Effect of Humidity on Light-Activated NO and NO2 Gas Sensing by Hybrid Materials
Abstract
1. Introduction
2. Materials and Methods
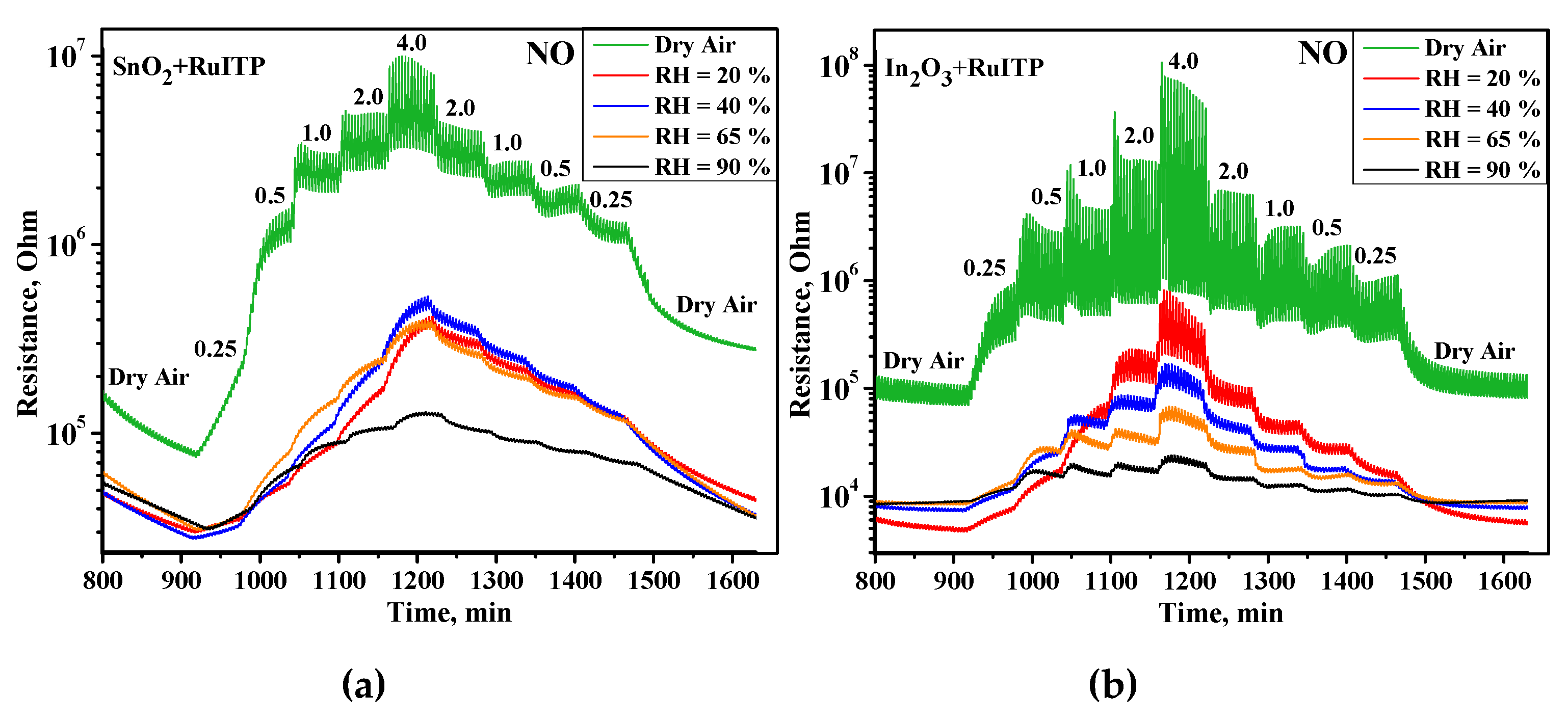
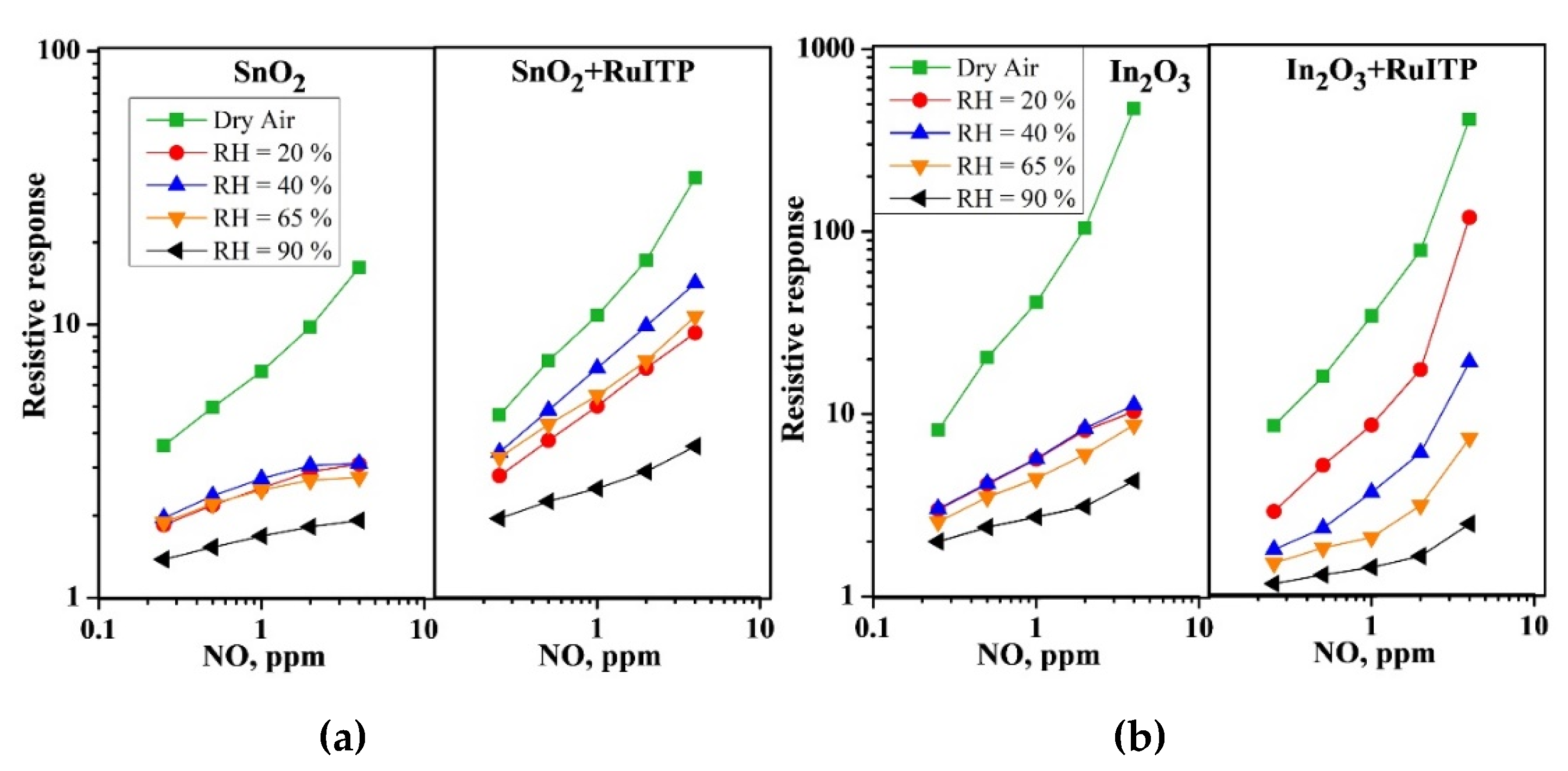
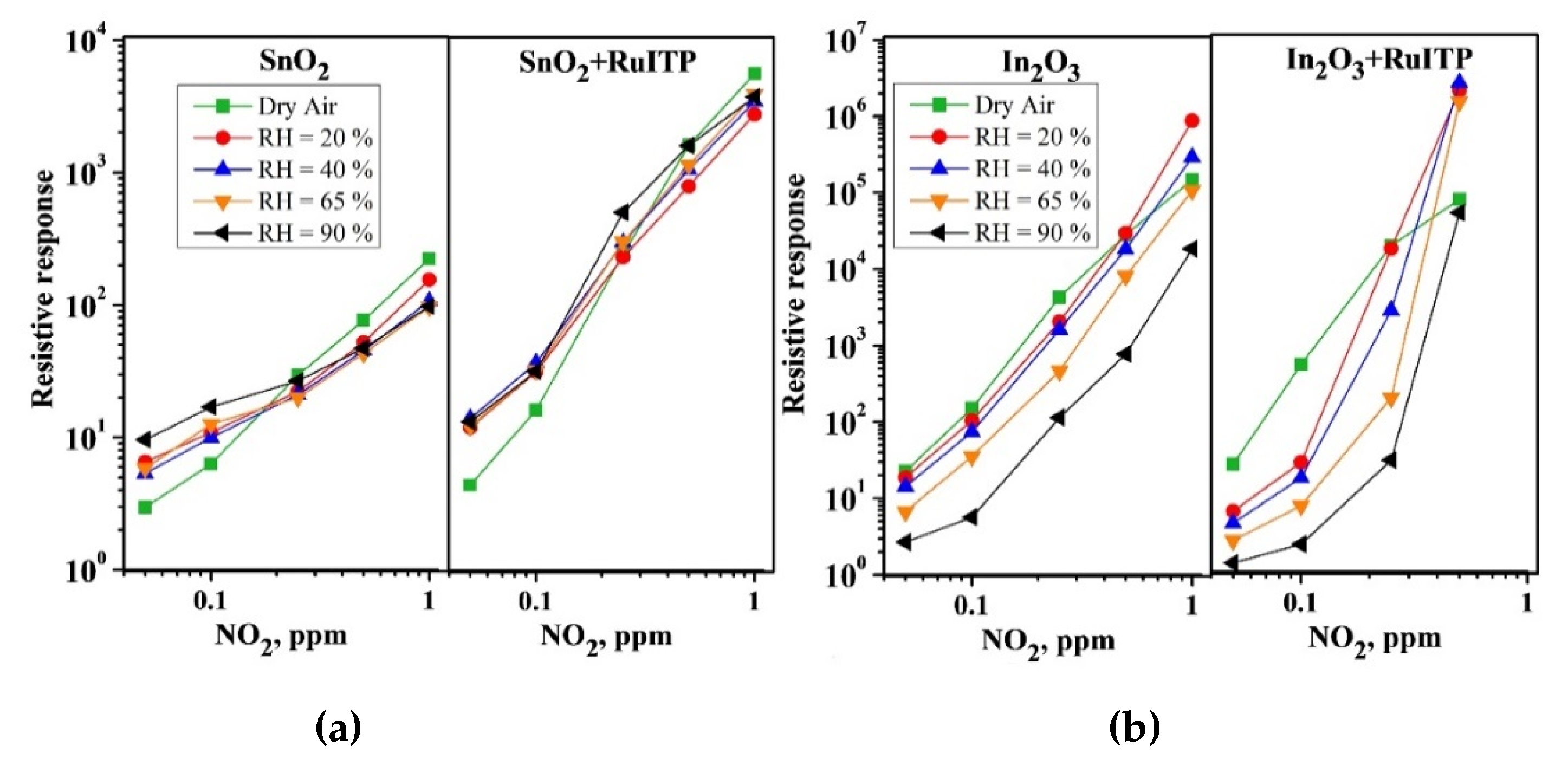
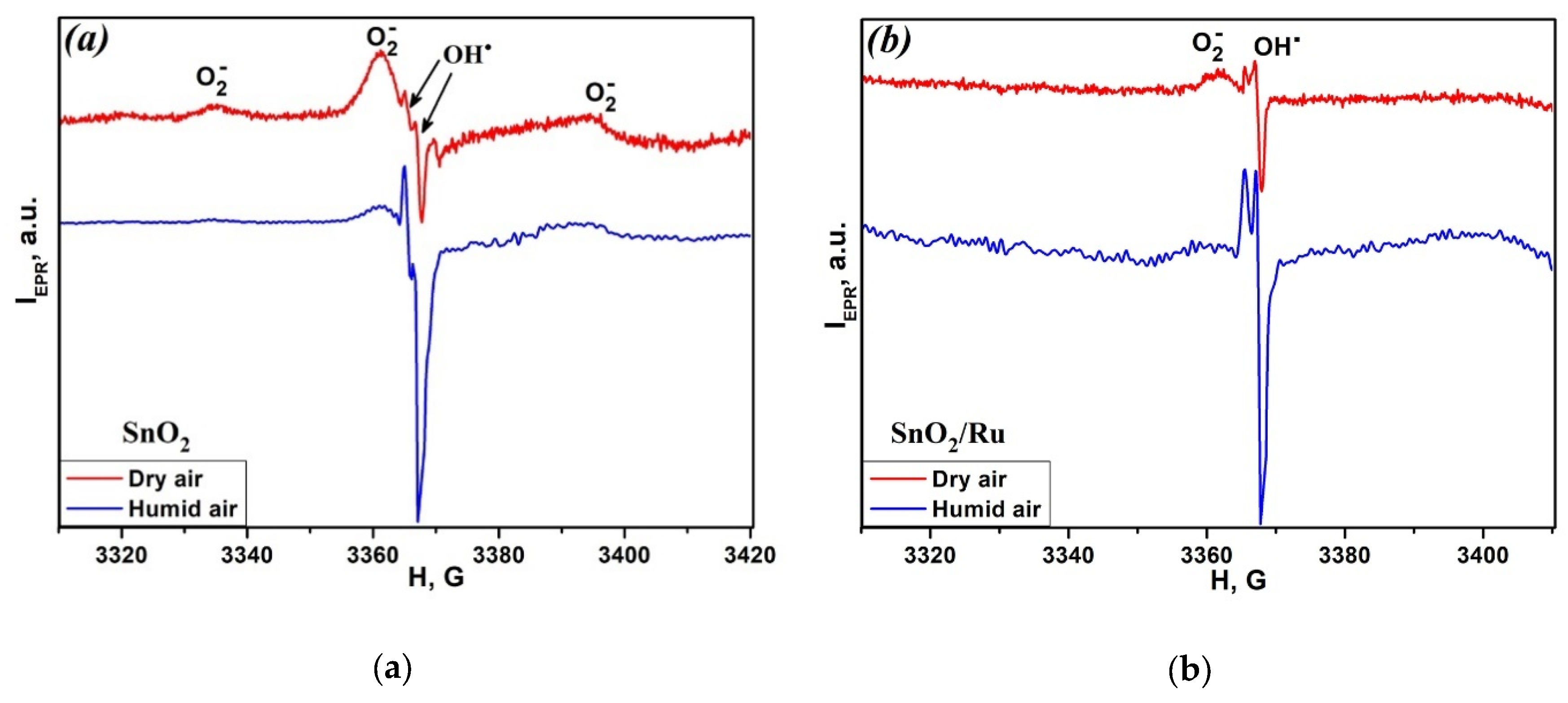
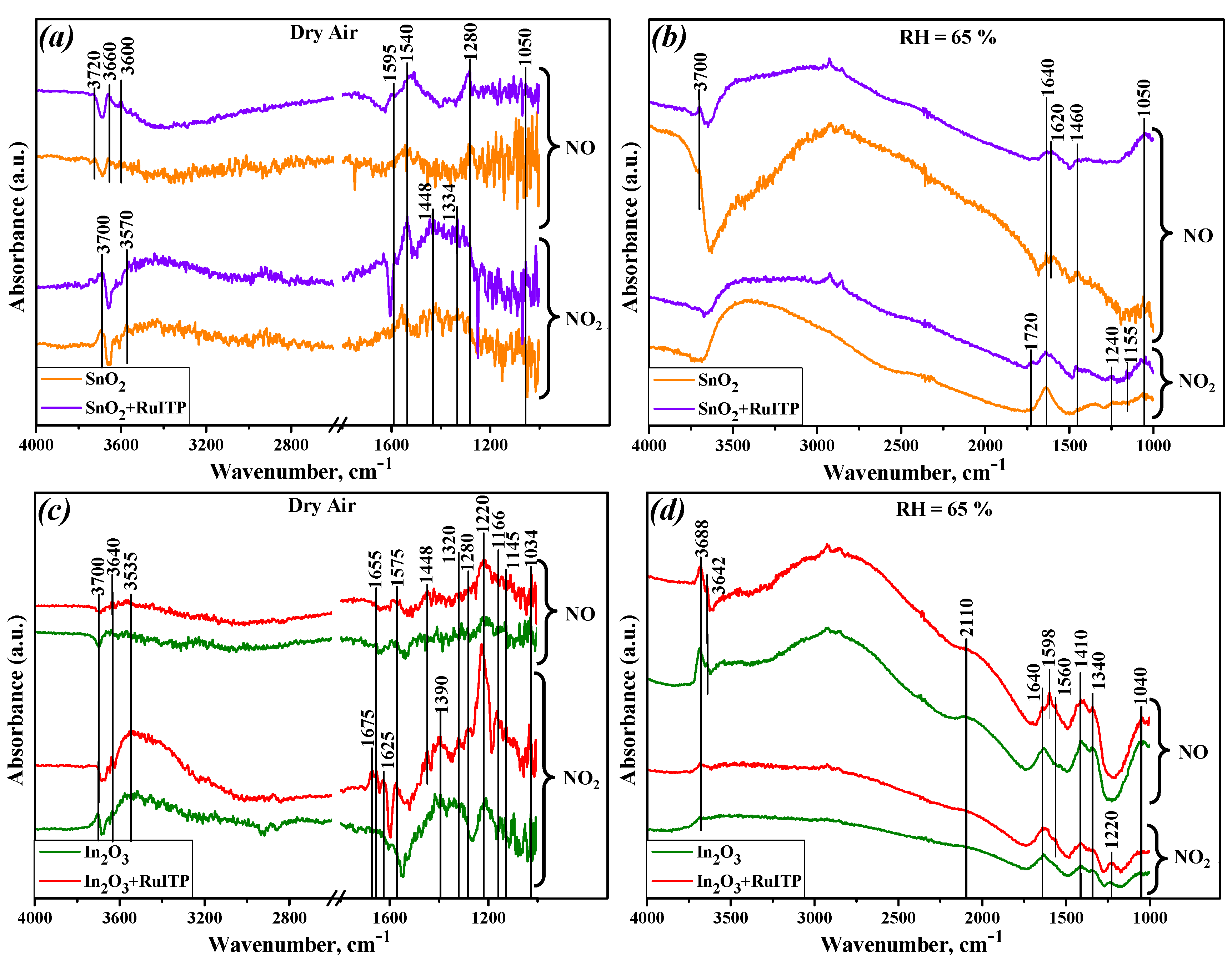
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). WHO Guidelines for Indoor Air Quality: Selected Pollutants. Available online: www.euro.who.int_data/assets/pdf_file/0009/128169/e94535/pdf (accessed on 30 March 2020).
- Menil, F.; Coillard, V.; Lucat, C. Critical Review of Nitrogen Monoxide Sensors for Exhaust Gases of Lean Burn Engines. Sens. Actuators B 2000, 67, 1–23. [Google Scholar] [CrossRef]
- Witschi, H. Ozone, nitrogen dioxide and lung cancer: A review of some recent issues and problems. Toxicology 1988, 48, 1–20. [Google Scholar] [CrossRef]
- Seinfeld, J.H.; Pandis, S.N. Chapter 20. Wet Deposition. In Atmospheric Chemistry and Physics: From Air Pollution to Climate Change, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; pp. 856–888. [Google Scholar]
- Barnes, P.J. Nitric Oxide and Airway Disease. Ann. Med. 1995, 27, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Lunt, A.; Ahmed, N.; Rafferty, G.F.; Dick, M.; Rees, D.; Height, S.; Thein, S.L.; Greenough, A. Airway and alveolar nitric oxide production, lung function, and pulmonary blood flow in sickle cell disease. Pediatri. Res. 2016, 79, 313–317. