Enhancement of Antibacterial Performance of Silver Nanowire Transparent Film by Post-Heat Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Material, Fabrication, and Heat Treatment
2.2. Characterization
2.3. Antibacterial Test
3. Results and Discussion
3.1. Surface Morphology
3.2. Oxidation State, Optical Characteristics, and Sheet Resistance
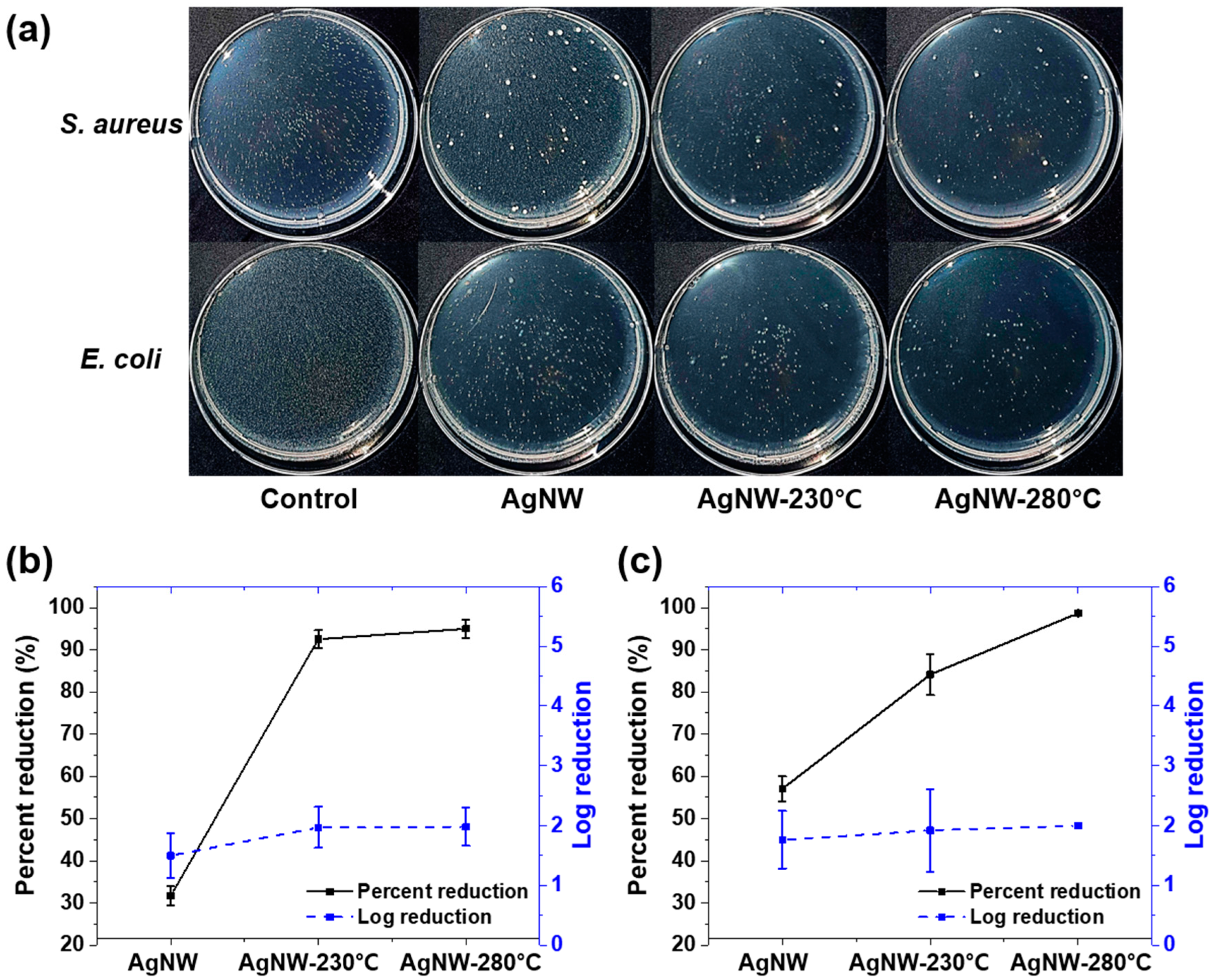
3.3. Antibacterial Activity
- Ag+ directly destroys the cell wall consisting of an outer membrane and a single-layered peptidoglycan, and eventually causes the cytoplasmic leakage of cellular contents [55].
