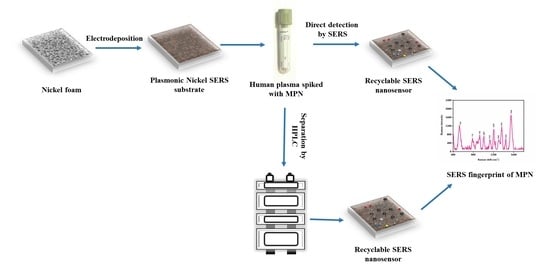
Gold-Deposited Nickel Foam as Recyclable Plasmonic Sensor for Therapeutic Drug Monitoring in Blood by Surface-Enhanced Raman Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
2.3. Pre-treatment of the Nickel Foam Substrate
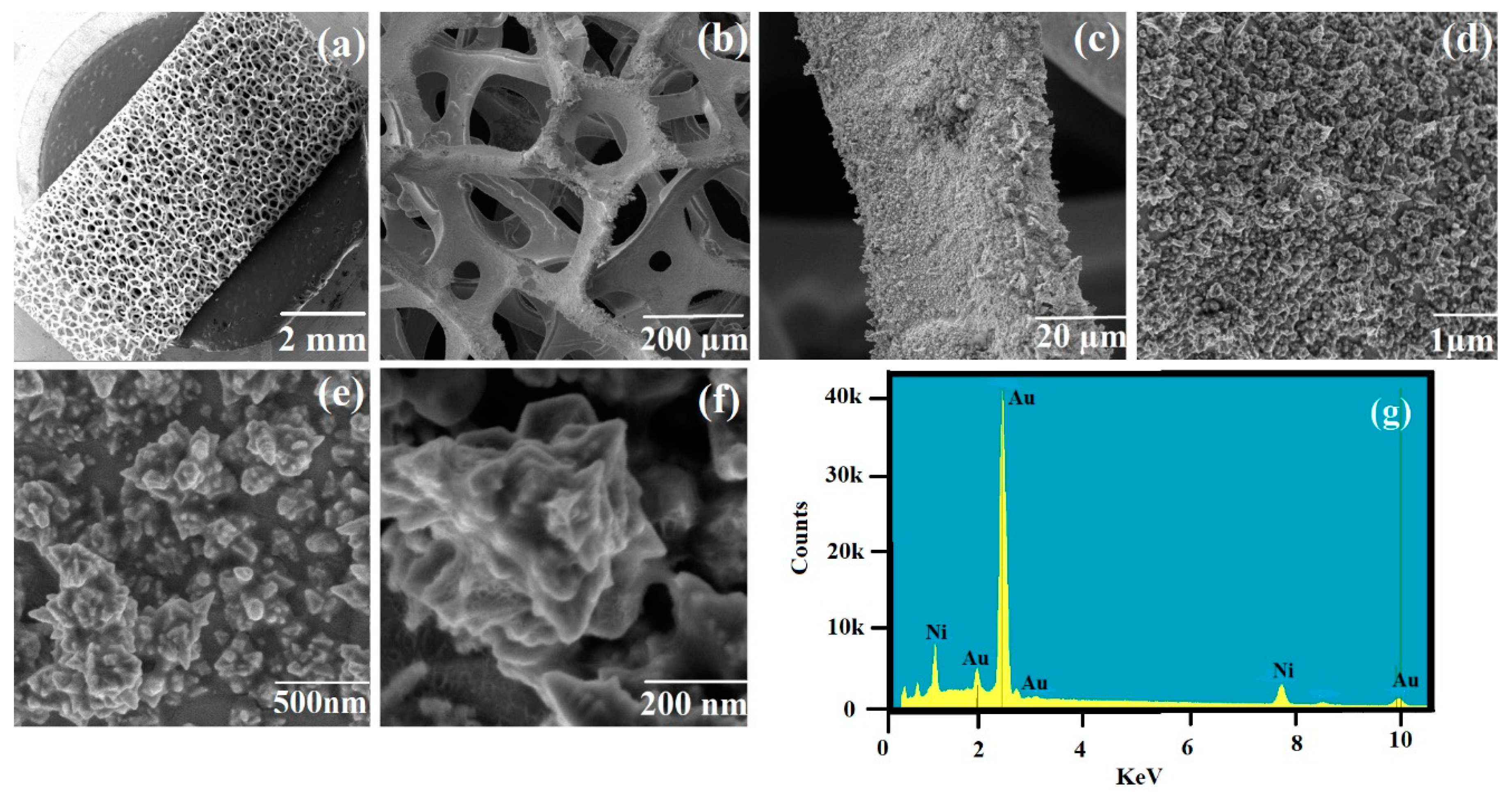
2.4. Synthesis of Plasmonic Nickel Foam by Chronoamperometry
2.5. Characterization of the Plasmonic Nickel Foam Material
2.6. Calculation of the Enhancement Factor (EF) of Plasmonic Nickel Foam Material
2.7. Preparation of Standard Solutions
2.8. Determination of MPN by SERS
2.9. Recycle of Plasmonic Nickel Foam
2.10. Cross-Validation of SERS Quantification of MPN with HPLC
2.11. Direct Detection of MPN in Spiked Blood Samples
2.12. Control Tests
2.13. Reproducibility of MPN Measurements by SERS
2.14. SERS Detection of MPN in Blood Plasma in the Presence of Paracetamol
2.15. Determination of MPN in Blood Plasma by HPLC-SERS
3. Results and Discussion
3.1. Synthesis and Characterisation of Plasmonic Nickel Foam
3.2. SERS Measurements of MPN
3.3. SERS Quantification of MPN in Aqueous Solution and Reproducibility of the Measurements
3.4. Direct SERS Quantification of MPN in Human Plasma
3.5. Cross Validation with HPLC-DAD
3.6. Determination of MPN by HPLC-SERS
3.7. Recycling of SERS Substrate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hassanain, W.A.; Izake, E.L.; Sivanesan, A.; Ayoko, G.A. Towards interference free HPLC-SERS for the trace analysis of drug metabolites in biological fluids. J. Pharm. Biomed. Anal. 2017, 136, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Rehm, S.; Rentsch, K. HILIC LC-MS/MS method for the quantification of cefepime, imipenem and meropenem. J. Pharm. Biomed. Anal. 2020, 186, 113289. [Google Scholar] [CrossRef]
- Trachta, G.; Schwarze, B.; Sägmüller, B.; Brehm, G.; Schneider, S. Combination of high-performance liquid chromatography and SERS detection applied to the analysis of drugs in human blood and urine. J. Mol. Struct. 2004, 693, 175–185. [Google Scholar] [CrossRef]
- Subaihi, A.; Trivedi, D.; Hollywood, K.; Bluett, J.; Xu, Y.; Muhamadali, H.; Ellis, D.I.; Goodacre, R. Quantitative Online Liquid Chromatography–Surface-Enhanced Raman Scattering (LC-SERS) of Methotrexate and its Major Metabolites. Anal. Chem. 2017, 89, 6702–6709. [Google Scholar] [CrossRef] [PubMed]
