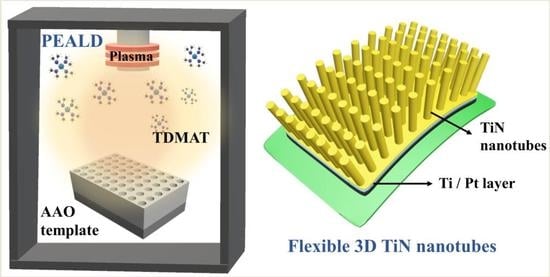
Flexible 3D Electrodes of Free-Standing TiN Nanotube Arrays Grown by Atomic Layer Deposition with a Ti Interlayer as an Adhesion Promoter
Abstract
1. Introduction
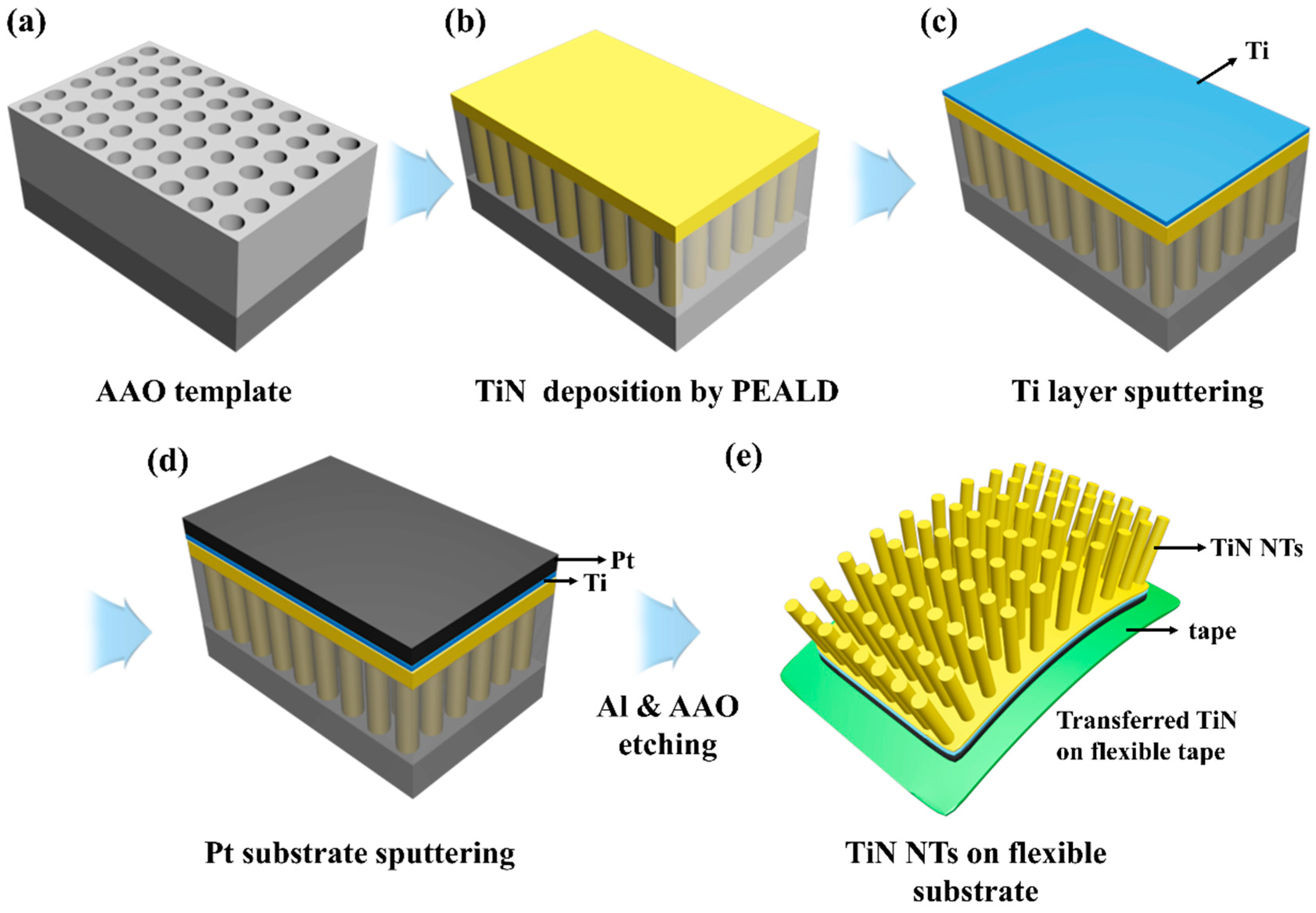
2. Materials and Methods
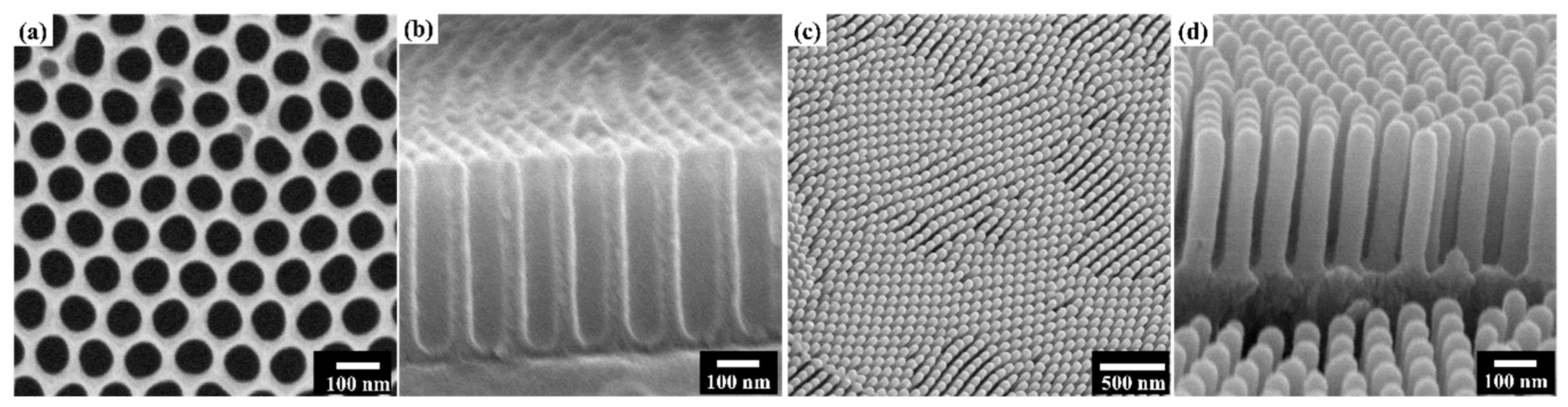
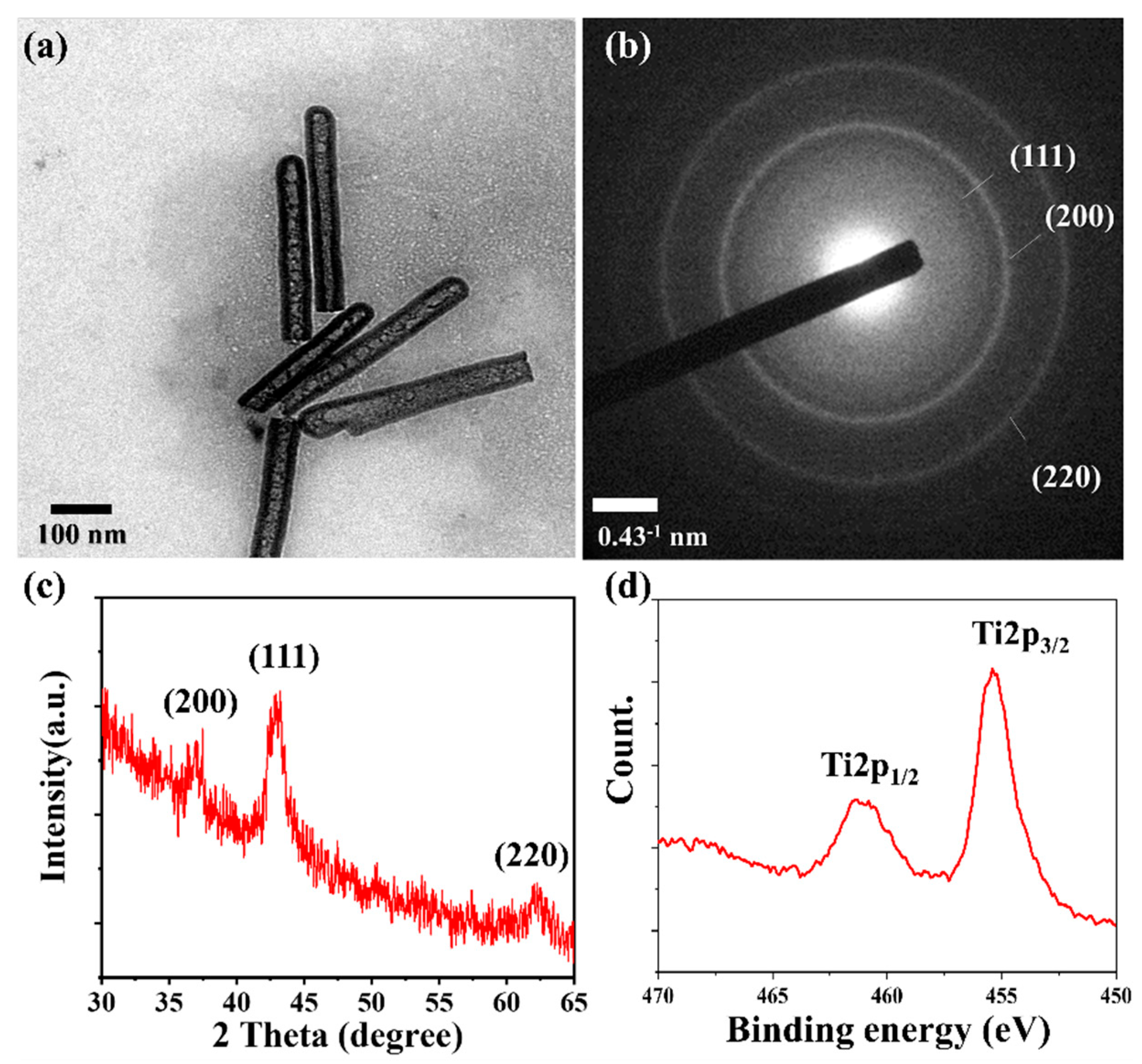
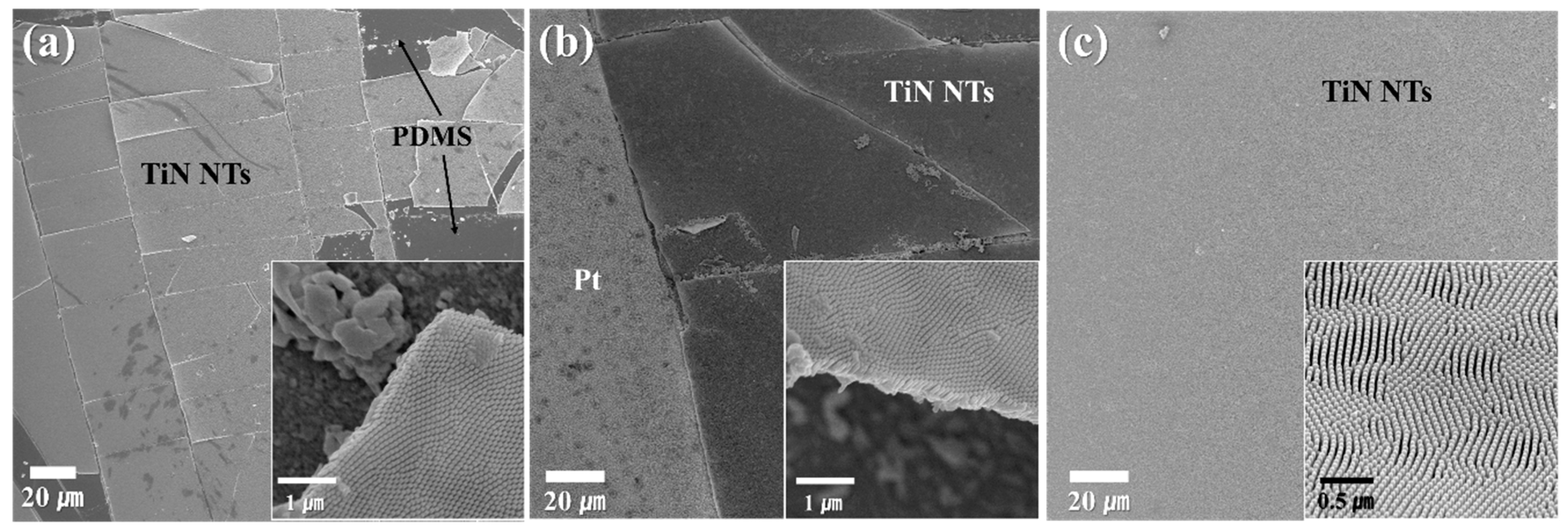
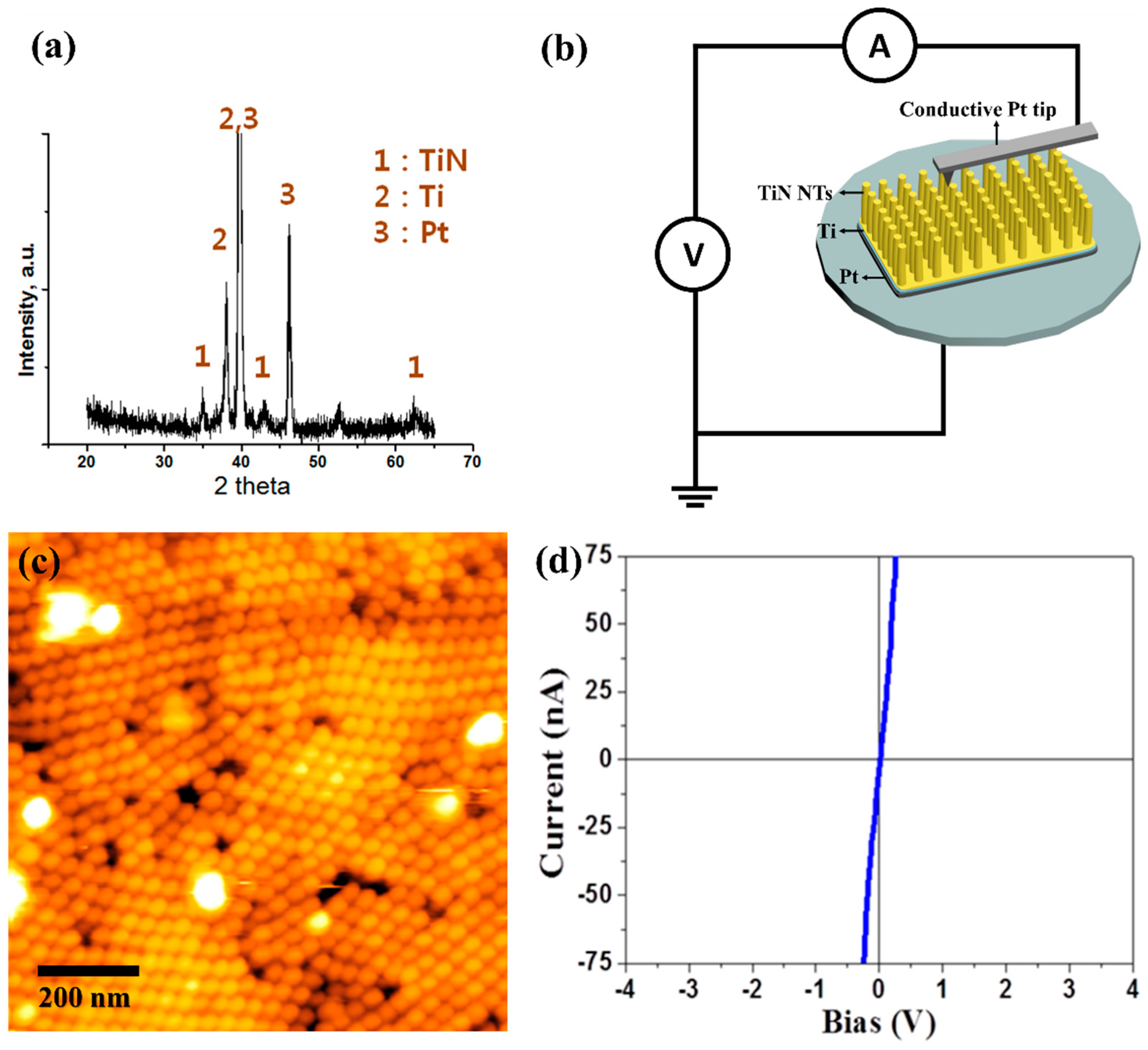
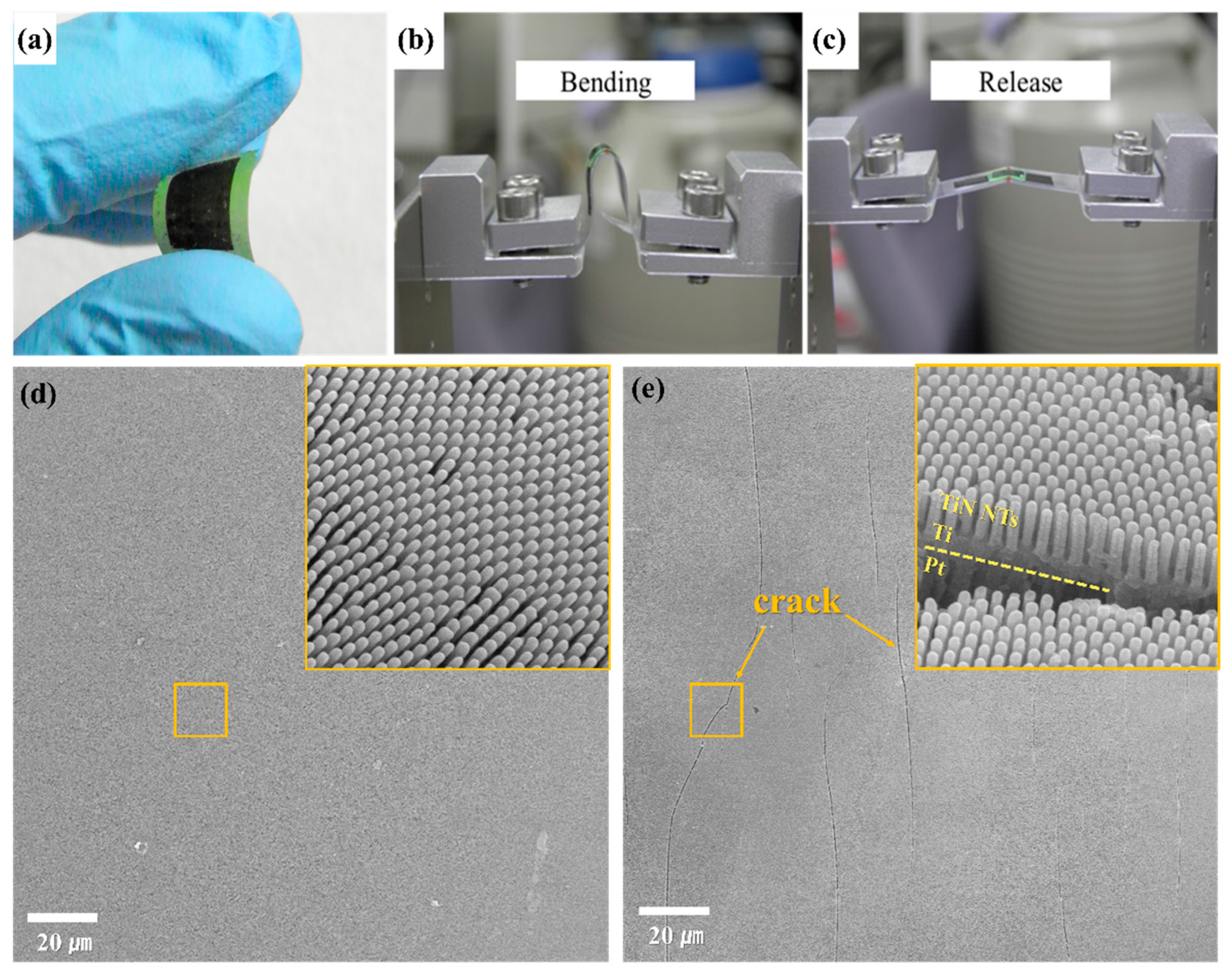
3. Results & Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shi, W.; Song, S.; Zhang, H. Hydrothermal synthetic strategies of inorganic semiconducting nanostructures. Chem. Soc. Rev. 2013, 42, 5714–5743. [Google Scholar] [CrossRef]
- Wang, Y.; Takahashi, K.; Lee, K.H.; Cao, G.Z. Nanostructured Vanadium Oxide Electrodes for Enhanced Lithium-Ion Intercalation. Adv. Funct. Mater. 2006, 16, 1133–1144. [Google Scholar] [CrossRef]
- Dong, X.C.; Xu, H.; Wang, X.W.; Huang, Y.X.; Chan-Park, M.B.; Zhang, H.; Wang, L.H.; Huang, W.; Chen, P. 3D graphene-cobalt oxide electrode for high-performance supercapacitor and enzymeless glucose detection. ACS Nano 2012, 6, 3206–3213. [Google Scholar] [CrossRef] [PubMed]
- Brezesinski, T.; Wang, J.; Tolbert, S.H.; Dunn, B. Ordered mesoporous alpha-MoO3 with iso-oriented nanocrystalline walls for thin-film pseudocapacitors. Nat. Mater. 2010, 9, 146–151. [Google Scholar] [CrossRef]
