Effective Gold Biosorption by Electrospun and Electrosprayed Bio-Composites with Immobilized Lysinibacillus sphaericus CBAM5
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Growth and Media
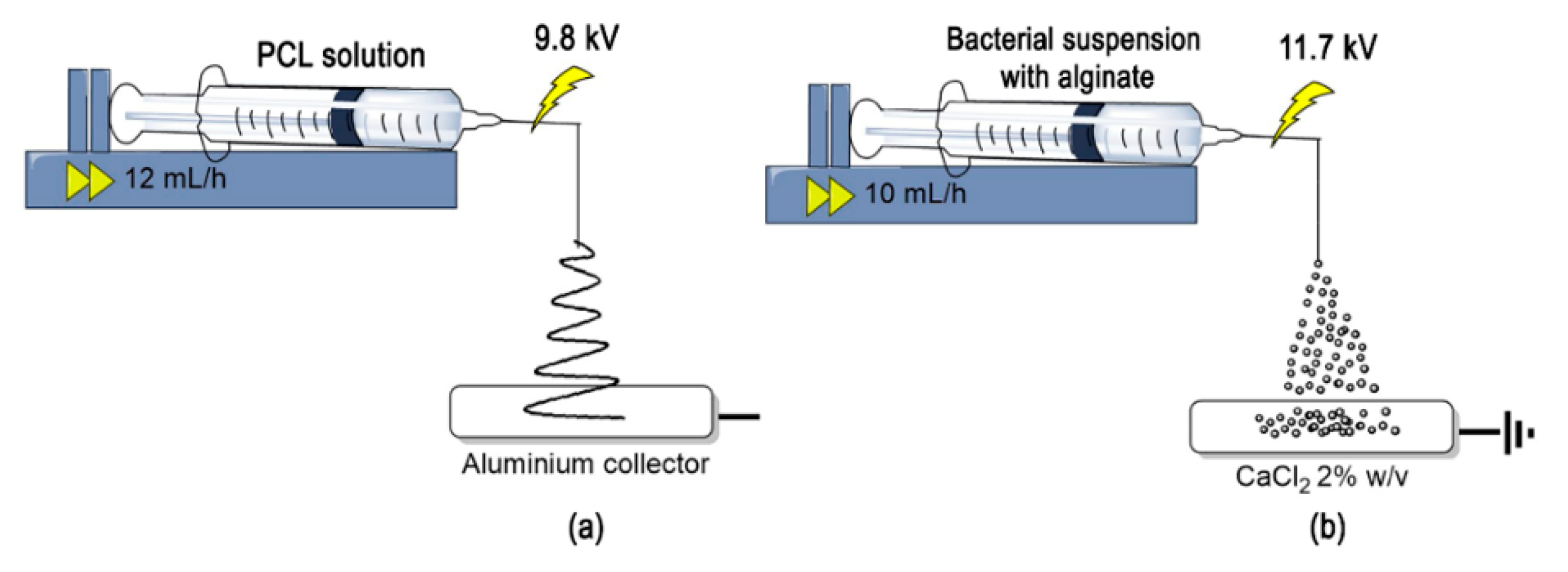
2.2. Production of Bio-Composite Microstructures
2.2.1. Electrospinning of Polycaprolactone (PCL) Micro-Fibrous Mats
2.2.2. Immobilization by Adhesion of L. sphaericus CBAM5 in PCL Micro-Fibrous Mats
2.2.3. Microencapsulation of L. sphaericus CBAM5 in An Alginate Matrix by Wet Electrospraying
2.3. Characterization of Electrospun and Electrosprayed Microstructures
2.3.1. Scanning Electron Microscopy (SEM) and Energy Dispersive X-Ray Spectroscopy (EDS) Analysis
2.3.2. Optical Microscopy
2.4. Gold Biosorption Assays with Electrospun and Electrosprayed Microstructures
2.4.1. Biosorption by Inclusion of Micro-Fibrous Mats with Immobilized L. sphaericus CBAM5 in a Synthetic Water with Gold
2.4.2. Gold Biosorption Performance of Micro-Fibrous Mats with Immobilized L. sphaericus CBAM5 in a Filtration System
2.4.3. Biosorption of Gold by Microencapsulated Cells of L. sphaericus CBAM5 in Alginate