- Muneer, S.; Ayoko, G.A.; Islam, N.; Izake, E.L. Utilizing the thiol chemistry of biomolecules for the rapid determination of anti-TNF-α drug in blood. Talanta 2020, 208, 120411. [Google Scholar] [CrossRef]
- Parachalil, D.R.; Commerford, D.; Bonnier, F.; Chourpa, I.; McIntyre, J.; Byrne, H.J.; Comerford, D. Raman spectroscopy as a potential tool for label free therapeutic drug monitoring in human serum: The case of busulfan and methotrexate. Analyst 2019, 144, 5207–5214. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.-B.; Li, P.; Liu, H.; Tang, X.; Liu, J. A dynamic surface enhanced Raman spectroscopy method for ultra-sensitive detection: From the wet state to the dry state. Chem. Soc. Rev. 2015, 44, 2837–2848. [Google Scholar] [CrossRef]
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A Review on Surface-Enhanced Raman Scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef] [Green Version]
- Cardinal, M.F.; Ende, E.V.; Hackler, R.A.; McAnally, M.O.; Stair, P.C.; Schatz, G.C.; Van Duyne, R.P. Expanding applications of SERS through versatile nanomaterials engineering. Chem. Soc. Rev. 2017, 46, 3886–3903. [Google Scholar] [CrossRef] [Green Version]
- Kleinman, S.L.; Frontiera, R.R.; Henry, A.-I.; Dieringer, J.A.; Van Duyne, R.P. Creating, characterizing, and controlling chemistry with SERS hot spots. Phys. Chem. Chem. Phys. 2013, 15, 21–36. [Google Scholar] [CrossRef]
- Langer, J.; De Aberasturi, D.J.; Aizpurua, J.; A Alvarez-Puebla, R.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2019, 14, 28–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jing, C.; Fang, Y. Simple method for electrochemical preparation of silver dendrites used as active and stable SERS substrate. J. Colloid Interface Sci. 2007, 314, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Cardinal, M.F.; Kleinman, S.L.; Greeneltch, N.G.; Frontiera, R.R.; Blaber, M.G.; Schatz, G.C.; Van Duyne, R.P. High-performance SERS substrates: Advances and challenges. MRS Bull. 2013, 38, 615–624. [Google Scholar] [CrossRef] [Green Version]
