Electrospun Active Biopapers of Food Waste Derived Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) with Short-Term and Long-Term Antimicrobial Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Solutions for Electrospinning
2.3. Preparation of the Electrospun Biopapers
2.4. Characterization
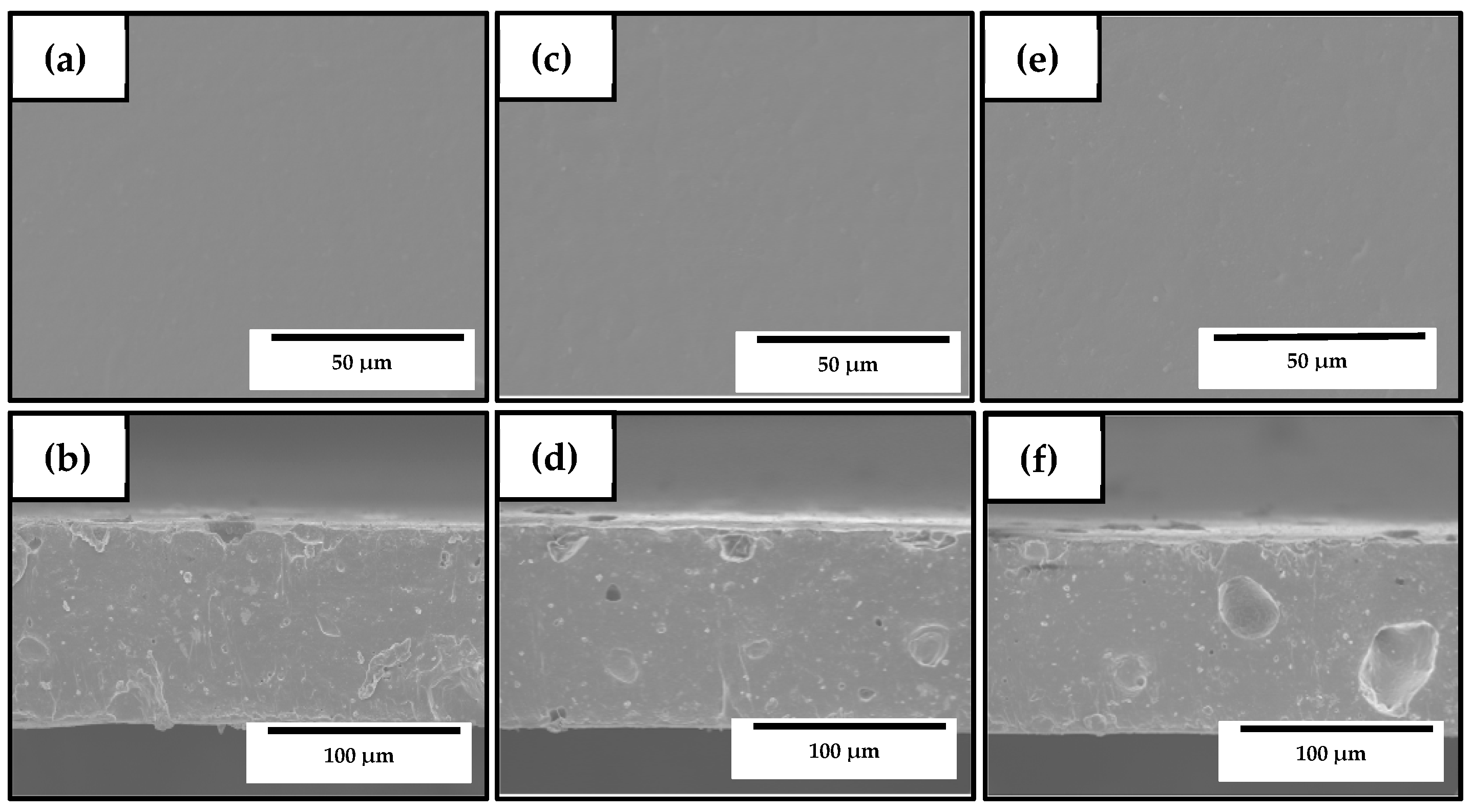
2.4.1. Thickness
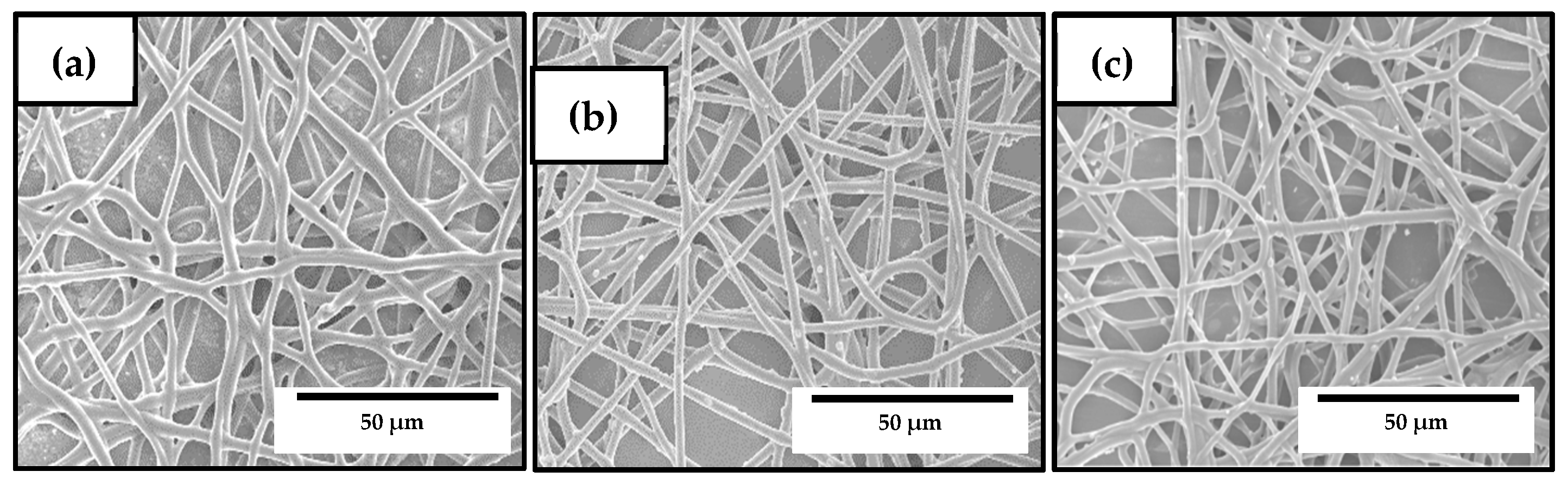
2.4.2. Morphology
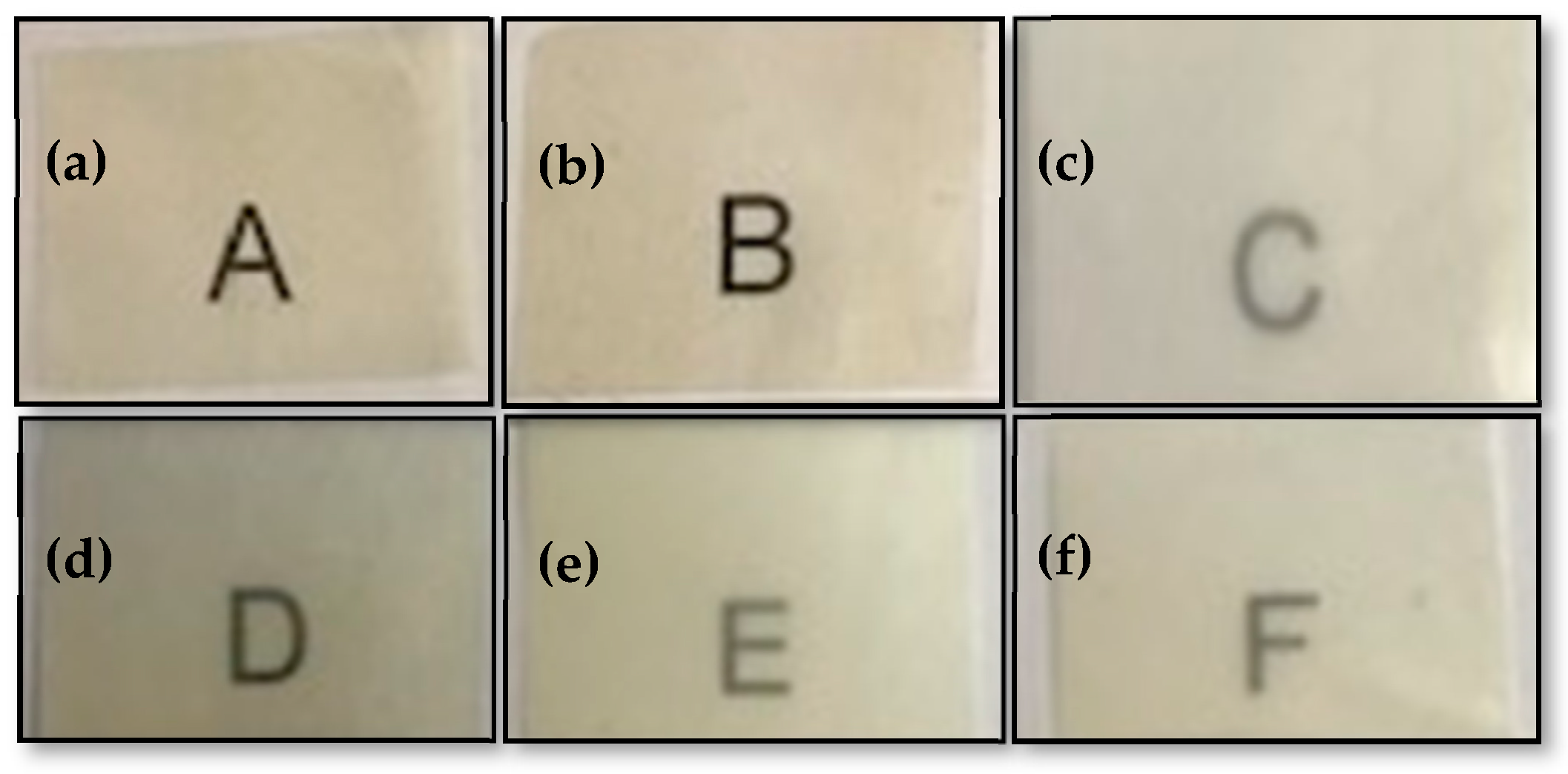
2.4.3. Transparency
2.4.4. Color
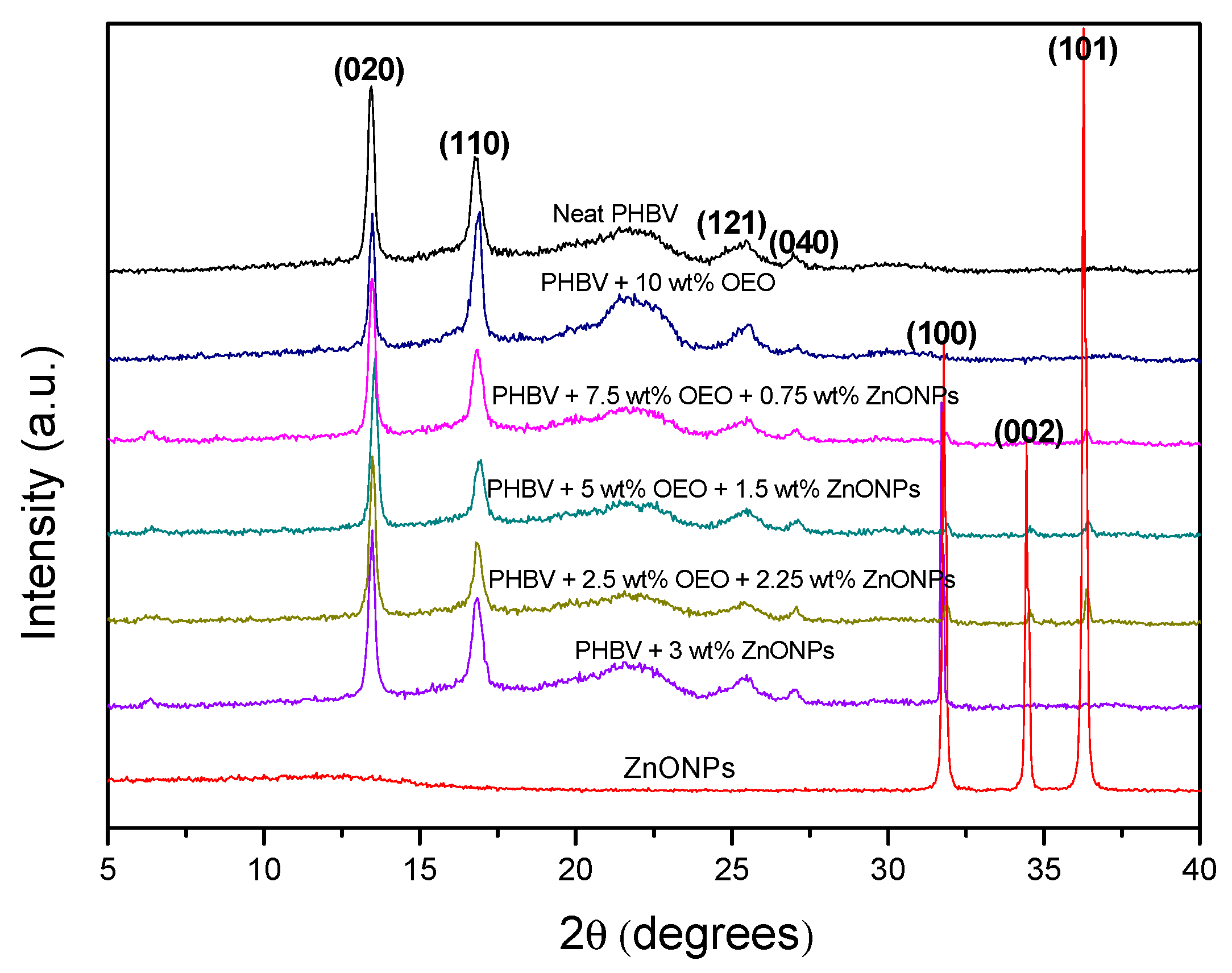
2.4.5. X-Ray Diffraction Analysis
2.4.6. Thermal Analysis
2.4.7. Mechanical Tests
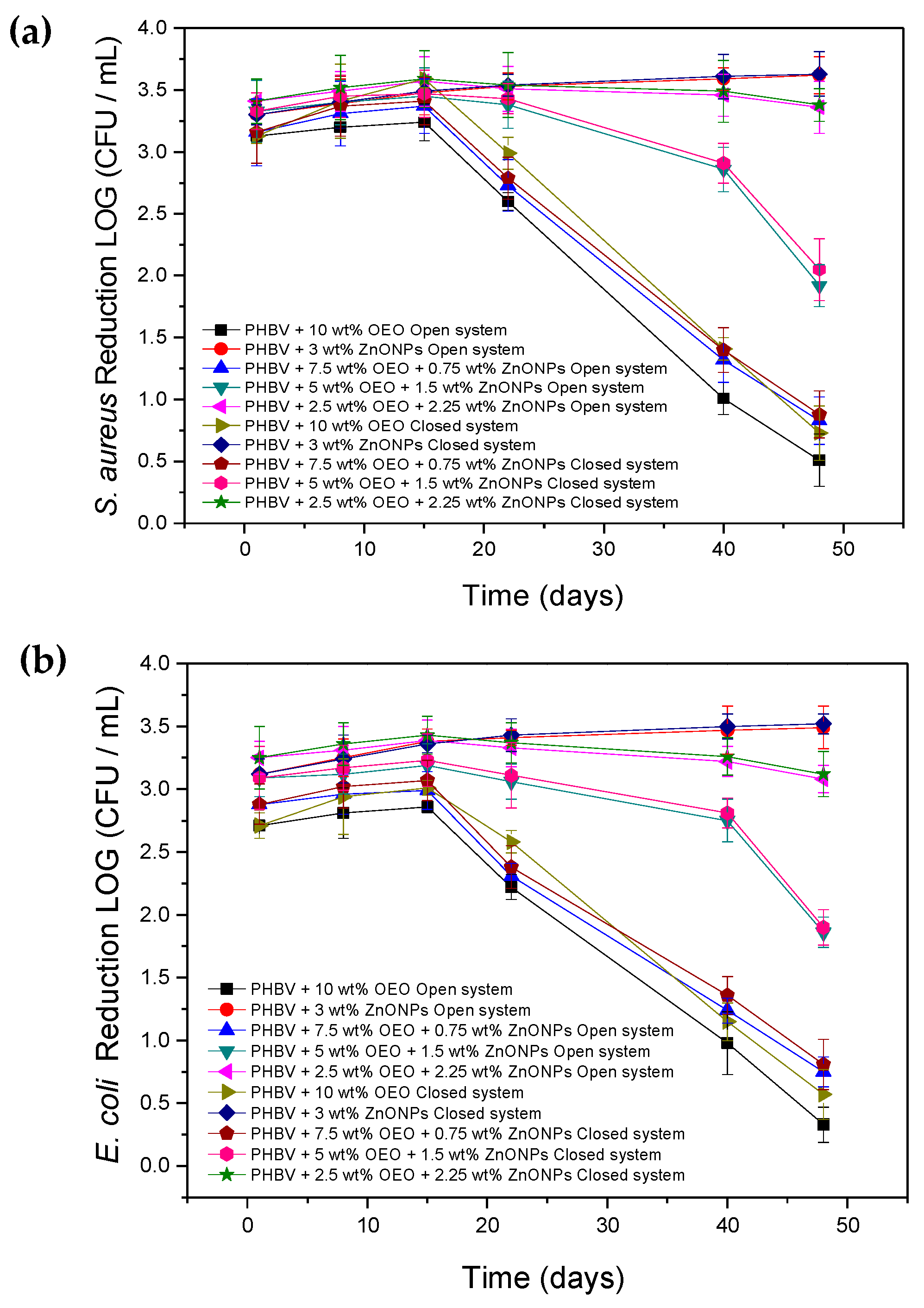
2.4.8. Antimicrobial Activity
2.4.9. Migration Tests
2.5. Statistical Analysis
3. Results and Discussion
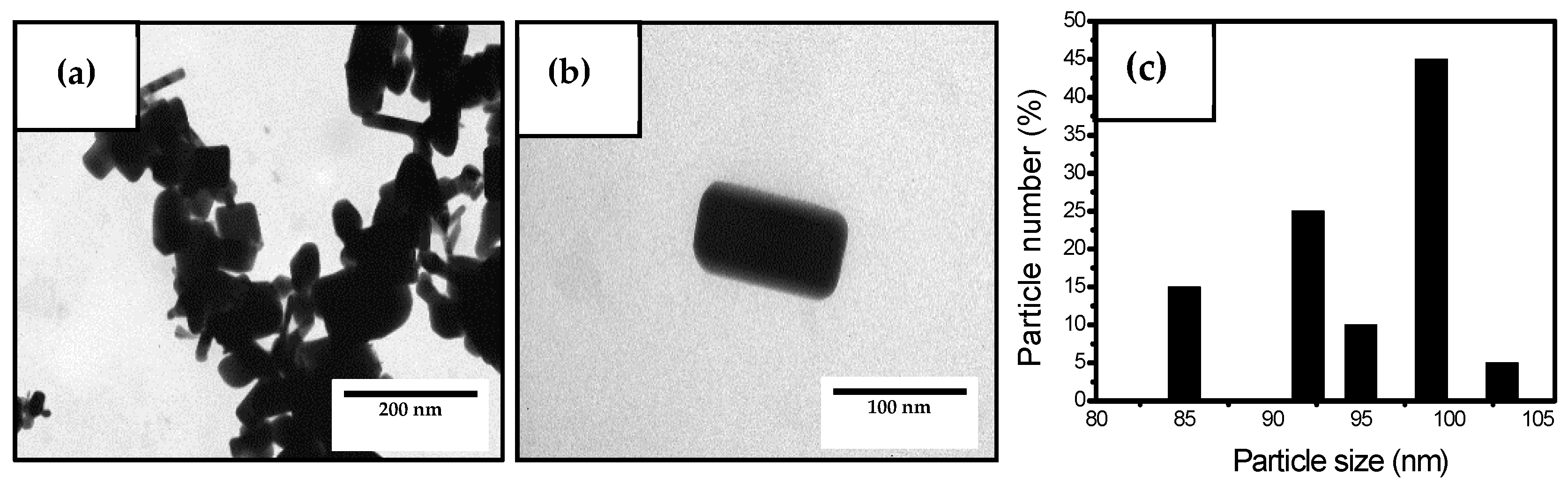
3.1. Characterization of the Antimicrobial Agents
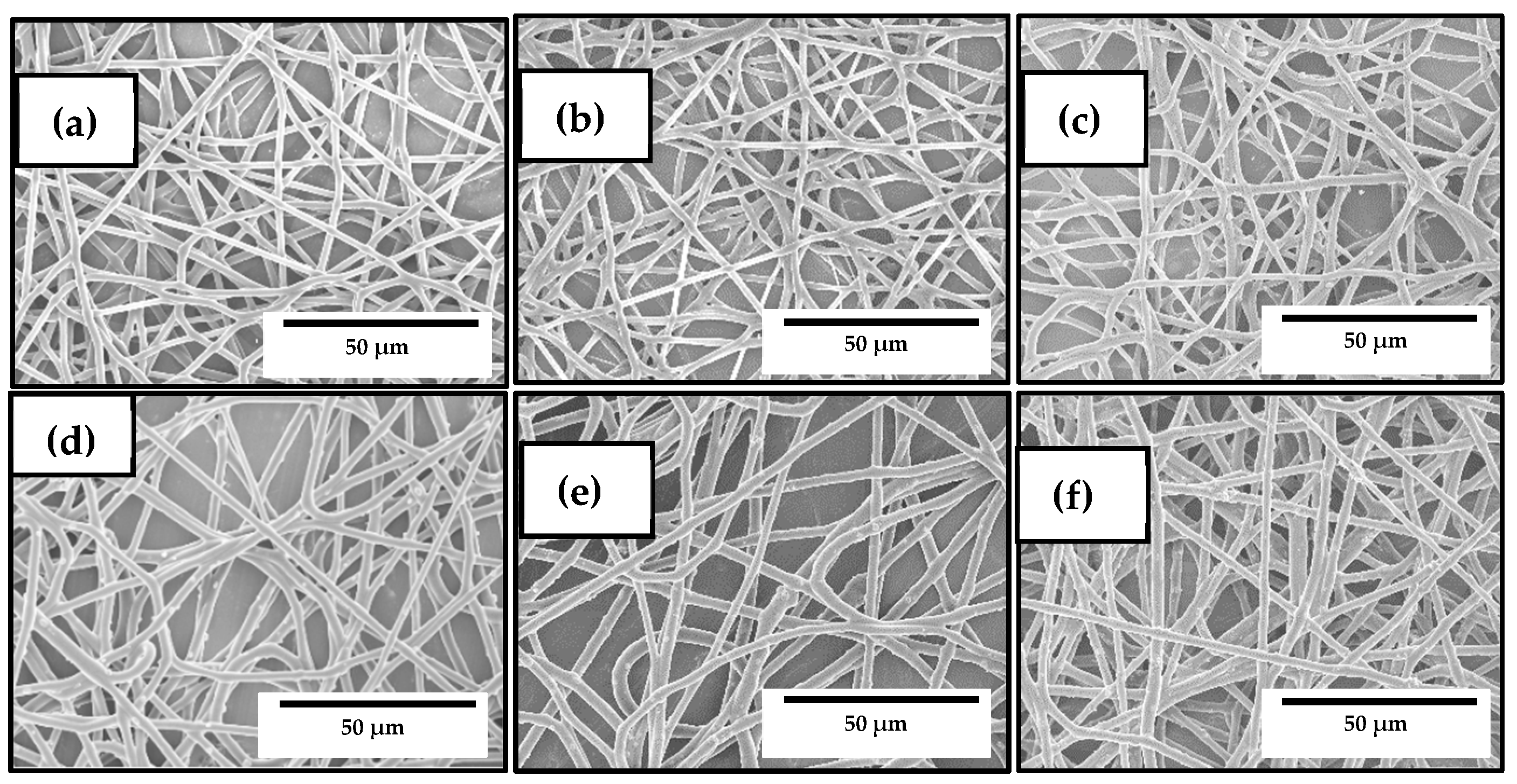
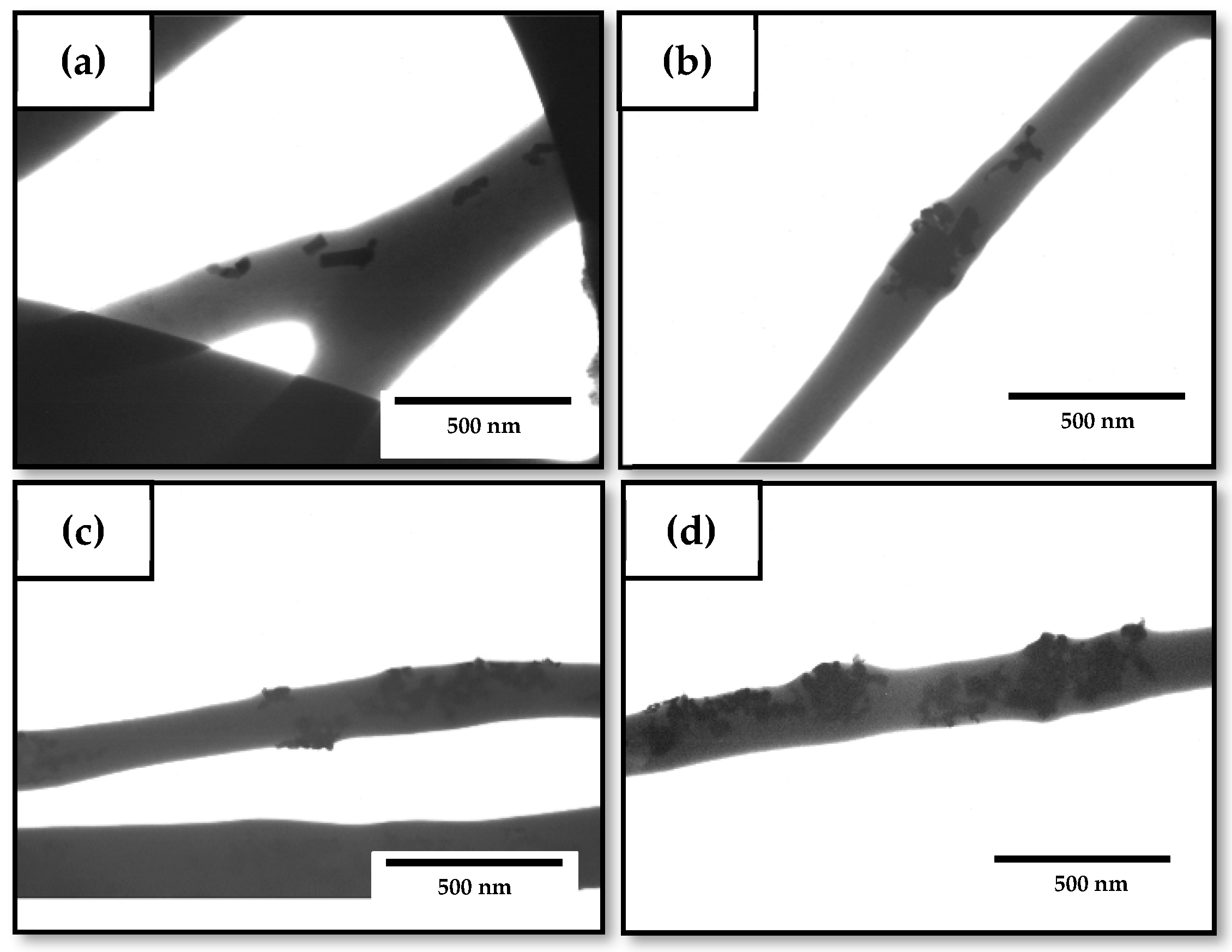
3.2. Optimization of the Electrospun PHBV/ZnONPs Biopapers
3.3. Development of the Electrospun Hybrid PHBV Biopapers
3.4. Optical Properties of the Electrospun Hybrid PHBV Biopapers
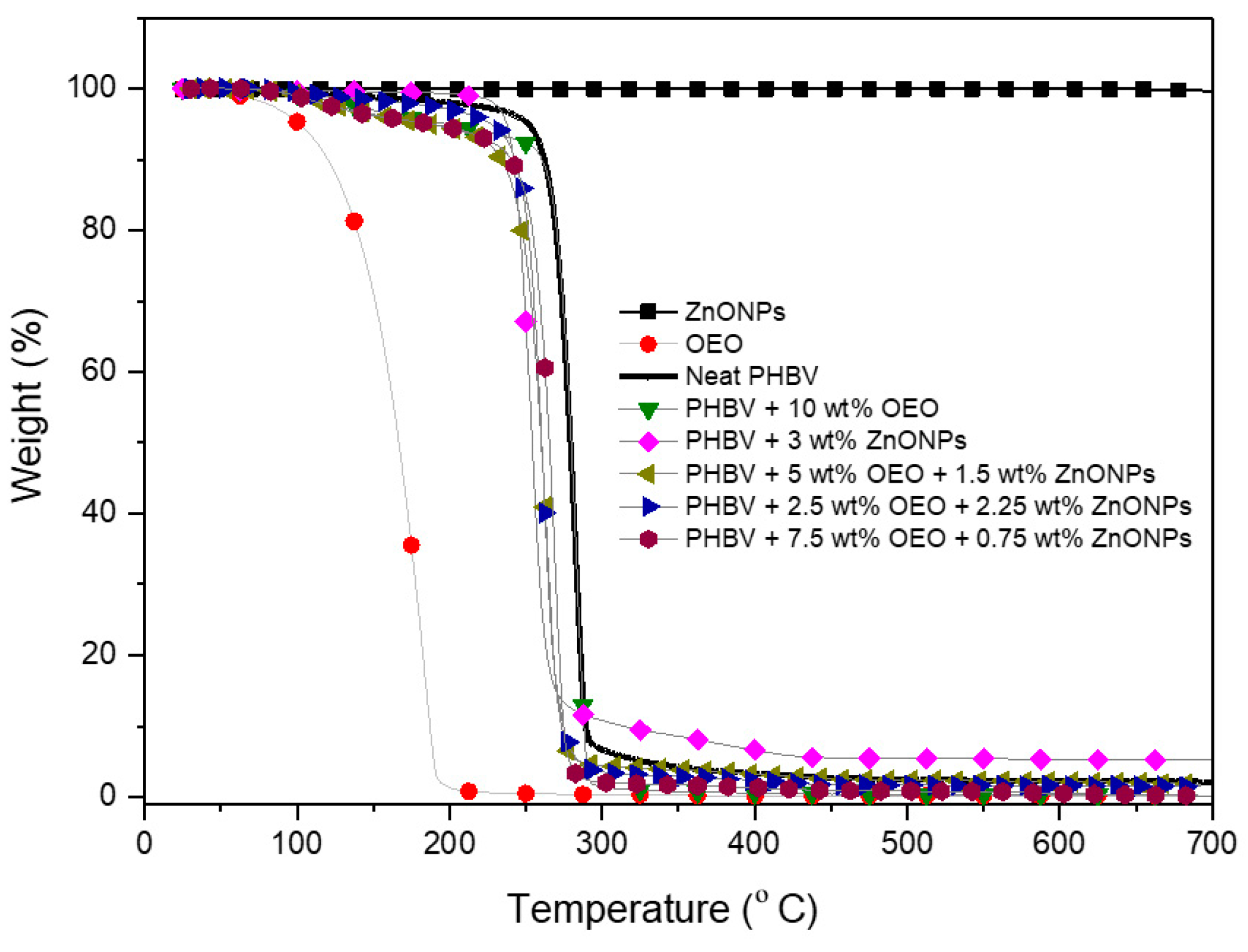
3.5. Thermal Stability of the Electrospun Hybrid PHBV Biopapers
3.6. Mechanical Properties of the Electrospun Hybrid PHBV Biopapers
3.7. Crystallinity of the Electrospun Hybrid PHBV Biopapers
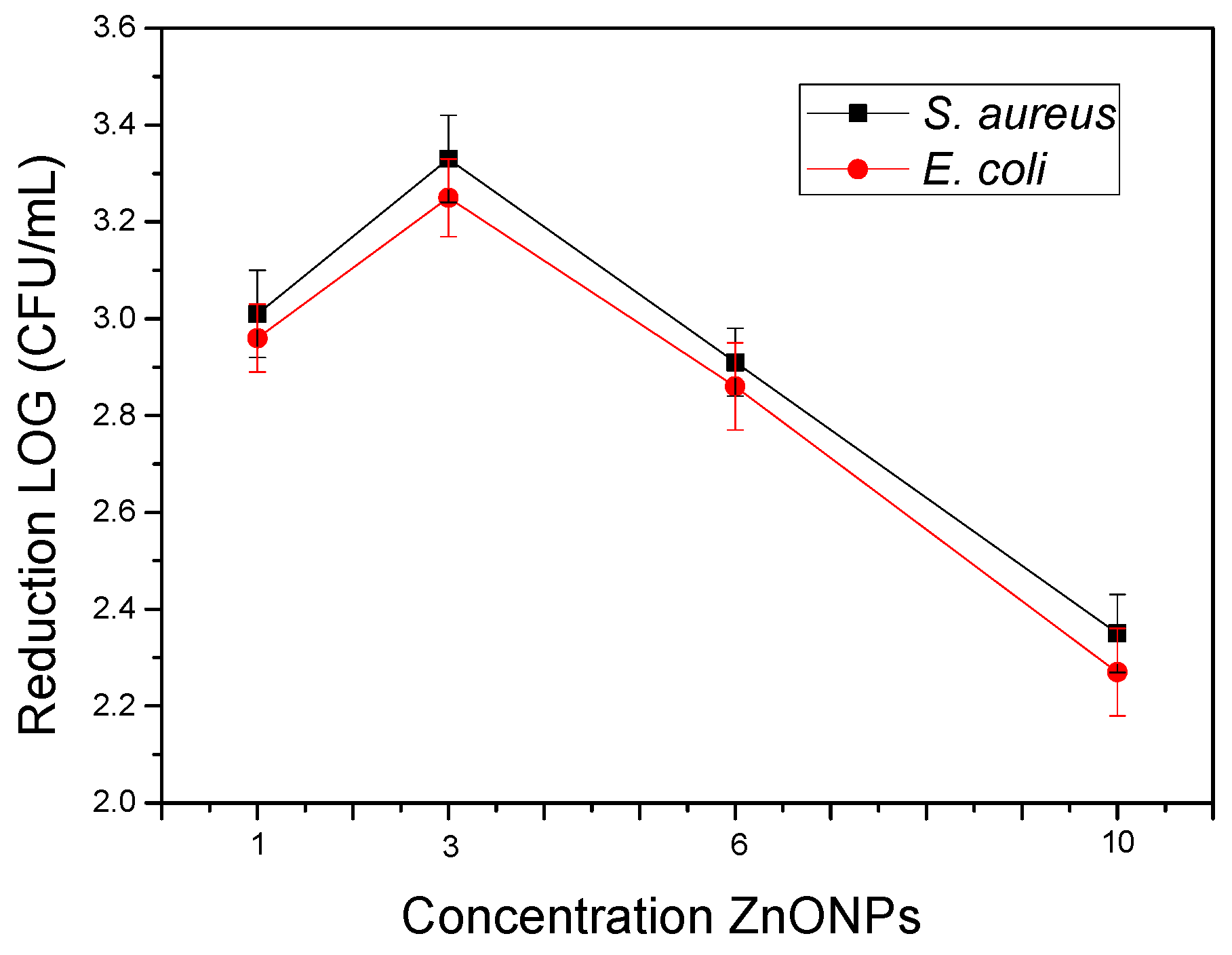
3.8. Antimicrobial Activity of the Electrospun Hybrid PHBV Biopapers
3.9. Migration Assessment of the Electrospun Hybrid PHBV Biopapers
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mihindukulasuriya, S.D.F.; Lim, L.T. Nanotechnology development in food packaging: A review. Trends Food Sci. Technol. 2014, 40, 149–167. [Google Scholar] [CrossRef]
- Singh, A.K.; Srivastava, J.K.; Chandel, A.K.; Sharma, L.; Mallick, N.; Singh, S.P. Biomedical applications of microbially engineered polyhydroxyalkanoates: An insight into recent advances, bottlenecks, and solutions. Appl. Microbiol. Biotechnol. 2019, 103, 2007–2032. [Google Scholar] [CrossRef] [PubMed]
- Bugnicourt, E.; Cinelli, P.; Lazzeri, A.; Alvarez, V. Polyhydroxyalkanoate (pha): Review of synthesis, characteristics, processing and potential applications in packaging. Express Polym. Lett. 2014, 8, 791–808. [Google Scholar] [CrossRef]
- Lemechko, P.; Le Fellic, M.; Bruzaud, S. Production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) using agro-industrial effluents with tunable proportion of 3-hydroxyvalerate monomer units. Int. J. Biol. Macromol. 2019, 128, 429–434. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Hilliou, L.; Melendez-Rodriguez, B.; Figueroa-Lopez, K.J.; Madalena, D.; Cabedo, L.; Covas, J.A.; Vicente, A.A.; Lagaron, J.M. Melt processability, characterization, and antibacterial activity of compression-molded green composite sheets made of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) reinforced with coconut fibers impregnated with oregano essential oil. Food Packag. Shelf Life 2018, 17, 39–49. [Google Scholar] [CrossRef]
- Garcia, C.V.; Shin, G.H.; Kim, J.T. Metal oxide-based nanocomposites in food packaging: Applications, migration, and regulations. Trends Food Sci. Technol. 2018, 82, 21–31. [Google Scholar] [CrossRef]
- Domínguez, R.; Barba, F.J.; Gómez, B.; Putnik, P.; Bursać Kovačević, D.; Pateiro, M.; Santos, E.M.; Lorenzo, J.M. Active packaging films with natural antioxidants to be used in meat industry: A review. Food Res. Int. 2018, 113, 93–101. [Google Scholar] [CrossRef]
