Synthesis of Magnetic Wires from Polyol-Derived Fe-Glycolate Wires
Abstract
1. Introduction
2. Experiment
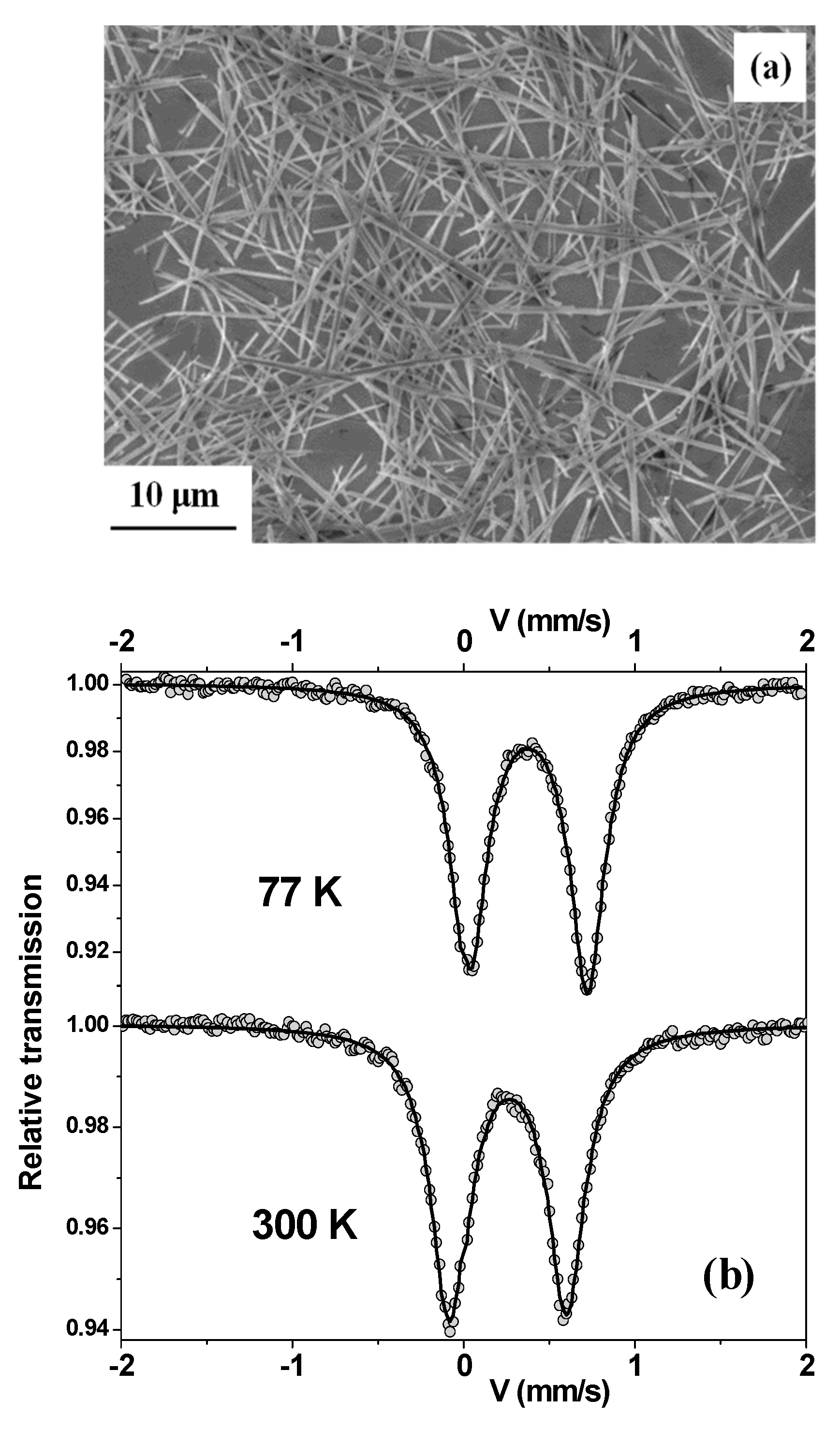
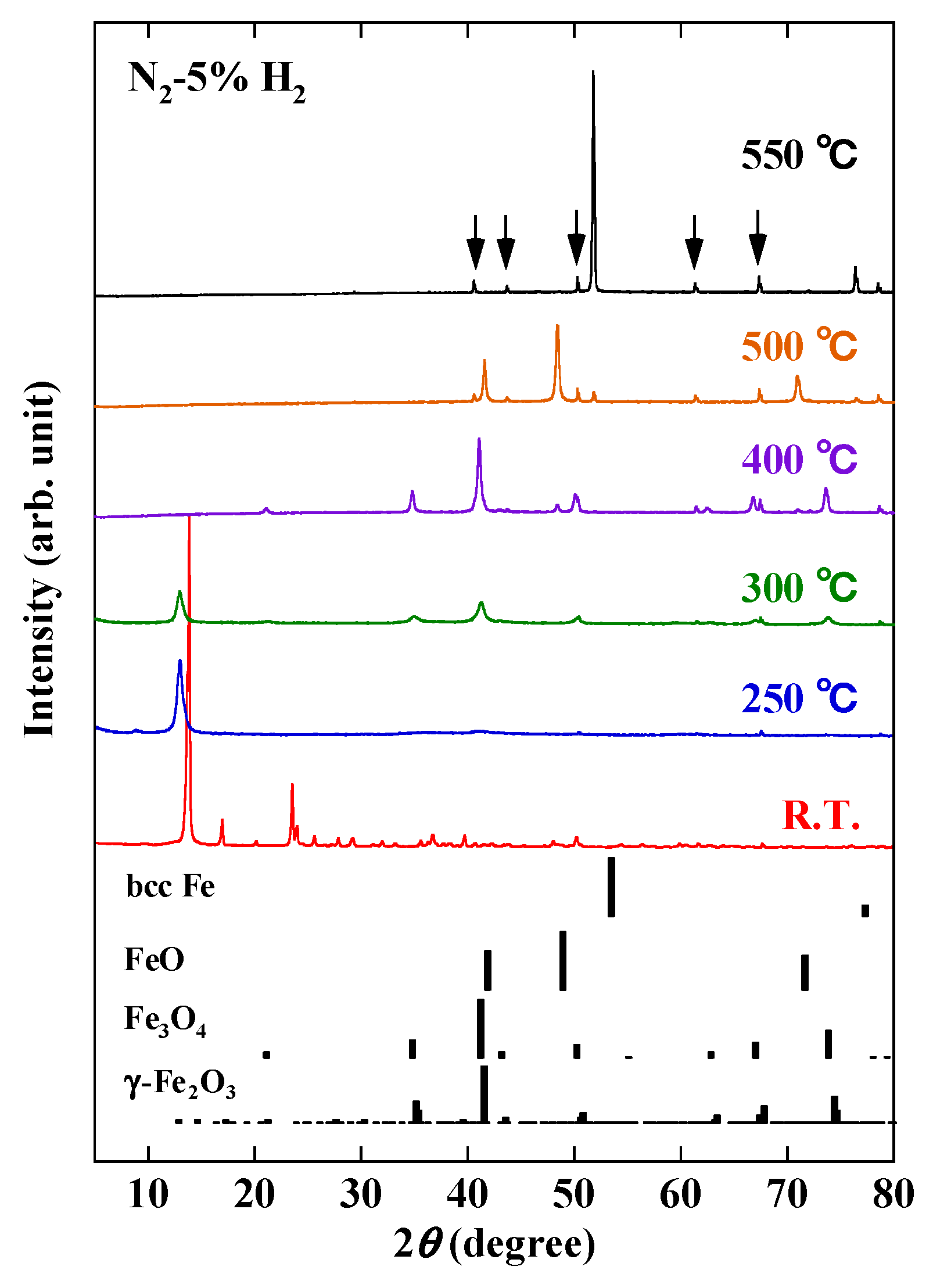
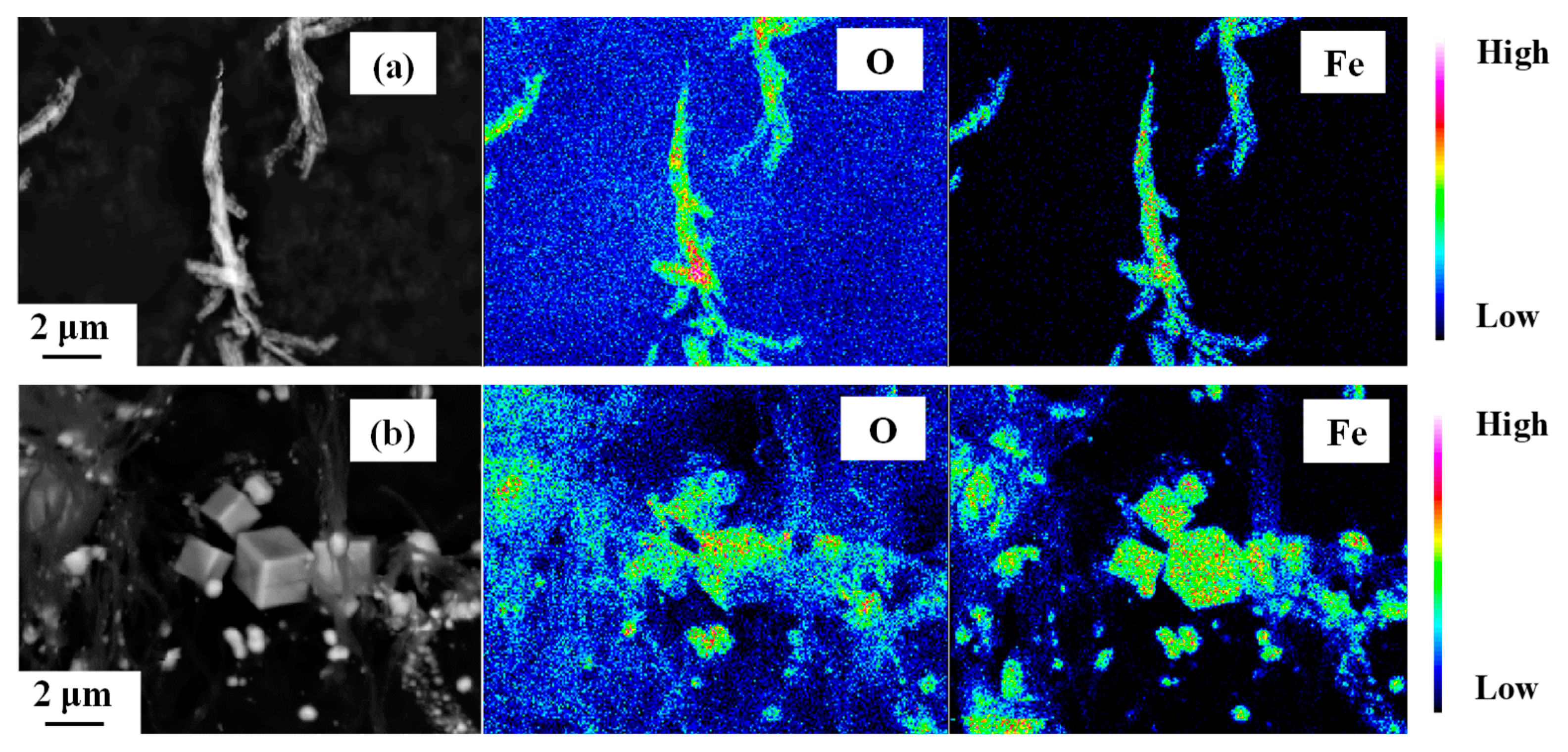
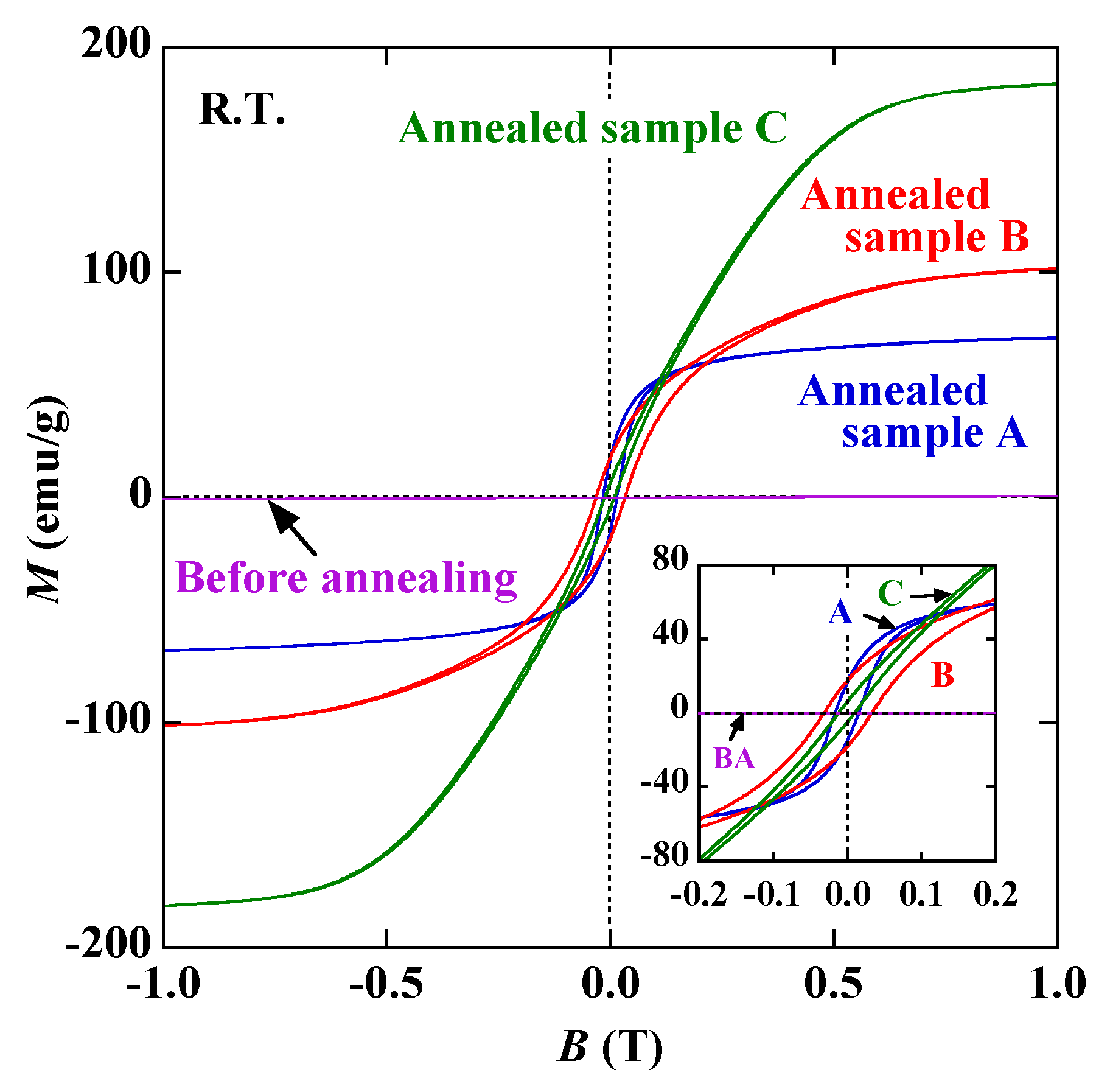
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jamal, M.; Hasan, M.; Mathewson, A.; Razeeb, K.M. Disposable sensor based on enzyme-free Ni nanowire array electrode to detect glutamate. Biosens. Bioelectron. 2013, 40, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Soumare, Y.; Garcia, C.; Maurer, T.; Chaboussant, G.; Ott, F.; Fiévet, F.; Piquemal, J.Y.; Viau, G. Kinetically controlled synthesis of hexagonally close-packed cobalt nanorods with high magnetic coercivity. Adv. Funct. Mater. 2009, 19, 1971–1977. [Google Scholar] [CrossRef]