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pijnenburg, M.W.H.; De Jongste, J.C. Exhaled nitric oxide in childhood asthma: A review. Clin. Exp. Allergy 2008, 38, 246–259. [Google Scholar] [CrossRef]
- Righettoni, M.; Amann, A.; Pratsinis, S.E. Breath analysis by nanostructured metal oxides as chemoresistive gas sensors. Mater. Today 2015, 18, 163–171. [Google Scholar] [CrossRef]
- Hyodo, T.; Urata, K.; Kamada, K.; Ueda, T.; Shimizu, Y. Semiconductor-type SnO2-based NO2 sensors operated at room temperature under UV-light irradiation. Sens. Actuators B Chem. 2017, 253, 630–640. [Google Scholar] [CrossRef]
- Liu, B.; Luo, Y.; Li, K.; Wang, H.; Gao, L.; Duan, G. Room-Temperature NO2 Gas Sensing with Ultra-Sensitivity Activated by Ultraviolet Light Based on SnO2 Monolayer Array Film. Adv. Mater. Interfaces 2019, 1900376, 1–10. [Google Scholar]
- Fomekong, R.L.; Saruhan, B. Influence of Humidity on NO2-Sensing and Selectivity of Spray-CVD Grown ZnO Thin Film above 400 °C. Chemosensors 2019, 7, 42. [Google Scholar] [CrossRef]
- Belghachi, A.; Collins, R.A. The effects of humidity on phthalocyanine NO2 and NH3 sensors. J. Phys. D Appl. Phys 1990, 23, 223–227. [Google Scholar] [CrossRef]
- Ling, Z.; Leach, C. The effect of relative humidity on the NO2 sensitivity of a SnO2/WO3 heterojunction gas sensor. Sens. Actuators B 2004, 102, 102–106. [Google Scholar] [CrossRef]