2.5. Flame Atomic Absorption Spectrometry (FAAS) Measurements
2.6. Statistical Analysis
3. Results
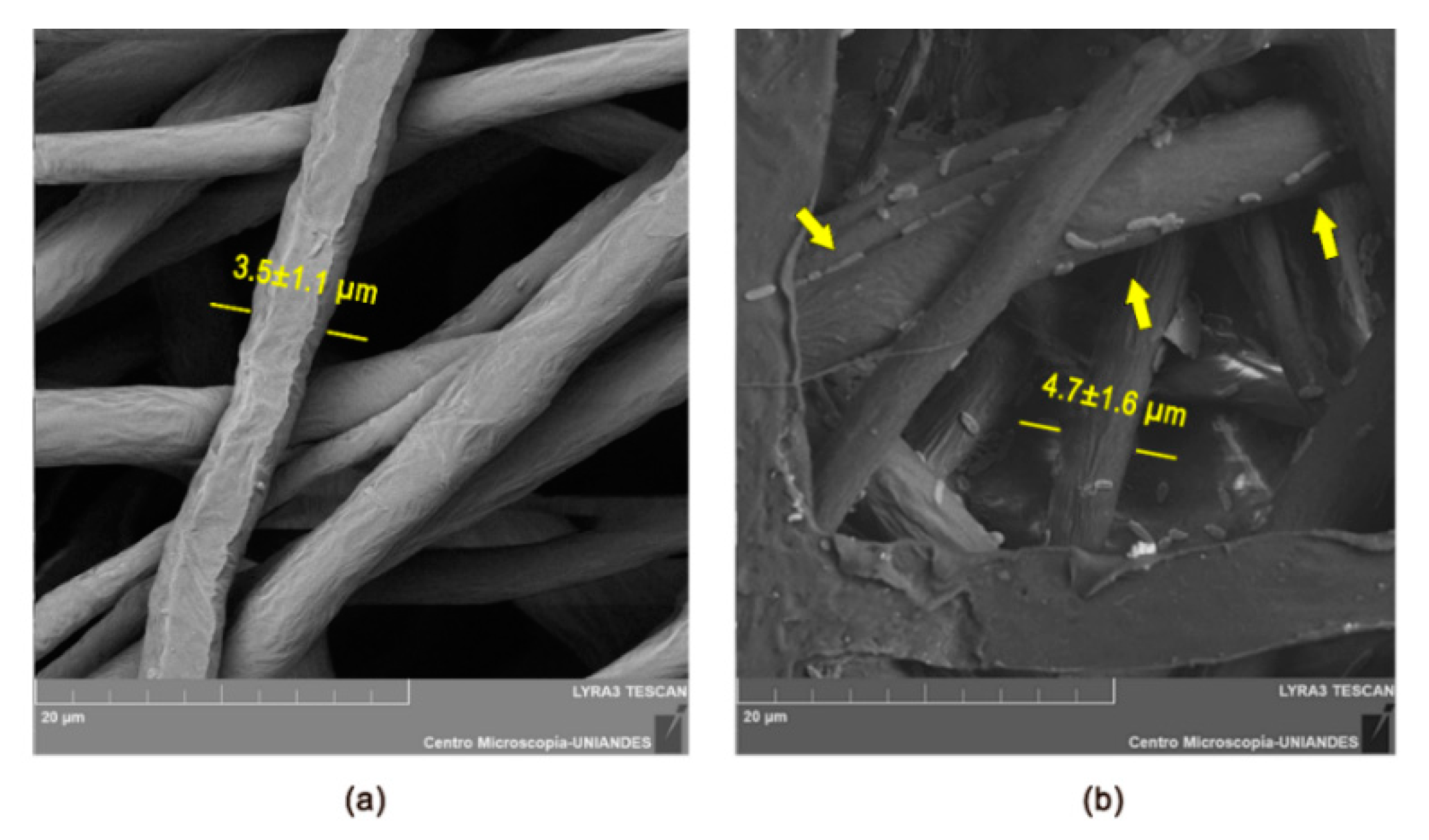
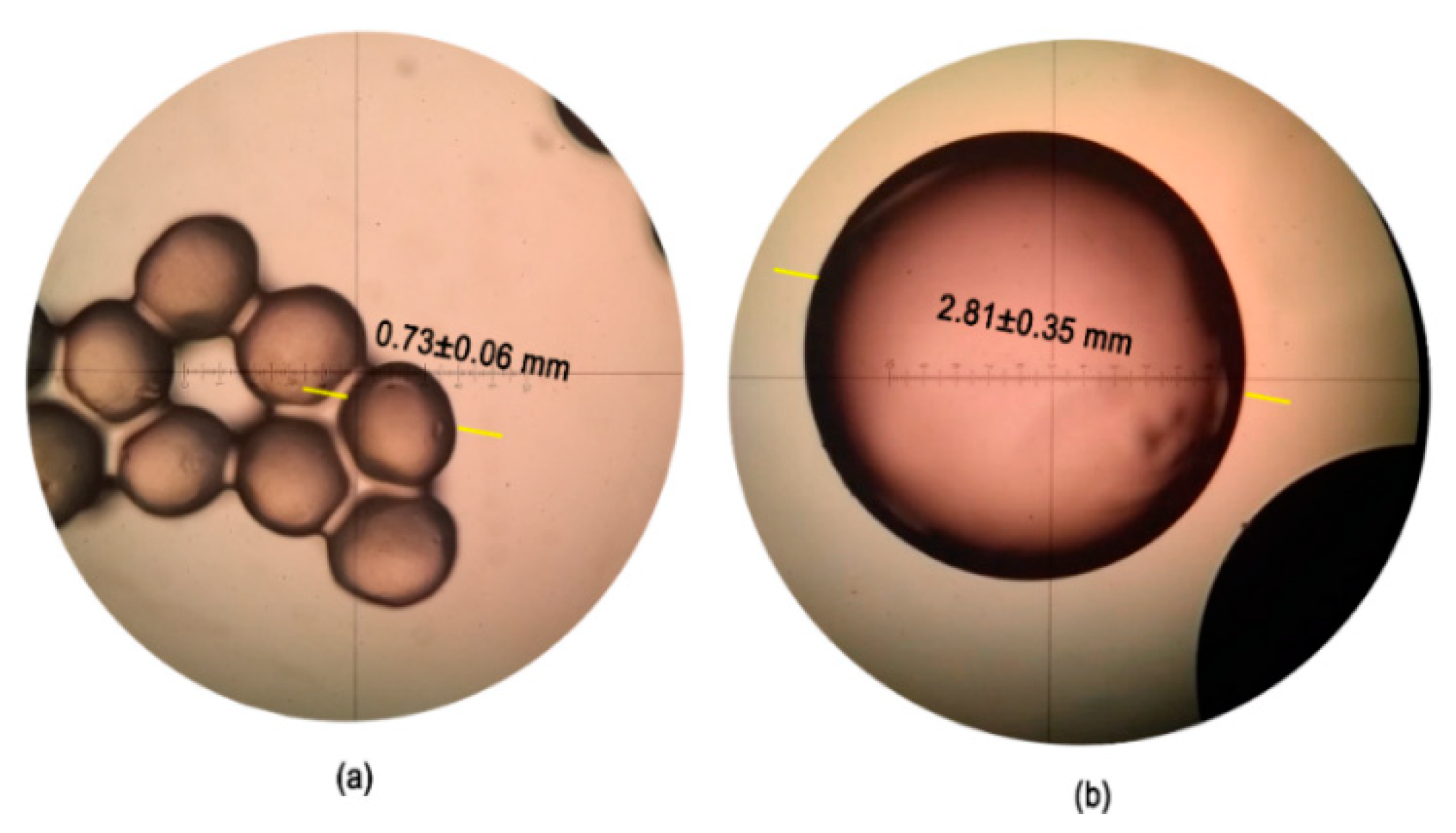
3.1. Morphological Characterization of Electrospun and Electrosprayed Microstructures
3.1.1. Characterization of Electrospun Fibers with and without Immobilized L. sphaericus CBAM5
3.1.2. Characterization of Electrosprayed Microcapsules with L. sphaericus CBAM5
3.2. Gold Biosorption Assays with Electrospun and Electrosprayed Microstructures
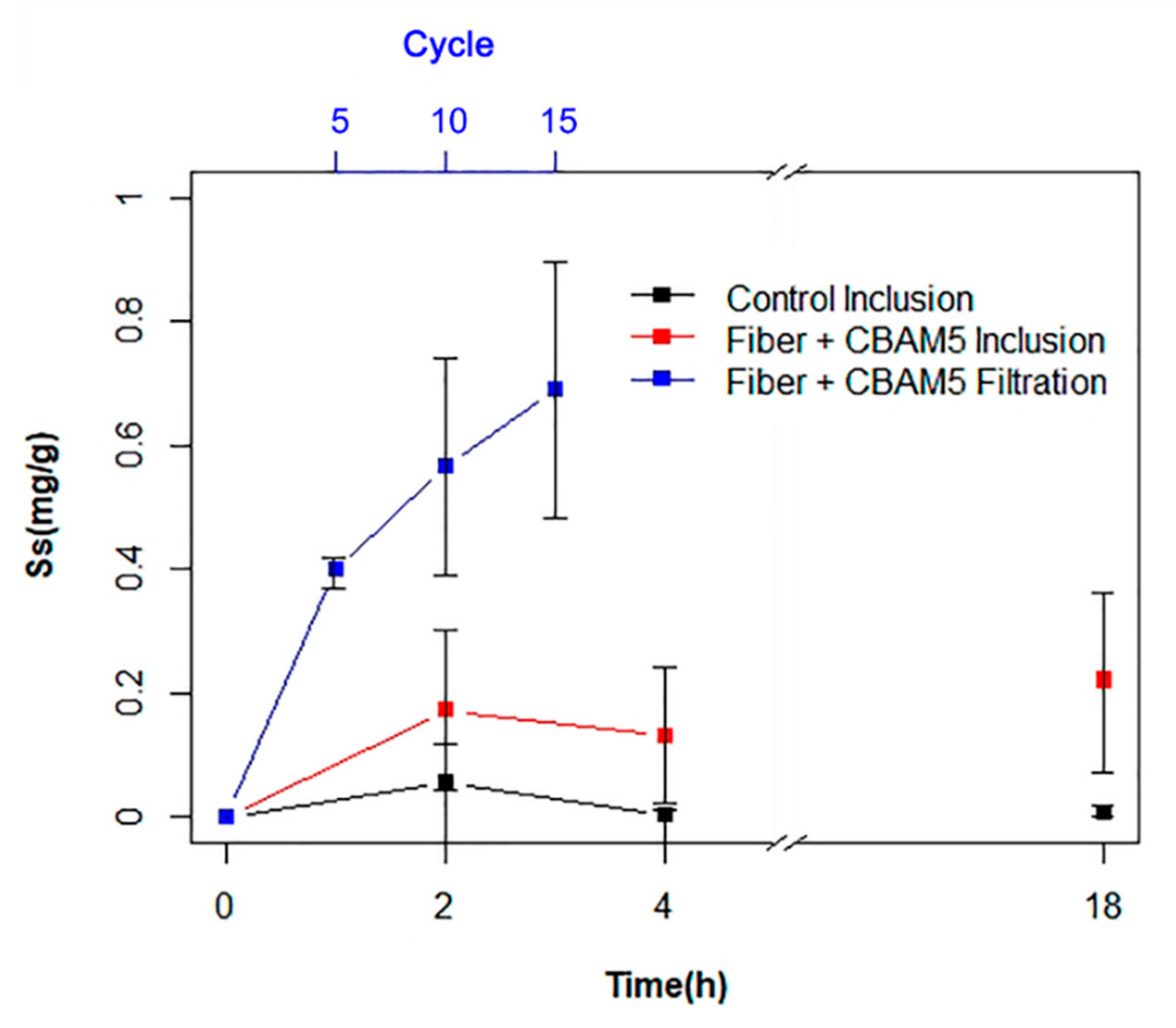
3.2.1. Biosorption of Gold by Inclusion of Micro-Fibrous Membranes with Immobilized L. sphaericus CBAM5
3.2.2. Gold Biosorption Performance of Micro-Fibrous Membranes with Immobilized L. sphaericus CBAM5 in a Filtration System
3.2.3. Biosorption of Gold by Microencapsulated Cells of L. sphaericus CBAM5 in Alginate
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khin, M.M.; Nair, A.S.; Babu, V.J.; Murugan, R.; Ramakrishna, S. A review on nanomaterials for environmental remediation. Energy Environ. Sci. 2012, 5, 8075–8109. [Google Scholar] [CrossRef]
