Synergistic Antibacterial Activity of Silver-Loaded Graphene Oxide towards Staphylococcus Aureus and Escherichia Coli
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bacterial Strains
2.3. GO Synthesis
2.4. Ag NPs Synthesis
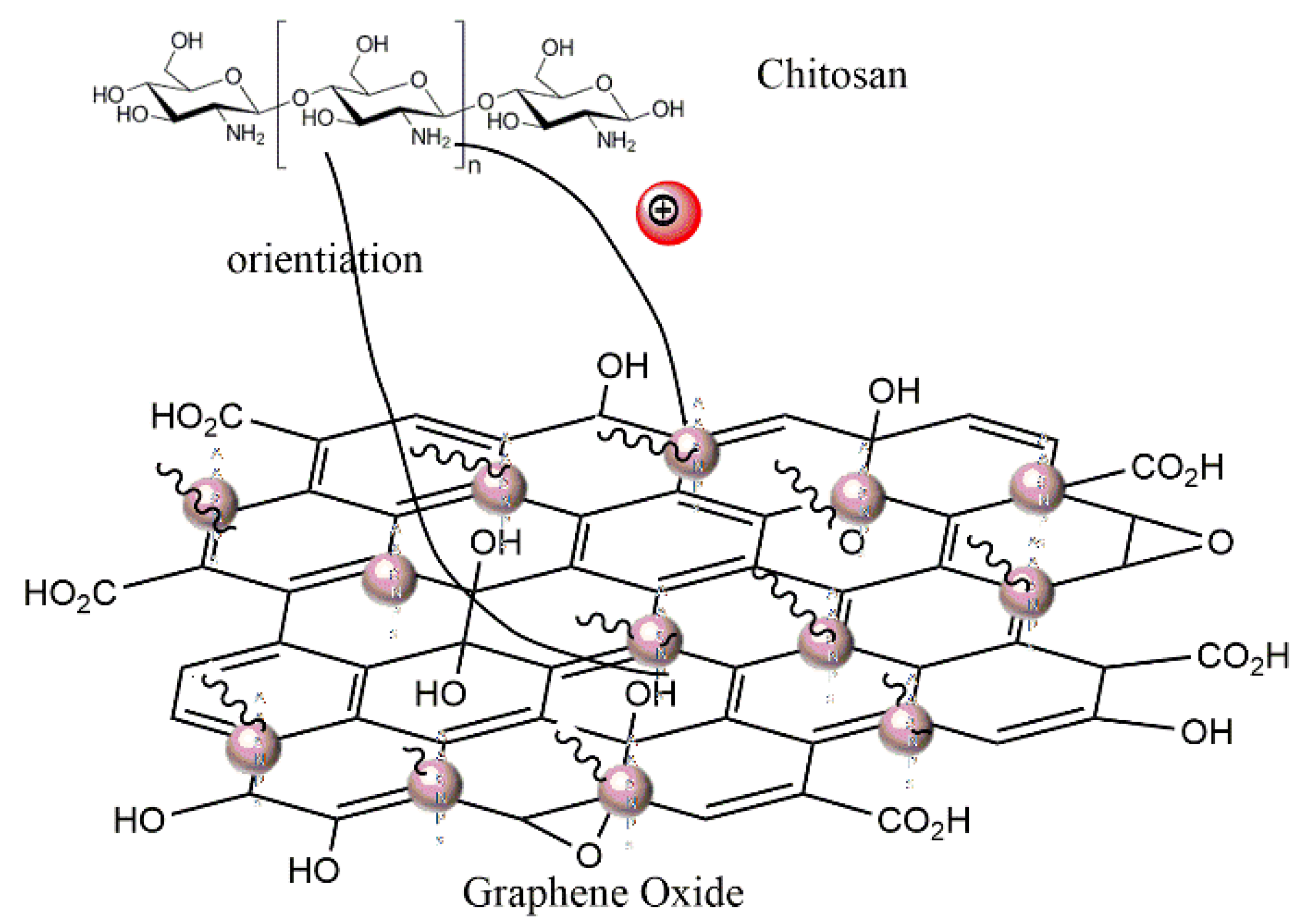
2.5. GO–Ag NP Synthesis
2.6. Characterizations
2.7. Cyclic Voltammetry Measurement
2.8. Antibacterial Test
2.8.1. Bacterial Growth Curve Assay
2.8.2. Disk Diffusion Assay
2.8.3. Change in Bacterial Morphology after Sample Exposure
2.8.4. Live/Dead Cell Staining Using Confocal Laser Scanning Microscopy
2.8.5. Reactive Oxygen Species Mechanism Applied to Bacteria Treatment
3. Results
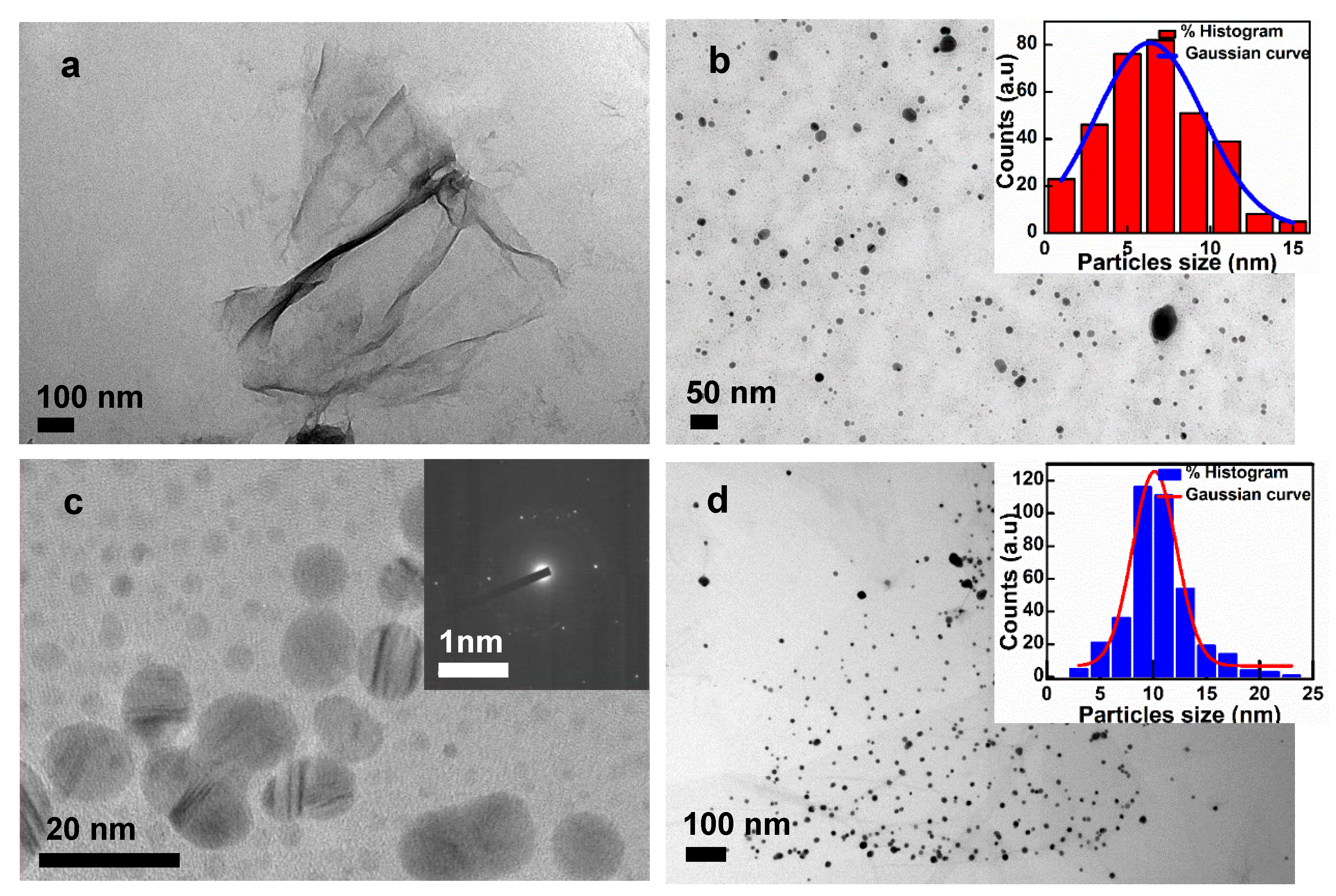
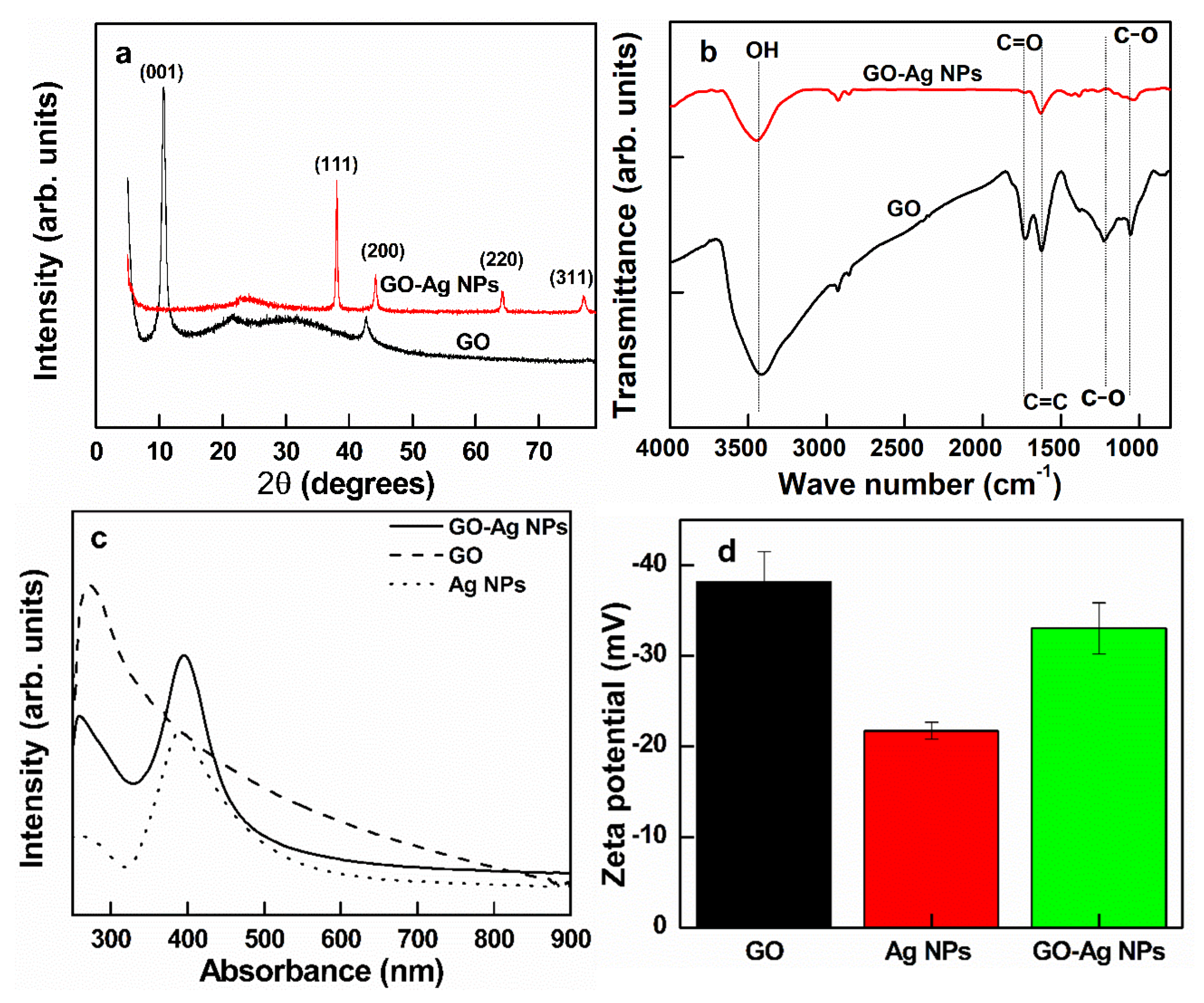
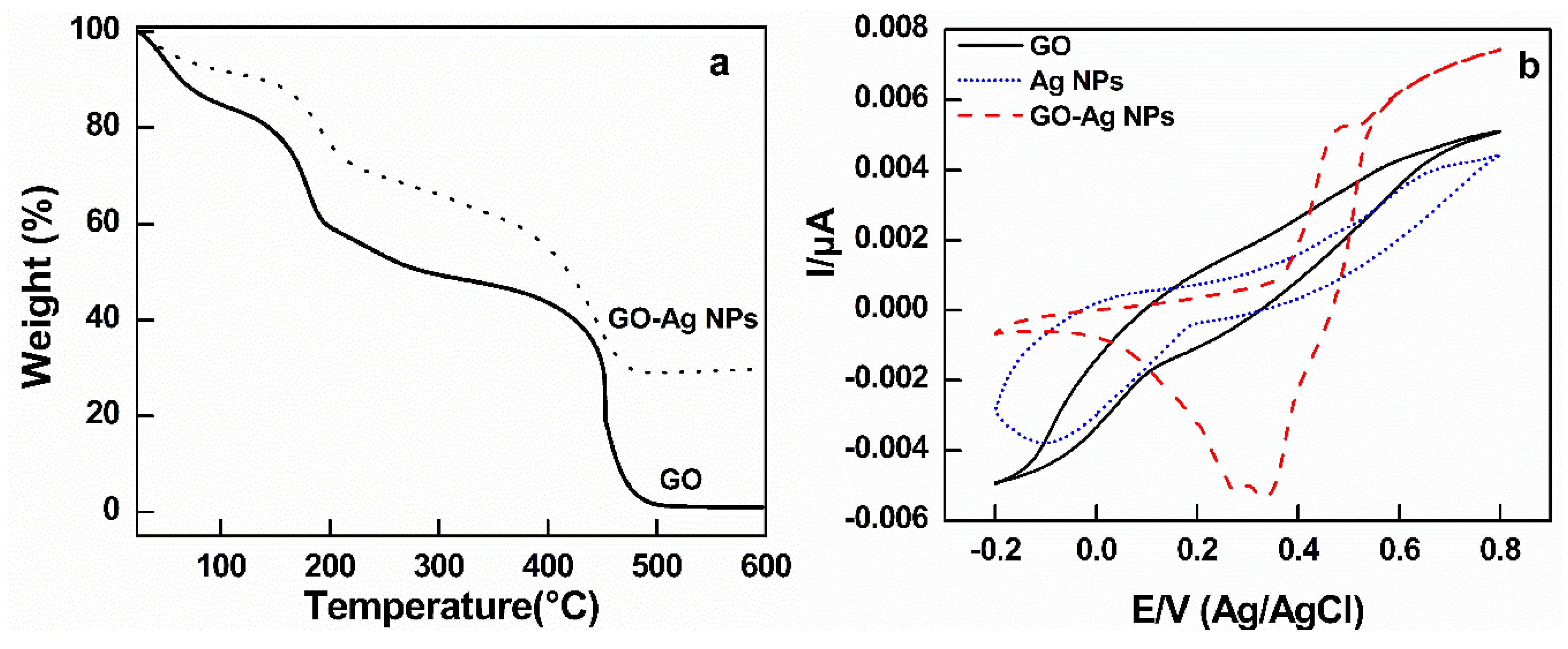
3.1. Synthesis of GO, Ag NPs, and GO–Ag NPs
3.2. Cyclic Voltammetry Test
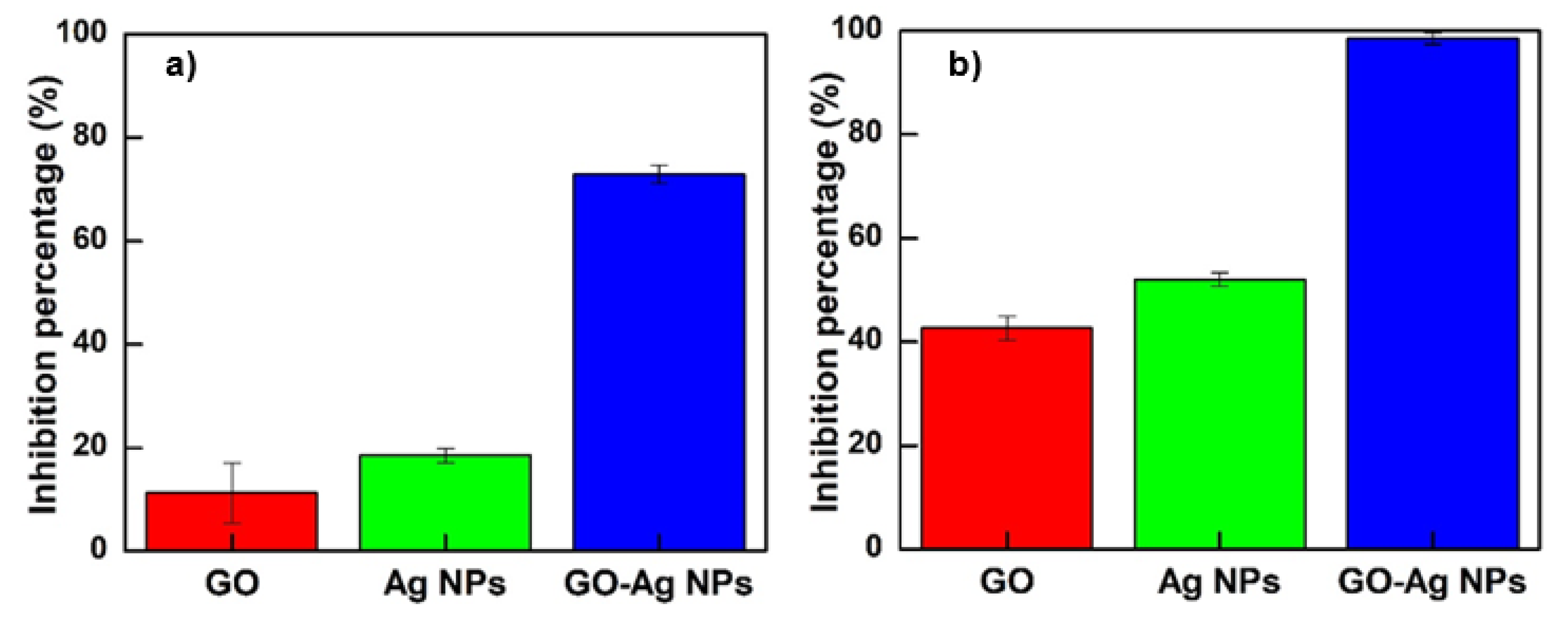
3.3. Antibacterial Test
3.3.1. Diffusion Disk Assay
3.3.2. Bacterial Growth Curve Inhibition Assay
3.3.3. Morphology Before and After Treatment
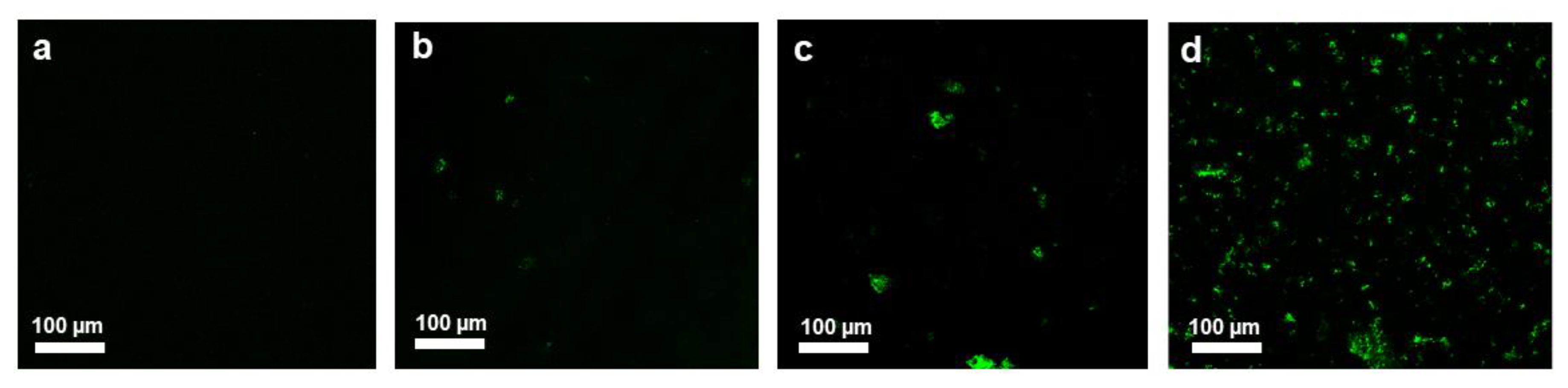
3.3.4. Live/Dead Cell Staining
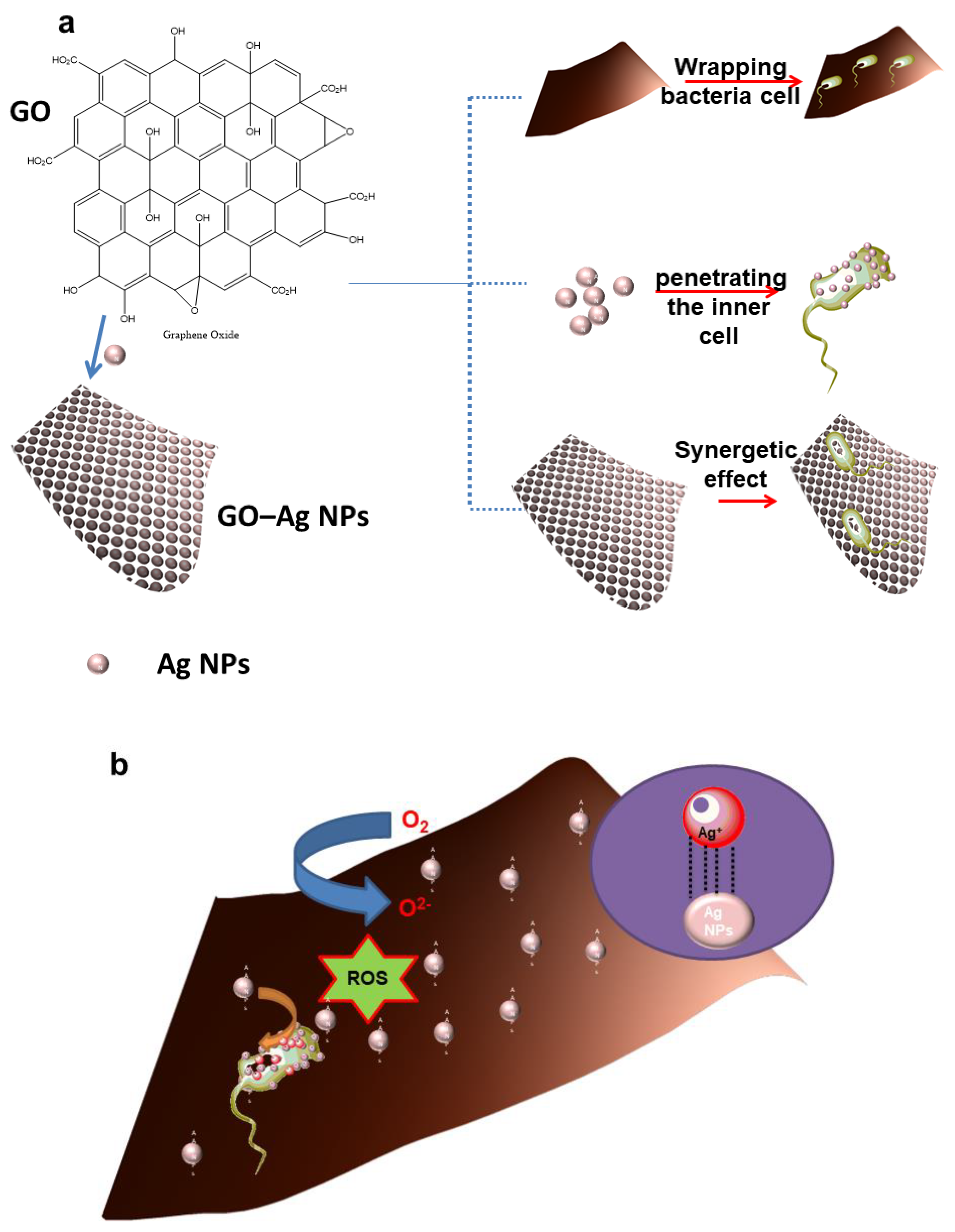
3.3.5. Reactive Oxygen Species Mechanism Applied for Bacterial Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- van der Meer, J.W.M. The infectious disease challenges of our time. Front. Public Health 2013, 1, 7. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Mumtaz, S.; Li, C.-H.; Hussain, I.; Rotello, V.M. Combatting antibiotic-resistant bacteria using nanomaterials. Chem. Soc. Rev. 2019, 48, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183. [Google Scholar] [CrossRef] [PubMed]
- Kapitanova, O.O.; Emelin, E.V.; Dorofeev, S.G.; Evdokimov, P.V.; Panin, G.N.; Lee, Y.; Lee, S. Direct patterning of reduced graphene oxide/graphene oxide memristive heterostructures by electron-beam irradiation. J. Mater. Sci. Technol. 2020, 38, 237–243. [Google Scholar] [CrossRef]
- Kumar, P.; Huo, P.; Zhang, R.; Liu, B. Antibacterial Properties of Graphene-Based Nanomaterials. Nanomaterials 2019, 9, 737. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial Activity of Graphite, Graphite Oxide, Graphene Oxide, and Reduced Graphene Oxide: Membrane and Oxidative Stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef]
- Efremova, L.V.; Vasilchenko, A.S.; Rakov, E.G.; Deryabin, D.G. Toxicity of Graphene Shells, Graphene Oxide, and Graphene Oxide Paper Evaluated with Escherichia coli Biotests. Biomed. Res. Int. 2015, 2015, 869361. [Google Scholar] [CrossRef]
- Tu, Y.; Lv, M.; Xiu, P.; Huynh, T.; Zhang, M.; Castelli, M.; Liu, Z.; Huang, Q.; Fan, C.; Fang, H.; et al. Destructive extraction of phospholipids from Escherichia coli membranes by graphene nanosheets. Nat. Nanotechnol. 2013, 8, 594–601. [Google Scholar] [CrossRef]