- Ling, T.; Da, P.; Zheng, X.; Ge, B.; Hu, Z.; Wu, M.; Du, X.W.; Hu, W.B.; Jaroniec, M.; Qiao, S.Z. Atomic-level structure engineering of metal oxides for high-rate oxygen intercalation pseudocapacitance. Sci. Adv. 2018, 4, eaau6261. [Google Scholar] [CrossRef]
- Liu, N.; Chortos, A.; Lei, T.; Jin, L.; Kim, T.R.; Bae, W.G.; Zhu, C.; Wang, S.; Pfattner, R.; Chen, X.; et al. Ultratransparent and stretchable graphene electrodes. Sci. Adv. 2017, 3, e1700159. [Google Scholar] [CrossRef]
- Lu, H.; Ren, X.; Ouyang, D.; Choy, W.C.H. Emerging Novel Metal Electrodes for Photovoltaic Applications. Small 2018, 14, e1703140. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Meng, G.; Zhou, F.; Song, L.; Li, X.; Hu, X.; Zhu, X.; Wu, B.; Wei, B. Dielectric capacitors with three-dimensional nanoscale interdigital electrodes for energy storage. Sci. Adv. 2015, 1, e1500605. [Google Scholar] [CrossRef]
- An, G.H.; Hong, J.; Pak, S.; Cho, Y.; Lee, S.; Hou, B.; Cha, S. 2D Metal Zn Nanostructure Electrodes for High-Performance Zn Ion Supercapacitors. Adv. Energy Mater. 2019, 10, 1902981. [Google Scholar] [CrossRef]
- Schnorr, J.M.; Swager, T.M. Emerging Applications of Carbon Nanotubes. Chem. Mater. 2011, 23, 646–657. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Kaskel, S.; Schlichte, K.; Kratzke, T. Catalytic properties of high surface area titanium nitride materials. J. Mol. Catal. A Chem. 2004, 208, 291–298. [Google Scholar] [CrossRef]
- Choi, D.; Kumta, P.N. Nanocrystalline TiN Derived by a Two-Step Halide Approach for Electrochemical Capacitors. J. Electrochem. Soc. 2006, 153. [Google Scholar] [CrossRef]