- Wu, Y.; Pang, H.; Liu, Y.; Wang, X.; Yu, S.; Fu, D.; Chen, J.; Wang, X. Environmental remediation of heavy metal ions by novel-nanomaterials: A review. Environ. Pollut. 2019, 246, 608–620. [Google Scholar] [CrossRef]
- Yeh, Y.C.; Creran, B.; Rotello, V.M. Gold nanoparticles: Preparation, properties, and applications in bionanotechnology. Nanoscale 2012, 4, 1871–1880. [Google Scholar] [CrossRef]
- Echegoyen, Y.; Fabra, M.J.; Castro-Mayorga, J.L.; Cherpinski, A.; Lagaron, J.M. High throughput electro-hydrodynamic processing in food encapsulation and food packaging applications: Viewpoint. Trends Food Sci. Technol. 2017, 60, 71–79. [Google Scholar] [CrossRef]
- Peng, S.; Jin, G.; Li, L.; Li, K.; Srinivasan, M.; Ramakrishna, S.; Chen, J. Multi-functional electrospun nanofibres for advances in tissue regeneration, energy conversion & storage, and water treatment. Chem. Soc. Rev. 2016, 45, 1225–1241. [Google Scholar]
- Santhosh, C.; Velmurugan, V.; Jacob, G.; Jeong, S.K.; Grace, A.N.; Bhatnagar, A. Role of nanomaterials in water treatment applications: A review. Chem. Eng. J. 2016, 306, 1116–1137. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Bashan, Y.; Hernandez, J.P.; Leyva, L.A.; Bacilio, M. Alginate microbeads as inoculant carriers for plant growth-promoting bacteria. Biol. Fertil. Soils 2002, 35, 359–368. [Google Scholar] [CrossRef]