- Nasriddinov, A.; Rumyantseva, M.; Shatalova, T.; Tokarev, S.; Yaltseva, P.; Fedorova, O.; Khmelevsky, N.; Gaskov, A. Organic-inorganic hybrid materials for room temperature light-activated sub-ppm NO detection. Nanomaterials 2020, 10, 70. [Google Scholar] [CrossRef]
- Rumyantseva, M.; Nasriddinov, A.; Vladimirova, S.; Fedorova, O.; Tokarev, S.; Krylov, I.; Drozdov, K.; Baranchikov, A.; Gaskov, A. Photosensitive organic-inorganic hybrid materials for room temperature gas sensor applications. Nanomaterials 2018, 8, 671. [Google Scholar] [CrossRef]
- Ruhland, B.; Becker, T.; Muller, G. Gas-kinetic interactions of nitrous oxides with SnO2 surfaces. Sens. Actuators B 1998, 50, 85–94. [Google Scholar] [CrossRef]
- Bârsan, N.; Weimar, U. Conduction model of metal oxide gas sensors. J. Electroceram. 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Rumyantseva, M.N.; Makeeva, E.A.; Badalyan, S.M.; Zhukova, A.A.; Gaskov, A.M. Nanocrystalline SnO2 and In2O3 as materials for gas sensors: The relationship between microstructure and oxygen chemisorption. Thin Solid Film. 2009, 518, 1283–1288. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Brinzari, V.; Golovanov, V.; Blinov, Y. Kinetics of gas response to reducing gases of SnO2 films, deposited by spray pyrolysis. Sens. Actuators B 2004, 98, 41–45. [Google Scholar] [CrossRef]
