Remarkable Stability Improvement of ZnO TFT with Al2O3 Gate Insulator by Yttrium Passivation with Spray Pyrolysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Device Fabrication
2.3. Characterization Techniques
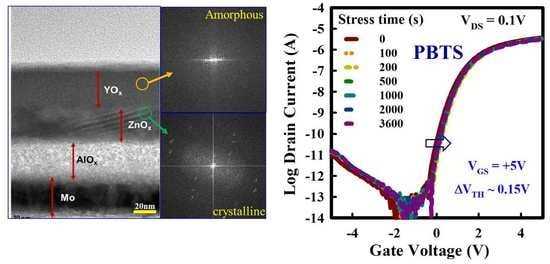
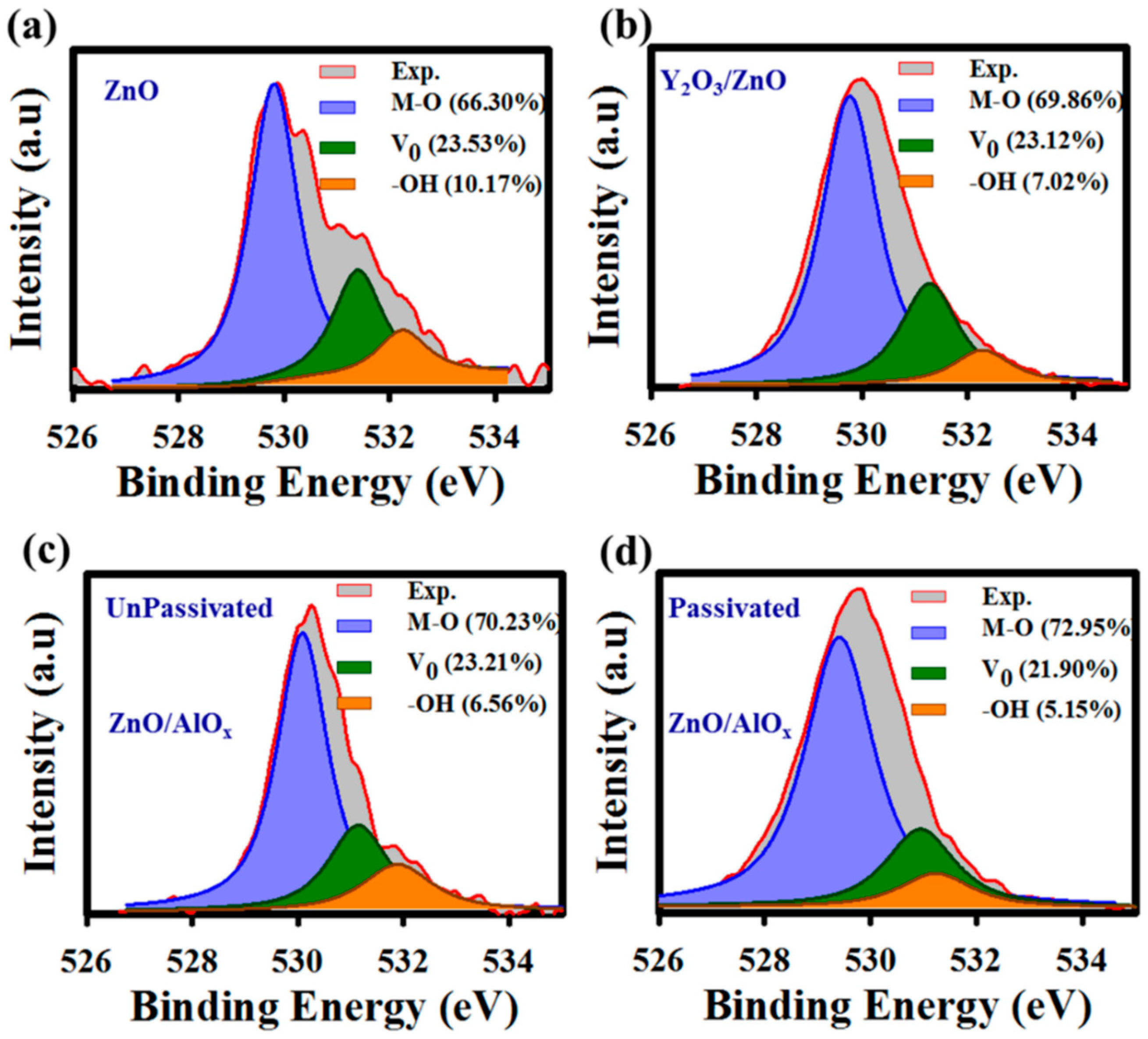
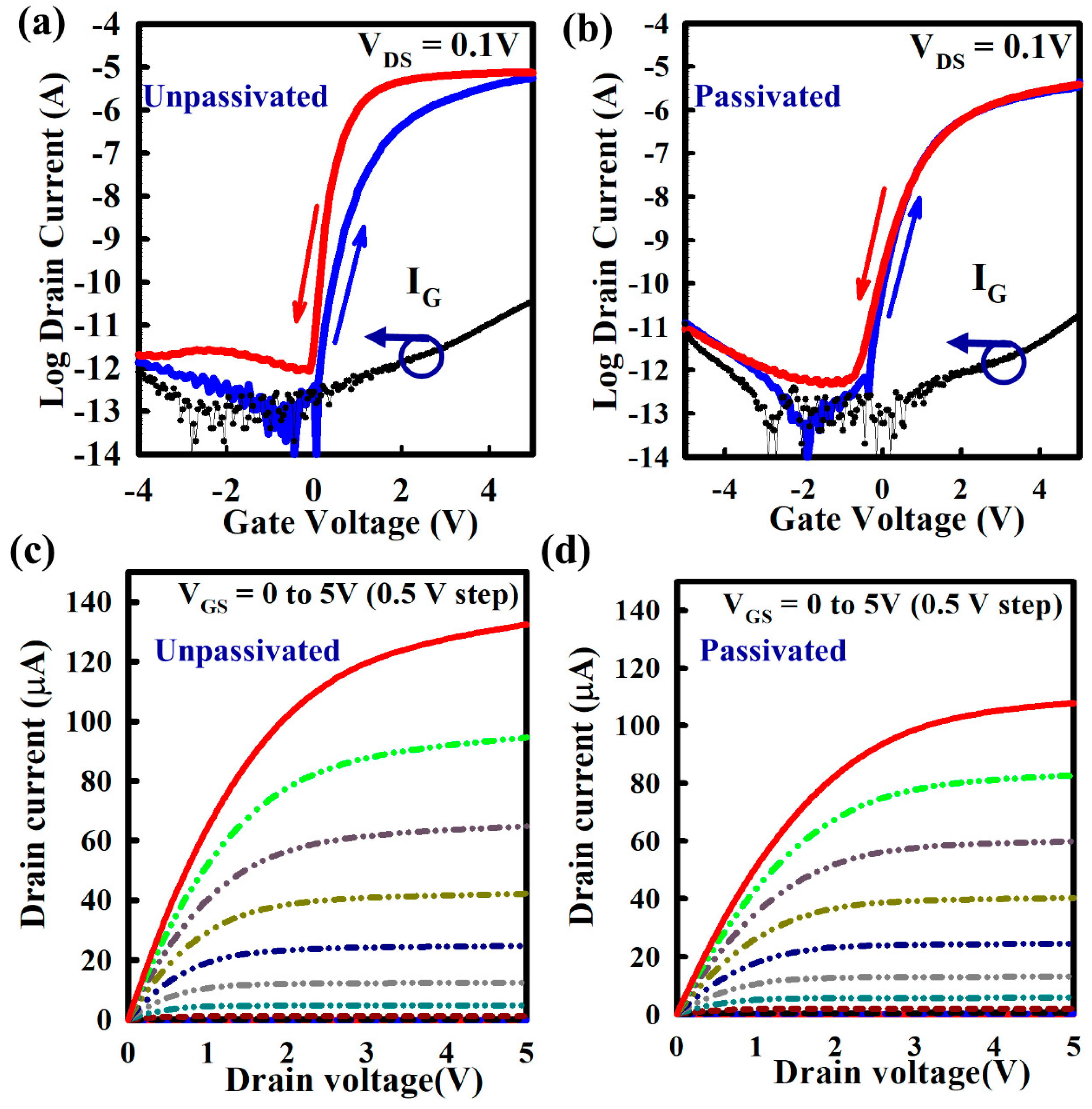
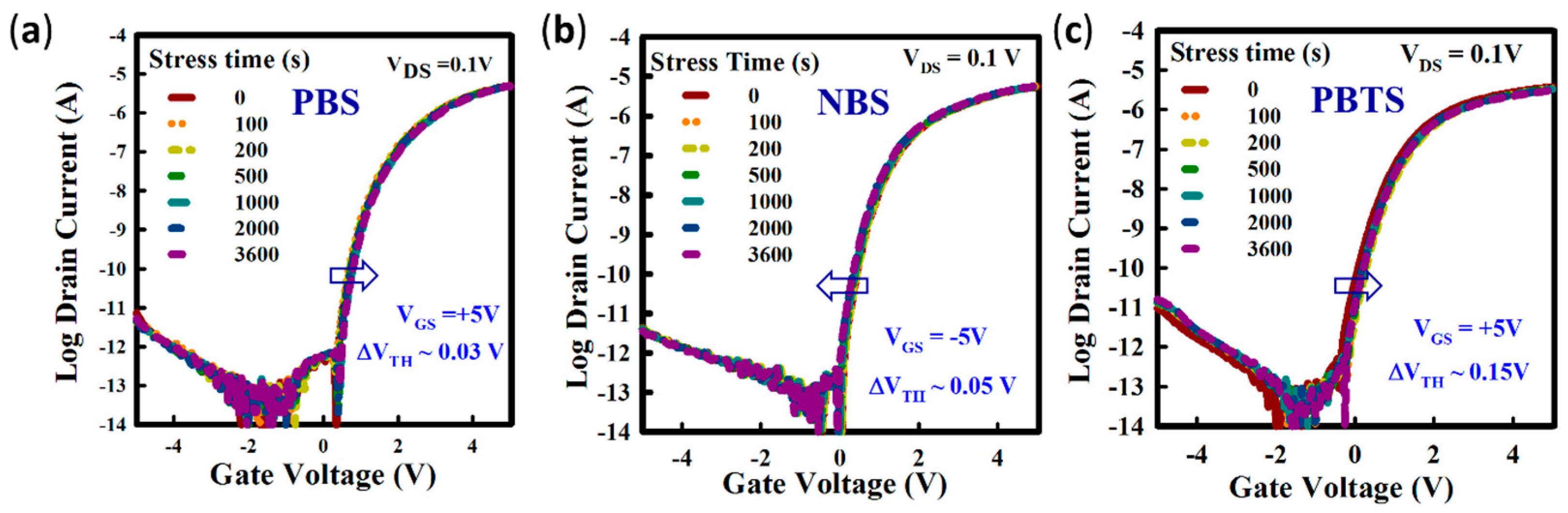
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Song, K.; Noh, J.; Jun, T.; Jung, Y.; Kang, H.Y.; Moon, J. Fully Flexible Solution-Deposited ZnO Thin-Film Transistors. Adv. Mater. 2010, 22, 4308–4312. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.Y.; Jeong, J.K. Recent progress in high performance and reliable n-type transition metal oxide-based thin film transistors. Semicond. Sci. Technol. 2015, 30, 024002. [Google Scholar] [CrossRef]
- Gleskova, H.; Wagner, S. Electron mobility in amorphous silicon thin-film transistors under compressive strain. Appl. Phys. Lett. 2001, 79, 3347–3349. [Google Scholar] [CrossRef]
- Fortunato, E.; Pimentel, A.; Pereira, L.; Goncalves, A.; Lavareda, G.; Aguas, H.; Ferreira, I.; Carvalho, C.N.; Martins, R. High field-effect mobility zinc oxide thin film transistors produced at room temperature. J. Non Cryst. Solids 2004, 338, 806–809. [Google Scholar] [CrossRef]
- Adamopoulos, G.; Bashir, A.; Gillin, W.P.; Georgakopoulos, S.; Shkunov, M.; Baklar, M.A.; Stingelin, N.; Bradley, D.D.C.; Anthopoulos, T.D. Structural and Electrical Characterization of ZnO Films Grown by Spray Pyrolysis and Their Application in Thin-Film Transistors. Adv. Funct. Mater. 2011, 21, 525–531. [Google Scholar] [CrossRef]
- Hirao, T.; Furuta, M.; Hiramatsu, T.; Matsuda, T.; Li, C.; Furuta, H.; Hokari, H.; Yoshida, M.; Ishii, H.; Kakegawa, M. Bottom-Gate Zinc Oxide Thin-Film Transistors for AMLCDs. IEEE Trans. Electron Devices. 2008, 55, 3136–3142. [Google Scholar] [CrossRef]
