Gold Nanoparticle Self-Aggregation on Surface with 1,6-Hexanedithiol Functionalization
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussions
3.1. Morphology Characterization
3.1.1. Atomic Force Microscope (AFM)
3.1.2. Scanning Electron Microscopy
3.1.3. X-Ray Reflectivity (XRR)
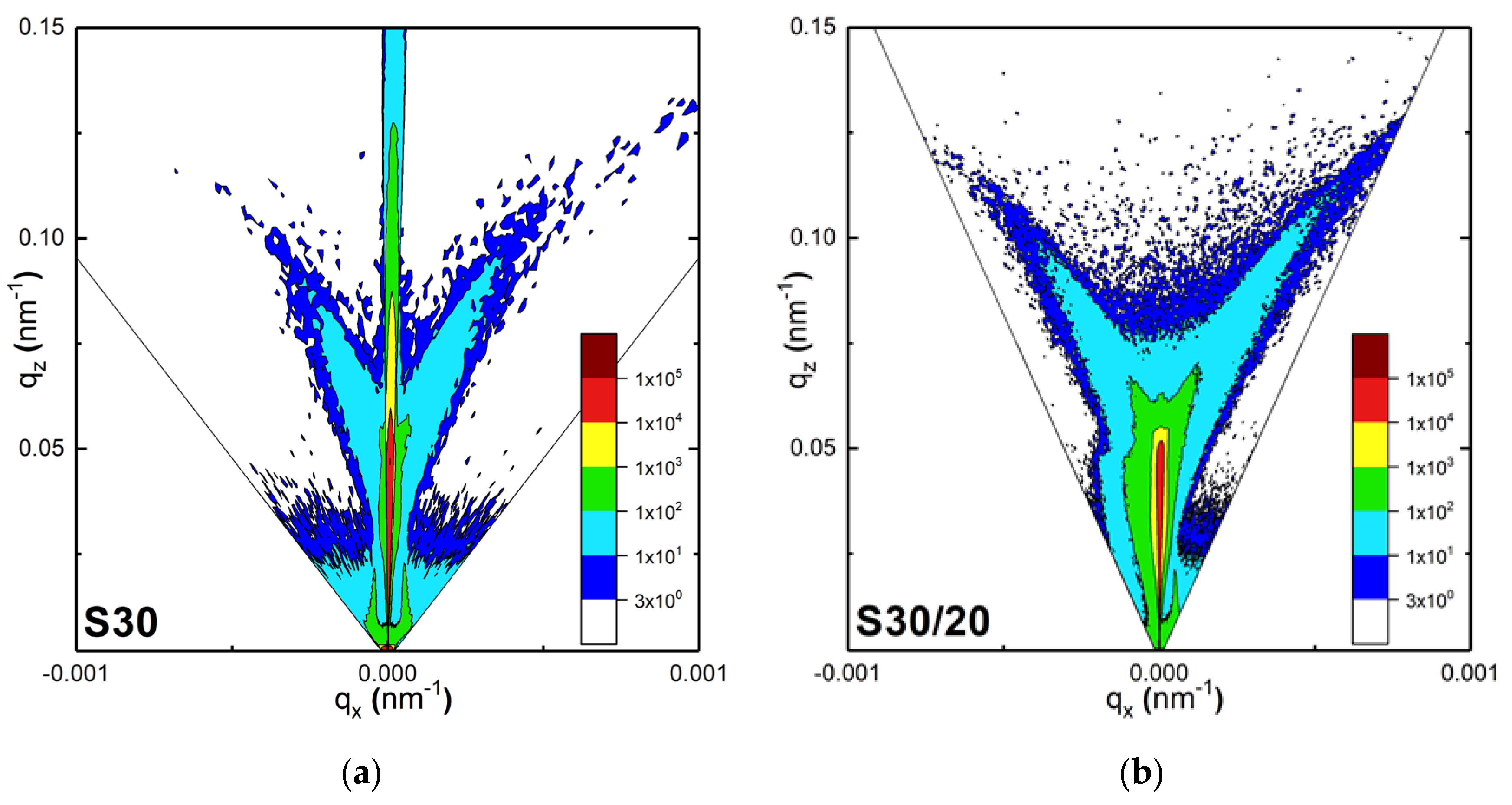
3.1.4. Reciprocal Space Maps (RSM)
3.2. Optical Characteristics of SAMs
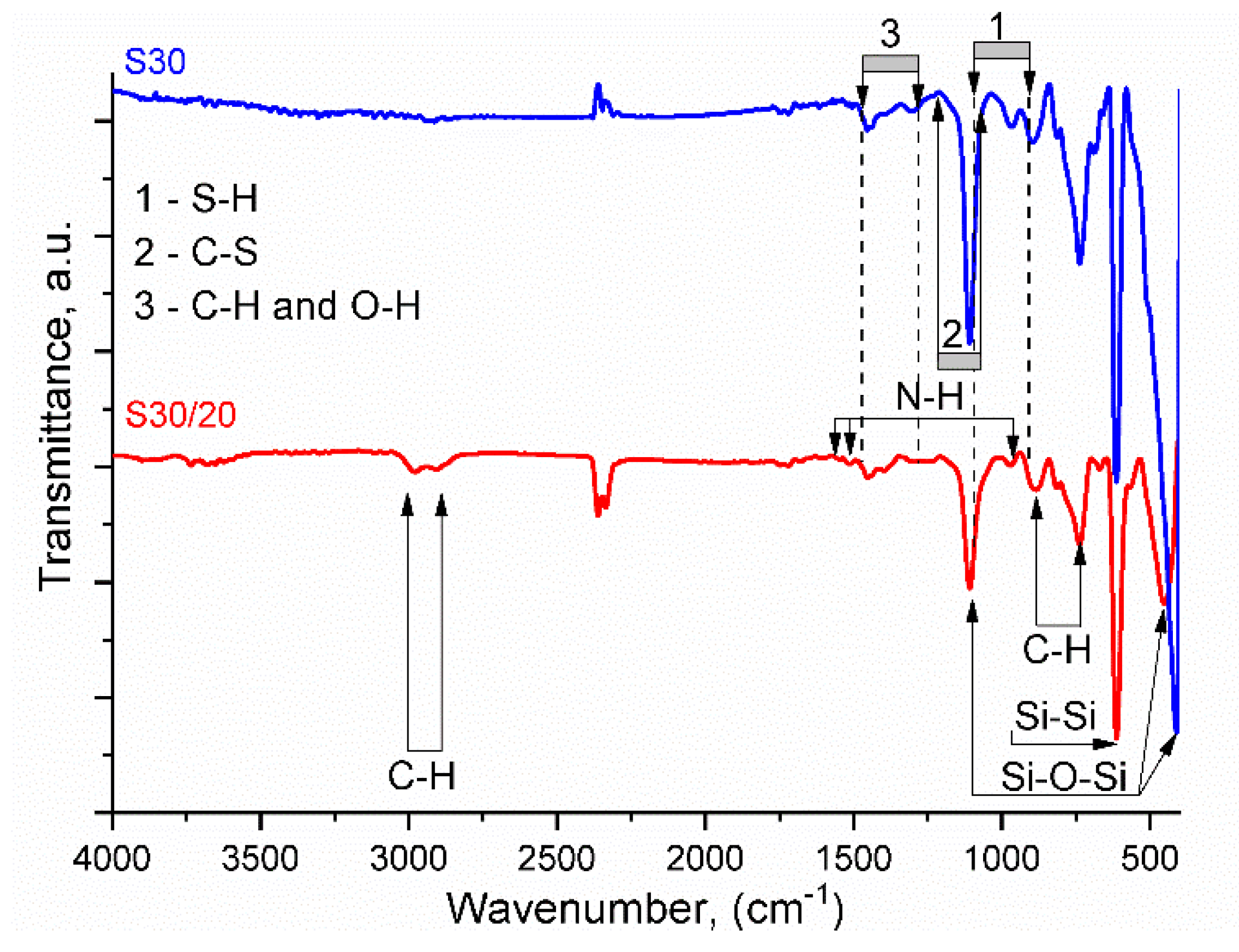
3.2.1. Fourier-Transform Infrared Spectroscopy (FTIR)
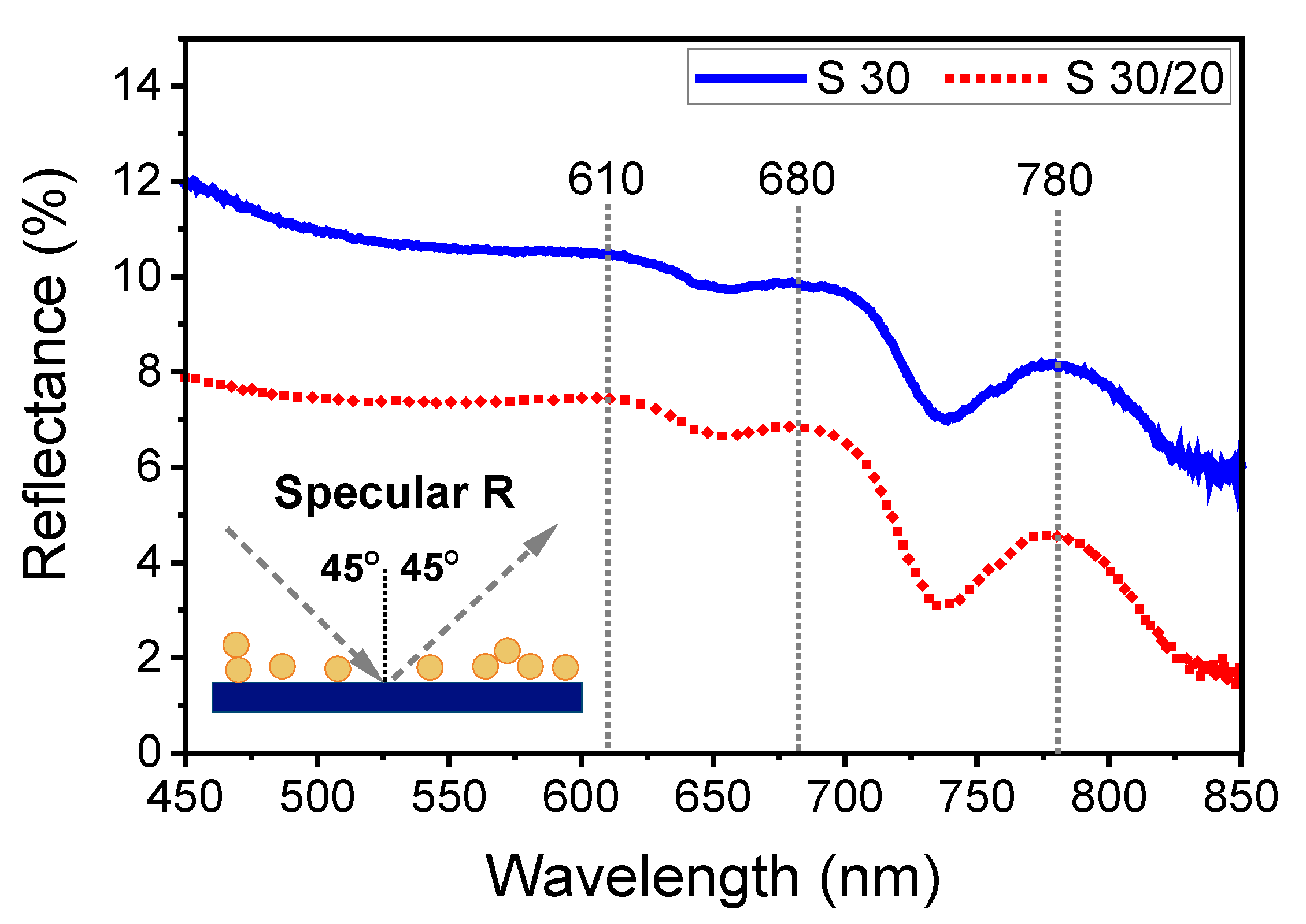
3.2.2. Specular Reflectance of Non-Polarized Light
3.2.3. Modulation Polarimetry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cao, L.; Wang, P.; Chen, L.; Wu, Y.; Di, J. A photoelectrochemical glucose sensor based on gold nanoparticles as a mimic enzyme of glucose oxidase. RSC Adv. 2019, 9, 15307–15313. [Google Scholar] [CrossRef]
- Zheng, S.; Zhang, H.; Lakshmipriya, T.; Gopinath, S.C.B.; Yang, N. Gold nanorod integrated electrochemical sensing for hyperglycaemia on interdigitated electrode. BioMed Res. Int. 2019, 2019, 9726967. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Y.; Lin, Z.; Liu, W.; Chen, X.; Liu, Y.; Tian, H.; Liu, Q.; Gillibert, R.; Spadavecchia, J.; et al. Femtomolar detection of nucleic acid based on functionalized gold nanoparticles. Nanophotonics 2019, 8, 1495–1503. [Google Scholar] [CrossRef]
- Guan, Z.; Zhang, T.; Zhu, H.; Lyu, D.; Sarangapani, S.; Xu, Q.-H.; Lang, M.J. Simultaneous imaging and selective photothermal therapy through aptamer-driven au nanosphere clustering. J. Phys. Chem. Lett. 2019, 10, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Chemla, Y.; Betzer, O.; Markus, A.; Farah, N.; Motiei, M.; Popovtzer, R.; Mandel, Y. Gold nanoparticles for multimodal high-resolution imaging of transplanted cells for retinal replacement therapy. Nanomedicine (Lond. U.K.) 2019, 14, 1857–1871. [Google Scholar] [CrossRef] [PubMed]
- McCold, C.E.; Fu, Q.; Howe, J.Y.; Hihath, J. Conductance based characterization of structure and hopping site density in 2D molecule-nanoparticle arrays. Nanoscale 2015, 7, 14937–14945. [Google Scholar] [CrossRef] [PubMed]
- Tie, M.; Dhirani, A.A. Conductance of molecularly linked gold nanoparticle films across an insulator-to-metal transition: From hopping to strong Coulomb electron-electron interactions and correlations. Phys. Rev. B 2015, 91, 155131. [Google Scholar] [CrossRef]