- Yousefi, M.; Dadashpour, M.; Hejazi, M.; Hasanzadeh, M.; Behnam, B.; de la Guardia, M.; Shadjou, N.; Mokhtarzadeh, A. Anti-bacterial activity of graphene oxide as a new weapon nanomaterial to combat multidrug-resistance bacteria. Mater. Sci. Eng. C 2017, 74, 568–581. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef] [PubMed]
- Abou El-Nour, K.M.M.; Eftaiha, A.A.; Al-Warthan, A.; Ammar, R.A.A. Synthesis and applications of silver nanoparticles. Arab. J. Chem. 2010, 3, 135–140. [Google Scholar] [CrossRef]
- Wong, K.K.Y.; Liu, X. Silver nanoparticles—The real “silver bullet” in clinical medicine? Med. Chem. Commun. 2010, 1, 125–131. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef]
- Zafar, N.; Shamaila, S.; Nazir, J.; Sharif, R.; Shahid Rafique, M.; Ul-Hasan, J.; Ammara, S.; Khalid, H. Antibacterial Action of Chemically Synthesized and Laser Generated Silver Nanoparticles against Human Pathogenic Bacteria. J. Mater. Sci. Technol. 2016, 32, 721–728. [Google Scholar] [CrossRef]
- D’Agostino, A.; Taglietti, A.; Desando, R.; Bini, M.; Patrini, M.; Dacarro, G.; Cucca, L.; Pallavicini, P.; Grisoli, P. Bulk Surfaces Coated with Triangular Silver Nanoplates: Antibacterial Action Based on Silver Release and Photo-Thermal Effect. Nanomaterials 2017, 7, 7. [Google Scholar] [CrossRef]
- D’Agostino, A.; Taglietti, A.; Grisoli, P.; Dacarro, G.; Cucca, L.; Patrini, M.; Pallavicini, P. Seed mediated growth of silver nanoplates on glass: Exploiting the bimodal antibacterial effect by near IR photo-thermal action and Ag+ release. RSC Adv. 2016, 6, 70414–70423. [Google Scholar] [CrossRef]
- López-Heras, M.; Theodorou, I.G.; Leo, B.F.; Ryan, M.P.; Porter, A.E. Towards understanding the antibacterial activity of Ag nanoparticles: Electron microscopy in the analysis of the materials-biology interface in the lung. Environ. Sci. Nano 2015, 2, 312–326. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Ghilini, F.; Rodríguez González, M.C.; Miñán, A.G.; Pissinis, D.; Creus, A.H.; Salvarezza, R.C.; Schilardi, P.L. Highly Stabilized Nanoparticles on Poly-l-Lysine-Coated Oxidized Metals: A Versatile Platform with Enhanced Antimicrobial Activity. ACS Appl. Mater. Interfaces 2018, 10, 23657–23666. [Google Scholar] [CrossRef]
- Pallavicini, P.; Dacarro, G.; Taglietti, A. Self-Assembled Monolayers of Silver Nanoparticles: From Intrinsic to Switchable Inorganic Antibacterial Surfaces. Eur. J. Inorg. Chem. 2018, 2018, 4846–4855. [Google Scholar] [CrossRef]