- Figueroa-Lopez, K.J.; Andrade-Mahecha, M.M.; Torres-Vargas, O.L. Spice oleoresins containing antimicrobial agents improve the potential use of bio-composite films based on gelatin. Food Packag. Shelf Life 2018, 17, 50–56. [Google Scholar] [CrossRef]
- Robledo, S.N.; Pierini, G.D.; Nieto, C.H.D.; Fernández, H.; Zon, M.A. Development of an electrochemical method to determine phenolic monoterpenes in essential oils. Talanta 2019, 196, 362–369. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, J.; Kong, W.; Zhao, G.; Yang, M. Mechanisms of antifungal and anti-aflatoxigenic properties of essential oil derived from turmeric (Curcuma longa L.) on aspergillus flavus. Food Chem. 2017, 220, 1–8. [Google Scholar] [CrossRef]
- da Rocha Neto, A.C.; Beaudry, R.; Maraschin, M.; Di Piero, R.M.; Almenar, E. Double-bottom antimicrobial packaging for apple shelf-life extension. Food Chem. 2019, 279, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Dutra, T.V.; Castro, J.C.; Menezes, J.L.; Ramos, T.R.; do Prado, I.N.; Machinski, M.; Mikcha, J.M.G.; Filho, B.A.d.A. Bioactivity of oregano (Origanum vulgare) essential oil against Alicyclobacillus spp. Ind. Crops Prod. 2019, 129, 345–349. [Google Scholar] [CrossRef]
- Mechergui, K.; Jaouadi, W.; Coelho, J.P.; Khouja, M.L. Effect of harvest year on production, chemical composition and antioxidant activities of essential oil of oregano (Origanum vulgare subsp glandulosum (desf.) ietswaart) growing in north africa. Ind. Crops Prod. 2016, 90, 32–37. [Google Scholar] [CrossRef]
- Sari, M.; Biondi, D.M.; Kaâbeche, M.; Mandalari, G.; D’Arrigo, M.; Bisignano, G.; Saija, A.; Daquino, C.; Ruberto, G. Chemical composition, antimicrobial and antioxidant activities of the essential oil of several populations of algerian origanum glandulosum desf. Flavour Fragr. J. 2006, 21, 890–898. [Google Scholar] [CrossRef]
- Reyes-Jurado, F.; Cervantes-Rincón, T.; Bach, H.; López-Malo, A.; Palou, E. Antimicrobial activity of mexican oregano (Lippia berlandieri), thyme (Thymus vulgaris), and mustard (Brassica nigra) essential oils in gaseous phase. Ind. Crops Prod. 2019, 131, 90–95. [Google Scholar] [CrossRef]
- Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential oils of oregano: Biological activity beyond their antimicrobial properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Deans, S.G.; Ritchie, G. Antibacterial properties of plant essential oils. Int. J. Food Microbiol. 1987, 5, 165–180. [Google Scholar] [CrossRef]
- Santurio, J.M.; Santurio, D.F.; Pozzatti, P.; Moraes, C.; Franchin, P.R.; Alves, S.H. Atividade antimicrobiana dos óleos essenciais de orégano, tomilho e canela frente a sorovares de salmonella enterica de origem avícola. Ciência Rural 2007, 37, 803–808. [Google Scholar] [CrossRef]
- Rodríguez-Meizoso, I.; Marin, F.R.; Herrero, M.; Señorans, F.J.; Reglero, G.; Cifuentes, A.; Ibáñez, E. Subcritical water extraction of nutraceuticals with antioxidant activity from oregano. Chemical and functional characterization. J. Pharm. Biomed. Anal. 2006, 41, 1560–1565. [Google Scholar] [CrossRef]
- Mechergui, K.; Coelho, J.A.; Serra, M.C.; Lamine, S.B.; Boukhchina, S.; Khouja, M.L. Essential oils of Origanum vulgare L. Subsp. Glandulosum (desf.) ietswaart from tunisia: Chemical composition and antioxidant activity. J. Sci. Food Agric. 2010, 90, 1745–1749. [Google Scholar] [CrossRef] [PubMed]
- Kulisic, T.; Radonic, A.; Katalinic, V.; Milos, M. Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem. 2004, 85, 633–640. [Google Scholar] [CrossRef]
