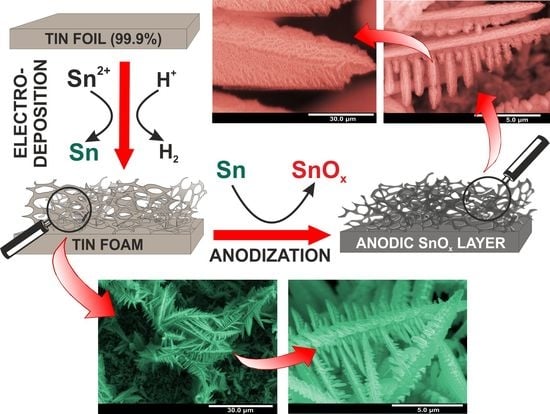
Hierarchical Nanoporous Sn/SnOx Systems Obtained by Anodic Oxidation of Electrochemically Deposited Sn Nanofoams
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrate Preparation
2.2. Fabrication of Sn Foams
2.3. Synthesis of SnOx Layers on Sn Foams
2.4. Materials Characterization
3. Results
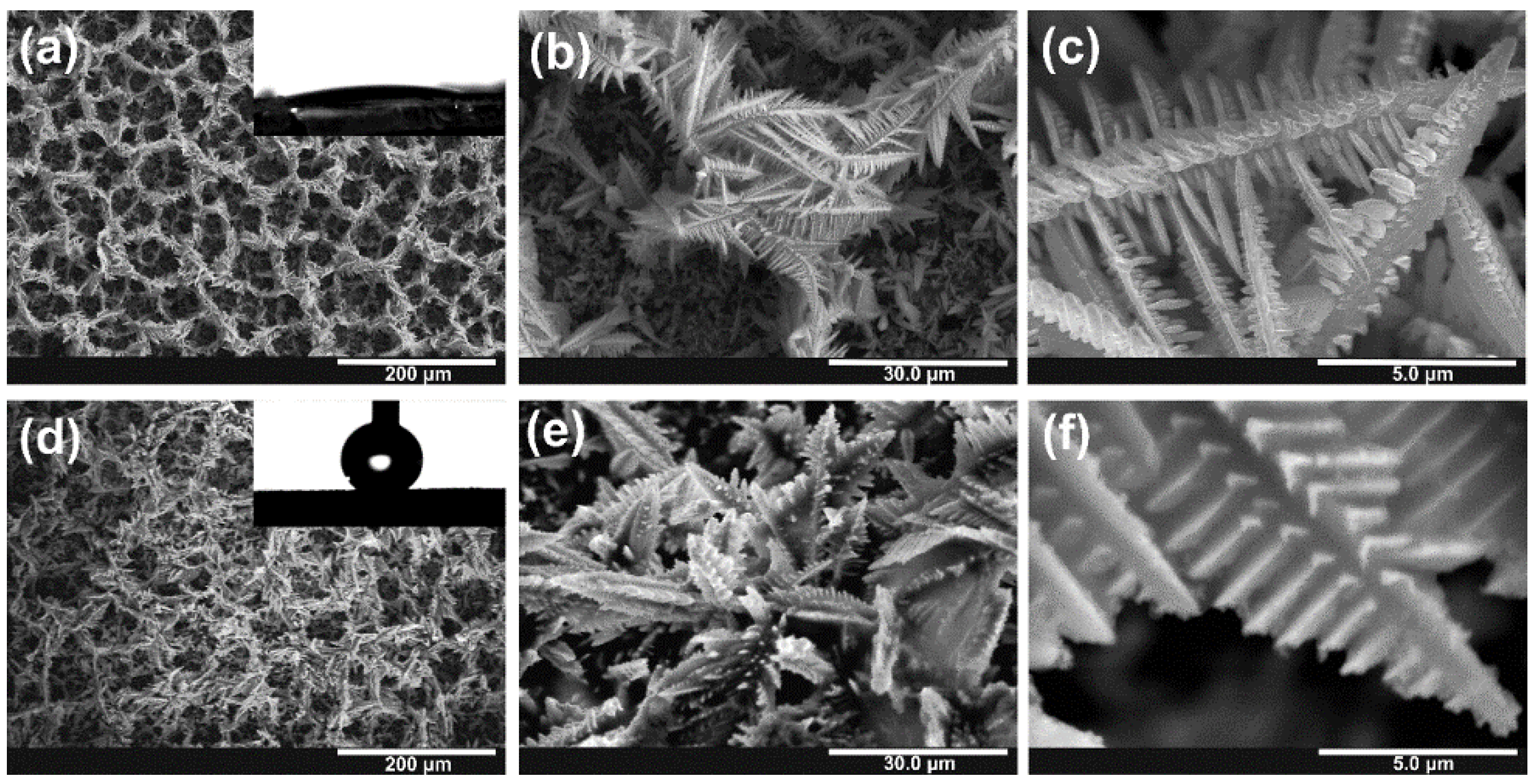
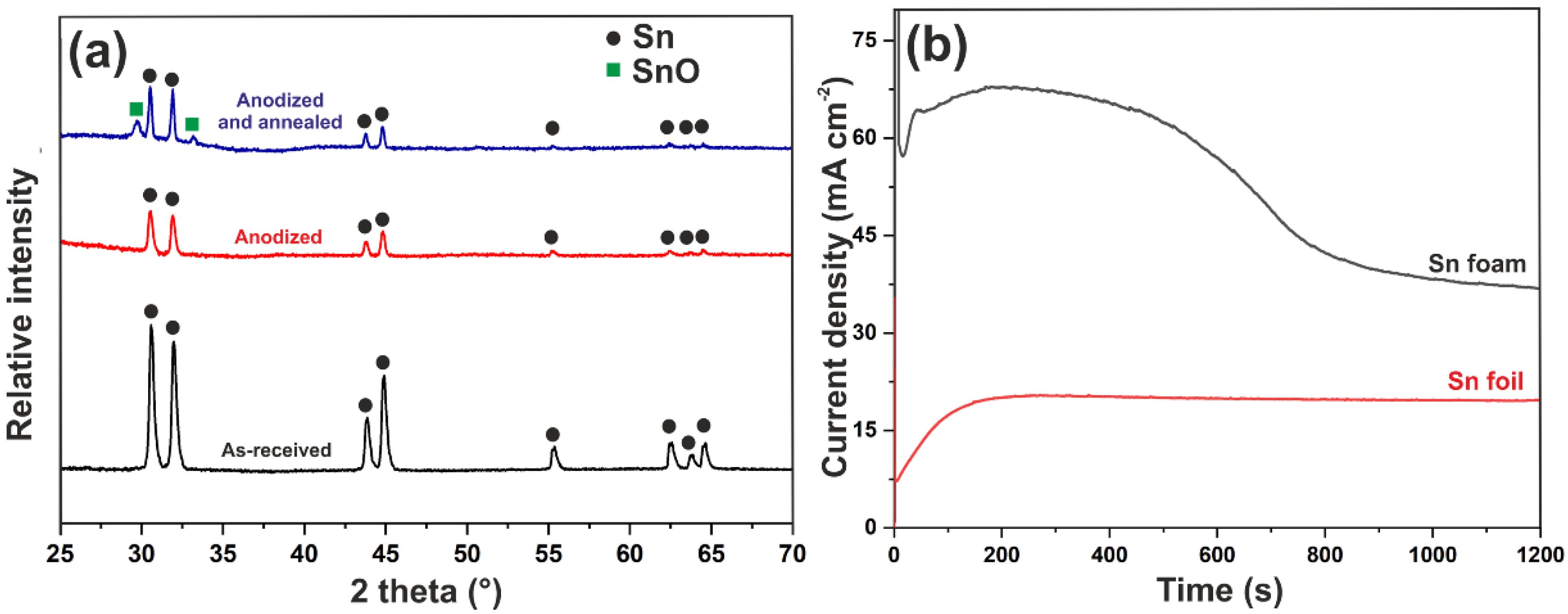
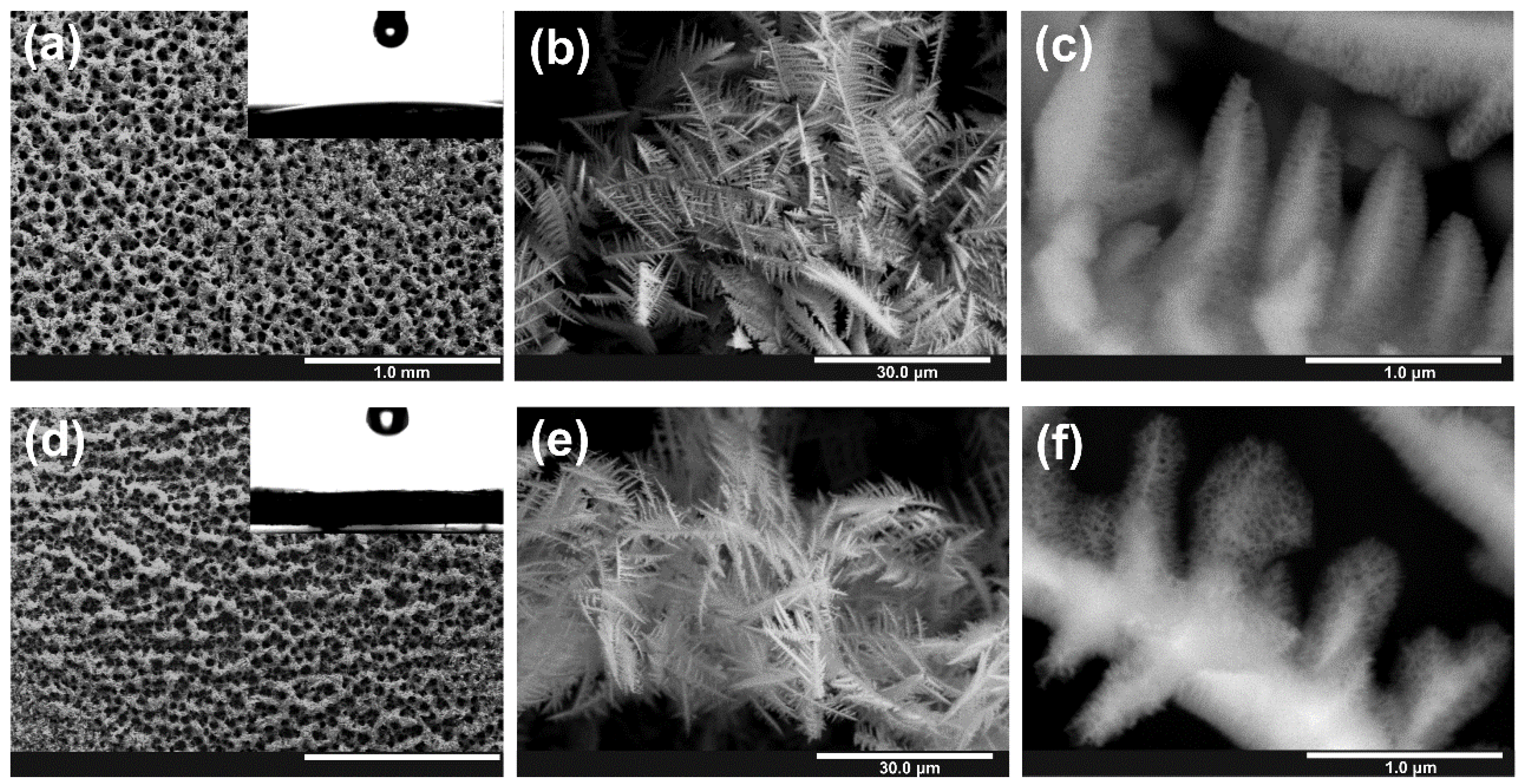
3.1. Electrodeposition of Sn Foams
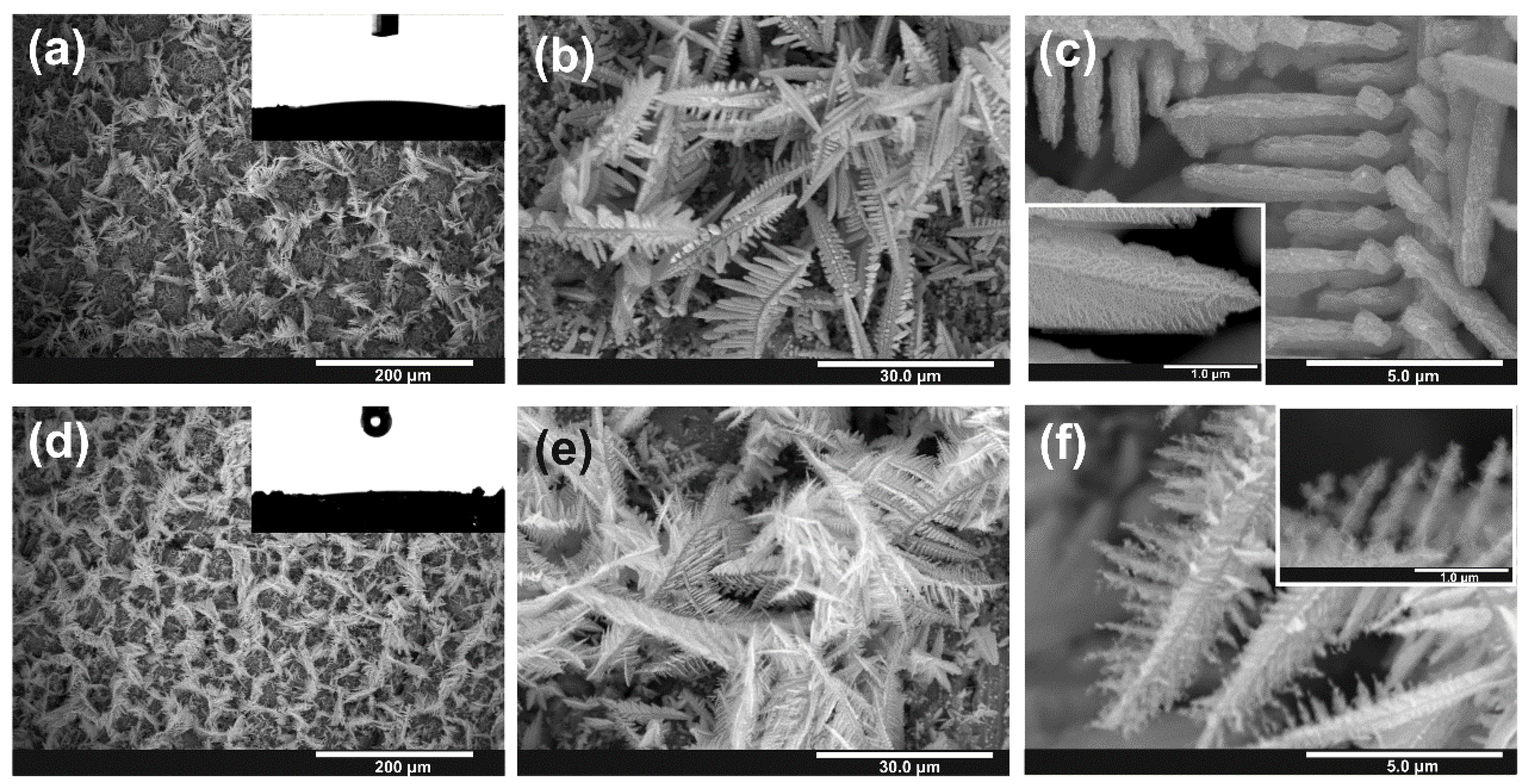
3.2. Anodic Oxidation of Sn Foams
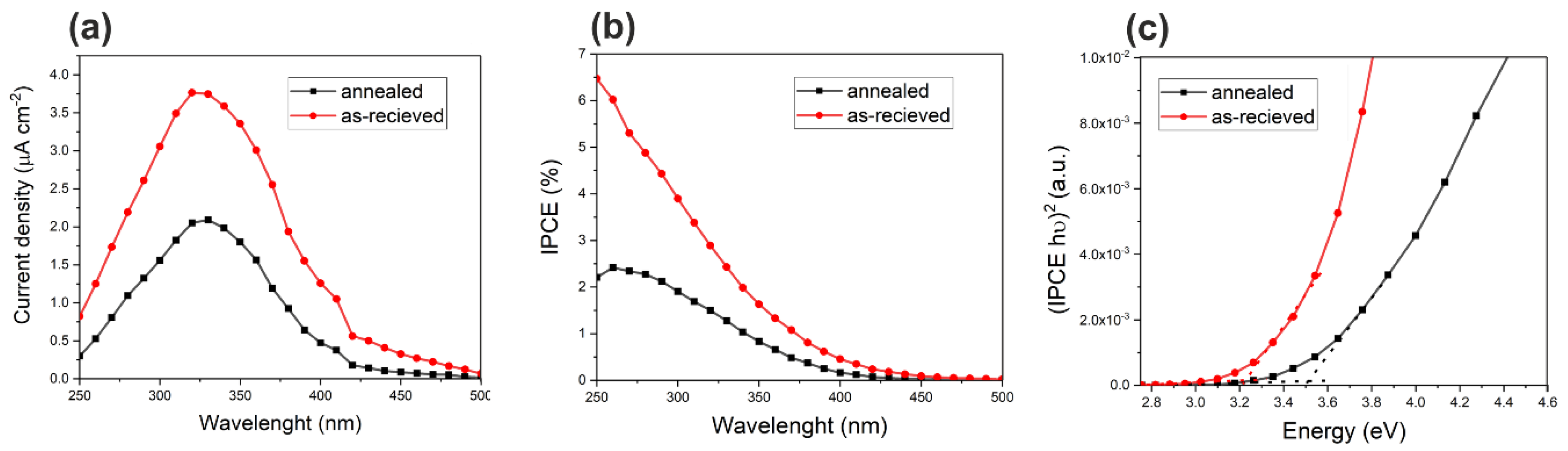
3.3. Photoelectrochemical Tests of Sn/SnOx Foams
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tappan, B.C.; Steiner, S.A., III; Luther, E.P. Nanoporous metal foams. Angew. Chem. Int. Ed. 2010, 49, 4544–4565. [Google Scholar] [CrossRef] [PubMed]
- Luc, W.; Jiao, F. Nanoporous metals as electrocatalysts: State-of-the-art, opportunities, and challenges. ACS Catal. 2017, 7, 5856–5861. [Google Scholar] [CrossRef]
- Jiang, B.; He, C.; Zhao, N.; Nash, P.; Shi, C.; Wang, Z. Ultralight metal foams. Sci. Rep. 2015, 5, 13825. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Ali, R.N.; Yang, Y.; Zheng, Z.; Xiang, B.; Cui, X. Fast synthesis of low density monolithic porous microstructure tin foam. J. Porous Mater. 2019, 26, 1363–1368. [Google Scholar] [CrossRef]
