondel, S.; Le Pape, A.; Bouakaz, A.; Perrotin, F. Real-Time Monitoring of Placental Oxygenation during Maternal Hypoxia and Hyperoxygenation Using Photoacoustic Imaging. PLoS ONE 2017, 12, e0169850. [Google Scholar] [CrossRef][Green Version]
- Su, R.; Ermilov, S.; Liopo, A.; Oraevsky, A. Laser optoacoustic tomography: Towards new technology for biomedical diagnostics. Nucl. Instrum. Methods Phys. Res. A 2013, 720, 58–61. [Google Scholar] [CrossRef][Green Version]
- Thompson, W.; Yu, A.; Dumani, D.S.; Cook, J.; Anastasio, M.A.; Emelianov, S.Y.; Ermilov, S.A. A preclinical small animal imaging platform combining multi-angle photoacoustic and fluorescence projections into co-registered 3D maps. Proc. SPIE 2020, 11240, 112400L. [Google Scholar] [CrossRef]
- Saijo, Y.; Ida, T.; Iwazaki, H.; Miyajima, J.; Tang, H.; Shintate, R.; Sato, K.; Hiratsuka, T.; Yoshizawa, S.; Umemura, S. Visualization of skin morphology and microcirculation with high frequency ultrasound and dual-wavelength photoacoustic microscope. Proc. SPIE 2019, 10878, 108783E. [Google Scholar] [CrossRef]
- Bauer, A.Q.; Nothdurft, R.E.; Culver, J.P.; Erpelding, T.N.; Wang, L.V. Quantitative photoacoustic imaging: Correcting for heterogeneous light fluence distributions using diffuse optical tomography. J. Biomed. Opt. 2011, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.K.A.; Jaeger, M.; Frenz, M.; Steenbergen, W. In vivo demonstration of reflection artifact reduction in photoacoustic imaging using synthetic aperture photoacoustic-guided focused ultrasound (PAFUSion). Biomed. Opt. Express 2016, 7, 2955–2972. [Google Scholar] [CrossRef]
- Li, M.; Tang, Y.; Yao, J. Photoacoustic tomography of blood oxygenation: A mini review. Photoacoustics 2018, 10, 65–73. [Google Scholar] [CrossRef]
- Jose, J.; Manohar, S.; Kolkman, R.G.M.; Steenbergen, W.; van Leeuwen, T.G. Imaging of tumor vasculature using Twente photoacoustic systems. J. Biophotonics 2009, 2, 701–717. [Google Scholar] [CrossRef]
- Diot, G.; Metz, S.; Noske, A.; Liapis, E.; Schroeder, B.; Ovsepian, S.V.; Meier, R.; Rummeny, E.; Ntziachristos, V. Multispectral Optoacoustic Tomography (MSOT) of Human Breast Cancer. Clin. Cancer Res. 2017, 23, 6912–6922. [Google Scholar] [CrossRef]
- Oraevsky, A.; Clingman, B.; Zalev, J.; Stavros, A.; Yang, W.; Parikh, J. Clinical optoacoustic imaging combined with ultrasound for coregistered functional and anatomical mapping of breast tumors. Photoacoustics 2018, 12, 30–45. [Google Scholar] [CrossRef]
- Wang, X.; Chamberland, D.L.; Xi, G. Noninvasive reflection mode photoacoustic imaging through infant skull toward imaging of neonatal brains. J. Neurosci. Methods 2008, 168, 412–421. [Google Scholar] [CrossRef]
- Guevara, E.; Berti, R.; Londono, I.; Xie, N.; Bellec, P.; Lesage, F.; Lodygensky, G.A. Imaging of an Inflammatory Injury in the Newborn Rat Brain with Photoacoustic Tomography. PLoS ONE 2013, 8, e83045. [Google Scholar] [CrossRef]
- Upputuri, P.K.; Pramanik, M. Photoacoustic imaging in the second near-infrared window: A review. J. Biomed. Opt. 2019, 24, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Sangha, G.S.; Phillips, E.H.; Goergen, C.J. In vivo photoacoustic lipid imaging in mice using the second near-infrared window. Biomed. Opt. Express 2017, 8, 736–742. [Google Scholar] [CrossRef]
- Park, E.; Lee, Y.J.; Lee, C.; Eom, T.J. Effective photoacoustic absorption spectrum for collagen-based tissue imaging. J. Biomed. Opt. 2020, 25, 1–8. [Google Scholar] [CrossRef]
- Klibanov, A.L. Microbubble Contrast Agents: Targeted Ultrasound Imaging and Ultrasound-Assisted Drug-Delivery Applications. Investig. Radiol. 2006, 41, 354–362. [Google Scholar] [CrossRef]
- Kim, J.; Park, S.; Lee, C.; Kim, J.Y.; Kim, C. Organic Nanostructures for Photoacoustic Imaging. ChemNanoMat 2016, 2, 156–166. [Google Scholar] [CrossRef]
- Weber, J.; Beard, P.; Bohndiek, S. Contrast agents for molecular photoacoustic imaging. Nat. Methods 2016, 13, 639–650. [Google Scholar] [CrossRef]
- Maturi, M.; Locatelli, E.; Monaco, I.; Comes Franchini, M. Current concepts in nanostructured contrast media development for in vivo photoacoustic imaging. Biomater. Sci. 2019, 7, 1746–1775. [Google Scholar] [CrossRef] [PubMed]
- Upputuri, P.K.; Pramanik, M. Recent advances in photoacoustic contrast agents for in vivo imaging. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1618. [Google Scholar] [CrossRef]
- Gong, H.; Dong, Z.; Liu, Y.; Yin, S.; Cheng, L.; Xi, W.; Xiang, J.; Liu, K.; Li, Y.; Liu, Z. Engineering of Multifunctional Nano-Micelles for Combined Photothermal and Photodynamic Therapy Under the Guidance of Multimodal Imaging. Adv. Funct. Mater. 2014, 24, 6492–6502. [Google Scholar] [CrossRef]
- Lovell, J.F.; Jin, C.S.; Huynh, E.; Jin, H.; Kim, C.; Rubinstein, J.L.; Chan, W.C.W.; Cao, W.; Wang, L.V.; Zheng, G. Porphysome nanovesicles generated by porphyrin bilayers for use as multimodal biophotonic contrast agents. Nat. Mater. 2011, 10, 324–332. [Google Scholar] [CrossRef]
- Sheng, Z.; Hu, D.; Zheng, M.; Zhao, P.; Liu, H.; Gao, D.; Gong, P.; Gao, G.; Zhang, P.; Ma, Y.; et al. Smart Human Serum Albumin-Indocyanine Green Nanoparticles Generated by Programmed Assembly for Dual-Modal Imaging-Guided Cancer Synergistic Phototherapy. ACS Nano 2014, 8, 12310–12322. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhao, P.; Luo, Z.; Zheng, M.; Tian, H.; Gong, P.; Gao, G.; Pan, H.; Liu, L.; Ma, A.; et al. Cancer Cell Membrane–Biomimetic Nanoparticles for Homologous-Targeting Dual-Modal Imaging and Photothermal Therapy. ACS Nano 2016, 10, 10049–10057. [Google Scholar] [CrossRef] [PubMed]
- Pu, K.; Shuhendler, A.J.; Jokerst, J.V.; Mei, J.; Gambhir, S.S.; Bao, Z.; Rao, J. Porphysome nanovesicles generated by porphyrin bilayers for use as multimodal biophotonic contrast agents. Nat. Nanotechnol. 2014, 9, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Luo, Z.; Men, Y.; Yang, P.; Peng, H.; Guo, R.; Tian, Y.; Pang, Z.; Yang, W. Red blood cell membrane-camouflaged melanin nanoparticles for enhanced photothermal therapy. Biomaterials 2017, 143, 29–45. [Google Scholar] [CrossRef]
- Lin, L.S.; Cong, Z.X.; Cao, J.B.; Ke, K.M.; Peng, Q.L.; Gao, J.; Yang, H.H.; Liu, G.; Chen, X. Multifunctional Fe3O4@Polydopamine Core–Shell Nanocomposites for Intracellular mRNA Detection and Imaging-Guided Photothermal Therapy. ACS Nano 2014, 8, 3876–3883. [Google Scholar] [CrossRef]
- Hauser, D.; Septiadi, D.; Turner, J.; Petri-Fink, A.; Rothen-Rutishauser, B. From Bioinspired Glue to Medicine: Polydopamine as a Biomedical Material. Materials 2020, 13, 1730. [Google Scholar] [CrossRef]