- Lu, F.; Yager, K.G.; Zhang, Y.; Xin, H.; Gang, O. Superlattices assembled through shape-induced directional binding. Nat. Commun. 2015, 6, 6912. [Google Scholar] [CrossRef]
- Hofmann, A.; Schmiel, P.; Stein, B.; Graf, C. Controlled formation of gold nanoparticle dimers using multivalent thiol ligands. Langmuir 2011, 27, 15165–15175. [Google Scholar] [CrossRef]
- Di, H.; Liu, H.; Li, M.; Li, J.; Liu, D. High-precision profiling of sialic acid expression in cancer cells and tissues using background-free surface-enhanced Raman scattering tags. Anal. Chem. 2017, 89, 5874–5881. [Google Scholar] [CrossRef]
- Song, S.; Kuang, Y.; Luo, L.; Sun, X. Asymmetric hetero-assembly of colloidal nanoparticles through "crash reaction" in a centrifugal field. Dalton Trans. 2014, 43, 5994–5997. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.T.; Zheng, L.Q.; Jia, H. Facile control of the self-assembly of gold nanoparticles by changing the capping agent structures. Colloids Surf. A 2014, 450, 9–14. [Google Scholar] [CrossRef]
- Li, M.; Johnson, S.; Guo, H.; Dujardin, E.; Mann, S. A generalized mechanism for ligand-induced dipolar assembly of plasmonic gold nanoparticle chain networks. Adv. Funct. Mater. 2011, 21, 851–859. [Google Scholar] [CrossRef]
- Sánchez-Iglesias, A.; Grzelczak, M.; Altantzis, T.; Goris, B.; Pérez-Juste, J.; Bals, S.; Van Tendeloo, G.; Donaldson, S.H.; Chmelka, B.F.; Israelachvili, J.N.; et al. Hydrophobic interactions modulate self-assembly of nanoparticles. ACS Nano 2012, 6, 11059–11065. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Zhou, Y.; Blaber, M.G.; Schatz, G.C.; Yoon, S. Surface plasmon coupling of compositionally heterogeneous core–satellite nanoassemblies. J. Phys. Chem. Lett. 2013, 4, 1371–1378. [Google Scholar] [CrossRef] [PubMed]
- Lermusiaux, L.; Funston, A.M. Plasmonic isomers via DNA-based self-assembly of gold nanoparticles. Nanoscale 2018, 10, 19557–19567. [Google Scholar] [CrossRef]
- Kutsenko, V.Y.; Lopatina, Y.Y.; Bossard-Giannesini, L.; Marchenko, O.A.; Pluchery, O.; Snegir, S.V. Alkylthiol self-assembled monolayers on Au(111) with tailored tail groups for attaching gold nanoparticles. Nanotechnology 2017, 28, 235603–235606. [Google Scholar] [CrossRef]
