Engineering Core-Shell Structures of Magnetic Ferrite Nanoparticles for High Hyperthermia Performance
Abstract
1. Introduction
2. Experimental Work
2.1. Materials
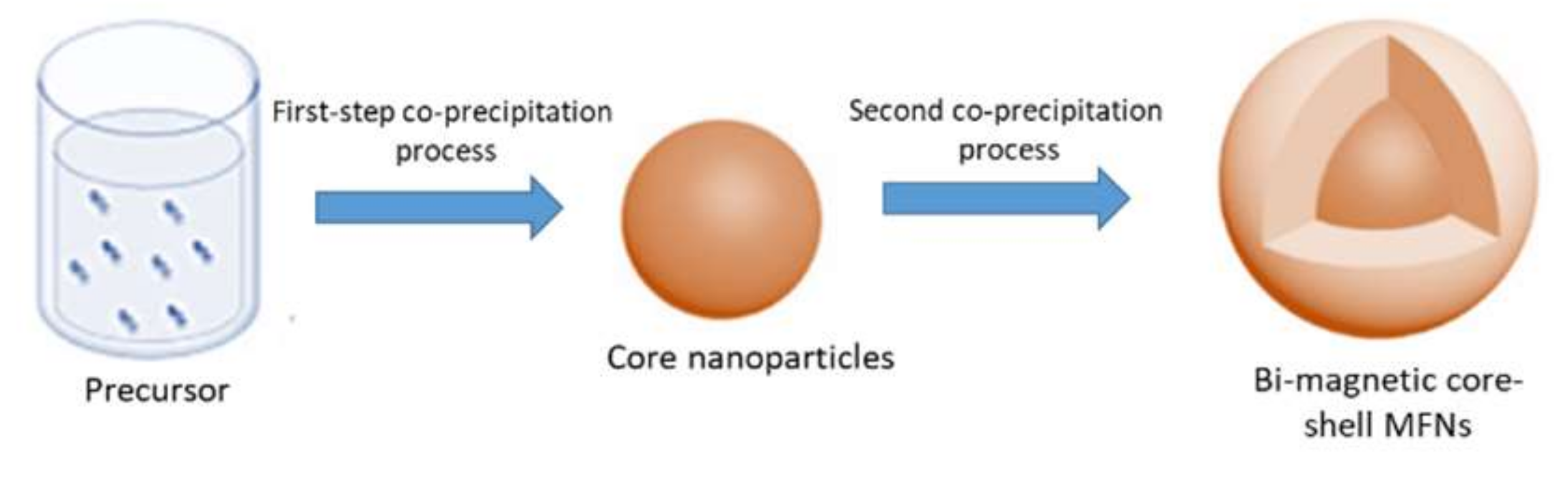
2.2. Synthesis of Bi-Magnetic Core-Shell MFNs
2.3. Characterization
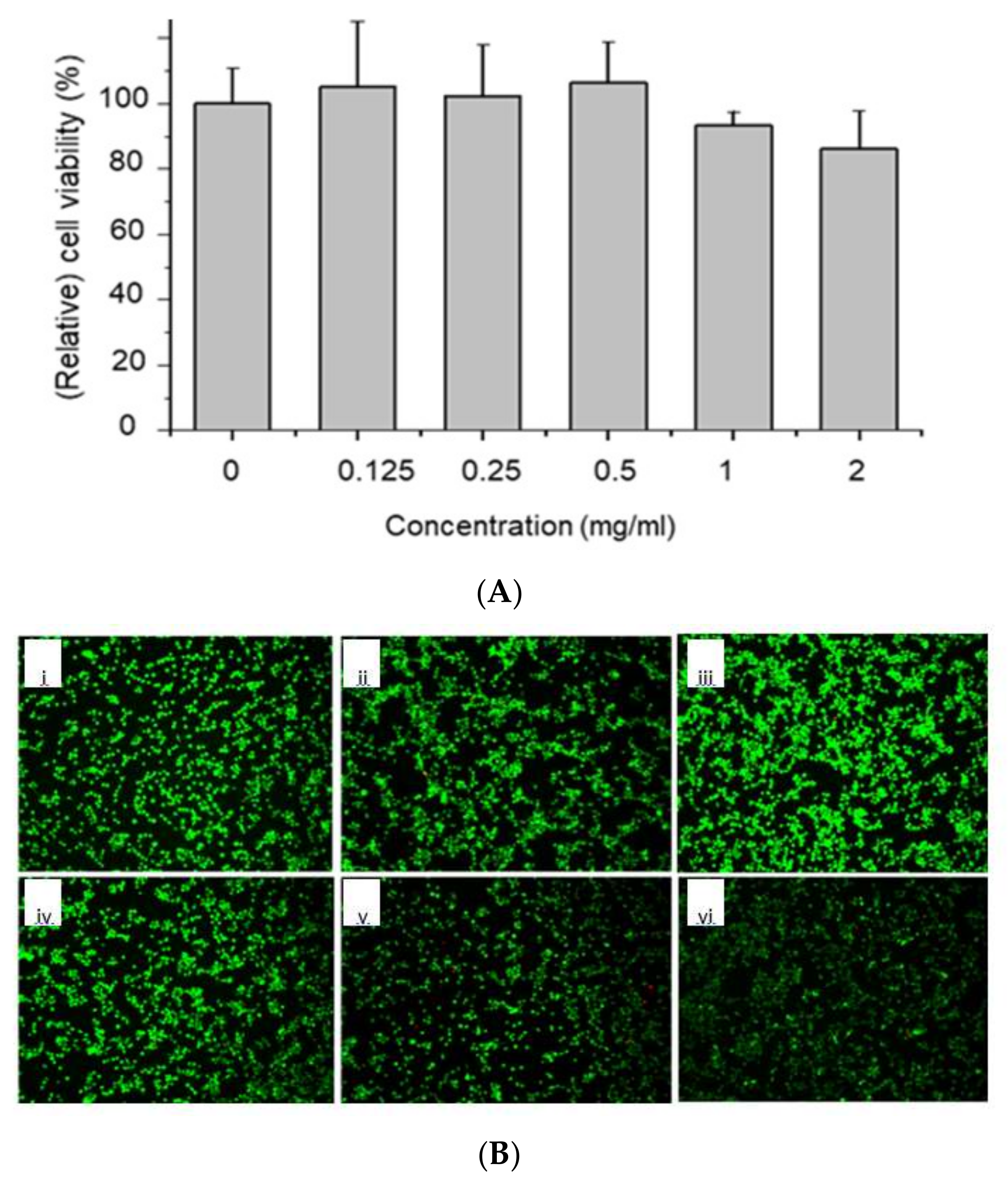
2.4. In Vitro Cytocompatibility Test
3. Results and Discussion
3.1. Synthesis of Core-Shell MFNs
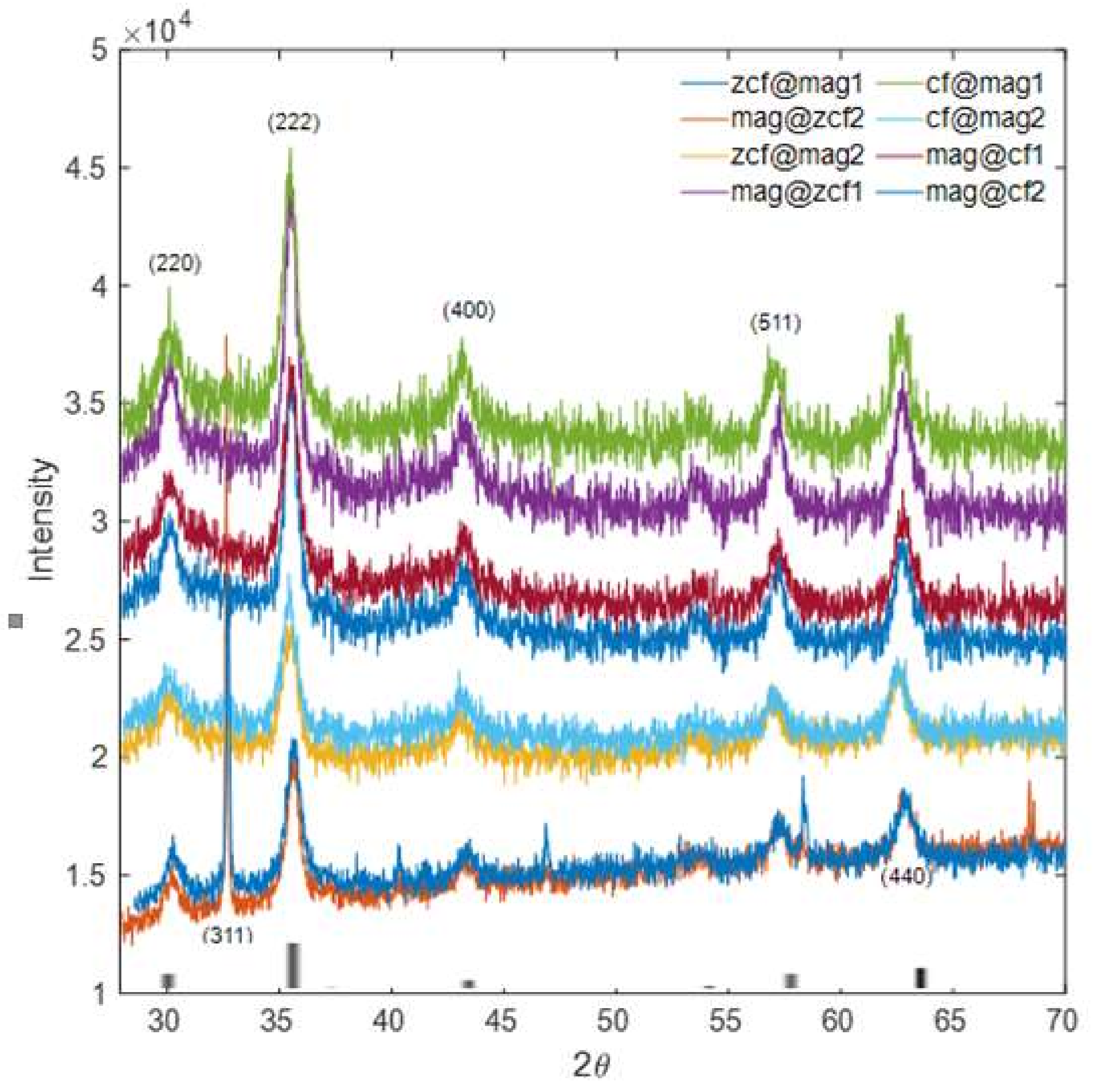
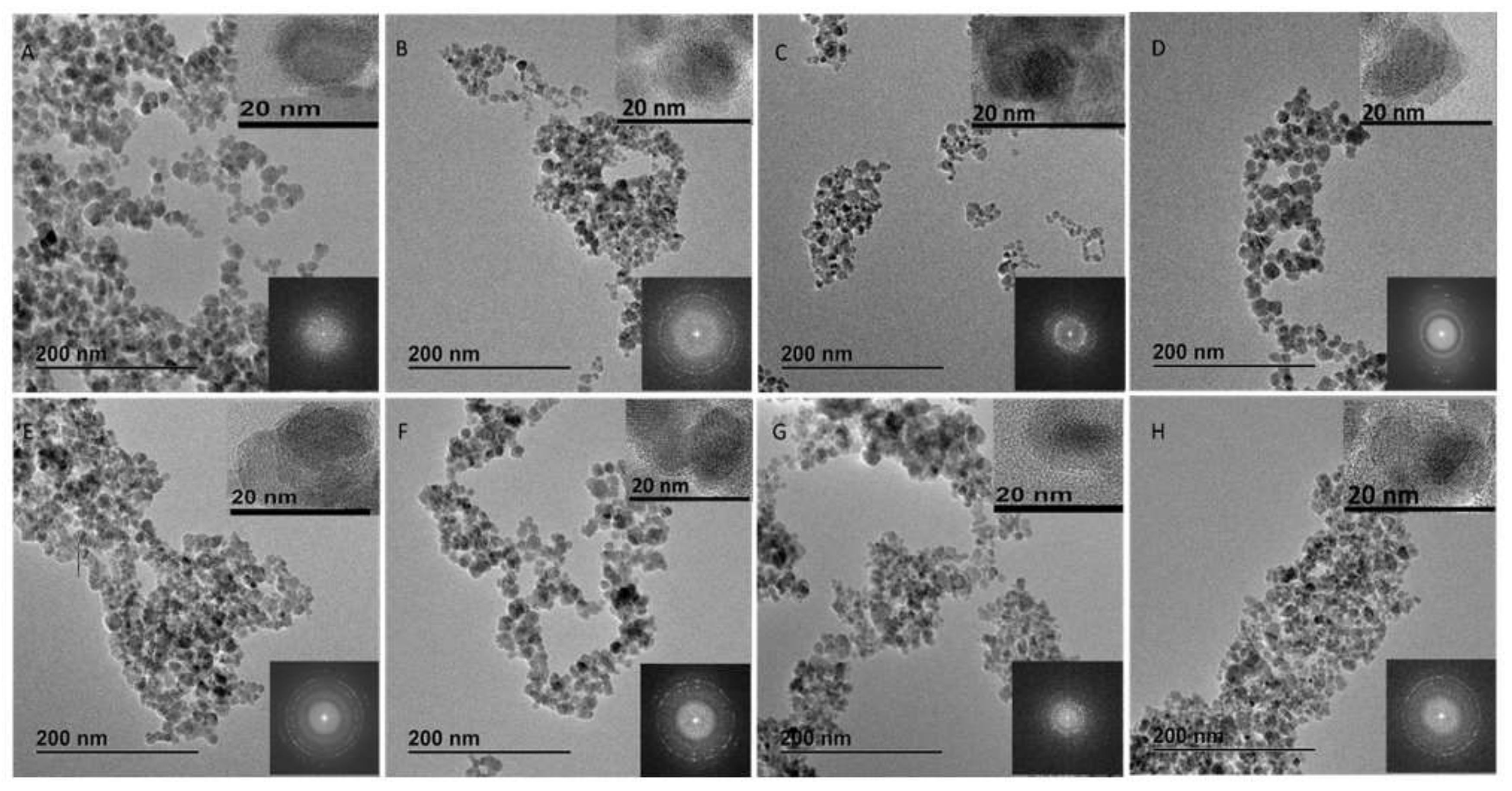
3.2. Characterization of the Prepared Core-Shell MFNs
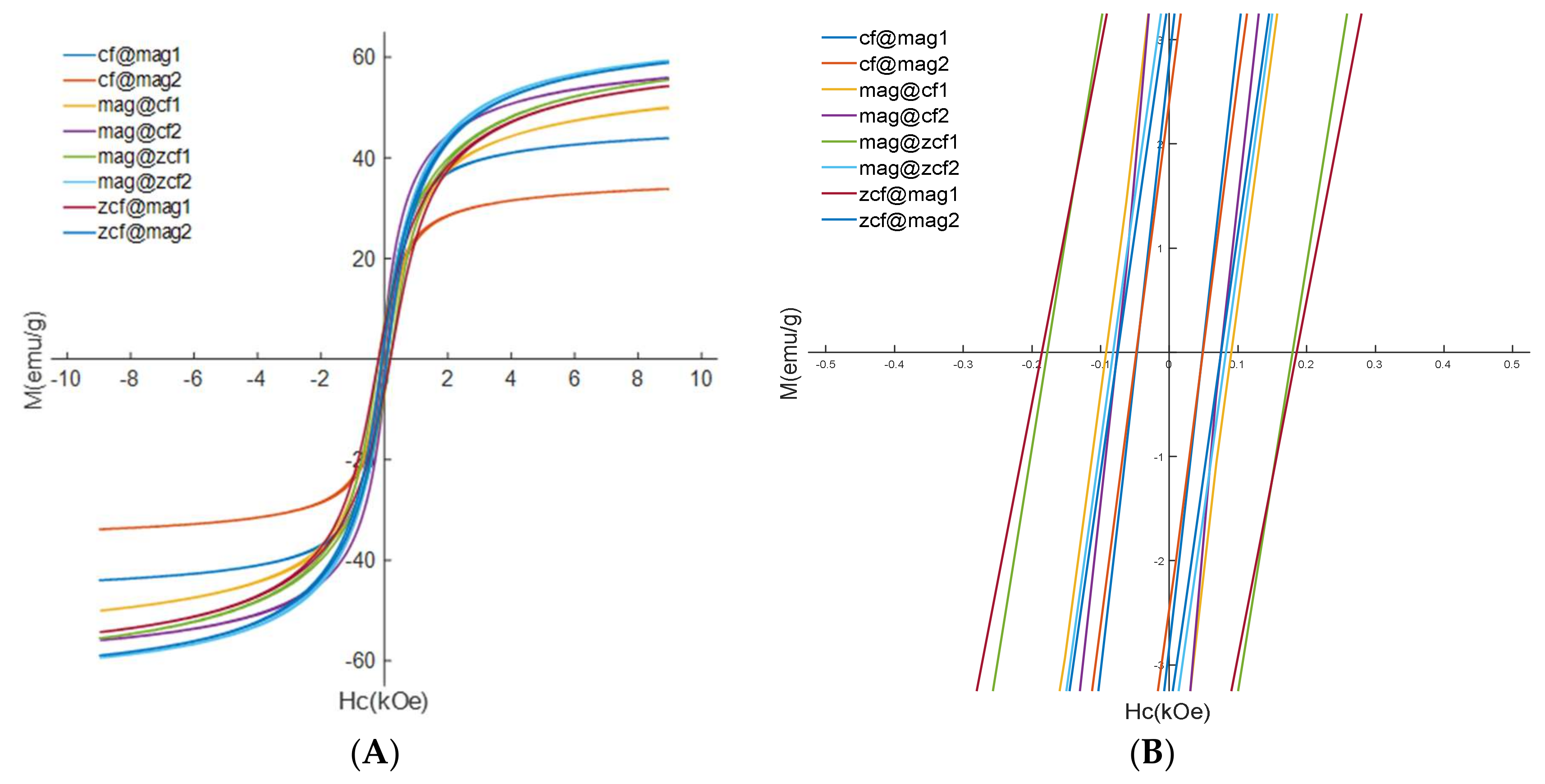
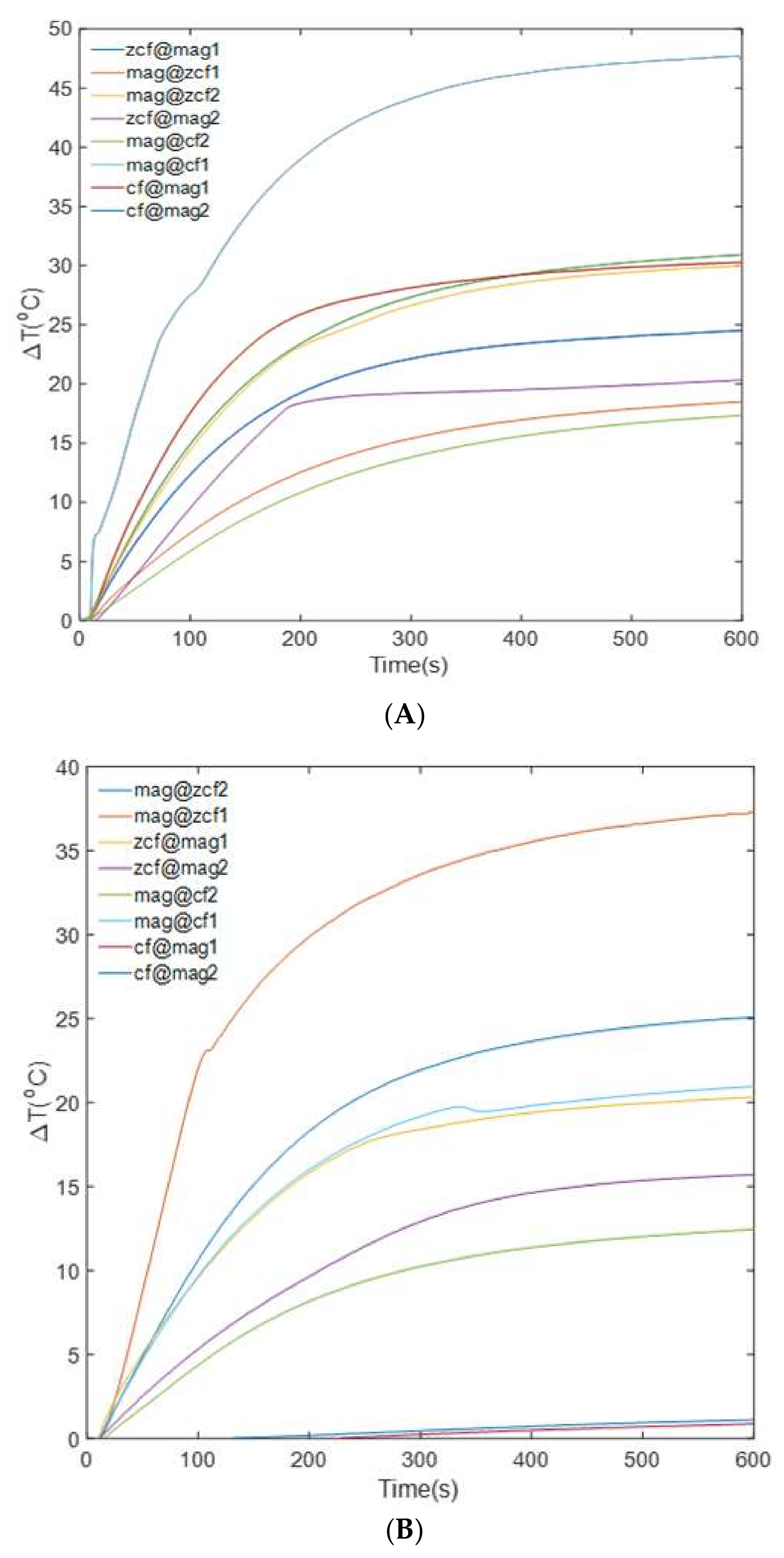
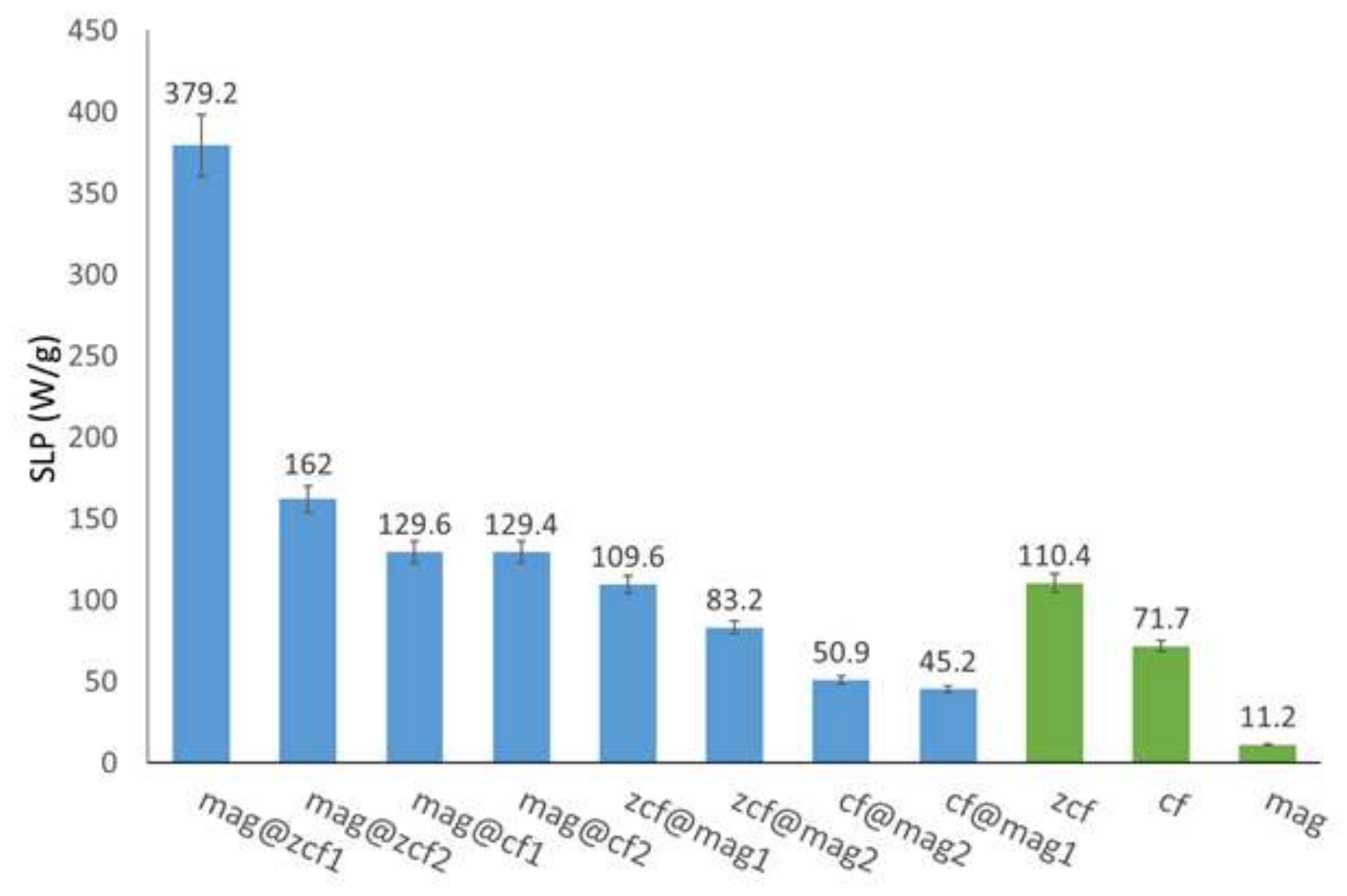
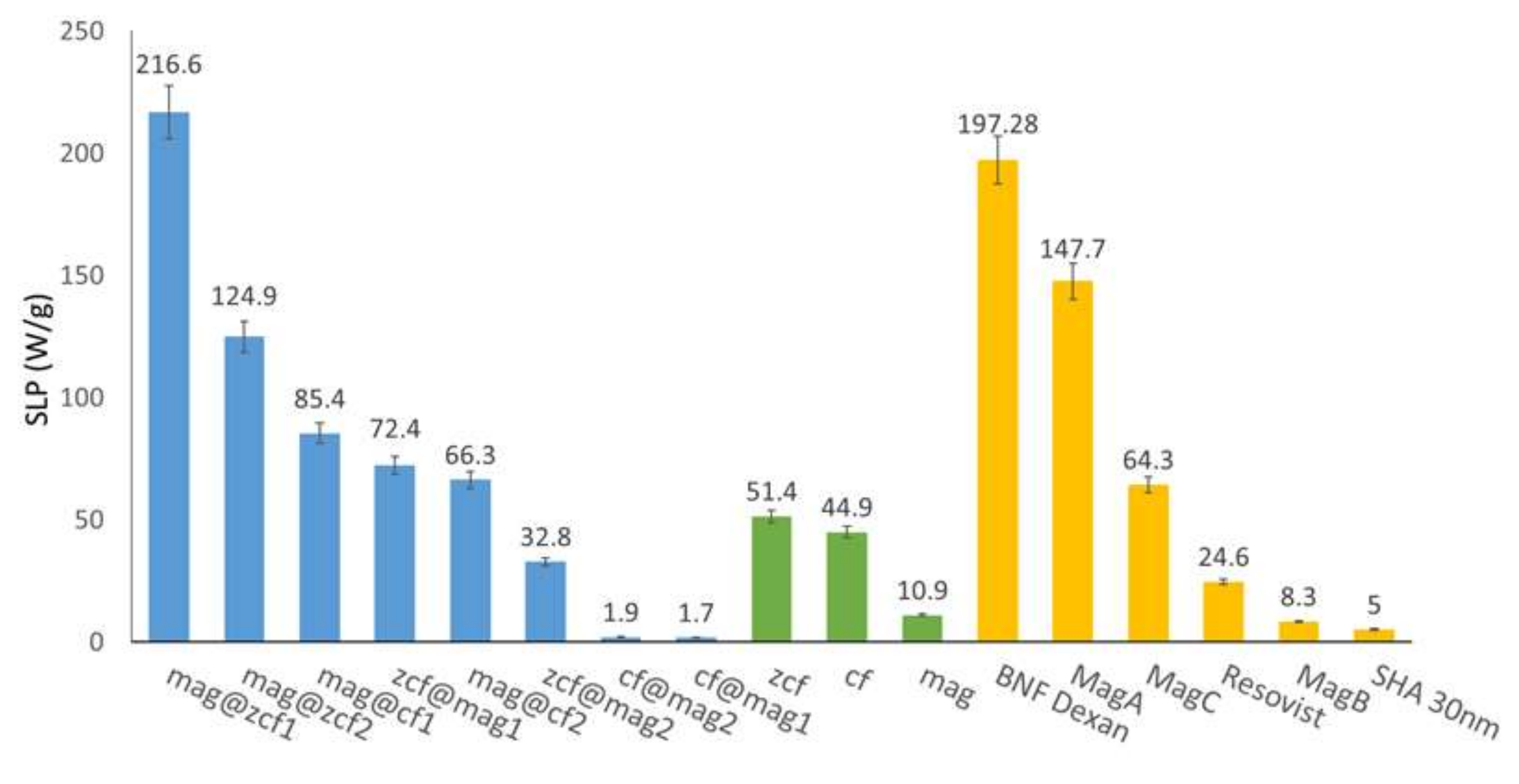
3.3. Heating Efficiency of the MFNs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mai, B.T.; Balakrishnan, P.B.; Barthel, M.J.; Piccardi, F.; Niculaes, D.; Marinaro, F.; Fernandes, S.; Curcio, A.; Kakwere, H.; Autret, G.; et al. Thermoresponsive Iron Oxide Nanocubes for an Effective Clinical Translation of Magnetic Hyperthermia and Heat-Mediated Chemotherapy. ACS Appl. Mater. Interfaces 2019, 11, 5727. [Google Scholar] [CrossRef] [PubMed]
- Makridis, A.; Curto, S.; van Rhoon, G.C.; Samaras, T.; Angelakeris, M. A standardization protocol for accurate evaluation of specific loss power in magnetic hyperthermia. J. Phys. D: Appl. Phys. 2019, 52, 255001. [Google Scholar] [CrossRef]
- Shaw, S.K.; Biswas, A.; Gangwar, A.; Maiti, P.; Prajapat, C.L.; Singh Meena, S.; Prasad, N.K. Synthesis of exchange coupled nanoflowers for efficient magnetic hyperthermia. J. Magn. Magn. Mater. 2019, 484, 437. [Google Scholar] [CrossRef]