- Ag+ promotes the formation of intracellular reactive oxygen species (ROSs). ROSs, involving superoxide-radical (O2−), hydroxyl radical (•OH), hydrogen peroxide (H2O2), and singlet oxygen (1O2), are short-lived strong oxidants [56]. Ag+ mainly generates O2− by two mechanisms: one is the inactivation of respiratory chain enzymes by interacting between Ag+ and the thiol group of the enzymes [56,57,58], and the other is Ag+-mediated mitochondria dysfunction [59,60]. The ROS-induced oxidative stress attacks the ribosome and DNA of bacterial cells, and furthermore oxidizes the membrane lipid excessively [61].
- Ag+ with high affinity for thiol groups favors a reaction with cysteine residues of respiration chain proteins [63], in a manner that strongly binds to the cysteine residues. As a consequence of the reaction, energy generation (i.e., ATP synthesis) is inhibited due to a decrease in the proton motive force by proton leakage into the cytoplasm [64].
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McBryde, E.S.; Bradley, L.C.; Whitby, M.; McElwain, D.L.S. An investigation of contact transmission of methicillin-resistant Staphylococcus aureus. J. Hosp. Infect. 2004, 58, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Klevens, R.M.; Edwards, J.R.; Richards, C.L.; Horan, T.C.; Gaynes, R.P.; Pollock, D.A.; Cardo, D.M. Estimating health care-associated infections and deaths in U.S. Hospitals, 2002. Public Health Rep. 2007, 122, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Rutledge-Taylor, K.; Matlow, A.; Gravel, D.; Embree, J.; Le Saux, N.; Johnston, L.; Suh, K.; Embil, J.; Henderson, E.; John, M.; et al. A point prevalence survey of health care-associated infections in Canadian pediatric inpatients. Am. J. Infect. Control. 2012, 40, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Zarb, P.; Coignard, B.; Griskeviciene, J.; Muller, A.; Vankerckhoven, V.; Weist, K.; Goossens, M.M.; Vaerenberg, S.; Hopkins, S.; Catry, B.; et al. The european centre for disease prevention and control (ECDC) pilot point prevalence survey of healthcare-associated infections and antimicrobial use. Eurosurveillance 2012, 17, 20316. [Google Scholar] [CrossRef]
- Allegranzi, B.; Nejad, S.B.; Combescure, C.; Graafmans, W.; Attar, H.; Donaldson, L.; Pittet, D. Burden of endemic health-care-associated infection in developing countries: Systematic review and meta-analysis. Lancet 2011, 377, 228–241. [Google Scholar] [CrossRef]
- Antibacterial agents in clinical development. Available online: https://apps.who.int/iris/bitstream/handle/10665/258965/WHO-EMP-IAU-2017.11-eng.pdf;jsessionid=E2B3EC3D8DEAED6A3D6E36CFB11D22C2?sequence=1 (accessed on 13 May 2020).
