Comparing the Effects of Intracellular and Extracellular Magnetic Hyperthermia on the Viability of BxPC-3 Cells
Abstract
1. Introduction
2. Materials
3. Methods
3.1. Characterisation
3.2. Iron Quantification
3.3. Heating Performance
3.4. In Vitro Cytotoxicity
3.5. Cell Uptake and Prussian Blue Staining
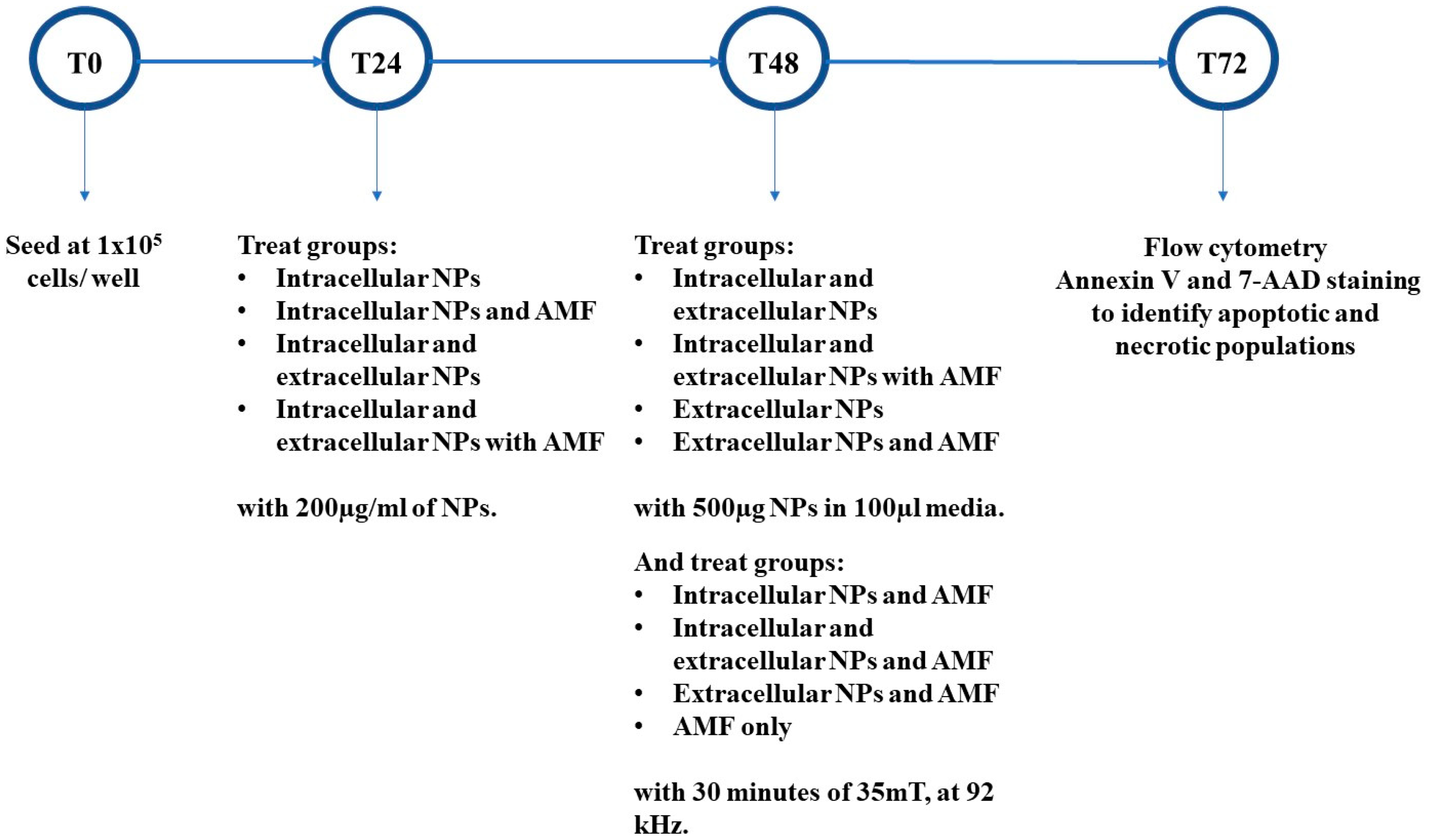
3.6. In Vitro Magnetic Hyperthermia
3.7. Apoptosis/Necrosis Detection
3.8. Caspase 3 Activity
3.9. Statistical Analysis
4. Results
4.1. Nanoparticle Characterization and Heating Performance
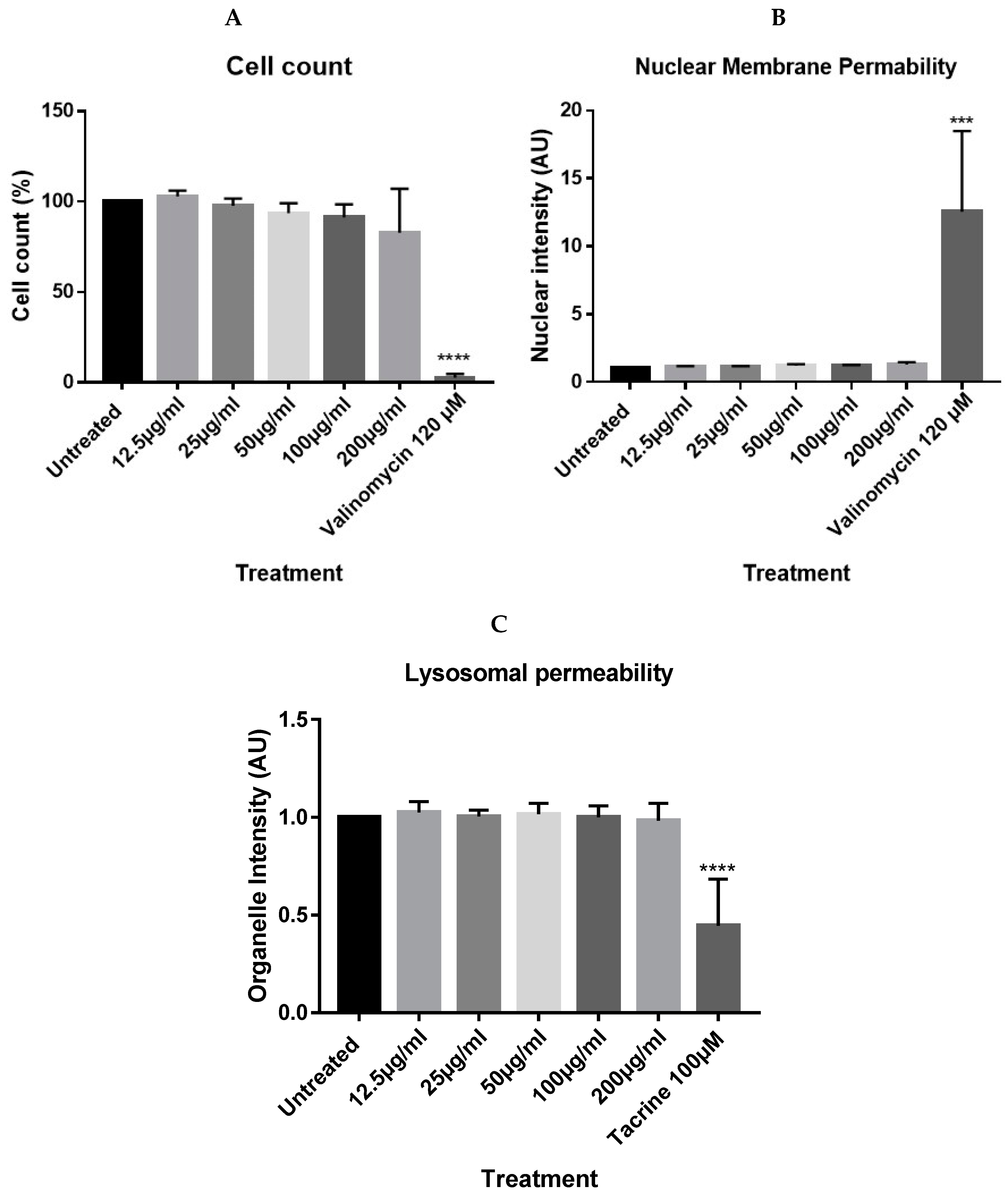
4.2. In Vitro Cytotoxicity
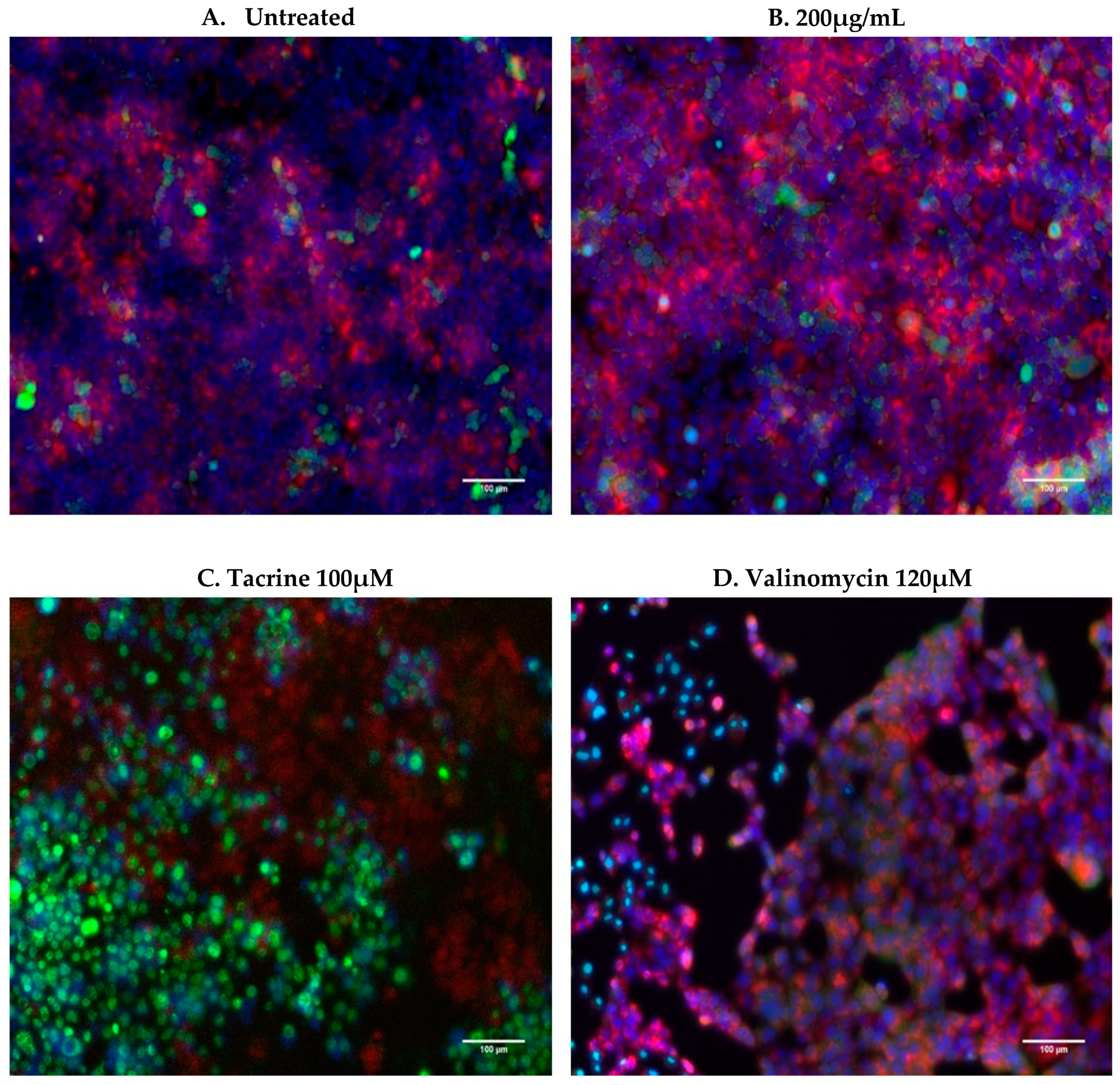
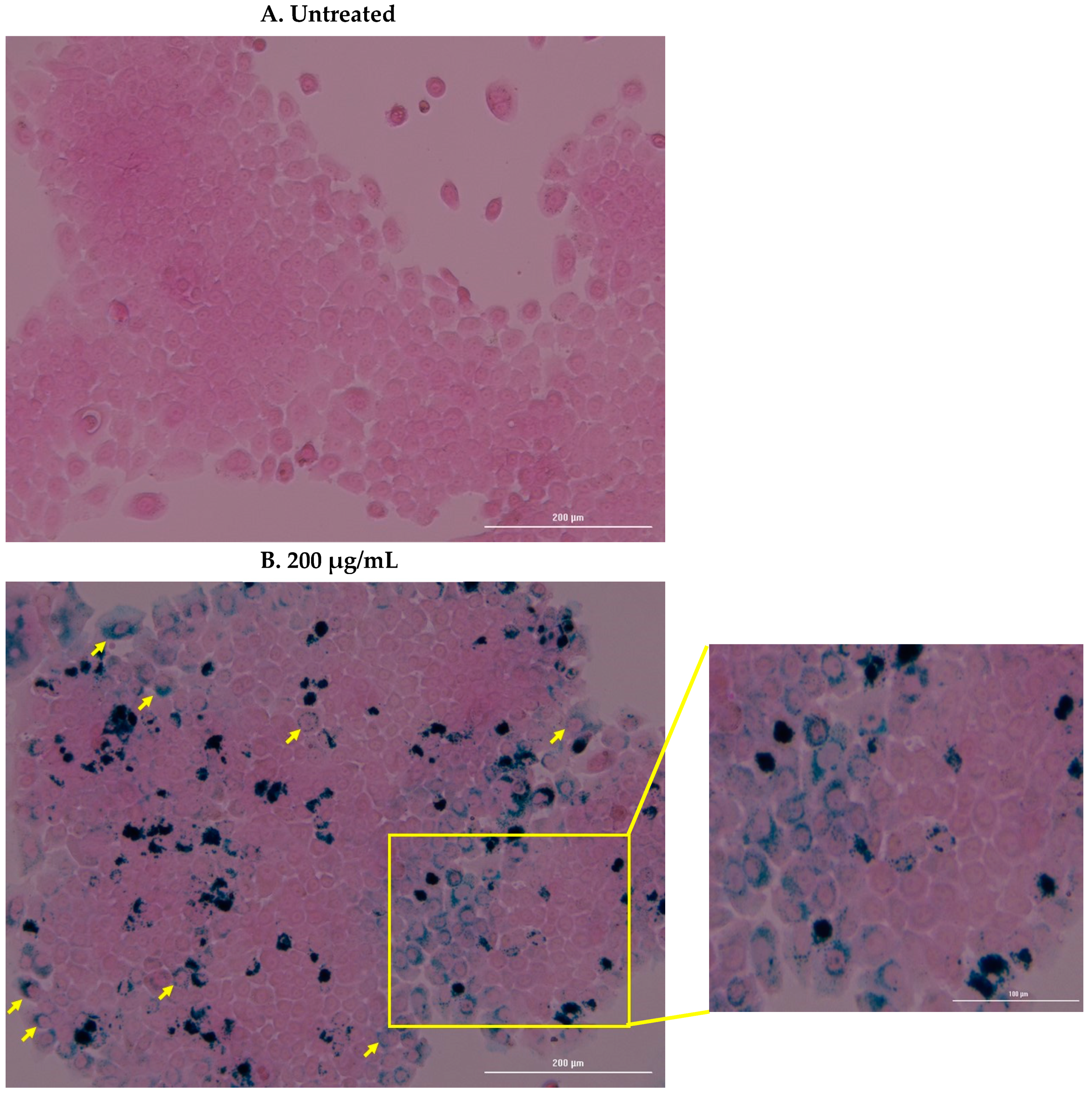
4.3. Cell Uptake and Prussian Blue Staining
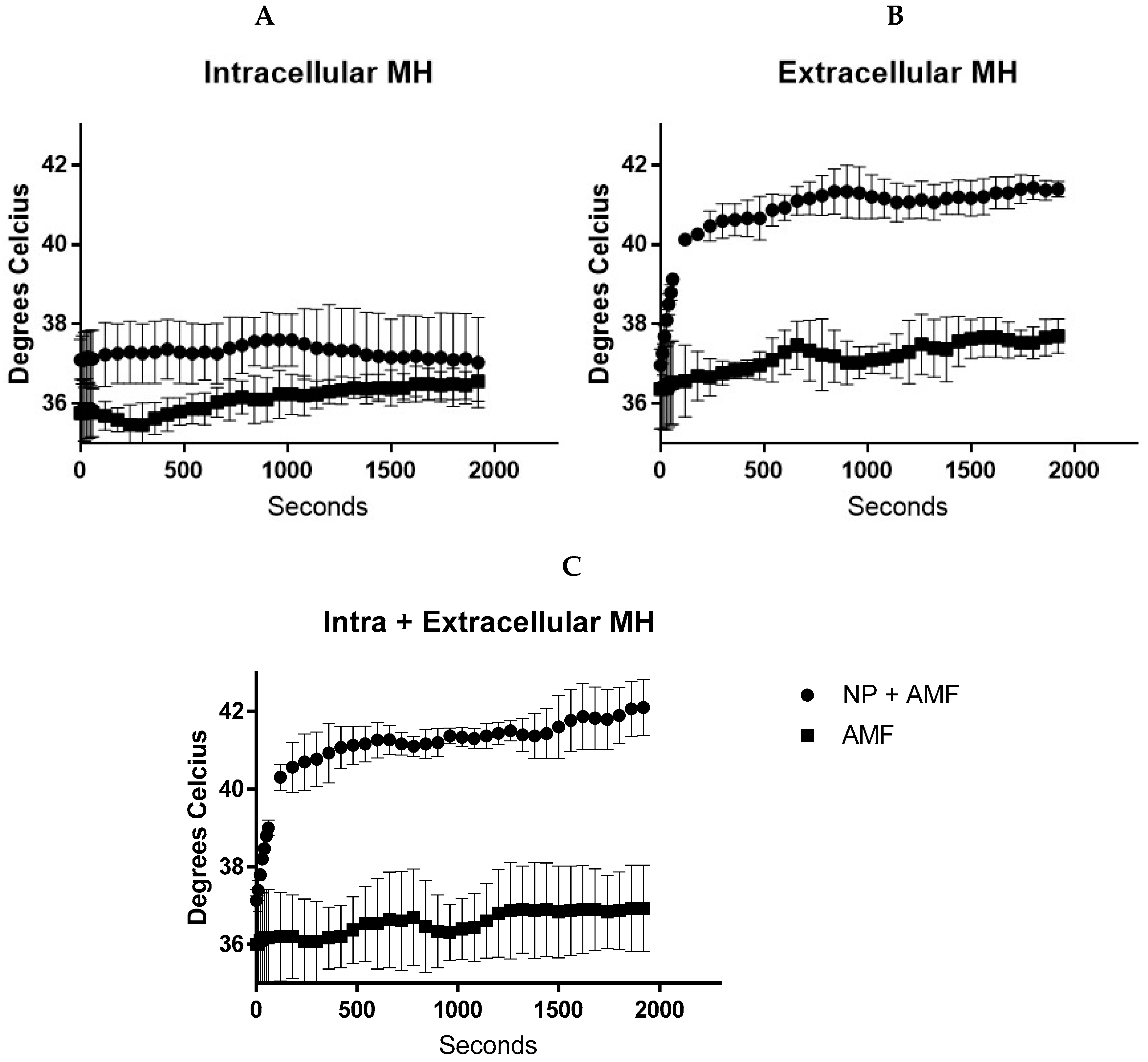
4.4. Temperature Generation during In Vitro Magnetic Hyperthermia
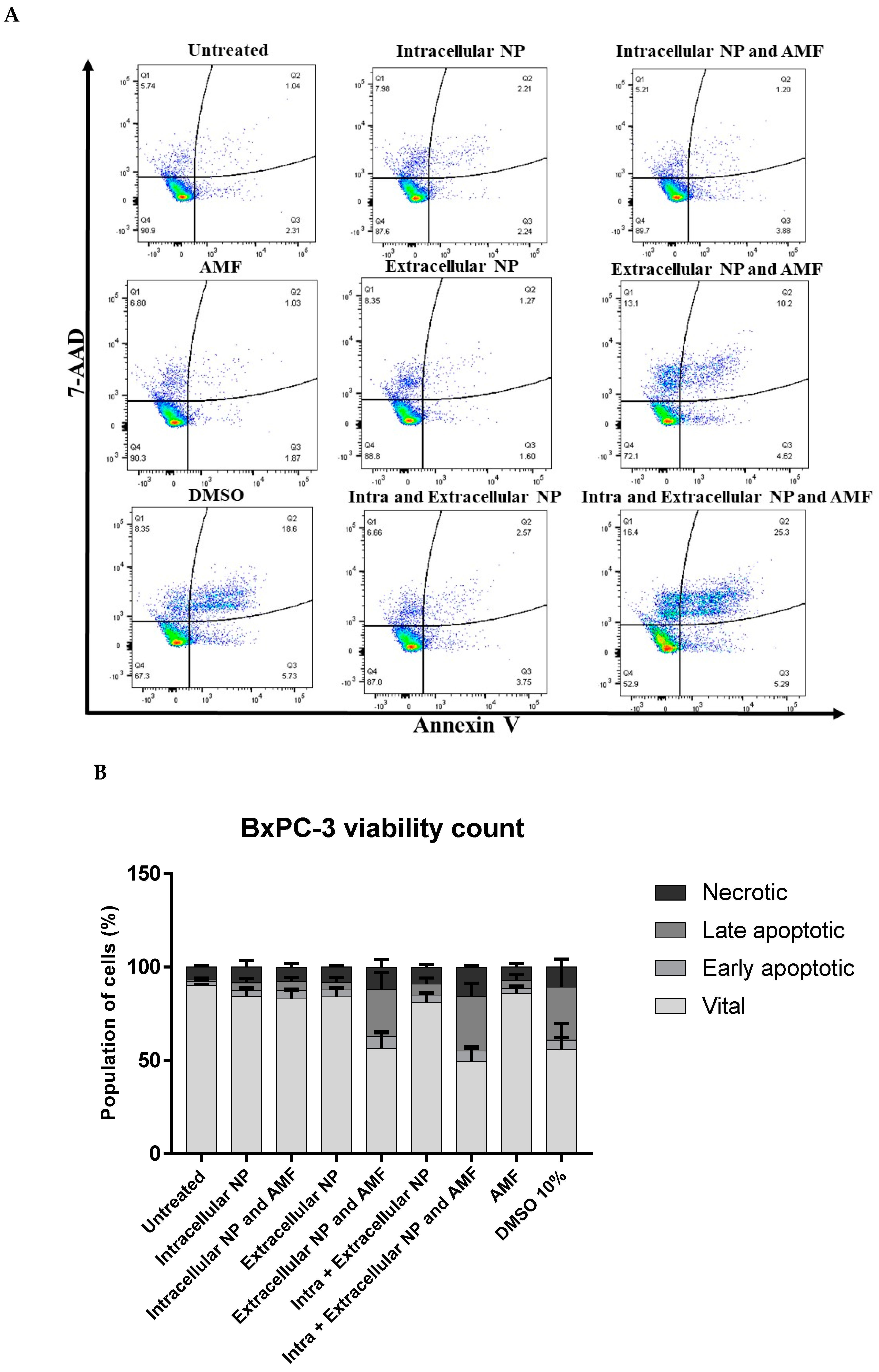
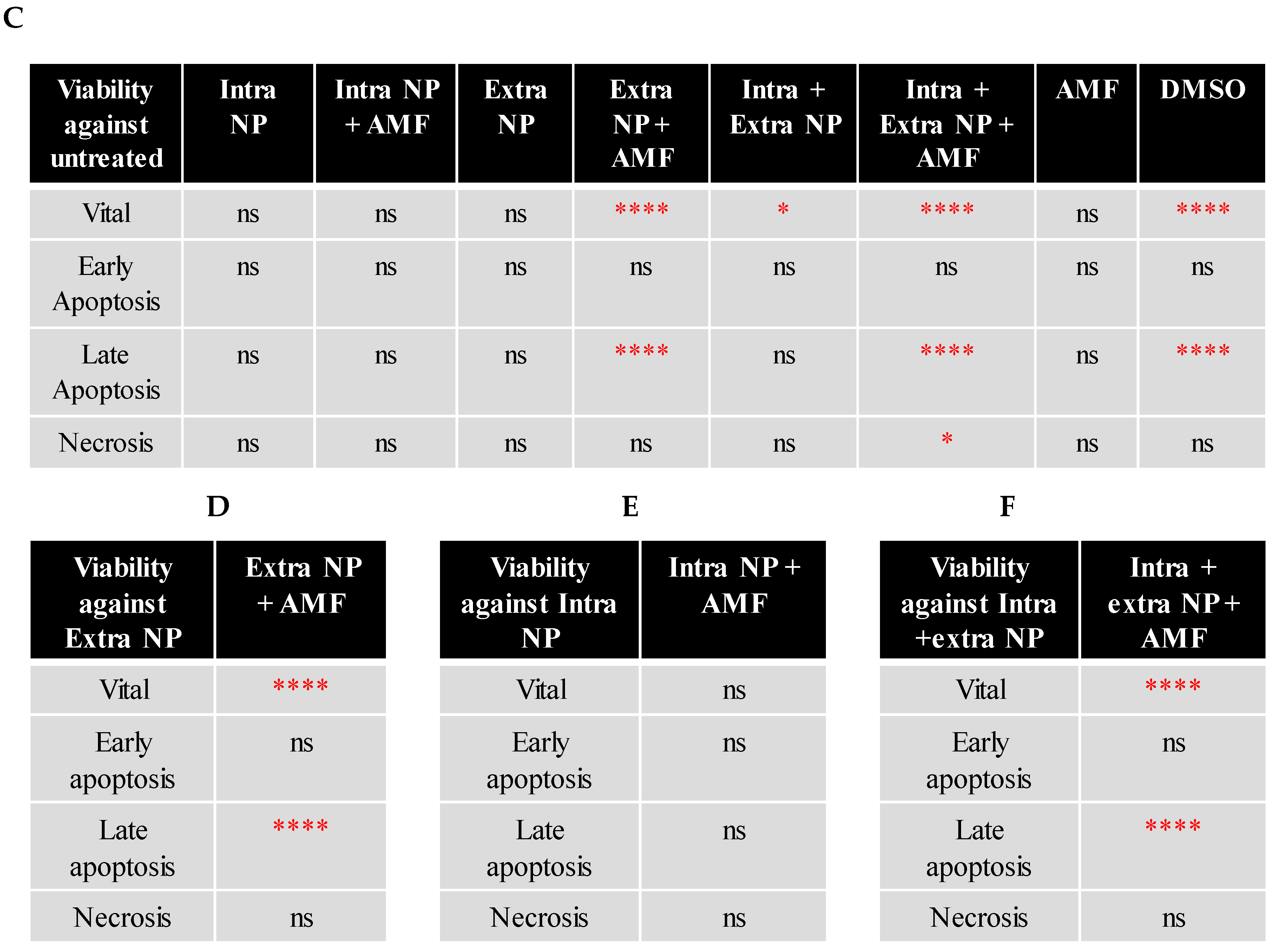