- Kulkarni, S.B.; Patil, U.M.; Salunkhe, R.R.; Joshi, S.S.; Lokhande, C.D. Temperature impact on morphological evolution of ZnO and its consequent effect on physico-chemical properties. J. Alloys Compd. 2011, 509, 3486–3492. [Google Scholar] [CrossRef]
- Özgür, U.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Doğan, S.; Avrutin, V.; Ch, S.J.; Morkoç, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 11. [Google Scholar] [CrossRef]
- Moezzi, A.; McDonagh, A.M.; Cortie, M.B. Zinc oxide particles: Synthesis, properties and applications. Chem. Eng. J. 2012, 185–186, 1–22. [Google Scholar] [CrossRef]
- Jones, N.; Ray, B.; Ranjit, K.T.; Manna, A.C. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol. Lett. 2008, 279, 71–76. [Google Scholar] [CrossRef]
- Bradley, E.L.; Castle, L.; Chaudhry, Q. Applications of nanomaterials in food packaging with a consideration of opportunities for developing countries. Trends Food Sci. Technol. 2011, 22, 604–610. [Google Scholar] [CrossRef]
- Emamifar, A.; Kadivar, M.; Shahedi, M.; Soleimanian-Zad, S. Effect of nanocomposite packaging containing ag and ZnO on inactivation of lactobacillus plantarum in orange juice. Food Control 2011, 22, 408–413. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Soares, N.d.F.F.; Coimbra, J.S.d.R.; de Andrade, N.J.; Cruz, R.S.; Medeiros, E.A.A. Zinc oxide nanoparticles: Synthesis, antimicrobial activity and food packaging applications. Food Bioprocess Technol. 2012, 5, 1447–1464. [Google Scholar] [CrossRef]
- Turner, S.; Tavernier, S.M.F.; Huyberechts, G.; Biermans, E.; Bals, S.; Batenburg, K.J.; Van Tendeloo, G. Assisted spray pyrolysis production and characterisation of ZnO nanoparticles with narrow size distribution. J. Nanopart. Res. 2010, 12, 615–622. [Google Scholar] [CrossRef]
- Johar Abdullah, M.; Putrus, G.A.; Chong, J.; Karim Mohamad, A. Nanostructure of ZnO fabricated via french process and its correlation to electrical properties of semiconducting varistor. Synth. React. Inorg. Metal Org. Nano Metal Chem. 2006, 36, 155–159. [Google Scholar]
- Eskandari, M.; Haghighi, N.; Ahmadi, V.; Haghighi, F.; Mohammadi, S.R. Growth and investigation of antifungal properties of ZnO nanorod arrays on the glass. Phys. B Condens. Matter 2011, 406, 112–114. [Google Scholar] [CrossRef]
- Sawai, J.; Yoshikawa, T. Quantitative evaluation of antifungal activity of metallic oxide powders (MgO, CaO and ZnO) by an indirect conductimetric assay. J. Appl. Microbiol. 2004, 96, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, Y.; Ding, Y.; Daskalakis, N.; Jeuken, L.; Povey, M.; O’Neill, A.J.; York, D.W. Mechanistic investigation into antibacterial behaviour of suspensions of ZnO nanoparticles against E. coli. J. Nanopart. Res. 2010, 12, 1625–1636. [Google Scholar] [CrossRef]
- Kumar, R.; Umar, A.; Kumar, G.; Nalwa, H.S. Antimicrobial properties of ZnO nanomaterials: A review. Ceram. Int. 2017, 43, 3940–3961. [Google Scholar] [CrossRef]
- Ramani, M.; Ponnusamy, S.; Muthamizhchelvan, C.; Cullen, J.; Krishnamurthy, S.; Marsili, E. Morphology-directed synthesis of ZnO nanostructures and their antibacterial activity. Coll. Surf. B Biointerfaces 2013, 105, 24–30. [Google Scholar] [CrossRef]
- Jain, A.; Bhargava, R.; Poddar, P. Probing interaction of gram-positive and gram-negative bacterial cells with ZnO nanorods. Mater. Sci. Eng. C 2013, 33, 1247–1253. [Google Scholar] [CrossRef]