- Zhu, Z.; Su, M.; Ma, L.; Ma, L.; Liu, D.; Wang, Z. Preparation of graphene oxide–silver nanoparticle nanohybrids with highly antibacterial capability. Talanta 2013, 117, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Bao, Q.; Zhang, D.; Qi, P. Synthesis and characterization of silver nanoparticle and graphene oxide nanosheet composites as a bactericidal agent for water disinfection. J. Colloid Interface Sci. 2011, 360, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Das, M.R.; Sarma, R.K.; Borah, S.C.; Kumari, R.; Saikia, R.; Deshmukh, A.B.; Shelke, M.V.; Sengupta, P.; Szunerits, S.; Boukherroub, R. The synthesis of citrate-modified silver nanoparticles in an aqueous suspension of graphene oxide nanosheets and their antibacterial activity. Colloids Surf. B Biointerfaces 2013, 105, 128–136. [Google Scholar] [CrossRef]
- Tai, Z.; Ma, H.; Liu, B.; Yan, X.; Xue, Q. Facile synthesis of Ag/GNS-g-PAA nanohybrids for antimicrobial applications. Colloids Surf. B Biointerfaces 2012, 89, 147–151. [Google Scholar] [CrossRef]
- Jaworski, S.; Wierzbicki, M.; Sawosz, E.; Jung, A.; Gielerak, G.; Biernat, J.; Jaremek, H.; Łojkowski, W.; Woźniak, B.; Wojnarowicz, J.; et al. Graphene Oxide-Based Nanocomposites Decorated with Silver Nanoparticles as an Antibacterial Agent. Nanoscale Res. Lett. 2018, 13, 116. [Google Scholar] [CrossRef]
- Rasoulzadehzali, M.; Namazi, H. Facile preparation of antibacterial chitosan/graphene oxide-Ag bio-nanocomposite hydrogel beads for controlled release of doxorubicin. Int. J. Biol. Macromol. 2018, 116, 54–63. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, J.; Xiong, Z.; Yong, Y.; Zhao, X.S. Preparation, characterization and antibacterial properties of silver-modified graphene oxide. J. Mater. Chem. 2011, 21, 3350–3352. [Google Scholar] [CrossRef]
- Tang, J.; Chen, Q.; Xu, L.; Zhang, S.; Feng, L.; Cheng, L.; Xu, H.; Liu, Z.; Peng, R. Graphene Oxide—Silver Nanocomposite As a Highly Effective Antibacterial Agent with Species-Specific Mechanisms. ACS Appl. Mater. Interfaces 2013, 5, 3867–3874. [Google Scholar] [CrossRef]
- No, H.K.; Young Park, N.; Ho Lee, S.; Meyers, S.P. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 2002, 74, 65–72. [Google Scholar] [CrossRef]
- Roller, S.; Covill, N. The antifungal properties of chitosan in laboratory media and apple juice. Int. J. Food Microbiol. 1999, 47, 67–77. [Google Scholar] [CrossRef]
- Fernandes, J.C.; Tavaria, F.K.; Soares, J.C.; Ramos, O.S.; Joao Monteiro, M.; Pintado, M.E.; Xavier Malcata, F. Antimicrobial effects of chitosans and chitooligosaccharides, upon Staphylococcus aureus and Escherichia coli, in food model systems. Food Microbiol. 2008, 25, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Moya, F.; Lopez-Llorca, L. Omics for Investigating Chitosan as an Antifungal and Gene Modulator. J. Fungi. 2016, 2, 11. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Marta, B.; Potara, M.; Iliut, M.; Jakab, E.; Radu, T.; Lucaci, F.; Gabriel, K.; Popescu, O.; Astilean, S. Designing chitosan-silver nanoparticles-graphene oxide nanohybrids with enhanced antibacterial activity against Staphylococcus aureus. Colloids Surf. A Physicochem. Eng. Asp. 2015, 487, 113–120. [Google Scholar] [CrossRef]