- Kumar, S.S.D.; Houreld, N.N.; Kroukamp, E.M.; Abrahamse, H. Cellular imaging and bactericidal mechanism of green-synthesized silver nanoparticles against human pathogenic bacteria. J. Photochem. Photobiol. B Biol. 2018, 178, 259–269. [Google Scholar] [CrossRef]
- Korshed, P.; Li, L.; Liu, Z.; Mironov, A.; Wang, T. Antibacterial mechanisms of a novel type picosecond laser-generated silver-titanium nanoparticles and their toxicity to human cells. Int. J. Nanomed. 2018, 13, 89. [Google Scholar] [CrossRef]
- Hornyak, G.L. Fundamentals of Nanotechnology; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Bachenheimer, L.; Scherzer, R.; Elliott, P.; Stagon, S.; Gasparov, L.; Huang, H. Degradation mechanism of Ag nanorods for surface enhanced Raman spectroscopy. Sci. Rep. 2017, 7, 16282. [Google Scholar] [CrossRef]
- Korte, K.E.; Skrabalak, S.E.; Xia, Y. Rapid synthesis of silver nanowires through a CuCl- or CuCl2-mediated polyol process. J. Mater. Chem. 2008, 18, 437–441. [Google Scholar] [CrossRef]
- Liang, H.; Wang, W.; Huang, Y.; Zhang, S.; Wei, H.; Xu, H. Controlled synthesis of uniform silver nanospheres. J. Phys. Chem. C 2010, 114, 7427–7431. [Google Scholar] [CrossRef]
- Hoop, M.; Shen, Y.; Chen, X.; Mushtaq, F.; Iuliano, L.M.; Sakar, M.S.; Petruska, A.; Loessner, M.J.; Nelson, B.J.; Pané, S. Magnetically Driven Silver-Coated Nanocoils for Efficient Bacterial Contact Killing. Adv. Funct. Mater. 2016, 26, 1063–1069. [Google Scholar] [CrossRef]
- Lok, C.-N.; Ho, C.-M.; Chen, R.; He, Q.-Y.; Yu, W.-Y.; Sun, H.; Tam, P.K.-H.; Chiu, J.-F.; Che, C.-M. Silver nanoparticles: Partial oxidation and antibacterial activities. JBIC J. Biol. Inorg. Chem. 2007, 12, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wyman, I.; Hu, J.; Lin, S.; Zhong, Z.; Tu, Y.; Huang, Z.; Wei, Y. Silver nanowires: Synthesis technologies, growth mechanism and multifunctional applications. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2017, 5, 2. [Google Scholar] [CrossRef]
- Zhang, R.; Engholm, M. Recent progress on the fabrication and properties of silver nanowire-based transparent electrodes. Nanomaterials 2018, 8, 628. [Google Scholar] [CrossRef] [PubMed]
- Duval, R.E.; Gouyau, J.; Lamouroux, E. Limitations of Recent Studies Dealing with the Antibacterial Properties of Silver Nanoparticles: Fact and Opinion. Nanomaterials 2019, 9, 1775. [Google Scholar] [CrossRef]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Lara, H.H.; Garza-Treviño, E.N.; Ixtepan-Turrent, L.; Singh, D.K. Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J. Nanobiotechnol. 2011, 9, 30. [Google Scholar] [CrossRef]
- Shahverdi, A.R.; Fakhimi, A.; Shahverdi, H.R.; Minaian, S. Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 168–171. [Google Scholar] [CrossRef]
- Visnapuu, M.; Joost, U.; Juganson, K.; Künnis-Beres, K.; Kahru, A.; Kisand, V.; Ivask, A. Dissolution of silver nanowires and nanospheres dictates their toxicity to escherichia coli. Biomed. Res. Int. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Helmlinger, J.; Sengstock, C.; Groß-Heitfeld, C.; Mayer, C.; Schildhauer, T.A.; Köller, M.; Epple, M. Silver nanoparticles with different size and shape: Equal cytotoxicity, but different antibacterial effects. Rsc Adv. 2016, 6, 18490–18501. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.T.; Lee, J.H.; Chae, Y.J.; Kim, Y.S.; Kim, B.C.; Sang, B.I.; Gu, M.B. Analysis of the toxic mode of action of silver nanoparticles using stress-specific bioluminescent bacteria. Small 2008, 4, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.S.; Draheim, R.R.; Roldo, M. Silver nanowires: Synthesis, antibacterial activity and biomedical applications. Appl. Sci. 2018, 8, 673. [Google Scholar] [CrossRef]
- Song, T.-B.; Rim, Y.S.; Liu, F.; Bob, B.; Ye, S.; Hsieh, Y.-T.; Yang, Y. Highly robust silver nanowire network for transparent electrode. ACS Appl. Mater. Interfaces 2015, 7, 24601–24607. [Google Scholar] [CrossRef]