- Kano, S.; Tada, T.; Majima, Y. Nanoparticle characterization based on STM and STS. Chem. Soc. Rev. 2015, 44, 970–987. [Google Scholar] [CrossRef]
- Kyaw, H.H.; Al-Harthi, S.H.; Sellai, A.; Dutta, J. Self-organization of gold nanoparticles on silanated surfaces. Beilstein J. Nanotechnol. 2015, 6, 2345–2353. [Google Scholar] [CrossRef]
- Stetsenko, M.O.; Rudenko, S.P.; Maksimenko, L.S.; Serdega, B.K.; Pluchery, O.; Snegir, S.V. Optical properties of gold nanoparticle assemblies on a glass surface. Nanoscale Res. Lett. 2017, 12, 348–358. [Google Scholar] [CrossRef]
- Snegir, S.; Mukha, I.; Sysoiev, D.; Lacaze, E.; Huhn, T.; Pluchery, O. Optically controlled properties of nanoparticles stabilised by photochromic difurylethene-base diarylethenes. Materialwiss. Werkstofftech. 2016, 47, 229–236. [Google Scholar] [CrossRef]
- Abu Bakar, N.; Mat Salleh, M.; Ali Umar, A.; Shapter, J.G. Direct deposition of silver nanoplates on quartz surface by sequence pre-treatment hydroxylation and silanisation. MethodsX 2017, 4, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Abu Bakar, N.; Shapter, J.; Mat Salleh, M.; Ali Umar, A. Self-assembly of high density of triangular silver nanoplate films promoted by 3-aminopropyltrimethoxysilane. Appl. Sci. 2015, 5, 209–221. [Google Scholar] [CrossRef]
- Cano, M.; de la Cueva-Méndez, G. Self-assembly of a superparamagnetic raspberry-like silica/iron oxide nanocomposite using epoxy–amine coupling chemistry. Chem. Commun. 2015, 51, 3620–3622. [Google Scholar] [CrossRef] [PubMed]
- Soliveri, G.; Annunziata, R.; Ardizzone, S.; Cappelletti, G.; Meroni, D. Multiscale rough titania films with patterned hydrophobic/oleophobic features. J. Phys. Chem. C 2012, 116, 26405–26413. [Google Scholar] [CrossRef]
- Shakeri, A.; Yip, D.; Badv, M.; Imani, S.M.; Sanjari, M.; Didar, T.F. Self-cleaning ceramic tiles produced via stable coating of TiO(2) nanoparticles. Materials (Basel) 2018, 11, 1003. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Li, X.M.; He, T. Kinetics of (3-aminopropyl) triethoxylsilane (APTES) silanization of superparamagnetic iron oxide nanoparticles. Langmuir 2013, 29, 15275–15282. [Google Scholar] [CrossRef]