- Appa Rao, P.; Srinivasa Rao, K.; Pydi Raju, T.R.K.; Kapusetti, G.; Choppadandi, M.; Chaitanya Varma, M.; Rao, K.H. A systematic study of cobalt-zinc ferrite nanoparticles for self-regulated magnetic hyperthermia. J. Alloys Compd. 2019, 794, 60. [Google Scholar] [CrossRef]
- Apostolov, A.; Apostolova, I.; Wesselinowa, J. Magnetic and dielectric properties of pure and ion doped RCrO3 nanoparticles. Eur. Phys. J. B. 2019, 92, 105. [Google Scholar] [CrossRef]
- Rani, S.; Varma, G.D. Superparamagnetism and metamagnetic transition in Fe3O4 nanoparticles synthesized via co-precipitation method at different pH. Phys. B: Conden. Mater. 2015, 472, 66. [Google Scholar] [CrossRef]
- Darwish, M.S.A.; Stibor, I. Preparation and characterization of magnetite-PDMS composites by magnetic induction heating. Mater. Chem. Phys. 2015, 164, 163. [Google Scholar] [CrossRef]
- Jana, N.R.; Chen, Y.; Peng, X. Size- and Shape-Controlled Magnetic (Cr, Mn, Fe, Co, Ni) Oxide Nanocrystals via a Simple and General Approach. Chem. Mater. 2004, 16, 3931. [Google Scholar] [CrossRef]
- Darwish, M.S.A.; Kunz, U.; Peuker, U. Preparation and catalytic use of platinum in magnetic core/shell nanocomposites. J. Appl. Polym. Sci. 2013, 129, 1806. [Google Scholar] [CrossRef]
- Obaidat, I.M.; Issa, B.; Haik, Y. Magnetic Properties of Magnetic Nanoparticles for Efficient Hyperthermia. Nanomaterials 2015, 5, 63. [Google Scholar] [CrossRef]
- Blanco-Andujar, C.; Ortega, D.; Southern, P.; Pankhurst, Q.A.; Thanh, N.T.K. High performance multi-core iron oxide nanoparticles for magnetic hyperthermia: Microwave synthesis, and the role of core-to-core interactions. Nanoscale 2015, 7, 1768. [Google Scholar] [CrossRef] [PubMed]