- Amani, Y.; Takahashi, A.; Chantrenne, P.; Maruyama, S.; Dancettem, S.; Maire, E. Thermal conductivity of highly porous metal foams: Experimental and image based finite element analysis. Int. J. Heat Mass Tran. 2018, 12, 1–10. [Google Scholar] [CrossRef]
- Zhuo, K.; Jeong, M.-G.; Shin, M.S.; Chun, W.W.; Bae, J.W.; Yoo, P.J.; Chung, C.-H. Morphological variation of highly porous Ni-Sn foams fabricated by electro-deposition in hydrogen bubble templates and their performance as pseudo-capacitors. Appl. Surf. Sci. 2014, 322, 15–20. [Google Scholar] [CrossRef]
- Jeun, J.-H.; Kim, W.-S.; Hong, S.-H. Electrochemical deposition of nanodendritic Sn/Cu6Sn5 foam. Mater. Lett. 2015, 138, 33–36. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Bond, A.M.; Zhang, J. Identification of a new substrate effect that enhances the electrocatalytic activity of dendritic tin in CO2 reduction. Phys. Chem. Chem. Phys. 2018, 20, 5936. [Google Scholar] [CrossRef]
- Shin, H.-C.; Dong, J.; Liu, M. Nanoporous structures prepared by an electrochemical deposition process. Adv. Mater. 2003, 15, 1610–1614. [Google Scholar] [CrossRef]
- Park, H.; Ahn, C.; Jo, H.; Choi, M.; Kim, D.S.; Kim, D.K.; Jeon, S.; Choe, H. Large-area metal foams with highly ordered sub-micrometer-scale pores for potential applications in energy areas. Mater. Lett. 2014, 129, 174–177. [Google Scholar] [CrossRef]
- Mishura, A.M.; Lytvynenko, A.S.; Gavrilenko, K.S.; Baranchikov, A.E.; Grabovaya, N.V.; Kiskin, M.A.; Kolotilov, S.V. Formation of hierarchically-ordered nanoporous silver foam and its electrocatalytic properties in reductive dehalogenation of organic compounds. New J. Chem. 2018, 42, 17499–17512. [Google Scholar] [CrossRef]