4.5. Cell Viability Detection Following Magnetic Hyperthermia
4.6. Caspase Activity
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ansari, M.O.; Ahmad, M.F.; Shadab, G.G.H.A.; Siddique, H.R. Superparamagnetic iron oxide nanoparticles based cancer theranostics: A double edge sword to fight against cancer. J. Drug Deliv. Sci. Technol. 2018, 45, 177–183. [Google Scholar] [CrossRef]
- Rosen, J.; Chan, L.; Shieh, D.-B.; Gu, F. Iron oxide nanoparticles for targeted cancer imaging and diagnostics. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Colombo, M.; Prosperi, D. Recent advances in magnetic fluid hyperthermia for cancer therapy. Colloids Surf. B Biointerfaces 2019, 174, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Aliru, M.L.; Chadha, A.S.; Asea, A.; Krishnan, S. Hyperthermia using nanoparticles--Promises and pitfalls. Int. J. Hyperthermia 2016, 32, 76–88. [Google Scholar] [CrossRef]
- Chang, D.; Lim, M.; Goos, J.A.; Qiao, R.; Ng, Y.Y.; Mansfeld, F.M.; Jackson, M.; Davis, T.P.; Kavallaris, M. Biologically Targeted Magnetic Hyperthermia: Potential and Limitations. Front. Pharmacol. 2018, 9, 831. [Google Scholar] [CrossRef]
- Hedayatnasab, Z.; Abnisa, F.; Daud, W.M.A.W. Review on magnetic nanoparticles for magnetic nanofluid hyperthermia application. Mater. Des. 2017, 123, 174–196. [Google Scholar] [CrossRef]
- Ortega, D.; Pankhurst, Q. Magnetic Hyperthermia. Nanoscience 2013, 1, e88. [Google Scholar]
- Kuncser, A.; Iacob, N.; Kuncser, V. On the relaxation time of interacting superparamagnetic nanoparticles and implications for magnetic fluid hyperthermia. Beilstein J. Nanotechnol. 2019, 10, 1280–1289. [Google Scholar] [CrossRef]
- Périgo Élio, A.; Hemery, G.; Sandre, O.; Ortega, D.; Garaio, E.; Plazaola, F.; Teran, F.J. Fundamentals and advances in magnetic hyperthermia. Appl. Phys. Rev. 2015, 2, 041302. [Google Scholar] [CrossRef]