- Shinde, V.V.; Dalavi, D.S.; Mali, S.S.; Hong, C.K.; Kim, J.H.; Patil, P.S. Surfactant free microwave assisted synthesis of ZnO microspheres: Study of their antibacterial activity. Appl. Surf. Sci. 2014, 307, 495–502. [Google Scholar] [CrossRef]
- Stanković, A.; Dimitrijević, S.; Uskoković, D. Influence of size scale and morphology on antibacterial properties of ZnO powders hydrothemally synthesized using different surface stabilizing agents. Coll. Surf. B Biointerfaces 2013, 102, 21–28. [Google Scholar] [CrossRef]
- Ng, Y.H.; Leung, Y.H.; Liu, F.Z.; Ng, A.M.C.; Gao, M.H.; Chan, C.M.N.; Djurišić, A.B.; Leung, F.C.C.; Chan, W.K. Antibacterial activity of ZnO nanoparticles under ambient illumination—The effect of nanoparticle properties. Thin Solid Films 2013, 542, 368–372. [Google Scholar] [CrossRef]
- Malini, M.; Thirumavalavan, M.; Yang, W.-Y.; Lee, J.-F.; Annadurai, G. A versatile chitosan/ZnO nanocomposite with enhanced antimicrobial properties. Int. J. Biol. Macromol. 2015, 80, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Kickelbick, G. Concepts for the incorporation of inorganic building blocks into organic polymers on a nanoscale. Prog. Polym. Sci. 2003, 28, 83–114. [Google Scholar] [CrossRef]
- Bumbudsanpharoke, N.; Choi, J.; Park, H.J.; Ko, S. Zinc migration and its effect on the functionality of a low density polyethylene-ZnO nanocomposite film. Food Packag. Shelf Life 2019, 20, 100301. [Google Scholar] [CrossRef]
- Mo, Z.; Lin, J.; Zhang, X.; Fan, Y.; Xu, X.; Xue, Y.; Liu, D.; Li, J.; Hu, L.; Tang, C. Morphology controlled synthesis zinc oxide and reinforcement in polyhydroxyalkanoates composites. Polym. Compos. 2014, 35, 1701–1706. [Google Scholar] [CrossRef]
- Heydari-Majd, M.; Ghanbarzadeh, B.; Shahidi-Noghabi, M.; Najafi, M.A.; Hosseini, M. A new active nanocomposite film based on PLA/ZnO nanoparticle/essential oils for the preservation of refrigerated otolithes ruber fillets. Food Packag. Shelf Life 2019, 19, 94–103. [Google Scholar] [CrossRef]
- Ejaz, M.; Arfat, Y.A.; Mulla, M.; Ahmed, J. Zinc oxide nanorods/clove essential oil incorporated type b gelatin composite films and its applicability for shrimp packaging. Food Packag. Shelf Life 2018, 15, 113–121. [Google Scholar] [CrossRef]
- Wu, H.; Lei, Y.; Zhu, R.; Zhao, M.; Lu, J.; Xiao, D.; Jiao, C.; Zhang, Z.; Shen, G.; Li, S. Preparation and characterization of bioactive edible packaging films based on pomelo peel flours incorporating tea polyphenol. Food Hydrocoll. 2019, 90, 41–49. [Google Scholar] [CrossRef]
- Chen, H.; Li, L.; Ma, Y.; McDonald, T.P.; Wang, Y. Development of active packaging film containing bioactive components encapsulated in β-cyclodextrin and its application. Food Hydrocoll. 2019, 90, 360–366. [Google Scholar] [CrossRef]
- Al-Jumaili, A.; Mulvey, P.; Kumar, A.; Prasad, K.; Bazaka, K.; Warner, J.; Jacob, M.V. Eco-friendly nanocomposites derived from geranium oil and zinc oxide in one step approach. Sci. Rep. 2019, 9, 5973. [Google Scholar] [CrossRef]
- Sani, I.K.; Pirsa, S.; Tağı, Ş. Preparation of chitosan/zinc oxide/melissa officinalis essential oil nano-composite film and evaluation of physical, mechanical and antimicrobial properties by response surface method. Polym. Test. 2019, 79, 106004. [Google Scholar] [CrossRef]
