Structural Modification of Nanomicelles through Phosphatidylcholine: The Enhanced Drug-Loading Capacity and Anticancer Activity of Celecoxib-Casein Nanoparticles for the Intravenous Delivery of Celecoxib
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Structural Modification of Casein Nanoparticles
2.3. Celecoxib Loading of PC-Casein Nanoparticles
2.4. Stability Study of PC-CX-Casein Nanoparticles
2.5. Preparation of CX-β-Casein Nanoparticles
2.6. Characterization of Casein Nanoparticle Dispersions
2.7. Cell Culture
2.8. Cell Viability Assay
2.9. In Vitro Cell Uptake Studies
2.10. Determination of Drug Concentration-Time Curve
2.11. Tumor Inhibition Experiments In Vivo
2.12. Statistical Analysis
3. Results and Discussion
3.1. Structural Modification of Casein Nanoparticles by PC
3.2. The Stability of PC-CX-Casein Nanoparticles
3.3. Resuspension of the Nanoparticles after Lyophilization
3.4. Inhibition on Tumor Cells In Vitro
3.5. In Vitro Cell Uptake of CX
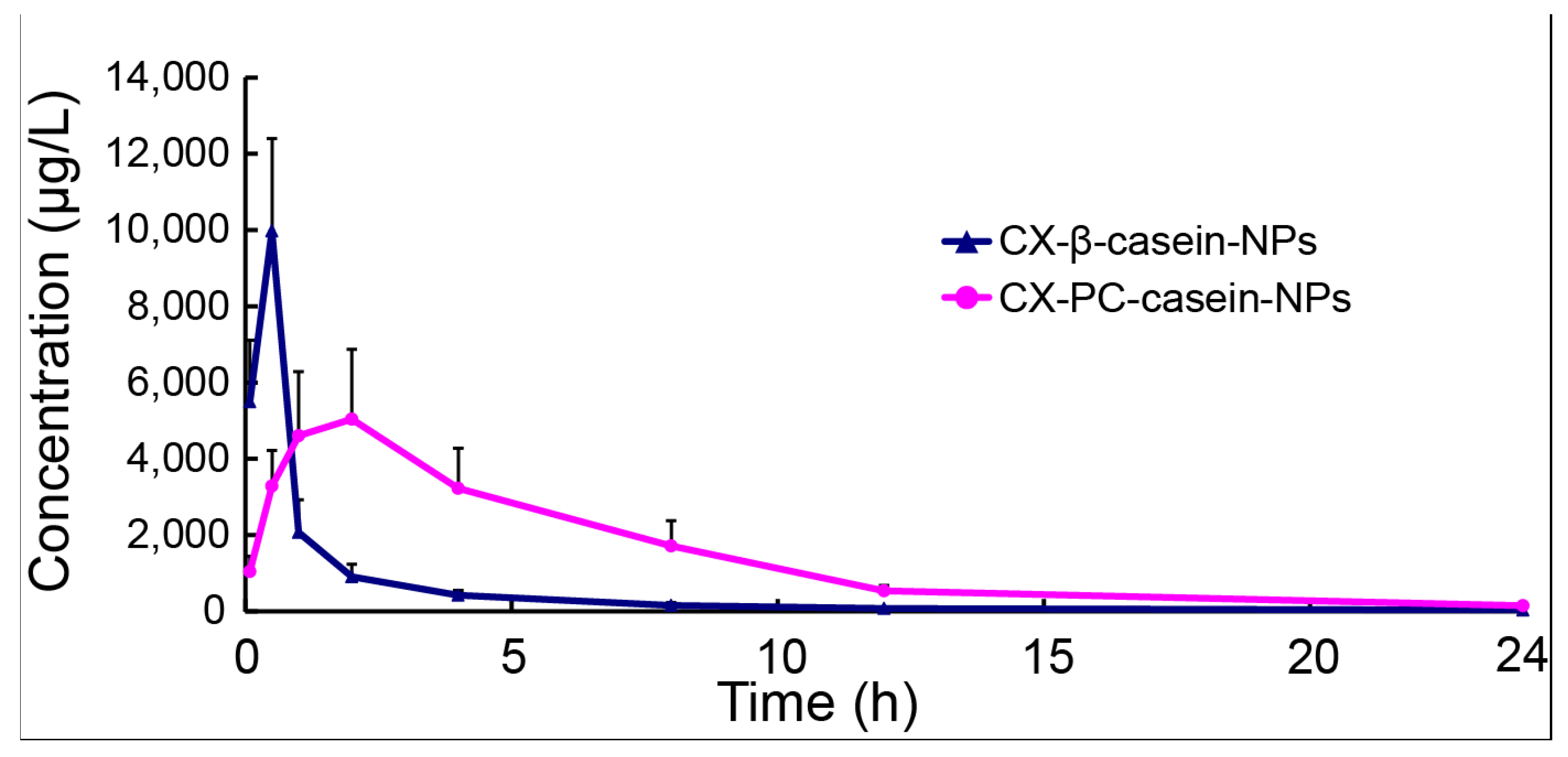
3.6. Pharmacokinetics of NP Formulations with Different Stability
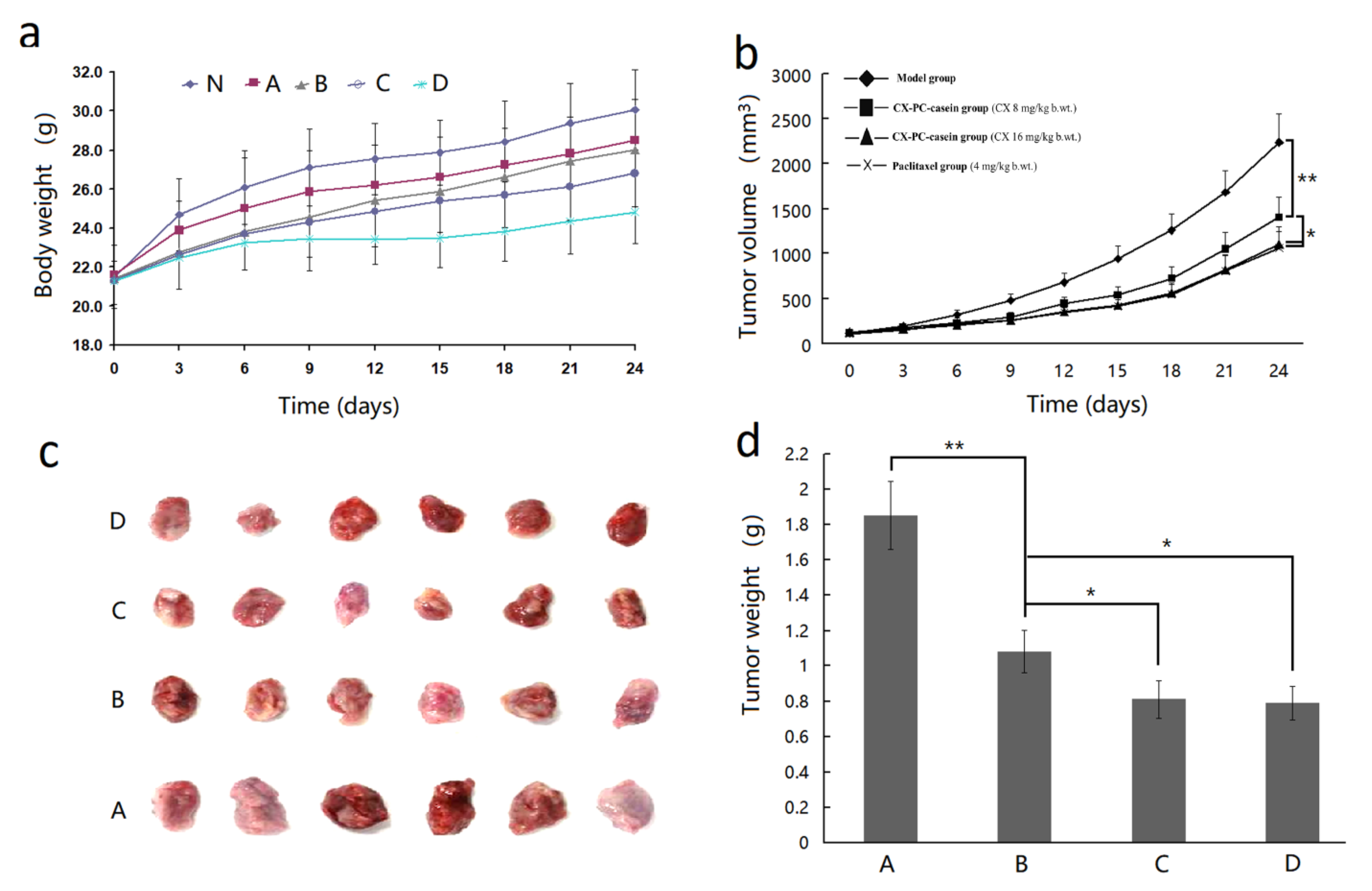
3.7. The Inhibition Effect of CX-PC-casein-NPs on Tumor Growth
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Semo, E.; Kesselman, E.; Danino, D.; Livney, Y.D. Casein micelle as a natural nano-capsular vehicle for nutraceuticals. Food Hydrocolloid. 2007, 21, 936–942. [Google Scholar] [CrossRef]