- Zhu, N.; Ji, H.; Yu, P.; Niu, J.; Farooq, M.U.; Akram, M.W.; Udego, I.O.; Li, H.; Niu, X. Surface modification of magnetic Iron Oxide nanoparticles. Nanomaterials (Basel) 2018, 8, 810. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Hassan, A.A.; Guyon, C.; Ognier, S.; Tatoulian, M. Plasma deposited high density amines on surface using (3-aminopropyl) triethoxysilane for assembling particles at near-nano size. Mater. Chem. Phys. 2020, 240, 121974. [Google Scholar] [CrossRef]
- Zheng, Y.; Thai, T.; Reineck, P.; Qiu, L.; Guo, Y.; Bach, U. DNA-directed self-assembly of core-satellite plasmonic nanostructures: A highly sensitive and reproducible near-IR SERS sensor. Adv. Funct. Mater. 2013, 23, 1519–1526. [Google Scholar] [CrossRef]
- Cha, H.; Yoon, J.H.; Yoon, S. Probing quantum plasmon coupling using gold nanoparticle dimers with tunable interparticle distances down to the subnanometer range. ACS Nano 2014, 8, 8554–8563. [Google Scholar] [CrossRef]
- Cha, H.; Lee, D.; Yoon, J.H.; Yoon, S. Plasmon coupling between silver nanoparticles: Transition from the classical to the quantum regime. J. Colloid Interface Sci. 2016, 464, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Klug, J.; Pérez, L.A.; Coronado, E.A.; Lacconi, G.I. Chemical and electrochemical oxidation of silicon surfaces functionalized with APTES: The role of surface roughness in the AuNPs anchoring kinetics. J Phys. Chem. C 2013, 117, 11317–11327. [Google Scholar] [CrossRef]
- Yadav, A.R.; Sriram, R.; Carter, J.A.; Miller, B.L. Comparative study of solution–phase and vapor–phase deposition of aminosilanes on silicon dioxide surfaces. Mater. Sci. Eng. C 2014, 35, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Pasternack, R.M.; Rivillon Amy, S.; Chabal, Y.J. Attachment of 3-(Aminopropyl) triethoxysilane on silicon Oxide surfaces: Dependence on solution temperature. Langmuir 2008, 24, 12963–12971. [Google Scholar] [CrossRef] [PubMed]
- Acres, R.G.; Ellis, A.V.; Alvino, J.; Lenahan, C.E.; Khodakov, D.A.; Metha, G.F.; Andersson, G.G. Molecular structure of 3-aminopropyltriethoxysilane layers formed on silanol-terminated silicon surfaces. J. Phys. Chem. C 2012, 116, 6289–6297. [Google Scholar] [CrossRef]
- García Raya, D.; Madueño, R.; Blázquez, M.; Pineda, T. Formation of a 1, 8-octanedithiol self-assembled monolayer on Au (111) prepared in a lyotropic liquid-crystalline medium. Langmuir 2010, 26, 11790–11796. [Google Scholar] [CrossRef]
- Kim, M.; Kwon, H.; Lee, S.; Yoon, S. Effect of nanogap morphology on plasmon coupling. ACS Nano 2019, 13, 12100–12108. [Google Scholar] [CrossRef]