- Ahmed, J.; Mulla, M.; Jacob, H.; Luciano, G.; T.B., B.; Almusallam, A. Polylactide/poly(ε-caprolactone)/zinc oxide/clove essential oil composite antimicrobial films for scrambled egg packaging. Food Packag. Shelf Life 2019, 21, 100355. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Pérez-Masiá, R.; Lagaron, J.M. A review on electrospun polymer nanostructures as advanced bioactive platforms. Polym. Eng. Sci. 2016, 56, 500–527. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Martinez-Abad, A.; Lagaron, J.M. Zein-based ultrathin fibers containing ceramic nanofillers obtained by electrospinning. II. Mechanical properties, gas barrier, and sustained release capacity of biocide thymol in multilayer polylactide films. J. Appl. Polym. Sci. 2014, 131, 9270–9276. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Montanes, N.; Lagaron, J.M.; Balart, R.; Torres-Giner, S. Bioactive multilayer polylactide films with controlled release capacity of gallic acid accomplished by incorporating electrospun nanostructured coatings and interlayers. Appl. Sci. 2019, 9, 533. [Google Scholar] [CrossRef]
- Torres-Giner, S. Novel antimicrobials obtained by electrospinning methods. In Antimicrobial Polymers; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 261–285. [Google Scholar]
- Cherpinski, A.; Torres-Giner, S.; Cabedo, L.; Lagaron, J.M. Post-processing optimization of electrospun submicron poly(3-hydroxybutyrate) fibers to obtain continuous films of interest in food packaging applications. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2017, 34, 1817–1830. [Google Scholar] [CrossRef]
- Figueroa-Lopez, K.J.; Vicente, A.A.; Reis, M.A.M.; Torres-Giner, S.; Lagaron, J.M. Antimicrobial and antioxidant performance of various essential oils and natural extracts and their incorporation into biowaste derived poly(3-hydroxybutyrate-co-3-hydroxyvalerate) layers made from electrospun ultrathin fibers. Nanomaterials 2019, 9, 144. [Google Scholar] [CrossRef]
- Melendez-Rodriguez, B.; Figueroa-Lopez, K.J.; Bernardos, A.; Martínez-Máñez, R.; Cabedo, L.; Torres-Giner, S.; Lagaron, J.M. Electrospun antimicrobial films of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) containing eugenol essential oil encapsulated in mesoporous silica nanoparticles. Nanomaterials 2019, 9, 227. [Google Scholar] [CrossRef]
- Castro-Mayorga, J.L.; Fabra, M.J.; Pourrahimi, A.M.; Olsson, R.T.; Lagaron, J.M. The impact of zinc oxide particle morphology as an antimicrobial and when incorporated in poly(3-hydroxybutyrate-co-3-hydroxyvalerate) films for food packaging and food contact surfaces applications. Food Bioprod. Process. 2017, 101, 32–44. [Google Scholar] [CrossRef]
- Enescu, D.; Cerqueira, M.A.; Fucinos, P.; Pastrana, L.M. Recent advances and challenges on applications of nanotechnology in food packaging. A literature review. Food Chem. Toxicol. 2019, 134, 110814. [Google Scholar] [CrossRef]
- Melendez-Rodriguez, B.; Castro-Mayorga, J.L.; Reis, M.A.M.; Sammon, C.; Cabedo, L.; Torres-Giner, S.; Lagaron, J.M. Preparation and characterization of electrospun food biopackaging films of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) derived from fruit pulp biowaste. Front. Sustain. Food Syst. 2018, 2, 38. [Google Scholar] [CrossRef]
- Figueroa-Lopez, K.J.; Andrade-Mahecha, M.M.; Torres-Varga, O.L. Development of antimicrobial biocomposite films to preserve the quality of bread. Molecules 2018, 23, 212. [Google Scholar] [CrossRef] [PubMed]
- Agüero, A.; Morcillo, M.d.C.; Quiles-Carrillo, L.; Balart, R.; Boronat, T.; Lascano, D.; Torres-Giner, S.; Fenollar, O. Study of the influence of the reprocessing cycles on the final properties of polylactide pieces obtained by injection molding. Polymers 2019, 11, 1908. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Lopez, K.J.; Castro-Mayorga, J.L.; Andrade-Mahecha, M.M.; Cabedo, L.; Lagaron, J.M. Antibacterial and barrier properties of gelatin coated by electrospun polycaprolactone ultrathin fibers containing black pepper oleoresin of interest in active food biopackaging applications. Nanomaterials 2018, 8, 199. [Google Scholar] [CrossRef] [PubMed]