- Cheng, L.; Gong, H.; Zhu, W.; Liu, J.; Wang, X.; Liu, G.; Liu, Z. PEGylated Prussian blue nanocubes as a theranostic agent for simultaneous cancer imaging and photothermal therapy. Biomaterials 2014, 35, 9844–9852. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, L. Multifunctional Prussian blue-based nanomaterials: Preparation, modification, and theranostic applications. Coord. Chem. Rev. 2020, 419, 213393. [Google Scholar] [CrossRef]
- De La Zerda, A.; Zavaleta, C.; Keren, S.; Vaithilingam, S.; Bodapati, S.; Liu, Z.; Levi, J.; Smith, B.R.; Ma, T.J.; Oralkan, O.; et al. Carbon nanotubes as photoacoustic molecular imaging agents in living mice. Nat. Nanotechnol. 2008, 3, 557–562. [Google Scholar] [CrossRef]
- Sheng, Z.; Song, L.; Zheng, J.; Hu, D.; He, M.; Zheng, M.; Gao, G.; Gong, P.; Zhang, P.; Ma, Y.; et al. Protein-assisted fabrication of nano-reduced graphene oxide for combined in vivo photoacoustic imaging and photothermal therapy. Biomaterials 2013, 34, 5236–5243. [Google Scholar] [CrossRef]
- Wang, L.; Hasanzadeh Kafshgari, M.; Meunier, M. Optical Properties and Applications of Plasmonic-Metal Nanoparticles. Adv. Funct. Mater. 2020, 30, 2005400. [Google Scholar] [CrossRef]
- Manohar, S.; Ungureanu, C.; Van Leeuwen, T.G. Gold nanorods as molecular contrast agents in photoacoustic imaging: The promises and the caveats. Contrast Media Mol. Imaging 2011, 6, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Liu, J.; Gu, X.; Gong, H.; Shi, X.; Liu, T.; Wang, C.; Wang, X.; Liu, G.; Xing, H.; et al. PEGylated WS2 Nanosheets as a Multifunctional Theranostic Agent for in vivo Dual-Modal CT/Photoacoustic Imaging Guided Photothermal Therapy. Adv. Mater. 2014, 26, 1886–1893. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yang, C.; Li, J.; Ding, Y.; Zhang, L.; Yousaf, M.Z.; Lin, J.; Pang, R.; Wei, L.; Xu, L.; et al. Multifunctional Fe5C2 Nanoparticles: A Targeted Theranostic Platform for Magnetic Resonance Imaging and Photoacoustic Tomography-Guided Photothermal Therapy. Adv. Mater. 2014, 26, 4114–4120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gao, S.; Zhang, F.; Yang, K.; Ma, Q.; Zhu, L. Activatable Hyaluronic Acid Nanoparticle as a Theranostic Agent for Optical/Photoacoustic Image-Guided Photothermal Therapy. ACS Nano 2014, 8, 12250–12258. [Google Scholar] [CrossRef]
- Song, X.R.; Wang, X.; Yu, S.X.; Cao, J.; Li, S.H.; Li, J.; Liu, G.; Yang, H.H.; Chen, X. Co9Se8 Nanoplates as a New Theranostic Platform for Photoacoustic/Magnetic Resonance Dual-Modal-Imaging-Guided Chemo-Photothermal Combination Therapy. Adv. Mater. 2015, 27, 3285–3291. [Google Scholar] [CrossRef]
- Yu, J.; Yin, W.; Zheng, X.; Tian, G.; Zhang, X.; Bao, T.; Dong, X.; Wang, Z.; Gu, Z.; Ma, X.; et al. Smart MoS2/Fe3O4 Nanotheranostic for Magnetically Targeted Photothermal Therapy Guided by Magnetic Resonance/Photoacoustic Imaging. Theranostics 2015, 5, 931–945. [Google Scholar] [CrossRef]
- Yang, W.; Guo, W.; Le, W.; Lv, G.; Zhang, F.; Shi, L.; Wang, X.; Wang, J.; Wang, S.; Chang, J.; et al. Albumin-Bioinspired Gd:CuS Nanotheranostic Agent for In Vivo Photoacoustic/Magnetic Resonance Imaging-Guided Tumor-Targeted Photothermal Therapy. ACS Nano 2016, 10, 10245–10257. [Google Scholar] [CrossRef]
- Borri, C.; Albino, M.; Innocenti, C.; Pineider, F.; Cavigli, L.; Centi, S.; Sangregorio, C.; Ratto, F.; Pini, R. A bionic shuttle carrying multi-modular particles and holding tumor-tropic features. Mater. Sci. Eng. C 2020, 117, 111338. [Google Scholar] [CrossRef]
- Liang, X.; Li, Y.; Li, X.; Jing, L.; Deng, Z.; Yue, X.; Li, C.; Dai, Z. PEGylated Polypyrrole Nanoparticles Conjugating Gadolinium Chelates for Dual-Modal MRI/Photoacoustic Imaging Guided Photothermal Therapy of Cancer. Adv. Funct. Mater. 2015, 25, 1451–1462. [Google Scholar] [CrossRef]
- Cai, Y.; Liang, P.; Tang, Q.; Yang, X.; Si, W.; Huang, W.; Zhang, Q.; Dong, X. Diketopyrrolopyrrole–Triphenylamine Organic Nanoparticles as Multifunctional Reagents for Photoacoustic Imaging-Guided Photodynamic/Photothermal Synergistic Tumor Therapy. ACS Nano 2017, 11, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Frey, W.; Kim, S.; Homan, K.; Kruizinga, P.; Sokolov, K.; Emelianov, S. Enhanced thermal stability of silica-coated gold nanorods for photoacoustic imaging and image-guided therapy. Opt. Express 2010, 18, 8867–8878. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Ge, J.; Liu, W.; Liu, S.; Niu, G.; Guo, L.; Zhang, H.; Wang, P. Gold nanorod@silica-carbon dots as multifunctional phototheranostics for fluorescence and photoacoustic imaging-guided synergistic photodynamic/photothermal therapy. Nanoscale 2016, 8, 13067–13077. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Chen, F.; Valdovinos, H.F.; Jiang, D.; Goel, S.; Yu, B.; Sun, H.; Barnhart, T.E.; Moon, J.J.; Cai, W. Bacteria-like mesoporous silica-coated gold nanorods for positron emission tomography and photoacoustic imaging-guided chemo-photothermal combined therapy. Biomaterials 2018, 165, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Wang, X.; Lin, L.; Song, T.; Sun, P.; Tian, H.; Liang, H.; Chen, X. Gold Nanorods Electrostatically Binding Nucleic Acid Probe for In Vivo MicroRNA Amplified Detection and Photoacoustic Imaging-Guided Photothermal Therapy. Adv. Funct. Mater. 2018, 28, 1800490. [Google Scholar] [CrossRef]
- Rouleau, L.; Berti, R.; Ng, V.W.K.; Matteau-Pelletier, C.; Lam, T.; Saboural, P.; Kakkar, A.K.; Lesage, F.; Rhéaume, E.; Tardif, J.C. VCAM-1-targeting gold nanoshell probe for photoacoustic imaging of atherosclerotic plaque in mice. Contrast Media Mol. Imaging 2013, 8, 27–39. [Google Scholar] [CrossRef]
- Kim, C.; Cho, E.C.; Chen, J.; Song, K.H.; Au, L.; Favazza, C.; Zhang, Q.; Cobley, C.M.; Gao, F.; Xia, Y.; et al. In Vivo Molecular Photoacoustic Tomography of Melanomas Targeted by Bioconjugated Gold Nanocages. ACS Nano 2010, 4, 4559–4564. [Google Scholar] [CrossRef]
- Xu, X.; Chong, Y.; Liu, X.; Fu, H.; Yu, C.; Huang, J.; Zhang, Z. Multifunctional nanotheranostic gold nanocages for photoacoustic imaging guided radio/photodynamic/photothermal synergistic therapy. Acta Biomater. 2019, 84, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Li, C.; Zhang, C.; Chen, Y.; Xu, L.; Bao, C.; Wang, X.; Liu, G.; Zhang, F.; Cui, D. CD44v6 Monoclonal Antibody-Conjugated Gold Nanostars for Targeted Photoacoustic Imaging and Plasmonic Photothermal Therapy of Gastric Cancer Stem-like Cells. Theranostics 2015, 5, 970–984. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, Y.; Teng, Z.; Tian, W.; Luo, S.; Kong, X.; Su, X.; Tang, Y.; Wang, S.; Lu, G. pH-Dependent Transmembrane Activity of Peptide-Functionalized Gold Nanostars for Computed Tomography/Photoacoustic Imaging and Photothermal Therapy. ACS Appl. Mater. Interfaces 2017, 9, 2114–2122. [Google Scholar] [CrossRef]