- Buhl, R.; Pulker, H.K.; Moll, E. Tin Coatings on Steel. Thin Solid Films 1981, 80, 265–270. [Google Scholar] [CrossRef]
- Andrievski, R.A. Physical-mechanical properties of nanostructured titanium nitride. Nanostruct. Mater. 1997, 9, 607–610. [Google Scholar] [CrossRef]
- Musschoot, J.; Xie, Q.; Deduytsche, D.; Van den Berghe, S.; Van Meirhaeghe, R.L.; Detavernier, C. Atomic layer deposition of titanium nitride from TDMAT precursor. Microelectron. Eng. 2009, 86, 72–77. [Google Scholar] [CrossRef]
- Kim, J.Y.; Seo, S.; Kim, D.Y.; Jeon, H.; Kim, Y. Remote plasma enhanced atomic layer deposition of TiN thin films using metalorganic precursor. J. Vac. Sci. Technol. A Vac. Surf. Films 2004, 22, 8–12. [Google Scholar] [CrossRef]
- Ma, J.; Wu, M.; Du, Y.; Chen, S.; Li, G.; Hu, J. Synthesis of nanocrystalline titanium nitride at low temperature and its thermal stability. J. Alloys Compd. 2009, 476, 603–605. [Google Scholar] [CrossRef]
- Park, M.H.; Lee, Y.H.; Kim, H.J.; Kim, Y.J.; Moon, T.; Kim, K.D.; Muller, J.; Kersch, A.; Schroeder, U.; Mikolajick, T.; et al. Ferroelectricity and antiferroelectricity of doped thin HfO2-based films. Adv. Mater. 2015, 27, 1811–1831. [Google Scholar] [CrossRef]
- Park, M.H.; Kim, H.J.; Kim, Y.J.; Lee, W.; Moon, T.; Kim, K.D.; Hwang, C.S. Study on the degradation mechanism of the ferroelectric properties of thin Hf0.5Zr0.5O2 films on TiN and Ir electrodes. Appl. Phys. Lett. 2014, 105. [Google Scholar] [CrossRef]