- Torres-Giner, S.; Torres, A.; Ferrándiz, M.; Fombuena, V.; Balart, R. Antimicrobial activity of metal cation-exchanged zeolites and their evaluation on injection-molded pieces of bio-based high-density polyethylene. J. Food Saf. 2017, 37, e12348. [Google Scholar] [CrossRef]
- Naphade, R.; Jog, J. Electrospinning of phbv/ZnO membranes: Structure and properties. Fibers Polym. 2012, 13, 692–697. [Google Scholar] [CrossRef]
- Ogunyemi, S.O.; Abdallah, Y.; Zhang, M.; Fouad, H.; Hong, X.; Ibrahim, E.; Masum, M.M.I.; Hossain, A.; Mo, J.; Li, B. Green synthesis of zinc oxide nanoparticles using different plant extracts and their antibacterial activity against Xanthomonas oryzae pv. oryzae. Artif. Cells Nanomed. Biotechnol. 2019, 47, 341–352. [Google Scholar] [CrossRef]
- Castilho, P.C.; Savluchinske-Feio, S.; Weinhold, T.S.; Gouveia, S.C. Evaluation of the antimicrobial and antioxidant activities of essential oils, extracts and their main components from oregano from Madeira island, Portugal. Food Control 2012, 23, 552–558. [Google Scholar] [CrossRef]
- Salah, N.; Al-Shawafi, W.M.; Alshahrie, A.; Baghdadi, N.; Soliman, Y.M.; Memic, A. Size controlled, antimicrobial ZnO nanostructures produced by the microwave assisted route. Mater.Sci. Eng. C 2019, 99, 1164–1173. [Google Scholar] [CrossRef]
- Kaushik, M.; Niranjan, R.; Thangam, R.; Madhan, B.; Pandiyarasan, V.; Ramachandran, C.; Oh, D.-H.; Venkatasubbu, G.D. Investigations on the antimicrobial activity and wound healing potential of ZnO nanoparticles. Appl. Surf. Sci. 2019, 479, 1169–1177. [Google Scholar] [CrossRef]
- Yang, T.; Oliver, S.; Chen, Y.; Boyer, C.; Chandrawati, R. Tuning crystallization and morphology of zinc oxide with polyvinylpyrrolidone: Formation mechanisms and antimicrobial activity. J. Coll. Interface Sci. 2019, 546, 43–52. [Google Scholar] [CrossRef]
- Padmavathy, N.; Vijayaraghavan, R. Enhanced bioactivity of ZnO nanoparticles—An antimicrobial study. Sci. Technol. Adv. Mater. 2008, 9, 035004. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.; Shahraki, F.; Aliabadi, M.; Haji, A.; Deuber, F.; Adlhart, C. Nanofiber immobilized ceo2/dendrimer nanoparticles: An efficient photocatalyst in the visible and the uv. Appl. Surf. Sci. 2019, 479, 608–618. [Google Scholar] [CrossRef]
- Cherpinski, A.; Gozutok, M.; Sasmazel, H.T.; Torres-Giner, S.; Lagaron, J.M. Electrospun oxygen scavenging films of poly(3-hydroxybutyrate) containing palladium nanoparticles for active packaging applications. Nanomaterials 2018, 8, 469. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Li, Y.; Si, Y.; Yin, X.; Yu, J.; Ding, B. Electrospun polyvinylidene fluoride/sio2 nanofibrous membranes with enhanced electret property for efficient air filtration. Compos. Commun. 2019, 13, 57–62. [Google Scholar] [CrossRef]
- Serrà, A.; Zhang, Y.; Sepúlveda, B.; Gómez, E.; Nogués, J.; Michler, J.; Philippe, L. Highly active ZnO-based biomimetic fern-like microleaves for photocatalytic water decontamination using sunlight. Appl. Catal. B Environ. 2019, 248, 129–146. [Google Scholar] [CrossRef]
- Wang, H.; Gong, X.; Miao, Y.; Guo, X.; Liu, C.; Fan, Y.Y.; Zhang, J.; Niu, B.; Li, W. Preparation and characterization of multilayer films composed of chitosan, sodium alginate and carboxymethyl chitosan-ZnO nanoparticles. Food Chem. 2019, 283, 397–403. [Google Scholar] [CrossRef]