- Isakov, I.; Faber, H.; Grell, M.; Moon, G.W.; Pliatsikas, N.; Kehagias, T.; Dimitrakopulos, G.P.; Patsalas, P.P.; Li, R.; Anthopoulos, T.D. Exploring the Leidenfrost Effect for the Deposition of High-Quality In2O3 Layers via Spray Pyrolysis at Low Temperatures and Their Application in High Electron Mobility Transistors. Adv. Funct. Mater. 2007, 27, 1606407. [Google Scholar] [CrossRef]
- Jun, T.; Jung, Y.; Song, K.; Moon, J. Influences of pH and Ligand Type on the Performance of Inorganic Aqueous Precursor-Derived ZnO Thin Film Transistors. ACS Appl. Mater. Interfaces 2011, 3, 774–781. [Google Scholar] [CrossRef]
- Cho, S.W.; Yun, M.G.; Ahn, C.H.; Kim, S.H.; Cho, H.K. Bilayer Channel Structure-Based Oxide Thin-Film Transistors Consisting of ZnO and Al-Doped ZnO with Different Al Compositions and Stacking Sequences. Electron. Mater. Lett. 2015, 11, 198–205. [Google Scholar] [CrossRef]
- Park, J.; Huh, J.E.; Lee, S.E.; Lee, J.; Lee, W.H.; Lim, K.H.; Kim, Y.S. Effective atmospheric-pressure plasma treatment toward high performance solution-processed oxide thin-film transistors. ACS Appl. Mater. Interfaces 2018, 10, 30581–30586. [Google Scholar] [CrossRef]
- Levy, D.H.; Freeman, D.; Nelson, S.F.; Corvan, P.J.C.; Irving, L.M. Stable ZnO thin film transistors by fast open air atomic layer deposition. Appl. Phys. Lett. 2008, 92, 2101. [Google Scholar] [CrossRef]
- Kim, Y.; Avis, C.; Hwang, H.R.; Kim, T.W.; Seol, Y.G.; Jang, J. Effect of Strontium Addition on Stability of Zinc-Tin-Oxide Thin-Film Transistors Fabricated by Solution Process. J. Display Technol. 2014, 10, 939–944. [Google Scholar] [CrossRef]
- Saha, J.K.; Billah, M.M.; Bukke, R.N.; Kim, Y.G.; Mude, N.N.; Siddik, A.B.; Islam, M.M.; Do, Y.; Choi, M.; Jang, J. Highly Stable, Nanocrystalline, ZnO Thin-Film Transistor by Spray Pyrolysis Using High-k Dielectric. IEEE Trans. Electron Devices 2020, 67, 1021–1026. [Google Scholar] [CrossRef]