- Snyder, M.Q.; Trebukhova, S.A.; Ravdel, B.; Wheeler, M.C.; DiCarlo, J.; Tripp, C.P.; DeSisto, W.J. Synthesis and characterization of atomic layer deposited titanium nitride thin films on lithium titanate spinel powder as a lithium-ion battery anode. J. Power Sources 2007, 165, 379–385. [Google Scholar] [CrossRef]
- Krogstrup, P.; Jørgensen, H.I.; Heiss, M.; Demichel, O.; Holm, J.V.; Aagesen, M.; Nygard, J.; Fontcuberta i Morral, A. Single-nanowire solar cells beyond the Shockley–Queisser limit. Nat. Photonics 2013, 7, 306–310. [Google Scholar] [CrossRef]
- Doiron, B.; Li, Y.; Mihai, A.; Bower, R.; Alford, N.M.; Petrov, P.K.; Maier, S.A.; Oulton, R.F. Plasmon-Enhanced Electron Harvesting in Robust Titanium Nitride Nanostructures. J. Phys. Chem. C 2019, 123, 18521–18527. [Google Scholar] [CrossRef]
- Jiang, Q.W.; Li, G.R.; Gao, X.P. Highly ordered TiN nanotube arrays as counter electrodes for dye-sensitized solar cells. Chem. Commun. (Camb.) 2009, 6720–6722. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhang, H.; Chen, Z.; Wang, T.; Wang, L.; Zeng, W.; Zhang, G.; Duan, H. Synthesis of TiN nanostructures by Mg-assisted nitriding TiO2 in N2 for lithium ion storage. Chem. Eng. J. 2018, 336, 12–19. [Google Scholar] [CrossRef]
- George, S.M. Atomic layer deposition: An overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef]
- Gelde, L.; Cuevas, A.; Martínez de Yuso, M.; Benavente, J.; Vega, V.; González, A.; Prida, V.; Hernando, B. Influence of TiO2-Coating Layer on Nanoporous Alumina Membranes by ALD Technique. Coatings 2018, 8, 60. [Google Scholar] [CrossRef]