- Khawaja, H.; Zahir, E.; Asghar, M.A.; Asghar, M.A. Graphene oxide, chitosan and silver nanocomposite as a highly effective antibacterial agent against pathogenic strains. Colloids Surf. A Physicochem. Eng. Asp. 2018, 555, 246–255. [Google Scholar] [CrossRef]
- de Faria, A.F.; Martinez, D.S.T.; Meira, S.M.M.; de Moraes, A.C.M.; Brandelli, A.; Filho, A.G.S.; Alves, O.L. Anti-adhesion and antibacterial activity of silver nanoparticles supported on graphene oxide sheets. Colloids Surf. B Biointerfaces 2014, 113, 115–124. [Google Scholar] [CrossRef]
- Vi, T.T.T.; Rajesh Kumar, S.; Rout, B.; Liu, C.-H.; Wong, C.-B.; Chang, C.-W.; Chen, C.-H.; Chen, D.W.; Lue, S.J. The Preparation of Graphene Oxide-Silver Nanocomposites: The Effect of Silver Loads on Gram-Positive and Gram-Negative Antibacterial Activities. Nanomaterials 2018, 8, 163. [Google Scholar] [CrossRef]
- Baskoro, F.; Wong, C.-B.; Kumar, S.R.; Chang, C.-W.; Chen, C.-H.; Chen, D.W.; Lue, S.J. Graphene oxide-cation interaction: Inter-layer spacing and zeta potential changes in response to various salt solutions. J. Membr. Sci. 2018, 554, 253–263. [Google Scholar] [CrossRef]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Adv. 2014, 4, 3974–3983. [Google Scholar] [CrossRef]
- Ravulapalli, S.; Mariserla, B.M.K.; Soma, V.R.; Saritha, R.; Rao, D. Biosynthesis of Silver Nanoparticles Using Coriandrum Sativum Leaf Extract and Their Application in Nonlinear Optics. Adv. Sci. Lett. 2010, 3, 138. [Google Scholar] [CrossRef]
- Regiel, A.; Irusta, S.; Kyzioł, A.; Arruebo, M.; Santamaria, J. Preparation and characterization of chitosan-silver nanocomposite films and their antibacterial activity against Staphylococcus aureus. Nanotechnology 2012, 24, 015101. [Google Scholar] [CrossRef] [PubMed]
- Honary, S.; Ghajar, K.; Khazaeli, P.; Shalchian, P. Preparation, Characterization and Antibacterial Properties of Silver-Chitosan Nanocomposites Using Different Molecular Weight Grades of Chitosan. Trop. J. Pharm. Res. 2011, 10. [Google Scholar] [CrossRef]
- Hien, N.; Phu, D.; Duy, N.; Quoc, L.; Lan, N.; Hoang, D.; Van, H.; Nu, P.; Thai Hoa, T. Influence of Chitosan Binder on the Adhesion of Silver Nanoparticles on Cotton Fabric and Evaluation of Antibacterial Activity. Adv. Nanopart. 2015, 4, 98–106. [Google Scholar] [CrossRef]
- Yang, Y.-K.; He, C.; He, W.-J.; Yu, L.-J.; Peng, R.-G.; Xie, X.-L.; Wang, X.-B.; Mai, Y.W. Reduction of silver nanoparticles onto graphene oxide nanosheets with N, N-dimethylformamide and SERS activities of GO/Ag composites. J. Nanopart. Res. 2011, 13, 1–11. [Google Scholar] [CrossRef]