- Skoropata, E.; Desautels, R.D.; Chi, C.C.; Ouyang, H.; Freeland, J.W.; van Lierop, J. Magnetism of iron oxide based core-shell nanoparticles from interface mixing with enhanced spin-orbit coupling. Phys. Rev. B. 2014, 89, 024410. [Google Scholar] [CrossRef]
- López-Ortega, A.; Estrader, M.; Salazar-Alvarez, G.; Roca, A.G.; Nogués, J. Applications of exchange coupled bi-magnetic hard/soft and soft/hard magnetic core/shell nanoparticles. Phys. Rep. 2015, 553, 1. [Google Scholar] [CrossRef]
- Guardia, P.; Di Corato, R.; Lartigue, L.; Wilhelm, C.; Espinosa, A.; Garcia-Hernandez, M.; Gazeau, F.; Manna, L.; Pellegrino, T. Water-Soluble Iron Oxide Nanocubes with High Values of Specific Absorption Rate for Cancer Cell Hyperthermia Treatment. ACS Nano 2012, 6, 3080. [Google Scholar] [CrossRef] [PubMed]
- Lavorato, G.; Das, R.; Xing, Y.; Robles, J.; Litterst, F.; Baggio-Saitovitch, E.; Phan, M.H.; Srikanth, H. Origin and Shell-Driven Optimization of the Heating Power in Core/Shell Bimagnetic Nanoparticles. ACS Appl. Nano Mater. 2020, 3, 1755. [Google Scholar] [CrossRef]
- Arami, H.; Khandhar, A.; Liggitt, D.; Krishnan, K.M. In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem. Soc. Rev. 2015, 44, 8576. [Google Scholar] [CrossRef]
- Petersen, E.J.; Nelson, B.C. Mechanisms and measurements of nanomaterial-induced oxidative damage to DNA. Anal. Bioanal. Chem. 2010, 398, 613. [Google Scholar] [CrossRef]
- Ling, D.; Hyeon, T. Chemical design of biocompatible iron oxide nanoparticles for medical applications. Small 2013, 9, 1450. [Google Scholar] [CrossRef]
- Leyssens, L.; Vinck, B.; Van Der Straeten, C.; Wuyts, F.; Maes, L. Cobalt Toxicity in humans-A Review of the Potential Sources and Systemic Health Effects. Toxicology 2017, 387, 43. [Google Scholar] [CrossRef]