- Mosier-Boss, P. Review of SERS Substrates for Chemical Sensing. Nanomaterials 2017, 7, 142. [Google Scholar] [CrossRef] [Green Version]
- Muneer, S.; Ayoko, G.A.; Islam, N.; Izake, E.L. Preconcentration and SERS-based determination of infliximab in blood by using a TNF-α-modified gold-coated copper oxide nanomaterial. Microchim. Acta 2019, 186, 780. [Google Scholar] [CrossRef]
- Pavliuk, G.; Pavlov, D.; Mitsai, E.; Vitrik, O.; Mironenko, A.; Zakharenko, A.; Kulinich, S.; Juodkazis, S.; Bratskaya, S.; Zhizhchenko, A. Ultrasensitive SERS-Based Plasmonic Sensor with Analyte Enrichment System Produced by Direct Laser Writing. Nanomaterials 2019, 10, 49. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; Lai, H.; Xu, H. Gold nanocone arrays directly grown on nickel foam for improved SERS detection of aromatic dyes. Anal. Methods 2018, 10, 3170–3177. [Google Scholar] [CrossRef]
- Mortensen, J.S.; Jensen, B.P.; Zhang, M.; Doogue, M. Preanalytical Stability of Piperacillin, Tazobactam, Meropenem, and Ceftazidime in Plasma and Whole Blood Using Liquid Chromatography–Tandem Mass Spectrometry. Ther. Drug Monit. 2019, 41, 538–543. [Google Scholar] [CrossRef]
- Al–Meshal, M.A.; Ramadan, M.A.; Lotfi, K.M.; Shibl, A.M. Determination of meropenem in plasma by high–performance liquid chromatography and a microbiological method. J. Clin. Pharm. Ther. 1995, 20, 159–163. [Google Scholar] [CrossRef]
- Trautmann, M.; Zick, R.; Rukavina, T.; Cross, A.S.; Marre, R. Antibiotic-induced release of endotoxin: In-vitro comparison of meropenem and other antibiotics. J. Antimicrob. Chemother. 1998, 41, 163–169. [Google Scholar] [CrossRef]
- Roth, T.; Fiedler, S.; Mihai, S.; Parsch, H. Determination of meropenem levels in human serum by high-performance liquid chromatography with ultraviolet detection. Biomed. Chromatogr. 2017, 31, e3880. [Google Scholar] [CrossRef] [PubMed]
- Hassanain, W.A.; Izake, E.L.; Ayoko, G.A. Spectroelectrochemical Nanosensor for the Determination of Cystatin C in Human Blood. Anal. Chem. 2018, 90, 10843–10850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarfo, D.K.; Izake, E.L.; O’Mullane, A.P.; Ayoko, G.A. Molecular recognition and detection of Pb(II) ions in water by aminobenzo-18-crown-6 immobilised onto a nanostructured SERS substrate. Sens. Actuators B 2018, 255, 1945–1952. [Google Scholar] [CrossRef]