- Egashira, M.; Nakashima, M.; Kawasuma, S.; Selyama, T. Temperature programmed desorption study of water adsorbed on metal oxides. Part 2. Tin oxide surfaces. J. Phys. Chem. 1981, 85, 4125–4130. [Google Scholar] [CrossRef]
- Bârsan, N.; Schweizer-Berberich, M.; Gopel, W. Fundamental and practical aspects in the design of nanoscaled SnO2 gas sensors: A status report. Fresenius J. Anal. Chem. 1999, 365, 287–304. [Google Scholar] [CrossRef]
- Yamazoe, N.; Sakai, G.; Shimanoe, K. Oxide semiconductor gas sensors. Catal. Surv. Asia 2003, 7, 63–75. [Google Scholar] [CrossRef]
- Davydov, A.A. Chapter 2. The Nature of Oxide Surface Centers. In Molecular Spectroscopy of Oxide Catalyst Surfaces; Sheppard, N.T., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2003. [Google Scholar]
- Heiland, G.; Kohl, D. Chapter 2. Physical and Chemical Aspects of Oxidic Semiconductor Gas Sensors. In Chemical Sensor Technology; Seiyama, T., Ed.; Elsevier Science: Amsterdam, The Netherlands, 1988; Volume 1, pp. 15–38. [Google Scholar]
- Morrison, S.R. Chapter 5. Bonding of Foreign Species at the Solid Surface. In The Chemical Physics of Surfaces, 2nd ed.; Springer Science+ Business Media: New York, NY, USA, 1990; pp. 173–220. [Google Scholar]
- Henrich, V.A.; Cox, P.A. Chapter 6. Molecular Adsorption on Oxides. In The Surface Science of Metal Oxides; Cambridge University Press: Cambridge, UK, 1994; pp. 247–370. [Google Scholar]