- Liu, C.; Yao, W.; Zhang, L.; Qian, H.; Wu, W.; Jiang, X. Cell-penetrating hollow spheres based on milk protein. Chem. Commun. 2010, 46, 7566–7568. [Google Scholar] [CrossRef] [PubMed]
- Fox, P.; Brodkorb, A. The casein micelle: Historical aspects, current concepts and significance. Int. Dairy J. 2008, 18, 677–684. [Google Scholar] [CrossRef]
- Trejo, R.; Dokland, T.; Jurat-Fuentes, J.; Harte, F. Cryo-transmission electron tomography of native casein micelles from bovine milk. J. Dairy Sci. 2011, 94, 5770–5775. [Google Scholar] [CrossRef]
- Shapira, A.; Assaraf, Y.G.; Livney, Y.D. Beta-casein nanovehicles for oral delivery of chemotherapeutic drugs. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 119–126. [Google Scholar] [CrossRef]
- Mcmahon, D.J.; Oommen, B.S. Supramolecular structure of the casein micelle. J. Dairy Sci. 2008, 91, 1709–1721. [Google Scholar] [CrossRef]
- Bachar, M.; Mandelbaum, A.; Portnaya, I.; Perlstein, H.; Simch, E.; Barenholz, Y.; Danino, D. Development and characterization of a novel drug nanocarrier for oral delivery, based on self-assembled β-casein micelles. J. Control. Release 2012, 160, 164–171. [Google Scholar] [CrossRef]
- Zimet, P.; Rosenberg, D.; Livney, Y.D. Re-assembled casein micelles and casein nanoparticles as nano-vehicles for ω-3 polyunsaturated fatty acids. Food Hydrocolloid. 2011, 25, 1270–1276. [Google Scholar] [CrossRef]
