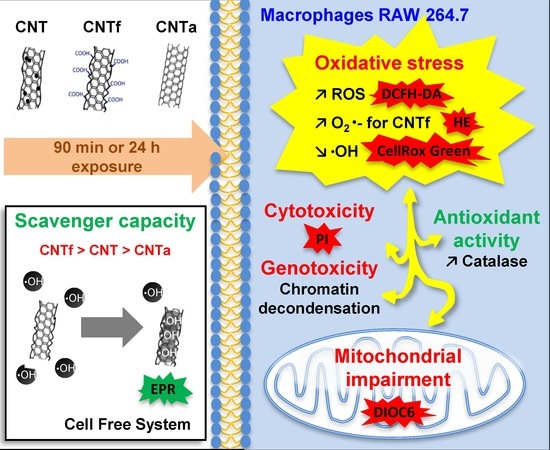
Quantitative Flow Cytometric Evaluation of Oxidative Stress and Mitochondrial Impairment in RAW 264.7 Macrophages after Exposure to Pristine, Acid Functionalized, or Annealed Carbon Nanotubes
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture of RAW 264.7 Macrophages
2.2. MWCNTs Powders Characteristics
- -
- CNT: pristine MWCNTs (NC7000TM, Nanocyl), with a diameter of 9.5 nm and lengths from 1.5 to 2 µm according to the manufacturer.
- -
- CNTa: same MWCNTs after an annealing treatment over 2125 °C for 1 h under argon, full procedure described in Figarol et al. [47].
- -
- CNTf: same MWCNTs after an acid functionalization by oxidation with refluxing in a solution of nitric and sulfuric acids (3:1 v/v during 6 h), as described in Figarol et al. [43].
- -
- MWCNTs suspensions preparation and physicochemical characterizations were carried out according to a methodology detailed in our previous studies [43,47,49]. The main physicochemical features of the three MWCNTs batches (CNT, CNTf, and CNTa) are summarized