- Bae, C.; Kim, H.; Kim, E.; Park, H.G.; Shin, H. Atomic-Layer Deposition into 2-versus 3-Dimensionally Ordered Nanoporous Media: Pore Size or Connectivity? Chem. Mater. 2018, 30, 4748–4754. [Google Scholar] [CrossRef]
- Bae, C.; Shin, H.; Nielsch, K. Surface modification and fabrication of 3D nanostructures by atomic layer deposition. MRS Bull. 2011, 36, 887–897. [Google Scholar] [CrossRef]
- Bae, C.; Yoo, H.; Kim, S.; Lee, K.; Kim, J.; Sung, M.A.; Shin, H. Template-directed synthesis of oxide nanotubes: Fabrication, characterization, and applications. Chem. Mater. 2008, 20, 756–767. [Google Scholar] [CrossRef]
- Tan, L.K.; Liu, X.; Gao, H. Vertically standing, highly ordered, and dimension and morphology controllable TiO2 nanotube arrays via template-assisted atomic layer deposition. J. Mater. Chem. 2011, 21, 11084–11087. [Google Scholar] [CrossRef]
- Lee, J.; Hong, K.S.; Shin, K.; Jho, J.Y. Fabrication of dye-sensitized solar cells using ordered and vertically oriented TiO2 nanotube arrays with open and closed ends. J. Ind. Eng. Chem. 2012, 18, 19–23. [Google Scholar] [CrossRef]
- Furneaux, R.C.; Rigby, W.R.; Davidson, A.P. The formation of controlled-porosity membranes from anodically oxidized aluminium. Nature 1989, 337, 147–149. [Google Scholar] [CrossRef]