- Vericat, C.; Vela, M.E.; Benitez, G.; Carro, P.; Salvarezza, R.C. Self-assembled monolayers of thiols and dithiols on gold: New challenges for a well-known system. Chem. Soc. Rev. 2010, 39, 1805–1834. [Google Scholar] [CrossRef]
- Hafezian, S.; Baloukas, B.; Martinu, L. Percolation threshold determination of sputtered silver films using Stokes parameters and in situ conductance measurements. Appl. Opt. 2014, 53, 5367–5374. [Google Scholar] [CrossRef]
- Fernández-Blanco, C.; Colina, Á.; Heras, A. UV/Vis spectroelectrochemistry as a tool for monitoring the fabrication of sensors based on silver nanoparticle modified electrodes. Sensors 2013, 13, 5700–5711. [Google Scholar] [CrossRef]
- Fiorillo, A.S.; Pullano, S.A.; Rudenko, S.P.; Stetsenko, M.O.; Maksimenko, L.S.; Krishchenko, I.M.; Synyuk, V.S. Antireflection properties of composite zeolite gold nanoparticles film. Electron. Lett. 2018, 54, 370–372. [Google Scholar] [CrossRef]
- Stetsenko, M.; Pullano, S.A.; Margitych, T.; Maksimenko, L.; Hassan, A.; Kryvyi, S.; Hu, R.; Huang, C.; Ziniuk, R.; Golovynskyi, S.; et al. Antireflection enhancement by composite nanoporous zeolite 3A-Carbon thin film. Nanomaterials (Basel) 2019, 9, 1641. [Google Scholar] [CrossRef] [PubMed]
- Stetsenko, M.O.; Matyash, I.E.; Rudenko, S.P.; Minailova, I.A.; Maksimenko, L.S.; Serdega, B.K. New type of plasmonic biosensors based on modulation polarimetry technique. In Proceedings of the 2017 IEEE International Young Scientists Forum on Applied Physics and Engineering (YSF), Lviv, Ukraine, 17–20 October 2017; pp. 100–103. [Google Scholar] [CrossRef]
- Parratt, L.G. Surface studies of solids by total reflection of X-rays. Phys. Rev. 1954, 95, 359–369. [Google Scholar] [CrossRef]
- Daillant, J.; Gibaud, A. X-ray and Neutron Reflectivity: Principles and Applications; Springer: Berlin/Heidelberg, Germany, 2009; Volume 770, pp. 1–348. [Google Scholar] [CrossRef]
- Rao, X.; Guyon, C.; Ognier, S.; Da Silva, B.; Chu, C.; Tatoulian, M.; Hassan, A.A. High density gold nanoparticles immobilized on surface via plasma deposited APTES film for decomposing organic compounds in microchannels. Appl. Surf. Sci. 2018, 439, 272–281. [Google Scholar] [CrossRef]
- Larkin, P. Infrared Raman Spectrosc; Elsevier: Amsterdam, The Netherlands, 2011; p. 230. [Google Scholar]