- Sarioglu, O.F.; San Keskin, N.O.; Celebioglu, A.; Tekinay, T.; Uyar, T. Bacteria immobilized electrospun polycaprolactone and polylactic acid fibrous webs for remediation of textile dyes in water. Chemosphere 2017, 184, 393–399. [Google Scholar] [CrossRef]
- Oya, N.; Keskin, S.; Celebioglu, A.; Faruk, O.; Uyar, T. Encapsulation of living bacteria in electrospun cyclodextrin ultrathin fibers for bioremediation of heavy metals and reactive dye from wastewater. Colloids Surfaces B Biointerfaces 2018, 161, 169–176. [Google Scholar]
- Sarioglu, O.F.; Celebioglu, A.; Tekinay, T.; Uyar, T. Evaluation of contact time and fiber morphology on bacterial immobilization for development of novel surfactant degrading nanofibrous webs. RSC Adv. 2015, 5, 102750–102758. [Google Scholar] [CrossRef]
- Tsuruta, T. Biosorption and recycling of gold using various microorganisms. J. Gen. Appl. Microbiol. 2004, 50, 221–228. [Google Scholar] [CrossRef]
- Lozano, L.C.; Dussán, J. Metal tolerance and larvicidal activity of Lysinibacillus sphaericus. World J. Microbiol. Biotechnol. 2013, 29, 1383–1389. [Google Scholar] [CrossRef]
- Velásquez, L.; Dussan, J. Biosorption and bioaccumulation of heavy metals on dead and living biomass of Bacillus sphaericus. J. Hazard. Mater. 2009, 167, 713–716. [Google Scholar] [CrossRef]
- Vega-Páez, J.D.; Rivas, R.E.; Dussán-Garzón, J. High efficiency mercury sorption by dead biomass of Lysinibacillus sphaericus-new insights into the treatment of contaminated water. Materials 2019, 12, 1296. [Google Scholar] [CrossRef]
- Bustos, M.C.; Ibarra, H.; Dussán, J. The golden activity of Lysinibacillus sphaericus: New insights on gold accumulation and possible nanoparticles biosynthesis. Materials 2018, 11, 1587. [Google Scholar] [CrossRef]
- Páez-Vélez, C.; Rivas, R.E.; Dussán, J. Enhanced Gold Biosorption of Lysinibacillus sphaericus CBAM5 by Encapsulation of Bacteria in an Alginate Matrix. Metals 2019, 9, 818. [Google Scholar] [CrossRef]
- Peña-Montenegro, T.; Lozano, L.; Dussán, J. Genome sequence and description of the mosquitocidal and heavy metal tolerant strain Lysinibacillus sphaericus CBAM5. Stand. Genom. Sci. 2015, 10, 2–10. [Google Scholar] [CrossRef]