- Yoon, J.; Kim, S.; Kim, D.; Kim, I.D.; Hong, S.; No, K. Fabrication of Highly Ordered and Well-Aligned PbTiO3/TiN Core-Shell Nanotube Arrays. Small 2015, 11, 3750–3754. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Kim, S.; No, K. Highly ordered and well aligned TiN nanotube arrays fabricated via template-assisted atomic layer deposition. Mater. Lett. 2012, 87, 124–126. [Google Scholar] [CrossRef]
- Tisone, T.C.; Drobek, J. Diffusion in Thin Film Ti–Au, Ti–Pd, and Ti–Pt Couples. J. Vac. Sci. Technol. 1972, 9, 271–275. [Google Scholar] [CrossRef]
- Kim, Y.H.; Chaug, Y.S.; Chou, N.J.; Kim, J. Adhesion of titanium thin film to oxide substrates. J. Vac. Sci. Technol. A Vac. Surf. Films 1987, 5, 2890–2893. [Google Scholar] [CrossRef]
- Ali, R.; Sebastiani, M.; Bemporad, E. Influence of Ti–TiN multilayer PVD-coatings design on residual stresses and adhesion. Mater. Des. 2015, 75, 47–56. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. X-ray photoelectron spectroscopy: Towards reliable binding energy referencing. Prog. Mater. Sci. 2020, 107. [Google Scholar] [CrossRef]
- Kim, G.S.; Lee, S.Y.; Hahn, J.H.; Lee, B.Y.; Han, J.G.; Lee, J.H.; Lee, S.Y. Effects of the thickness of Ti buffer layer on the mechanical properties of TiN coatings. Surf. Coat. Technol. 2003, 171, 83–90. [Google Scholar] [CrossRef]
- Mardare, C.C.; Joanni, E.; Mardare, A.I.; Fernandes, J.R.A.; Sá, C.P.M.d.; Tavares, P.B. Effects of adhesion layer (Ti or Zr) and Pt deposition temperature on the properties of PZT thin films deposited by RF magnetron sputtering. Appl. Surf. Sci. 2005, 243, 113–124. [Google Scholar] [CrossRef]