- Gurlo, A. Interplay between O2 and SnO2: Oxygen ionosorption and spectroscopic evidence for adsorbed oxygen. Chem. Phys. Chem. 2006, 7, 2041–2052. [Google Scholar] [CrossRef]
- Konstantinova, E.A.; Pentegov, I.S.; Marikutsa, A.V.; Rumyantseva, M.N.; Gaskov, A.M.; Kashkarov, P.K. EPR study of nanocrystalline tin dioxide. Phys. Status Solidi C 2011, 8, 1957–1960. [Google Scholar] [CrossRef]
- Konstantinova, E.A.; Weidmann, J.; Dittrich, T. Influence of adsorbed water and oxygen on the photoluminescence and EPR of por-TiO2 (anatase). J. Porous Mater. 2000, 7, 389–392. [Google Scholar] [CrossRef]
- Weidmann, J.; Dittrich, T.; Konstantinova, E.A.; Lauermann, I.; Uhlendorf, I.; Koch, F. Influence of oxygen and water related surface defects on the sensitized TiO2 solar cell. Sol. Energy Mater. Sol. Cells 1999, 56, 153–165. [Google Scholar] [CrossRef]
- Yang, L.; Marikutsa, A.; Rumyantseva, M.; Konstantinova, E.; Khmelevsky, N.; Gaskov, A. Quasi similar routes of NO2 and NO sensing by nanocrystalline WO3: Evidence by in situ drift spectroscopy. Sensors 2019, 19, 3405. [Google Scholar] [CrossRef]
- Leblanc, E.; Perier-Camby, L.; Thomas, G.; Gibert, R.; Primet, M.; Gelin, P. NOx adsorption onto dehydroxylated or hydroxylated tin dioxide surface. Application to SnO2-based sensors. Sens. Actuators B 2000, 62, 67–72. [Google Scholar] [CrossRef]