in Table 1. All samples showed similar diameters and lengths. Traces of iron, a metallic catalytic impurity, were found in pristine CNT. Its level was dramatically reduced after acid functionalization or annealing treatment. Acid functionalization increased the amount of structural defects established with Raman spectroscopy (Id/Ig ratio: corresponding to the intensity of the D- to G-band ratio from the Raman spectra, respectively linked to sp3 and sp2 carbons), while annealing has the reverse effect. MWCNTs suspensions in culture medium at 37°C were stable over 48 h [43,47,49].
2.3. Experimental In Vitro Exposure Conditions
2.4. Flow Cytometry Toxicity Evaluation
2.4.1. Quantitative Analysis of Radical Species
2.4.2. Cytotoxicity Evaluation: PI Permeability Versus Mitochondrial Membrane Potential (∆Ψm)
2.4.3. Genotoxicological Evaluation: DNA Strand Breaks and Chromatin Decondensation
2.5. Analysis of Superoxide Dismutase (SOD) and Catalase Anti-Oxidant Activities
2.6. Cell Free System
2.6.1. Analysis of Bioavailable Iron Impurities Accessibility on MWCNTs Surface
2.6.2. MWCNTs Hydroxyl Radical Scavenging Activity Analysis
2.7. Statistical Analysis
3. Results
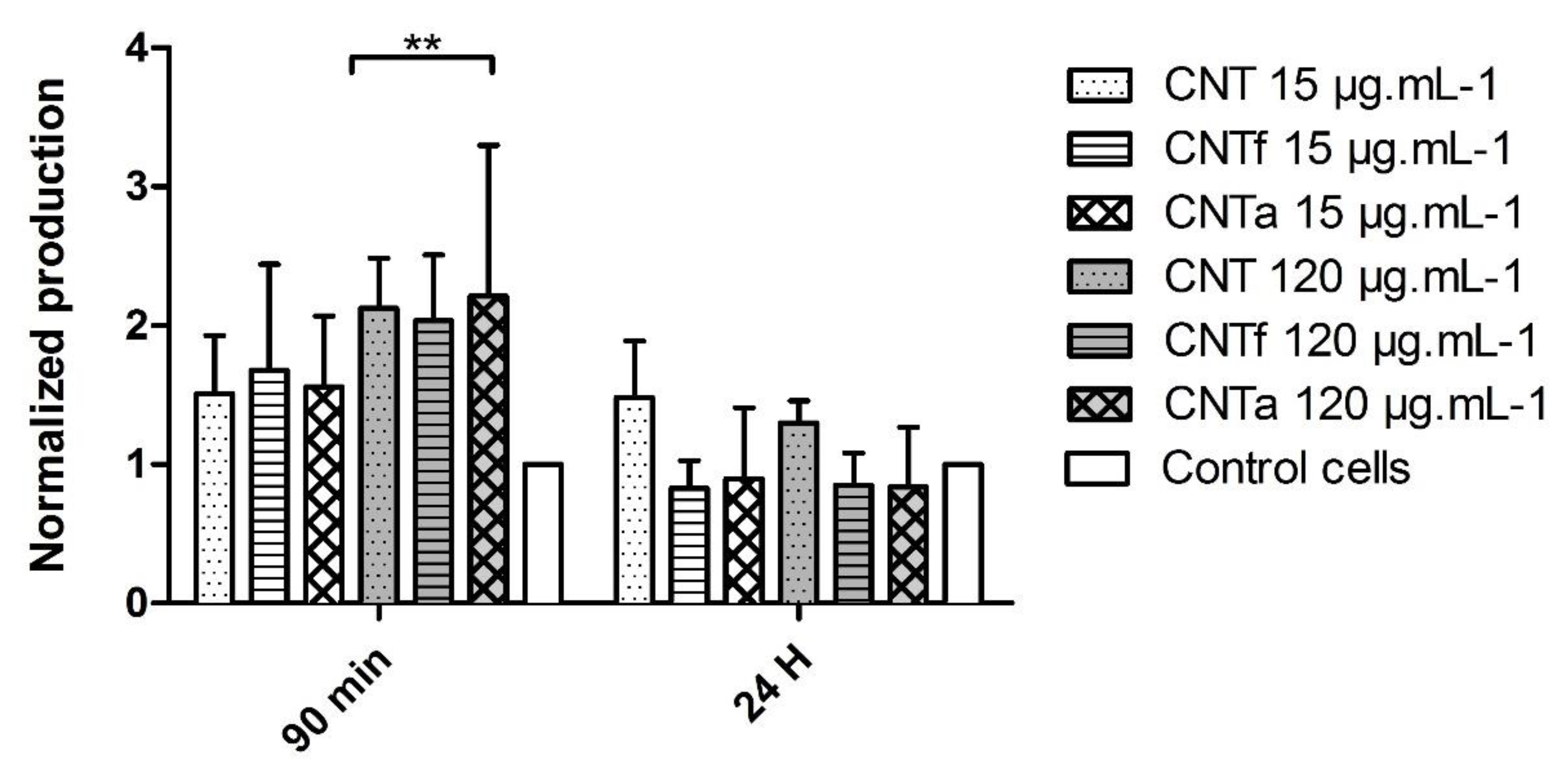
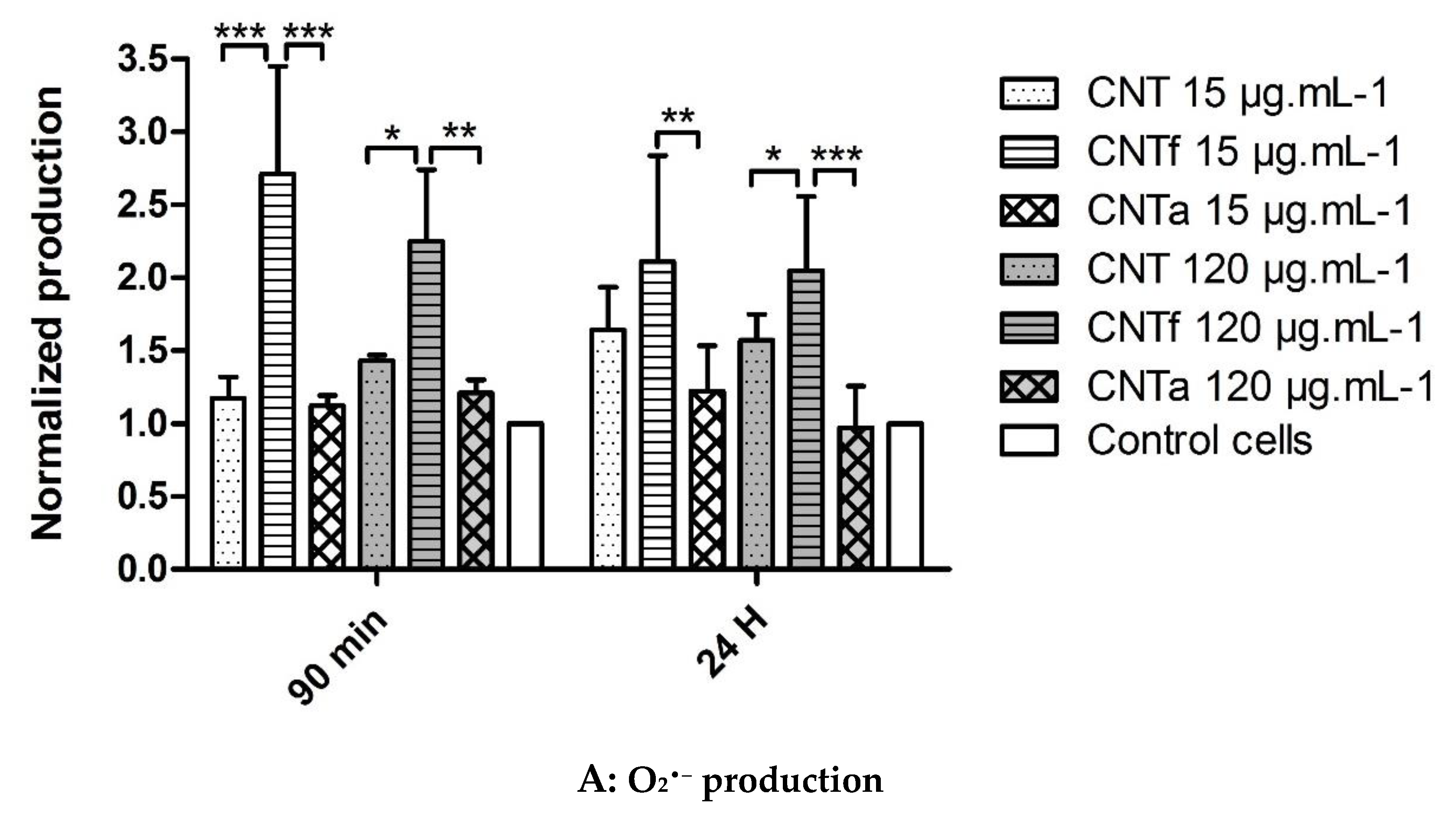
3.1. Multi-Sided Quantification of Oxidative Stress by FCM
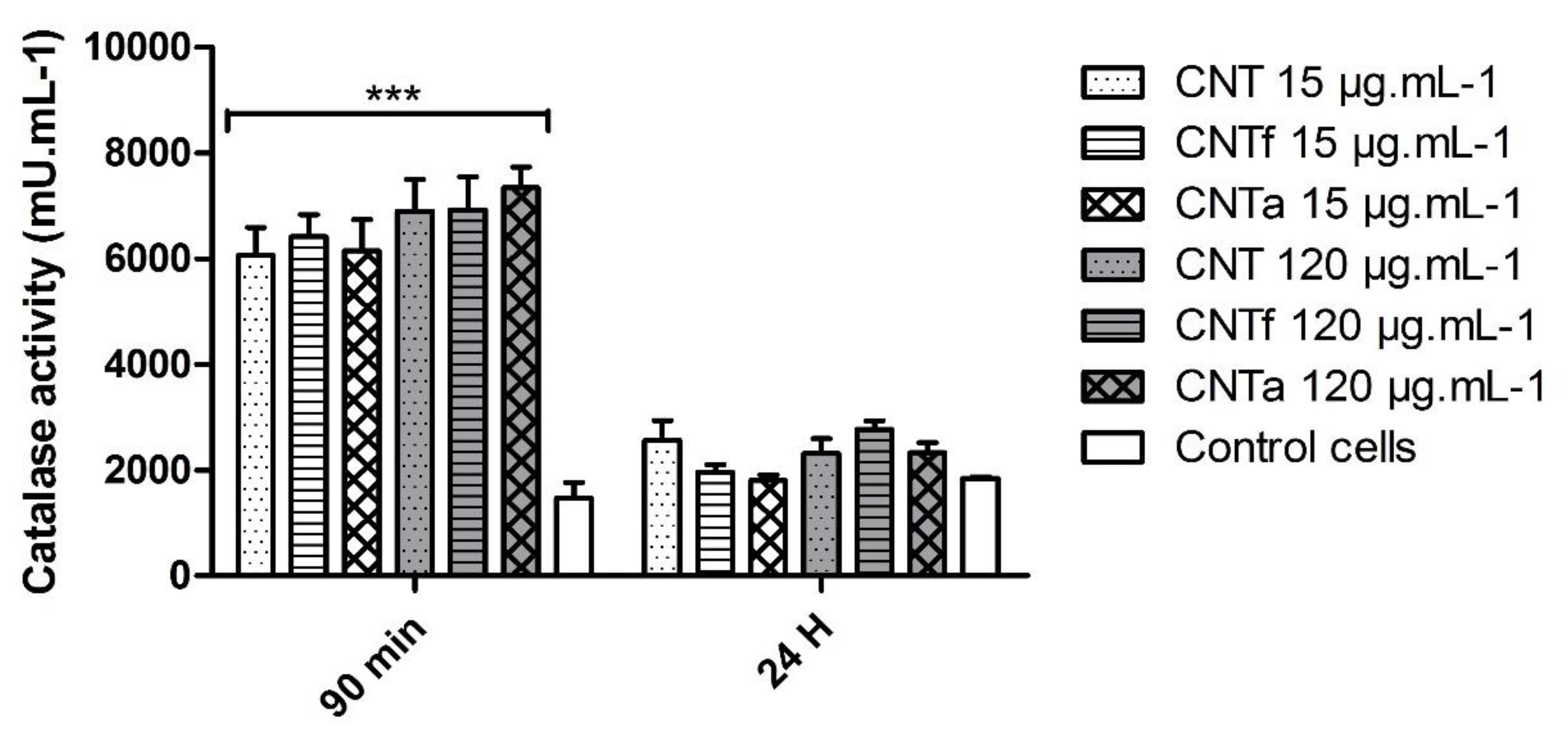
3.2. Anti-Oxidant Activity
3.3. Additional Oxidative Stress Analysis in a Cell-Free System
3.3.1. MWCNT Surface Iron Accessibility
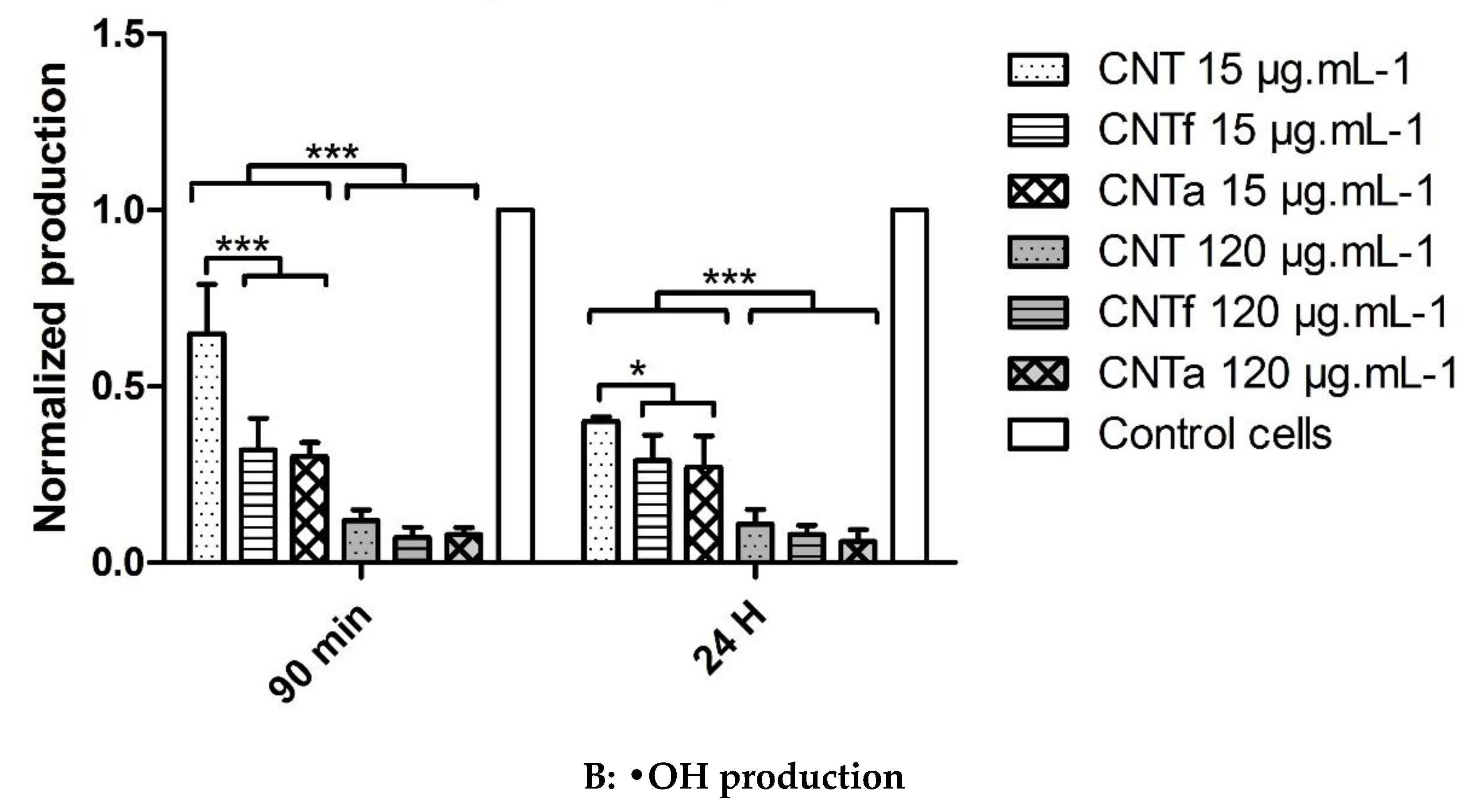
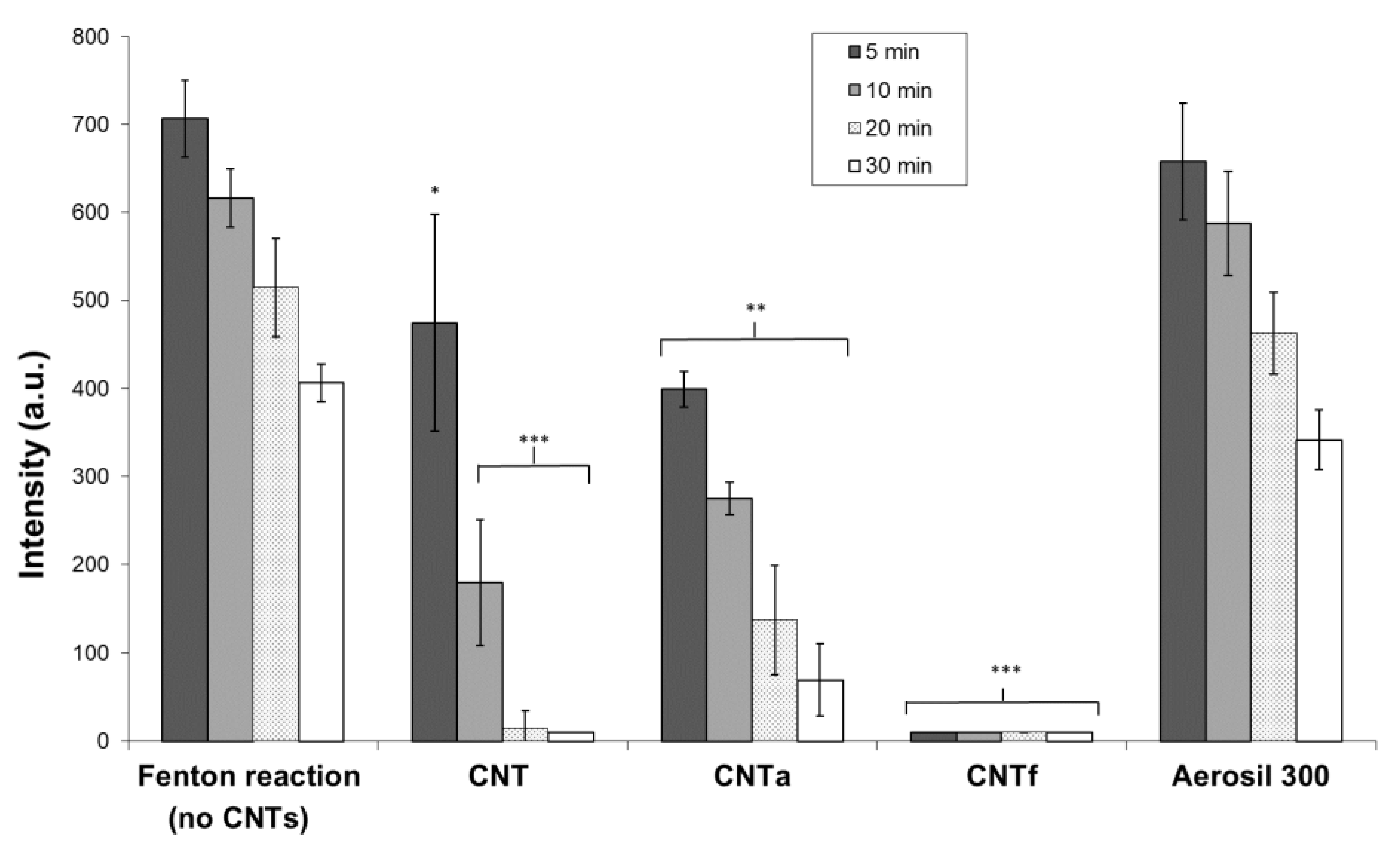
3.3.2. Scavenging Activity toward Hydroxyl Radicals •OH
3.4. Cytotoxicity and Genotoxicity Analysis by FCM
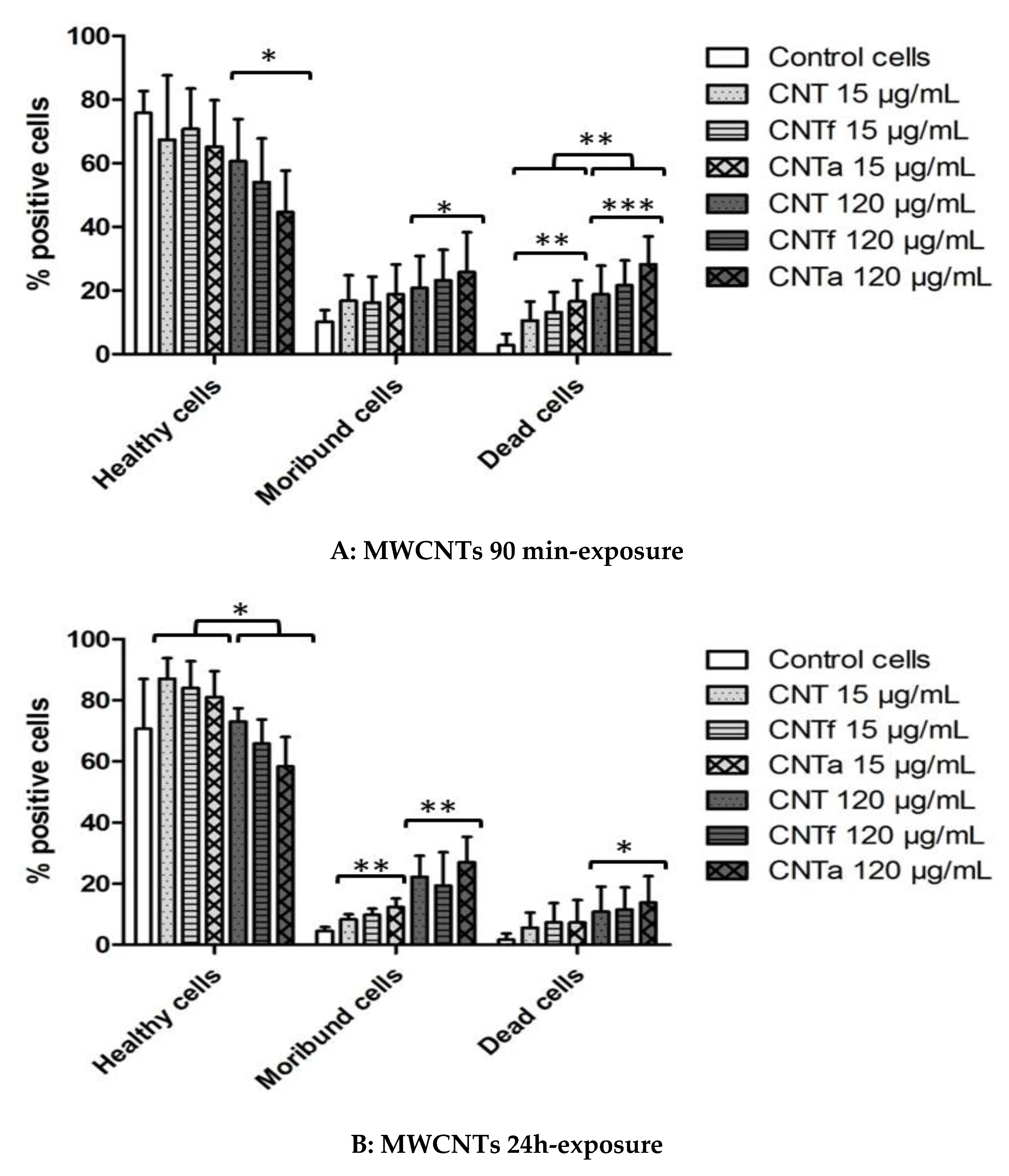
3.4.1. Death rate: PI Permeability
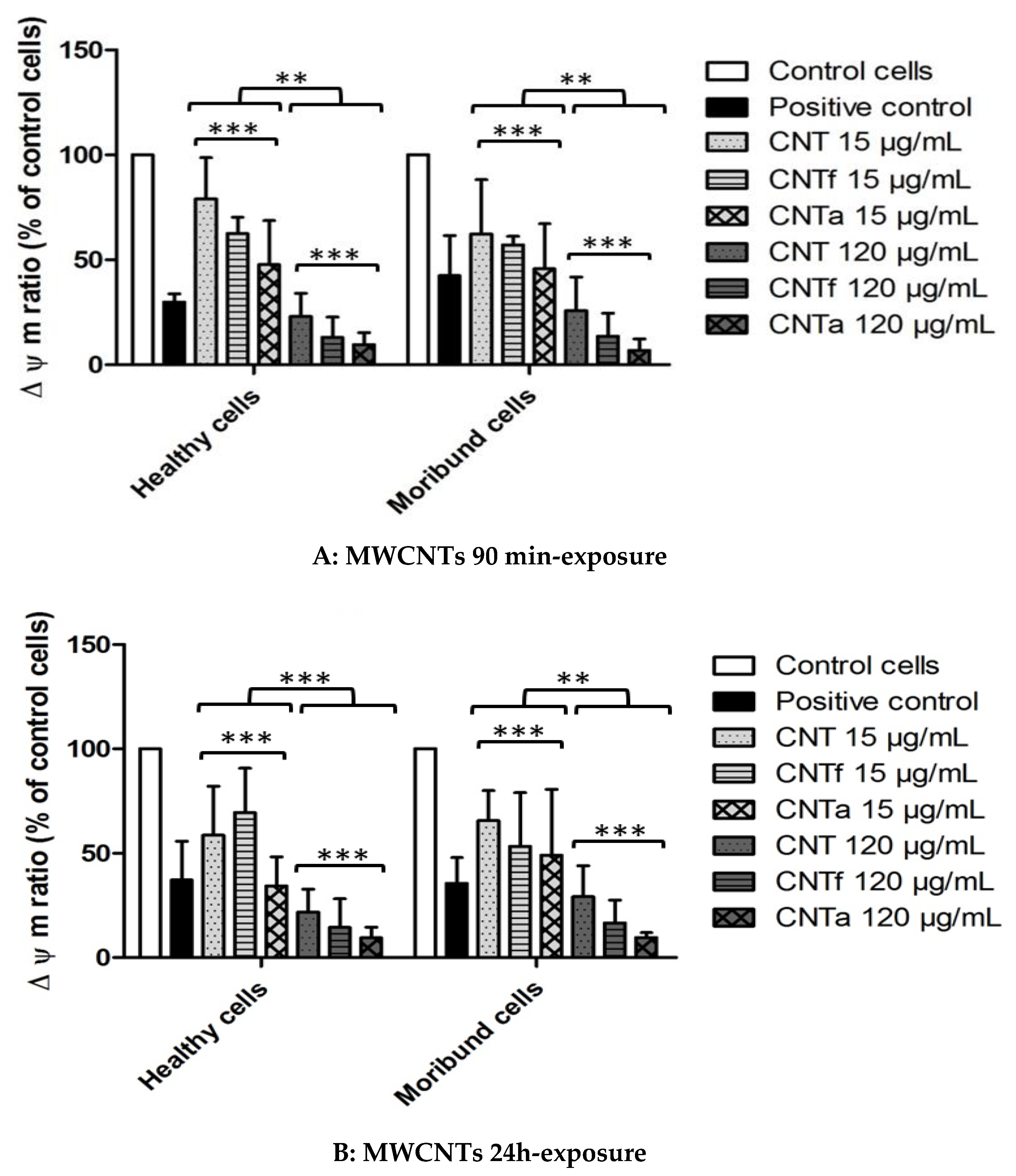
3.4.2. Mitochondrial Membrane Potential Analysis
3.4.3. Genotoxicity: DNA Fragmentation and Chromatin Decondensation
4. Discussion
5. Conclusions
- First, no significant differences in large spectrum ROS production were observed between the MWCNTs types. Moreover, ROS enhancement was only detectable after short-term exposure, while over the long term (24 h), the antioxidant mechanisms such as catalase activity counterbalanced it.
- Then, using our FCM multiparametric methodology for a more specific analysis of O2•− and •OH production, it was discovered that acid functionalization and annealing actually affected the MWCNTs related to oxidative stress. Iron impurities, only detected in pristine CNT, were not the main cause of the oxidative stress.
- CNTf, purified by acid functionalization, was characterized by a stronger increased scavenging capacity and an enhanced specific O2•− production, compared to CNTa and CNT; meanwhile, CNTa, purified by annealing, showed the most reduced scavenger capacity.
- Both acid functionalization and annealing treatment had deleterious impacts on the cytotoxicity i.e., the mitochondrial membrane impairment and cell death, and genotoxic effect i.e., chromatin decondensation (Table S1).
- This study underlined that a full benefit–risk balance evaluation of the MWCNTs post-treatments on bio-toxicity should be carried out if a “safer by design” approach is attempted.