- Klissurska, R.D. Microstructure of PZT sol-gel fllms on Pt substrates with different adhesion layers. Microelectron. Eng. 1995, 29, 297–300. [Google Scholar] [CrossRef]
- Pingree, L.S.C.; Reid, O.G.; Ginger, D.S. Electrical Scanning Probe Microscopy on Active Organic Electronic Devices. Adv. Mater. 2009, 21, 19–28. [Google Scholar] [CrossRef]
- Yang, C.H.; Seidel, J.; Kim, S.Y.; Rossen, P.B.; Yu, P.; Gajek, M.; Chu, Y.H.; Martin, L.W.; Holcomb, M.B.; He, Q.; et al. Electric modulation of conduction in multiferroic Ca-doped BiFeO3 films. Nat. Mater. 2009, 8, 485–493. [Google Scholar] [CrossRef]
- Guyonnet, J.; Gaponenko, I.; Gariglio, S.; Paruch, P. Conduction at domain walls in insulating Pb(Zr0.2 Ti0.8)O3 thin films. Adv. Mater. 2011, 23, 5377–5382. [Google Scholar] [CrossRef] [PubMed]
- D’Aquila, K.; Phatak, C.; Holt, M.V.; Stripe, B.D.; Tong, S.; Park, W.I.; Hong, S.; Petford-Long, A.K. Bipolar resistance switching in Pt/CuOx/Pt via local electrochemical reduction. Appl. Phys. Lett. 2014, 104, 242902. [Google Scholar] [CrossRef]
- Hong, S. Effect of metal–insulator–semiconductor structure derived space charge field on the tip vibration signal in electrostatic force microscopy. J. Vac. Sci. Technol. B 2000, 18, 2688–2691. [Google Scholar] [CrossRef]
- Choi, S.-J.; Kim, S.-J.; Kim, I.-D. Ultrafast optical reduction of graphene oxide sheets on colorless polyimide film for wearable chemical sensors. NPG Asia Mater. 2016, 8, e315. [Google Scholar] [CrossRef]