- Hadjiivanov, K.I. Identification of neutral and charged NxOy surface species by IR spectroscopy. Catal. Rev. Sci. Eng. 2000, 42, 71–144. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds. Part A: Theory and Applications in Inorganic Chemistry, 6th ed.; John Wiley & Sons, Inc.: New Jersey, NJ, USA, 2009. [Google Scholar]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2001. [Google Scholar]
- Guglielminotti, E.; Boccuzzi, F. Nitric Oxide Adsorption and Nitric Oxide-Carbon Monoxide Interaction on Ru/ZnO Catalyst. J. Catal. 1993, 141, 486–493. [Google Scholar] [CrossRef]
- Valden, M.; Keiski, R.; Xiang, N.; Pere, J.; Aaltonen, J.; Pessa, M.; Maunula, T.; Savimaki, A.; Lahti, A.; Harkonen, M. Reactivity of Pd/Al2O3, Pd/La2O3–Al2O3 and Pd/LaAlO3 Catalysts for the Reduction of NO by CO: CO and NO Adsorption. J. Catal. 1996, 161, 614–625. [Google Scholar] [CrossRef]
- Chao, C.C.; Lunsford, J.H. Adsorption of nitric oxide on Y-type zeolites. Low-temperature infrared study. J. Am. Chem. Soc. 1971, 93, 6794–6800. [Google Scholar] [CrossRef]
- Kantcheva, M.; Bushev, V.; Hadjiivanov, K. Nitrogen dioxide adsorption on deuteroxylated titania (anatase). J. Chem. Soc. Faraday Trans. 1992, 88, 3087–3089. [Google Scholar] [CrossRef]
- Gil, B.; Datka, J.; Kubacka, A.; Janas, J.; Sulikowski, B. NO adsorption on the active sites of Co- and/or in-containing ferrierite catalysts for the CH4-SCR-NO process. Stud. Surf. Sci. Catal. 2005, 158, 1137–1144. [Google Scholar]