- Sen, S.; Liu, D.; Palmore, G.T.R. Electrochemical reduction of CO2 at copper nanofoams. ACS Catal. 2014, 4, 3091–3095. [Google Scholar] [CrossRef]
- Yang, G.-M.; Chen, X.; Li, J.; Guo, Z.; Liu, J.-H.; Huang, X.-J. Bubble dynamic templated deposition of three-dimensional palladium nanostructure catalysts: Approach to oxygen reduction using macro-, micro-, and nano-architectures on electrode surfaces. Electrochim. Acta 2011, 56, 6771–6778. [Google Scholar] [CrossRef]
- Ott, A.; Jones, L.A.; Bhargava, S.K. Direct electrodeposition of porous platinum honeycomb structures. Electrochem. Commun. 2011, 13, 1248–1251. [Google Scholar] [CrossRef]
- Li, D.; Wu, J.; Liu, T.; Liu, J.; Yan, Z.; Zhen, L.; Feng, Y. Tuning the pore structure of porous tin foam electrodes for enhanced electrochemical reduction of carbon dioxide to formate. Chem. Eng. J. 2019, 375, 122024. [Google Scholar] [CrossRef]
- Han, X.; Jin, M.; Xie, S.; Kuang, Q.; Jiang, Z.; Jiang, Y.; Xie, Z.; Zheng, L. Synthesis of tin dioxide octahedral nanoparticles with exposed high-energy {221} facets and enhanced gas-sensing properties. Angew. Chem. Int. Ed. 2009, 48, 9180–9183. [Google Scholar] [CrossRef] [PubMed]
- Schleife, A.; Varley, J.B.; Fuchs, F.; Rödl, C.; Bechstedt, F.; Rinke, P.; Janotti, A.; Van de Walle, C.G. Tin dioxide from first principles: Quasiparticle electronic states and optical properties. Phys. Rev. B 2011, 83, 035116. [Google Scholar] [CrossRef]