- Nasrin, S.; Chowdhury, F.U.Z.; Hoque, S.M. Study of hyperthermia temperature of manganese-substituted cobalt nano ferrites prepared by chemical co-precipitation method for biomedical application. J. Magn. Magn. Mater. 2019, 479, 126. [Google Scholar] [CrossRef]
- Thirunavukkarasu, G.K.; Cherukula, K.; Lee, H.; Yeon Jeong, Y.; Park, I.; Young Lee, J. Magnetic field-inducible drug-eluting nanoparticles for image-guided thermo-chemotherapy. Biomaterials 2018, 180, 240. [Google Scholar] [CrossRef] [PubMed]
- Darwish, M.S.A.; Kim, H.; Lee, H.; Ryu, C.; Lee, J.Y.; Yoon, J. Synthesis of magnetic ferrite nanoparticles with high hyperthermia performance via a controlled co-precipitation method. Nanomaterials 2019, 9, 1176. [Google Scholar] [CrossRef]
- Angotzi, M.S.; Musinu, A.; Mameli, V.; Ardu, A.; Cara, C.; Niznansky, D.; Xin, H.L.; Cannas, C. Spinel Ferrite Core–Shell Nanostructures by a Versatile Solvothermal Seed-Mediated Growth Approach and Study of Their Nanointerfaces. ACS Nano 2017, 11, 7889. [Google Scholar] [CrossRef] [PubMed]
- Noh, S.H.; Na, W.; Jang, J.T.; Lee, J.H.; Lee, E.J.; Moon, S.H.; Lim, Y.; Shin, J.S.; Cheon, J. Nanoscale Magnetism Control via Surface and Exchange Anisotropy for Optimized Ferrimagnetic Hysteresis. Nano Lett. 2012, 12, 3716. [Google Scholar] [CrossRef] [PubMed]
- Jun, Y.W.; Huh, Y.M.; Choi, J.S.; Lee, J.H.; Song, H.T.; Kim, K.; Yoon, S.; Kim, K.S.; Shin, J.S.; Suh, J.S.; et al. Nanoscale Size Effect of Magnetic Nanocrystals and Their Utilization for Cancer Diagnosis via Magnetic Resonance Imaging. J. Am. Chem. Soc. 2005, 127, 5732. [Google Scholar] [CrossRef]