- Djonev, B.; Tsyntsarski, B.; Klissurski, D.; Hadjiivanov, K. IR spectroscopic study of NOx adsorption and NOx–O2 coadsorption on Co2+/SiO2 catalysts. J. Chem. Soc. Faraday Trans. 1997, 93, 4055–4063. [Google Scholar] [CrossRef]
- Chen, E.S.; Wentworth, W.E.; Chen, E.C.M. The electron affinities of NO and O2. J. Mol. Struct. 2002, 606, 1–7. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, L.; He, C.; Ma, D.; Lu, Z. Adsorption and oxidation of NO on various SnO2(110) surfaces: A density functional theory study. Sens. Actuators B 2015, 221, 717–722. [Google Scholar] [CrossRef]
- Sergent, N.; Epifani, M.; Comini, E.; Faglia, G.; Pagnier, T. Interactions of nanocrystalline tin oxide powder with NO2: A Raman spectroscopic study. Sens. Actuators B 2007, 126, 1–5. [Google Scholar] [CrossRef]
| Sample | Phase Composition | dXRD1, nm | dTEM2, nm | Ssurf3, m2/g | Average Pore Diameter, nm | , at. % | Rav5, Ohm Pure Air | SPh6, in Pure Air (λ = 470 nm) |
|---|---|---|---|---|---|---|---|---|
| SnO2 | SnO2, cassiterite In2O3, bixbyite | 4 ± 1 | 4 ± 1 | 115 ± 5 | 3–5; 70–80 | - | 7.8·104 | 1.00 |
| SnO2+RuITP | 7 ± 1 | 7 ± 2 | 90 ± 5 | 3–4 | 1.2 ± 0.1 | 6.9·105 | 1.22 | |
| In2O3 | - | 1.7·104 | 1.25 | |||||
| In2O3+RuITP | 2.1 ± 0.2 | 5.8·105 | 1.95 |
| Conditions | SnO2 | SnO2/Ru | ||||
|---|---|---|---|---|---|---|
