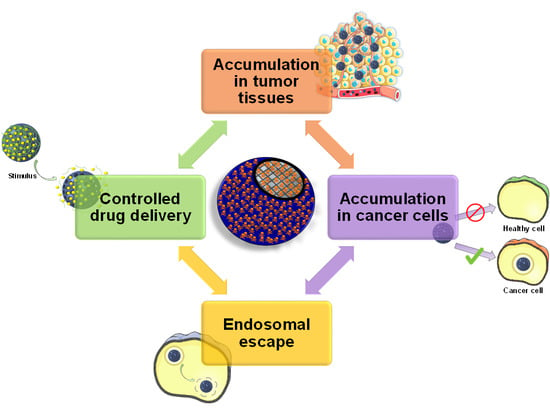
Influence of the Surface Functionalization on the Fate and Performance of Mesoporous Silica Nanoparticles
Abstract
1. Introduction
2. Targeting Tumor Tissues
2.1. Passive Targeting of Tumor Tissues
2.1.1. Enhanced Permeability and Retention Effect
2.1.2. Nanoparticle Features Affecting the Biodistribution of MSNs
2.2. Active Targeting of Tumor Tissues
2.2.1. Tumor-Tropic Peptides
2.2.2. Cells with Migratory Properties
2.3. Enhanced Penetration in the Tumoral Mass
2.3.1. Proteolytic Enzymes
2.3.2. Ultrasounds
3. Targeting Cancer Cells
3.1. Single Targeting
3.1.1. Antibodies
3.1.2. Aptamers
3.1.3. Peptides
3.1.4. Proteins
3.1.5. Carbohydrates
3.1.6. Small Molecules
3.2. Dual Targeting
3.2.1. Membrane Dual Targeting
3.2.2. Sequential Dual Targeting
3.2.3. Janus Dual Targeting
3.3. Hierarchical Targeting
3.3.1. pH-Responsive Hierarchical Targeting
3.3.2. Enzyme-Responsive Hierarchical Targeting
4. Achieving Endosomal Escape
4.1. Internally-Triggered Endosomal Escape
4.1.1. Proton Sponge Effect
4.1.2. Other Mechanisms for Destabilizing the Endo-Lysosomal Membrane
4.2. Externally-Triggered Endosomal Escape
5. Functional Groups Determine Drug Loading and Release
6. Stimuli-Responsive Drug Delivery
6.1. pH-Responsive MSNs
6.1.1. Acid-Labile Bonds
6.1.2. pH-Degradable Gatekeepers
6.1.3. pH-Operated Nanovalves
6.1.4. Conformation-Changing Polymers
6.1.5. pH-Responsive Biomolecules
6.1.6. Polyelectrolytes
6.2. Redox-Responsive MSNs
6.2.1. Proteins
6.2.2. Small Nanoparticles and Nanovalves
6.2.3. Polymers and Small Molecules
6.3. Enzyme-Responsive MSNs
6.3.1. Cathepsin B
6.3.2. Metalloproteinases
6.3.3. Other Enzymes
6.4. Light-Responsive MSNs
6.4.1. Light-Induced Cleavable Bonds
6.4.2. Light-Induced Conformational Changes
6.5. Ultrasound-Responsive MSNs
6.5.1. US-Enhanced Drug Release
6.5.2. US-Cleavable Bonds
6.6. Thermo-Responsive MSNs
6.6.1. Thermo-Responsive Disassembling Gatekeepers
6.6.2. Thermo-Responsive Polymers
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jain, K.K. Future of nanomedicine: Impact on healthcare & society. Nanomedicine 2015, 10, 3199–3202. [Google Scholar]
- Fornaguera, C.; García-Celma, M.J. Personalized nanomedicine: A revolution at the nanoscale. J. Pers. Med. 2017, 7, 12. [Google Scholar] [CrossRef]
- Pautler, M.; Brenner, S. Nanomedicine: Promises and challenges for the future of public health. Int. J. Nanomed. 2010, 5, 803–809. [Google Scholar]
- Zhu, X.; Radovic-Moreno, A.F.; Wu, J.; Langer, R.; Shi, J. Nanomedicine in the management of microbial infection—Overview and perspectives. Nano Today 2014, 9, 478–498. [Google Scholar] [CrossRef]
- Doane, T.L.; Burda, C. The unique role of nanoparticles in nanomedicine: Imaging, drug delivery and therapy. Chem. Soc. Rev. 2012, 41, 2885. [Google Scholar] [CrossRef]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef]
- Oerlemans, C.; Bult, W.; Bos, M.; Storm, G.; Nijsen, J.F.W.; Hennink, W.E. Polymeric Micelles in Anticancer Therapy: Targeting, Imaging and Triggered Release. Pharm. Res. 2010, 27, 2569–2589. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef]
- Azharuddin, M.; Zhu, G.H.; Das, D.; Ozgur, E.; Uzun, L.; Turner, A.P.F.; Patra, H.K. A repertoire of biomedical applications of noble metal nanoparticles. Chem. Commun. 2019, 55, 6964–6996. [Google Scholar] [CrossRef]
- Maiti, D.; Tong, X.; Mou, X.; Yang, K. Carbon-Based Nanomaterials for Biomedical Applications: A Recent Study. Front. Pharmacol. 2019, 9, 1401. [Google Scholar] [CrossRef]
- Narayan, R.; Nayak, Y.U.; Raichur, M.A.; Garg, S. Mesoporous Silica Nanoparticles: A Comprehensive Review on Synthesis and Recent Advances. Pharmaceutics 2018, 10, 118. [Google Scholar] [CrossRef]
- Yanagisawa, T.; Shimizu, T.; Kuroda, K.; Kato, C. The preparation of alkyltrimethylammonium-kanemite complexes and their conversion to microporous materials. Bull. Chem. Soc. Jpn. 1990, 63, 988–992. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Yan, Z.; Meng, H.; Shi, L.; Li, Z.; Kang, P. Synthesis of mesoporous hollow carbon hemispheres as highly efficient Pd electrocatalyst support for ethanol oxidation. Electrochem. Commun. 2010, 12, 689–692. [Google Scholar] [CrossRef]
- Serrano, E.; Linares, N.; García-Martínez, J.; Berenguer, J.R. Sol–Gel Coordination Chemistry: Building Catalysts from the Bottom-Up. ChemCatChem 2013, 5, 844–860. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, S.; Zhu, S.; Ma, J.; Sun, Z.; Farid, M. Evaluation of paraffin infiltrated in various porous silica matrices as shape-stabilized phase change materials for thermal energy storage. Energy Convers. Manag. 2018, 171, 361–370. [Google Scholar] [CrossRef]
- Mitran, R.A.; Berger, D.; Munteanu, C.; Matei, C. Evaluation of Different Mesoporous Silica Supports for Energy Storage in Shape-Stabilized Phase Change Materials with Dual Thermal Responses. J. Org. Chem. C 2015, 119, 15177–15184. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Rámila, A.; del Real, R.P.; Pérez-Pariente, J. A new property of MCM-41: Drug delivery system. Chem. Mater. 2001, 13, 308–311. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Balas, F.; Arcos, D. Mesoporous materials for drug delivery. Angew. Chem. Int. Ed. 2007, 46, 7548–7558. [Google Scholar] [CrossRef]
- Manzano, M.; Vallet-Regí, M. Mesoporous silica nanoparticles for drug delivery. Adv. Funct. Mater. 2019, 1902634. [Google Scholar] [CrossRef]
- Rosenholm, J.M.; Mamaeva, V.; Sahlgren, C.; Lindén, M. Nanoparticles in targeted cancer therapy: Mesoporous silica nanoparticles entering preclinical development stage. Nanomedicine 2012, 7, 111–120. [Google Scholar] [CrossRef]
- Napierska, D.; Thomassen, L.C.J.; Lison, D.; Martens, J.A.; Hoet, P.H. The nanosilica hazard: Another variable entity. Part. Fibre Toxicol. 2010, 7, 39. [Google Scholar] [CrossRef]
- Croissant, J.G.; Fatieiev, Y.; Khashab, N.M. Degradability and clearance of silicon, organosilica, silsesquioxane, silica mixed oxide, and mesoporous silica nanoparticles. Adv. Mater. 2017, 29, 1604634. [Google Scholar] [CrossRef]
- Yamada, H.; Urata, C.; Aoyama, Y.; Osada, S.; Yamauchi, Y.; Kuroda, K. Preparation of colloidal mesoporous silica nanoparticles with different diameters and their unique degradation behavior in static aqueous systems. Chem. Mater. 2012, 24, 1462–1471. [Google Scholar] [CrossRef]
- Paris, J.L.; Colilla, M.; Izquierdo-barba, I.; Manzano, M.; Vallet-Regí, M. Tuning mesoporous silica dissolution in physiological environments: A review. J. Mater. Sci. 2017, 52, 8761–8771. [Google Scholar] [CrossRef]
- Kempen, P.J.; Greasley, S.; Parker, K.A.; Campbell, J.L.; Chang, H.-Y.; Jones, J.R.; Sinclair, R.; Gambhir, S.S.; Jokerst, J. V Theranostic mesoporous silica nanoparticles biodegrade after pro-survival drug delivery and ultrasound/magnetic resonance imaging of stem cells. Theranostics 2015, 5, 631–642. [Google Scholar] [CrossRef]
- Chen, G.; Teng, Z.; Su, X.; Liu, Y.; Lu, G. Unique biological degradation behavior of stöber mesoporous silica nanoparticles from their interiors to their exteriors. J. Biomed. Nanotechnol. 2015, 11, 722–729. [Google Scholar] [CrossRef]
- Zhai, W.; He, C.; Wu, L.; Zhou, Y.; Chen, H.; Chang, J.; Zhang, H. Degradation of hollow mesoporous silica nanoparticles in human umbilical vein endothelial cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100B, 1397–1403. [Google Scholar] [CrossRef]
- Gisbert-Garzarán, M.; Manzano, M.; Vallet-Regí, M. Mesoporous silica nanoparticles for the treatment of complex bone diseases: Bone cancer, bone infection and osteoporosis. Pharmaceutics 2020, 12, 83. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; González, B.; Izquierdo-Barba, I. Nanomaterials as promising alternative in the infection treatment. Int. J. Mol. Sci. 2019, 20, 3806. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Y.; Teh, C.; Ang, C.Y.; Li, M.; Li, P.; Korzh, V.; Zhao, Y. Responsive mesoporous silica nanoparticles for sensing of hydrogen peroxide and simultaneous treatment toward heart failure. Nanoscale 2017, 9, 2253–2261. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Ding, Q.; Wang, J.; Deng, L.; Yang, L.; Tao, L.; Lei, H.; Lu, S. 5-Azacytidine delivered by mesoporous silica nanoparticles regulates the differentiation of P19 cells into cardiomyocytes. Nanoscale 2016, 8, 2011–2021. [Google Scholar] [CrossRef] [PubMed]
- Doadrio, A.L.; Sánchez-Montero, J.M.; Doadrio, J.C.; Salinas, A.J.; Vallet-Regí, M. Mesoporous silica nanoparticles as a new carrier methodology in the controlled release of the active components in a polypill. Eur. J. Pharm. Sci. 2017, 97, 1–8. [Google Scholar] [CrossRef]
- Liao, Y.-T.; Lee, C.-H.; Chen, S.-T.; Lai, J.-Y.; Wu, K.C.-W. Gelatin-functionalized mesoporous silica nanoparticles with sustained release properties for intracameral pharmacotherapy of glaucoma. J. Mater. Chem. B 2017, 5, 7008–7013. [Google Scholar] [CrossRef]
- Hu, C.; Sun, J.; Zhang, Y.; Chen, J.; Lei, Y.; Sun, X.; Deng, Y. Local delivery and sustained-release of nitric oxide donor loaded in mesoporous silica particles for efficient treatment of primary open-angle glaucoma. Adv. Healthc. Mater. 2018, 7, 1801047. [Google Scholar] [CrossRef]
- Hou, L.; Zheng, Y.; Wang, Y.; Hu, Y.; Shi, J.; Liu, Q.; Zhang, H.; Zhang, Z. Self-regulated carboxyphenylboronic acid-modified mesoporous silica nanoparticles with “touch switch” releasing property for insulin delivery. ACS Appl. Mater. Interfaces 2018, 10, 21927–21938. [Google Scholar] [CrossRef]
- Xu, B.; Jiang, G.; Yu, W.; Liu, D.; Zhang, Y.; Zhou, J.; Sun, S.; Liu, Y. H2O2-Responsive mesoporous silica nanoparticles integrated with microneedle patches for the glucose-monitored transdermal delivery of insulin. J. Mater. Chem. B 2017, 5, 8200–8208. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar] [PubMed]
- Grodzinski, P.; Kircher, M.; Goldberg, M.; Gabizon, A. Integrating nanotechnology into cancer care. ACS Nano 2019, 13, 7370–7376. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Nakamura, H.; Maeda, H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 2011, 63, 136–151. [Google Scholar] [CrossRef]
- Dogra, P.; Adolphi, N.L.; Wang, Z.; Lin, Y.S.; Butler, K.S.; Durfee, P.N.; Croissant, J.G.; Noureddine, A.; Coker, E.N.; Bearer, E.L.; et al. Establishing the effects of mesoporous silica nanoparticle properties on in vivo disposition using imaging-based pharmacokinetics. Nat. Commun. 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Etheridge, M.L.; Campbell, S.A.; Erdman, A.G.; Haynes, C.L.; Wolf, S.M.; McCullough, J. The big picture on nanomedicine: The state of investigational and approved nanomedicine products. Nanomedicine Nanotechnol. Biol. Med. 2013, 9, 1–14. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Ran, F.; Cui, Y.; Liu, C.; Zhao, Q.; Gao, Y.; Wang, D.; Wang, S. A comparison between sphere and rod nanoparticles regarding their in vivo biological behavior and pharmacokinetics. Sci. Rep. 2017, 7, 4131. [Google Scholar] [CrossRef]
- Toy, R.; Peiris, P.M.; Ghaghada, K.B. Shaping cancer nanomedicine: The effect of particle shape on the in vivo journey of nanoparticles. Nanomedicine 2014, 9, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Stenzel, M.H. Entry of Nanoparticles into Cells: The Importance of Nanoparticle Properties. Polym. Chem. 2018, 9, 259–272. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- Ge, C.; Tian, J.; Zhao, Y.; Chen, C.; Zhou, R.; Chai, Z. Towards understanding of nanoparticle–protein corona. Arch. Toxicol. 2015, 89, 519–539. [Google Scholar] [CrossRef]
- Jokerst, J.V.; Lobovkina, T.; Zare, R.N.; Gambhir, S.S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine 2011, 6, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Clemments, A.M.; Muniesa, C.; Landry, C.C.; Botella, P. Effect of surface properties in protein corona development on mesoporous silica nanoparticles. RSC Adv. 2014, 4, 29134–29138. [Google Scholar] [CrossRef]
- Yildirim, A.; Ozgur, E.; Bayindir, M. Impact of mesoporous silica nanoparticle surface functionality on hemolytic activity, thrombogenicity and non-specific protein adsorption. J. Mater. Chem. B 2013, 1, 1909–1920. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, L.; Zhao, C.; Zheng, J. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymer (Guildf.) 2010, 51, 5283–5293. [Google Scholar] [CrossRef]
- Encinas, N.; Angulo, M.; Astorga, C.; Colilla, M.; Izquierdo-Barba, I.; Vallet-Regí, M. Mixed-charge pseudo-zwitterionic mesoporous silica nanoparticles with low-fouling and reduced cell uptake properties. Acta Biomater. 2019, 84, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Salcedo, S.; Vallet-Regí, M.; Shahin, S.A.; Glackin, C.A.; Zink, J.I. Mesoporous core-shell silica nanoparticles with anti-fouling properties for ovarian cancer therapy. Chem. Eng. J. 2018, 340, 114–124. [Google Scholar] [CrossRef]
- Rosen, J.E.; Gu, F.X. Surface functionalization of silica nanoparticles with cysteine: A low-fouling zwitterionic surface. Langmuir 2011, 27, 10507–10513. [Google Scholar] [CrossRef]
- Xuan, M.; Shao, J.; Zhao, J.; Li, Q.; Dai, L.; Li, J. Magnetic mesoporous silica nanoparticles cloaked by red blood cell membranes: Applications in cancer therapy. Angew. Chem. Int. Ed. 2018, 57, 6049–6053. [Google Scholar] [CrossRef]
- Cai, D.; Liu, L.; Han, C.; Ma, X.; Qian, J.; Zhou, J.; Zhu, W. Cancer cell membrane-coated mesoporous silica loaded with superparamagnetic ferroferric oxide and Paclitaxel for the combination of Chemo/Magnetocaloric therapy on MDA-MB-231 cells. Sci. Rep. 2019, 9, 14475. [Google Scholar] [CrossRef]
- Yue, J.; Wang, Z.; Shao, D.; Chang, Z.; Hu, R.; Li, L.; Luo, S.; Dong, W. Cancer cell membrane-modified biodegradable mesoporous silica nanocarriers for berberine therapy of liver cancer. RSC Adv. 2018, 8, 40288–40297. [Google Scholar] [CrossRef]
- Yang, J.; Teng, Y.; Fu, Y.; Zhang, C. Chlorins e6 loaded silica nanoparticles coated with gastric cancer cell membrane for tumor specific photodynamic therapy of gastric cancer. Int. J. Nanomedicine 2019, 14, 5061–5071. [Google Scholar] [CrossRef] [PubMed]
- van Rijt, S.H.; Bölükbas, D.A.; Argyo, C.; Wipplinger, K.; Naureen, M.; Datz, S.; Eickelberg, O.; Meiners, S.; Bein, T.; Schmid, O.; et al. Applicability of avidin protein coated mesoporous silica nanoparticles as drug carriers in the lung. Nanoscale 2016, 8, 8058–8069. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Hu, Y.; Xin, R.; Zhang, B.; Li, J.; Ding, X.; Hou, Y.; Yang, L.; Cai, K. Surface functionalized mesoporous silica nanoparticles with natural proteins for reduced immunotoxicity. J. Biomed. Mater. Res. Part A 2014, 102, 3781–3794. [Google Scholar] [CrossRef] [PubMed]