- Haham, M.; Ish-Shalom, S.; Nodelman, M.; Duek, I.; Segal, E.; Kustanovich, M.; Livney, Y.D. Stability and bioavailability of vitamin D nanoencapsulated in casein micelles. Food Funct. 2012, 3, 737–744. [Google Scholar] [CrossRef]
- Shapira, A.; Davidson, I.; Avni, N.; Assaraf, Y.G.; Livney, Y.D. β-Casein nanoparticle-based oral drug delivery system for potential treatment of gastric carcinoma: Stability, target-activated release and cytotoxicity. Eur. J. Pharm. Biopharm. 2012, 80, 298–305. [Google Scholar] [CrossRef]
- Steinbach, G.; Lynch, P.M.; Phillips, R.K.; Wallace, M.H.; Hawk, E.; Gordon, G.B.; Wakabayashi, N.; Saunders, B.; Shen, Y.; Fujimura, T. The Effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N. Engl. J. Med. 2000, 342, 1946–1952. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.L.; Velmurugan, B.K.; Chung, C.M.; Lin, S.H.; Wang, Z.H.; Hua, C.H.; Tsai, M.H.; Kuo, T.M.; Yeh, K.T.; Chang, P.Y. Preventive effect of celecoxib use against cancer progression and occurrence of oral squamous cell carcinoma. Sci. Rep. 2017, 7, 6235. [Google Scholar] [CrossRef] [PubMed]
- Hsu, A.L.; Ching, T.T.; Wang, D.S.; Song, X.; Rangnekar, V.M.; Chen, C.S. The cyclooxygenase-2 inhibitor celecoxib induces apoptosis by blocking Akt activation in human prostate cancer cells independently of Bcl-2. J. Biol. Chem. 2000, 275, 11397. [Google Scholar] [CrossRef] [PubMed]