- Rosensweig, R.E. Heating magnetic fluid with alternating magnetic field. J. Magn. Magn. Mater. 2002, 252, 370. [Google Scholar] [CrossRef]
- Xu, Y.; Qin, Y.; Palchoudhury, S.; Bao, Y. Water-soluble Iron Oxide Nanoparticles with High Stability and Selective Surface Functionality. Langmuir 2011, 27, 8990. [Google Scholar] [CrossRef] [PubMed]
- Kmita, A.; Lachowicz, D.; Żukrowski, J.; Gajewska, M.; Szczerba, W.; Kuciakowski, J.; Zapotoczny, S.; Sikora, M. One-Step Synthesis of Long Term Stable Superparamagnetic Colloid of Zinc Ferrite Nanorods in Water. Materials 2019, 12, 1048. [Google Scholar] [CrossRef]
- Ayyappan, S.; Mahadevan, S.; Chandramohan, P.; Srinivasan, M.P.; Philip, J.; Raj, B. Influence of Co2+ Ion concentration on the size, magnetic properties, and purity of CoFe2O4 Spinel ferrite nanoparticles. J. Phys. Chem. C. 2010, 114, 6334. [Google Scholar] [CrossRef]
- Chinnasamy, C.N.; Senoue, M.; Jeyadevan, B.; Perales-Perez, O.; Shinoda, K.; Tohji, K. Synthesis of size-controlled cobalt ferrite particles with high coercivity and squareness ratio. J. Colloid Interface Sci. 2003, 263, 80. [Google Scholar] [CrossRef]
- Choi, H.; An, M.; Eom, W.; Lim, S.; Shim, I.; Kim, C.; Kim, S. Crystallographic and magnetic properties of the hyperthermia material CoFe2O4@AlFe2O4. J. Korean Phys. Soc. 2017, 70, 173. [Google Scholar] [CrossRef]
- Zhen, G.B.; Muir, W.; Moffat, B.A.; Harbour, P.; Murray, K.S.; Moubaraki, B.; Suzuki, K.; Madsen, I.; Agron-Olshina, N.; Waddington, L.; et al. Comparative Study of the Magnetic Behavior of Spherical and Cubic Superparamagnetic Iron Oxide Nanoparticles. J. Phys. Chem. C. 2011, 115, 327. [Google Scholar] [CrossRef]