- Natfji, A.A.; Ravishankar, D.; Osborn, H.M.I.; Greco, F. Parameters affecting the enhanced permeability and retention effect: The need for patient selection. J. Pharm. Sci. 2017, 106, 3179–3187. [Google Scholar] [CrossRef] [PubMed]
- Villaverde, G.; Baeza, A. Targeting strategies for improving the efficacy of nanomedicine in oncology. Beilstein J. Nanotechnol. 2019, 10, 168–181. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Y.; Kilchrist, K.V.; Li, J.; Duvall, C.L.; Oupický, D. Endosomolytic and tumor-penetrating mesoporous silica nanoparticles for siRNA/miRNA combination cancer therapy. ACS Appl. Mater. Interfaces 2020, 12, 4308–4322. [Google Scholar] [CrossRef]
- Liu, X.; Lin, P.; Perrett, I.; Lin, J.; Liao, Y.-P.; Chang, C.H.; Jiang, J.; Wu, N.; Donahue, T.; Wainberg, Z.; et al. Tumor-penetrating peptide enhances transcytosis of silicasome-based chemotherapy for pancreatic cancer. J. Clin. Invest. 2017, 127, 2007–2018. [Google Scholar] [CrossRef]
- Kuang, J.; Song, W.; Yin, J.; Zeng, X.; Han, S.; Zhao, Y.-P.; Tao, J.; Liu, C.-J.; He, X.-H.; Zhang, X.-Z. iRGD modified chemo-immunotherapeutic nanoparticles for enhanced immunotherapy against glioblastoma. Adv. Funct. Mater. 2018, 28, 1800025. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, J.; Ji, Y.; Lu, J.; Chan, R.; Meng, H. Targeted drug delivery using iRGD peptide for solid cancer treatment. Mol. Syst. Des. Eng. 2017, 2, 370–379. [Google Scholar] [CrossRef]
- Ruoslahti, E. Tumor penetrating peptides for improved drug delivery. Adv. Drug Deliv. Rev. 2017, 110–111, 3–12. [Google Scholar] [CrossRef]
- Kang, T.; Gao, X.; Hu, Q.; Jiang, D.; Feng, X.; Zhang, X.; Song, Q.; Yao, L.; Huang, M.; Jiang, X.; et al. iNGR-modified PEG-PLGA nanoparticles that recognize tumor vasculature and penetrate gliomas. Biomaterials 2014, 35, 4319–4332. [Google Scholar] [CrossRef]
- Pawelek, J.M.; Low, K.B.; Bermudes, D. Bacteria as tumour-targeting vectors. Lancet Oncol. 2003, 4, 548–556. [Google Scholar] [CrossRef]
- Li, Z.; Fan, D.; Xiong, D. Mesenchymal stem cells as delivery vectors for anti-tumor therapy. Stem Cell Investig. 2015, 2, 6. [Google Scholar] [PubMed]
- Vallet-Regí, M.; Paris, J.L.; de la Torre, P.; Cabañas, M.V.; Manzano, M.; Flores, A.I. Mesenchymal stem cells from human placenta as nanoparticle delivery vectors. Insights Stem Cells 2018, 4, 1. [Google Scholar]
- Das, S.; Raj, R. Prospects of bacteriotherapy with nanotechnology in nanoparticledrug conjugation approach for cancer therapy. Curr. Med. Chem. 2016, 23, 1477–1494. [Google Scholar]
- Suh, S.B.; Jo, A.; Traore, M.A.; Zhan, Y.; Coutermarsh-Ott, S.L.; Ringel-Scaia, V.M.; Allen, I.C.; Davis, R.M.; Behkam, B. Nanoscale bacteria-enabled autonomous drug delivery system (NanoBEADS) enhances intratumoral transport of nanomedicine. Adv. Sci. 2019, 6, 1801309. [Google Scholar] [CrossRef]
- Moreno, V.M.; Álvarez, E.; Izquierdo-Barba, I.; Baeza, A.; Serrano-López, J.; Vallet-Regí, M. Bacteria as Nanoparticles Carrier for Enhancing Penetration in a Tumoral Matrix Model. Adv. Mater. Interfaces 2020, in press. [Google Scholar] [CrossRef]
- Paris, J.L.; de la Torre, P.; Cabañas, M.V.; Manzano, M.; Grau, M.; Flores, A.I.; Vallet-Regí, M. Vectorization of ultrasound-responsive nanoparticles in placental mesenchymal stem cells for cancer therapy. Nanoscale 2017, 9, 5528–5537. [Google Scholar] [CrossRef]
- Paris, J.L.; de la Torre, P.; Manzano, M.; Cabañas, M.V.; Flores, A.I.; Vallet-Regí, M. Decidua-derived mesenchymal stem cells as carriers of mesoporous silica nanoparticles. In vitro and in vivo evaluation on mammary tumors. Acta Biomater. 2016, 33, 275–282. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, F.; Wang, H.; Niu, G.; Choi, K.Y.; Swierczewska, M.; Zhang, G.; Gao, H.; Wang, Z.; Zhu, L.; et al. Mesenchymal stem cell-based cell engineering with multifunctional mesoporous silica nanoparticles for tumor delivery. Biomaterials 2013, 34, 1772–1780. [Google Scholar] [CrossRef]
- Netti, P.A.; Berk, D.A.; Swartz, M.A.; Grodzinsky, A.J.; Jain, R.K. Role of Extracellular Matrix Assembly in Interstitial Transport in Solid Tumors. Cancer Res. 2000, 60, 2497–2503. [Google Scholar]
- Walker, C.; Mojares, E.; Del Río Hernández, A. Role of extracellular matrix in development and cancer progression. Int. J. Mol. Sci. 2018, 19, 3028. [Google Scholar] [CrossRef]
- Parodi, A.; Haddix, S.G.; Taghipour, N.; Scaria, S.; Taraballi, F.; Cevenini, A.; Yazdi, I.K.; Corbo, C.; Palomba, R.; Khaled, S.Z.; et al. Bromelain surface modification increases the diffusion of silica nanoparticles in the tumor extracellular matrix. ACS Nano 2014, 8, 9874–9883. [Google Scholar] [CrossRef]
- Villegas, M.R.; Baeza, A.; Noureddine, A.; Durfee, P.N.; Butler, K.S.; Agola, J.O.; Brinker, C.J.; Vallet-Regí, M. Multifunctional Protocells for Enhanced Penetration in 3D Extracellular Tumoral Matrices. Chem. Mater. 2018, 30, 112–120. [Google Scholar] [CrossRef]
- Villegas, M.R.; Baeza, A.; Vallet-Regí, M. Hybrid Collagenase Nanocapsules for Enhanced Nanocarrier Penetration in Tumoral Tissues. ACS Appl. Mater. Interfaces 2015, 7, 24075–24081. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-R.; Lin, R.; Li, H.-J.; He, W.; Du, J.-Z.; Wang, J. Strategies to improve tumor penetration of nanomedicines through nanoparticle design. WIREs Nanomed. Nanobiotechnol. 2019, 11, e1519. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, D.; Chen, P.; Qiao, Y.; Yang, T.; Yu, Z.; Wang, C.; Wu, H. Construction of a novel “ball-and-rod” MSNs-pp-PEG system: A promising antitumor drug delivery system with a particle size switchable function. Chem. Commun. 2020, in press. [Google Scholar] [CrossRef]
- Coussios, C.C.; Roy, R.A. Applications of acoustics and cavitation to noninvasive therapy and drug delivery. Annu. Rev. Fluid Mech. 2008, 40, 395–420. [Google Scholar] [CrossRef]
- Arvanitis, C.D.; Bazan-Peregrino, M.; Rifai, B.; Seymour, L.W.; Coussios, C.C. Cavitation-enhanced extravasation for drug delivery. Ultrasound Med. Biol. 2011, 37, 1838–1852. [Google Scholar] [CrossRef]
- Ho, Y.-J.; Wu, C.-H.; Jin, Q.; Lin, C.-Y.; Chiang, P.-H.; Wu, N.; Fan, C.-H.; Yang, C.-M.; Yeh, C.-K. Superhydrophobic drug-loaded mesoporous silica nanoparticles capped with β-cyclodextrin for ultrasound image-guided combined antivascular and chemo-sonodynamic therapy. Biomaterials 2020, 232, 119723. [Google Scholar] [CrossRef]
- Paris, J.L.; Mannaris, C.; Cabañas, M.V.; Carlisle, R.; Manzano, M.; Vallet-Regí, M.; Coussios, C.C. Ultrasound-mediated cavitation-enhanced extravasation of mesoporous silica nanoparticles for controlled-release drug delivery. Chem. Eng. J. 2018, 340, 2–8. [Google Scholar] [CrossRef]
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.W.; Ghandehari, H. Nanoparticle uptake: The phagocyte problem. Nano Today 2015, 10, 487–510. [Google Scholar] [CrossRef] [PubMed]
- Arvizo, R.R.; Miranda, O.R.; Moyano, D.F.; Walden, C.A.; Giri, K.; Robertson, J.D.; Rotello, V.M.; Reid, J.M.; Mukherjee, P. Modulating pharmacokinetics, tumor uptake and biodistribution by engineered nanoparticles. PLoS ONE 2011, 6, 24374. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Stenzel, M.H. Multicellular tumor spheroids (MCTS) as a 3D in vitro evaluation tool of nanoparticles. Small 2018, 14, 1702858. [Google Scholar] [CrossRef]
- Alkilany, A.M.; Zhu, L.; Weller, H.; Mews, A.; Parak, W.J.; Barz, M.; Feliu, N. Ligand density on nanoparticles: A parameter with critical impact on nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 22–36. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Hayama, K.; Sasagawa, I.; Okada, Y.; Kawase, T.; Tsubokawa, N.; Tsuchimochi, M. HER2-targeted multifunctional silica nanoparticles specifically enhance the radiosensitivity of HER2-overexpressing breast cancer cells. Int. J. Mol. Sci. 2018, 19, 908. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lu, Y.; Jiang, C.; Zhu, Y.; Yang, X.; Hu, X.; Lin, Z.; Zhang, Y.; Peng, M.; Xia, H.; et al. Actively targeted deep tissue imaging and photothermal-chemo therapy of breast cancer by antibody-functionalized drug-loaded X-ray-responsive bismuth sulfide@mesoporous silica core–shell nanoparticles. Adv. Funct. Mater. 2018, 28, 1704623. [Google Scholar] [CrossRef]
- Ngamcherdtrakul, W.; Morry, J.; Gu, S.; Castro, D.J.; Goodyear, S.M.; Sangvanich, T.; Reda, M.M.; Lee, R.; Mihelic, S.A.; Beckman, B.L.; et al. Cationic polymer modified mesoporous silica nanoparticles for targeted siRNA delivery to HER2+ breast cancer. Adv. Funct. Mater. 2015, 25, 2646–2659. [Google Scholar] [CrossRef]
- Tsai, C.-P.; Chen, C.-Y.; Hung, Y.; Chang, F.-H.; Mou, C.-Y. Monoclonal antibody-functionalized mesoporous silica nanoparticles (MSN) for selective targeting breast cancer cells. J. Mater. Chem. 2009, 19, 5737–5743. [Google Scholar] [CrossRef]
- Ngamcherdtrakul, W.; Sangvanich, T.; Reda, M.; Gu, S.; Bejan, D.; Yantasee, W. Lyophilization and stability of antibody-conjugated mesoporous silica nanoparticle with cationic polymer and PEG for siRNA delivery. Int. J. Nanomed. 2018, 13, 4015–4027. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Wang, S.; Shi, D.; Zhou, X.; Wang, C.; Wu, J.; Zeng, Z.; Li, Y.; Sun, J.; et al. CD44-engineered mesoporous silica nanoparticles for overcoming multidrug resistance in breast cancer. Appl. Surf. Sci. 2015, 332, 308–317. [Google Scholar] [CrossRef]
- Chen, F.; Hong, H.; Shi, S.; Goel, S.; Valdovinos, H.F.; Hernandez, R.; Theuer, C.P.; Barnhart, T.E.; Cai, W. Engineering of hollow mesoporous silica nanoparticles for remarkably enhanced tumor active targeting efficacy. Sci. Rep. 2014, 4, 5080. [Google Scholar] [CrossRef]
- Goel, S.; Chen, F.; Luan, S.; Valdovinos, H.F.; Shi, S.; Graves, S.A.; Ai, F.; Barnhart, T.E.; Theuer, C.P.; Cai, W. Engineering intrinsically zirconium-89 radiolabeled self-destructing mesoporous silica nanostructures for in vivo biodistribution and tumor targeting studies. Adv. Sci. 2016, 3, 1600122. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Hong, H.; Zhang, Y.; Valdovinos, H.F.; Shi, S.; Kwon, G.S.; Theuer, C.P.; Barnhart, T.E.; Cai, W. In vivo tumor targeting and image-guided drug delivery with antibody-conjugated, radiolabeled mesoporous silica nanoparticles. ACS Nano 2013, 7, 9027–9039. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Meng, B.; Yu, Y.; Wang, S. EpCAM antibody-conjugated mesoporous silica nanoparticles to enhance the anticancer efficacy of carboplatin in retinoblastoma. Mater. Sci. Eng. C 2017, 76, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Dréau, D.; Moore, L.J.; Alvarez-Berrios, M.P.; Tarannum, M.; Mukherjee, P.; Vivero-Escoto, J.L. Mucin-1-antibody-conjugated mesoporous silica nanoparticles for selective breast cancer detection in a Mucin-1 transgenic murine mouse model. J. Biomed. Nanotechnol. 2016, 12, 2172–2184. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Wei, M.; Liu, C.; Yu, T.; Yang, J. Cetuximab-modified silica nanoparticle loaded with ICG for tumor-targeted combinational therapy of breast cancer. Drug Deliv. 2019, 26, 129–136. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, H.-Y.; Yang, L.; Zhang, Z.; Ji, H. Cetuximab-modified mesoporous silica nano-medicine specifically targets EGFR-mutant lung cancer and overcomes drug resistance. Sci. Rep. 2016, 6, 25468. [Google Scholar] [CrossRef]
- Er, Ö.; Colak, G.S.; Ocakoglu, K.; Ince, M.; Bresolí-Obach, R.; Mora, M.; Sagristá, L.M.; Yurt, F.; Nonell, S. Selective photokilling of human pancreatic cancer cells using cetuximab-targeted mesoporous silica nanoparticles for delivery of zinc phthalocyanine. Molecules 2018, 23, 2749. [Google Scholar] [CrossRef]
- Farokhzad, O.C.; Karp, J.M.; Langer, R. Nanoparticle–aptamer bioconjugates for cancer targeting. Expert Opin. Drug Deliv. 2006, 3, 311–324. [Google Scholar] [CrossRef]
- Dineshkumar, S.; Raj, A.; Srivastava, A.; Mukherjee, S.; Pasha, S.S.; Kachwal, V.; Fageria, L.; Chowdhury, R.; Laskar, I.R. Facile incorporation of “aggregation-induced emission”-active conjugated polymer into mesoporous silica hollow nanospheres: Synthesis, characterization, photophysical studies, and application in bioimaging. ACS Appl. Mater. Interfaces 2019, 11, 31270–31282. [Google Scholar] [CrossRef]
- Babaei, M.; Abnous, K.; Taghdisi, S.M.; Amel Farzad, S.; Peivandi, M.T.; Ramezani, M.; Alibolandi, M. Synthesis of theranostic epithelial cell adhesion molecule targeted mesoporous silica nanoparticle with gold gatekeeper for hepatocellular carcinoma. Nanomedicine 2017, 12, 1261–1279. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Li, F.; Zhang, H.; Lu, Y.; Lian, S.; Lin, H.; Gao, Y.; Jia, L. EpCAM aptamer-functionalized mesoporous silica nanoparticles for efficient colon cancer cell-targeted drug delivery. Eur. J. Pharm. Sci. 2016, 83, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Duo, Y.; Bao, S.; He, L.; Ling, K.; Luo, J.; Zhang, Y.; Huang, H.; Zhang, H.; Yu, X. EpCAM aptamer-functionalized polydopamine-coated mesoporous silica nanoparticles loaded with DM1 for targeted therapy in colorectal cancer. Int. J. Nanomedicine 2017, 12, 6239–6257. [Google Scholar] [CrossRef] [PubMed]
- Pascual, L.; Cerqueira-Coutinho, C.; García-Fernández, A.; de Luis, B.; Bernardes, E.S.; Albernaz, M.S.; Missailidis, S.; Martínez-Máñez, R.; Santos-Oliveira, R.; Orzaez, M.; et al. MUC1 aptamer-capped mesoporous silica nanoparticles for controlled drug delivery and radio-imaging applications. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- Hanafi-Bojd, M.Y.; Moosavian Kalat, S.A.; Taghdisi, S.M.; Ansari, L.; Abnous, K.; Malaekeh-Nikouei, B. MUC1 aptamer-conjugated mesoporous silica nanoparticles effectively target breast cancer cells. Drug Dev. Ind. Pharm. 2018, 44, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Cheng, F.; Zhou, R.; Cao, J.; Li, J.; Burda, C.; Min, Q.; Zhu, J.-J. DNA-hybrid-gated multifunctional mesoporous silica nanocarriers for dual-targeted and microRNA-responsive controlled drug delivery. Angew. Chem. Int. Ed. 2014, 53, 2371–2375. [Google Scholar] [CrossRef]
- Alizadeh, L.; Alizadeh, E.; Zarebkohan, A.; Ahmadi, E.; Rahmati-Yamchi, M.; Salehi, R. AS1411 aptamer-functionalized chitosan-silica nanoparticles for targeted delivery of epigallocatechin gallate to the SKOV-3 ovarian cancer cell lines. J. Nanoparticle Res. 2020, 22, 5. [Google Scholar] [CrossRef]