- Chirea, M.; Borges, J.; Pereira, C.M.; Silva, A.F. Density-dependent electrochemical properties of vertically aligned gold nanorods. J. Phys. Chem. C 2010, 114, 9478–9488. [Google Scholar] [CrossRef]
- Temple, T.L.; Mahanama, G.D.K.; Reehal, H.S.; Bagnall, D.M. Influence of localized surface plasmon excitation in silver nanoparticles on the performance of silicon solar cells. Sol. Energy Mater. Sol. Cells 2009, 93, 1978–1985. [Google Scholar] [CrossRef]
- Yang, S.C.; Kobori, H.; He, C.L.; Lin, M.H.; Chen, H.Y.; Li, C.; Kanehara, M.; Teranishi, T.; Gwo, S. Plasmon hybridization in individual gold nanocrystal dimers: Direct observation of bright and dark modes. Nano Lett. 2010, 10, 632–637. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, Q.; Hou, M.; Ma, L.; Zhang, Z. Nanogap effects on near- and far-field plasmonic behaviors of metallic nanoparticle dimers. Phys. Chem. Chem. Phys. 2015, 17, 29293–29298. [Google Scholar] [CrossRef]
- Schmid, M.; Grandidier, J.; Atwater, H.A. Scanning near-field optical microscopy on dense random assemblies of metal nanoparticles. J. Opt. 2013, 15, 125001. [Google Scholar] [CrossRef][Green Version]
- Zito, G.; Rusciano, G.; Vecchione, A.; Pesce, G.; Girolamo, R.; Malafronte, A.; Sasso, A. Nanometal skin of plasmonic heterostructures for highly efficient near-field scattering probes. Sci. Rep. 2016, 6, 31113. [Google Scholar] [CrossRef]
- Hoeing, D.; Schulz, F.; Mueller, N.S.; Reich, S.; Lange, H. Dark plasmon modes for efficient hot electron generation in multilayers of gold nanoparticles. Chem. Phys. 2020, 152, 064710. [Google Scholar] [CrossRef] [PubMed]
- Humbert, C.; Pluchery, O.; Lacaze, E.; Tadjeddine, A.; Busson, B. Optical spectroscopy of functionalized gold nanoparticles assemblies as a function of the surface coverage. Gold Bull. (Berl. Ger.) 2013, 46, 299–309. [Google Scholar] [CrossRef]
- Burrows, C.P.; Barnes, W.L. Large spectral extinction due to overlap of dipolar and quadrupolar plasmonic modes of metallic nanoparticles in arrays. Opt. Express 2010, 18, 3187–3198. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, T.P.; Liu, Z.; Liu, Y.C.; Liu, Y.; Yu, S.F. Optical properties of gold nanoparticles on heavily-doped Si substrate synthesized with an electrochemical process. J. Electrochem. Soc. 2011, 158, K152–K155. [Google Scholar] [CrossRef][Green Version]