- Branquinho, R.; Salgueiro, D.; Santa, A.; Kiazadeh, A.; Barquinha, P.; Pereira, L.; Martins, R.; Fortunato, E. Towards environmental friendly solution-based ZTO/AlOx TFTs. Semicond. Sci. Technol. 2015, 30, 024007. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, H.; Bai, Y.; Ma, J.W.; Cao, J.; Jiang, X.; Zhang, Z.L. Enhanced performances of ZnO-TFT by improving surface properties of channel layer. Solid State Commun. 2008, 146, 387–390. [Google Scholar] [CrossRef]
- Bashir, A.; Wöbkenberg, P.H.; Smith, J.; Ball, J.M.; Adamopoulos, G.; Bradley, D.D.C.; Anthopoulos, T.D. High-Performance Zinc Oxide Transistors and Circuits Fabricated by Spray Pyrolysis in Ambient Atmosphere. Adv. Mater. 2009, 21, 2226–2231. [Google Scholar] [CrossRef]
- Kim, D.H.; Yoon, S.B.; Jeong, Y.T.; Kim, Y.M.; Kim, B.S.; Hong, M.P. Role of adsorbed H2O on transfer characteristics of solution-processed zinc tin oxide thin-film transistors. Appl. Phys. Express 2012, 5, 021101. [Google Scholar] [CrossRef]
- Xu, X.; Cui, Q.; Jin, Y.; Guo, X. Low-voltage zinc oxide thin-film transistors with solution-processed channel and dielectric layers below 150 °C. Appl. Phys. Lett. 2012, 101, 2114–2116. [Google Scholar] [CrossRef]
- An, S.; Mativenga, M.; Kim, Y.; Jang, J. Improvement of bias-stability in amorphous-indium-gallium-zinc-oxide thin-film transistors by using solution-processed Y2O3 passivation. Appl. Phys. Lett. 2014, 105, 053507–053511. [Google Scholar] [CrossRef]
- Bukke, R.N.; Avis, C.; Jang, J. Solution-Processed Amorphous In–Zn–Sn Oxide Thin-Film Transistor Performance Improvement by Solution-Processed Y2O3 Passivation. IEEE Electron Device Lett. 2016, 37, 433–436. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, T.; Lee, J.; Avis, C.; Jang, J. Solution-Processed Gadolinium Doped Indium-Oxide Thin-Film Transistors with Oxide Passivation. Appl. Phys. Lett. 2017, 110, 122102. [Google Scholar] [CrossRef]
- Guo, X.; Silva, S.R.P.; Ishii, T. Current percolation in ultrathin channel nano-crystalline silicon transistors. Appl. Phys. Lett. 2008, 93, 042105. [Google Scholar] [CrossRef]