- Çiplak, Z.; Yildiz, N.; Çalimli, A. Investigation of Graphene/Ag Nanocomposites Synthesis Parameters for Two Different Synthesis Methods. Fuller. Nanotub. Carbon Nanostructures 2014, 23, 361–370. [Google Scholar] [CrossRef]
- Ţucureanu, V.; Matei, A.; Avram, A.M. FTIR Spectroscopy for Carbon Family Study. Crit. Rev. Anal. Chem. 2016, 46, 502–520. [Google Scholar] [CrossRef]
- Raza, M.A.; Kanwal, Z.; Rauf, A.; Sabri, A.N.; Riaz, S.; Naseem, S. Size- and Shape-Dependent Antibacterial Studies of Silver Nanoparticles Synthesized by Wet Chemical Routes. Nanomaterials 2016, 6, 74. [Google Scholar] [CrossRef]
- Al-Gaashani, R.; Najjar, A.; Zakaria, Y.; Mansour, S.; Atieh, M.A. XPS and structural studies of high quality graphene oxide and reduced graphene oxide prepared by different chemical oxidation methods. Ceram. Int. 2019, 45, 14439–14448. [Google Scholar] [CrossRef]
- Rawson, F.J.; Hicks, J.; Dodd, N.; Abate, W.; Garrett, D.J.; Yip, N.; Fejer, G.; Downard, A.J.; Baronian, K.H.R.; Jackson, S.K.; et al. Fast, Ultrasensitive Detection of Reactive Oxygen Species Using a Carbon Nanotube Based-Electrocatalytic Intracellular Sensor. ACS Appl. Mater. Interfaces 2015, 7, 23527–23537. [Google Scholar] [CrossRef]
- Yang, D.; Ni, N.; Cao, L.; Song, X.; Alhamoud, Y.; Yu, G.; Zhao, J.; Zhou, H. Silver Doped Mesoporous Silica Nanoparticles Based Electrochemical Enzyme-Less Sensor for Determination of H2O2 Released from Live Cells. Micromachines 2019, 10, 268. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Hsu, J.H.; Hsu, Y.K. Branched silver nanowires on fluorine-doped tin oxide glass for simultaneous amperometric detection of H2O2 and of 4-aminothiophenol by SERS. Mikrochim. Acta 2018, 185, 106. [Google Scholar] [CrossRef] [PubMed]
- Rudzka, D.A.; Cameron, J.M.; Olson, M.F. Reactive oxygen species and hydrogen peroxide generation in cell migration. Commun. Integr. Biol. 2015, 8, e1074360. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Singh, S.P.; Hader, D.P.; Sinha, R.P. Detection of reactive oxygen species (ROS) by the oxidant-sensing probe 2′, 7′-dichlorodihydrofluorescein diacetate in the cyanobacterium Anabaena variabilis PCC 7937. Biochem. Biophys. Res. Commun. 2010, 397, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Barbolina, I.; Woods, C.R.; Lozano, N.; Kostarelos, K.; Novoselov, K.S.; Roberts, I.S. Purity of graphene oxide determines its antibacterial activity. Materials 2016, 3, 025025. [Google Scholar] [CrossRef]
- Anand, A.; Unnikrishnan, B.; Wei, S.-C.; Chou, C.P.; Zhang, L.-Z.; Huang, C.-C. Graphene oxide and carbon dots as broad-spectrum antimicrobial agents—A minireview. Nanoscale Horiz. 2019, 4, 117–137. [Google Scholar] [CrossRef]