- Singh, M.; Rana, T.R.; Kim, S.; Kim, K.; Yun, J.H.; Kim, J. Silver nanowires binding with sputtered ZnO to fabricate highly conductive and thermally stable transparent electrode for solar cell applications. ACS Appl. Mater. Interfaces 2016, 8, 12764–12771. [Google Scholar] [CrossRef]
- Chu, X.; Wang, K.; Tao, J.; Li, S.; Ji, S.; Ye, C. Tackling the Stability Issues of Silver Nanowire Transparent Conductive Films through FeCl3 Dilute Solution Treatment. Nanomaterials 2019, 9, 533. [Google Scholar] [CrossRef]
- Hwang, B.; An, Y.; Lee, H.; Lee, E.; Becker, S.; Kim, Y.-H.; Kim, H. Highly flexible and transparent Ag nanowire electrode encapsulated with ultra-thin Al2O3: Thermal, ambient, and mechanical stabilities. Sci. Rep. 2017, 7, 41336. [Google Scholar] [CrossRef]
- Chen, D.; Liang, J.; Liu, C.; Saldanha, G.; Zhao, F.; Tong, K.; Liu, J.; Pei, Q. Thermally stable silver nanowire–polyimide transparent electrode based on atomic layer deposition of zinc oxide on silver nanowires. Adv. Funct. Mater. 2015, 25, 7512–7520. [Google Scholar] [CrossRef]
- Bid, A.; Bora, A.; Raychaudhuri, A.K. Experimental study of Rayleigh instability in metallic nanowires using resistance fluctuations measurements from 77 K to 375 K. In Proceedings of the Fluctuations and Noise in Materials II., International Society for Optics and Photonics, Austin, TX, USA, 24 May 2005; Volume 5843, pp. 147–154. [Google Scholar]
- Jeong, Y.-C.; Nam, J.; Kim, J.; Kim, C.S.; Jo, S. Enhancing Thermal Oxidation Stability of Silver Nanowire Transparent Electrodes by Using a Cesium Carbonate-Incorporated Overcoating Layer. Materials 2019, 12, 1140. [Google Scholar] [CrossRef] [PubMed]
- Lagrange, M.; Langley, D.P.; Giusti, G.; Jiménez, C.; Bréchet, Y.; Bellet, D. Optimization of silver nanowire-based transparent electrodes: Effects of density, size and thermal annealing. Nanoscale 2015, 7, 17410–17423. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.S.; Oh, J.S.; Shin, J.H.; Yeom, G.Y.; Kim, K.N. Nano-welding of Ag nanowires using rapid thermal annealing for transparent conductive films. J. Nanosci. Nanotechnol. 2015, 15, 8647–8651. [Google Scholar] [CrossRef]
- Japanese Standards Association JIS Z 2801:2010 Antibacterial products–test for antibacterial activity and efficacy; Japanese Standards Association: Tokyo, Japan, 2010.
- Wang, Y.; Du, D.; Yang, X.; Zhang, X.; Zhao, Y. Optoelectronic and Electrothermal Properties of Transparent Conductive Silver Nanowires Films. Nanomaterials 2019, 9, 904. [Google Scholar] [CrossRef] [PubMed]
- Fantanas, D.; Brunton, A.; Henley, S.J.; Dorey, R.A. Investigation of the mechanism for current induced network failure for spray deposited silver nanowires. Nanotechnology 2018, 29, 465705. [Google Scholar] [CrossRef] [PubMed]
- Molares, M.E.T.; Balogh, A.G.; Cornelius, T.W.; Neumann, R.; Trautmann, C. Fragmentation of nanowires driven by Rayleigh instability. Appl. Phys. Lett. 2004, 85, 5337–5339. [Google Scholar] [CrossRef]
- Huang, X.J. Nanotechnology Research: New Nanostructures, Nanotubes and Nanofibers; Nova Publishers: Hauppauge, NY, USA, 2008; ISBN 1600219020. [Google Scholar]
- Zhang, R.; Hummelgård, M.; Olin, H. Single layer porous gold films grown at different temperatures. Phys. B Condens. Matter 2010, 405, 4517–4522. [Google Scholar] [CrossRef]
- Mayoral, A.; Allard, L.F.; Ferrer, D.; Esparza, R.; Jose-Yacaman, M. On the behavior of Ag nanowires under high temperature: In situ characterization by aberration-corrected STEM. J. Mater. Chem. 2011, 21, 893–898. [Google Scholar] [CrossRef]
- Wagner, C.D.; Naumkin, A.V.; Kraut-Vass, A.; Allison, J.W.; Powell, C.J.; Rumble, J.R., Jr. NIST standard reference database 20, Version 3.4 (Web version). Available online: https://srdata.nist.gov/xps/Version_his.aspx (accessed on 13 May 2020).