- Choi, S.J.; Kim, S.J.; Jang, J.S.; Lee, J.H.; Kim, I.D. Silver Nanowire Embedded Colorless Polyimide Heater for Wearable Chemical Sensors: Improved Reversible Reaction Kinetics of Optically Reduced Graphene Oxide. Small 2016, 12, 5826–5835. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, S.; Kim, S.-J.; Youn, J.; Kim, H.; Ryu, J.; Bae, C.; No, K.; Hong, S. Flexible 3D Electrodes of Free-Standing TiN Nanotube Arrays Grown by Atomic Layer Deposition with a Ti Interlayer as an Adhesion Promoter. Nanomaterials 2020, 10, 409. https://doi.org/10.3390/nano10030409
Yun S, Kim S-J, Youn J, Kim H, Ryu J, Bae C, No K, Hong S. Flexible 3D Electrodes of Free-Standing TiN Nanotube Arrays Grown by Atomic Layer Deposition with a Ti Interlayer as an Adhesion Promoter. Nanomaterials. 2020; 10(3):409. https://doi.org/10.3390/nano10030409
Chicago/Turabian StyleYun, Seokjung, Sang-Joon Kim, Jaesung Youn, Hoon Kim, Jeongjae Ryu, Changdeuck Bae, Kwangsoo No, and Seungbum Hong. 2020. "Flexible 3D Electrodes of Free-Standing TiN Nanotube Arrays Grown by Atomic Layer Deposition with a Ti Interlayer as an Adhesion Promoter" Nanomaterials 10, no. 3: 409. https://doi.org/10.3390/nano10030409
APA StyleYun, S., Kim, S.-J., Youn, J., Kim, H., Ryu, J., Bae, C., No, K., & Hong, S. (2020). Flexible 3D Electrodes of Free-Standing TiN Nanotube Arrays Grown by Atomic Layer Deposition with a Ti Interlayer as an Adhesion Promoter. Nanomaterials, 10(3), 409. https://doi.org/10.3390/nano10030409