- Adhim, Z.; Matsuoka, T.; Bito, T.; Shigemura, K.; Lee, K.M.; Kawabata, M.; Fujisawa, M.; Nibu, K.; Shirakawa, T. In vitro and in vivo inhibitory effect of three Cox-2 inhibitors and epithelial-to-mesenchymal transition in human bladder cancer cell lines. Brit. J. Cancer 2011, 105, 393–402. [Google Scholar] [CrossRef]
- Bocca, C.; Bozzo, F.; Cannito, S.; Parola, M.; Miglietta, A. Celecoxib inactivates epithelial-mesenchymal transition stimulated by hypoxia and/or epidermal growth factor in colon cancer cells. Mol. Carcinog. 2012, 51, 783–795. [Google Scholar] [CrossRef]
- Chen, C.; Xu, W.; Wang, C.-M. Combination of celecoxib and doxorubicin increases growth inhibition and apoptosis in acute myeloid leukemia cells. Leuk. Lymphoma 2013, 54, 2517–2522. [Google Scholar] [CrossRef]
- Verena, J. Targeting apoptosis pathways by Celecoxib in cancer. Cancer Lett. 2013, 332, 313–324. [Google Scholar]
- Melina, S.; Maria, H.; Marcus, F.; Michael, L.; Burkhard, H. Celecoxib increases lung cancer cell lysis by lymphokine-activated killer cells via upregulation of ICAM-1. Oncotarget 2015, 6, 39342–39356. [Google Scholar]