- Dennis, C.L.; Ivkov, R. Physics of heat generation using magnetic nanoparticles for hyperthermia. Int. J. Hyperthermia 2013, 29, 715–729. [Google Scholar] [CrossRef]
- Shaterabadi, Z.; Nabiyouni, G.; Soleymani, M. Physics responsible for heating efficiency and self-controlled temperature rise of magnetic nanoparticles in magnetic hyperthermia therapy. Prog. Biophys. Mol. Biol. 2018, 133, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, R.K.; Medal, R.; Shorey, W.D.; Hanselman, R.C.; Parrott, J.C.; Taylor, C.B. Selective inductive heating of lymph nodes. Ann. Surg. 1957, 146, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, R.K.; Shorey, W.D.; Hanselman, R.C.; Depeyster, F.A.; Yang, J.; Medal, R. Effects of electromagnetic heating on internal viscera: A preliminary to the treatment of human tumors. Ann. Surg. 1965, 161, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Wang, L.F.; Ding, W.J.; Wang, H.; Zhou, J.M.; Jin, H.K.; Su, S.F.; Ouyang, W.W. Clinical trials of magnetic induction hyperthermia for treatment of tumours. OA Cancer 2014, 18, 1–6. [Google Scholar]
- MagForce. Available online: https://www.magforce.com/home/ (accessed on 23 March 2020).
- Kossatz, S.; Grandke, J.; Couleaud, P.; Latorre, A.; Aires, A.; Crosbie-Staunton, K.; Ludwig, R.; Dähring, H.; Ettelt, V.; Lazaro-Carrillo, A.; et al. Efficient treatment of breast cancer xenografts with multifunctionalized iron oxide nanoparticles combining magnetic hyperthermia and anti-cancer drug delivery. Breast Cancer Res. BCR 2015, 17, 66. [Google Scholar] [CrossRef] [PubMed]
- Sanhaji, M.; Göring, J.; Couleaud, P.; Aires, A.; Cortajarena, A.L.; Courty, J.; Prina-Mello, A.; Stapf, M.; Ludwig, R.; Volkov, Y.; et al. The phenotype of target pancreatic cancer cells influences cell death by magnetic hyperthermia with nanoparticles carrying gemicitabine and the pseudo-peptide NucAnt. Nanomed. Nanotechnol. Biol. Med. 2019, 20, 101983. [Google Scholar] [CrossRef]
- Maguire, C.M.; Sillence, K.; Roesslein, M.; Hannell, C.; Suarez, G.; Sauvain, J.J.; Capracotta, S.; Contal, S.; Cambier, S.; El Yamani, N.; et al. Benchmark of Nanoparticle Tracking Analysis on Measuring Nanoparticle Sizing and Concentration. J. Micro Nano-Manuf. 2017, 5, 041002. [Google Scholar] [CrossRef]
- Hole, P.; Sillence, K.; Hannell, C.; Maguire, C.M.; Roesslein, M.; Suarez, G.; Capracotta, S.; Magdolenova, Z.; Horev-Azaria, L.; Dybowska, A.; et al. Interlaboratory comparison of size measurements on nanoparticles using nanoparticle tracking analysis (NTA). J. Nanopart. Res. 2013, 15, 2101. [Google Scholar] [CrossRef]
- Maguire, C. Particle Tracking Analysis. 2018. Available online: http://www.euncl.eu/about-us/assay-cascade/PDFs/PCC/EUNCL_PCC_023.pdf?m=1526712237& (accessed on 23 March 2020).