- Akgul, F.A.; Gumus, C.; Er, A.O.; Farha, A.H.; Akgul, G.; Ufuktepe, Y.; Liu, Z. Structural and electronic properties of SnO2. J. Alloys Compd. 2013, 579, 50–56. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, C.; Wu, Z.; Han, W.; Wang, Y.; Fu, W.; Li, X.; Xie, E. Toward efficient photoelectrochemical water-splitting by using screw-like SnO2 nanostructures as photoanode after being decorated with CdS quantum dots. Nano Energy 2016, 19, 318–327. [Google Scholar] [CrossRef]
- Park, N.-G.; Kang, M.G.; Ryu, K.S.; Kim, K.M.; Chang, S.H. Photovoltaic characteristics of dye-sensitized surface-modified nanocrystalline SnO2 solar cells. J. Photochem. Photobiol. A 2004, 161, 105–110. [Google Scholar] [CrossRef]
- Faramarzi, M.S.; Abnavi, A.; Ghasemi, S.; Sanaee, Z. Nanoribbons of SnO2 as a high performance Li-ion battery anode material. Mater. Res. Express 2018, 5, 065040. [Google Scholar] [CrossRef]
- Ma, D.; Li, Y.; Zhang, P.; Lin, Z. Oxygen vacancy engineering in SnO2-based anode materials toward advanced sodium-ion batteries. ChemSusChem 2018, 11, 3693–3703. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, D.; Ahmaruzzaman, M. Tin oxide nanostructured materials: An overview of recent developments in synthesis, modifications and potential applications. RSC Adv. 2016, 6, 110996–111015. [Google Scholar] [CrossRef]