- Huang, B.; Tai, K.P.; Zhang, M.G.; Xiao, Y.R.; Dillon, S.J. Comparative study of Li and Na electrochemical reactions with iron oxide nanowires. Electrochim. Acta 2014, 118, 143–149. [Google Scholar] [CrossRef]
- Hong, H.Y.; Hu, L.; Li, M.; Zheng, J.W.; Sun, X.H.; Lu, X.H.; Cao, X.Q.; Lu, J.M.; Gu, H.W. Preparation of Pt@Fe2O3 nanowires and their catalysis of selective oxidation of olefins and alcohols. Chem. Eur. J. 2011, 17, 8726–8730. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Wang, J. Synthetic micro/nanomotors in drug delivery. Nanoscale 2014, 6, 10486–10494. [Google Scholar] [CrossRef] [PubMed]
- Fiévet, F.; Lagier, J.P.; Figlarz, M. Preparing monodisperse metal powders in micrometer and submicrometer sizes by the polyol process. MRS Bull. 1989, 14, 29–34. [Google Scholar] [CrossRef]
- Fiévet, F.; Ammer-Merah, S.; Brayner, R.; Chau, F.; Giraud, M.; Mammeri, F.; Peron, J.; Piquemal, J.Y.; Sicard, L.; Viau, G. The polyol process: A unique method for easy access to metal nanoparticles with tailored sizes, shapes and compositions. Chem. Soc. Rev. 2018, 47, 5187–5233. [Google Scholar] [CrossRef] [PubMed]
- Wiley, B.; Sun, Y.; Mayers, B.; Xia, Y. Shape-controlled synthesis of metal nanostructures: The case of silver. Chem. Eur. J. 2005, 11, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Lee, C.; Cho, S.; Yoo, J.; Piao, Y.; Kim, Y.S. Facile synthesis of oxidation-resistant copper nanowires toward solution-processable, flexible, foldable, and free-standing electrodes. Small 2014, 10, 5047–5052. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kim, K.S. Large-scale polyol synthesis of single-crystal bismuth nanowires and the role of NaOH in the synthesis process. Nanotechnology 2008, 19, 265303. [Google Scholar] [CrossRef] [PubMed]
- Ung, D.; Viau, G.; Ricolleau, C.; Warmont, F.; Gredin, P.; Fiévet, F. CoNi nanowires synthesized by heterogeneous nucleation in liquid polyo. Adv. Mater. 2005, 17, 338–344. [Google Scholar] [CrossRef]
- Anagnostopoulou, E.; Grindi, B.; Lacroix, L.M.; Ott, F.; Panagiotopoulos, I.; Viau, G. Dense arrays of cobalt nanorods as rare-earth free permanent magnets. Nanoscale 2016, 8, 4020–4029. [Google Scholar] [CrossRef] [PubMed]
- Greneche, J.M.; Varret, F.J. On the texture problem in Mössbauer spectroscopy. J. Phys. C: Solid State Phys. 1982, 15, 5333–5344. [Google Scholar] [CrossRef]
- Greneche, J.M.; Varret, F. A new method of general use to obtain random powder spectra in 57Fe Mössbauer spectroscopy: the rotating sample recording. J. Phys. Lett. 1982, 43, L233–L237. [Google Scholar] [CrossRef]
- Arizaga, G.G.C.; Satyanarayana, K.G.; Wypych, F. Layered hydroxide salts: Synthesis, properties and potential applications. Solid State Ion. 2007, 178, 1143–1162. [Google Scholar] [CrossRef]
- Millan, A.; Urtizberea, A.; Silva, N.J.O.; Palacio, F.; Amaral, S.; Snoeck, E.; Serin, V. Surface effects in maghemite nanoparticles. J. Magn. Magn. Mater. 2007, 312, L5–L9. [Google Scholar] [CrossRef]