- The methodology developed for this work could be relevant for acute or chronic nanotoxicological studies of other nanomaterials, and further thorough determination of surface properties impact on oxidative stress, cytotoxicity, and genotoxicity.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tsuzuki, T. Commercial scale production of inorganic nanoparticles. Int. J. Nanotechnol. 2009, 6, 567–578. [Google Scholar] [CrossRef]
- Roco, M.C. The long view of nanotechnology development: The National Nanotechnology Initiative at 10 years. J. Nanopart. Res. 2011, 13, 427–445. [Google Scholar] [CrossRef]
- De Volder, M.F.L.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon nanotubes: Present and future commercial applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Baughman, R.H.; Zakhidov, A.A.; de Heer, W.A. Carbon nanotubes—The route toward applications. Science 2002, 297, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Tabish, T.A.; Zhang, S.; Winyard, P.G. Developing the next generation of graphene-based platforms for cancer therapeutics: The potential role of reactive oxygen species. Redox Biol. 2018, 15, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Sims, C.M.; Hanna, S.K.; Heller, D.A.; Horoszko, C.P.; Johnson, M.E.; Montoro Bustos, A.R.; Reipa, V.; Riley, K.R.; Nelson, B.C. Redox-active nanomaterials for nanomedicine applications. Nanoscale 2017, 9, 15226–15251. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Kostarelos, K.; Prato, M. Applications of carbon nanotubes in drug delivery. Curr. Opin. Chem. Biol. 2005, 9, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Mundra, R.V.; Wu, X.; Sauer, J.; Dordick, J.S.; Kane, R.S. Nanotubes in biological applications. Curr. Opin. Biotechnol. 2014, 28, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Ostiguy, C.; Roberge, B.; Woods, C.; Soucy, B. Engineered Nanoparticles: Current Knowledge about Occupational Health and Safety Risks and Prevention Measures. Studies and Research Projects/Report R-656, Montréal, IRSST. 2010. Available online: http://www.irsst.qc.ca/media/documents/PubIRSST/R-656.pdf (accessed on 15 December 2019).
- Ostiquy, C.; Lapointe, G.; Troltier, M.; Menard, L.; Cloutier, Y.; Boutin, M.; Antoun, M.; Normand, C. Health effect nanoparticles. Studies and Research Projects/Report R-469 Montréal, IRSST. 2006. Available online: https://www.irsst.qc.ca/media/documents/PubIRSST/R-469.pdf (accessed on 15 December 2019).
- ANSES Evaluation des risques liés aux nanomatériaux. ANSES, Edition scientifique, April 2014. Report. Available online: http://www.alimentation-sante.org/wp-content/uploads/2014/05/AP2012sa0273Ra.pdf (accessed on 15 December 2019).
- Madannejad, R.; Shoaie, N.; Jahanpeyma, F.; Darvishi, M.H.; Azimzadeh, M.; Javadi, H. Toxicity of carbon-based nanomaterials: Reviewing recent reports in medical and biological systems. Chem. Biol. Interact. 2019, 307, 206–222. [Google Scholar] [CrossRef]
- Reilly, R.M. Carbon nanotubes: Potential benefits and risks of nanotechnology in nuclear medicine. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2007, 48, 1039–1042. [Google Scholar] [CrossRef]
- Ganesh, E.N. Single Walled and Multi Walled Carbon Nanotube Structure, Synthesis and Applications. Int. J. Innov. Technol. Explor. Eng. (IJITEE). 2013, 2, 311–320. [Google Scholar]
- Higashisaka, K.; Nagano, K.; Yoshioka, Y.; Tsutsumi, Y. Nano-safety Research: Examining the Associations among the Biological Effects of Nanoparticles and Their Physicochemical Properties and Kinetics. Biol. Pharm. Bull. 2017, 40, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.P.; Xia, Q.; Hwang, H.-M.; Ray, P.C.; Yu, H. Mechanisms of nanotoxicity: Generation of reactive oxygen species. J. Food Drug Anal. 2014, 22, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Lanone, S.; Andujar, P.; Kermanizadeh, A.; Boczkowski, J. Determinants of carbon nanotube toxicity. Adv. Drug Deliv. Rev. 2013, 65, 2063–2069. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, K.; Aitken, R.; Tran, L.; Stone, V.; Duffin, R.; Forrest, G.; Alexander, A. Carbon nanotubes: A review of their properties in relation to pulmonary toxicology and workplace safety. Toxicol. Sci. Off. J. Soc. Toxicol. 2006, 92, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Simon-Deckers, A. Effets biologiques de nanoparticules manufacturées: Influence de leurs caractéristiques. Ph.D. Thesis, AgroParisTech, Paris, France, 2008. [Google Scholar]
- Fraczek-Szczypta, A.; Menaszek, E.; Syeda, T.B.; Misra, A.; Alavijeh, M.; Adu, J.; Blazewicz, S. Effect of MWCNT surface and chemical modification on in vitro cellular response. J. Nanopart. Res. Interdiscip. Forum Nanoscale Sci. Technol. 2012, 14, 1181. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Yoshioka, Y.; Higashisaka, K.; Morishita, Y.; Yoshida, T.; Fujimura, M.; Kayamuro, H.; Nabeshi, H.; Yamashita, T.; Nagano, K.; et al. Carbon nanotubes elicit DNA damage and inflammatory response relative to their size and shape. Inflammation 2010, 33, 276–280. [Google Scholar] [CrossRef]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef]
- Khanna, P.; Ong, C.; Bay, B.H.; Baeg, G.H. Nanotoxicity: An Interplay of Oxidative Stress, Inflammation and Cell Death. Nanomaterials. 2015, 5, 1163–1180. [Google Scholar] [CrossRef]
- Ahmadi, H.; Ramezani, M.; Yazdian-Robati, R.; Behnam, B.; Razavi Azarkhiavi, K.; Hashem Nia, A.; Mokhtarzadeh, A.; Matbou Riahi, M.; Razavi, B.M.; Abnous, K. Acute toxicity of functionalized single wall carbon nanotubes: A biochemical, histopathologic and proteomics approach. Chem. Biol. Interact. 2017, 275, 196–209. [Google Scholar] [CrossRef]
- Nahle, S.; Safar, R.; Grandemange, S.; Foliguet, B.; Lovera-Leroux, M.; Doumandji, Z.; Le Faou, A.; Joubert, O.; Rihn, B.; Ferrari, L. Single wall and multiwall carbon nanotubes induce different toxicological responses in rat alveolar macrophages. J. Appl. Toxicol. JAT 2019, 39, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Liu, Y.; Song, W.M.; Hayashi, Y.; Ding, X.C.; Li, W.H. In vitro evaluation of cytotoxicity and oxidative stress induced by multiwalled carbon nanotubes in murine RAW 264.7 macrophages and human A549 lung cells. Biomed. Environ. Sci. BES 2011, 24, 593–601. [Google Scholar] [PubMed]