- Zaraska, L.; Gilek, D.; Gawlak, K.; Jaskuła, M.; Sulka, G.D. Formation of crack-free nanoporous tin oxide layers via simple one-step anodic oxidation in NaOH at low applied voltages. Appl. Surf. Sci. 2016, 390, 31–37. [Google Scholar] [CrossRef]
- Zaraska, L.; Czopik, N.; Bobruk, M.; Sulka, G.D.; Mech, J.; Jaskuła, M. Synthesis of nanoporous tin oxide layers by electrochemical anodization. Electrochim. Acta 2013, 104, 549–557. [Google Scholar] [CrossRef]
- Shin, H.-C.; Dong, J.; Liu, M. Porous tin oxides prepared using an anodic oxidation process. Adv. Mater. 2004, 16, 237–240. [Google Scholar] [CrossRef]
- Lee, J.-W.; Park, S.-J.; Choi, W.-S.; Shin, H.-C. Well-defined meso-to macro-porous film of tin oxides formed by an anodization process. Electrochim. Acta 2011, 56, 5919–5925. [Google Scholar] [CrossRef]
- Wang, M.; Liu, Y.; Xie, D.; Zhang, D.; Yang, H. Preparation of nanoporous tin oxide by electrochemical anodization in alkaline electrolytes. Electrochim. Acta 2011, 56, 8797–8801. [Google Scholar] [CrossRef]
- Palacios-Padros, A.; Altomare, M.; Lee, K.; Diez-Perez, I.; Sanz, F.; Schmuki, P. Controlled thermal annealing tunes the photoelectrochemical properties of nanochanneled tin-oxide structures. ChemElectroChem 2014, 1, 1133–1137. [Google Scholar] [CrossRef]
- Zaraska, L.; Bobruk, M.; Jaskuła, M.; Sulka, G.D. Growth and complex characterization of nanoporous oxide layers on metallic tin during one-step anodic oxidation in oxalic acid at room temperature. Appl. Surf. Sci. 2015, 351, 1034–1042. [Google Scholar] [CrossRef]