- Romero-Vargas Castrillón, S.; Perreault, F.; de Faria, A.F.; Elimelech, M. Interaction of Graphene Oxide with Bacterial Cell Membranes: Insights from Force Spectroscopy. Environ. Sci. Technol. Lett. 2015, 2, 112–117. [Google Scholar] [CrossRef]
- Hui, L.; Piao, J.-G.; Auletta, J.; Hu, K.; Zhu, Y.; Meyer, T.; Liu, H.; Yang, L. Availability of the Basal Planes of Graphene Oxide Determines Whether It Is Antibacterial. ACS Appl. Mater. Interfaces 2014, 6, 13183–13190. [Google Scholar] [CrossRef]
- Sengupta, I.; Bhattacharya, P.; Talukdar, M.; Neogi, S.; Pal, S.K.; Chakraborty, S. Bactericidal effect of graphene oxide and reduced graphene oxide: Influence of shape of bacteria. Colloid Interface Sci. Commun. 2019, 28, 60–68. [Google Scholar] [CrossRef]
- Samberg, M.E.; Orndorff, P.E.; Monteiro-Riviere, N.A. Antibacterial efficacy of silver nanoparticles of different sizes, surface conditions and synthesis methods. Nanotoxicology 2011, 5, 244–253. [Google Scholar] [CrossRef]
- Gao, L.; Wang, L.; Yang, L.; Zhao, Y.; Shi, N.; An, C.; Sun, Y.; Xie, J.; Wang, H.; Song, Y.; et al. Preparation, characterization and antibacterial activity of silver nanoparticle/graphene oxide/diatomite composite. Appl. Surf. Sci. 2019, 484, 628–636. [Google Scholar] [CrossRef]
- Zhao, R.; Lv, M.; Li, Y.; Sun, M.; Kong, W.; Wang, L.; Song, S.; Fan, C.; Jia, L.; Qiu, S.; et al. Stable Nanocomposite Based on PEGylated and Silver Nanoparticles Loaded Graphene Oxide for Long-Term Antibacterial Activity. ACS Appl. Mater. Interfaces 2017, 9, 15328–15341. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Gong, J.-L.; Zeng, G.-M.; Ou, X.-M.; Chang, Y.-N.; Deng, C.-H.; Zhang, J.; Liu, H.-Y.; Huang, S.-Y. Magnetic chitosan–graphene oxide composite for anti-microbial and dye removal applications. Int. J. Biol. Macromol. 2016, 82, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Gao, Y.; Sun, Z.; Chang, D.; Gao, G.; Dong, A. Synthesis, Characterization, and Bactericidal Evaluation of Chitosan/Guanidine Functionalized Graphene Oxide Composites. Molecules 2016, 22, 12. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Tu, Y.; Li, L.; Shang, S.; Tao, X.-M. Well-Dispersed Chitosan/Graphene Oxide Nanocomposites. ACS Appl. Mater. Interfaces 2010, 2, 1707–1713. [Google Scholar] [CrossRef]
- Chook, S.W.; Chia, C.H.; Zakaria, S.; Ayob, M.K.; Chee, K.L.; Huang, N.M.; Neoh, H.M.; Lim, H.N.; Jamal, R.; Rahman, R. Antibacterial performance of Ag nanoparticles and AgGO nanocomposites prepared via rapid microwave-assisted synthesis method. Nanoscale Res. Lett. 2012, 7, 541. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Geetha Bai, R.; Muthoosamy, K.; Shipton, F.N.; Pandikumar, A.; Rameshkumar, P.; Huang, N.M.; Manickam, S. The biogenic synthesis of a reduced graphene oxide-silver (RGO–Ag) nanocomposite and its dual applications as an antibacterial agent and cancer biomarker sensor. RSC Adv. 2016, 6, 36576–36587. [Google Scholar] [CrossRef]