- Losurdo, M.; Giangregorio, M.M.; Bianco, G.V.; Suvorova, A.A.; Kong, C.; Rubanov, S.; Capezzuto, P.; Humlicek, J.; Bruno, G. Size dependence of the dielectric function of silicon-supported plasmonic gold nanoparticles. Phys. Rev. B 2010, 82, 155451. [Google Scholar] [CrossRef]
- Xiaoli, W.; Philippe, G.; Edmond, C.; Bruno, P. Near- and far-field effects on the plasmon coupling in gold nanoparticle arrays. J. Phys. Chem. C 2012, 116, 24741. [Google Scholar] [CrossRef]
- Diaz-Egea, C.; Abargues, R.; Martínez-Pastor, J.P.; Sigle, W.; van Aken, P.A.; Molina, S.I. High spatial resolution mapping of individual and collective localized surface plasmon resonance modes of silver nanoparticle aggregates: Correlation to optical measurements. Nanoscale Res. Lett. 2015, 10, 310. [Google Scholar] [CrossRef]
- Herrmann, L.O.; Valev, V.K.; Tserkezis, C.; Barnard, J.S.; Kasera, S.; Scherman, O.A.; Aizpurua, J.; Baumberg, J.J. Threading plasmonic nanoparticle strings with light. Nat. Commun. 2014, 5, 4568. [Google Scholar] [CrossRef]
- Barrow, S.J.; Funston, A.M.; Gómez, D.E.; Davis, T.J.; Mulvaney, P. Surface plasmon resonances in strongly coupled gold nanosphere chains from monomer to hexamer. Nano Lett. 2011, 11, 4180–4187. [Google Scholar] [CrossRef]
- Maksimenko, L.S.; Rudenko, S.P.; Stetsenko, M.O.; Matyash, I.E.; Mischuk, O.M.; Kolomzarov, Y.V.; Serdega, B.K. Diagnostic of resonant properties of Au-PTFE nanostructures for sensor applications. In Nanomaterials for Security; Bonča, J., Kruchinin, S., Eds.; NATO Science for Peace and Security Series A: Chemistry and Biology; Springer: Dordrecht, The Netherlands, 2016; pp. 267–280. [Google Scholar] [CrossRef]
- Chandel, S.; Soni, J.; Ray, S.K.; Das, A.; Ghosh, A.; Raj, S.; Ghosh, N. Complete polarization characterization of single plasmonic nanoparticle enabled by a novel Dark-field Mueller matrix spectroscopy system. Sci. Rep. 2016, 6, 26466. [Google Scholar] [CrossRef] [PubMed]
- Kravets, V.G.; Kabashin, A.V.; Barnes, W.L.; Grigorenko, A.N. Plasmonic surface lattice resonances: A review of properties and applications. Chem. Rev. 2018, 118, 5912–5951. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-D.; Yue, P.; Zhang, S.; Wang, M.; Dai, H.; Chen, Y.; Nie, Z.-Q.; Cui, Y.; Han, J.-B.; Duan, H. Metasurfaces composed of plasmonic molecules: Hybridization between parallel and orthogonal surface lattice resonances. Adv. Opt. Mater. 2019, 8, 1901109. [Google Scholar] [CrossRef]