- Tang, Y.; Hu, H.; Zhang, M.G.; Song, J.; Nie, L.; Wang, S.; Niu, G.; Huang, P.; Lu, G.; Chen, X. An aptamer-targeting photoresponsive drug delivery system using “off–on” graphene oxide wrapped mesoporous silica nanoparticles. Nanoscale 2015, 7, 6304–6310. [Google Scholar] [CrossRef]
- Li, L.-L.; Yin, Q.; Cheng, J.; Lu, Y. Polyvalent mesoporous silica nanoparticle-aptamer bioconjugates target breast cancer cells. Adv. Healthc. Mater. 2012, 1, 567–572. [Google Scholar] [CrossRef]
- Nejabat, M.; Mohammadi, M.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Fabrication of acetylated carboxymethylcellulose coated hollow mesoporous silica hybrid nanoparticles for nucleolin targeted delivery to colon adenocarcinoma. Carbohydr. Polym. 2018, 197, 157–166. [Google Scholar] [CrossRef]
- Tan, J.; Yang, N.; Zhong, L.; Tan, J.; Hu, Z.; Zhao, Q.; Gong, W.; Zhang, Z.; Zheng, R.; Lai, Z.; et al. A new theranostic system based on endoglin aptamer conjugated fluorescent silica nanoparticles. Theranostics 2017, 7, 4862–4876. [Google Scholar] [CrossRef]
- Wang, K.; Yao, H.; Meng, Y.; Wang, Y.; Yan, X.; Huang, R. Specific aptamer-conjugated mesoporous silica-carbon nanoparticles for HER2-targeted chemo-photothermal combined therapy. Acta Biomater. 2015, 16, 196–205. [Google Scholar] [CrossRef]
- Habault, J.; Poyet, J.L. Recent advances in cell penetrating peptide-based anticancer therapies. Molecules 2019, 24, 927. [Google Scholar] [CrossRef]
- Guidotti, G.; Brambilla, L.; Rossi, D. Cell-penetrating peptides: From basic research to clinics. Trends Pharmacol. Sci. 2017, 38, 406–424. [Google Scholar] [CrossRef]
- Pan, L.; He, Q.; Liu, J.; Chen, Y.; Ma, M.; Zhang, L.; Shi, J. Nuclear-targeted drug delivery of tat peptide-conjugated monodisperse mesoporous silica nanoparticles. J. Am. Chem. Soc. 2012, 134, 5722–5725. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Wang, M.; Ma, Y.; Xia, W.; Gu, H. A mesoporous silica nanoparticle – PEI – Fusogenic peptide system for siRNA delivery in cancer therapy. Biomaterials 2013, 34, 1391–1401. [Google Scholar] [CrossRef]
- Wu, X.; Han, Z.; Schur, R.M.; Lu, Z. Targeted Mesoporous Silica Nanoparticles Delivering Arsenic Trioxide with Environment Sensitive Drug Release for E ffective Treatment of Triple Negative Breast Cancer. ACS Biomater. Sci. 2016, 2, 501–507. [Google Scholar] [CrossRef]
- Paris, J.L.; Villaverde, G.; Cabañas, M.V.; Manzano, M.; Vallet-Regí, M. From proof-of-concept material to PEGylated and modularly targeted ultrasound-responsive mesoporous silica nanoparticles. J. Mater. Chem. B 2018, 6, 2785–2794. [Google Scholar] [CrossRef]
- Lu, Y.; Li, L.; Lin, Z.; Li, M.; Hu, X.; Zhang, Y.; Peng, M. Enhancing osteosarcoma killing and CT imaging using ultrahigh drug loading and nir-responsive bismuth sulfide@mesoporous silica nanoparticles. Adv. Healthc. Mater. 2018, 7, 1800602. [Google Scholar] [CrossRef]
- Li, H.; Li, K.; Zeng, Q.; Zeng, Y.; Chen, D.; Pang, L.; Chen, X.; Zhan, Y. Novel vinyl-modified RGD conjugated silica nanoparticles based on photo click chemistry for in vivo prostate cancer targeted fluorescence imaging. RSC Adv. 2019, 9, 25318–25325. [Google Scholar] [CrossRef]
- Xu, X.; Li, H.; Li, K.; Zeng, Q.; Liu, Y.; Zeng, Y.; Chen, D.; Liang, J.; Chen, X.; Zhan, Y. A photo-triggered conjugation approach for attaching RGD ligands to biodegradable mesoporous silica nanoparticles for the tumor fluorescent imaging. Nanomed. Nanotechnol. Biol. Med. 2019, 19, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Paris, J.L.; Villaverde, G.; Gómez-Graña, S.; Vallet-Regí, M. Nanoparticles for multimodal antivascular therapeutics: Dual drug release, photothermal and photodynamic therapy. Acta Biomater. 2020, 101, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Tang, M.; Huang, Q.; Zhao, G.; Huang, N.; Zhang, X.; Tan, Y.; Cheng, Y. Combination of 3-methyladenine therapy and Asn-Gly-Arg (NGR)-modified mesoporous silica nanoparticles loaded with temozolomide for glioma therapy in vitro. Biochem. Biophys. Res. Commun. 2019, 509, 549–556. [Google Scholar] [CrossRef]
- Lee, J.; Oh, E.-T.; Han, Y.; Kim, H.G.; Park, H.J.; Kim, C. Mesoporous silica nanocarriers with cyclic peptide gatekeeper: Specific targeting of aminopeptidase n and triggered drug release by stimuli-responsive conformational transformation. Chem. Eur. J. 2017, 23, 16966–16971. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, X.; Wen, Z.; Tan, Y.; Huang, N.; Cheng, S.; Zheng, H.; Cheng, Y. Asn-Gly-Arg-modified polydopamine-coated nanoparticles for dual-targeting therapy of brain glioma in rats. Oncotarget 2016, 7, 73681–73696. [Google Scholar] [CrossRef]
- Villaverde, G.; Gómez-Graña, S.; Guisasola, E.; García, I.; Hanske, C.; Liz-Marzán, L.M.; Baeza, A.; Vallet-Regí, M. Targeted chemo-photothermal therapy: A nanomedicine approximation to selective melanoma treatment. Part. Part. Syst. Charact. 2018, 35, 1800148. [Google Scholar] [CrossRef]
- Wei, Y.; Gao, L.; Wang, L.; Shi, L.; Wei, E.; Zhou, B.; Zhou, L.; Ge, B. Polydopamine and peptide decorated doxorubicin-loaded mesoporous silica nanoparticles as a targeted drug delivery system for bladder cancer therapy. Drug Deliv. 2017, 24, 681–691. [Google Scholar] [CrossRef]
- Shi, J.; Hou, S.; Huang, J.; Wang, S.; Huan, W.; Huang, C.; Liu, X.; Jiang, R.; Qian, W.; Lu, J.; et al. An MSN-PEG-IP drug delivery system and IL13Rα2 as targeted therapy for glioma. Nanoscale 2017, 9, 8970–8981. [Google Scholar] [CrossRef]
- Martínez-Carmona, M.; Baeza, A.; Rodríguez-Milla, M.A.; García-Castro, J.; Vallet-Regí, M. Mesoporous silica nanoparticles grafted with a light-responsive protein shell for highly cytotoxic antitumoral therapy. J. Mater. Chem. B 2015, 3, 5746–5752. [Google Scholar] [CrossRef]
- Montalvo-Quiros, S.; Aragoneses-Cazorla, G.; Garcia-Alcalde, L.; Vallet-Regí, M.; González, B.; Luque-Garcia, J.L. Cancer cell targeting and therapeutic delivery of silver nanoparticles by mesoporous silica nanocarriers: Insights into the action mechanisms using quantitative proteomics. Nanoscale 2019, 11, 4531–4545. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sun, H.; Hu, J.; Han, X.; Liu, H.; Hu, Y. Transferrin gated mesoporous silica nanoparticles for redox-responsive and targeted drug delivery. Colloids Surf., B 2017, 152, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, M.; Lim, W.Q.; Luo, Z.; Phua, S.Z.F.; Huo, R.; Li, L.; Li, K.; Dai, L.; Liu, J.; et al. A transferrin-conjugated hollow nanoplatform for redox-controlled and targeted chemotherapy of tumor with reduced inflammatory reactions. Theranostics 2018, 8, 518–532. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Shen, S.; Sun, Y.; Jiang, X.; Yang, W. A functionalized hollow mesoporous silica nanoparticles-based controlled dual-drug delivery system for improved tumor cell cytotoxicity. Part. Part. Syst. Char. 2015, 32, 222–233. [Google Scholar] [CrossRef]
- Saini, K.; Bandyopadhyaya, R. Transferrin-conjugated polymer-coated mesoporous silica nanoparticles loaded with gemcitabine for killing pancreatic cancer cells. ACS Appl. Nano Mater. 2020, 3, 229–240. [Google Scholar] [CrossRef]
- Gurka, M.K.; Pender, D.; Chuong, P.; Fouts, B.L.; Sobelov, A.; McNally, M.W.; Mezera, M.; Woo, S.Y.; McNally, L.R. Identification of pancreatic tumors in vivo with ligand-targeted, pH responsive mesoporous silica nanoparticles by multispectral optoacoustic tomography. J. Control. Release 2016, 231, 60–67. [Google Scholar] [CrossRef]
- Martínez-Carmona, M.; Lozano, D.; Colilla, M.; Vallet-Regí, M. Lectin-conjugated pH-responsive mesoporous silica nanoparticles for targeted bone cancer treatment. Acta Biomater. 2018, 65, 393–404. [Google Scholar] [CrossRef]
- Bhat, R.; García, I.; Aznar, E.; Arnaiz, B.; Martínez-Bisbal, M.C.; Liz-Marzán, L.M.; Penadés, S.; Martínez-Máñez, R. Lectin-gated and glycan functionalized mesoporous silica nanocontainers for targeting cancer cells overexpressing Lewis X antigen. Nanoscale 2018, 10, 239–249. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, Z.; Sun, W.; Yang, Y.; Jin, H.; Qiu, L.; Chen, J.; Chen, J. Co-responsive smart cyclodextrin-gated mesoporous silica nanoparticles with ligand-receptor engagement for anti-cancer treatment. Mater. Sci. Eng. C 2019, 103, 109831. [Google Scholar] [CrossRef]
- Zhao, R.; Li, T.; Zheng, G.; Jiang, K.; Fan, L.; Shao, J. Simultaneous inhibition of growth and metastasis of hepatocellular carcinoma by co-delivery of ursolic acid and sorafenib using lactobionic acid modified and pH-sensitive chitosan-conjugated mesoporous silica nanocomplex. Biomaterials 2017, 143, 1–16. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, P.; He, Z.; He, H.; Rong, W.; Li, J.; Zhou, D.; Huang, Y. Mesoporous silica nanoparticles with lactose-mediated targeting effect to deliver platinum(iv) prodrug for liver cancer therapy. J. Mater. Chem. B 2017, 5, 7591–7597. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Cai, K.; Hu, Y.; Zhao, L.; Liu, P.; Duan, L.; Yang, W. Mesoporous silica nanoparticles end-capped with collagen: Redox-responsive nanoreservoirs for targeted drug delivery. Angew. Chem. Int. Ed. 2011, 50, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhang, Q.; Dai, L.; Shen, X.; Chen, W.; Cai, K. Phenylboronic acid-modified hollow silica nanoparticles for dual-responsive delivery of doxorubicin for targeted tumor therapy. Regen. Biomater. 2017, 4, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Sodagar Taleghani, A.; Ebrahimnejad, P.; Heidarinasab, A.; Akbarzadeh, A. Sugar-conjugated dendritic mesoporous silica nanoparticles as pH-responsive nanocarriers for tumor targeting and controlled release of deferasirox. Mater. Sci. Eng. C 2019, 98, 358–368. [Google Scholar] [CrossRef]
- Niemelä, E.; Desai, D.; Nkizinkiko, Y.; Eriksson, J.E.; Rosenholm, J.M. Sugar-decorated mesoporous silica nanoparticles as delivery vehicles for the poorly soluble drug celastrol enables targeted induction of apoptosis in cancer cells. Eur. J. Pharm. Biopharm. 2015, 96, 11–21. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Z.; Lin, Y.; Yin, M.; Ren, J.; Qu, X. Bioresponsive hyaluronic acid-capped mesoporous silica nanoparticles for targeted drug delivery. Chem. Eur. J. 2013, 19, 1778–1783. [Google Scholar] [CrossRef]
- Yu, M.; Jambhrunkar, S.; Thorn, P.; Chen, J.; Gu, W.; Yu, C. Hyaluronic acid modified mesoporous silica nanoparticles for targeted drug delivery to CD44-overexpressing cancer cells. Nanoscale 2013, 5, 178–183. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, J.; Zhu, W.; Sun, C.; Di, D.; Zhang, Y.; Wang, P.; Wang, Z.; Wang, S. Dual-stimuli responsive hyaluronic acid-conjugated mesoporous silica for targeted delivery to CD44-overexpressing cancer cells. Acta Biomater. 2015, 23, 147–156. [Google Scholar] [CrossRef]
- Chen, C.; Tang, W.; Jiang, D.; Yang, G.; Wang, X.; Zhou, L.; Zhang, W.; Wang, P. Hyaluronic acid conjugated polydopamine functionalized mesoporous silica nanoparticles for synergistic targeted chemo-photothermal therapy. Nanoscale 2019, 11, 11012–11024. [Google Scholar] [CrossRef]
- Radhakrishnan, K.; Tripathy, J.; Datey, A.; Chakravortty, D.; Raichur, A.M. Mesoporous silica–chondroitin sulphate hybrid nanoparticles for targeted and bio-responsive drug delivery. New J. Chem. 2015, 39, 1754–1760. [Google Scholar] [CrossRef]
- Oommen, O.P.; Duehrkop, C.; Nilsson, B.; Hilborn, J.; Varghese, O.P. Multifunctional hyaluronic acid and chondroitin sulfate nanoparticles: Impact of glycosaminoglycan presentation on receptor mediated cellular uptake and immune activation. ACS Appl. Mater. Interfaces 2016, 8, 20614–20624. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Du, H.; Khan, A.R.; Ji, J.; Yu, A.; Zhai, G. Redox/enzyme sensitive chondroitin sulfate-based self-assembled nanoparticles loading docetaxel for the inhibition of metastasis and growth of melanoma. Carbohydr. Polym. 2018, 184, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Russell-Jones, G.; Mctavish, K.; Mcewan, J.; Rice, J.; Nowotnik, D. Vitamin-mediated targeting as a potential mechanism to increase drug uptake by tumours. J. Inorg. Biochem. 2004, 98, 1625–1633. [Google Scholar] [CrossRef] [PubMed]
- Liong, M.; Zink, J.I.; Lu, J.; Tamanoi, F.; Kovochich, M.; Xia, T.; Nel, A.E.; Ruehm, S.G. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano 2008, 2, 889–896. [Google Scholar] [CrossRef]
- Sun, X.; Wang, N.; Yang, L.-Y.; Ouyang, X.-K.; Huang, F. Folic acid and PEI modified mesoporous silica for targeted delivery of curcumin. Pharmaceutics 2019, 11, 430. [Google Scholar] [CrossRef]
- Cabañas, M.V.; Lozano, D.; Torres-Pardo, A.; Sobrino, C.; González-Calbet, J.; Arcos, D.; Vallet-Regí, M. Features of aminopropyl modified mesoporous silica nanoparticles. Implications on the active targeting capability. Mater. Chem. Phys. 2018, 220, 260–269. [Google Scholar] [CrossRef]
- Datz, S.; Argyo, C.; Gattner, M.; Weiss, V.; Brunner, K.; Bretzler, J.; von Schirnding, C.; Torrano, A.A.; Spada, F.; Vrabel, M.; et al. Genetically designed biomolecular capping system for mesoporous silica nanoparticles enables receptor-mediated cell uptake and controlled drug release. Nanoscale 2016, 8, 8101–8110. [Google Scholar] [CrossRef]
- Martínez-Carmona, M.; Lozano, D.; Colilla, M.; Vallet-Regí, M. Selective topotecan delivery to cancer cells by targeted pH-sensitive mesoporous silica nanoparticles. RSC Adv. 2016, 6, 50923–50932. [Google Scholar] [CrossRef]
- Cheng, W.; Nie, J.; Xu, L.; Liang, C.; Peng, Y.; Liu, G.; Wang, T.; Mei, L.; Huang, L.; Zeng, X. pH-sensitive delivery vehicle based on folic acid-conjugated polydopamine-modified mesoporous silica nanoparticles for targeted cancer therapy. ACS Appl. Mater. Interfaces 2017, 9, 18462–18473. [Google Scholar] [CrossRef]
- Qu, W.; Meng, B.; Yu, Y.; Wang, S. Folic acid-conjugated mesoporous silica nanoparticles for enhanced therapeutic efficacy of topotecan in retina cancers. Int. J. Nanomedicine 2018, 13, 4379–4389. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Song, F.X.; Zhang, L.; Yang, W.; Wang, H.X.; Chen, Q.L. A pH-sensitive drug delivery system based on folic acid-targeted HBP-modified mesoporous silica nanoparticles for cancer therapy. Colloids Surf., A. 2020, 590, 124470. [Google Scholar] [CrossRef]