- Visalli, G.; Facciolà, A.; Currò, M.; Laganà, P.; La Fauci, V.; Iannazzo, D.; Pistone, A.; Di Pietro, A. Mitochondrial Impairment Induced by Sub-Chronic Exposure to Multi-Walled Carbon Nanotubes. Int. J. Environ. Res. Public Health 2019, 16, 792. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; Sharma, P.K.; Tiwari, R.; Rayavarapu, R.G.; Shankar, J.; Chauhan, L.K.S.; Pandey, A.K. Impaired lysosomal activity mediated autophagic flux disruption by graphite carbon nanofibers induce apoptosis in human lung epithelial cells through oxidative stress and energetic impairment. Part. Fibre Toxicol. 2017, 14, 15. [Google Scholar] [CrossRef] [PubMed]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. BioMed Res. Int. 2013, 2013, 942916. [Google Scholar] [CrossRef] [PubMed]
- Gardès-Albert, M.; Bonnefont-Rousselot, D.; Abedinzadeh, Z. Espèces réactives de l’oxygène. Actual. Chim. Méc. Biochim. 2003, 91–96. [Google Scholar]
- Kagan, V.E.; Tyurina, Y.Y.; Tyurin, V.A.; Konduru, N.V.; Potapovich, A.I.; Osipov, A.N.; Kisin, E.R.; Schwegler-Berry, D.; Mercer, R.; Castranova, V.; et al. Direct and indirect effects of single walled carbon nanotubes on RAW 264.7 macrophages: Role of iron. Toxicol. Lett. 2006, 165, 88–100. [Google Scholar] [CrossRef]
- Turci, F.; Tomatis, M.; Lesci, I.G.; Roveri, N.; Fubini, B. The iron-related molecular toxicity mechanism of synthetic asbestos nanofibres: A model study for high-aspect-ratio nanoparticles. Chem. Weinh. Bergstr. Ger. 2011, 17, 350–358. [Google Scholar] [CrossRef]
- Bussy, C.; Paineau, E.; Cambedouzou, J.; Brun, N.; Mory, C.; Fayard, B.; Salomé, M.; Pinault, M.; Huard, M.; Belade, E.; et al. Intracellular fate of carbon nanotubes inside murine macrophages: pH-dependent detachment of iron catalyst nanoparticles. Part. Fibre Toxicol. 2013, 10, 24. [Google Scholar] [CrossRef]
- Meng, L.; Jiang, A.; Chen, R.; Li, C.; Wang, L.; Qu, Y.; Wang, P.; Zhao, Y.; Chen, C. Inhibitory effects of multiwall carbon nanotubes with high iron impurity on viability and neuronal differentiation in cultured PC12 cells. Toxicology 2013, 313, 49–58. [Google Scholar] [CrossRef]
- Girardello, R.; Baranzini, N.; Tettamanti, G.; de Eguileor, M.; Grimaldi, A. Cellular responses induced by multi-walled carbon nanotubes: In vivo and in vitro studies on the medicinal leech macrophages. Sci. Rep. 2017, 7, 8871. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.-X.; Wan, B.; Guo, L.-H. In vitro toxicity of acid-functionalized single-walled carbon nanotubes: Effects on murine macrophages and gene expression profiling. Nanotoxicology 2012, 6, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Montes-Fonseca, S.L.; Orrantia-Borunda, E.; Aguilar-Elguezabal, A.; González Horta, C.; Talamás-Rohana, P.; Sánchez-Ramírez, B. Cytotoxicity of functionalized carbon nanotubes in J774A macrophages. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Fu, Y.; Wei, T.; Le Guyader, L.; Gao, G.; Liu, R.-S.; Chang, Y.-Z.; Chen, C. The triggering of apoptosis in macrophages by pristine graphene through the MAPK and TGF-beta signaling pathways. Biomaterials 2012, 33, 402–411. [Google Scholar] [CrossRef]
- Ursini, C.L.; Maiello, R.; Ciervo, A.; Fresegna, A.M.; Buresti, G.; Superti, F.; Marchetti, M.; Iavicoli, S.; Cavallo, D. Evaluation of uptake, cytotoxicity and inflammatory effects in respiratory cells exposed to pristine and -OH and -COOH functionalized multi-wall carbon nanotubes. J. Appl. Toxicol. JAT 2016, 36, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E.; Meindl, C.; Höfler, A.; Leitinger, G.; Roblegg, E. Combination of small size and carboxyl functionalisation causes cytotoxicity of short carbon nanotubes. Nanotoxicology 2013, 7, 1211–1224. [Google Scholar] [CrossRef] [PubMed]
- Schrurs, F.; Lison, D. Focusing the research efforts. Nat. Nanotechnol. 2012, 7, 546–548. [Google Scholar] [CrossRef]
- Cha, C.; Shin, S.R.; Annabi, N.; Dokmeci, M.R.; Khademhosseini, A. Carbon-based nanomaterials: Multifunctional materials for biomedical engineering. ACS Nano 2013, 7, 2891–2897. [Google Scholar] [CrossRef]
- Figarol, A.; Pourchez, J.; Boudard, D.; Forest, V.; Tulliani, J.-M.; Lecompte, J.-P.; Cottier, M.; Bernache-Assollant, D.; Grosseau, P. Biological response to purification and acid functionalization of carbon nanotubes. J. Nanopart. Res. 2014, 16, 1–12. [Google Scholar] [CrossRef]
- Bussy, C.; Pinault, M.; Cambedouzou, J.; Landry, M.J.; Jegou, P.; Mayne-L’hermite, M.; Launois, P.; Boczkowski, J.; Lanone, S. Critical role of surface chemical modifications induced by length shortening on multi-walled carbon nanotubes-induced toxicity. Part. Fibre Toxicol. 2012, 9, 46. [Google Scholar] [CrossRef]
- Jain, S.; Thakare, V.S.; Das, M.; Godugu, C.; Jain, A.K.; Mathur, R.; Chuttani, K.; Mishra, A.K. Toxicity of multiwalled carbon nanotubes with end defects critically depends on their functionalization density. Chem. Res. Toxicol. 2011, 24, 2028–2039. [Google Scholar] [CrossRef] [PubMed]
- Fenoglio, I.; Greco, G.; Tomatis, M.; Muller, J.; Raymundo-Piñero, E.; Béguin, F.; Fonseca, A.; Nagy, J.B.; Lison, D.; Fubini, B. Structural defects play a major role in the acute lung toxicity of multiwall carbon nanotubes: Physicochemical aspects. Chem. Res. Toxicol. 2008, 21, 1690–1697. [Google Scholar] [CrossRef] [PubMed]
- Figarol, A.; Pourchez, J.; Boudard, D.; Forest, V.; Berhanu, S.; Tulliani, J.-M.; Lecompte, J.-P.; Cottier, M.; Bernache-Assollant, D.; Grosseau, P. Thermal annealing of carbon nanotubes reveals a toxicological impact of the structural defects. J. Nanopart. Res. 2015, 17, 1–14. [Google Scholar] [CrossRef]
- Zhang, T.; Tang, M.; Kong, L.; Li, H.; Zhang, T.; Zhang, S.; Xue, Y.; Pu, Y. Comparison of cytotoxic and inflammatory responses of pristine and functionalized multi-walled carbon nanotubes in RAW 264.7 mouse macrophages. J. Hazard. Mater. 2012, 219–220, 203–212. [Google Scholar] [CrossRef]
- Figarol, A.; Pourchez, J.; Boudard, D.; Forest, V.; Akono, C.; Tulliani, J.-M.; Lecompte, J.-P.; Cottier, M.; Bernache-Assollant, D.; Grosseau, P. In vitro toxicity of carbon nanotubes, nano-graphite and carbon black, similar impacts of acid functionalization. Toxicol. Vitro Int. J. Publ. Assoc. BIBRA 2015, 30, 476–485. [Google Scholar] [CrossRef]
- Dhawan, A.; Sharma, V. Toxicity assessment of nanomaterials: Methods and challenges. Anal. Bioanal. Chem. 2010, 398, 589–605. [Google Scholar] [CrossRef]
- Kroll, A.; Pillukat, M.H.; Hahn, D.; Schnekenburger, J. Current in vitro methods in nanoparticle risk assessment: Limitations and challenges. Eur. J. Pharm. Biopharm. 2009, 72, 370–377. [Google Scholar] [CrossRef]