- Kim, S.H.; Choi, W.I.; Kim, K.H.; Yang, D.J.; Heo, S.; Yun, D.J. Nanoscale Chemical and Electrical Stabilities of Graphene-covered Silver Nanowire Networks for Transparent Conducting Electrodes. Sci. Rep. 2016, 6, 33074. [Google Scholar] [CrossRef]
- Wei, J.; Lei, Y.; Jia, H.; Cheng, J.; Hou, H.; Zheng, Z. Controlled in situ fabrication of Ag2O/AgO thin films by a dry chemical route at room temperature for hybrid solar cells. Dalt. Trans. 2014, 43, 11333–11338. [Google Scholar] [CrossRef]
- Li, C.M.; Robertson, I.M.; Jenkins, M.L.; Hutchison, J.L.; Doole, R.C. In situ TEM observation of the nucleation and growth of silver oxide nanoparticles. Micron 2005, 36, 9–15. [Google Scholar] [CrossRef]
- Dannenberg, R.; Stach, E.; Groza, J.R.; Dresser, B.J. TEM annealing study of normal grain growth in silver thin films. Thin Solid Films 2000, 379, 133–138. [Google Scholar] [CrossRef]
- Tomsia, A.P.; Glaeser, A.M. Ceramic Microstructures: Control at the Atomic Level; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; ISBN 1461553938. [Google Scholar]
- Albregtsen, F. Reflection, refraction, diffraction, and scattering; University of Oslo: Oslo, Norway, 2008. [Google Scholar]
- McDermott, W. The Comprehensive Pbr Guide by Allegorithmic-Vol. 1. Available online: https://academy.substance3d.com/courses/the-pbr-guide-part-1 (accessed on 2 February 2020).
- Wolfe, M.K.; Lantagne, D.S. A method to test the efficacy of handwashing for the removal of emerging infectious pathogens. JoVE J. Vis. Exp. 2017, 124, e55604. [Google Scholar] [CrossRef] [PubMed]
- Allegranzi, B.; Pittet, D. Role of hand hygiene in healthcare-associated infection prevention. J. Hosp. Infect. 2009, 73, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Ivask, A.; Kurvet, I.; Kasemets, K.; Blinova, I.; Aruoja, V.; Suppi, S.; Vija, H.; Käkinen, A.; Titma, T.; Heinlaan, M.; et al. Size-dependent toxicity of silver nanoparticles to bacteria, yeast, algae, crustaceans and mammalian cells in vitro. PLoS ONE 2014, 9, e102108. [Google Scholar] [CrossRef] [PubMed]
- Jose, M.; Sienkiewicz, P.; Szymańska, K.; Darowna, D.; Moszyński, D.; Lendzion-Bieluń, Z.; Szymański, K.; Mozia, S. Influence of preparation procedure on physicochemical and antibacterial properties of titanate nanotubes modified with silver. Nanomaterials 2019, 9, 795. [Google Scholar] [CrossRef]
- Nielloud, F.; Marti-Mestres, G. Pharmaceutical Emulsions and Suspensions: Second Edition, Revised and Expanded; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Chaloupka, K.; Malam, Y.; Seifalian, A.M. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010, 28, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Kim, J.Y.; Kim, J.; Lee, J.H.; Hahn, J.S.; Gu, M.B.; Yoon, J. Silver-ion-mediated reactive oxygen species generation affecting bactericidal activity. Water Res. 2009, 43, 1027–1032. [Google Scholar] [CrossRef]
- Holt, K.B.; Bard, A.J. Interaction of silver(I) ions with the respiratory chain of Escherichia coli: An electrochemical and scanning electrochemical microscopy study of the antimicrobial mechanism of micromolar Ag. Biochemistry 2005, 44, 13214–13223. [Google Scholar] [CrossRef]