- Lin, H.C.; Hung, C.H.; Chen, W.C.; Lin, Z.M.; Hsu, H.H.; Hunag, T.Y. Origin of hysteresis in current-voltage characteristics of polycrystalline silicon thin-film transistors. J. Appl. Phys. 2009, 105, 054502. [Google Scholar] [CrossRef]
- Abliz, A.; Xu, L.; Wan, D.; Duan, H.; Wang, J.; Wang, C.; Luo, S.; Liu, C. Effects of yttrium doping on the electrical performances and stability of ZnO thin-film transistors. Appl. Surf. Sci. 2019, 475, 565–570. [Google Scholar] [CrossRef]
- Kim, J.H.; Rim, Y.H.; Kim, H.J. Homojunction Solution-Processed Metal Oxide Thin-Film Transistors Using Passivation-Induced Channel Definition. ACS Appl. Mater. Interfaces 2014, 6, 4819–4822. [Google Scholar] [CrossRef]
- Xu, X.; Feng, L.; He, S.; Jin, Y.; Guo, X. Solution-Processed Zinc Oxide Thin-Film Transistors with a Low-Temperature Polymer Passivation Laye. IEEE Electron Device Lett. 2012, 33, 1420–1422. [Google Scholar] [CrossRef]
- Nomura, K.; Kamiya, T.; Hosono, H. Stability and high-frequency operation of amorphous In–Ga–Zn–O thin-film transistors with various passivation layers. Thin Solid Films 2012, 520, 3778–3782. [Google Scholar] [CrossRef]
- Wan, L.; He, F.; Qin, Y.; Lin, Z.; Su, J.; Chang, J.; Hao, Y. Effects of Interfacial Passivation on the Electrical Performance, Stability, and Contact Properties of Solution Process Based ZnO Thin Film Transistors. Materials 2018, 11, 1761. [Google Scholar] [CrossRef]
- Hong, S.; Park, S.P.; Kim, Y.G.; Kang, B.H.; Na, J.W.; Kim, H.J. Low-temperature fabrication of an HfO2 passivation layer for amorphous indium-gallium-zinc oxide thin film transistors using a solution process. Sci. Rep. 2017, 7, 16265. [Google Scholar] [CrossRef]
- Zhou, Y.; Dong, C. Influence of passivation layers on positive gate bias-stress stability of amorphous InGaZnO thin-film transistors. Micromachines 2018, 9, 603. [Google Scholar] [CrossRef]