- Song, Y.; Zhou, B.; Du, X.; Wang, Y.; Zhang, J.; Ai, Y.; Xia, Z.; Zhao, G. Folic acid (FA)-conjugated mesoporous silica nanoparticles combined with MRP-1 siRNA improves the suppressive effects of myricetin on non-small cell lung cancer (NSCLC). Biomed. Pharmacother. 2020, 125, 109561. [Google Scholar] [CrossRef] [PubMed]
- Lv, G.; Qiu, L.; Liu, G.; Wang, W.; Li, K.; Zhao, X.; Lin, J. pH sensitive chitosan-mesoporous silica nanoparticles for targeted delivery of a ruthenium complex with enhanced anticancer effects. Dalt. Trans. 2016, 45, 18147–18155. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Zhang, K.; Wu, Z.; Feng, N. Biotinylated-lipid bilayer coated mesoporous silica nanoparticles for improving the bioavailability and anti-leukaemia activity of Tanshinone IIA. Artif. Cells Nanomed. Biotechnol. 2018, 46, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Lv, G.; Li, K.; Qiu, L.; Peng, Y.; Zhao, X.; Li, X.; Liu, Q.; Wang, S.; Lin, J. Enhanced tumor diagnostic and therapeutic effect of mesoporous silica nanoparticle-mediated pre-targeted strategy. Pharm. Res. 2018, 35, 63. [Google Scholar] [CrossRef] [PubMed]
- Thepphankulngarm, N.; Wonganan, P.; Sapcharoenkun, C.; Tuntulani, T.; Leeladee, P. Combining vitamin B12 and cisplatin-loaded porous silica nanoparticles via coordination: A facile approach to prepare a targeted drug delivery system. New J. Chem. 2017, 41, 13823–13829. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, M.; Lu, Q.; Qing, G.; Sun, T. Sialic acid-targeted biointerface materials and bio-applications. Polymers 2017, 9, 249. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, B.; Luo, Z.; Ding, X.; Li, J.; Dai, L.; Zhou, J.; Zhao, X.; Ye, J.; Cai, K. Enzyme responsive mesoporous silica nanoparticles for targeted tumor therapy in vitro and in vivo. Nanoscale 2015, 7, 3614–3626. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Y.; Zhang, L.; Yan, G.; Yao, J.; Yang, P.; Lu, H. Highly specific revelation of rat serum glycopeptidome by boronic acid-functionalized mesoporous silica. Anal. Chim. Acta 2012, 753, 64–72. [Google Scholar] [CrossRef]
- Villaverde, G.; Baeza, A.; Melen, G.J.; Alfranca, A.; Ramírez, M.; Vallet-Regí, M. A new targeting agent for the selective drug delivery of nanocarriers for treating neuroblastoma. J. Mater. Chem. B 2015, 3, 4831–4842. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, Z.; Li, Y.; Guo, Y.; Zhou, H.; Li, Y.; Wu, F.; Zhang, L.; Yang, X.; Lu, B.; et al. Preparation and characterization of a dual-receptor mesoporous silica nanoparticle–hyaluronic acid–RGD peptide targeting drug delivery system. RSC Adv. 2016, 6, 40427–40435. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, H.; Li, X.; Lv, Y.; Ma, T.; Guo, S.; Huang, Z.; Wang, X.; Xu, P. Dual targeting hyaluronic acid—RGD mesoporous silica coated gold nanorods for chemo-photothermal cancer therapy. Mater. Sci. Eng. C 2017, 81, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Daglioglu, C. Environmentally Responsive Dual-Targeting Nanoparticles: Improving Drug Accumulation in Cancer Cells as a Way of Preventing Anticancer Drug Efflux. J. Pharm. Sci. 2018, 107, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Duo, Y.; Zhai, P.; He, L.; Zhong, K.; Zhang, Y.; Huang, K.; Luo, J.; Zhang, H.; Yu, X. Dual targeting delivery of miR-328 by functionalized mesoporous silica nanoparticles for colorectal cancer therapy. Nanomedicine 2018, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Villaverde, G.; Alfranca, A.; Gonzalez-Murillo, Á.; Melen, G.J.; Castillo, R.R.; Ramírez, M.; Baeza, A.; Vallet-Regí, M. Molecular scaffolds as double-targeting agents for the diagnosis and treatment of neuroblastoma. Angew. Chem. Int. Ed. 2019, 58, 3067–3072. [Google Scholar] [CrossRef]
- Pan, L.; Liu, J.; He, Q.; Shi, J. MSN-mediated sequential vascular-to-cell nuclear-targeted drug delivery for efficient tumor regression. Adv. Mater. 2014, 26, 6742–6748. [Google Scholar] [CrossRef]
- Xiong, L.; Du, X.; Kleitz, F.; Qiao, S.Z. Cancer-cell-specific nuclear-targeted drug delivery by dual-ligand-modified mesoporous silica nanoparticles. Small 2015, 11, 5919–5926. [Google Scholar] [CrossRef]
- Naz, S.; Wang, M.; Han, Y.; Hu, B.; Teng, L.; Zhou, J.; Zhang, H.; Chen, J. Enzyme-responsive mesoporous silica nanoparticles for tumor cells and mitochondria multistage-targeted drug delivery. Int. J. Nanomed. 2019, 14, 2533–2542. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, R.; Wei, Q.; Li, W.; Zhu, G.; Chi, H.; Guo, Z.; Wang, L.; Cui, C.; Xu, J.; et al. Dual-functionalized janus mesoporous silica nanoparticles with active targeting and charge reversal for synergistic tumor-targeting therapy. ACS Appl. Mater. Interfaces 2019, 11, 44582–44592. [Google Scholar] [CrossRef]
- López, V.; Villegas, M.R.; Rodríguez, V.; Villaverde, G.; Lozano, D.; Baeza, A.; Vallet-Regí, M. Janus mesoporous silica nanoparticles for dual targeting of tumor cells and mitochondria. ACS Appl. Mater. Interfaces 2017, 9, 26697–26706. [Google Scholar] [CrossRef]
- Fang, Y.; Xue, J.; Gao, S.; Lu, A.; Yang, D.; Jiang, H.; He, Y.; Shi, K. Cleavable PEGylation: A strategy for overcoming the “PEG dilemma” in efficient drug delivery. Drug Deliv. 2017, 24, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Yang, Z. Benzoic-Imine-Based Physiological-pH-Responsive Materials for Biomedical Applications. Chem. Asian J. 2016, 11, 2633–2641. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Luo, Z.; Zhang, J.; Luo, T.; Zhou, J.; Zhao, X.; Cai, K. Hollow mesoporous silica nanoparticles facilitated drug delivery via cascade pH stimuli in tumor microenvironment for tumor therapy. Biomaterials 2016, 83, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Kuang, Y.; Liu, R.; Chen, Z.; Jiang, B.; Sun, Z.; Chen, X.; Li, C. Dual-pH-sensitive mesoporous silica nanoparticle-based drug delivery system for tumor-triggered intracellular drug release. J. Mater. Sci. 2018, 53, 10653–10665. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, C.; Liu, X.; Ma, R.; Kong, D.; Shi, L. A Multifunctional nanocarrier based on nanogated mesoporous silica for enhanced tumor-specific uptake and intracellular delivery. Macromol. Biosci. 2012, 12, 251–259. [Google Scholar] [CrossRef]
- Xiao, D.; Jia, H.Z.; Zhang, J.; Liu, C.W.; Zhuo, R.X.; Zhang, X.Z. A dual-responsive mesoporous silica nanoparticle for tumor-triggered targeting drug delivery. Small 2014, 10, 591–598. [Google Scholar] [CrossRef]
- Paris, J.L.; Manzano, M.; Cabañas, M.V.; Vallet-Regí, M. Mesoporous silica nanoparticles engineered for ultrasound-induced uptake by cancer cells. Nanoscale 2018, 10, 6402–6408. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J. Mesoporous silica nanoparticle-based intelligent drug delivery system for bienzyme-responsive tumour targeting and controlled release. R. Soc. open Sci. 2018, 5, 170986. [Google Scholar] [CrossRef]
- Zou, Z.; He, X.; He, D.; Wang, K.; Qing, Z.; Yang, X.; Wen, L.; Xiong, J.; Li, L.; Cai, L. Programmed packaging of mesoporous silica nanocarriers for matrix metalloprotease 2-triggered tumor targeting and release. Biomaterials 2015, 58, 35–45. [Google Scholar] [CrossRef]
- Lei, Q.; Qiu, W.-X.; Hu, J.-J.; Cao, P.-X.; Zhu, C.-H.; Cheng, H.; Zhang, X.-Z. Multifunctional mesoporous silica nanoparticles with thermal-responsive gatekeeper for nir light-triggered chemo/photothermal-therapy. Small 2016, 12, 4286–4298. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.-F.; Chen, W.-H.; Zhang, X.-Z.; Zhuo, R.-X.; Zhang, J.; Wang, Y.; Cheng, S.-X.; Luo, G.-F. Multifunctional envelope-type mesoporous silica nanoparticles for tumor-triggered targeting drug delivery. J. Am. Chem. Soc. 2013, 135, 5068–5073. [Google Scholar]
- Smith, S.A.; Selby, L.I.; Johnston, A.P.R.; Such, G.K. The endosomal escape of nanoparticles: Toward more efficient cellular delivery. Bioconjug. Chem. 2019, 30, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Gisbert-Garzarán, M.; Manzano, M.; Vallet-Regí, M. pH-responsive mesoporous silica and carbon nanoparticles for drug delivery. Bioengineering 2017, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Weiss, V.; Argyo, C.; Torrano, A.A.; Strobel, C.; Mackowiak, S.A.; Schmidt, A.; Datz, S.; Gatzenmeier, T.; Hilger, I.; Bräuchle, C.; et al. Dendronized mesoporous silica nanoparticles provide an internal endosomal escape mechanism for successful cytosolic drug release. Microporous Mesoporous Mater. 2016, 227, 242–251. [Google Scholar] [CrossRef]
- Tu, J.; Wang, T.; Shi, W.; Wu, G.; Tian, X.; Wang, Y.; Ge, D.; Ren, L. Multifunctional ZnPc-loaded mesoporous silica nanoparticles for enhancement of photodynamic therapy efficacy by endolysosomal escape. Biomaterials 2012, 33, 7903–7914. [Google Scholar] [CrossRef]
- Rosenholm, J.M.; Peuhu, E.; Eriksson, J.E.; Sahlgren, C.; Lindén, M. Targeted intracellular delivery of hydrophobic agents using mesoporous hybrid silica nanoparticles as carrier systems. Nano Lett. 2009, 9, 3308–3311. [Google Scholar] [CrossRef]
- Benjaminsen, R.V.; Mattebjerg, M.A.; Henriksen, J.R.; Moghimi, S.M.; Andresen, T.L. The possible "proton sponge " effect of polyethylenimine (PEI) does not include change in lysosomal pH. Mol. Ther. 2013, 21, 149–157. [Google Scholar] [CrossRef]
- Shen, J.; Kim, H.-C.; Su, H.; Wang, F.; Wolfram, J.; Kirui, D.; Mai, J.; Mu, C.; Ji, L.-N.; Mao, Z.-W.; et al. Cyclodextrin and polyethylenimine functionalized mesoporous silica nanoparticles for delivery of siRNA cancer therapeutics. Theranostics 2014, 4, 487–497. [Google Scholar] [CrossRef]
- Prabhakar, N.; Zhang, J.; Desai, D.; Casals, E.; Gulin-Sarfraz, T.; Näreoja, T.; Westermarck, J.; Rosenholm, J.M. Stimuli-responsive hybrid nanocarriers developed by controllable integration of hyperbranched PEI with mesoporous silica nanoparticles for sustained intracellular siRNA delivery. Int. J. Nanomedicine 2016, 11, 6591–6608. [Google Scholar] [CrossRef]
- Hom, C.; Lu, J.; Liong, M.; Luo, H.; Li, Z.; Zink, J.I.; Tamanoi, F. Mesoporous silica nanoparticles facilitate delivery of siRNA to shutdown signaling pathways in mammalian cells. Small 2010, 6, 1185–1190. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, P.; Zhang, Y.; Liu, Y.; He, Y.; Zhang, L.; Gao, Y. Enhanced sensitivity of cancer stem cells to chemotherapy using functionalized mesoporous silica nanoparticles. Mol. Pharm. 2016, 13, 2749–2759. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.M.; Symonds, P.; Murray, J.C.; Hunter, A.C.; Debska, G.; Szewczyk, A. A two-stage poly(ethylenimine)-mediated cytotoxicity: Implications for gene transfer/therapy. Mol. Ther. 2005, 11, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, M.-A.; Almeida, P.V.; Mäkilä, E.M.; Kaasalainen, M.H.; Salonen, J.J.; Hirvonen, J.T.; Santos, H.A. Augmented cellular trafficking and endosomal escape of porous silicon nanoparticles via zwitterionic bilayer polymer surface engineering. Biomaterials 2014, 35, 7488–7500. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hong, M. Protonation, tautomerization, and rotameric structure of histidine: A comprehensive study by magic-angle-spinning solid-state NMR. J. Am. Chem. Soc. 2011, 133, 1534–1544. [Google Scholar] [CrossRef]
- Bilalis, P.; Tziveleka, L.-A.; Varlas, S.; Iatrou, H. pH-Sensitive nanogates based on poly(L-histidine) for controlled drug release from mesoporous silica nanoparticles. Polym. Chem. 2016, 7, 1475–1485. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, L.; Tang, C.; Yin, C. Co-delivery of doxorubicin and survivin shRNA-expressing plasmid via microenvironment-responsive dendritic mesoporous silica nanoparticles for synergistic cancer therapy. Pharm. Res. 2017, 34, 2829–2841. [Google Scholar] [CrossRef]
- Ashley, C.E.; Carnes, E.C.; Phillips, G.K.; Padilla, D.; Durfee, P.N.; Brown, P.A.; Hanna, T.N.; Liu, J.; Phillips, B.; Carter, M.B.; et al. The targeted delivery of multicomponent cargos to cancer cells by nanoporous particle-supported lipid bilayers. Nat. Mater. 2011, 10, 389–397. [Google Scholar] [CrossRef]
- Ashley, C.E.; Carnes, E.C.; Epler, K.E.; Padilla, D.P.; Phillips, G.K.; Castillo, R.E.; Wilkinson, D.C.; Wilkinson, B.S.; Burgard, C.A.; Kalinich, R.M.; et al. Delivery of small interfering RNA by peptide-targeted mesoporous silica nanoparticle-supported lipid bilayers. ACS Nano 2012, 6, 2174–2188. [Google Scholar] [CrossRef]
- Sun, X.; Luo, Y.; Huang, L.; Yu, B.Y.; Tian, J. A peptide-decorated and curcumin-loaded mesoporous silica nanomedicine for effectively overcoming multidrug resistance in cancer cells. RSC Adv. 2017, 7, 16401–16409. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, D.; Li, M.F.; Feng, J. Multifunctional mesoporous silica nanoparticles based on charge-reversal plug-gate nanovalves and acid-decomposable ZnO quantum dots for intracellular drug delivery. ACS Appl. Mater. Interfaces 2015, 7, 26666–26673. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-Y.; Liu, Y.; Hu, J.-J.; Xu, Q.; Liu, L.-H.; Jia, H.-Z.; Chen, W.-H.; Lei, Q.; Rong, L.; Zhang, X.-Z. Stepwise-acid-active multifunctional mesoporous silica nanoparticles for tumor-specific nucleus-targeted drug delivery. ACS Appl. Mater. Interfaces 2014, 6, 14568–14575. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Han, S.; Lee, J.; Choi, M.; Kim, C. Stimuli-responsive α-helical peptide gatekeepers for mesoporous silica nanocarriers. New J. Chem. 2017, 41, 6969–6972. [Google Scholar] [CrossRef]
- Qu, Q.; Ma, X.; Zhao, Y. Anticancer effect of α-tocopheryl succinate delivered by mitochondria-targeted mesoporous silica nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 34261–34269. [Google Scholar] [CrossRef] [PubMed]
- Qu, Q.; Ma, X.; Zhao, Y. Targeted delivery of doxorubicin to mitochondria using mesoporous silica nanoparticle nanocarriers. Nanoscale 2015, 7, 16677–16686. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.K.; Kumar, S.; Choi, E.-H.; Chaudhary, S.; Kim, M.-H. Molecular dynamic simulations of oxidized skin lipid bilayer and permeability of reactive oxygen species. Sci. Rep. 2019, 9, 4496. [Google Scholar] [CrossRef] [PubMed]
- Dobay, M.P.; Schmidt, A.; Mendoza, E.; Bein, T.; Rädler, J.O. Cell type determines the light-induced endosomal escape kinetics of multifunctional mesoporous silica nanoparticles. Nano Lett. 2013, 13, 1047–1052. [Google Scholar] [CrossRef]
- Schloßbauer, A.; Sauer, A.M.; Cauda, V.; Schmidt, A.; Engelke, H.; Rothbauer, U.; Zolghadr, K.; Leonhardt, H.; Bräuchle, C.; Bein, T. Cascaded photoinduced drug delivery to cells from multifunctional core–shell mesoporous silica. Adv. Healthc. Mater. 2012, 1, 316–320. [Google Scholar] [CrossRef]