- Matsumura, Y.; Yoshikata, K.; Kunisaki, S.; Tsuchido, T. Mode of bactericidal action of silver zeolite and its comparison with that of silver nitrate. Appl. Environ. Microbiol. 2003, 69, 4278–4281. [Google Scholar] [CrossRef]
- Song, B.; Zhang, C.; Zeng, G.; Gong, J.; Chang, Y.; Jiang, Y. Antibacterial properties and mechanism of graphene oxide-silver nanocomposites as bactericidal agents for water disinfection. Arch. Biochem. Biophys. 2016, 604, 167–176. [Google Scholar] [CrossRef]
- Jin, J.; Zhang, L.; Shi, M.; Zhang, Y.; Wang, Q. Ti-GO-Ag nanocomposite: The effect of content level on the antimicrobial activity and cytotoxicity. Int. J. Nanomed. 2017, 12, 4209. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. Transl. Integr. 1997, 82, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Noronha, V.T.; Paula, A.J.; Durán, G.; Galembeck, A.; Cogo-Müller, K.; Franz-Montan, M.; Durán, N. Silver nanoparticles in dentistry. Dent. Mater. 2017, 33, 1110–1126. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, A.; Chandran, P.; Khan, S.S. Biofunctionalized silver nanoparticles: Advances and prospects. Colloids Surf. B Biointerfaces 2013, 105, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Schreurs, W.J.A.; Rosenberg, H. Effect of silver ions on transport and retention of phosphate by Escherichia coli. J. Bacteriol. 1982, 152, 7–13. [Google Scholar]
- Molleman, B.; Hiemstra, T. Surface structure of silver nanoparticles as a model for understanding the oxidative dissolution of silver ions. Langmuir 2015, 31, 13361–13372. [Google Scholar] [CrossRef]
- Förster, T. Handbook of Chemistry and Physics. Z. fur Phys. Chem. 1962, 34, 405. [Google Scholar] [CrossRef]
- Liu, J.; Sonshine, D.A.; Shervani, S.; Hurt, R.H. Controlled release of biologically active silver from nanosilver surfaces. ACS Nano 2010, 4, 6903–6913. [Google Scholar] [CrossRef]
- Wu, S.; Zuber, F.; Maniura-Weber, K.; Brugger, J.; Ren, Q. Nanostructured surface topographies have an effect on bactericidal activity. J. Nanobiotechnol. 2018, 16, 20. [Google Scholar] [CrossRef]
- Tripathy, A.; Sen, P.; Su, B.; Briscoe, W.H. Natural and bioinspired nanostructured bactericidal surfaces. Adv. Colloid Interface Sci. 2017, 248, 85–104. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-H.; Ma, J.; Jo, S.; Lee, S.; Kim, C.S. Enhancement of Antibacterial Performance of Silver Nanowire Transparent Film by Post-Heat Treatment. Nanomaterials 2020, 10, 938. https://doi.org/10.3390/nano10050938
Kim J-H, Ma J, Jo S, Lee S, Kim CS. Enhancement of Antibacterial Performance of Silver Nanowire Transparent Film by Post-Heat Treatment. Nanomaterials. 2020; 10(5):938. https://doi.org/10.3390/nano10050938
Chicago/Turabian StyleKim, Ji-Hyeon, Junfei Ma, Sungjin Jo, Seunghun Lee, and Chang Su Kim. 2020. "Enhancement of Antibacterial Performance of Silver Nanowire Transparent Film by Post-Heat Treatment" Nanomaterials 10, no. 5: 938. https://doi.org/10.3390/nano10050938
APA StyleKim, J.-H., Ma, J., Jo, S., Lee, S., & Kim, C. S. (2020). Enhancement of Antibacterial Performance of Silver Nanowire Transparent Film by Post-Heat Treatment. Nanomaterials, 10(5), 938. https://doi.org/10.3390/nano10050938