- Zaraska, L.; Syrek, K.; Hnida, K.E.; Bobruk, M.; Krzysik, A.; Łojewski, T.; Jaskuła, M.; Sulka, G.D. Nanoporous tin oxides synthesized via electrochemical anodization in oxalic acid and their photoelectrochemical activity. Electrochim. Acta 2016, 205, 273–280. [Google Scholar] [CrossRef]
- Zaraska, L.; Gawlak, K.; Gilek, D.; Sulka, G.D. Electrochemical growth of multisegment nanoporous tin oxide layers by applying periodically changed anodizing potential. Appl. Surf. Sci. 2018, 455, 1005–1009. [Google Scholar] [CrossRef]
- Wu, K.-H.; Lu, S.-Y. Fabrication of array of nanoporous tin oxide nanorods with electrochemical processes. Electrochem. Solid State Lett. 2005, 8, D9–D11. [Google Scholar] [CrossRef]
- Cheng, C.; Fan, H.J. Branched nanowires: Synthesis and energy applications. Nano Today 2012, 7, 327–343. [Google Scholar] [CrossRef]
- Jung, H.-R.; Kim, E.-J.; Park, Y.J.; Shin, H.-C. Nickel-foam with nanostructured walls for rechargeable lithium battery. J. Power Sources 2011, 196, 5122–5127. [Google Scholar] [CrossRef]
- Ye, B.; Kim, S. Formation of nanocrystalline surface of Cu-Sn alloy foam electrochemically produced for Li-ion battery electrode. J. Nanosci. Nanotechnol. 2015, 15, 8217–8221. [Google Scholar] [CrossRef]
- Cao, L.; Liu, J.; Xu, S.; Xia, Y.; Huang, W.; Li, Z. Inherent superhydrophobicity of Sn/SnOx films prepared by surface self-passivation of electrodeposited porous dendritic Sn. Mater. Res. Bull. 2013, 48, 4804–4810. [Google Scholar] [CrossRef]