- Fu, L.; Lai, G.; Mahon, P.; Wang, J.; Zhu, D.; Jia, B.; Malherbe, F.; Yu, A. Carbon Nanotube and Graphene Oxide Directed Electrochemical Synthesis of Silver Dendrites. RSC Adv. 2014. [Google Scholar] [CrossRef]
- Jones, A.M.; Garg, S.; He, D.; Pham, A.N.; Waite, T.D. Superoxide-Mediated Formation and Charging of Silver Nanoparticles. Environ. Sci. Technol. 2011, 45, 1428–1434. [Google Scholar] [CrossRef]
- Lakshmi, V.; Balavijayalakshmi, J. Silver Nanocomposites Decorated Reduced Graphene Oxide Nanosheets for Electrochemical Sensor Applications. Orient. J. Chem. 2018, 34, 2872–2877. [Google Scholar] [CrossRef]
- Wimmerstedt, A.; Kahlmeter, G. Associated antimicrobial resistance in Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Streptococcus pneumoniae and Streptococcus pyogenes. Clin. Microbiol. Infect. 2008, 14, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Pulingam, T.; Thong, K.L.; Ali, M.E.; Appaturi, J.N.; Dinshaw, I.J.; Ong, Z.Y.; Leo, B.F. Graphene oxide exhibits differential mechanistic action towards Gram-positive and Gram-negative bacteria. Colloids Surf. B Biointerfaces 2019, 181, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E. Toxicity of Graphene and Graphene Oxide Nanowalls Against Bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef] [PubMed]
- Cabiscol, E.; Tamarit, J.; Ros, J. Oxidative stress in bacteria and protein damage by reactive oxygen species. Int. Microbiol. Off. J. Span. Soc. Microbiol. 2000, 3, 3–8. [Google Scholar]
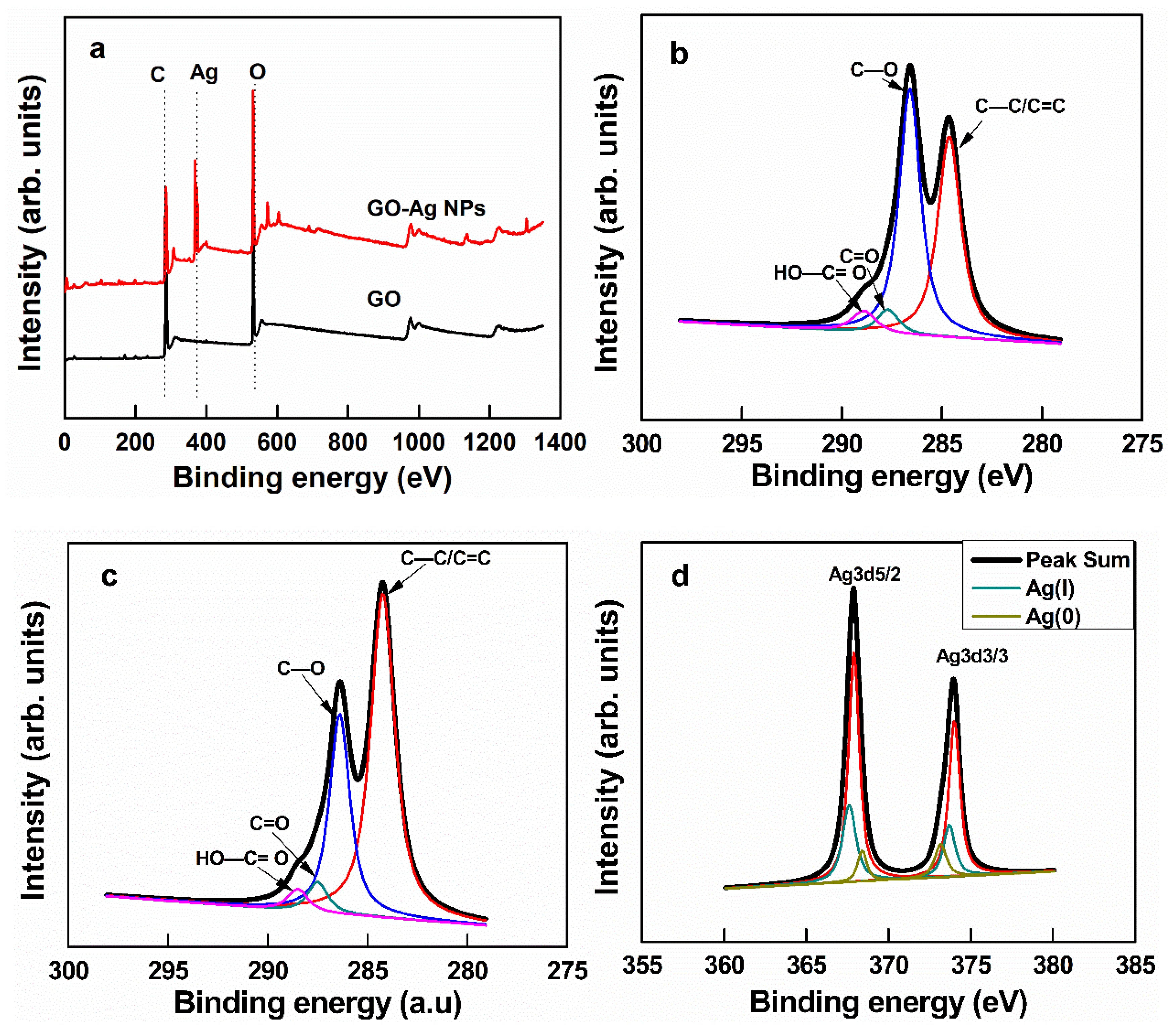



| C1s | C=C | C–O | C=O | O–C–O |
|---|---|---|---|---|
| GO | 42.29 | 48.11 | 4.81 | 4.79 |
| GO–Ag NPs | 69.60 | 22.70 | 4.79 | 2.61 |
| Inhibition Zone (mm) | ||||||
|---|---|---|---|---|---|---|
| Concentration (μg mL−1) | GO | Ag NPs | GO–Ag NPs | |||
| E. coli | S. Aureus | E. coli | S. Aureus | E. coli | S. Aureus | |
| 10 | 9.5 | 9.0 | 9.5 | 9.5 | 11.0 | 11.5 |
| 50 | 10.0 | 10.5 | 11.0 | 11.5 | 13.0 | 15.0 |
| 100 | 15.0 | 18.0 | 16.0 | 20.0 | 18.0 | 28.0 |
| Linker | Reducing Agents | Bacteria Model | Ag NPs Size | Evaluation Method | Inhibition | Compared with Pure GO or Ag NPs | Mechanism of Action | Reference |
|---|---|---|---|---|---|---|---|---|
| None | Hydroquinone | E. coli and S. Aureus | 80 nm | - Plate counting - Disk diffusion | - 100 and 87.6% - 18 and 21 mm. | - 51.9 and 61.3% by GO - 9 and 10 mm by GO | None | [23] |
| None | PVP | E. coli, S. Aureus, S. epidermidis, and C. Albicans | 80–230 nm | GCI | - 77.5–88.6% | - None | ROS production (by fluorescence) | [26] |