- Calzolai, L. Measuring Batch Mode DLS. 2015. Available online: http://www.euncl.eu/about-us/assay-cascade/PDFs/Prescreening/EUNCL-PCC-001.pdf?m=1468937875& (accessed on 23 March 2020).
- Kallumadil, M.; Tada, M.; Nakagawa, T.; Abe, M.; Southern, P.; Pankhurst, Q.A. Suitability of commercial colloids for magnetic hyperthermia. J. Magn. Magn. Mater. 2009, 321, 1509–1513. [Google Scholar] [CrossRef]
- Gao, C.; Ding, Y.; Zhong, L.; Jiang, L.; Geng, C.; Yao, X.; Cao, J. Tacrine induces apoptosis through lysosome- and mitochondria-dependent pathway in HepG2 cells. Toxicol. In Vitro 2014, 28, 667–674. [Google Scholar] [CrossRef]
- Dise, C.A.; Goodman, D.B. The relationship between valinomycin-induced alterations in membrane phospholipid fatty acid turnover, membrane potential, and cell volume in the human erythrocyte. J. Biol. Chem. 1985, 260, 2869–2874. [Google Scholar] [PubMed]
- Klein, B.; Wörndl, K.; Lütz-Meindl, U.; Kerschbaum, H.H. Perturbation of intracellular K+ homeostasis with valinomycin promotes cell death by mitochondrial swelling and autophagic processes. Apoptosis 2011, 16, 1101. [Google Scholar] [CrossRef] [PubMed]
- Calero, M.; Chiappi, M.; Lazaro-Carrillo, A.; Rodríguez, M.J.; Chichón, F.J.; Crosbie-Staunton, K.; Prina-Mello, A.; Volkov, Y.; Villanueva, Á.; Carrascosa, J.L. Characterization of interaction of magnetic nanoparticles with breast cancer cells. J. Nanobiotechnol. 2015, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Prina-Mello, A.; Crosbie-Staunton, K.; Salas, G.; Morales, M.D.P.; Volkov, Y. Multiparametric Toxicity Evaluation of SPIONs by High Content Screening Technique: Identification of Biocompatible Multifunctional Nanoparticles for Nanomedicine. IEEE Trans. Magn. 2013, 49, 377–382. [Google Scholar] [CrossRef]
- Miyamoto, R.; Oda, T.; Hashimoto, S.; Kurokawa, T.; Inagaki, Y.; Shimomura, O.; Ohara, Y.; Yamada, K.; Akashi, Y.; Enomoto, T.; et al. Cetuximab delivery and antitumor effects are enhanced by mild hyperthermia in a xenograft mouse model of pancreatic cancer. Cancer Sci. 2016, 107, 514–520. [Google Scholar] [CrossRef]
- Hilger, I. In vivo applications of magnetic nanoparticle hyperthermia. Int. J. Hyperthermia 2013, 29, 828–834. [Google Scholar] [CrossRef]
- Blanco-Andujar, C.; Ortega, D.; Southern, P.; Nesbitt, S.A.; Thanh, N.T.; Pankhurst, Q.A. Real-time tracking of delayed-onset cellular apoptosis induced by intracellular magnetic hyperthermia. Nanomedicine 2016, 11, 121–136. [Google Scholar] [CrossRef]
- Ludwig, R.; Teran, F.J.; Teichgraeber, U.; Hilger, I. Nanoparticle-based hyperthermia distinctly impacts production of ROS, expression of Ki-67, TOP2A, and TPX2, and induction of apoptosis in pancreatic cancer. Int. J. Nanomed. 2017, 12, 1009–1018. [Google Scholar] [CrossRef]
- Rego, G.N.A.; Mamani, J.B.; Souza, T.K.F.; Nucci, M.P.; Da Silva, H.R.; Gamarra, L.F. Therapeutic evaluation of magnetic hyperthermia using Fe3O4-aminosilane-coated iron oxide nanoparticles in glioblastoma animal model. Einstein (Sao Paulo) 2019, 17, eAO4786. [Google Scholar] [CrossRef]
- Asin, L.; Ibarra, M.R.; Tres, A.; Goya, G.F. Controlled Cell Death by Magnetic Hyperthermia: Effects of Exposure Time, Field Amplitude, and Nanoparticle Concentration. Pharm. Res. 2012, 29, 1319–1327. [Google Scholar] [CrossRef]
- Oh, Y.; Lee, N.; Kang, H.W. In vitro study on apoptotic cell death by effective magnetic hyperthermia with chitosan-coated MnFe2O4. Nanotechnology 2016, 27, 115101. [Google Scholar] [CrossRef] [PubMed]