- Yao, X.; Song, Y.; Jiang, L. Applications of bio-inspired special wettable surfaces. Adv. Mater. 2011, 23, 719–734. [Google Scholar] [CrossRef]
- Zhang, Y.-P.; Yang, J.-H.; Li, L.-L.; Cui, C.-X.; Li, Y.; Liu, S.-Q.; Zhou, X.-M.; Qu, L.-B. Facile fabrication of superhydrophobic copper-foam and electrospinning polystyrene fiber for combinational oil-water separation. Polymers 2019, 11, 97. [Google Scholar] [CrossRef]
- Zaraska, L.; Gawlak, K.; Wiercigroch, E.; Małek, K.; Kozieł, M.; Andrzejczuk, M.; Marzec, M.M.; Jarosz, M.; Brzózka, A.; Sulka, G.D. The effect of anodizing potential and annealing conditions on the morphology, composition and photoelectrochemical activity of porous anodic tin oxide films. Electrochim. Acta 2019, 319, 18–30. [Google Scholar] [CrossRef]
- Horcas, I.; Fernández, R.; Gómez-Rodríguez, J.M.; Colchero, J.; Gómez-Herrero, J.; Baro, A.M. WSxM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wang, M.; Wang, Z.; Gong, X.; Guo, Z. The structure evolution mechanism of electrodeposited porous Ni films on NH4Cl concentration. Appl. Surf. Sci. 2016, 360, 502–509. [Google Scholar] [CrossRef]
- Peraldo Bicelli, L.; Bozzini, B.; Mele, C.; D’Urzo, L. A review of nanostructural aspects of metal electrodeposition. Int. J. Electrochem. Sci. 2008, 3, 356–408. [Google Scholar]
- Zaraska, L.; Gawlak, K.; Gurgul, M.; Chlebda, D.K.; Socha, R.P.; Sulka, G.D. Controlled synthesis of nanoporous tin oxide layers with various pore diameters and their photoelectrochemical properties. Electrochim. Acta 2017, 254, 238–245. [Google Scholar] [CrossRef]