- Kroll, A.; Pillukat, M.H.; Hahn, D.; Schnekenburger, J. Interference of engineered nanoparticles with in vitro toxicity assays. Arch. Toxicol. 2012, 86, 1123–1136. [Google Scholar] [CrossRef]
- Monteiro-Riviere, N.A.; Inman, A.O.; Zhang, L.W. Limitations and relative utility of screening assays to assess engineered nanoparticle toxicity in a human cell line. Toxicol. Appl. Pharmacol. 2009, 234, 222–235. [Google Scholar] [CrossRef]
- Ali-Boucetta, H.; Al-Jamal, K.T.; Kostarelos, K. Cytotoxic assessment of carbon nanotube interaction with cell cultures. Methods Mol. Biol. Clifton NJ 2011, 726, 299–312. [Google Scholar]
- Forest, V.; Figarol, A.; Boudard, D.; Cottier, M.; Grosseau, P.; Pourchez, J. Adsorption of Lactate Dehydrogenase Enzyme on Carbon Nanotubes: How to Get Accurate Results for the Cytotoxicity of These Nanomaterials. Langmuir 2015, 31, 3635–3643. [Google Scholar] [CrossRef]
- Wörle-Knirsch, J.M.; Pulskamp, K.; Krug, H.F. Oops they did it again! Carbon nanotubes hoax scientists in viability assays. Nano Lett. 2006, 6, 1261–1268. [Google Scholar] [CrossRef]
- Casey, E.H. Spectroscopic analysis confirms the interaction between SWCNT and various dyes commonly used to assess cytotoxicity. Carbon 2007, 45, 1425–1432. [Google Scholar] [CrossRef]
- Belyanskaya, L.; Manser, P.; Spohn, P.; Bruinink, A.; Wick, P. The reliability and limits of the MTT reduction assay for carbon nanotubes–cell interaction. Carbon 2007, 45, 2643–2648. [Google Scholar] [CrossRef]
- Shapiro, H.M. Practical Flow Cytometry; John Wiley & Sons: Hoboken, NJ, USA, 2005; ISBN 978-0-471-43403-0. [Google Scholar]
- Reardon, A.J.F.; Elliott, J.A.W.; McGann, L.E. Fluorescence as an alternative to light-scatter gating strategies to identify frozen-thawed cells with flow cytometry. Cryobiology 2014, 69, 91–99. [Google Scholar] [CrossRef]
- Al-Rubeai, M.; Welzenbach, K.; Lloyd, D.R.; Emery, A.N. A rapid method for evaluation of cell number and viability by flow cytometry. Cytotechnology 1997, 24, 161–168. [Google Scholar] [CrossRef]
- Kermanizadeh, A.; Jantzen, K.; Brown, D.M.; Møller, P.; Loft, S. A Flow Cytometry-based Method for the Screening of Nanomaterial-induced Reactive Oxygen Species Production in Leukocytes Subpopulations in Whole Blood. Basic Clin. Pharmacol. Toxicol. 2018, 122, 149–156. [Google Scholar] [CrossRef]
- Al-Jamal, K.T.; Kostarelos, K. Assessment of cellular uptake and cytotoxicity of carbon nanotubes using flow cytometry. Methods Mol. Biol. Clifton NJ 2010, 625, 123–134. [Google Scholar]
- Cai, D.; Blair, D.; Dufort, F.J.; Gumina, M.R.; Huang, Z.; Hong, G.; Wagner, D.; Canahan, D.; Kempa, K.; Ren, Z.F.; et al. Interaction between carbon nanotubes and mammalian cells: Characterization by flow cytometry and application. Nanotechnology 2008, 19, 1–10. [Google Scholar] [CrossRef]
- Zeinabad, H.A.; Zarrabian, A.; Saboury, A.A.; Alizadeh, A.M.; Falahati, M. Interaction of single and multi wall carbon nanotubes with the biological systems: Tau protein and PC12 cells as targets. Sci. Rep. 2016, 6, 26508. [Google Scholar] [CrossRef]
- Gomes, A.; Fernandes, E.; Lima, J.L.F.C. Fluorescence probes used for detection of reactive oxygen species. J. Biochem. Biophys. Methods 2005, 65, 45–80. [Google Scholar] [CrossRef] [PubMed]
- Eruslanov, E.; Kusmartsev, S. Identification of ROS using oxidized DCFDA and flow-cytometry. Methods Mol. Biol. Clifton NJ 2010, 594, 57–72. [Google Scholar]
- ThermoFisher Scientific Fluorescence Response of APF, HPF and H2DCFDA to Various Reactive Oxygen Species (ROS)—Table 18.4 – FR. Available online: https://www.thermofisher.com/fr/fr/home/references/molecular-probes-the-handbook/tables/fluorescence-response-of-3-p-aminophenyl-fluorescein-apf-3-p-hydroxyphenyl-fluorescein-hpf-and-dichlorodihydrofluorescein-diacetate-h2dcfda-to-various-reactive-oxygen-species-ros.html (accessed on 24 December 2019).
- ThermoFisher scientific Generating and Detecting Reactive Oxygen Species—Section 18.2 – FR. Available online: https://www.thermofisher.com/fr/fr/home/references/molecular-probes-the-handbook/probes-for-reactive-oxygen-species-including-nitric-oxide/generating-and-detecting-reactive-oxygen-species.html (accessed on 24 December 2019).
- ThermoFisher Scientific Reactive Oxygen Species—Table 18.1 – FR. Available online: https://www.thermofisher.com/fr/fr/home/references/molecular-probes-the-handbook/tables/reactive-oxygen-species.html (accessed on 24 December 2019).
- Marchetti, C.; Obert, G.; Deffosez, A.; Formstecher, P.; Marchetti, P. Study of mitochondrial membrane potential, reactive oxygen species, DNA fragmentation and cell viability by flow cytometry in human sperm. Hum. Reprod. Oxf. Engl. 2002, 17, 1257–1265. [Google Scholar] [CrossRef]
- Fenoglio, I.; Tomatis, M.; Lison, D.; Muller, J.; Fonseca, A.; Nagy, J.B.; Fubini, B. Reactivity of carbon nanotubes: Free radical generation or scavenging activity? Free Radic. Biol. Med. 2006, 40, 1227–1233. [Google Scholar] [CrossRef]
- Galano, A. Carbon nanotubes: Promising agents against free radicals. Nanoscale 2010, 2, 373–380. [Google Scholar] [CrossRef]
- Czarny, B.; Georgin, D.; Berthon, F.; Plastow, G.; Pinault, M.; Patriarche, G.; Thuleau, A.; L’Hermite, M.M.; Taran, F.; Dive, V. Carbon nanotube translocation to distant organs after pulmonary exposure: Insights from in situ (14)C-radiolabeling and tissue radioimaging. ACS Nano 2014, 8, 5715–5724. [Google Scholar] [CrossRef]
- Shvedova, A.A.; Kisin, E.R.; Mercer, R.; Murray, A.R.; Johnson, V.J.; Potapovich, A.I.; Tyurina, Y.Y.; Gorelik, O.; Arepalli, S.; Schwegler-Berry, D.; et al. Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 289, L698–L708. [Google Scholar] [CrossRef]
- Lin, J.; Litster, A. Fluorescence flow cytometry methodology to exclude platelet aggregate interference when measuring feline CD4 and CD8 lymphocyte counts. Vet. J. 2013, 198, 275–278. [Google Scholar] [CrossRef]
- Jahnke, V.E.; Sabido, O.; Freyssenet, D. Control of mitochondrial biogenesis, ROS level, and cytosolic Ca2+ concentration during the cell cycle and the onset of differentiation in L6E9 myoblasts. Am. J. Physiol. Cell Physiol. 2009, 296, C1185–C1194. [Google Scholar] [CrossRef]
- ThermoFisher Scientific Probes for Mitochondria—Section 12.2 – FR. Available online: https://www.thermofisher.com/fr/fr/home/references/molecular-probes-the-handbook/probes-for-organelles/probes-for-mitochondria.html (accessed on 24 December 2019).