- Naghili, H.; Tajik, H.; Mardani, K.; Razavi Rouhani, S.M.; Ehsani, A.; Zare, P. Validation of drop plate technique for bacterial enumeration by parametric and nonparametric tests. Vet. Res. Forum Int. Q. J. 2013, 4, 179–183. [Google Scholar]
- Devi, S.A.; Harshiny, M. Role of Nanofibers in Bioremediation. In Bioremediation: Applications for Environmental Protection and Management; Springer: Singapore, 2018; pp. 99–114. ISBN 9789811074851. [Google Scholar]
- Huang, L.; Arena, J.T.; Manickam, S.S.; Jiang, X.; Willis, B.G.; McCutcheon, J.R. Improved mechanical properties and hydrophilicity of electrospun nanofiber membranes for filtration applications by dopamine modification. J. Membr. Sci. 2014, 460, 241–249. [Google Scholar] [CrossRef]
- Li, X.; Zhang, C.; Zhao, R.; Lu, X.; Xu, X.; Jia, X.; Wang, C.; Li, L. Efficient adsorption of gold ions from aqueous systems with thioamide-group chelating nanofiber membranes. Chem. Eng. J. 2013, 229, 420–428. [Google Scholar] [CrossRef]
- Gómez-Garzón, C.; Hernández-Santana, A.; Dussan, J. A genome-scale metabolic reconstruction of Lysinibacillus sphaericus unveils unexploited biotechnological potentials. PLoS ONE 2017, 12, e0179666. [Google Scholar] [CrossRef] [PubMed]