- Khlebtsov, N.G. T-matrix method in plasmonics: An overview. J. Quant. Spectrosc. Radiat. Transf. 2013, 123, 184–217. [Google Scholar] [CrossRef]
- Amendola, V.; Pilot, R.; Frasconi, M.; Maragò, O.M.; Iatì, M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Phys. Condens. Matter 2017, 29, 203002. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.Y.; Chen, C.T.; Yeh, C.S. Comparative efficiencies of photothermal destruction of malignant cells using antibody-coated silica@Au nanoshells, hollow Au/Ag nanospheres and Au nanorods. Nanotechnology 2009, 20, 425104. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.R.; Mirin, N.A.; Knight, M.W.; Goodrich, G.P.; Halas, N.J. Photothermal Efficiencies of Nanoshells and Nanorods for Clinical Therapeutic Applications. J. Phys. Chem. C 2009, 113, 12090–12094. [Google Scholar] [CrossRef]
- Richardson, H.H.; Carlson, M.T.; Tandler, P.J.; Hernandez, P.; Govorov, A.O. Experimental and Theoretical Studies of Light-to-Heat Conversion and Collective Heating Effects in Metal Nanoparticle Solutions. Nano Lett. 2009, 9, 1139–1146. [Google Scholar] [CrossRef]
- Chen, H.; Shao, L.; Ming, T.; Sun, Z.; Zhao, C.; Yang, B.; Wang, J. Understanding the Photothermal Conversion Efficiency of Gold Nanocrystals. Small 2010, 6, 2272–2280. [Google Scholar] [CrossRef]
- Pattani, V.P.; Tunnell, J.W. Nanoparticle-mediated photothermal therapy: A comparative study of heating for different particle types. Lasers Surg. Med. 2012, 44, 675–684. [Google Scholar] [CrossRef]
- Pérez-Juste, J.; Pastoriza-Santos, I.; Liz-Marzán, L.M.; Mulvaney, P. Gold nanorods: Synthesis, characterization and applications. Coord. Chem. Rev. 2005, 249, 1870–1901. [Google Scholar] [CrossRef]
- Gans, R. Über die Form ultramikroskopischer Goldteilchen. Ann. Phys. 1912, 342, 881–900. [Google Scholar] [CrossRef]
- Khlebtsov, N.G. Optics and biophotonics of nanoparticles with a plasmon resonance. Quantum Electron. 2008, 38, 504–529. [Google Scholar] [CrossRef]
- Chang, S.S.; Shih, C.W.; Chen, C.D.; Lai, W.C.; Wang, C.R.C. The Shape Transition of Gold Nanorods. Langmuir 1999, 15, 701–709. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Chang, S.S.; Lee, C.L.; Wang, C.R.C. Gold Nanorods: Electrochemical Synthesis and Optical Properties. J. Phys. Chem. B 1997, 101, 6661–6664. [Google Scholar] [CrossRef]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and Growth Mechanism of Gold Nanorods (NRs) Using Seed-Mediated Growth Method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- Wang, Z.; Mohamed, M.; Link, S.; El-Sayed, M. Crystallographic facets and shapes of gold nanorods of different aspect ratios. Surf. Sci. 1999, 440, L809–L814. [Google Scholar] [CrossRef]
- Requejo, K.I.; Liopo, A.V.; Zubarev, E.R. Gold Nanorod Synthesis with Small Thiolated Molecules. Langmuir 2020, 36, 3758–3769. [Google Scholar] [CrossRef]
- Martin, C.R. Membrane-Based Synthesis of Nanomaterials. Chem. Mater. 1996, 8, 1739–1746. [Google Scholar] [CrossRef]
- Wurtz, G.A.; Pollard, R.; Hendren, W.; Wiederrecht, G.P.; Gosztola, D.J.; Podolskiy, V.A.; Zayats, A.V. Designed ultrafast optical nonlinearity in a plasmonic nanorod metamaterial enhanced by nonlocality. Nat. Nanotechnol. 2011, 6, 107–111. [Google Scholar] [CrossRef]
- Wang, S.; Xi, W.; Cai, F.; Zhao, X.; Xu, Z.; Qian, J.; He, S. Three-Photon Luminescence of Gold Nanorods and Its Applications for High Contrast Tissue and Deep in vivo Brain Imaging. Theranostics 2015, 5, 251–266. [Google Scholar] [CrossRef]
- Khlebtsov, N.G.; Melnikov, A.G.; Bogatyrev, V.A.; Dykman, L.A.; Alekseeva, A.V.; Trachuk, L.A.; Khlebtsov, B.N. Can the Light Scattering Depolarization Ratio of Small Particles Be Greater Than 1/3? J. Phys. Chem. B 2005, 109, 13578–13584. [Google Scholar] [CrossRef]
- Khlebtsov, B.N.; Khanadeev, V.A.; Ye, J.; Sukhorukov, G.B.; Khlebtsov, N.G. Overgrowth of Gold Nanorods by Using a Binary Surfactant Mixture. Langmuir 2014, 30, 1696–1703. [Google Scholar] [CrossRef]
- Khanadeev, V.A.; Khlebtsov, N.G.; Burov, A.M.; Khlebtsov, B.N. Tuning of plasmon resonance of gold nanorods by controlled etching. Colloid J. 2015, 77, 652–660. [Google Scholar] [CrossRef]
- Xu, X.; Cortie, M. Shape Change and Color Gamut in Gold Nanorods, Dumbbells, and Dog Bones. Adv. Funct. Mater. 2006, 16, 2170–2176. [Google Scholar] [CrossRef]
- Wang, P.; Liu, M.; Gao, G.; Zhang, S.; Shi, H.; Li, Z.; Zhang, L.; Fang, Y. From gold nanorods to nanodumbbells: A different way to tailor surface plasmon resonances by a chemical route. J. Mater. Chem. 2012, 22, 24006–24011. [Google Scholar] [CrossRef]
- Vigderman, L.; Khanal, B.P.; Zubarev, E.R. Functional Gold Nanorods: Synthesis, Self-Assembly, and Sensing Applications. Adv. Mater. 2012, 24, 4811–4841. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated Absorption and Scattering Properties of Gold Nanoparticles of Different Size, Shape, and Composition: Applications in Biological Imaging and Biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef] [PubMed]
- Khlebtsov, B.; Zharov, V.; Melnikov, A.; Tuchin, V.; Khlebtsov, N. Optical amplification of photothermal therapy with gold nanoparticles and nanoclusters. Nanotechnology 2006, 17, 5167–5179. [Google Scholar] [CrossRef]
- Stefan Kooij, E.; Poelsema, B. Shape and size effects in the optical properties of metallic nanorods. Phys. Chem. Chem. Phys. 2006, 8, 3349–3357. [Google Scholar] [CrossRef]
- Baffou, G.; Quidant, R.; Girard, C. Heat generation in plasmonic nanostructures: Influence of morphology. Appl. Phys. Lett. 2009, 94, 153109. [Google Scholar] [CrossRef]
- Kessentini, S.; Barchiesi, D. Quantitative comparison of optimized nanorods, nanoshells and hollow nanospheres for photothermal therapy. Biomed. Opt. Express 2012, 3, 590–604. [Google Scholar] [CrossRef]
- Maestro, L.M.; Camarillo, E.; Sánchez-Gil, J.A.; Rodríguez-Oliveros, R.; Ramiro-Bargueño, J.; Caamaño, A.J.; Jaque, F.; Solé, J.G.; Jaque, D. Gold nanorods for optimized photothermal therapy: The influence of irradiating in the first and second biological windows. RSC Adv. 2014, 4, 54122–54129. [Google Scholar] [CrossRef]
- Khlebtsov, B.N.; Khanadeev, V.A.; Maksimova, I.L.; Terentyuk, G.S.; Khlebtsov, N.G. Silver nanocubes and gold nanocages: Fabrication and optical and photothermal properties. Nanotechnol. Russ. 2010, 5, 454–468. [Google Scholar] [CrossRef]
- Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Bajaj, A.; Mout, R.; Rotello, V.M. Monolayer coated gold nanoparticles for delivery applications. Adv. Drug Deliv. Rev. 2012, 64, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mei, Z.; Wang, Y.; Tang, L. Gold nanorod biochip functionalization by antibody thiolation. Talanta 2015, 136, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Servos, M.R.; Liu, J. Instantaneous and Quantitative Functionalization of Gold Nanoparticles with Thiolated DNA Using a pH-Assisted and Surfactant-Free Route. J. Am. Chem. Soc. 2012, 134, 7266–7269. [Google Scholar] [CrossRef]
- Wilson, C.G.; Sisco, P.N.; Gadala-Maria, F.A.; Murphy, C.J.; Goldsmith, E.C. Polyelectrolyte-coated gold nanorods and their interactions with type I collagen. Biomaterials 2009, 30, 5639–5648. [Google Scholar] [CrossRef]
- Lai, S.; Centi, S.; Borri, C.; Ratto, F.; Cavigli, L.; Micheletti, F.; Kemper, B.; Ketelhut, S.; Kozyreva, T.; Gonnelli, L.; et al. A multifunctional organosilica cross-linker for the bio-conjugation of gold nanorods. Colloids Surf. B 2017, 157, 174–181. [Google Scholar] [CrossRef]
- Azab, M.M.; Cherif, R.; Finnie, A.L.; Abou El-Alamin, M.M.; Sultan, M.A.; Wark, A.W. Optimized polydopamine coating and DNA conjugation onto gold nanorods for single nanoparticle bioaffinity measurements. Analyst 2018, 143, 1635–1643. [Google Scholar] [CrossRef]
- Khlebtsov, B.N.; Burov, A.M.; Pylaev, T.E.; Khlebtsov, N.G. Polydopamine-coated Au nanorods for targeted fluorescent cell imaging and photothermal therapy. Beilstein J. Nanotechnol. 2019, 10, 794–803. [Google Scholar] [CrossRef]
- Danhier, F.; Feron, O.; Préat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar] [CrossRef]
- Prabhakar, U.; Maeda, H.; Jain, R.K.; Sevick-Muraca, E.M.; Zamboni, W.; Farokhzad, O.C.; Barry, S.T.; Gabizon, A.; Grodzinski, P.; Blakey, D.C. Challenges and Key Considerations of the Enhanced Permeability and Retention Effect for Nanomedicine Drug Delivery in Oncology. Cancer. Res. 2013, 73, 2412–2417. [Google Scholar] [CrossRef]
- Byrne, J.D.; Betancourt, T.; Brannon-Peppas, L. Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv. Drug Deliv. Rev. 2008, 60, 1615–1626. [Google Scholar] [CrossRef]
- Huang, X.; Peng, X.; Wang, Y.; Wang, Y.; Shin, D.M.; El-Sayed, M.A.; Nie, S. A Reexamination of Active and Passive Tumor Targeting by Using Rod-Shaped Gold Nanocrystals and Covalently Conjugated Peptide Ligands. ACS Nano 2010, 4, 5887–5896. [Google Scholar] [CrossRef]
- Ratto, F.; Witort, E.; Tatini, F.; Centi, S.; Lazzeri, L.; Carta, F.; Lulli, M.; Vullo, D.; Fusi, F.; Supuran, C.T.; et al. Plasmonic Particles that Hit Hypoxic Cells. Adv. Funct. Mater. 2015, 25, 316–323. [Google Scholar] [CrossRef]
- Mooney, R.; Roma, L.; Zhao, D.; Van Haute, D.; Garcia, E.; Kim, S.U.; Annala, A.J.; Aboody, K.S.; Berlin, J.M. Neural Stem Cell-Mediated Intratumoral Delivery of Gold Nanorods Improves Photothermal Therapy. ACS Nano 2014, 8, 12450–12460. [Google Scholar] [CrossRef]
- Borri, C.; Centi, S.; Ratto, F.; Pini, R. Polylysine as a functional biopolymer to couple gold nanorods to tumor-tropic cells. J. Nanobiotechnol. 2018, 16, 50. [Google Scholar] [CrossRef]
- Ratto, F.; Centi, S.; Avigo, C.; Borri, C.; Tatini, F.; Cavigli, L.; Kusmic, C.; Lelli, B.; Lai, S.; Colagrande, S.; et al. A Robust Design for Cellular Vehicles of Gold Nanorods for Multimodal Imaging. Adv. Funct. Mater. 2016, 26, 7954. [Google Scholar] [CrossRef]
- Alkilany, A.M.; Nagaria, P.K.; Hexel, C.R.; Shaw, T.J.; Murphy, C.J.; Wyatt, M.D. Cellular Uptake and Cytotoxicity of Gold Nanorods: Molecular Origin of Cytotoxicity and Surface Effects. Small 2009, 5, 701–708. [Google Scholar] [CrossRef]
- Ali, M.R.K.; Rahman, M.A.; Wu, Y.; Han, T.; Peng, X.; Mackey, M.A.; Wang, D.; Shin, H.J.; Chen, Z.G.; Xiao, H.; et al. Efficacy, long-term toxicity, and mechanistic studies of gold nanorods photothermal therapy of cancer in xenograft mice. Proc. Natl. Acad. Sci. USA 2017, 114, E3110–E3118. [Google Scholar] [CrossRef]
- Kolosnjaj-Tabi, J.; Javed, Y.; Lartigue, L.; Volatron, J.; Elgrabli, D.; Marangon, I.; Pugliese, G.; Caron, B.; Figuerola, A.; Luciani, N.; et al. The One Year Fate of Iron Oxide Coated Gold Nanoparticles in Mice. ACS Nano 2015, 9, 7925–7939. [Google Scholar] [CrossRef]
- Balfourier, A.; Luciani, N.; Wang, G.; Lelong, G.; Ersen, O.; Khelfa, A.; Alloyeau, D.; Gazeau, F.; Carn, F. Unexpected intracellular biodegradation and recrystallization of gold nanoparticles. Proc. Natl. Acad. Sci. USA 2020, 117, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Fales, A.M.; Vogt, W.C.; Wear, K.A.; Ilev, I.K.; Pfefer, T.J. Pulsed laser damage of gold nanorods in turbid media and its impact on multi-spectral photoacoustic imaging. Biomed. Opt. Express 2019, 10, 1919–1934. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xia, Z.; Cheng, Y.B.; Gu, M. High-capacity optical long data memory based on enhanced Young’s modulus in nanoplasmonic hybrid glass composites. Nat. Commun. 2018, 9, 1183. [Google Scholar] [CrossRef]
- Ratto, F.; Matteini, P.; Cini, A.; Centi, S.; Rossi, F.; Fusi, F.; Pini, R. CW laser-induced photothermal conversion and shape transformation of gold nanodogbones in hydrated chitosan films. J. Nanopart. Res. 2011, 13, 4337–4348. [Google Scholar] [CrossRef]
- Tong, W.; Katz-Boon, H.; Walsh, M.J.; Weyland, M.; Etheridge, J.; Funston, A.M. The evolution of size, shape, and surface morphology of gold nanorods. Chem. Commun. 2018, 54, 3022–3025. [Google Scholar] [CrossRef]
- González-Rubio, G.; Díaz-Núñez, P.; Rivera, A.; Prada, A.; Tardajos, G.; González-Izquierdo, J.; Bañares, L.; Llombart, P.; Macdowell, L.G.; Alcolea Palafox, M.; et al. Femtosecond laser reshaping yields gold nanorods with ultranarrow surface plasmon resonances. Science 2017, 358, 640–644. [Google Scholar] [CrossRef]
- Kennedy, W.J.; Izor, S.; Anderson, B.D.; Frank, G.; Varshney, V.; Ehlert, G.J. Thermal Reshaping Dynamics of Gold Nanorods: Influence of Size, Shape, and Local Environment. ACS Appl. Mater. Interfaces 2018, 10, 43865–43873. [Google Scholar] [CrossRef]
- Link, S.; Burda, C.; Mohamed, M.B.; Nikoobakht, B.; El-Sayed, M.A. Laser Photothermal Melting and Fragmentation of Gold Nanorods: Energy and Laser Pulse-Width Dependence. J. Phys. Chem. A 1999, 103, 1165–1170. [Google Scholar] [CrossRef]
- Plech, A.; Kotaidis, V.; Lorenc, M.; Boneberg, J. Femtosecond laser near-field ablation from gold nanoparticles. Nat. Phys. 2006, 2, 44–47. [Google Scholar] [CrossRef]
- Ekici, O.; Harrison, R.K.; Durr, N.J.; Eversole, D.S.; Lee, M.; Ben-Yakar, A. Thermal analysis of gold nanorods heated with femtosecond laser pulses. J. Phys. D 2008, 41, 185501. [Google Scholar] [CrossRef]
- Wu, X.; Ni, Y.; Zhu, J.; Burrows, N.D.; Murphy, C.J.; Dumitrica, T.; Wang, X. Thermal Transport across Surfactant Layers on Gold Nanorods in Aqueous Solution. ACS Appl. Mater. Interfaces 2016, 8, 10581–10589. [Google Scholar] [CrossRef] [PubMed]