- Le Ru, E.C.; Blackie, E.; Meyer, M.; Etchegoin, P.G. Surface Enhanced Raman Scattering Enhancement Factors: A Comprehensive Study. J. Phys. Chem. C 2007, 111, 13794–13803. [Google Scholar] [CrossRef]
- Rao, L.V.; Ramu, G.; Kumar, M.S.; Rambabu, C. Reverse Phase HPLC and Visible Spectrophotometric Methods for the Determination of Meropenem in Pure and Pharmaceutical Dosage Form. Int. J. PharmTech. Res. 2012, 4, 957–962. [Google Scholar]
- Sarfo, D.K.; Izake, E.L.; O’Mullane, A.P.; Wang, T.; Wang, H.; Tesfamichael, T.; Ayoko, G.A. Fabrication of dual function disposable substrates for spectroelectrochemical nanosensing. Sens. Actuators B 2019, 287, 9–17. [Google Scholar] [CrossRef]
- Vu, T.; Duy, P.K.; Chung, H. Nickel foam-caged Ag-Au bimetallic nanostructure as a highly rugged and durable SERS substrate. Sens. Actuators B 2019, 282, 535–540. [Google Scholar] [CrossRef]
- Ding, Q.; Liu, H.; Yang, L.-B.; Liu, J. Speedy and surfactant-free in situ synthesis of nickel/Ag nanocomposites for reproducible SERS substrates. J. Mater. Chem. 2012, 22, 19932. [Google Scholar] [CrossRef]
- Hammes, K.; Smernik, R.J.; Skjemstad, J.O.; Schmidt, M.W.I. Characterisation and evaluation of reference materials for black carbon analysis using elemental composition, colour, BET surface area and 13C NMR spectroscopy. Appl. Geochem. 2008, 23, 2113–2122. [Google Scholar] [CrossRef]
- Wang, G.; Cao, D.; Yin, C.; Gao, Y.; Yin, J.; Cheng, L. Nickel Foam Supported-Co3O4 Nanowire Arrays for H2O2 Electroreduction. Chem. Mater. 2009, 21, 5112–5118. [Google Scholar] [CrossRef]
- Cielecka-Piontek, J.; Paczkowska, M.; Lewandowska, K.; Barszcz, B.; Zalewski, P.; Garbacki, P. Solid-state stability study of meropenem—solutions based on spectrophotometric analysis. Chem. Cent. J. 2013, 7, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendez, A.S.; Dalomo, J.; Steppe, M.; Schapoval, E.E.S. Stability and degradation kinetics of meropenem in powder for injection and reconstituted sample. J. Pharm. Biomed. Anal. 2006, 41, 1363–1366. [Google Scholar] [CrossRef] [PubMed]