- Egashira, I.; Takahashi-Yanaga, F.; Nishida, R.; Arioka, M.; Igawa, K.; Tomooka, K.; Nakatsu, Y.; Tsuzuki, T.; Nakabeppu, Y.; Kitazono, T. Celecoxib and 2,5-dimethylcelecoxib inhibit intestinal cancer growth by suppressing the Wnt/β-catenin signaling pathway. Cancer Sci. 2017, 108, 108–115. [Google Scholar] [CrossRef]
- Solomon, D.H.; Husni, M.E.; Libby, P.A.; Yeomans, N.D.; Lincoff, A.; Lüscher, T.F.; Menon, V.; Brennan, D.M.; Wisniewski, L.M.; Nissen, S.E. The risk of major NSAID toxicity with celecoxib, ibuprofen or naproxen: A secondary analysis of the precision randomized controlled clinical trial. Am. J. Med. 2017, 130, 1415–1422. [Google Scholar] [CrossRef]
- Perlstein, H.; Turovsky, T.; Gimeson, P.; Cohen, R.; Rubinstein, A.; Danino, D.; Barenholz, Y. Thermotropic behavior of celecoxib-loaded beta-casein micelles: Relevance to the improved bioavailability. Eur. J. Nanomed. 2015, 7, 303–312. [Google Scholar] [CrossRef]
- Turovsky, T.; Khalfin, R.; Kababya, S.; Schmidt, A.; Barenholz, Y.; Danino, D. Celecoxib encapsulation in β-casein micelles: Structure, interactions, and conformation. Langmuir 2015, 31, 7183–7192. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Xu, Y.; Yin, J.; Jin, J.; Jiang, Y.; Du, Q. Improving the effectiveness of (−)-epigallocatechin gallate (EGCG) against rabbit atherosclerosis by EGCG-loaded nanoparticles prepared from chitosan and polyaspartic acid. J. Agric. Food Chem. 2014, 62, 12603–12609. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wen, J.; Liu, T.; Ou-yang, Q.; Cai, J.; Zhou, H. Genistein exposure interferes with pharmacokinetics of celecoxib in SD male rats by UPLC-MS/MS. Biochem. Res. Int. 2017, 2017, 6510232. [Google Scholar] [CrossRef]
- Lewis, B.A.; Engelman, D.M. Lipid bilayer thickness varies linearly with acyl chain length in fluid phosphatidylcholine vesicles. J. Mol. Biol. 1983, 166, 211–217. [Google Scholar] [CrossRef]
- Fettiplace, R.; Andrews, D.M.; Haydon, D. The thickness, composition and structure of some lipid bilayers and natural membranes. J. Membr. Biol. 1971, 5, 277–296. [Google Scholar] [CrossRef]
- Pan, Y.; Hui-Yuan, L.I.; Zhao, Y.F.; Han, Y.; Guo, S.Z. To study the height and topography of lipid bilayer by AFM. Chin. J. Med. Phy. 2007, 24, 420–424. [Google Scholar]
- Kurihara, Y.; Hatori, M.; Ando, Y.; Ito, D.; Toyoshima, T.; Tanaka, M.; Shintani, S. Inhibition of cyclooxygenase-2 suppresses the invasiveness of oral squamous cell carcinoma cell lines via down-regulation of matrix metalloproteinase-2 production and activation. Clin. Exp. Metas. 2009, 26, 425–432. [Google Scholar] [CrossRef]
- Yu, C.; Li, W.; Liu, J.; Lu, J.; Feng, J. Autophagy: Novel applications of nonsteroidal anti-inflammatory drugs for primary cancer. Cancer Med. 2018, 7, 471–484. [Google Scholar] [CrossRef]