- Link, S.; Burda, C.; Nikoobakht, B.; El-Sayed, M. How long does it take to melt a gold nanorod? A femtosecond pump–probe absorption spectroscopic study. Chem. Phys. Lett. 1999, 315, 12–18. [Google Scholar] [CrossRef]
- Blumm, J.; Lindemann, A. Characterization of the thermophysical properties of molten polymers and liquids using the flash technique. High Temp. High Press. 2003, 35, 627–632. [Google Scholar] [CrossRef]
- Cavigli, L.; Cini, A.; Centi, S.; Borri, C.; Lai, S.; Ratto, F.; de Angelis, M.; Pini, R. Photostability of Gold Nanorods upon Endosomal Confinement in Cultured Cells. J. Phys. Chem. C 2017, 121, 6393–6400. [Google Scholar] [CrossRef]
- Zijlstra, P.; Chon, J.W.M.; Gu, M. Five-dimensional optical recording mediated by surface plasmons in gold nanorods. Nature 2009, 459, 410–413. [Google Scholar] [CrossRef]
- Plech, A.; Cerna, R.; Kotaidis, V.; Hudert, F.; Bartels, A.; Dekorsy, T. A Surface Phase Transition of Supported Gold Nanoparticles. Nano Lett. 2007, 7, 1026–1031. [Google Scholar] [CrossRef]
- Taylor, A.B.; Siddiquee, A.M.; Chon, J.W.M. Below Melting Point Photothermal Reshaping of Single Gold Nanorods Driven by Surface Diffusion. ACS Nano 2014, 8, 12071–12079. [Google Scholar] [CrossRef]
- Jaklevic, R.C.; Elie, L. Scanning-Tunneling-Microscope Observation of Surface Diffusion on an Atomic Scale: Au on Au(111). Phys. Rev. Lett. 1988, 60, 120–123. [Google Scholar] [CrossRef]
- Huber, S.E.; Warakulwit, C.; Limtrakul, J.; Tsukuda, T.; Probst, M. Thermal stabilization of thin gold nanowires by surfactant-coating: A molecular dynamics study. Nanoscale 2012, 4, 585–590. [Google Scholar] [CrossRef]
- He, X.; Cheng, F.; Chen, Z.X. The Lattice Kinetic Monte Carlo Simulation of Atomic Diffusion and Structural Transition for Gold. Sci. Rep. 2016, 6, 33128. [Google Scholar] [CrossRef]
- Zou, R.; Zhang, Q.; Zhao, Q.; Peng, F.; Wang, H.; Yu, H.; Yang, J. Thermal stability of gold nanorods in an aqueous solution. Colloids Surf. A 2010, 372, 177–181. [Google Scholar] [CrossRef]
- Link, S.; Burda, C.; Nikoobakht, B.; El-Sayed, M.A. Laser-Induced Shape Changes of Colloidal Gold Nanorods Using Femtosecond and Nanosecond Laser Pulses. J. Phys. Chem. B 2000, 104, 6152–6163. [Google Scholar] [CrossRef]
- Takahata, R.; Yamazoe, S.; Warakulwit, C.; Limtrakul, J.; Tsukuda, T. Rayleigh Instability and Surfactant-Mediated Stabilization of Ultrathin Gold Nanorods. J. Phys. Chem. C 2016, 120, 17006–17010. [Google Scholar] [CrossRef]
- Karim, S.; Toimil-Molares, M.E.; Balogh, A.G.; Ensinger, W.; Cornelius, T.W.; Khan, E.U.; Neumann, R. Morphological evolution of Au nanowires controlled by Rayleigh instability. Nanotechnology 2006, 17, 5954–5959. [Google Scholar] [CrossRef]
- González-Rubio, G.; Guerrero-Martínez, A.; Liz-Marzán, L.M. Reshaping, Fragmentation, and Assembly of Gold Nanoparticles Assisted by Pulse Lasers. Acc. Chem. Res. 2016, 49, 678–686. [Google Scholar] [CrossRef]
- Mercatelli, R.; Ratto, F.; Centi, S.; Soria, S.; Romano, G.; Matteini, P.; Quercioli, F.; Pini, R.; Fusi, F. Quantitative readout of optically encoded gold nanorods using an ordinary dark-field microscope. Nanoscale 2013, 5, 9645–9650. [Google Scholar] [CrossRef]
- Conversano, F.; Soloperto, G.; Greco, A.; Ragusa, A.; Casciaro, E.; Chiriacò, F.; Demitri, C.; Gigli, G.; Maffezzoli, A.; Casciaro, S. Echographic detectability of optoacoustic signals from low-concentration PEG-coated gold nanorods. Int. J. Nanomed. 2012, 7, 373–4389. [Google Scholar] [CrossRef]
- Ungureanu, C.; Kroes, R.; Petersen, W.; Groothuis, T.A.M.; Ungureanu, F.; Janssen, H.; van Leeuwen, F.W.B.; Kooyman, R.P.H.; Manohar, S.; van Leeuwen, T.G. Light Interactions with Gold Nanorods and Cells: Implications for Photothermal Nanotherapeutics. Nano Lett. 2011, 11, 1887–1894. [Google Scholar] [CrossRef]
- Yoon, S.J.; Murthy, A.; Johnston, K.P.; Sokolov, K.V.; Emelianov, S.Y. Thermal stability of biodegradable plasmonic nanoclusters in photoacoustic imaging. Opt. Express 2012, 20, 29479–29487. [Google Scholar] [CrossRef]
- Knights, O.B.; Ye, S.; Ingram, N.; Freear, S.; McLaughlan, J.R. Optimising gold nanorods for photoacoustic imaging in vitro. Nanoscale Adv. 2019, 1, 1472–1481. [Google Scholar] [CrossRef]
- Cavigli, L.; Tatini, F.; Borri, C.; Ratto, F.; Centi, S.; Cini, A.; Lelli, B.; Matteini, P.; Pini, R. Preparation and Photoacoustic Analysis of Cellular Vehicles Containing Gold Nanorods. J. Vis. Exp. 2016, 111, e53328. [Google Scholar] [CrossRef] [PubMed]
- Cavigli, L.; Micheletti, F.; Tortoli, P.; Centi, S.; Lai, S.; Borri, C.; Rossi, F.; Ratto, F.; Pini, R. Light activated microbubbles for imaging and microsurgery. Proc. SPIE 2017, 10064, 1006457. [Google Scholar] [CrossRef]
- Frigenti, G.; Cavigli, L.; Fernández-Bienes, A.; Ratto, F.; Centi, S.; García-Fernández, T.; Nunzi Conti, G.; Soria, S. Resonant Microbubble as a Microfluidic Stage for All-Optical Photoacoustic Sensing. Phys. Rev. Appl. 2019, 12, 014062. [Google Scholar] [CrossRef]
- Frigenti, G.; Cavigli, L.; Fernández-Bienes, A.; Ratto, F.; Centi, S.; García-Fernández, T.; Nunzi Conti, G.; Soria, S. Microbubble Resonators for All-Optical Photoacoustics of Flowing Contrast Agents. Sensors 2020, 20, 1696. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.C.; Wei, C.W.; Souris, J.S.; Cheng, S.H.; Chen, C.T.; Yang, C.S.; Li, P.C.; Lo, L.W. Enhanced photoacoustic stability of gold nanorods by silica matrix confinement. J. Biomed. Opt. 2010, 15, 1–6. [Google Scholar] [CrossRef][Green Version]
- Hauck, T.; Ghazani, A.; Chan, W. Assessing the Effect of Surface Chemistry on Gold Nanorod Uptake, Toxicity, and Gene Expression in Mammalian Cells. Small 2008, 4, 153–159. [Google Scholar] [CrossRef]
- Mazzoni, M.; Ratto, F.; Fortunato, C.; Centi, S.; Tatini, F.; Pini, R. Partial Decoupling in Aggregates of Silanized Gold Nanorods. J. Phys. Chem. C 2014, 118, 20018–20025. [Google Scholar] [CrossRef]
- Slowing, I.I.; Vivero-Escoto, J.L.; Wu, C.W.; Lin, V.S.Y. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Deliv. Rev. 2008, 60, 1278–1288, Inorganic Nanoparticles in Drug Delivery. [Google Scholar] [CrossRef]
- Liu, J.; Kan, C.; Cong, B.; Xu, H.; Ni, Y.; Li, Y.; Shi, D. Plasmonic Property and Stability of Core-Shell Au@SiO2 Nanostructures. Plasmonics 2014, 9, 1007–1014. [Google Scholar] [CrossRef]
- Gergely-Fülöp, E.; Zámbó, D.; Deák, A. Thermal stability of mesoporous silica-coated gold nanorods with different aspect ratios. Mater. Chem. Phys. 2014, 148, 909–913. [Google Scholar] [CrossRef][Green Version]