- Khurshid, H.; Alonso, J.; Nemati, Z.; Phan, M.H.; Mukherjee, P.; Fdez-Gubieda, M.L.; Barandiaran, J.M.; Srikanth, H.J. Anisotropy effects in magnetic hyperthermia: A comparison between spherical and cubic exchange-coupled FeO/Fe3O4 nanoparticles. Appl. Phys. 2015, 117, 17A337. [Google Scholar] [CrossRef]
- Pereira, C.; Pereira, A.M.; Fernandes, C.; Rocha, M.; Mendes, R.; Fernández-García, M.P.; Guedes, A.; Tavares, P.B.; Grenèche, J.M.; Araújo, J.P.; et al. Superparamagnetic MFe2O4 (M = Fe, Co, Mn) Nanoparticles: Tuning the Particle Size and Magnetic Properties through a Novel One-Step Coprecipitation Route. Chem. Mater. 2012, 24, 1496. [Google Scholar] [CrossRef]
- Song, Q.; Zhang, Z.J. Controlled Synthesis and Magnetic Properties of Bimagnetic Spinel Ferrite CoFe2O4 and MnFe2O4 Nanocrystals with Core-Shell Architecture. J. Am. Chem. Soc. 2012, 134, 10182. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K. Complex Magnetic Nanostructures: Synthesis, Assembly and Applications, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 10 3319520865. [Google Scholar]
- Lee, J.H.; Jang, J.T.; Choi, J.S.; Moon, S.H.; Noh, S.H.; Kim, J.W.; Kim, J.G.; Kim, I.S.; Park, K.I.; Cheon, J. Exchange-coupled Magnetic Nanoparticles for Efficient Heat Induction. Nat. Nanotechnol. 2011, 6, 418. [Google Scholar] [CrossRef]
- Phadatare, M.R.; Meshram, J.V.; Gurav, K.V.; Kim, J.H.; Pawar, S.H. Enhancement of specific absorption rate by exchange coupling of the core–shell structure of magnetic nanoparticles for magnetic hyperthermia. J. Phys. D Appl. Phys. 2016, 49, 095004. [Google Scholar] [CrossRef]
- Cheon, J.W.; Jang, J.T. Heat Generating Nanomaterials. Patent No.: US 8,778,411 B2, 15 July 2014. [Google Scholar]