- Hao, F.; Sonnefraud, Y.; Dorpe, P.V.; Maier, S.A.; Halas, N.J.; Nordlander, P. Symmetry breaking in plasmonic nanocavities: Subradiant LSPR sensing and a tunable fano resonance. Nano Lett. 2008, 8, 3983–3988. [Google Scholar] [CrossRef]
- Gerrard, A.; Burch, J.M. Introduction to Matrix Methods in Optics; Dover Publications: Mineola, NY, USA, 2012; p. 384. [Google Scholar]
- Ma, W.; Xu, L.; de Moura, A.F.; Wu, X.; Kuang, H.; Xu, C.; Kotov, N.A. Chiral inorganic nanostructures. Chem. Rev. 2017, 117, 8041–8093. [Google Scholar] [CrossRef]
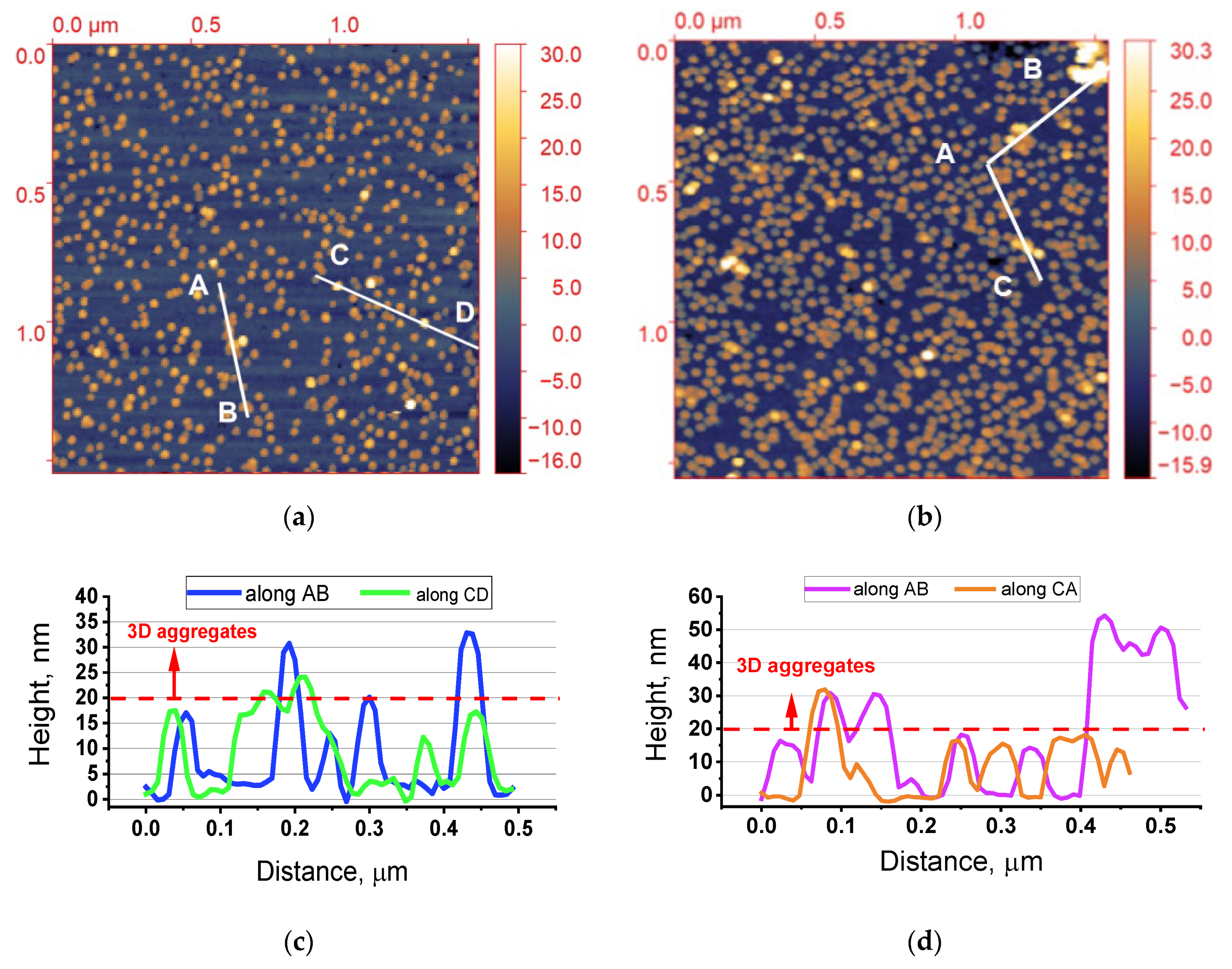
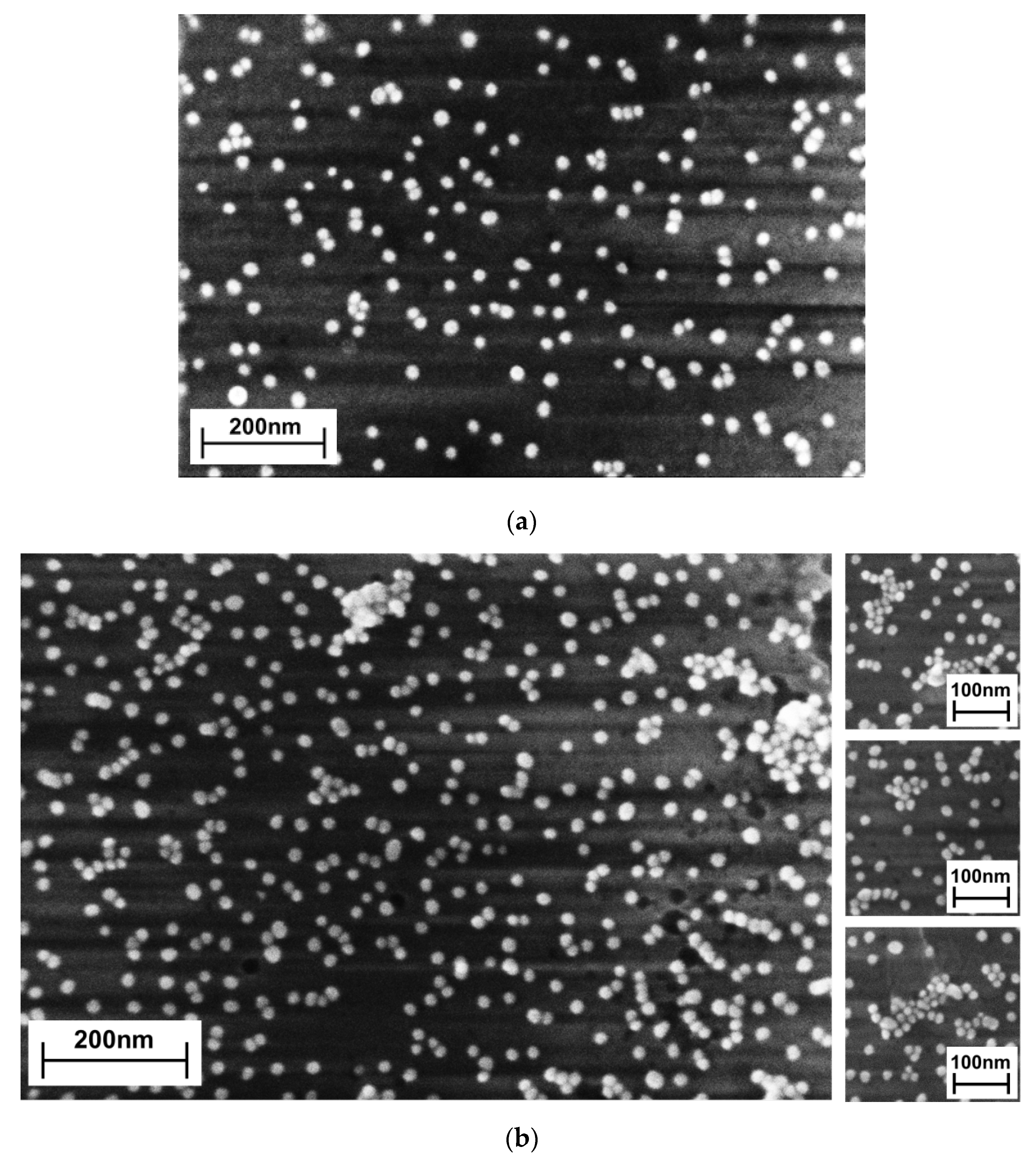



| Types of Nanoobjects | Sample S30, per 1 μm2 | Sample S30/20, per 1 μm2 | Increase Rate, % |
|---|---|---|---|
| Monomers | 172 | 291 | 69 |
| Dimers | 16 | 45 | 180 |
| Agglomerates | 8 | 49 | 512 |
| Sample | ρ, g/cm3 | Au, % vol. | Thickness d, nm | Roughness σ, nm |
|---|---|---|---|---|
| S30 | 1 layer: 2.08 | 6 ± 1 | 18 ± 2 | 1.3 ± 0.1 |
| S30/20 | 1 layer: 1.17 2 layer: 2.19 | 1 ± 1 6.6 ± 1 | - - | 5 ± 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stetsenko, M.; Margitych, T.; Kryvyi, S.; Maksimenko, L.; Hassan, A.; Filonenko, S.; Li, Β.; Qu, J.; Scheer, E.; Snegir, S. Gold Nanoparticle Self-Aggregation on Surface with 1,6-Hexanedithiol Functionalization. Nanomaterials 2020, 10, 512. https://doi.org/10.3390/nano10030512
Stetsenko M, Margitych T, Kryvyi S, Maksimenko L, Hassan A, Filonenko S, Li Β, Qu J, Scheer E, Snegir S. Gold Nanoparticle Self-Aggregation on Surface with 1,6-Hexanedithiol Functionalization. Nanomaterials. 2020; 10(3):512. https://doi.org/10.3390/nano10030512
Chicago/Turabian StyleStetsenko, Maksym, Tetiana Margitych, Serhii Kryvyi, Lidia Maksimenko, Ali Hassan, Svitlana Filonenko, Βaikui Li, Junle Qu, Elke Scheer, and Sergii Snegir. 2020. "Gold Nanoparticle Self-Aggregation on Surface with 1,6-Hexanedithiol Functionalization" Nanomaterials 10, no. 3: 512. https://doi.org/10.3390/nano10030512
APA StyleStetsenko, M., Margitych, T., Kryvyi, S., Maksimenko, L., Hassan, A., Filonenko, S., Li, Β., Qu, J., Scheer, E., & Snegir, S. (2020). Gold Nanoparticle Self-Aggregation on Surface with 1,6-Hexanedithiol Functionalization. Nanomaterials, 10(3), 512. https://doi.org/10.3390/nano10030512