- Martínez-Carmona, M.; Lozano, D.; Baeza, A.; Colilla, M.; Vallet-Regí, M. A novel visible light responsive nanosystem for cancer treatment. Nanoscale 2017, 9, 15967–15973. [Google Scholar] [CrossRef]
- Mackowiak, S.A.; Schmidt, A.; Weiss, V.; Argyo, C.; von Schirnding, C.; Bein, T.; Bräuchle, C. Targeted drug delivery in cancer cells with red-light photoactivated mesoporous silica nanoparticles. Nano Lett. 2013, 13, 2576–2583. [Google Scholar] [CrossRef]
- Niedermayer, S.; Weiss, V.; Herrmann, A.; Schmidt, A.; Datz, S.; Müller, K.; Wagner, E.; Bein, T.; Bräuchle, C. Multifunctional polymer-capped mesoporous silica nanoparticles for pH-responsive targeted drug delivery. Nanoscale 2015, 7, 7953–7964. [Google Scholar] [CrossRef]
- Hai, L.; Jia, X.; He, D.; Zhang, A.; Wang, T.; Cheng, H.; He, X.; Wang, K. DNA-functionalized hollow mesoporous silica nanoparticles with dual cargo loading for near-infrared-responsive synergistic chemo-photothermal treatment of cancer cells. ACS Appl. Nano Mater. 2018, 1, 3486–3497. [Google Scholar] [CrossRef]
- Horcajada, P.; Rámila, A.; Férey, G.; Vallet-Regí, M. Influence of superficial organic modification of MCM-41 matrices on drug delivery rate. Solid State Sci. 2006, 8, 1243–1249. [Google Scholar] [CrossRef]
- Balas, F.; Manzano, M.; Horcajada, P.; Vallet-Regí, M. Confinement and controlled release of bisphosphonates on ordered mesoporous silica-based materials. J. Am. Chem. Soc. 2006, 128, 8116–8117. [Google Scholar] [CrossRef] [PubMed]
- Nieto, A.; Balas, F.; Manzano, M.; Vallet-Regí, M. Functionalization degree of SBA-15 as key factor to modulate sodium alendronate dosage. Microporous Mesoporous Mater. 2008, 116, 4–13. [Google Scholar] [CrossRef]
- Doadrio, J.C.; Sousa, E.M.B.; Izquierdo-Barba, I.; Doadrio, A.L.; Perez-Pariente, J.; Vallet-Regí, M. Functionalization of mesoporous materials with long alkyl chains as a strategy for controlling drug delivery pattern. J. Mater. Chem. 2006, 16, 462–466. [Google Scholar] [CrossRef]
- López-Noriega, A.; Arcos, D.; Vallet-Regí, M. Functionalizing mesoporous bioglasses for long-term anti-osteoporotic drug delivery. Chem. A Eur. J. 2010, 16, 10879–10886. [Google Scholar] [CrossRef]
- Varache, M.; Bezverkhyy, I.; Weber, G.; Saviot, L.; Chassagnon, R.; Baras, F.; Bouyer, F. Loading of cisplatin into mesoporous silica nanoparticles: Effect of surface functionalization. Langmuir 2019, 35, 8984–8995. [Google Scholar] [CrossRef]
- Wani, A.; Muthuswamy, E.; Savithra, G.H.L.; Mao, G.; Brock, S.; Oupický, D. Surface functionalization of mesoporous silica nanoparticles controls loading and release behavior of mitoxantrone. Pharm. Res. 2012, 29, 2407–2418. [Google Scholar] [CrossRef]
- Chang, B.; Guo, J.; Liu, C.; Qian, J.; Yang, W. Surface functionalization of magnetic mesoporous silica nanoparticles for controlled drug release. J. Mater. Chem. 2010, 20, 9941–9947. [Google Scholar] [CrossRef]
- She, X.; Chen, L.; Li, C.; He, C.; He, L.; Kong, L. Functionalization of hollow mesoporous silica nanoparticles for improved 5-FU loading. J. Nanomater. 2015, 2015, 872035. [Google Scholar] [CrossRef]
- Bahrami, Z.; Badiei, A.; Atyabi, F.; Darabi, H.R.; Mehravi, B. Piperazine and its carboxylic acid derivatives-functionalized mesoporous silica as nanocarriers for gemcitabine: Adsorption and release study. Mater. Sci. Eng. C 2015, 49, 66–74. [Google Scholar] [CrossRef]
- Aggad, D.; Jimenez, C.M.; Dib, S.; Croissant, J.G.; Lichon, L.; Laurencin, D.; Richeter, S.; Maynadier, M.; Alsaiari, S.K.; Boufatit, M.; et al. Gemcitabine delivery and photodynamic therapy in cancer cells via porphyrin-ethylene-based periodic mesoporous organosilica nanoparticles. ChemNanoMat 2018, 4, 46–51. [Google Scholar] [CrossRef]
- Croissant, J.; Cattoën, X.; Man, M.W.C.; Gallud, A.; Raehm, L.; Trens, P.; Maynadier, M.; Durand, J.-O. Biodegradable ethylene-bis(propyl)disulfide-based periodic mesoporous organosilica nanorods and nanospheres for efficient in-vitro drug delivery. Adv. Mater. 2014, 26, 6174–6180. [Google Scholar] [CrossRef]
- Croissant, J.G.; Fatieiev, Y.; Julfakyan, K.; Lu, J.; Emwas, A.-H.; Anjum, D.H.; Omar, H.; Tamanoi, F.; Zink, J.I.; Khashab, N.M. Biodegradable oxamide-phenylene-based mesoporous organosilica nanoparticles with unprecedented drug payloads for delivery in cells. Chem. Eur. J. 2016, 22, 14806–14811. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Kato, Y.; Ozawa, S.; Miyamoto, C.; Maehata, Y.; Suzuki, A.; Maeda, T.; Baba, Y. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 2013, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Casey, J.R.; Grinstein, S.; Orlowski, J. Sensors and regulators of intracellular pH. Nat. Rev. Mol. Cell Biol. 2010, 11, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Lin, H.; Yang, C.; Han, X.; Zhang, T.; Qu, F. Synthesis of multifunctional Fe3O4@mSiO2@Au core-shell nanocomposites for pH-responsive drug delivery. Eur. J. Inorg. Chem. 2014, 2014, 6156–6164. [Google Scholar] [CrossRef]
- Chen, S.; Yang, Y.; Li, H.; Zhou, X.; Liu, M. pH-Triggered Au-fluorescent mesoporous silica nanoparticles for 19F MR/fluorescent multimodal cancer cellular imaging. Chem. Commun. 2014, 50, 283–285. [Google Scholar] [CrossRef]
- Dai, L.; Zhang, Q.; Shen, X.; Sun, Q.; Mu, C.; Gu, H.; Cai, K. pH-responsive nanocontainer based on hydrazone-bearing hollow silica nanoparticles for targeting tumor therapy. J. Mater. Chem. B 2016, 4, 4594–4604. [Google Scholar] [CrossRef]
- Yuan, X.; Peng, S.; Lin, W.; Wang, J.; Zhang, L. Multistage pH-responsive mesoporous silica nanohybrids with charge reversal and intracellular release for efficient anticancer drug delivery. J. Colloid Interface Sci. 2019, 555, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhang, Y.; Zhao, X.; Agarwal, A.; Mueller, L.J.; Feng, P. pH-responsive nanogated ensemble based on gold-capped mesoporous silica through an acid-labile acetal linker. J. Am. Chem. Soc. 2010, 132, 1500–1501. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ai, K.; Liu, J.; Sun, G.; Yin, Q.; Lu, L. Multifunctional envelope-type mesoporous silica nanoparticles for pH-responsive drug delivery and magnetic resonance imaging. Biomaterials 2015, 60, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Yu, H.; Yang, N.; Wang, M.; Ding, C.; Fu, J. Graphene quantum dot-capped mesoporous silica nanoparticles through an acid-cleavable acetal bond for intracellular drug delivery and imaging. J. Mater. Chem. B 2014, 2, 4979–4982. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; He, X.; Wang, K.; He, D.; Yang, S.; Qiu, P.; Chen, S. A pH-responsive polymer/mesoporous silica nano-container linked through an acid cleavable linker for intracellular controlled release and tumor therapy in vivo. J. Mater. Chem. B 2014, 2, 428–436. [Google Scholar] [CrossRef]
- Yang, K.; Luo, H.; Zeng, M.; Jiang, Y.; Li, J.; Fu, X. Intracellular pH-triggered, targeted drug delivery to cancer cells by multifunctional envelope-type mesoporous silica nanocontainers. ACS Appl. Mater. Interfaces 2015, 7, 17399–17447. [Google Scholar] [CrossRef]
- Bull, S.D.; Davidson, M.G.; Van Den Elsen, J.M.H.; Fossey, J.S.; Jenkins, A.T.A.; Jiang, Y.B.; Kubo, Y.; Marken, F.; Sakurai, K.; Zhao, J.; et al. Exploiting the reversible covalent bonding of boronic acids: Recognition, sensing, and assembly. Acc. Chem. Res. 2013, 46, 312–326. [Google Scholar] [CrossRef]
- Aznar, E.; Marcos, M.D.; Martínez-Máñez, R.; Sancenón, F.; Soto, J.; Amorós, P.; Guillem, C. pH- and photo-switched release of guest molecules from mesoporous silica supports. J. Am. Chem. Soc. 2009, 131, 6833–6843. [Google Scholar] [CrossRef]
- Gan, Q.; Lu, X.; Yuan, Y.; Qian, J.; Zhou, H.; Lu, X.; Shi, J.; Liu, C. A magnetic, reversible pH-responsive nanogated ensemble based on Fe3O4 nanoparticles-capped mesoporous silica. Biomaterials 2011, 32, 1932–1942. [Google Scholar] [CrossRef]
- Salinas, Y.; Hoerhager, C.; García-Fernández, A.; Resmini, M.; Sancenón, F.; Matínez-Máñez, R.; Brueggemann, O. Biocompatible phenylboronic-acid-capped ZnS nanocrystals designed As caps in mesoporous silica hybrid materials for on-demand pH-triggered release in cancer cells. ACS Appl. Mater. Interfaces 2018, 10, 34029–34038. [Google Scholar] [CrossRef]
- Luo, Z.; Cai, K.; Hu, Y.; Zhang, B.; Xu, D. Cell-specific intracellular anticancer drug delivery from mesoporous silica nanoparticles with pH sensitivity. Adv. Healthc. Mater. 2012, 1, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, H.; Leng, F.; Zheng, L.; Yang, J.; Wang, W.; Huang, C.Z. Autofluorescent and pH-responsive mesoporous silica for cancer-targeted and controlled drug release. Microporous Mesoporous Mater. 2014, 186, 187–193. [Google Scholar] [CrossRef]
- Chen, H.; Zheng, D.; Liu, J.; Kuang, Y.; Li, Q.; Zhang, M.; Ye, H.; Qin, H.; Xu, Y.; Li, C.; et al. pH-Sensitive drug delivery system based on modified dextrin coated mesoporous silica nanoparticles. Int. J. Biol. Macromol. 2016, 85, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.; Lan, J.; Zhang, Y.; Wu, Z.L.; Li, C.M.; Wang, J.; Huang, C.Z. Reduced graphene oxide gated mesoporous silica nanoparticles as a versatile chemo-photothermal therapy system through pH controllable release. J. Mater. Chem. B 2015, 3, 6377–6384. [Google Scholar] [CrossRef]
- Muhammad, F.; Guo, M.; Qi, W.; Sun, F.; Wang, A.; Guo, Y.; Zhu, G. pH-triggered controlled drug release from mesoporous silica nanoparticles via intracellular dissolution of ZnO nanolids. J. Am. Chem. Soc. 2011, 133, 8778–8781. [Google Scholar] [CrossRef]
- Wu, S.; Huang, X.; Du, X. pH- and redox-triggered synergistic controlled release of a ZnO-gated hollow mesoporous silica drug delivery system. J. Mater. Chem. B 2015, 3, 1426–1432. [Google Scholar] [CrossRef]
- Muhammad, F.; Wang, A.; Guo, M.; Zhao, J.; Qi, W.; Yingjie, G.; Gu, J.; Zhu, G. PH dictates the release of hydrophobic drug cocktail from mesoporous nanoarchitecture. ACS Appl. Mater. Interfaces 2013, 5, 11828–11835. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Liu, L.; You, X.; Zhang, C.; Wang, Y. A pH-sensitive nanocarrier for co-delivery of doxorubicin and camptothecin to enhance chemotherapeutic efficacy and overcome multidrug resistance in vitro. RSC Adv. 2015, 5, 77097–77105. [Google Scholar] [CrossRef]
- Chen, Y.; Yin, Q.; Ji, X.; Zhang, S.; Chen, H.; Zheng, Y.; Sun, Y.; Qu, H.; Wang, Z.; Li, Y.; et al. Manganese oxide-based multifunctionalized mesoporous silica nanoparticles for pH-responsive MRI, ultrasonography and circumvention of MDR in cancer cells. Biomaterials 2012, 33, 7126–7137. [Google Scholar] [CrossRef]
- Zhang, S.; Qian, X.; Zhang, L.; Peng, W.; Chen, Y. Composition-property relationships in multifunctional hollow mesoporous carbon nanosystems for PH-responsive magnetic resonance imaging and on-demand drug release. Nanoscale 2015, 7, 7632–7643. [Google Scholar] [CrossRef]
- Jin, L.; Huang, Q.-J.; Zeng, H.-Y.; Du, J.-Z.; Xu, S.; Chen, C.-R. Hydrotalcite-gated hollow mesoporous silica delivery system for controlled drug release. Microporous Mesoporous Mater. 2019, 274, 304–312. [Google Scholar] [CrossRef]
- Shao, M.; Chang, C.; Liu, Z.; Chen, K.; Zhou, Y.; Zheng, G.; Huang, Z.; Xu, H.; Xu, P.; Lu, B. Polydopamine coated hollow mesoporous silica nanoparticles as pH-sensitive nanocarriers for overcoming multidrug resistance. Colloids Surfaces B Biointerfaces 2019, 183, 110427. [Google Scholar] [CrossRef] [PubMed]
- Gisbert-Garzarán, M.; Manzano, M.; Vallet-Regí, M. Self-immolative chemistry in nanomedicine. Chem. Eng. J. 2017, 340, 24–31. [Google Scholar] [CrossRef]
- Juárez, L.A.; Añón, E.; Giménez, C.; Sancenón, F.; Martínez-Máñez, R.; Costero, A.M.; Gaviña, P.; Parra, M.; Bernardos, A. Self-immolative linkers as caps for the design of gated silica mesoporous supports. Chem. Eur. J. 2016, 22, 14126–14130. [Google Scholar] [CrossRef] [PubMed]
- Gisbert-Garzarán, M.; Lozano, D.; Vallet-Regí, M.; Manzano, M. Self-Immolative Polymers as novel pH-responsive gate keepers for drug delivery. RSC Adv. 2017, 7, 132–136. [Google Scholar] [CrossRef]
- Gisbert-Garzarán, M.; Berkmann, J.C.; Giasafaki, D.; Lozano, D.; Spyrou, K.; Manzano, M.; Steriotis, T.; Duda, G.N.; Schmidt-Bleek, K.; Charalambopoulou, G.; et al. Engineered pH-responsive mesoporous carbon nanoparticles for drug delivery. ACS Appl. Mater. Interfaces 2020, 12, 14946–14957. [Google Scholar] [CrossRef]
- Birault, A.; Giret, S.; Théron, C.; Gallud, A.; Da Silva, A.; Durand, D.; Nguyen, C.; Bettache, N.; Gary-Bobo, M.; Bartlett, J.R.; et al. Sequential delivery of synergistic drugs by silica nanocarriers for enhanced tumour treatment. J. Mater. Chem. B 2020, 8, 1472–1480. [Google Scholar] [CrossRef]
- Meng, H.; Xue, M.; Xia, T.; Zhao, Y.L.; Tamanoi, F.; Stoddart, J.F.; Zink, J.I.; Nel, A.E. Autonomous in vitro anticancer drug release from mesoporous silica nanoparticles by pH-sensitive nanovalves. J. Am. Chem. Soc. 2010, 132, 12690–12697. [Google Scholar] [CrossRef]
- Li, Z.; Clemens, D.L.; Lee, B.Y.; Dillon, B.J.; Horwitz, M.A.; Zink, J.I. Mesoporous silica nanoparticles with pH-sensitive nanovalves for delivery of moxifloxacin provide improved treatment of lethal pneumonic tularemia. ACS Nano 2015, 9, 10778–10789. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Tehrani, Z.M. Poly(N-isopropylacrylamide)-coated β-cyclodextrin–capped magnetic mesoporous silica nanoparticles exhibiting thermal and pH dual response for triggered anticancer drug delivery. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 336–348. [Google Scholar] [CrossRef]
- Théron, C.; Gallud, A.; Carcel, C.; Gary-Bobo, M.; Maynadier, M.; García, M.; Lu, J.; Tamanoi, F.; Zink, J.I.; Wong Chi Man, M. Hybrid mesoporous silica nanoparticles with pH-operated and complementary H-bonding caps as an autonomous drug-delivery system. Chem. Eur. J. 2014, 20, 9372–9380. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.T.; Hong, C.Y.; Pan, C.Y. Fabrication of PDEAEMA-coated mesoporous silica nanoparticles and pH-responsive controlled release. J. Phys. Chem. C 2010, 114, 12481–12486. [Google Scholar] [CrossRef]
- Yu, F.; Tang, X.; Pei, M. Facile synthesis of PDMAEMA-coated hollow mesoporous silica nanoparticles and their pH-responsive controlled release. Microporous Mesoporous Mater. 2013, 173, 64–69. [Google Scholar] [CrossRef]