- García-Moreno, P.J.; Mendes, A.C.; Jacobsen, C.; Chronakis, I.S. Biopolymers for the Nano-microencapsulation of Bioactive Ingredients by Electrohydrodynamic Processing. In Polymers for Food Applications; Springer International Publishing AG: Berlin/Heidelberg, Germany, 2018; pp. 447–479. ISBN 9783319946252. [Google Scholar]
- Covarrubias, S.A.; De-Bashan, L.E.; Moreno, M.; Bashan, Y. Alginate beads provide a beneficial physical barrier against native microorganisms in wastewater treated with immobilized bacteria and microalgae. Appl. Microbiol. Biotechnol. 2012, 93, 2669–2680. [Google Scholar] [CrossRef] [PubMed]
- Hui, B.; Zhang, Y.; Ye, L. Preparation of PVA hydrogel beads and adsorption mechanism for advanced phosphate removal. Chem. Eng. J. 2014, 235, 207–214. [Google Scholar] [CrossRef]
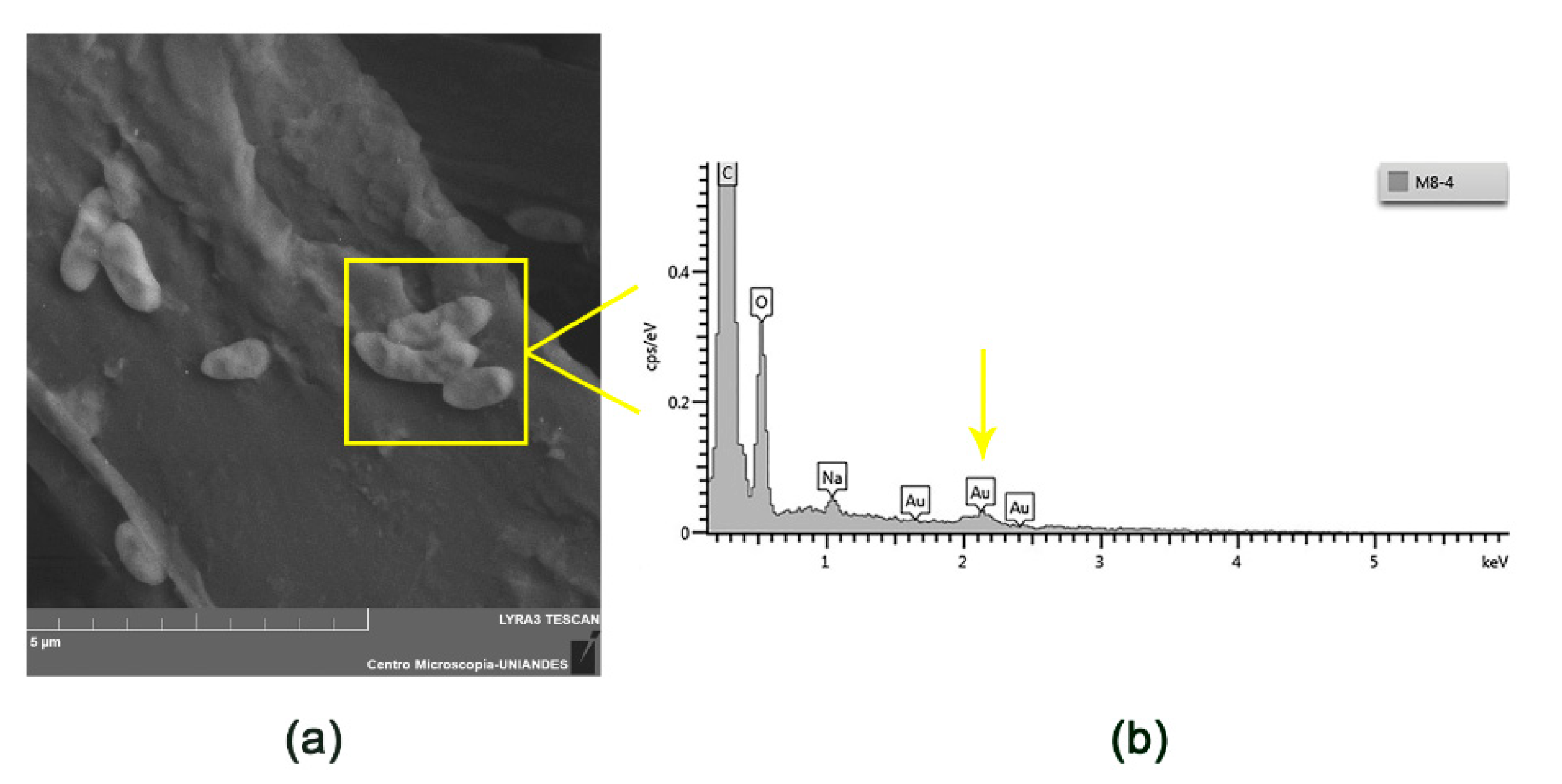
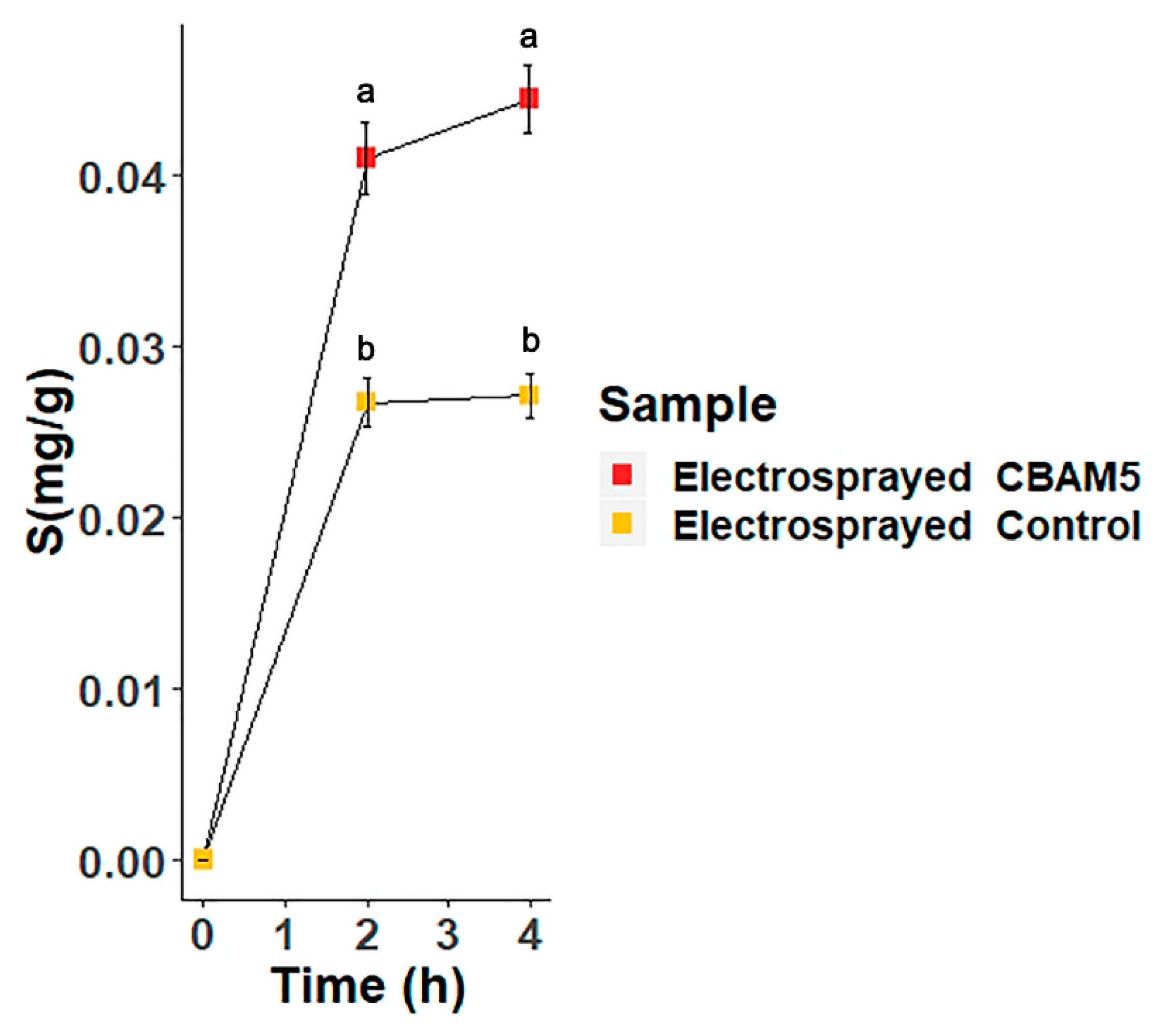
| Material | Size | Captured Au/(Weight Dry Material) |
|---|---|---|
| µm | mg/g | |
| PCL sub-micro-fibrous mat (Inclusion) | 2.91 ± 0.36 | 0.17 ± 0.13 |
| PCL sub-micro-fibrous mat (Filtration) | 2.91 ± 0.36 | 0.56 ± 0.17 |