- Zaraska, L.; Gawlak, K.; Gurgul, M.; Dziurka, M.; Nowak, M.; Gilek, D.; Sulka, G.D. Influence of anodizing conditions on generation of internal cracks in anodic porous tin oxide films grown in NaOH electrolyte. Appl. Surf. Sci. 2018, 439, 672–680. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurgul, M.; Lytvynenko, A.S.; Jarosz, M.; Gawlak, K.; Sulka, G.D.; Zaraska, L. Hierarchical Nanoporous Sn/SnOx Systems Obtained by Anodic Oxidation of Electrochemically Deposited Sn Nanofoams. Nanomaterials 2020, 10, 410. https://doi.org/10.3390/nano10030410
Gurgul M, Lytvynenko AS, Jarosz M, Gawlak K, Sulka GD, Zaraska L. Hierarchical Nanoporous Sn/SnOx Systems Obtained by Anodic Oxidation of Electrochemically Deposited Sn Nanofoams. Nanomaterials. 2020; 10(3):410. https://doi.org/10.3390/nano10030410
Chicago/Turabian StyleGurgul, Magdalena, Anton S. Lytvynenko, Magdalena Jarosz, Karolina Gawlak, Grzegorz D. Sulka, and Leszek Zaraska. 2020. "Hierarchical Nanoporous Sn/SnOx Systems Obtained by Anodic Oxidation of Electrochemically Deposited Sn Nanofoams" Nanomaterials 10, no. 3: 410. https://doi.org/10.3390/nano10030410
APA StyleGurgul, M., Lytvynenko, A. S., Jarosz, M., Gawlak, K., Sulka, G. D., & Zaraska, L. (2020). Hierarchical Nanoporous Sn/SnOx Systems Obtained by Anodic Oxidation of Electrochemically Deposited Sn Nanofoams. Nanomaterials, 10(3), 410. https://doi.org/10.3390/nano10030410