- Chang, C.S.; Rothberg, L.J. Plasmon-Enhanced Photoconductivity in Amorphous Silicon Thin Films by Use of Thermally Stable Silica-Coated Gold Nanorods. Chem. Mater. 2015, 27, 3211–3215. [Google Scholar] [CrossRef]
- Chen, Y.S.; Frey, W.; Kim, S.; Kruizinga, P.; Homan, K.; Emelianov, S. Silica-Coated Gold Nanorods as Photoacoustic Signal Nanoamplifiers. Nano Lett. 2011, 11, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Gao, X. Multilayer coating of gold nanorods for combined stability and biocompatibility. Phys. Chem. Chem. Phys. 2011, 13, 10028–10035. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, W.; Deng, T.S.; Goris, B.; van Huis, M.A.; Bals, S.; van Blaaderen, A. Single Particle Deformation and Analysis of Silica-Coated Gold Nanorods before and after Femtosecond Laser Pulse Excitation. Nano Lett. 2016, 16, 1818–1825. [Google Scholar] [CrossRef]
- Canpean, V.; Gabudean, A.; Astilean, S. Enhanced thermal stability of gelatin coated gold nanorods in water solution. Colloids Surf. A 2013, 433, 9–13. [Google Scholar] [CrossRef]
- Gole, A.; Murphy, C.J. Polyelectrolyte-Coated Gold Nanorods: Synthesis, Characterization and Immobilization. Chem. Mater. 2005, 17, 1325–1330. [Google Scholar] [CrossRef]
- Alkilany, A.M.; Thompson, L.B.; Murphy, C.J. Polyelectrolyte Coating Provides a Facile Route to Suspend Gold Nanorods in Polar Organic Solvents and Hydrophobic Polymers. ACS Appl. Mater. Interfaces 2010, 2, 3417–3421. [Google Scholar] [CrossRef]
- Centi, S.; Cavigli, L.; Borri, C.; Milanesi, A.; Banchelli, M.; Chioccioli, S.; Khlebtsov, B.N.; Khlebtsov, N.G.; Matteini, P.; Bogani, P.; et al. Small Thiols Stabilize the Shape of Gold Nanorods. J. Phys. Chem. C 2020, 124, 11132–11140. [Google Scholar] [CrossRef]
- Milanesi, A.; Magni, G.; Centi, S.; Schifino, G.; Aluigi, A.; Khlebtsov, B.N.; Cavigli, L.; Barucci, A.; Khlebtsov, N.G.; Ratto, F.; et al. Optically activated and interrogated plasmonic hydrogels for applications in wound healing. J. Biophotonics 2020, 13, e202000135. [Google Scholar] [CrossRef]
- Gorelikov, I.; Matsuura, N. Single-Step Coating of Mesoporous Silica on Cetyltrimethyl Ammonium Bromide-Capped Nanoparticles. Nano Lett. 2008, 8, 369–373. [Google Scholar] [CrossRef]
- Inose, T.; Oikawa, T.; Shibuya, K.; Tokunaga, M.; Hatoyama, K.; Nakashima, K.; Kamei, T.; Gonda, K.; Kobayashi, Y. Fabrication of silica-coated gold nanorods and investigation of their property of photothermal conversion. Biochem. Biophys. Res. Commun. 2017, 484, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Santana Vega, M.; Guerrero Martínez, A.; Cucinotta, F. Facile Strategy for the Synthesis of Gold@Silica Hybrid Nanoparticles with Controlled Porosity and Janus Morphology. Nanomaterials 2019, 9, 348. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, X.; Wang, S.; Qian, J.; He, S. Biologically Inspired Polydopamine Capped Gold Nanorods for Drug Delivery and Light-Mediated Cancer Therapy. ACS Appl. Mater. Interfaces 2016, 8, 24368–24384. [Google Scholar] [CrossRef] [PubMed]
- Khlebtsov, B.; Khanadeev, V.; Khlebtsov, N. Surface-enhanced Raman scattering inside Au@Ag core/shell nanorods. Nano Res. 2016, 9, 2303–2318. [Google Scholar] [CrossRef]
- Tsai, M.F.; Chang, S.H.G.; Cheng, F.Y.; Shanmugam, V.; Cheng, Y.S.; Su, C.H.; Yeh, C.S. Au Nanorod Design as Light-Absorber in the First and Second Biological Near-Infrared Windows for in Vivo Photothermal Therapy. ACS Nano 2013, 7, 5330–5342. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Fernández-López, C.; Mateo-Mateo, C.; Álvarez Puebla, R.A.; Pérez-Juste, J.; Pastoriza-Santos, I.; Liz-Marzán, L.M. Highly Controlled Silica Coating of PEG-Capped Metal Nanoparticles and Preparation of SERS-Encoded Particles. Langmuir 2009, 25, 13894–13899. [Google Scholar] [CrossRef]
- Liu, J.; Chang, M.J.; Gao, B.; Xu, Z.G.; Zhang, H.L. Sonication-assisted synthesis of multi-functional gold nanorod/silica core–shell nanostructures. J. Alloys Compd. 2013, 551, 405–409. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, M.; Chen, Q.; Guan, G.; Hu, W.; Zhao, X.; Qiao, M.; Hu, H.; Liang, Y.; Zhu, H.; et al. Gold nanorods/mesoporous silica-based nanocomposite as theranostic agents for targeting near-infrared imaging and photothermal therapy induced with laser. Int. J. Nanomed. 2015, 10, 4747–4761. [Google Scholar] [CrossRef]
- Hinman, J.G.; Stork, A.J.; Varnell, J.A.; Gewirth, A.A.; Murphy, C.J. Seed mediated growth of gold nanorods: Towards nanorod matryoshkas. Faraday Discuss. 2016, 191, 9–33. [Google Scholar] [CrossRef]
- Sendroiu, I.E.; Warner, M.E.; Corn, R.M. Fabrication of Silica-Coated Gold Nanorods Functionalized with DNA for Enhanced Surface Plasmon Resonance Imaging Biosensing Applications. Langmuir 2009, 25, 11282–11284. [Google Scholar] [CrossRef] [PubMed]
- Malinsky, M.D.; Kelly, K.L.; Schatz, G.C.; Van Duyne, R.P. Chain Length Dependence and Sensing Capabilities of the Localized Surface Plasmon Resonance of Silver Nanoparticles Chemically Modified with Alkanethiol Self-Assembled Monolayers. J. Am. Chem. Soc. 2001, 123, 1471–1482. [Google Scholar] [CrossRef]
- Khlebtsov, N.G.; Dykman, L.A.; Bogatyrev, V.A.; Khlebtsov, B.N. Two-Layer Model of Colloidal Gold Bioconjugates and Its Application to the Optimization of Nanosensors. Colloid. J. 2003, 65, 508–518. [Google Scholar] [CrossRef]
- Ryu, J.H.; Messersmith, P.B.; Lee, H. Polydopamine Surface Chemistry: A Decade of Discovery. ACS Appl. Mater. Interfaces 2018, 10, 7523–7540. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zeng, G.; Wang, K.; Wan, Q.; Tao, L.; Zhang, X.; Wei, Y. Recent developments in polydopamine: An emerging soft matter for surface modification and biomedical applications. Nanoscale 2016, 8, 16819–16840. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chem. Rev. 2014, 114. [Google Scholar] [CrossRef]
- Ding, Y.; Floren, M.; Tan, W. Mussel-inspired polydopamine for bio-surface functionalization. Biosurf. Biotribol. 2016, 2, 121–136. [Google Scholar] [CrossRef]
- Khlebtsov, B.N.; Burov, A.M.; Khlebtsov, N.G. Polydopamine coating decreases longitudinal plasmon of Au nanorods: Experiment and simulations. Appl. Mater. Today 2019, 15, 67–76. [Google Scholar] [CrossRef]
- Ah, C.S.; Hong, S.D.; Jang, D.J. Preparation of AucoreAgshell Nanorods and Characterization of Their Surface Plasmon Resonances. J. Phys. Chem. B 2001, 105, 7871–7873. [Google Scholar] [CrossRef]
- Okuno, Y.; Nishioka, K.; Kiya, A.; Nakashima, N.; Ishibashi, A.; Niidome, Y. Uniform and controllable preparation of Au–Ag core–shell nanorods using anisotropic silver shell formation on gold nanorods. Nanoscale 2010, 2, 1489–1493. [Google Scholar] [CrossRef]