- Xiao, P.; Wang, W.; Ye, Y.; Dong, T.; Yuan, S.; Deng, J.; Zhang, L.; Chen, J.; Yuan, J. Back-Channel-Etched InGaZnO Thin-Film Transistors with Au Nanoparticles on the Back Channel Surface. Electron. Mater. Lett. 2020, 16, 115–122. [Google Scholar] [CrossRef]
- Shaw, A.; Wrench, J.S.; Jin, J.D.; Whittles, T.J.; Mitrovic, I.Z.; Raja, M.; Dhanak, V.R.; Chalker, P.R.; Hall, S. Atomic layer deposition of Nb-doped ZnO for thin film transistors. Appl. Phys. Lett. 2016, 109, 222103. [Google Scholar] [CrossRef]
- Mude, N.N.; Bukke, R.N.; Saha, J.K.; Avis, C.; Jang, J. Highly Stable, Solution Processed Ga Doped IZTO Thin Film Transistor by Ar/O2 Plasma Treatment. Adv. Electron. Mater. 2019, 6, 1900768. [Google Scholar] [CrossRef]
- Afouxenidis, D.; Mazzocco, R.; Vourlias, G.; Livesley, P.J.; Krier, A.; Milne, W.I.; Kolosov, O.; Adamopoulos, G. ZnO-based Thin Film Transistors Employing Aluminum Titanate Gate Dielectrics Deposited by Spray Pyrolysis at Ambient Air. ACS Appl. Mater. Interfaces 2015, 7, 7334. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.; Qin, C.; Ding, X.; Zhang, J. Low-Voltage Hf-ZnO Thin Film Transistors with Ag Nanowires Gate Electrode and Their Application in Logic Circuit. IEEE Trans. Elec. Dev. 2019, 66, 1760. [Google Scholar] [CrossRef]
- Kim, D.N.; Kim, D.L.; Kim, G.H.; Kim, S.J.; Rim, Y.S.; Jeong, W.H.; Kim, H.J. The effect of La in InZnO systems for solution-processed amorphous oxide thin-film transistors. Appl. Phys. Lett. 2010, 97, 192105. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Zhang, X.; Jiang, X.; Zhang, Z. High performance ZnO-thin-film transistor with TaO5 dielectrics fabricated at room temperature. Appl. Phys. Lett. 2009, 95, 072112–072114. [Google Scholar] [CrossRef]



| Active/GI | Passivation Layer | Processing Method | Substrate Temp. (°C) | μFE [cm2V−1s−1] | SS [mV/dec] | Ion/Ioff | Year |
|---|---|---|---|---|---|---|---|
| InOx/SiO2 | AlOx | SC | 400 | 0.02 | 730 | 106 | 2014 (25) |