| None | Glucose | E. coli | 10–70 nm | ADA | - N/A | - No antibacterial effect by GO | Morphology observation | [28] |
| None | Sodium citrate | P. aeruginosa | 7.5 nm | ADA | - 100% | - No antibacterial effect by GO | None | [37] |
| None | Sodium citrate | E. coli and S. Aureus | 50–70 nm | GCI | - 60% for GCI both bacteria strain - ADA: 74% towards S. Aureus and 94% towards E. coli | - No antibacterial effect by GO and Ag NPs | Morphology observation, cell division disorder. | [29] |
| None | NaBH4 | S. Aureus and B. subtilis | 2–25 nm | GCI and plate counting | - 100% | - 96–97% by Ag NPs | None | [24] |
| PAA | None | E. coli and S. Aureus | 4–8 nm | Disk diffusion | - 9.9 and 11.4 mm | - N/A | None | [25] |
| PDDA | Sodium citrate | E. coli and B. subtilis | 14 nm | ADA | - 54 and 60% | - No antibacterial effect by Ag NPs | None | [22] |
| STPP | CS | E. coli and S. aureus | 20 ± 1 nm | Disk diffusion | - 18.5 and 17 mm | - 5 and 6 mm | None | [27] |
| NaSH | NaSH | S. Aureus and P. aeruginosa | 2 nm | GCI | - 100% | - 27.2% and 32. 7% induced by GO | None | [38] |
| CS | Green tea leaves | S. Aureus, S. mutans, E. coli, K. pneumoniae, P. aeruginosa, S. typhi | 15–25 nm | Disk diffusion | - 19–22 mm | - 7–11 mm by GO | None | [36] |
| CS | NaBH4 | S. Aureus | 10–30 nm | MBC and MIC | - 1.09 and 4.05 µg mL−1 | - 100% | None | [35] |
| CS | NaBH4 | E. coli and S. Aureus | 10.1 nm | GCI and disk diffusion | - 73 and 98.5% - 28 and 18 mm. | - 11 and 18% by GO; 43 and 52 by Ag NPs. - 15 and 18 mm by GO; 16 and 20 mm by Ag NPs | Morphology observation, ROS production (by CV test and fluorescence) | This study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vi, T.T.T.; Kumar, S.R.; Pang, J.-H.S.; Liu, Y.-K.; Chen, D.W.; Lue, S.J. Synergistic Antibacterial Activity of Silver-Loaded Graphene Oxide towards Staphylococcus Aureus and Escherichia Coli. Nanomaterials 2020, 10, 366. https://doi.org/10.3390/nano10020366
Vi TTT, Kumar SR, Pang J-HS, Liu Y-K, Chen DW, Lue SJ. Synergistic Antibacterial Activity of Silver-Loaded Graphene Oxide towards Staphylococcus Aureus and Escherichia Coli. Nanomaterials. 2020; 10(2):366. https://doi.org/10.3390/nano10020366
Chicago/Turabian StyleVi, Truong Thi Tuong, Selvaraj Rajesh Kumar, Jong-Hwei Su Pang, Yu-Kuo Liu, Dave W. Chen, and Shingjiang Jessie Lue. 2020. "Synergistic Antibacterial Activity of Silver-Loaded Graphene Oxide towards Staphylococcus Aureus and Escherichia Coli" Nanomaterials 10, no. 2: 366. https://doi.org/10.3390/nano10020366
APA StyleVi, T. T. T., Kumar, S. R., Pang, J.-H. S., Liu, Y.-K., Chen, D. W., & Lue, S. J. (2020). Synergistic Antibacterial Activity of Silver-Loaded Graphene Oxide towards Staphylococcus Aureus and Escherichia Coli. Nanomaterials, 10(2), 366. https://doi.org/10.3390/nano10020366