- Khlebtsov, B.N.; Liu, Z.; Ye, J.; Khlebtsov, N.G. Au@Ag core/shell cuboids and dumbbells: Optical properties and SERS response. J. Quant. Spectrosc. Radiat. Transf. 2015, 167, 64–75. [Google Scholar] [CrossRef]
- Wang, S.; Xu, H.; Ye, J. Plasmonic rod-in-shell nanoparticles for photothermal therapy. Phys. Chem. Chem. Phys. 2014, 16, 12275–12281. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.C.; Ding, S.J. Structure, Properties and Applications of Mussel-Inspired Polydopamine. J. Biomed. Nanotechnol. 2014, 10, 3063–3084. [Google Scholar] [CrossRef] [PubMed]
- Sahiner, N.; Sagbas, S.; Sahiner, M.; Blake, D.A.; Reed, W.F. Polydopamine particles as nontoxic, blood compatible, antioxidant and drug delivery materials. Colloids Surf. B 2018, 172, 618–626. [Google Scholar] [CrossRef]
- Von Maltzahn, G.; Centrone, A.; Park, J.H.; Ramanathan, R.; Sailor, M.J.; Hatton, T.A.; Bhatia, S.N. SERS-Coded Gold Nanorods as a Multifunctional Platform for Densely Multiplexed Near-Infrared Imaging and Photothermal Heating. Adv. Mater. 2009, 21, 3175–3180. [Google Scholar] [CrossRef] [PubMed]
- Khlebtsov, B.N.; Khanadeev, V.A.; Panfilova, E.V.; Bratashov, D.N.; Khlebtsov, N.G. Gold Nanoisland Films as Reproducible SERS Substrates for Highly Sensitive Detection of Fungicides. ACS Appl. Mater. Interfaces 2015, 7, 6518–6529. [Google Scholar] [CrossRef]
- Cao, J.; Sun, T.; Grattan, K.T. Gold nanorod-based localized surface plasmon resonance biosensors: A review. Sens. Actuator B-Chem. 2014, 195, 332–351. [Google Scholar] [CrossRef]
- Wang, Z.; Zong, S.; Yang, J.; Li, J.; Cui, Y. Dual-mode probe based on mesoporous silica coated gold nanorods for targeting cancer cells. Biosens. Bioelectron. 2011, 26, 2883–2889. [Google Scholar] [CrossRef]
- Zhang, Y.; Qian, J.; Wang, D.; Wang, Y.; He, S. Multifunctional Gold Nanorods with Ultrahigh Stability and Tunability for In Vivo Fluorescence Imaging, SERS Detection, and Photodynamic Therapy. Angew. Chem. 2013, 52, 1148–1151. [Google Scholar] [CrossRef]
- Gao, Z.; Burrows, N.D.; Valley, N.A.; Schatz, G.C.; Murphy, C.J.; Haynes, C.L. In solution SERS sensing using mesoporous silica-coated gold nanorods. Analyst 2016, 141, 5088–5095. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, L.; Wang, J.; Jiang, X.; Li, X.; Hu, Z.; Ji, Y.; Wu, X.; Chen, C. Mesoporous Silica-Coated Gold Nanorods as a Light-Mediated Multifunctional Theranostic Platform for Cancer Treatment. Adv. Mater. 2012, 24, 1418–1423. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Rosei, F.; Vetrone, F. A single multifunctional nanoplatform based on upconversion luminescence and gold nanorods. Nanoscale 2015, 7, 5178–5185. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Detrembleur, C.; De Pauw-Gillet, M.C.; Mornet, S.; Jérôme, C.; Duguet, E. Gold Nanorods Coated with Mesoporous Silica Shell as Drug Delivery System for Remote Near Infrared Light-Activated Release and Potential Phototherapy. Small 2015, 11, 2323–2332. [Google Scholar] [CrossRef] [PubMed]
- Dykman, L.A.; Khlebtsov, N.G. Multifunctional gold-based nanocomposites for theranostics. Biomaterials 2016, 108, 13–34. [Google Scholar] [CrossRef]
- Song, Z.; Liu, Y.; Shi, J.; Ma, T.; Zhang, Z.; Ma, H.; Cao, S. Hydroxyapatite/mesoporous silica coated gold nanorods with improved degradability as a multi-responsive drug delivery platform. Mater. Sci. Eng. C 2018, 83, 90–98. [Google Scholar] [CrossRef]
- Huang, H.C.; Barua, S.; Kay, D.B.; Rege, K. Simultaneous Enhancement of Photothermal Stability and Gene Delivery Efficacy of Gold Nanorods Using Polyelectrolytes. ACS Nano 2009, 3, 2941–2952. [Google Scholar] [CrossRef]
- Huang, J.; Jackson, K.S.; Murphy, C.J. Polyelectrolyte Wrapping Layers Control Rates of Photothermal Molecular Release from Gold Nanorods. Nano Lett. 2012, 12, 2982–2987. [Google Scholar] [CrossRef]
- Haine, A.T.; Niidome, T. Gold Nanorods as Nanodevices for Bioimaging, Photothermal Therapeutics, and Drug Delivery. Chem. Pharm. Bull. 2017, 65, 625–628. [Google Scholar] [CrossRef]
- Peng, D.; Du, Y.; Shi, Y.; Mao, D.; Jia, X.; Li, H.; Zhu, Y.; Wang, K.; Tian, J. Precise diagnosis in different scenarios using photoacoustic and fluorescence imaging with dual-modality nanoparticles. Nanoscale 2016, 8, 14480–14488. [Google Scholar] [CrossRef]
- Jokerst, J.V.; Cole, A.J.; Van de Sompel, D.; Gambhir, S.S. Gold Nanorods for Ovarian Cancer Detection with Photoacoustic Imaging and Resection Guidance via Raman Imaging in Living Mice. ACS Nano 2012, 6, 10366–10377. [Google Scholar] [CrossRef]
- Yang, H.W.; Liu, H.L.; Li, M.L.; Hsi, I.W.; Fan, C.T.; Huang, C.Y.; Lu, Y.J.; Hua, M.Y.; Chou, H.Y.; Liaw, J.W.; et al. Magnetic gold-nanorod/ PNIPAAmMA nanoparticles for dual magnetic resonance and photoacoustic imaging and targeted photothermal therapy. Biomaterials 2013, 34, 5651–5660. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Lin, Y.; Li, Z.; Chen, S.; Huang, G.; Lin, H.; Wang, J.; Liu, G.; Yang, H.H. Gadolinium oxysulfide-coated gold nanorods with improved stability and dual-modal magnetic resonance/photoacoustic imaging contrast enhancement for cancer theranostics. Nanoscale 2017, 9, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Vera, J.; Bayazitoglu, Y. Temperature and heat flux dependence of thermal resistance of water/metal nanoparticle interfaces at sub-boiling temperatures. J. Heat Mass Transf. 2015, 86, 433–442. [Google Scholar] [CrossRef]
- Hu, H.; Sun, Y. Effect of nanopatterns on Kapitza resistance at a water-gold interface during boiling: A molecular dynamics study. J. Appl. Phys. 2012, 112, 053508. [Google Scholar] [CrossRef]
- Gonzalez, M.G.; Acosta, E.O.; Santiago, G.D. Determination of the thermal boundary conductance of gold nanoparticles in aqueous solution using a method based on nanobubble generation. Appl. Opt. 2018, 57, 6229–6232. [Google Scholar] [CrossRef]
- Chen, X.; Munjiza, A.; Zhang, K.; Wen, D. Molecular Dynamics Simulation of Heat Transfer from a Gold Nanoparticle to a Water Pool. J. Phys. Chem. C 2014, 118, 1285–1293. [Google Scholar] [CrossRef]
- Alper, J.; Hamad-Schifferli, K. Effect of Ligands on Thermal Dissipation from Gold Nanorods. Langmuir 2010, 26, 3786–3789. [Google Scholar] [CrossRef]
- Lombard, J.; Biben, T.; Merabia, S. Kinetics of Nanobubble Generation Around Overheated Nanoparticles. Phys. Rev. Lett. 2014, 112, 105701. [Google Scholar] [CrossRef]
- Stoll, T.; Maioli, P.; Crut, A.; Rodal-Cedeira, S.; Pastoriza-Santos, I.; Vallée, F.; Del Fatti, N. Time-Resolved Investigations of the Cooling Dynamics of Metal Nanoparticles: Impact of Environment. J. Phys. Chem. C 2015, 119, 12757–12764. [Google Scholar] [CrossRef]