| Electrosprayed microcapsules | 730 ± 60 | 0.26 ± 0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Páez-Vélez, C.; Castro-Mayorga, J.L.; Dussán, J. Effective Gold Biosorption by Electrospun and Electrosprayed Bio-Composites with Immobilized Lysinibacillus sphaericus CBAM5. Nanomaterials 2020, 10, 408. https://doi.org/10.3390/nano10030408
Páez-Vélez C, Castro-Mayorga JL, Dussán J. Effective Gold Biosorption by Electrospun and Electrosprayed Bio-Composites with Immobilized Lysinibacillus sphaericus CBAM5. Nanomaterials. 2020; 10(3):408. https://doi.org/10.3390/nano10030408
Chicago/Turabian StylePáez-Vélez, Carolina, J. L. Castro-Mayorga, and Jenny Dussán. 2020. "Effective Gold Biosorption by Electrospun and Electrosprayed Bio-Composites with Immobilized Lysinibacillus sphaericus CBAM5" Nanomaterials 10, no. 3: 408. https://doi.org/10.3390/nano10030408
APA StylePáez-Vélez, C., Castro-Mayorga, J. L., & Dussán, J. (2020). Effective Gold Biosorption by Electrospun and Electrosprayed Bio-Composites with Immobilized Lysinibacillus sphaericus CBAM5. Nanomaterials, 10(3), 408. https://doi.org/10.3390/nano10030408