| O2− | OH∙ | OH∙/O2− | O2− | OH∙ | OH∙/O2− | |
| Dry air | 8 × 1014 | 1.9 × 1014 | 0.24 | 2.5 × 1014 | 4.4 × 1014 | 1.2 |
| Humid air | 2.8 × 1014 | 4.3 × 1014 | 1.54 | 1014 | 1.1 × 1015 | 11 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasriddinov, A.; Rumyantseva, M.; Konstantinova, E.; Marikutsa, A.; Tokarev, S.; Yaltseva, P.; Fedorova, O.; Gaskov, A. Effect of Humidity on Light-Activated NO and NO2 Gas Sensing by Hybrid Materials. Nanomaterials 2020, 10, 915. https://doi.org/10.3390/nano10050915
Nasriddinov A, Rumyantseva M, Konstantinova E, Marikutsa A, Tokarev S, Yaltseva P, Fedorova O, Gaskov A. Effect of Humidity on Light-Activated NO and NO2 Gas Sensing by Hybrid Materials. Nanomaterials. 2020; 10(5):915. https://doi.org/10.3390/nano10050915
Chicago/Turabian StyleNasriddinov, Abulkosim, Marina Rumyantseva, Elizaveta Konstantinova, Artem Marikutsa, Sergey Tokarev, Polina Yaltseva, Olga Fedorova, and Alexander Gaskov. 2020. "Effect of Humidity on Light-Activated NO and NO2 Gas Sensing by Hybrid Materials" Nanomaterials 10, no. 5: 915. https://doi.org/10.3390/nano10050915
APA StyleNasriddinov, A., Rumyantseva, M., Konstantinova, E., Marikutsa, A., Tokarev, S., Yaltseva, P., Fedorova, O., & Gaskov, A. (2020). Effect of Humidity on Light-Activated NO and NO2 Gas Sensing by Hybrid Materials. Nanomaterials, 10(5), 915. https://doi.org/10.3390/nano10050915