- Poller, J.M.; Zaloga, J.; Schreiber, E.; Unterweger, H.; Janko, C.; Radon, P.; Eberbeck, D.; Trahms, L.; Alexiou, C.; Friedrich, R.P. Selection of potential iron oxide nanoparticles for breast cancer treatment based on in vitro cytotoxicity and cellular uptake. Int. J. Nanomed. 2017, 12, 3207–3220. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Xie, Q.; Zhang, B.; Chen, H.; Tang, J.; Lei, Z.; Wu, M.; Zhang, D.; Hu, J. Neural Induction Potential and MRI of ADSCs Labeled Cationic Superparamagnetic Iron Oxide Nanoparticle In Vitro. Contrast Media Mol. Imaging 2018, 2018, 6268437. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Zhang, B.; Huang, Y.; Fan, Z.; Zhao, Y. Enhanced cellular uptake of iron oxide nanoparticles modified with 1,2-dimyristoyl-sn-glycero-3-phosphocholine. RSC Adv. 2017, 7, 38001–38007. [Google Scholar] [CrossRef]
- Zembruski, N.C.; Stache, V.; Haefeli, W.E.; Weiss, J. 7-Aminoactinomycin D for apoptosis staining in flow cytometry. Anal. Biochem. 2012, 429, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Fadok, V.A.; Bratton, D.L.; Frasch, S.C.; Warner, M.L.; Henson, P.M. The role of phosphatidylserine in recognition of apoptotic cells by phagocytes. Cell Death Differ. 1998, 5, 551–562. [Google Scholar] [CrossRef]
- Berghe, T.V.; Vanlangenakker, N.; Parthoens, E.; Deckers, W.; Devos, M.; Festjens, N.; Guerin, C.J.; Brunk, U.T.; Declercq, W.; Vandenabeele, P. Necroptosis, necrosis and secondary necrosis converge on similar cellular disintegration features. Cell Death Differ. 2009, 17, 922. [Google Scholar] [CrossRef]
- Krysko, D.V.; Berghe, T.V.; D’Herde, K.; Vandenabeele, P. Apoptosis and necrosis: Detection, discrimination and phagocytosis. Methods 2008, 44, 205–221. [Google Scholar] [CrossRef]
- Porter, A.G.; Jänicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef]
- Ludwig, R.; Stapf, M.; Dutz, S.; Müller, R.; Teichgräber, U.; Hilger, I. Structural properties of magnetic nanoparticles determine their heating behavior—An estimation of the in vivo heating potential. Nanoscale Res. Lett. 2014, 9, 602. [Google Scholar] [CrossRef]
- Ludwig, R.; Stapf, M.; Dutz, S.; Müller, R.; Teichgräber, U.; Hilger, I. Combined Magnetic Hyperthermia and Immune Therapy for Primary and Metastatic Tumor Treatments. ACS Nano 2020, 14, 1033–1044. [Google Scholar]
- Chen, L.; Chen, C.; Wang, P.; Song, T. Mechanisms of Cellular Effects Directly Induced by Magnetic Nanoparticles under Magnetic Fields. J. Nanomater. 2017, 2017, 1564634. [Google Scholar] [CrossRef]


| Stain | Excitation/Emission (nm) | Stock Concentration |
|---|---|---|
| Lysotracker® red | 577/590 | 1 mM |
| YO-PRO®-1 | 491/509 | 1 mM |
| Hoechst 33342 | 350/461 | 16.2 mM |
| Measured Parameter | Value (Technique) |
|---|---|
| Mean hydrodynamic size | 100.0 ± 2.6 nm (NTA) 91.2 nm (DLS) |
| Nanoparticle number | 9.48 × 1013 ± 4.60 × 1012 NP/mL (NTA) |
| Polydispersity index | 0.145 (DLS) |
| Zeta potential | −21.0 ± 5.86 mV (DLS) |
| Mean dry size | 11 ± 3 nm (TEM) |
| Specific absorbance rate | 98 W/gFe |
| Intrinsic loss power | 1.4 nHm2/kg |
| Fe content | 0.736 ± 0.01 mg Fe/mg NP (AAS) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hannon, G.; Bogdanska, A.; Volkov, Y.; Prina-Mello, A. Comparing the Effects of Intracellular and Extracellular Magnetic Hyperthermia on the Viability of BxPC-3 Cells. Nanomaterials 2020, 10, 593. https://doi.org/10.3390/nano10030593
Hannon G, Bogdanska A, Volkov Y, Prina-Mello A. Comparing the Effects of Intracellular and Extracellular Magnetic Hyperthermia on the Viability of BxPC-3 Cells. Nanomaterials. 2020; 10(3):593. https://doi.org/10.3390/nano10030593
Chicago/Turabian StyleHannon, Gary, Anna Bogdanska, Yuri Volkov, and Adriele Prina-Mello. 2020. "Comparing the Effects of Intracellular and Extracellular Magnetic Hyperthermia on the Viability of BxPC-3 Cells" Nanomaterials 10, no. 3: 593. https://doi.org/10.3390/nano10030593
APA StyleHannon, G., Bogdanska, A., Volkov, Y., & Prina-Mello, A. (2020). Comparing the Effects of Intracellular and Extracellular Magnetic Hyperthermia on the Viability of BxPC-3 Cells. Nanomaterials, 10(3), 593. https://doi.org/10.3390/nano10030593