- Ten, E.; Turtle, J.; Bahr, D.; Jiang, L.; Wolcott, M. Thermal and mechanical properties of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/cellulose nanowhiskers composites. Polymer 2010, 51, 2652–2660. [Google Scholar] [CrossRef]
- Fabra, M.J.; Lopez-Rubio, A.; Lagaron, J.M. Nanostructured interlayers of zein to improve the barrier properties of high barrier polyhydroxyalkanoates and other polyesters. J. Food Eng. 2014, 127, 1–9. [Google Scholar] [CrossRef]
- Ke, Y.; Qu, Z.; Wu, G.; Wang, Y. Thermal and in vitro degradation properties of the nh2-containing phbv films. Polym. Degrad. Stab. 2014, 105, 59–67. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. Poly(3-hydroxybutyrate)/ZnO bionanocomposites with improved mechanical, barrier and antibacterial properties. Int. J. Mol. Sci. 2014, 15, 10950–10973. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T. Physico-chemical properties, morphology and antioxidant activity of film from fish skin gelatin incorporated with root essential oils. J. Food Eng. 2013, 117, 350–360. [Google Scholar] [CrossRef]
- Chu, Z.; Zhao, T.; Li, L.; Fan, J.; Qin, Y. Characterization of antimicrobial poly (lactic acid)/nano-composite films with silver and zinc oxide nanoparticles. Materials 2017, 10, 659. [Google Scholar] [CrossRef] [PubMed]
- Marschessault, R.H.; Monasterios, C.J.; Morin, F.G.; Sundararajan, P.R. Chiral poly(β-hydroxyalkanoates): An adaptable helix influenced by the alkane side-chain. Int. J. Biol. Macromol. 1990, 12, 158–165. [Google Scholar] [CrossRef]
- Skrbić, Z.; Divjaković, V. Temperature influence on changes of parameters of the unit cell of biopolymer phb. Polymer 1996, 37, 505–507. [Google Scholar] [CrossRef]
- Rodríguez-Tobías, H.; Morales, G.; Grande, D. Comprehensive review on electrospinning techniques as versatile approaches toward antimicrobial biopolymeric composite fibers. Mater. Sci. Eng. C 2019, 101, 306–322. [Google Scholar] [CrossRef]
- Amna, T.; Hassan, M.S.; Barakat, N.A.M.; Pandeya, D.R.; Hong, S.T.; Khil, M.-S.; Kim, H.Y. Antibacterial activity and interaction mechanism of electrospun zinc-doped titania nanofibers. Appl. Microbiol. Biotechnol. 2012, 93, 743–751. [Google Scholar] [CrossRef]
- Saha, R.; Subramani, K.; Petchi Muthu Raju, S.A.K.; Rangaraj, S.; Venkatachalam, R. Psidium guajava leaf extract-mediated synthesis of ZnO nanoparticles under different processing parameters for hydrophobic and antibacterial finishing over cotton fabrics. Prog. Org. Coat. 2018, 124, 80–91. [Google Scholar] [CrossRef]
- EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF). Scientific opinion on the safety evaluation of the substance zinc oxide, nanoparticles, uncoated and coated with [3-(methacryloxy)propyl] trimethoxysilane, for use in food contact materials. EFSA J. 2015, 13, 4063. [Google Scholar]
- European Commission (EC). Commission Regulation (EU): Plastic Materials and Articles Intended to Come Into Contact with Food 2016/1416 of 24 august 2016; European Commission: Brussels, Belgium, 2016; Volume 2019. [Google Scholar]


| Active Agent | Bacteria | MIC (µL/mL) | MBC (µL/mL) |
|---|---|---|---|
| OEO | E. coli | 0.625 | 0.625 |
| OEO | S. aureus | 0.312 | 0.312 |
| ZnONPs | E. coli | 0.156 | 0.156 |
| ZnONPs | S. aureus | 0.156 |
| Biopaper | L* | a* | b* | ΔE* | T | O |
|---|---|---|---|---|---|---|
| PHBV | 89.82 ± 0.06 a | 0.87 ± 0.07 a | −0.38 ± 0.02 a | --- | 3.13 ± 0.02 a | 0.016 ± 0.06 a |