- Gao, Q.; Xu, Y.; Wu, D.; Shen, W.; Deng, F. Synthesis, characterization, and in vitro pH-controllable drug release from mesoporous silica spheres with switchable gates. Langmuir 2010, 26, 17133–17138. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lü, S.; Gao, C.; Wang, X.; Bai, X.; Gao, N.; Liu, M. Facile preparation of pH-sensitive and self-fluorescent mesoporous silica nanoparticles modified with PAMAM dendrimers for label-free imaging and drug delivery. Chem. Eng. J. 2015, 266, 171–178. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Tehrani, Z.M.; Moghanaki, A.A. Folate-Conjugated pH-Responsive Nanocarrier Designed for Active Tumor Targeting and Controlled Release of Gemcitabine. Pharm. Res. 2015, 417–432. [Google Scholar] [CrossRef]
- Liu, R.; Liao, P.; Liu, J.; Feng, P. Responsive polymer-coated mesoporous silica as a pH-sensitive nanocarrier for controlled release. Langmuir 2011, 27, 3095–3099. [Google Scholar] [CrossRef]
- Rafi, A.A.; Mahkam, M.; Davaran, S.; Hamishehkar, H. A Smart pH-responsive Nano-Carrier as a Drug Delivery System: A hybrid system comprised of mesoporous nanosilica MCM-41 (as a nano-container) & a pH-sensitive polymer (as smart reversible gatekeepers): Preparation, characterization and in vitro release. Eur. J. Pharm. Sci. 2016, 93, 64–73. [Google Scholar]
- Peng, H.; Dong, R.; Wang, S.; Zhang, Z.; Luo, M.; Bai, C.; Zhao, Q.; Li, J.; Chen, L.; Xiong, H. A pH-responsive nano-carrier with mesoporous silica nanoparticles cores and poly(acrylic acid) shell-layers: Fabrication, characterization and properties for controlled release of salidroside. Int. J. Pharm. 2013, 446, 153–159. [Google Scholar] [CrossRef]
- Samart, C.; Prawingwong, P.; Amnuaypanich, S.; Zhang, H.; Kajiyoshi, K.; Reubroycharoen, P. Preparation of poly(Acrylic Acid) grafted-mesoporous silica as pH responsive releasing material. J. Ind. Eng. Chem. 2014, 20, 2153–2158. [Google Scholar] [CrossRef]
- Hong, C.-Y.; Li, X.; Pan, C.-Y. Fabrication of smart nanocontainers with a mesoporous core and a pH-responsive shell for controlled uptake and release. J. Mater. Chem. 2009, 19, 5155–5160. [Google Scholar] [CrossRef]
- Choi, J.Y.; Gupta, B.; Ramasamy, T.; Jeong, J.-H.; Jin, S.G.; Choi, H.-G.; Yong, C.S.; Kim, J.O. PEGylated polyaminoacid-capped mesoporous silica nanoparticles for mitochondria-targeted delivery of celastrol in solid tumors. Colloids Surfaces B Biointerfaces 2018, 165, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.T.H.; Webb, R.I.; Lambert, L.K.; Strounina, E.; Lee, E.C.; Parat, M.-O.; McGuckin, M.A.; Popat, A.; Cabot, P.J.; Ross, B.P. Bifunctional succinylated ε-polylysine-coated mesoporous silica nanoparticles for pH-responsive and intracellular drug delivery targeting the colon. ACS Appl. Mater. Interfaces 2017, 9, 9470–9483. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Di, J.; Cao, C.; Zhao, Y.; Ma, Y.; Luo, J.; Wen, Y.; Song, W.; Song, Y.; Jiang, L. A pH-driven DNA nanoswitch for responsive controlled release. Chem. Commun. 2011, 47, 2850–2852. [Google Scholar] [CrossRef]
- Chen, M.; Yang, S.; He, X.; Wang, K.; Qiu, P.; He, D. Co-loading of coralyne and indocyanine green into adenine DNA-functionalized mesoporous silica nanoparticles for pH- and near-infrared-responsive chemothermal treatment of cancer cells. J. Mater. Chem. B 2014, 2, 6064–6071. [Google Scholar] [CrossRef] [PubMed]
- He, D.G.; He, X.X.; Wang, K.M.; Chen, M.A.; Zhao, Y.X.; Zou, Z. Intracellular acid-triggered drug delivery system using mesoporous silica nanoparticles capped with T-Hg2+-T base pairs mediated duplex DNA. J. Mater. Chem. B 2013, 1, 1552–1560. [Google Scholar] [CrossRef] [PubMed]
- Murai, K.; Higuchi, M.; Kinoshita, T.; Nagata, K.; Kato, K. Design of a nanocarrier with regulated drug release ability utilizing a reversible conformational transition of a peptide, responsive to slight changes in pH. Phys. Chem. Chem. Phys. 2013, 15, 11454–11460. [Google Scholar] [CrossRef]
- Chen, C.; Pu, F.; Huang, Z.; Liu, Z.; Ren, J.; Qu, X. Stimuli-responsive controlled-release system using quadruplex DNA-capped silica nanocontainers. Nucleic Acids Res. 2011, 39, 1638–1644. [Google Scholar] [CrossRef]
- Xue, M.; Findenegg, G.H. Lysozyme as a pH-responsive valve for the controlled release of guest molecules from mesoporous silica. Langmuir 2012, 28, 17578–17584. [Google Scholar] [CrossRef]
- Mishra, A.K.; Pandey, H.; Agarwal, V.; Ramteke, P.W.; Pandey, A.C. Nanoengineered mesoporous silica nanoparticles for smart delivery of doxorubicin. J. Nanoparticle Res. 2014, 16, 2515. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Tehrani, Z.M.; Bennett, C. PEG-co-polyvinyl pyridine coated magnetic mesoporous silica nanoparticles for pH-responsive controlled release of doxorubicin. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 570–577. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Tehrani, Z.M. Mesoporous silica nanoparticles with bilayer coating of poly(acrylic acid-co-itaconic acid) and human serum albumin (HSA): A pH-sensitive carrier for gemcitabine delivery. Mater. Sci. Eng. C 2016, 61, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Pourjavadi, A.; Mazaheri Tehrani, Z.; Jokar, S. Chitosan based supramolecular polypseudorotaxane as a pH-responsive polymer and their hybridization with mesoporous silica-coated magnetic graphene oxide for triggered anticancer drug delivery. Polym. (United Kingdom) 2015, 76, 52–61. [Google Scholar] [CrossRef]
- Liu, W.T.; Yang, Y.; Shen, P.H.; Gao, X.J.; He, S.Q.; Liu, H.; Zhu, C.S. Facile and simple preparation of pH-sensitive chitosan-mesoporous silica nanoparticles for future breast cancer treatment. Express Polym. Lett. 2015, 9, 1068–1075. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Tehrani, Z.M. Mesoporous silica nanoparticles (MCM-41) coated PEGylated chitosan as a pH-responsive nanocarrier for triggered release of erythromycin. Int. J. Polym. Mater. Polym. Biomater. 2014, 63, 692–697. [Google Scholar] [CrossRef]
- Chen, F.; Zhu, Y. Chitosan enclosed mesoporous silica nanoparticles as drug nano-carriers: Sensitive response to the narrow pH range. Microporous Mesoporous Mater. 2012, 150, 83–89. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, Y.L.; Wang, L.; Ma, J.; Yang, Y.W.; Gao, H. Nanoassembles constructed from mesoporous silica nanoparticles and surface-coated multilayer polyelectrolytes for controlled drug delivery. Microporous Mesoporous Mater. 2014, 185, 245–253. [Google Scholar] [CrossRef]
- Wan, X.; Zhang, G.; Liu, S. PH-disintegrable polyelectrolyte multilayer-coated mesoporous silica nanoparticles exhibiting triggered co-release of cisplatin and model drug molecules. Macromol. Rapid Commun. 2011, 32, 1082–1089. [Google Scholar] [CrossRef]
- Du, P.; Zhao, X.; Zeng, J.; Guo, J.; Liu, P. Layer-by-layer engineering fluorescent polyelectrolyte coated mesoporous silica nanoparticles as pH-sensitive nanocarriers for controlled release. Appl. Surf. Sci. 2015, 345, 90–98. [Google Scholar] [CrossRef]
- Feng, W.; Nie, W.; He, C.; Zhou, X.; Chen, L.; Qiu, K.; Wang, W.; Yin, Z. Effect of pH-responsive alginate/chitosan multilayers coating on delivery efficiency, cellular uptake and biodistribution of mesoporous silica nanoparticles based nanocarriers. ACS Appl. Mater. Interfaces 2014, 6, 8447–8460. [Google Scholar] [CrossRef]
- Traverso, N.; Ricciarelli, R.; Nitti, M.; Marengo, B.; Furfaro, A.L.; Pronzato, M.A.; Marinari, U.M.; Domenicotti, C. Role of glutathione in cancer progression and chemoresistance. Oxid. Med. Cell. Longev. 2013, 972913. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Feng, F.; Meng, F.; Deng, C.; Feijen, J.; Zhong, Z. Glutathione-responsive nano-vehicles as a promising platform for targeted intracellular drug and gene delivery. J. Control. Release 2011, 152, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Estrela, J.M.; Ortega, A.; Obrador, E. Glutathione in cancer biology and therapy. Crit. Rev. Clin. Lab. Sci. 2006, 43, 143–181. [Google Scholar] [CrossRef] [PubMed]
- Balendiran, G.K.; Dabur, R.; Fraser, D. The role of glutathione in cancer. Cell Biochem. Funct. 2004, 22, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Cheng, Y.; Zhao, X.; Luo, Y.; Chen, J.; Yuan, W.E. Advances in redox-responsive drug delivery systems of tumor microenvironment. J. Nanobiotechnol. 2018, 16, 74–83. [Google Scholar] [CrossRef]
- Maggini, L.; Cabrera, I.; Ruiz-Carretero, A.; Prasetyanto, E.A.; Robinet, E.; De Cola, L. Breakable mesoporous silica nanoparticles for targeted drug delivery. Nanoscale 2016, 8, 7240–7247. [Google Scholar] [CrossRef] [PubMed]
- Mai, N.X.D.; Birault, A.; Matsumoto, K.; Ta, H.K.T.; Intasa-ard, S.G.; Morrison, K.; Thang, P.B.; Doan, T.L.H.; Tamanoi, F. Biodegradable periodic mesoporous organosilica (BPMO) loaded with daunorubicin: A promising nanoparticle-based anticancer drug. ChemMedChem 2020, 15, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhou, X.; Nie, W.; Zhang, Q.; Wang, W.; Zhang, Y.; He, C. Multifunctional redox-responsive mesoporous silica nanoparticles for efficient targeting drug delivery and magnetic resonance imaging. ACS Appl. Mater. Interfaces 2016, 8, 33829–33841. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Luo, Z.; Liu, J.; Ding, X.; Li, J.; Cai, K. Cytochrome c end-capped mesoporous silica nanoparticles as redox-responsive drug delivery vehicles for liver tumor-targeted triplex therapy in vitro and in vivo. J. Control. Release 2014, 192, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wang, S.; Yang, Y.; Li, X.; Di, D.; Zhang, C.; Jiang, T.; Wang, S. Hyaluronic acid and carbon dots-gated hollow mesoporous silica for redox and enzyme-triggered targeted drug delivery and bioimaging. Mater. Sci. Eng. C 2017, 78, 475–484. [Google Scholar] [CrossRef]
- Jiao, J.; Liu, C.; Li, X.; Liu, J.; Di, D.; Zhang, Y.; Zhao, Q.; Wang, S. Fluorescent carbon dot modified mesoporous silica nanocarriers for redox-responsive controlled drug delivery and bioimaging. J. Colloid Interface Sci. 2016, 483, 343–352. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, Y.; Di, D.; Zhang, X.; Wang, D.; Zhao, Q.; Wang, S. Gold nanoparticle-gated mesoporous silica as redox-triggered drug delivery for chemo-photothermal synergistic therapy. J. Colloid Interface Sci. 2017, 508, 323–331. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, C.; Bai, J.; Wu, C.; Xiao, Y.; Li, Y.; Zheng, J.; Yang, R.; Tan, W. Silver nanoparticle gated, mesoporous silica coated gold nanorods (AuNR@MS@AgNPs): Low premature release and multifunctional cancer theranostic platform. ACS Appl. Mater. Interfaces 2015, 7, 6211–6219. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, F.; Wang, A.; Qi, W.; Zhang, S.; Zhu, G. Intracellular antioxidants dissolve man-made antioxidant nanoparticles: Using redox vulnerability of nanoceria to develop a responsive drug delivery system. ACS Appl. Mater. Interfaces 2014, 6, 19424–19433. [Google Scholar] [CrossRef]
- Kim, H.; Kim, S.; Park, C.; Lee, H.; Park, H.J.; Kim, C. Glutathione-induced intracellular release of guests from mesoporous silica nanocontainers with cyclodextrin gatekeepers. Adv. Mater. 2010, 22, 4280–4283. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, F.; Nguyen, K.T.; Ma, X.; Wang, X.; Xing, B.; Zhao, Y. Multifunctional mesoporous silica nanoparticles for cancer-targeted and controlled drug delivery. Adv. Funct. Mater. 2012, 22, 5144–5156. [Google Scholar] [CrossRef]
- Nguyen Thi, T.T.; Tran, T.V.; Tran, N.Q.; Nguyen, C.K.; Nguyen, D.H. Hierarchical self-assembly of heparin-PEG end-capped porous silica as a redox sensitive nanocarrier for doxorubicin delivery. Mater. Sci. Eng. C 2017, 70, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Ding, X.; Hu, Y.; Wu, S.; Xiang, Y.; Zeng, Y.; Zhang, B.; Yan, H.; Zhang, H.; Zhu, L.; et al. Engineering a Hollow Nanocontainer Platform with Multifunctional Molecular Machines for Tumor-Targeted Therapy in Vitro and in Vivo. ACS Nano 2013, 7, 10271–10284. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, X.; Yuan, Y.; Li, Q.; Chang, B.; Xu, L.; Cai, B.; Qi, C.; Li, C.; Jiang, X.; et al. Supramolecular modular approach toward conveniently constructing and multifunctioning a pH/redox dual-responsive drug delivery nanoplatform for improved cancer chemotherapy. ACS Appl. Mater. Interfaces 2018, 10, 26473–26484. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-T.; Liu, Z.-K.; Zhu, Q.-L.; Rong, X.-H.; Liang, C.-L.; Wang, J.; Ma, D.; Sun, J.; Wang, G.-H. Redox-responsive nanocarriers for drug and gene co-delivery based on chitosan derivatives modified mesoporous silica nanoparticles. Colloids Surfaces B Biointerfaces 2017, 155, 41–50. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, L.; Nie, W.; Wang, W.; Qin, M.; Mo, X.; Wang, H.; He, C. Dual-Responsive Mesoporous Silica Nanoparticles Mediated Codelivery of Doxorubicin and Bcl-2 SiRNA for Targeted Treatment of Breast Cancer. J. Phys. Chem. C 2016, 120, 22375–22387. [Google Scholar] [CrossRef]
- Palanikumar, L.; Kim, J.; Oh, J.Y.; Choi, H.; Park, M.-H.; Kim, C.; Ryu, J.-H. Hyaluronic Acid-Modified Polymeric Gatekeepers on Biodegradable Mesoporous Silica Nanoparticles for Targeted Cancer Therapy. ACS Biomater. Sci. Eng. 2018, 4, 1716–1722. [Google Scholar] [CrossRef]
- Li, H.; Zhang, J.Z.; Tang, Q.; Du, M.; Hu, J.; Yang, D. Reduction-responsive drug delivery based on mesoporous silica nanoparticle core with crosslinked poly(acrylic acid) shell. Mater. Sci. Eng. C 2013, 33, 3426–3431. [Google Scholar] [CrossRef]
- Li, Q.L.; Xu, S.H.; Zhou, H.; Wang, X.; Dong, B.; Gao, H.; Tang, J.; Yang, Y.W. PH and Glutathione Dual-Responsive Dynamic Cross-Linked Supramolecular Network on Mesoporous Silica Nanoparticles for Controlled Anticancer Drug Release. ACS Appl. Mater. Interfaces 2015, 7, 28656–28664. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Guo, W.; Cui, L.; An, N.; Zhang, T.; Guo, G.; Lin, H.; Qu, F. Fe3O4@mSiO2 core–shell nanocomposite capped with disulfide gatekeepers for enzyme-sensitive controlled release of anti-cancer drugs. J. Mater. Chem. B 2015, 3, 1010–1019. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-Y.; Hu, J.-J.; Xu, Q.; Chen, S.; Jia, H.-Z.; Sun, Y.-X.; Zhuo, R.-X.; Zhang, X.-Z. A redox-responsive drug delivery system based on RGD containing peptide-capped mesoporous silica nanoparticles. J. Mater. Chem. B 2015, 3, 39–44. [Google Scholar] [CrossRef]
- Cheng, Y.J.; Zhang, A.Q.; Hu, J.J.; He, F.; Zeng, X.; Zhang, X.Z. Multifunctional peptide-amphiphile end-capped mesoporous silica nanoparticles for tumor targeting drug delivery. ACS Appl. Mater. Interfaces 2017, 9, 2093–2103. [Google Scholar] [CrossRef]