- Moon, H.; Kumar, D.; Kim, H.; Sim, C.; Chang, J.H.; Kim, J.M.; Kim, H.; Lim, D.K. Amplified Photoacoustic Performance and Enhanced Photothermal Stability of Reduced Graphene Oxide Coated Gold Nanorods for Sensitive Photoacoustic Imaging. ACS Nano 2015, 9, 2711–2719. [Google Scholar] [CrossRef]
- Chen, Y.S.; Zhao, Y.; Yoon, S.J.; Gambhir, S.S.; Emelianov, S. Miniature gold nanorods for photoacoustic molecular imaging in the second near-infrared optical window. Nat. Nanotechnol. 2019, 14, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.H.; Murphy, C.J. Mini Gold Nanorods with Tunable Plasmonic Peaks beyond 1000 nm. Chem. Mater. 2018, 30, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Sau, T.K.; Murphy, C.J. Seeded High Yield Synthesis of Short Au Nanorods in Aqueous Solution. Langmuir 2004, 20, 6414–6420. [Google Scholar] [CrossRef] [PubMed]
- Lohse, S.E.; Eller, J.R.; Sivapalan, S.T.; Plews, M.R.; Murphy, C.J. A Simple Millifluidic Benchtop Reactor System for the High-Throughput Synthesis and Functionalization of Gold Nanoparticles with Different Sizes and Shapes. ACS Nano 2013, 7, 4135–4150. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Fang, C.; Zhu, X.M.; Ruan, Q.; Wang, Y.X.J.; Wang, J. Synthesis of Absorption-Dominant Small Gold Nanorods and Their Plasmonic Properties. Langmuir 2015, 31, 7418–7426. [Google Scholar] [CrossRef]
- Ali, M.R.K.; Snyder, B.; El-Sayed, M.A. Synthesis and Optical Properties of Small Au Nanorods Using a Seedless Growth Technique. Langmuir 2012, 28, 9807–9815. [Google Scholar] [CrossRef]
- Zhang, L.; Xia, K.; Lu, Z.; Li, G.; Chen, J.; Deng, Y.; Li, S.; Zhou, F.; He, N. Efficient and Facile Synthesis of Gold Nanorods with Finely Tunable Plasmonic Peaks from Visible to Near-IR Range. Chem. Mater. 2014, 26, 1794–1798. [Google Scholar] [CrossRef]
- Zijlstra, P.; Bullen, C.; Chon, J.W.M.; Gu, M. High-Temperature Seedless Synthesis of Gold Nanorods. J. Phys. Chem. C 2006, 110, 19315–19318. [Google Scholar] [CrossRef]
- Tatini, F.; Landini, I.; Scaletti, F.; Massai, L.; Centi, S.; Ratto, F.; Nobili, S.; Romano, G.; Fusi, F.; Messori, L.; et al. Size dependent biological profiles of PEGylated gold nanorods. J. Mater. Chem. B 2014, 2, 6072–6080. [Google Scholar] [CrossRef]
- Juvé, V.; Cardinal, M.F.; Lombardi, A.; Crut, A.; Maioli, P.; Pérez-Juste, J.; Liz-Marzán, L.M.; Del Fatti, N.; Vallée, F. Size-Dependent Surface Plasmon Resonance Broadening in Nonspherical Nanoparticles: Single Gold Nanorods. Nano Lett. 2013, 13, 2234–2240. [Google Scholar] [CrossRef]
- Pan, Y.; Neuss, S.; Leifert, A.; Fischler, M.; Wen, F.; Simon, U.; Schmid, G.; Brandau, W.; Jahnen-Dechent, W. Size-Dependent Cytotoxicity of Gold Nanoparticles. Small 2007, 3, 1941–1949. [Google Scholar] [CrossRef] [PubMed]
- Schmid, G.; Kreyling, W.G.; Simon, U. Toxic effects and biodistribution of ultrasmall gold nanoparticles. Arch. Toxicol. 2017, 91, 3011–3037. [Google Scholar] [CrossRef] [PubMed]
- Cavigli, L.; Centi, S.; Borri, C.; Tortoli, P.; Panettieri, I.; Streit, I.; Ciofini, D.; Magni, G.; Rossi, F.; Siano, S.; et al. 1064-nm-resonant gold nanorods for photoacoustic theranostics within permissible exposure limits. J. Biophotonics 2019, 0, e201900082. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.M.; Tam, J.O.; Murthy, A.; Ingram, D.R.; Ma, L.L.; Travis, K.; Johnston, K.P.; Sokolov, K.V. Controlled Assembly of Biodegradable Plasmonic Nanoclusters for Near-Infrared Imaging and Therapeutic Applications. ACS Nano 2010, 4, 2178–2184. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yu, M.; Zhou, C.; Yang, S.; Ning, X.; Zheng, J. Passive Tumor Targeting of Renal-Clearable Luminescent Gold Nanoparticles: Long Tumor Retention and Fast Normal Tissue Clearance. J. Am. Chem. Soc. 2013, 135, 4978–4981. [Google Scholar] [CrossRef]
- Cassano, D.; Summa, M.; Pocoví-Martínez, S.; Mapanao, A.K.; Catelani, T.; Bertorelli, R.; Voliani, V. Biodegradable Ultrasmall-in-Nano Gold Architectures: Mid-Period In Vivo Distribution and Excretion Assessment. Part. Part. Syst. Charact. 2019, 36, 1800464. [Google Scholar] [CrossRef]
- Cavigli, L.; Centi, S.; Lai, S.; Borri, C.; Micheletti, F.; Tortoli, P.; Panettieri, I.; Streit, I.; Rossi, F.; Ratto, F.; et al. Light-activated microbubbles around gold nanorods for photoacoustic microsurgery. Proc. SPIE 2018, 10494, 104942D. [Google Scholar] [CrossRef]
- DeSantis, C.J.; Huang, D.; Zhang, H.; Hogan, N.J.; Zhao, H.; Zhang, Y.; Manjavacas, A.; Zhang, Y.; Chang, W.S.; Nordlander, P.; et al. Laser-Induced Spectral Hole-Burning through a Broadband Distribution of Au Nanorods. J. Phys. Chem. C 2016, 120, 20518–20524. [Google Scholar] [CrossRef]


| Device | MSOT Acuity [19] | Vevo LAZR-X [20] | LOIS3D [21] | TriTom [22] | Hadatomo Z [23] |
|---|---|---|---|---|---|
| Company | iThera Medical | FUJIFILM | TomoWave | PST | Advantest Corp. |
| Pulse duration * | <10 ns | 4–6 ns | 3–5 ns | 5 ns | 10 ns |
| Rep rate * | 25 Hz | 20 Hz | 10 Hz | 10 Hz | 1000 Hz |
| Wavelength * | 660–1300 nm | 680–970; 1200–2000 nm | 680–1064 nm | 670–2600 nm | 532; 556 nm |
| Peak energy * | 30 mJ | 45 mJ | up to 200 mJ | 150 mJ | 16 J |
| Imaging depth | up to 4 cm | 1 cm | >3 cm | n.a. | 3 mm |
| Lateral resolution | 200 m | 45 m | 250 m | 150 m | 15 m |
| Applications | clinical | pre-clinical | pre-clinical | pre-clinical | clinical |
| Chromophore | Wavelenght Window | Clinical Interest |
|---|---|---|
| Melanin | NIR-I and NIR-II | Skin cancer |
| Hemoglobin (Oxy-Deoxy) | NIR-I | Ischemia, hypoxia or hypoxemia, tumor angiogenesis |
| Lipids [33] | NIR-II | Arterial plaques monitoring, diabetes, obesity, fatty liver disease |
| Collagen [34] | NIR-II | Orthopedics, dermatology, and cardiology |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavigli, L.; Khlebtsov, B.N.; Centi, S.; Khlebtsov, N.G.; Pini, R.; Ratto, F. Photostability of Contrast Agents for Photoacoustics: The Case of Gold Nanorods. Nanomaterials 2021, 11, 116. https://doi.org/10.3390/nano11010116
Cavigli L, Khlebtsov BN, Centi S, Khlebtsov NG, Pini R, Ratto F. Photostability of Contrast Agents for Photoacoustics: The Case of Gold Nanorods. Nanomaterials. 2021; 11(1):116. https://doi.org/10.3390/nano11010116
Chicago/Turabian StyleCavigli, Lucia, Boris N. Khlebtsov, Sonia Centi, Nikolai G. Khlebtsov, Roberto Pini, and Fulvio Ratto. 2021. "Photostability of Contrast Agents for Photoacoustics: The Case of Gold Nanorods" Nanomaterials 11, no. 1: 116. https://doi.org/10.3390/nano11010116
APA StyleCavigli, L., Khlebtsov, B. N., Centi, S., Khlebtsov, N. G., Pini, R., & Ratto, F. (2021). Photostability of Contrast Agents for Photoacoustics: The Case of Gold Nanorods. Nanomaterials, 11(1), 116. https://doi.org/10.3390/nano11010116