- Pasteur, X.; Sabido, O.; Maubon, I.; Perrin-Cottier, M.; Laurent, J.L. Quantitative assessment of chromatin stability alteration in human spermatozoa induced by freezing and thawing. A flow cytometric study. Anal. Quant. Cytol. Histol. Int. Acad. Cytol. Am. Soc. Cytol. 1991, 13, 383–390. [Google Scholar]
- Aldieri, E.; Fenoglio, I.; Cesano, F.; Gazzano, E.; Gulino, G.; Scarano, D.; Attanasio, A.; Mazzucco, G.; Ghigo, D.; Fubini, B. The role of iron impurities in the toxic effects exerted by short multiwalled carbon nanotubes (MWCNT) in murine alveolar macrophages. J. Toxicol. Environ. Health A 2013, 76, 1056–1071. [Google Scholar] [CrossRef] [PubMed]
- Pulskamp, K.; Diabaté, S.; Krug, H.F. Carbon nanotubes show no sign of acute toxicity but induce intracellular reactive oxygen species in dependence on contaminants. Toxicol. Lett. 2007, 168, 58–74. [Google Scholar] [CrossRef]
- Ruipérez, F.; Mujika, J.I.; Ugalde, J.M.; Exley, C.; Lopez, X. Pro-oxidant activity of aluminum: Promoting the Fenton reaction by reducing Fe(III) to Fe(II). J. Inorg. Biochem. 2012, 117, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.-S.; Wu, R.; Jafvert, C.T. Light-Independent Reactive Oxygen Species (ROS) Formation through Electron Transfer from Carboxylated Single-Walled Carbon Nanotubes in Water. Environ. Sci. Technol. 2014, 48, 11330–11336. [Google Scholar] [CrossRef]
- Snyder, R.J.; Verhein, K.C.; Vellers, H.L.; Burkholder, A.B.; Garantziotis, S.; Kleeberger, S.R. Multi-walled carbon nanotubes upregulate mitochondrial gene expression and trigger mitochondrial dysfunction in primary human bronchial epithelial cells. Nanotoxicology 2019, 13, 1344–1361. [Google Scholar] [CrossRef]
- Pescatori, M.; Bedognetti, D.; Venturelli, E.; Ménard-Moyon, C.; Bernardini, C.; Muresu, E.; Piana, A.; Maida, G.; Manetti, R.; Sgarrella, F.; et al. Functionalized carbon nanotubes as immunomodulator systems. Biomaterials 2013, 34, 4395–4403. [Google Scholar] [CrossRef]
- Doak, S.H.; Manshian, B.; Jenkins, G.J.S.; Singh, N. In vitro genotoxicity testing strategy for nanomaterials and the adaptation of current OECD guidelines. Mutat. Res. Toxicol. Environ. Mutagen. 2012, 745, 104–111. [Google Scholar] [CrossRef]
- Siegrist, K.J.; Reynolds, S.H.; Porter, D.W.; Mercer, R.R.; Bauer, A.K.; Lowry, D.; Cena, L.; Stueckle, T.A.; Kashon, M.L.; Wiley, J.; et al. Mitsui-7, heat-treated, and nitrogen-doped multi-walled carbon nanotubes elicit genotoxicity in human lung epithelial cells. Part. Fibre Toxicol. 2019, 16. [Google Scholar] [CrossRef]
| PHYSICOCHEMICAL CHARACTERISTICS | CNT | CNTf | CNTa |
|---|---|---|---|
| Diameter (nm) with FEG-SEM and TEM | 17 ± 5 | 18 ± 5 | 17 ± 5 |
| O (% atomic proportion) by XPS | 8.7 | 7.7 | 3.5 |
| SSA (m2 g−1) using BET method | 212 ± 2 | 279 ± 1 | 209 ± 3 |
| Catalytic iron metallic impurities (wt%) by XPS and ICP-AES | 0.19 | 0.02 | 0.01 |
| Structural defects indication (Id/Ig ratio) with Raman spectroscopy | 1.18 ± 0.10 | 1.33 | 0.77 ± 0.13 |
| Zeta potential in water (mV) | −23.3 ± 7 | −28.7 ± 2.1 | −36.4 ± 4.3 |
| Isoelectric point in water (pH) | 3.12 ± 0.32 | 2.6 ± 0.1 | 2.82 ± 0.02 |
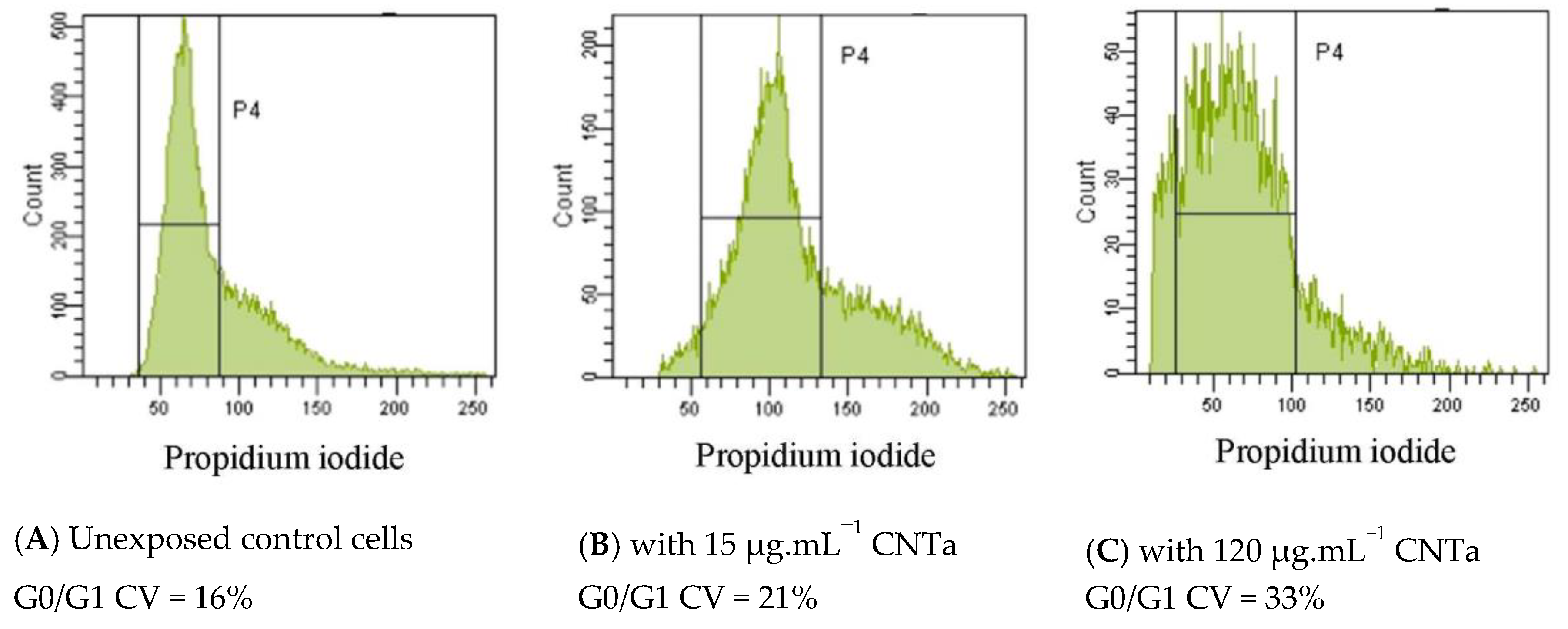
| CV Ratio (mean + SD) | |
|---|---|
| CNT 15 μg·mL−1 | 1.3 ± 0.2 |
| CNT 120 μg·mL−1 | 1.5 ± 0.3 * |
| CNTf 15 μg·mL−1 | 1.4 ± 0.3 |
| CNTf 120 μg·mL−1 | 1.8 ± 0.3 * |
| CNTa 15 μg·mL−1 | 1.4 ± 0.1 |
| CNTa 120 μg·mL−1 | 1.9 ± 0.3 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabido, O.; Figarol, A.; Klein, J.-P.; Bin, V.; Forest, V.; Pourchez, J.; Fubini, B.; Cottier, M.; Tomatis, M.; Boudard, D. Quantitative Flow Cytometric Evaluation of Oxidative Stress and Mitochondrial Impairment in RAW 264.7 Macrophages after Exposure to Pristine, Acid Functionalized, or Annealed Carbon Nanotubes. Nanomaterials 2020, 10, 319. https://doi.org/10.3390/nano10020319
Sabido O, Figarol A, Klein J-P, Bin V, Forest V, Pourchez J, Fubini B, Cottier M, Tomatis M, Boudard D. Quantitative Flow Cytometric Evaluation of Oxidative Stress and Mitochondrial Impairment in RAW 264.7 Macrophages after Exposure to Pristine, Acid Functionalized, or Annealed Carbon Nanotubes. Nanomaterials. 2020; 10(2):319. https://doi.org/10.3390/nano10020319
Chicago/Turabian StyleSabido, Odile, Agathe Figarol, Jean-Philippe Klein, Valérie Bin, Valérie Forest, Jérémie Pourchez, Bice Fubini, Michèle Cottier, Maura Tomatis, and Delphine Boudard. 2020. "Quantitative Flow Cytometric Evaluation of Oxidative Stress and Mitochondrial Impairment in RAW 264.7 Macrophages after Exposure to Pristine, Acid Functionalized, or Annealed Carbon Nanotubes" Nanomaterials 10, no. 2: 319. https://doi.org/10.3390/nano10020319
APA StyleSabido, O., Figarol, A., Klein, J.-P., Bin, V., Forest, V., Pourchez, J., Fubini, B., Cottier, M., Tomatis, M., & Boudard, D. (2020). Quantitative Flow Cytometric Evaluation of Oxidative Stress and Mitochondrial Impairment in RAW 264.7 Macrophages after Exposure to Pristine, Acid Functionalized, or Annealed Carbon Nanotubes. Nanomaterials, 10(2), 319. https://doi.org/10.3390/nano10020319