- Sha, L.; Wang, D.; Mao, Y.; Shi, W.; Gao, T.; Zhao, Q.; Wang, S. Hydrophobic interaction mediated coating of pluronics on mesoporous silica nanoparticle with stimuli responsiveness for cancer therapy. Nanotechnology 2018, 29, 345101. [Google Scholar] [CrossRef]
- Xiao, D.; Jia, H.-Z.; Ma, N.; Zhuo, R.-X.; Zhang, X.-Z. A redox-responsive mesoporous silica nanoparticle capped with amphiphilic peptides by self-assembly for cancer targeting drug delivery. Nanoscale 2015, 7, 10071–10077. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.; Hao, S.; Young, P.; Zhang, H. Targeting cathepsin B for cancer therapies. Horizons Cancer Res. 2015, 56, 23–40. [Google Scholar]
- de la Torre, C.; Mondragón, L.; Coll, C.; Sancenón, F.; Marcos, M.D.; Martínez-Máñez, R.; Amorós, P.; Pérez-Payá, E.; Orzáez, M. Cathepsin-B induced controlled release from peptide-capped mesoporous silica nanoparticles. Chem. Eur. J. 2014, 20, 15309–15314. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, F.; Shao, Q.; Min, Y.; Costa, M.; Yeow, E.K.L.; Xing, B. Enzyme-responsive cell-penetrating peptide conjugated mesoporous silica quantum dot nanocarriers for controlled release of nucleus-targeted drug molecules and real-time intracellular fluorescence imaging of tumor cells. Adv. Healthc. Mater. 2014, 3, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.J.; Luo, G.F.; Zhu, J.Y.; Xu, X.D.; Zeng, X.; Cheng, D.B.; Li, Y.M.; Wu, Y.; Zhang, X.Z.; Zhuo, R.X.; et al. Enzyme-induced and tumor-targeted drug delivery system based on multifunctional mesoporous silica nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 9078–9087. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Zhang, P.; Xi, Y.; Huang, K.; Min, Q.; Zhu, J.-J. Peptide-mediated core/satellite/shell multifunctional nanovehicles for precise imaging of cathepsin B activity and dual-enzyme controlled drug release. NPG Asia Mater. 2017, 9, e366. [Google Scholar] [CrossRef]
- Tukappa, A.; Ultimo, A.; de la Torre, C.; Pardo, T.; Sancenón, F.; Martínez-Máñez, R. Polyglutamic acid-gated mesoporous silica nanoparticles for enzyme-controlled drug delivery. Langmuir 2016, 32, 8507–8515. [Google Scholar] [CrossRef]
- Li, E.; Yang, Y.; Hao, G.; Yi, X.; Zhang, S.; Pan, Y.; Xing, B.; Gao, M. Multifunctional magnetic mesoporous silica nanoagents for in vivo enzyme-responsive drug delivery and MR imaging. Nanotheranostics 2018, 2, 233–242. [Google Scholar] [CrossRef]
- Hu, J.-J.; Liu, L.-H.; Li, Z.-Y.; Zhuo, R.-X.; Zhang, X.-Z. MMP-responsive theranostic nanoplatform based on mesoporous silica nanoparticles for tumor imaging and targeted drug delivery. J. Mater. Chem. B 2016, 4, 1932–1940. [Google Scholar] [CrossRef]
- Eskandari, P.; Bigdeli, B.; Porgham Daryasari, M.; Baharifar, H.; Bazri, B.; Shourian, M.; Amani, A.; Sadighi, A.; Goliaei, B.; Khoobi, M.; et al. Gold-capped mesoporous silica nanoparticles as an excellent enzyme-responsive nanocarrier for controlled doxorubicin delivery. J. Drug Target. 2019, 27, 1084–1093. [Google Scholar] [CrossRef]
- van Rijt, S.H.; Bölükbas, D.A.; Argyo, C.; Datz, S.; Lindner, M.; Eickelberg, O.; Königshoff, M.; Bein, T.; Meiners, S. Protease-mediated release of chemotherapeutics from mesoporous silica nanoparticles to ex vivo human and mouse lung tumors. ACS Nano 2015, 9, 2377–2389. [Google Scholar] [CrossRef]
- Xu, J.-H.; Gao, F.-P.; Li, L.-L.; Ma, H.L.; Fan, Y.-S.; Liu, W.; Guo, S.-S.; Zhao, X.-Z.; Wang, H. Gelatin–mesoporous silica nanoparticles as matrix metalloproteinases-degradable drug delivery systems in vivo. Microporous Mesoporous Mater. 2013, 182, 165–172. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, X.; Li, J.; Luo, Z.; Hu, Y.; Liu, J.; Dai, L.; Zhou, J.; Hou, C.; Cai, K. Enzyme responsive drug delivery system based on mesoporous silica nanoparticles for tumor therapy in vivo. Nanotechnology 2015, 26, 145102. [Google Scholar] [CrossRef]
- Ding, J.; Liang, T.; Zhou, Y.; He, Z.; Min, Q.; Jiang, L.; Zhu, J. Hyaluronidase-triggered anticancer drug and siRNA delivery from cascaded targeting nanoparticles for drug-resistant breast cancer therapy. Nano Res. 2017, 10, 690–703. [Google Scholar] [CrossRef]
- Srivastava, P.; Hira, S.K.; Srivastava, D.N.; Gupta, U.; Sen, P.; Singh, R.A.; Manna, P.P. Protease-Responsive Targeted Delivery of Doxorubicin from Bilirubin-BSA-Capped Mesoporous Silica Nanoparticles against Colon Cancer. ACS Biomater. Sci. Eng. 2017, 3, 3376–3385. [Google Scholar] [CrossRef]
- Polo, L.; Gómez-Cerezo, N.; Aznar, E.; Vivancos, J.L.; Sancenón, F.; Arcos, D.; Vallet-Regí, M.; Martínez-Máñez, R. Molecular gates in mesoporous bioactive glasses for the treatment of bone tumors and infection. Acta Biomater. 2017, 50, 114–126. [Google Scholar] [CrossRef]
- Fernando, I.R.; Ferris, D.P.; Frasconi, M.; Malin, D.; Strekalova, E.; Yilmaz, M.D.; Ambrogio, M.W.; Algaradah, M.M.; Hong, M.P.; Chen, X.; et al. Esterase- and pH-responsive poly(β-amino ester)-capped mesoporous silica nanoparticles for drug delivery. Nanoscale 2015, 7, 7178–7183. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-L.; Zhou, Y.; Li, Q.-L.; Yang, Y.-W. Enzyme-responsive supramolecular nanovalves crafted by mesoporous silica nanoparticles and choline-sulfonatocalix[4]arene [2]pseudorotaxanes for controlled cargo release. Chem. Commun. 2013, 49, 9033–9035. [Google Scholar] [CrossRef]
- Patel, K.; Angelos, S.; Dichtel, W.R.; Coskun, A.; Yang, Y.-W.; Zink, J.I.; Stoddart, J.F. Enzyme-responsive snap-top covered silica nanocontainers. J. Am. Chem. Soc. 2008, 130, 2382–2383. [Google Scholar] [CrossRef]
- Linsley, C.S.; Wu, B.M. Recent advances in light-responsive on-demand drug-delivery systems. Ther. Deliv. 2017, 8, 89–107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ang, C.Y.; Li, M.; Tan, S.Y.; Qu, Q.; Luo, Z.; Zhao, Y. Polymer-coated hollow mesoporous silica nanoparticles for triple-responsive drug delivery. ACS Appl. Mater. Interfaces 2015, 7, 18179–18187. [Google Scholar] [CrossRef]
- Lin, X.; Wu, M.; Li, M.; Cai, Z.; Sun, H.; Tan, X.; Li, J.; Zeng, Y.; Liu, X.; Liu, J. Photo-responsive hollow silica nanoparticles for light-triggered genetic and photodynamic synergistic therapy. Acta Biomater. 2018, 76, 178–192. [Google Scholar] [CrossRef]
- Knežević, N.Ž.; Trewyn, B.G.; Lin, V.S.-Y. Functionalized mesoporous silica nanoparticle-based visible light responsive controlled release delivery system. Chem. Commun. 2011, 47, 2817–2819. [Google Scholar] [CrossRef] [PubMed]
- He, D.; He, X.; Wang, K.; Cao, J.; Zhao, Y. A light-responsive reversible molecule-gated system using thymine-modified mesoporous silica nanoparticles. Langmuir 2012, 28, 4003–4008. [Google Scholar] [CrossRef] [PubMed]
- Chai, S.; Guo, Y.; Zhang, Z.; Chai, Z.; Ma, Y.; Qi, L. Cyclodextrin-gated mesoporous silica nanoparticles as drug carriers for red light-induced drug release. Nanotechnology 2017, 28, 145101. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Sun, X.; Liu, J.; Feng, L.; Liu, Z. Light-responsive, singlet-oxygen-triggered on-demand drug release from photosensitizer-doped mesoporous silica nanorods for cancer combination therapy. Adv. Funct. Mater. 2016, 26, 4722–4732. [Google Scholar] [CrossRef]
- Zhang, T.; Lin, H.; Cui, L.; An, N.; Tong, R.; Chen, Y.; Yang, C.; Li, X.; Liu, J.; Qu, F. Near infrared light triggered reactive oxygen species responsive upconversion nanoplatform for drug delivery and photodynamic therapy. Eur. J. Inorg. Chem. 2016, 2016, 1206–1213. [Google Scholar] [CrossRef]
- Wang, G.; Dong, J.; Yuan, T.; Zhang, J.; Wang, L.; Wang, H. Visible light and pH responsive polymer-coated mesoporous silica nanohybrids for controlled release. Macromol. Biosci. 2016, 16, 990–994. [Google Scholar] [CrossRef]
- Xing, Q.; Li, N.; Chen, D.; Sha, W.; Jiao, Y.; Qi, X.; Xu, Q.; Lu, J. Light-responsive amphiphilic copolymer coated nanoparticles as nanocarriers and real-time monitors for controlled drug release. J. Mater. Chem. B 2014, 2, 1182–1189. [Google Scholar] [CrossRef]
- Lai, J.; Mu, X.; Xu, Y.; Wu, X.; Wu, C.; Li, C.; Chen, J.; Zhao, Y. Light-responsive nanogated ensemble based on polymer grafted mesoporous silica hybrid nanoparticles. Chem. Commun. 2010, 46, 7370–7372. [Google Scholar] [CrossRef]
- Sun, Y.-L.; Yang, B.-J.; Zhang, S.X.-A.; Yang, Y.-W. Cucurbit[7]uril Pseudorotaxane-Based Photoresponsive Supramolecular Nanovalve. Chem.—A Eur. J. 2012, 18, 9212–9216. [Google Scholar] [CrossRef]
- Wang, M.; Wang, T.; Wang, D.; Jiang, W.; Fu, J. Acid and light stimuli-responsive mesoporous silica nanoparticles for controlled release. J. Mater. Sci. 2019, 54, 6199–6211. [Google Scholar] [CrossRef]
- Zhao, J.; He, Z.; Li, B.; Cheng, T.; Liu, G. AND logic-like pH- and light-dual controlled drug delivery by surface modified mesoporous silica nanoparticles. Mater. Sci. Eng. C 2017, 73, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Yang, S.; Chen, D.; Li, N.; Li, H.; Xu, Q.; Ge, J.; Lu, J. Light-triggered reversible assemblies of azobenzene-containing amphiphilic copolymer with β-cyclodextrin-modified hollow mesoporous silica nanoparticles for controlled drug release. Chem. Commun. 2012, 48, 10010–10012. [Google Scholar] [CrossRef] [PubMed]
- Bian, Q.; Xue, Z.; Sun, P.; Shen, K.; Wang, S.; Jia, J. Visible-light-triggered supramolecular valves based on β-cyclodextrin-modified mesoporous silica nanoparticles for controlled drug release. RSC Adv. 2019, 9, 17179–17182. [Google Scholar] [CrossRef]
- Lu, J.; Choi, E.; Tamanoi, F.; Zink, J.I. Light-activated nanoimpeller-controlled drug release in cancer cells. Small 2008, 4, 421–426. [Google Scholar] [CrossRef]
- Croissant, J.; Maynadier, M.; Gallud, A.; Peindy N’Dongo, H.; Nyalosaso, J.L.; Derrien, G.; Charnay, C.; Durand, J.-O.; Raehm, L.; Serein-Spirau, F.; et al. Two-photon-triggered drug delivery in cancer cells using nanoimpellers. Angew. Chem. Int. Ed. 2013, 52, 13813–13817. [Google Scholar] [CrossRef]
- Kim, H.-J.; Matsuda, H.; Zhou, H.; Honma, I. Ultrasound-triggered smart drug release from a poly(dimethylsiloxane)– Mesoporous silica composite. Adv. Mater. 2006, 18, 3083–3088. [Google Scholar] [CrossRef]
- Li, X.; Xie, C.; Xia, H.; Wang, Z. pH and ultrasound dual-responsive polydopamine-coated mesoporous silica nanoparticles for controlled drug delivery. Langmuir 2018, 34, 9974–9981. [Google Scholar] [CrossRef]
- Wang, J.; Jiao, Y.; Shao, Y. Mesoporous silica nanoparticles for dual-mode chemo-sonodynamic therapy by low-energy ultrasound. Materials 2018, 11, 2041. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Xia, H. Ultrasound reversible response nanocarrier based on sodium alginate modified mesoporous silica nanoparticles. Front. Chem. 2019, 7, 59. [Google Scholar] [CrossRef]
- Lee, S.-F.; Zhu, X.-M.; Wang, Y.-X.J.; Xuan, S.-H.; You, Q.; Chan, W.-H.; Wong, C.-H.; Wang, F.; Yu, J.C.; Cheng, C.H.K.; et al. Ultrasound, pH, and magnetically responsive crown-ether-coated core/shell nanoparticles as drug encapsulation and release systems. ACS Appl. Mater. Interfaces 2013, 5, 1566–1574. [Google Scholar] [CrossRef]
- Cheng, C.-A.; Chen, W.; Zhang, L.; Wu, H.H.; Zink, J.I. A responsive mesoporous silica nanoparticle platform for magnetic resonance imaging-guided high-intensity focused ultrasound-stimulated cargo delivery with controllable location, time, and dose. J. Am. Chem. Soc. 2019, 141, 17670–17684. [Google Scholar] [CrossRef] [PubMed]
- Paris, J.L.; Cabañas, M.V.; Manzano, M.; Vallet-Regí, M. Polymer-grafted mesoporous silica nanoparticles as ultrasound-responsive drug carriers. ACS Nano 2015, 9, 11023–11033. [Google Scholar] [CrossRef] [PubMed]
- Jose, J.; Kumar, R.; Harilal, S.; Mathew, G.E.; Parambi, D.G.T.; Prabhu, A.; Uddin, M.S.; Aleya, L.; Kim, H.; Mathew, B. Magnetic nanoparticles for hyperthermia in cancer treatment: An emerging tool. Environ. Sci. Pollut. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tao, C. DNA-capped Fe3O4/SiO2 magnetic mesoporous silica nanoparticles for potential controlled drug release and hyperthermia. RSC Adv. 2015, 5, 22365–22372. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Hua, M.; Yu, H.; Wei, S.; Wang, A.; Zhou, J. Photothermal-triggered controlled drug release from mesoporous silica nanoparticles based on base-pairing rules. ACS Biomater. Sci. Eng. 2019, 5, 2399–2408. [Google Scholar] [CrossRef]
- Yu, Z.; Li, N.; Zheng, P.; Pan, W.; Tang, B. Temperature-responsive DNA-gated nanocarriers for intracellular controlled release. Chem. Commun. 2014, 50, 3494–3497. [Google Scholar] [CrossRef]
- Schlossbauer, A.; Warncke, S.; Gramlich, P.M.E.; Kecht, J.; Manetto, A.; Carell, T.; Bein, T. A programmable DNA-based molecular valve for colloidal mesoporous silica. Angew. Chem. Int. Ed. 2010, 49, 4734–4737. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, G.; Wang, Y.; Liu, J.; Zhang, Y.; Ma, Y. AuNP and ssDNA capped mesoporous silica nanoparticles for laser controlled drug release. RSC Adv. 2019, 9, 34958–34962. [Google Scholar] [CrossRef]
- Ruiz-Hernández, E.; Baeza, A.; Vallet-Regí, M. Smart drug delivery through DNA/magnetic nanoparticle gates. ACS Nano 2011, 5, 1259–1266. [Google Scholar] [CrossRef]
- Ruan, L.; Chen, W.; Wang, R.; Lu, J.; Zink, J.I. Magnetically stimulated drug release using nanoparticles capped by self-assembling peptides. ACS Appl. Mater. Interfaces 2019, 11, 43835–43842. [Google Scholar] [CrossRef]
- Shi, Z.; Yang, C.; Li, R.; Ruan, L. Microwave thermal-triggered drug delivery using thermosensitive peptide-coated core–shell mesoporous silica nanoparticles. J. Mater. Sci. 2020, 55, 6118–6129. [Google Scholar] [CrossRef]
- Cui, Y.; Deng, R.; Li, X.; Wang, X.; Jia, Q.; Bertrand, E.; Meguellati, K.; Yang, Y.-W. Temperature-sensitive polypeptide brushes-coated mesoporous silica nanoparticles for dual-responsive drug release. Chinese Chem. Lett. 2019, 30, 2291–2294. [Google Scholar] [CrossRef]
- Martelli, G.; Zope, H.R.; Bròvia Capell, M.; Kros, A. Coiled-coil peptide motifs as thermoresponsive valves for mesoporous silica nanoparticles. Chem. Commun. 2013, 49, 9932–9934. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.R.; Ferris, D.P.; Lee, J.-H.; Choi, E.; Cho, M.H.; Kim, E.S.; Stoddart, J.F.; Shin, J.-S.; Cheon, J.; Zink, J.I. Noninvasive remote-controlled release of drug molecules in vitro using magnetic actuation of mechanized nanoparticles. J. Am. Chem. Soc. 2010, 132, 10623–10625. [Google Scholar] [CrossRef] [PubMed]