- Sudhakar, B.; NagaJyothi, K.; Ramana Murthy, K. Nanosuspensions as a versatile carrier based drug delivery system–an overview. Curr. Drug Del. 2014, 11, 299–305. [Google Scholar] [CrossRef]
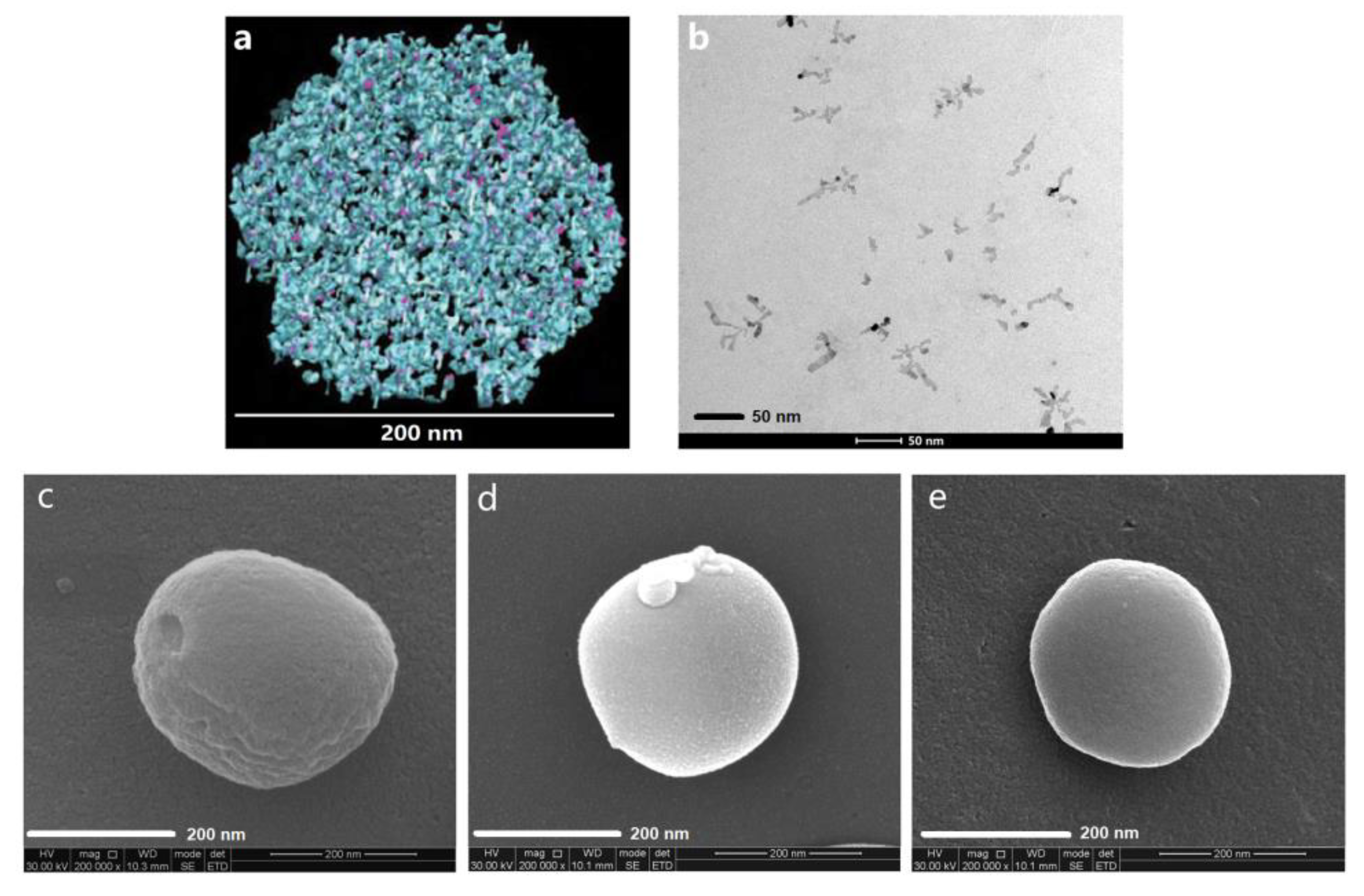
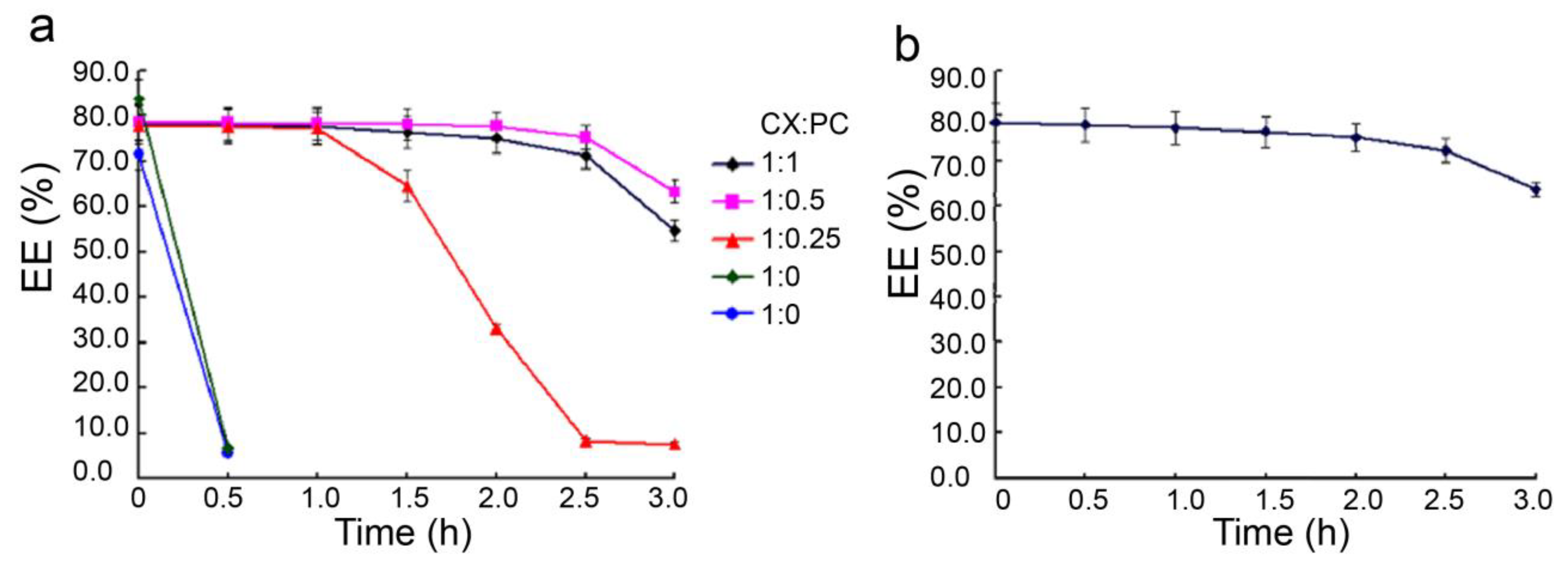
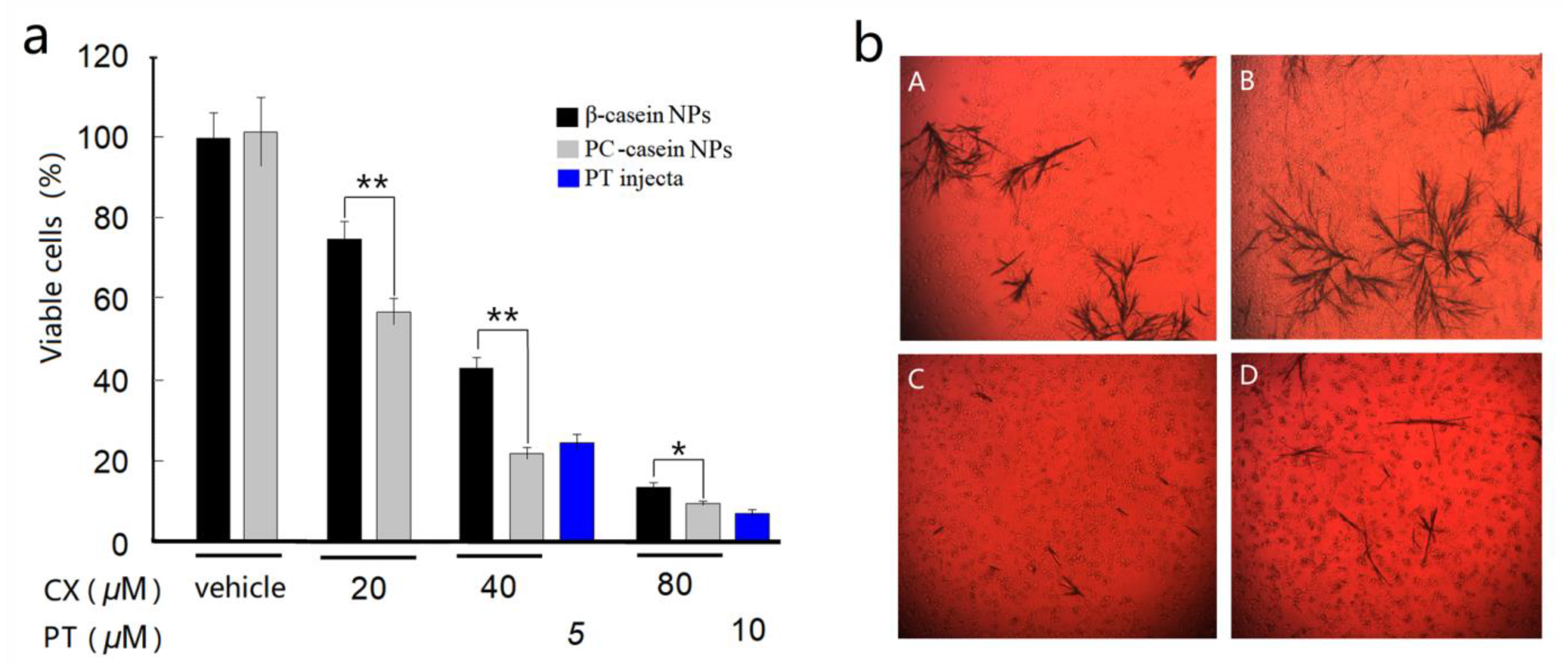
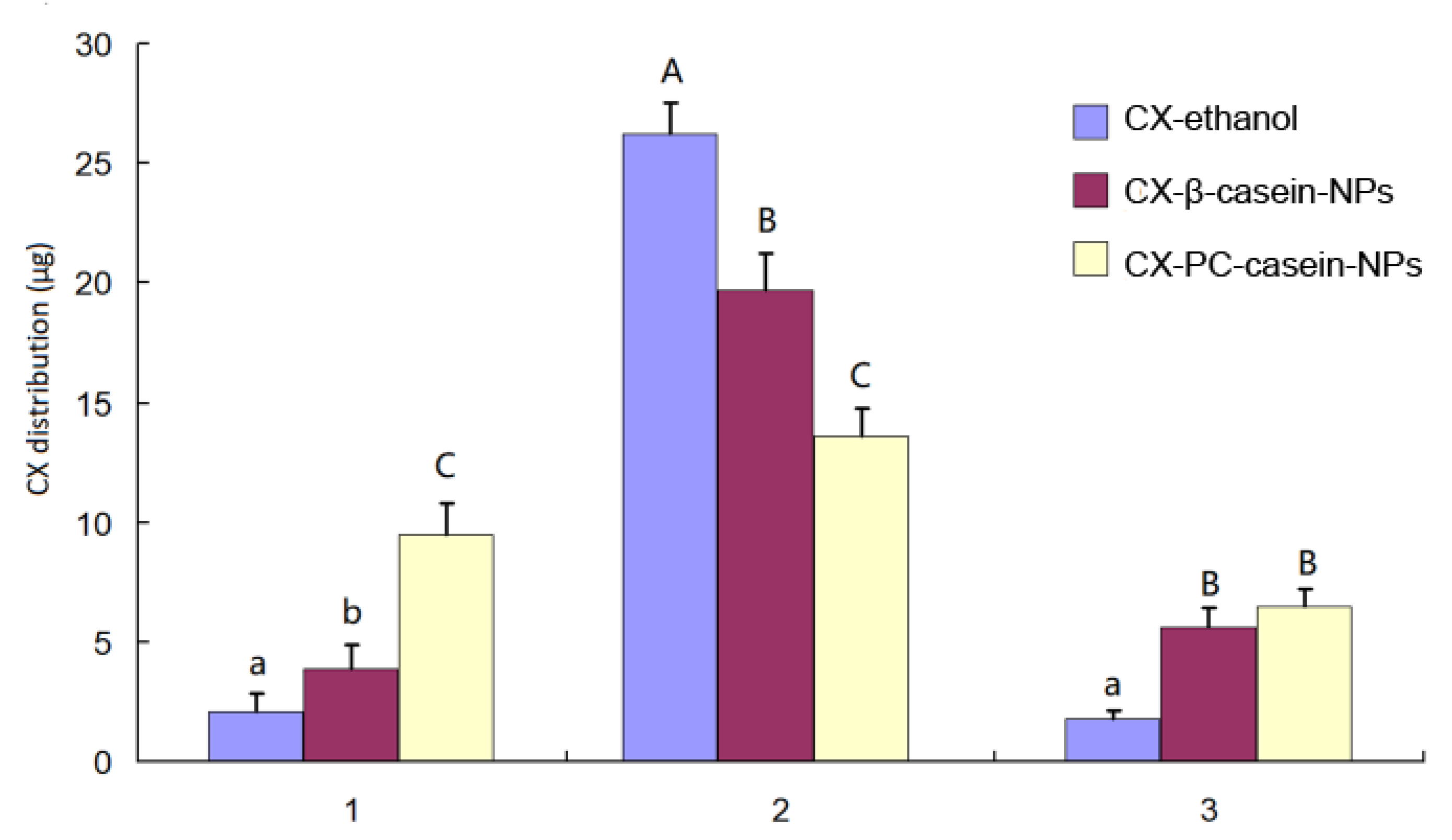
| Nanoparticles. | Mean Diameter (nm) | Polydispersity Index (PDI) | Zeta Potential (mV) | CX in Dispersion (mg/mL) |
|---|---|---|---|---|
| Fresh nanoparticle dispersion | ||||
| CX-casein nanoparticles | 254.4 ± 5.4 | 0.340 ± 0.29 | −44.57 ± 2.11 | 7.3 |
| CX-PC-casein-NPs | 192.6 ± 4.3 | 0.175 ± 0.09 | −37.21 ± 1.47 | 8.5 |
| Reconstructed nanoparticle dispersion from the corresponding lyophilized powder | ||||
| CX-casein nanoparticles | /a | / | / | / |
| CX-PC-casein-NPs | 202.4 ± 2.9 | 0.188 ± 0.18 | -36.87 ± 1.77 | 8.4 |
| Parameter | Formulation | |
|---|---|---|
| CX-β-casein-NPs | CX-PC-casein-NPs | |
| AUC(0-24) (μg/L h) | 11882.6 ± 1545.6 | 34574.3 ± 7844.9 |
| t1/2 (h) | 2.91 ± 0.40 | 4.51 ± 0.37 |
| Cmax (μg/L) | 9974.2 ± 2426.3 | 6165.8 ± 1063.5 |
| Tmax (h) | 0.5 ± 0 | 1.6 ± 0.5 |
| CL (L/h/kg) | 0.42 ± 0.06 | 0.15 ± 0.04 |