- Muneer, S.; Muhammad, I.N.; Abrar, M.A.; Muneer, S.; Kaukab, I.; Sagheer, A.; Zafar, H.; Sultana, K. High Performance Liquid Chromatographic Determination of Naproxen in Prepared Pharmaceutical Dosage Form and Human Plasma and its Application to Pharmacokinetic Study. J. Chromatogr. Sep. Tech. 2017, 8, 1–5. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, V.B. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young. Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]



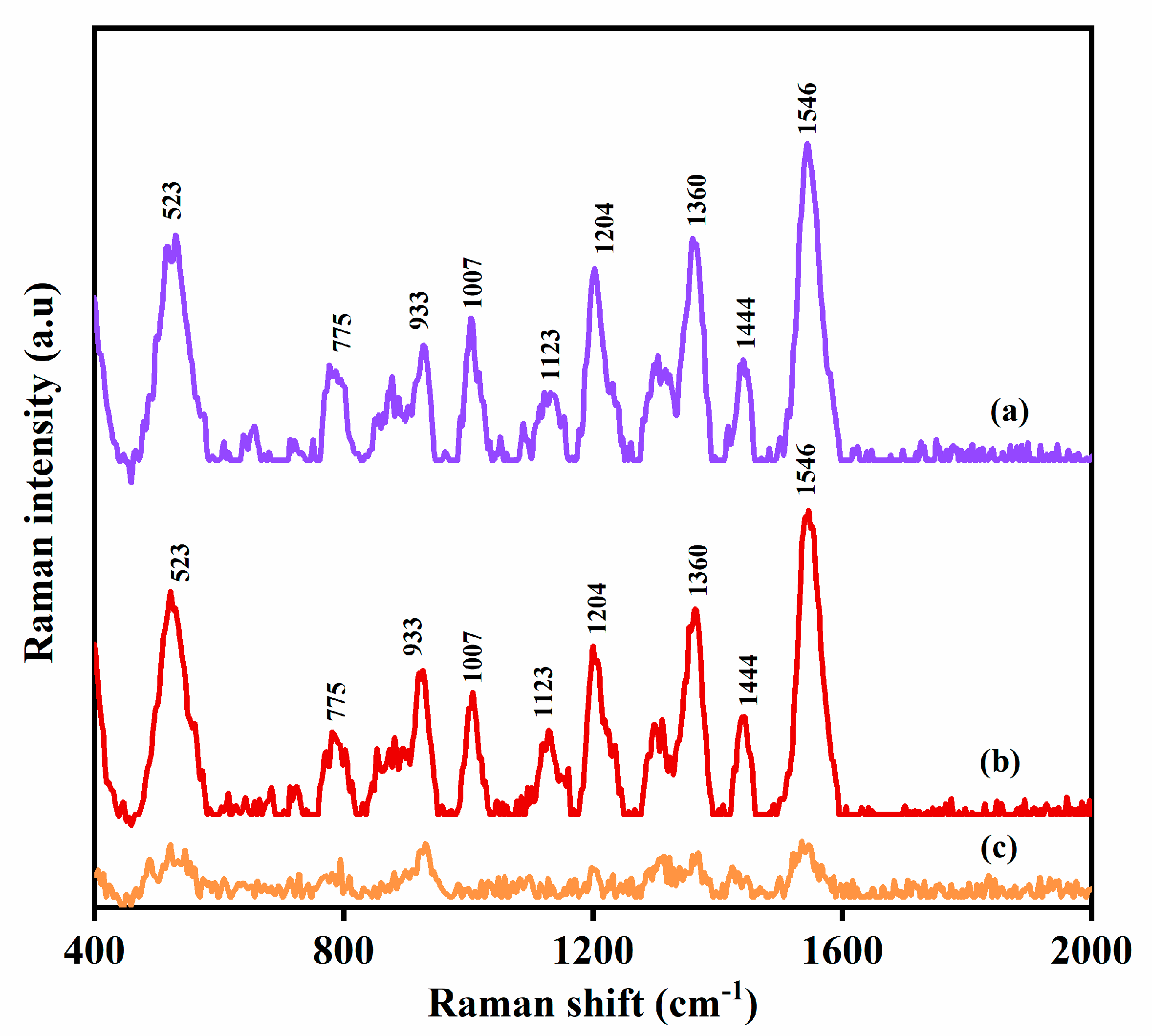
| Raman Shift (cm−1) | Band Assignment | Reference |
|---|---|---|
| 1546 | CH3 in dimethylcarbamoyl group | [31] |
| 1444 | C-N in dimethylcarbamoyl group | [31] |
| 1360 | CH in hydroxyethyl group, CH in pyrrolidine ring | [31] |
| 1123 | CH twist, C-N stretch in pyrrolidine ring | [31] |
| 1007 | C-C in β-lactam ring, CH in trans-hydroxyethyl group | [31] |
| 933 | -CO symmetric stretch C-C between β-lactam and dimethylcarbamoyl group CN in β-lactam | [5] |
| 570 | O-H b in carboxylic group | [31] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muneer, S.; Sarfo, D.K.; Ayoko, G.A.; Islam, N.; Izake, E.L. Gold-Deposited Nickel Foam as Recyclable Plasmonic Sensor for Therapeutic Drug Monitoring in Blood by Surface-Enhanced Raman Spectroscopy. Nanomaterials 2020, 10, 1756. https://doi.org/10.3390/nano10091756
Muneer S, Sarfo DK, Ayoko GA, Islam N, Izake EL. Gold-Deposited Nickel Foam as Recyclable Plasmonic Sensor for Therapeutic Drug Monitoring in Blood by Surface-Enhanced Raman Spectroscopy. Nanomaterials. 2020; 10(9):1756. https://doi.org/10.3390/nano10091756
Chicago/Turabian StyleMuneer, Saiqa, Daniel K. Sarfo, Godwin A. Ayoko, Nazrul Islam, and Emad L. Izake. 2020. "Gold-Deposited Nickel Foam as Recyclable Plasmonic Sensor for Therapeutic Drug Monitoring in Blood by Surface-Enhanced Raman Spectroscopy" Nanomaterials 10, no. 9: 1756. https://doi.org/10.3390/nano10091756
APA StyleMuneer, S., Sarfo, D. K., Ayoko, G. A., Islam, N., & Izake, E. L. (2020). Gold-Deposited Nickel Foam as Recyclable Plasmonic Sensor for Therapeutic Drug Monitoring in Blood by Surface-Enhanced Raman Spectroscopy. Nanomaterials, 10(9), 1756. https://doi.org/10.3390/nano10091756