| Name | Core | Shell and Precursors | ||||||
|---|---|---|---|---|---|---|---|---|
| Shell | Fe+3 (Mole) | Fe+2 (Mole) | DW (mL) | Co+2 (Mole) | Amm (mL) | Zn+2 (Mole) | ||
| mag | Fe3O4 | - | 0.59 | 0.399 | 50 | - | 20 | - |
| cf | CoFe2O4 | - | 0.59 | 0.399 | 50 | 0.199 | 20 | - |
| zcf | ZnCoFe2O4 | - | 0.59 | 0.399 | 50 | 0.199 | 20 | 0.199 |
| mag@cf1 | CoFe2O4 (0.3 g) | Fe3O4 | 0.149 | 0.098 | 25 | - | 5 | - |
| mag@cf2 | CoFe2O4 (0.1 g) | Fe3O4 | 1.19 | 0.195 | 25 | - | 20 | - |
| cf@mag1 | Fe3O4 (0.3 g) | CoFe2O4 | 0.074 | 0.049 | 50 | 0.024 | 5 | - |
| cf@mag2 | Fe3O4 (0.1 g) | CoFe2O4 | 0.149 | 0.195 | 25 | 0.048 | 5 | - |
| mag@zcf1 | ZnCoFe2O4 (0.3 g) | Fe3O4 | 0.149 | 0.098 | 25 | - | 5 | - |
| mag@zcf2 | ZnCoFe2O4 (0.1 g) | Fe3O4 | 0.599 | 0.097 | 50 | - | 20 | - |
| zcf@mag1 | Fe3O4 (0.3 g) | ZnCoFe2O4 | 0.149 | 0.098 | 25 | 0.048 | 5 | 0.049 |
| zcf@mag2 | Fe3O4 (0.1 g) | ZnCoFe2O4 | 0.149 | 0.195 | 25 | 0.048 | 5 | 0.049 |
| Mag | cf | zcf | mag@cf1 | mag@cf2 | cf@mag1 | cf@mag2 | mag@zcf1 | mag@zcf2 | zcf@mag1 | zcf@mag2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystallite size ( nm) | 9.8 | 8.7 | 9.7 | 11.13 | 10.71 | 9.44 | 10.16 | 12.32 | 12.38 | 9.44 | 9.45 |
| Lattice parameter (Å) | - | - | - | 8.35 | 8.36 | 8.38 | 8.39 | 8.39 | 8.25 | 8.40 | 8.36 |
| Core-shell diameter (nm)) | 10 ± 0.3 | 8 ± 2 | 25 ± 5 | 12 ± 1.7 | 16 ± 0.46 | 14 ± 0.5 | 16 ± 0.25 | 9 ± 1.8 | 13 ± 0.9 | 8 ± 1.3 | 9 ± 2.5 |
| Core diameter (nm) | - | - | - | 7.74 ± 1.1 | 10.6 ± 0.3 | 7.56 ± 0.27 | 10.6 ± 0.16 | 7.9 ± 1.5 | 11.9 ± 0.8 | 7.2 ± 1.1 | 8.01 ± 2.2 |