| Vd (L/kg) | 3.1 ± 1.78 | 0.97 ± 0.29 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xv, L.; Qian, X.; Wang, Y.; Yu, C.; Qin, D.; Zhang, Y.; Jin, P.; Du, Q. Structural Modification of Nanomicelles through Phosphatidylcholine: The Enhanced Drug-Loading Capacity and Anticancer Activity of Celecoxib-Casein Nanoparticles for the Intravenous Delivery of Celecoxib. Nanomaterials 2020, 10, 451. https://doi.org/10.3390/nano10030451
Xv L, Qian X, Wang Y, Yu C, Qin D, Zhang Y, Jin P, Du Q. Structural Modification of Nanomicelles through Phosphatidylcholine: The Enhanced Drug-Loading Capacity and Anticancer Activity of Celecoxib-Casein Nanoparticles for the Intravenous Delivery of Celecoxib. Nanomaterials. 2020; 10(3):451. https://doi.org/10.3390/nano10030451
Chicago/Turabian StyleXv, Liuli, Xinxin Qian, Yan Wang, Chenghuan Yu, Dingkui Qin, Yahui Zhang, Peng Jin, and Qizhen Du. 2020. "Structural Modification of Nanomicelles through Phosphatidylcholine: The Enhanced Drug-Loading Capacity and Anticancer Activity of Celecoxib-Casein Nanoparticles for the Intravenous Delivery of Celecoxib" Nanomaterials 10, no. 3: 451. https://doi.org/10.3390/nano10030451
APA StyleXv, L., Qian, X., Wang, Y., Yu, C., Qin, D., Zhang, Y., Jin, P., & Du, Q. (2020). Structural Modification of Nanomicelles through Phosphatidylcholine: The Enhanced Drug-Loading Capacity and Anticancer Activity of Celecoxib-Casein Nanoparticles for the Intravenous Delivery of Celecoxib. Nanomaterials, 10(3), 451. https://doi.org/10.3390/nano10030451