| PHBV + 10 wt% OEO | 85.35 ± 0.07 b | 1.13 ± 0.05 b | 6.67 ± 0.03 b | 8.36 ± 0.08 a | 3.55 ± 0.03 a | 0.019 ± 0.08 a |
| PHBV + 3 wt% ZnONPs | 88.11 ± 0.06 a | 0.56 ± 0.04 a | −0.96 ± 0.05 c | 1.83 ± 0.05 b | 9.60 ± 0.06 b | 0.052 ± 0.05 b |
| PHBV +2.5 wt% OEO +2.25 wt% ZnONPs | 86.80 ± 0.03 c | −0.92 ± 0.03 c | 4.32 ± 0.02 d | 5.87 ± 0.03 c | 8.08 ± 0.04 c | 0.041 ± 0.05 c |
| PHBV + 5 wt% OEO + 1.5 wt% ZnONPs | 84.10 ± 0.07 d | −0.65 ± 0.06 d | 3.14 ± 0.09 e | 6.88 ± 0.09 d | 7.17 ± 0.05 d | 0.037 ± 0.05 c |
| PHBV + 7.5 wt% OEO + 0.75 wt% ZnONPs | 84.96 ± 0.06 bd | −0.55 ± 0.08 e | 5.35 ± 0.06 f | 7.64 ± 0.08 e | 5.03 ± 0.06 e | 0.027 ± 0.05 d |
| Sample | T5% (°C) | Tdeg (°C) | Mass Loss (%) | Residual Mass (%) |
|---|---|---|---|---|
| OEO | 101.5 ± 2.3 | 178.4 ± 0.89 | 74.16 ± 0.6 | 0.14 ± 0.5 |
| ZnONPs | --- | --- | 0.030 ± 0.5 | 99.97 ± 1.6 |
| PHBV | 251.5 ± 1.6 | 279.0 ± 1.1 | 55.23 ± 0.8 | 2.10 ± 0.7 |
| PHBV + 10 wt% OEO | 197.5 ± 2.7 | 283.6 ± 0.9 | 69.58 ± 1.0 | 2.16 ± 1.3 |
| PHBV + 3 wt% ZnONPs | 261.7 ± 2.3 | 275.6 ± 0.8 | 32.91 ± 1.4 | 4.35 ± 1.4 |
| PHBV + 2.5 wt% OEO + 2.25 wt% ZnONPs | 227.8 ± 2.1 | 261.4 ± 1.1 | 33.46 ± 0.7 | 3.77 ± 1.0 |
| PHBV + 5 wt% OEO + 1.5 wt% ZnONPs | 211.8 ± 1.3 | 263.3 ± 0.9 | 36.36 ± 1.9 | 3.44 ± 1.7 |
| PHBV + 7.5 wt% OEO + 0.75 wt% ZnONPs | 205.7 ± 1.9 | 268.9 ± 1.7 | 38.82 ± 1.5 | 3.15 ± 0.8 |
| Biopaper | E (MPa) | (MPa) | (%) | Toughness (mJ/mm3) |
|---|---|---|---|---|
| PHBV | 1125 ± 441 a | 12.6 ± 2.7 a | 1.71 ± 0.35 a | 0.124 ± 0.005 a |
| PHBV + 10 wt% OEO | 814 ± 82 b | 18.5 ± 0.5 b | 4.53 ± 0.41 b | 0.560 ± 0.076 b |
| PHBV + 3 wt% ZnONPs | 1286 ± 142c | 17.1 ± 0.9c | 1.26 ± 0.23 c | 0.244 ± 0.048 c |
| PHBV + 2.5 wt% OEO + 2.25 wt% ZnONPs | 855 ± 118 d | 14.5 ± 2.4 d | 3.28 ± 0.32 d | 0.303 ± 0.061 d |
| PHBV + 5 wt% OEO + 1.5 wt% ZnONPs | 801 ± 93 b | 14.8 ± 4.5 d | 4.35 ± 1.28 b | 0.446 ± 0.273 e |
| PHBV + 7.5 wt% OEO + 0.75 wt% ZnONPs | 778 ± 102 e | 14.1 ± 3.8 d | 5.01 ± 1.34 e | 0.485 ± 0.249 e |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueroa-Lopez, K.J.; Torres-Giner, S.; Enescu, D.; Cabedo, L.; Cerqueira, M.A.; Pastrana, L.M.; Lagaron, J.M. Electrospun Active Biopapers of Food Waste Derived Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) with Short-Term and Long-Term Antimicrobial Performance. Nanomaterials 2020, 10, 506. https://doi.org/10.3390/nano10030506
Figueroa-Lopez KJ, Torres-Giner S, Enescu D, Cabedo L, Cerqueira MA, Pastrana LM, Lagaron JM. Electrospun Active Biopapers of Food Waste Derived Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) with Short-Term and Long-Term Antimicrobial Performance. Nanomaterials. 2020; 10(3):506. https://doi.org/10.3390/nano10030506
Chicago/Turabian StyleFigueroa-Lopez, Kelly J., Sergio Torres-Giner, Daniela Enescu, Luis Cabedo, Miguel A. Cerqueira, Lorenzo M. Pastrana, and Jose M. Lagaron. 2020. "Electrospun Active Biopapers of Food Waste Derived Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) with Short-Term and Long-Term Antimicrobial Performance" Nanomaterials 10, no. 3: 506. https://doi.org/10.3390/nano10030506
APA StyleFigueroa-Lopez, K. J., Torres-Giner, S., Enescu, D., Cabedo, L., Cerqueira, M. A., Pastrana, L. M., & Lagaron, J. M. (2020). Electrospun Active Biopapers of Food Waste Derived Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) with Short-Term and Long-Term Antimicrobial Performance. Nanomaterials, 10(3), 506. https://doi.org/10.3390/nano10030506