| Shell thickness (nm) | - | - | - | 4.26 ± 0.6 | 5.3 ± 0.16 | 6.44 ± 0.23 | 5.3 ± 0.08 | 1.05 ± 0.3 | 1.07 ± 0.07 | 0.71±0.2 | 0.99 ± 0.3 |
| Ms (emu/g) | 41.93 | 50.61 | 50.71 | 49.84 | 43.93 | 55.45 | 54.25 | 55.75 | 33.77 | 59.30 | 58.89 |
| Mr (emu/g) | 3.8 | 10.75 | 10.71 | 5 | 5.5 | 3.4 | 2.4 | 6.8 | 3.7 | 6.3 | 2.7 |
| Hc (Oe) | 40.5 | 159.8 | 225 | 90 | 70 | 50 | 50 | 180 | 80 | 190 | 70 |
| SQ | 0.090 | 0.212 | 0.211 | 0.10 | 0.12 | 0.06 | 0.04 | 0.12 | 0.010 | 0.106 | 0.045 |
| Zeta potential (mV) | −34 ± 0.6 | −30 ± 0.5 | +14 ± 0.6 | −29.8 ± 0.9 | −13.1 ± 2.5 | −25 ± 0.9 | −25.1 ± 0.3 | −26.9 ± 0.7 | −20.5 ± 1.8 | +4.8 ± 0.4 | −4 ± 0.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darwish, M.S.A.; Kim, H.; Lee, H.; Ryu, C.; Young Lee, J.; Yoon, J. Engineering Core-Shell Structures of Magnetic Ferrite Nanoparticles for High Hyperthermia Performance. Nanomaterials 2020, 10, 991. https://doi.org/10.3390/nano10050991
Darwish MSA, Kim H, Lee H, Ryu C, Young Lee J, Yoon J. Engineering Core-Shell Structures of Magnetic Ferrite Nanoparticles for High Hyperthermia Performance. Nanomaterials. 2020; 10(5):991. https://doi.org/10.3390/nano10050991
Chicago/Turabian StyleDarwish, Mohamed S. A., Hohyeon Kim, Hwangjae Lee, Chiseon Ryu, Jae Young Lee, and Jungwon Yoon. 2020. "Engineering Core-Shell Structures of Magnetic Ferrite Nanoparticles for High Hyperthermia Performance" Nanomaterials 10, no. 5: 991. https://doi.org/10.3390/nano10050991
APA StyleDarwish, M. S. A., Kim, H., Lee, H., Ryu, C., Young Lee, J., & Yoon, J. (2020). Engineering Core-Shell Structures of Magnetic Ferrite Nanoparticles for High Hyperthermia Performance. Nanomaterials, 10(5), 991. https://doi.org/10.3390/nano10050991