- Rühle, B.; Datz, S.; Argyo, C.; Bein, T.; Zink, J.I. A molecular nanocap activated by superparamagnetic heating for externally stimulated cargo release. Chem. Commun. 2016, 52, 1843–1846. [Google Scholar] [CrossRef] [PubMed]
- Guisasola, E.; Baeza, A.; Talelli, M.; Arcos, D.; Vallet-Regí, M. Design of thermoresponsive polymeric gates with opposite controlled release behaviors. RSC Adv. 2016, 6, 42510–42516. [Google Scholar] [CrossRef]
- Song, Z.; Shi, J.; Zhang, Z.; Qi, Z.; Han, S.; Cao, S. Mesoporous silica-coated gold nanorods with a thermally responsive polymeric cap for near-infrared-activated drug delivery. J. Mater. Sci. 2018, 53, 7165–7179. [Google Scholar] [CrossRef]
- Ribeiro, T.; Coutinho, E.; Rodrigues, A.S.; Baleizão, C.; Farinha, J.P.S. Hybrid mesoporous silica nanocarriers with thermovalve-regulated controlled release. Nanoscale 2017, 9, 13485–13494. [Google Scholar] [CrossRef]
- Su, Y.; Ojo, O.F.; Tsengam, I.K.M.; He, J.; McPherson, G.L.; John, V.T.; Valla, J.A. Thermoresponsive coatings on hollow particles with mesoporous shells serve as stimuli-responsive gates to species encapsulation and release. Langmuir 2018, 34, 14608–14616. [Google Scholar] [CrossRef]
- Hegazy, M.; Zhou, P.; Wu, G.; Wang, L.; Rahoui, N.; Taloub, N.; Huang, X.; Huang, Y. Construction of polymer coated core–shell magnetic mesoporous silica nanoparticles with triple responsive drug delivery. Polym. Chem. 2017, 8, 5852–5864. [Google Scholar] [CrossRef]
- Brunella, V.; Jadhav, S.A.; Miletto, I.; Berlier, G.; Ugazio, E.; Sapino, S.; Scalarone, D. Hybrid drug carriers with temperature-controlled on–off release: A simple and reliable synthesis of PNIPAM-functionalized mesoporous silica nanoparticles. React. Funct. Polym. 2016, 98, 31–37. [Google Scholar] [CrossRef]
- Peralta, M.E.; Jadhav, S.A.; Magnacca, G.; Scalarone, D.; Mártire, D.O.; Parolo, M.E.; Carlos, L. Synthesis and in vitro testing of thermoresponsive polymer-grafted core-shell magnetic mesoporous silica nanoparticles for efficient controlled and targeted drug delivery. J. Colloid Interface Sci. 2019, 544, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Sha, X.; Guo, J.; Jiao, Y.; Wang, C.; Yang, W. Thermo and pH dual responsive, polymer shell coated, magnetic mesoporous silica nanoparticles for controlled drug release. J. Mater. Chem. 2011, 21, 9239–9247. [Google Scholar] [CrossRef]
- Tian, Z.; Yu, X.; Ruan, Z.; Zhu, M.; Zhu, Y.; Hanagata, N. Magnetic mesoporous silica nanoparticles coated with thermo-responsive copolymer for potential chemo- and magnetic hyperthermia therapy. Microporous Mesoporous Mater. 2018, 256, 1–9. [Google Scholar] [CrossRef]
- Guisasola, E.; Baeza, A.; Talelli, M.; Arcos, D.; Moros, M.; de la Fuente, J.M.; Vallet-Regí, M. Magnetic-responsive release controlled by hot spot effect. Langmuir 2015, 31, 12777–12782. [Google Scholar] [CrossRef]
- Guisasola, E.; Asín, L.; Beola, L.; de la Fuente, J.M.; Baeza, A.; Vallet-Regí, M. Beyond traditional hyperthermia: In vivo cancer treatment with magnetic-responsive mesoporous silica nanocarriers. ACS Appl. Mater. Interfaces 2018, 10, 12518–12525. [Google Scholar] [CrossRef]
- Hei, M.; Wang, J.; Wang, K.; Zhu, W.; Ma, P.X. Dually responsive mesoporous silica nanoparticles regulated by upper critical solution temperature polymers for intracellular drug delivery. J. Mater. Chem. B 2017, 5, 9497–9501. [Google Scholar] [CrossRef]
- Hu, W.; Bai, X.; Wang, Y.; Lei, Z.; Luo, H.; Tong, Z. Upper critical solution temperature polymer-grafted hollow mesoporous silica nanoparticles for near-infrared-irradiated drug release. J. Mater. Chem. B 2019, 7, 5789–5796. [Google Scholar] [CrossRef]
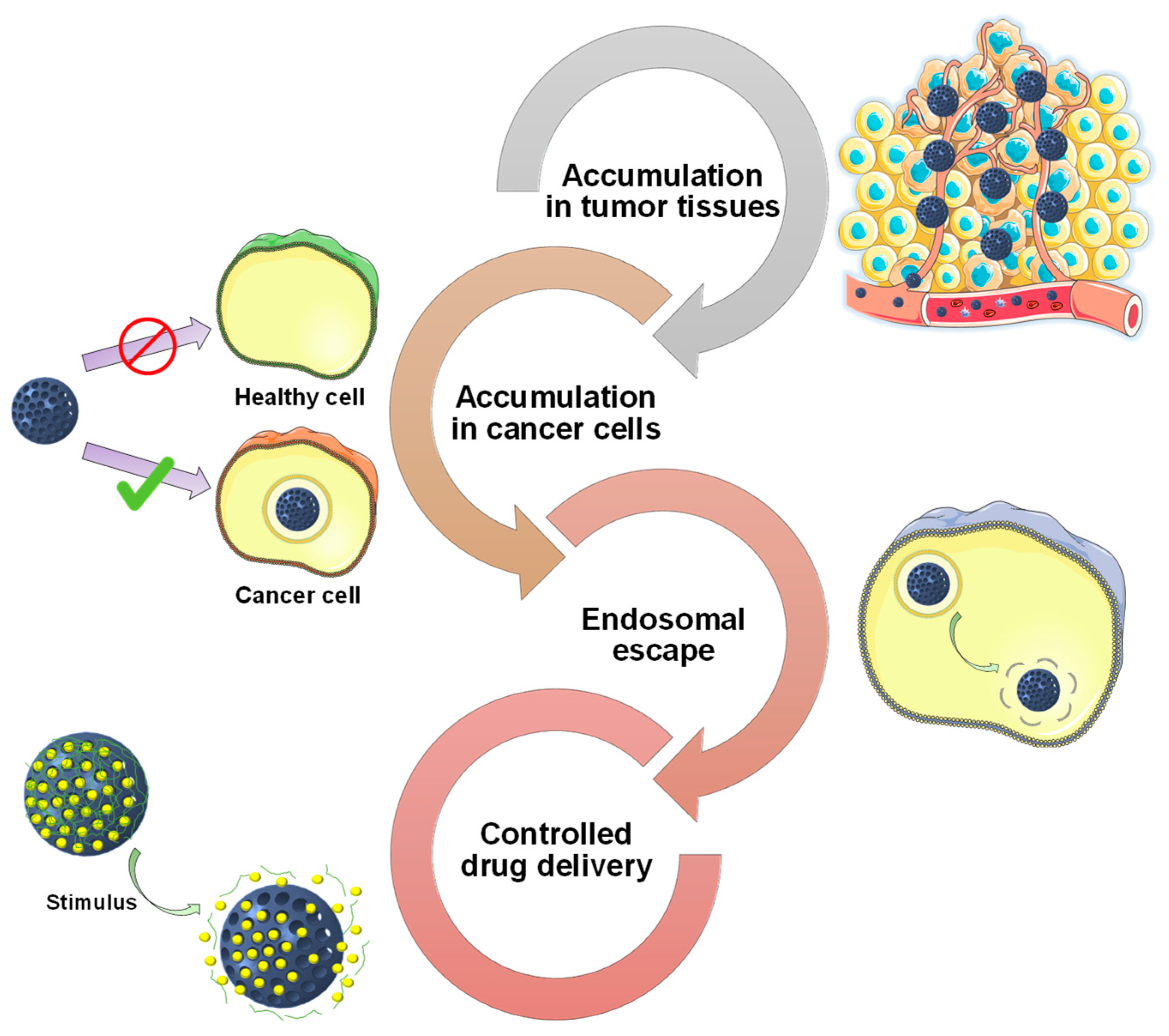
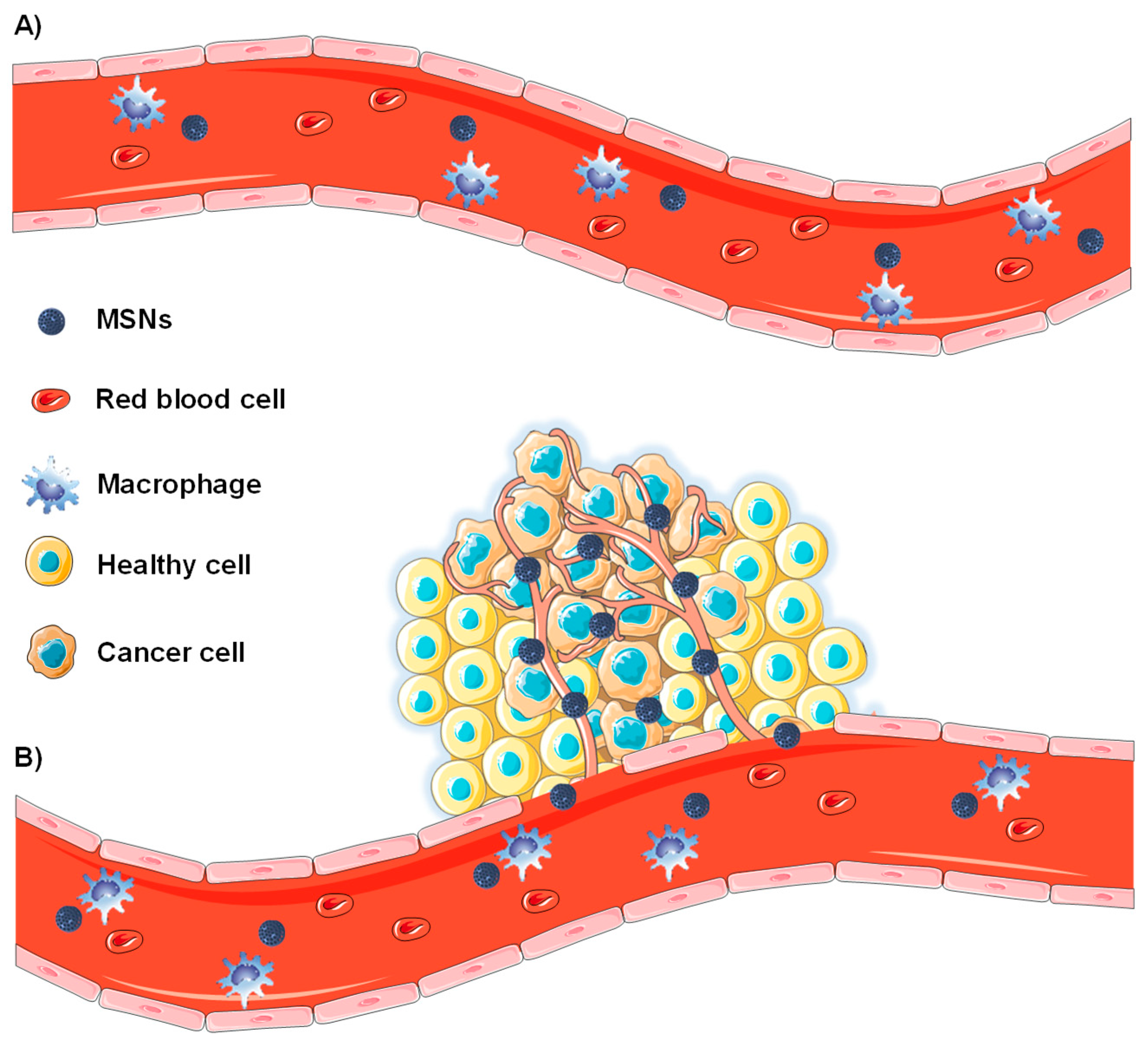
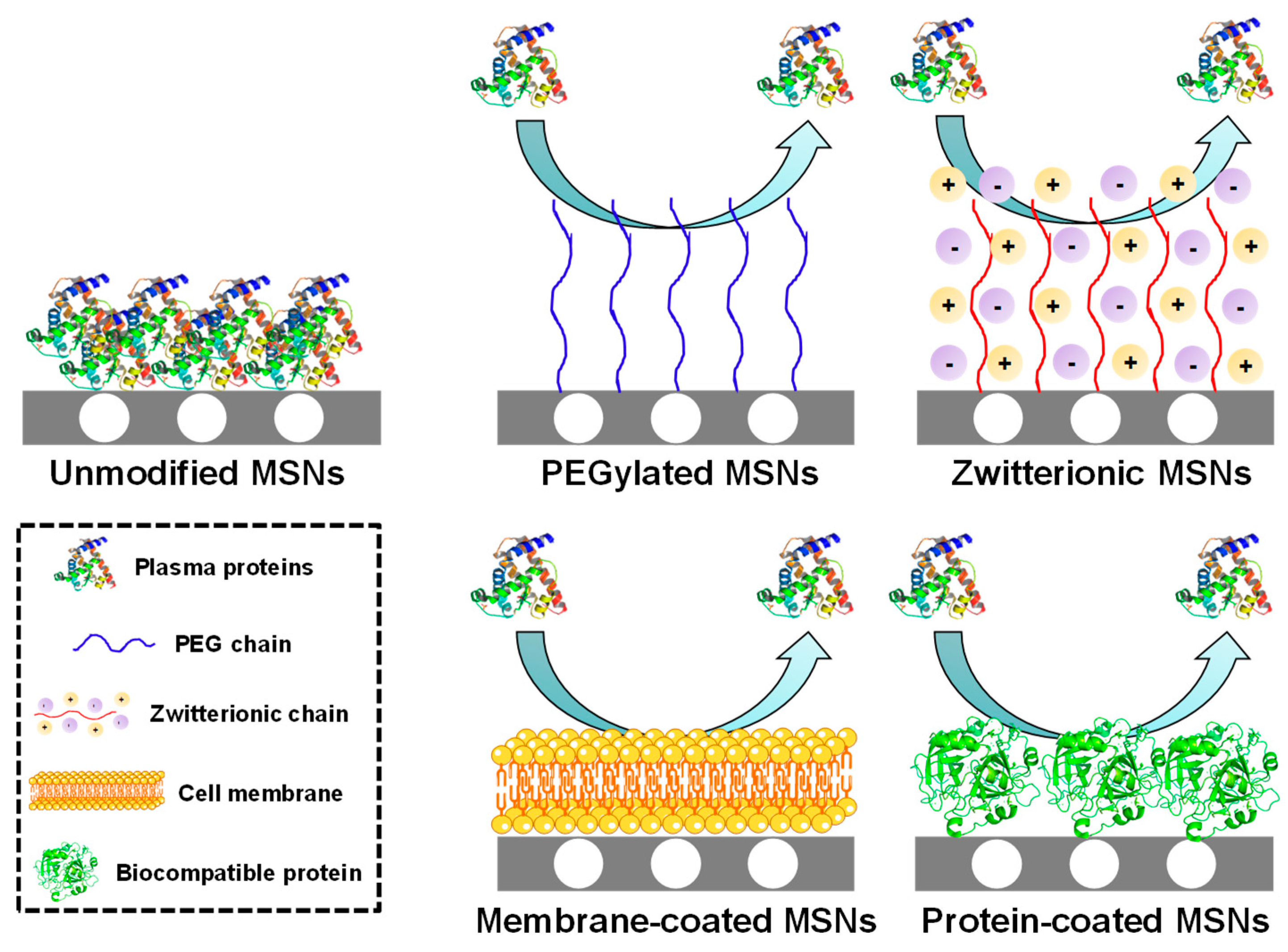

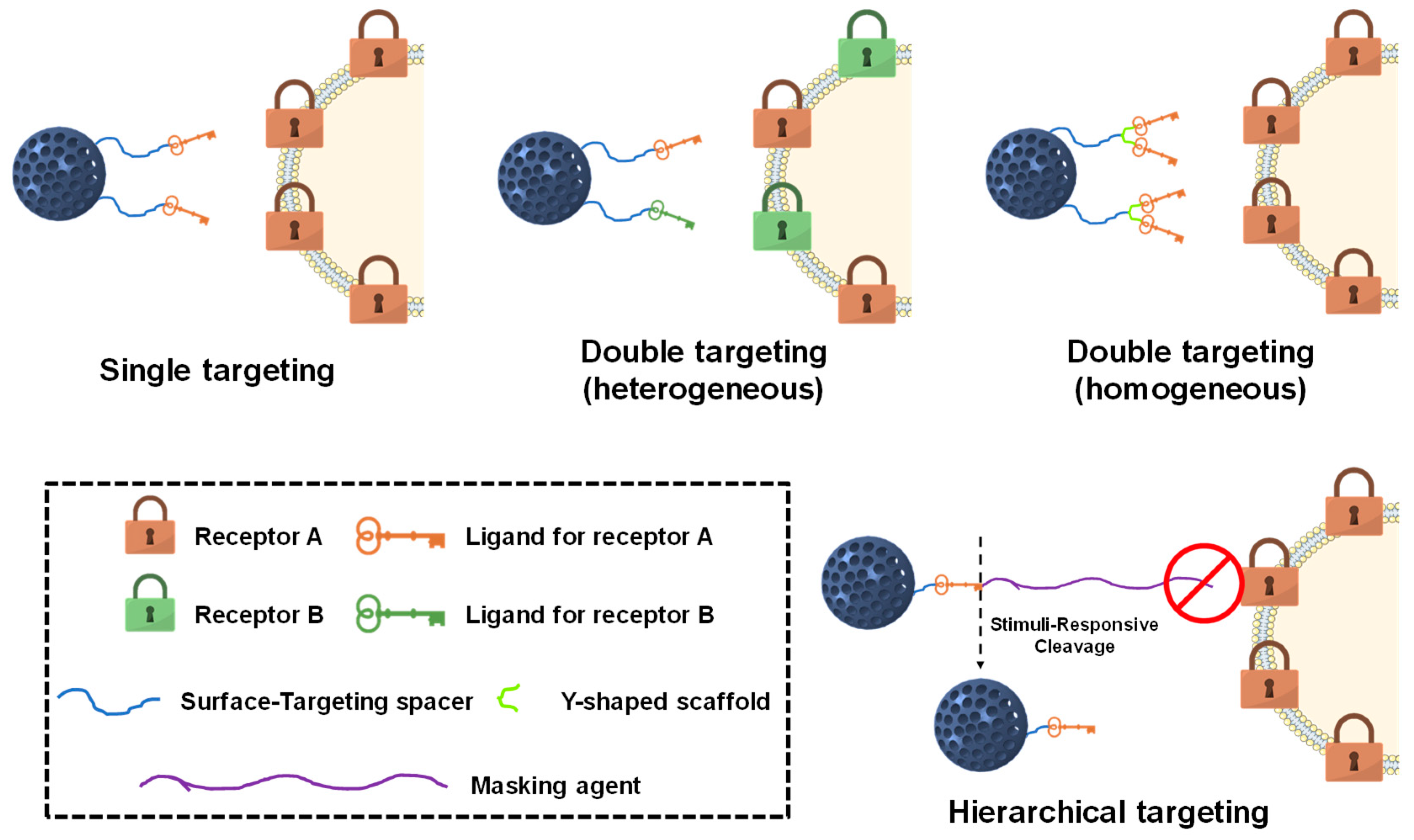
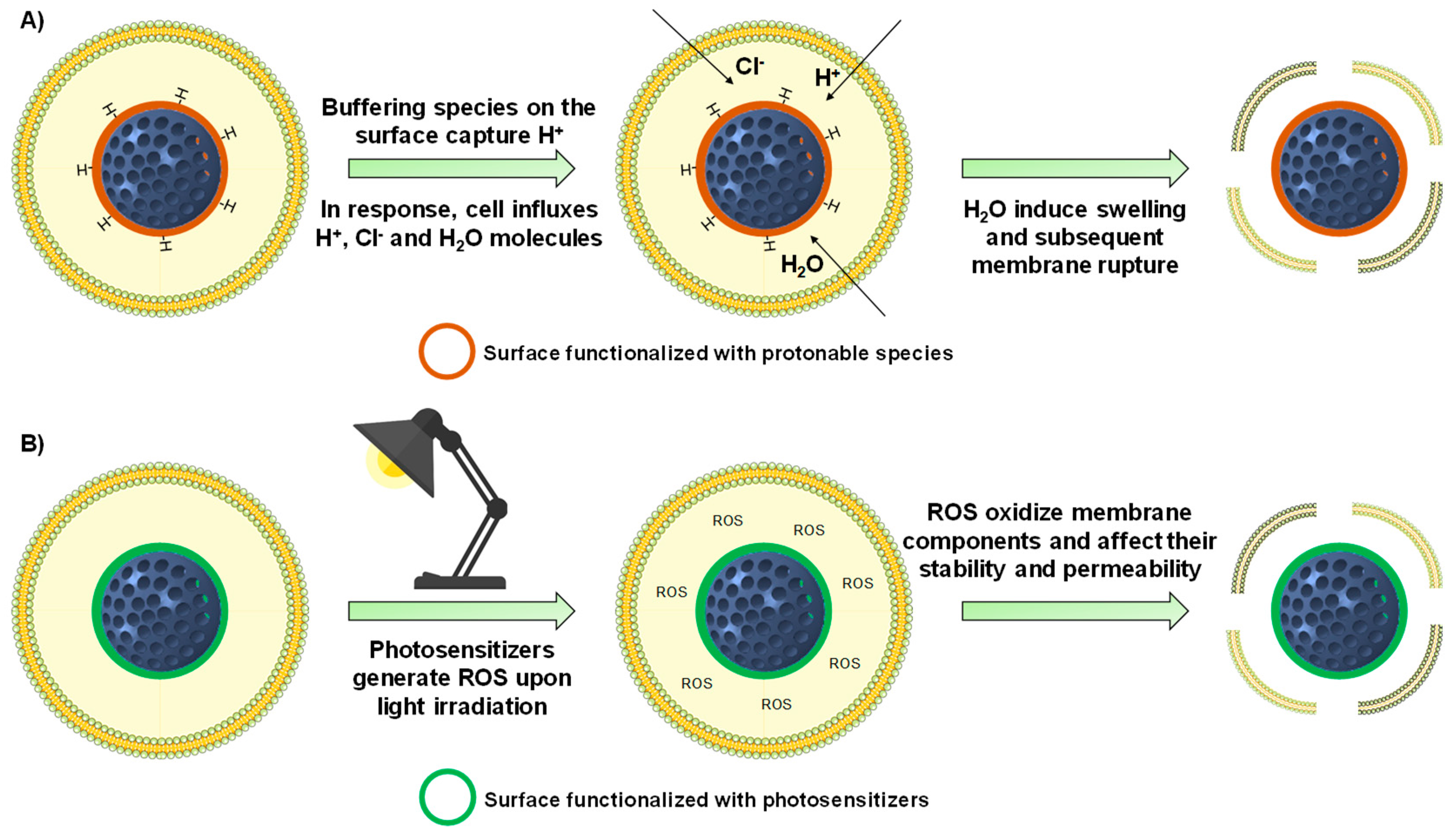
| Targeting Agent | Membrane Receptor | Cell Line | Reference |
|---|---|---|---|
| Antibodies | |||
| Trastuzumab | HER2 | SK-BR3, BT-474 | [96,97,98,99,100] |
| Anti-CD44 | CD44 | MCF-7 | [101] |
| TRC105 | CD105 | 4T1 | [102,103,104] |
| Anti-EpCAM | EpCAM | Y79 | [105] |
| TAB-004 | MUC1 | MMT | [106] |
| Cetuximab | EGFR | MCF-7, PC9, AsPC-1, PANC-1, MIA PaCa-2 | [107,108,109] |
| Aptamers | |||
| EpCAM | EpCAM | Huh-7, HepG2, SW620, SW480 | [111,112,113,114] |
| MUC1 | MUC1 | MDA-MB-231, MCF-7 | [115,116] |
| AS1411 | NCL | HeLa, SKOV-3, MCF-7 | [117,118,119,120,121] |
| YQ26 | END | HEK293 | [122] |
| HB5 | HER2 | SK-BR-3 | [123] |
| Peptides | |||
| TAT | Importin α/β | HeLa | [126] |
| KALA | - | A549, HeLa | [127] |
| RGD | αvβ3-integrin | MDA-MB-231, HeLa, UMR-106, PC-3, 4T1, HUVEC | [128,129,130,131,132,133,181] |
| NGR | CD13 | C6, NCI-H1299, BCEC | [134,135] |
| NAPamide | Melanocortin-1 | #17 (melanoma cancer cells) | [137] |
| Bld-1 | FPR-1 | HT-1376 | [138] |
| IL-13 | IL-13R-α2 | U87 | [139] |
| Proteins | |||
| Transferrin | TfR | HT1080, HepG2, Huh-7, MDA-MB-231, C6, MIA PaCa-2 | [140,141,142,143,144,145] |
| Urokinase plasminogen activator | UPAR | S2-VP10 | [146] |
| Concanavalin A | Sialic acids | HOS | [147] |
| Aleuria Auranti | Sialyl-Lewis X antigen | DLD-1 | [148] |
| Carbohydrates | |||
| Galactose | ASGPR | HepG2, SMMC-7721 | [149] |
| Lactobionic acid | [150,151,152,153] | ||
| Glucose derivatives | GLUT | Y79, HeLa, A549 | [154,155] |
| Hyaluronic acid | CD44 | MDA-MB-231, HCT-116, HeLa, MCF-7 | [156,157,158,159] |
| Chondroitin sulfate | [160,161,162] | ||
| Small Molecules | |||
| Folic acid | FR-α | PANC-1, LS174T, LnCAP, KB, HeLa, Y79, A549, NCI-H1299 | [164,165,166,167,168,169,170,171,172] |
| Biotin | BR | A549, HeLa, NB-4 | [173,174,175] |
| Boronic acid | Sialic acids | HepG2 | [178] |
| Benzylguanidine derivatives | NET | NB-1691 | [180] |
| Targeting Agents | Approach | Cell line | Reference |
|---|---|---|---|
| Dual Targeting | |||
| Hyaluronic acid + RGD | Two different membrane targeting agents | SKOV-3 | [181,182] |
| Biotin + Folic acid | HeLa | [183] | |
| Bevacizumab + EpCAM aptamer | SW480 | [184] | |
| Benzylguanidine derivatives | Y-shaped scaffold using the same membrane targeting agent | NB-1691 | [185] |
| RGD + TAT | Sequential vascular-membrane-organelle targeting | HeLa | [186] |
| Folic acid + Dexamethasone | Sequential membrane-organelle targeting | HeLa | [187] |
| Hyaluronic acid + Triphenylphosphonium | MGC-803 | [188] | |
| Hyaluronic acid + Positive charge | Janus dual membrane targeting | A549 | [189] |
| Folic acid + TPP | Janus membrane-organelle targeting | LnCAP | [190] |
| Hierarchical Targeting | |||
| Positive charge | pH-responsive benzoic imine bond | HepG2, HeLa | [193,194,195] |
| RGD | U87 | [196] | |
| Positive charge | Thermally-cleavable bond | HOS | [197] |
| Hyaluronic acid | MMP-2-degradable gelatin | MDA-MB-231 | [198] |
| Folic acid | HT-29 | [199] | |
| RGD | RGD masked with MMP-2-clevable peptide sequence | 4T1, HT-29 | [200,201] |
| Approach | Description | Reference |
|---|---|---|
| Acid-Labile Bonds | ||
| Hydrazone bond | pH-responsive bonds that find application as linkers between MSNs and different gatekeepers | [248,249,251] |
| Acetal bond | [147,252,253,254,255,256] | |
| Boronate ester bond | [258,259,260,261] | |
| Imine bond | pH-responsive bond useful as cross-linking agent | [262,263,264] |
| pH-Degradable Gatekeepers | ||
| Inorganic nanoparticles | Small nanoparticles that degrade at acid pH, generating different ions with therapeutic applications | [265,266,267,268,269,270,271] |
| Polymers | Polymeric coatings that decompose into their building blocks upon changes in pH | [272,274,275,276] |
| pH-Operated Nanovalves | ||
| Stalk + Cap | Supramolecular structures that close and open the pores thanks to the interaction with stalks grafted on the surface | [277,278,279,280,281] |
| Conformation-Changing Polymers | ||
| Cationic | Polymers that are collapsed on the surface of the particles when deprotonated (pores closed) and undergo a conformational change when pH varies (pores open) | [230,282,283,284,285,286,287,288] |
| Anionic | [289,290,291] | |
| pH-Responsive Biomolecules | ||
| Polypeptides | Large peptidic chains containing protonable groups that exhibit collapsed-to-extended behavior upon pH variations | [215,292,293] |
| Nucleic acids and proteins | Macromolecules that modify their 3D structure upon variations in pH | [294,295,296,297,298,299] |
| Polyelectrolytes | ||
| Monolayers | Charged polymers that close the pores by forming a monolayer through electrostatic interactions | [300,301,302,303,304,305,306] |
| Multilayers | Arrangement of multiple charged layers on the surface of the particles to close the pore entrances | [307,308,309,310] |
| Enzyme | Description | Reference |
|---|---|---|
| Peptides as Gatekeepers | ||
| CatB | Large peptidic sequences that close the mesopores and allow drug release upon enzymatic degradation | [341,345] |
| MMP-2 | [346,347] | |
| Peptide as Linkers | ||
| CatB | Short peptidic sequences employed to graft different types of bulky gatekeepers (small nanoparticles, proteins, nanovalves) to the surface of MSNs | [342,343,344] |
| MMP-2 | [348] | |
| MMP-9 | [349] | |
| MMP-13 | [351] | |
| Other Enzyme-Degradable Gatekeepers | ||
| MMP-9 | MSNs covered with a gelatin that degrade in the presence of MMP-9 | [350] |
| Hyaluronidase | MSNs functionalized with large carbohydrates (hyaluronic acid, chondroitin sulfate) that act simultaneously targeting agents and gatekeepers | [156,158,160,162,352] |
| Trypsin | MSNs gated with BSA, which can be degraded by overexpressed trypsin in liver cancer | [353] |
| Alkaline phosphatase | ATP-capped mesoporous silica-based materials that allow drug release upon enzymatic degradation of ATP | [354] |
| Esterases | MSNs functionalized with ester-containing gatekeepers that are degraded in the presence of such enzymes | [355,356,357] |
| Approach | Description | Reference |
|---|---|---|
| Light-Responsive Bonds | ||
| o-nitrobenzyl group | Cleavable using 365 nm light. Used as linker for the grafting of proteins and pH-responsive polymers | [140,359] |
| Coumarin group | Cleavable using 405 nm light. Used as linker for the grafting of cationic polymers for gene delivery | [360] |
| Thiolated coordination bond | Cleavable using 455 nm light. Coordination bond formed by ruthenium bipyridine-based compounds that act as gatekeepers | [361] |
| Thymine derivatives | Reversible formation (365 nm) and cleavage (240 nm) of a cyclobutane dimer. Used as on-off gatekeeper | [362] |
| ROS-Responsive Bonds (ROS Generation upon Light Application) | ||
| Aminoacrylate bond | Used as linker for the grafting of a porphyrin acting simultaneously as gatekeeper and ROS generator upon visible light irradiation | [228] |
| (alkylthio)alkene-based bond | Used as linker for the grafting of nanovalves and proteins. Cleaved when loaded photosensitizer chlorin e6 generates ROS upon NIR light irradiation | [363,364] |
| Thioketal group | Used as gatekeeper. Cleaved when chlorin e6 generates ROS upon NIR light irradiation | [365] |
| Double bonds | Photosensitizer AsPCs2a generates ROS upon NIR light irradiation that oxidize double bonds of the lipids and increase membrane permeability | [229] |
| Light-Induced Conformational Changes | ||
| Perylene group | Their removal from a polymer chain upon light irradiation, 450 nm (perylene) or 365 nm (spiropyran and o-nitrobenzyl), induce a conformational change that open the pores and triggers drug release | [366] |
| Spiropyran group | [367] | |
| o-nitrobenzyl group | [368] | |
| Cinnamamide derivative | Used as stalks for grafting of nanovalves (grafted on the surface or as pendant groups in polymers). Reversible trans-to-cis conformation change when irradiated, in general, with UV light | [369] |
| Azobenzene derivatives | [370,371,372,373,374,375] | |
| Approach | Description | Reference | |
|---|---|---|---|
| Thermo-Responsive Disassembling Gatekeepers | |||
| DNA | Large DNA strands acting as gatekeepers that dehybridize above a certain temperature, triggering drug release | [231,384,385,386] | |
| Short DNA strands used as linkers for grafting bulky gatekeepers (proteins, small nanoparticles) that allow drug release when strands dehybridize upon heating | [387,388,389] | ||
| Peptides | Peptide sequences used as gatekeepers that self-assemble at physiological temperature and undergo disassembly when heated | [390,391,392] | |
| Heterodimeric peptide acting as gatekeeper that present a coiled coil conformation at physiological temperature. That 3D structure is lost when heat is applied, triggering drug release | [393] | ||
| Nanovalves | Supramolecular nanovalves attached to the surface through thermo-sensitive stalks | [394,395] | |
| LCST Polymers (Hydrophobic if T > LCST) | |||
| poly(urethane-amine) | LCST ca. 50 °C | Polymers form a polymeric network. Below LCTS hydrophilic chains hamper drug release. When T > LCTS, polymers become hydrophobic and the polymeric network shrinks, facilitating drug release. | [397] |
| p(MEO2MA-co-OEGMA) | LCST ca. 37 °C | [398] | |
| p(NIPAM) | LCST ca. 32 °C | [399,400] | |
| p(NIPAM-co-MPS) | LCST ca. 36 °C | [401,402] | |
| p(NIPAM-co-MAA) | LCST ca. 44 °C | [403,404] | |
| p(NIPAM-co-NHMA) | LCST ca. 42 °C | [137,405,406] | |
| UCST Polymers (Hydrophilic if T > UCST) | |||
| poly(acrylamide-co-acrylonitrile) | UCST ca. 42 °C | Polymers become hydrophilic above UCST, adopting an extended conformation that triggers drug release | [407] |
| p(NAGAm-co-NPhAm) | UCST ca. 45 °C | [408] | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gisbert-Garzarán, M.; Vallet-Regí, M. Influence of the Surface Functionalization on the Fate and Performance of Mesoporous Silica Nanoparticles. Nanomaterials 2020, 10, 916. https://doi.org/10.3390/nano10050916
Gisbert-Garzarán M, Vallet-Regí M. Influence of the Surface Functionalization on the Fate and Performance of Mesoporous Silica Nanoparticles. Nanomaterials. 2020; 10(5):916. https://doi.org/10.3390/nano10050916
Chicago/Turabian StyleGisbert-Garzarán, Miguel, and María Vallet-Regí. 2020. "Influence of the Surface Functionalization on the Fate and Performance of Mesoporous Silica Nanoparticles" Nanomaterials 10, no. 5: 916. https://doi.org/10.3390/nano10050916
APA StyleGisbert-Garzarán, M., & Vallet-Regí, M. (2020). Influence of the Surface Functionalization on the Fate and Performance of Mesoporous Silica Nanoparticles. Nanomaterials, 10(5), 916. https://doi.org/10.3390/nano10050916