| ZnO/SiOx | PDMS | SC | 150 | 0.50 | 240 | 106 | 2012 (26) |
| IGZO/SiO2 | Y2O3 | SC | 250 | 21.31 | 160 | 108 | 2014 (19) |
| IZTO/ZrOx | Y2O3 | SC | 350 | 4.75 | 114 | 109 | 2016 (20) |
| IGZO/SiO2 | Y2O3 | SC | 350 | 11.10 | 140 | 108 | 2012 (27) |
| GdInOx/AlOx | Y2O3 | SC | 350 | 9.47 | 79 | 107 | 2017 (21) |
| ZnO/SiO2 | PCBA | SC | 150 | 4.50 | - | 106 | 2018 (28) |
| IGZO/SiO2 | HfOx | SC | 250 | 9.60 | 350 | 108 | 2017 (29) |
| IGZO/SiO2 | Y2O3 | SC | 250 | 11.30 | 267 | 103 | 2017 (29) |
| IGZO/SiO2 | PMMA | SC | 250 | 9.51 | 320 | 108 | 2017 (29) |
| ZnO/AlOx | Y2O3 | SP | 350 | 35.36 | 128 | 108 | This work |
| GI: Gate Insulator, SC: Spin Coating, SP: Spray Coating, Temp.: temperature | |||||||
| ZnO TFT | µFE (cm2V−1 s−1) | Vth (V) | SS (mV/dec) |
|---|---|---|---|
| Without passivation | 43.13 ± 3.48 | 0.61 ± 0.05 | 170.40 ± 9.22 |
| With passivation | 36.88 ± 3.75 | 0.50 ± 0.04 | 126.40 ± 10.98 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saha, J.K.; Bukke, R.N.; Mude, N.N.; Jang, J. Remarkable Stability Improvement of ZnO TFT with Al2O3 Gate Insulator by Yttrium Passivation with Spray Pyrolysis. Nanomaterials 2020, 10, 976. https://doi.org/10.3390/nano10050976
Saha JK, Bukke RN, Mude NN, Jang J. Remarkable Stability Improvement of ZnO TFT with Al2O3 Gate Insulator by Yttrium Passivation with Spray Pyrolysis. Nanomaterials. 2020; 10(5):976. https://doi.org/10.3390/nano10050976
Chicago/Turabian StyleSaha, Jewel Kumer, Ravindra Naik Bukke, Narendra Naik Mude, and Jin Jang. 2020. "Remarkable Stability Improvement of ZnO TFT with Al2O3 Gate Insulator by Yttrium Passivation with Spray Pyrolysis" Nanomaterials 10, no. 5: 976. https://doi.org/10.3390/nano10050976
APA StyleSaha, J. K., Bukke, R. N., Mude, N. N., & Jang, J. (2020). Remarkable Stability Improvement of ZnO TFT with Al2O3 Gate Insulator by Yttrium Passivation with Spray Pyrolysis. Nanomaterials, 10(5), 976. https://doi.org/10.3390/nano10050976