- O’Handley, R.C. Modern Magnetic Materials; Wiley: New York, NY, USA, 2000. [Google Scholar]

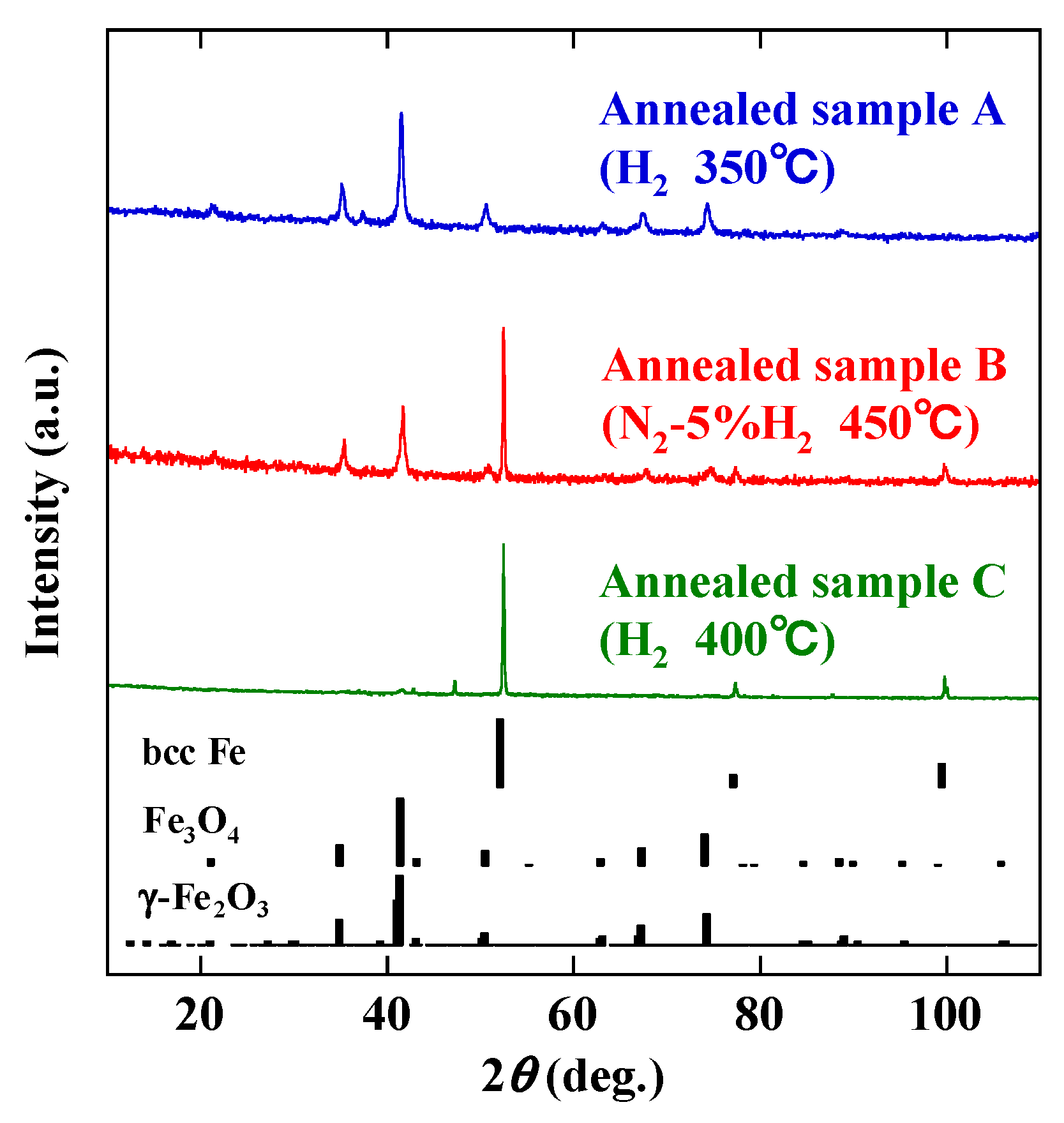
| Sample | Atmosphere | Temperature (°C) | Time (h) |
|---|---|---|---|
| A | H2 | 350 | 1 |
| B | N2-5% H2 | 450 | 1 |
| C | H2 | 400 | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujieda, S.; Gaudisson, T.; Grenèche, J.-M.; François, M.; Ammar, S. Synthesis of Magnetic Wires from Polyol-Derived Fe-Glycolate Wires. Nanomaterials 2020, 10, 318. https://doi.org/10.3390/nano10020318
Fujieda S, Gaudisson T, Grenèche J-M, François M, Ammar S. Synthesis of Magnetic Wires from Polyol-Derived Fe-Glycolate Wires. Nanomaterials. 2020; 10(2):318. https://doi.org/10.3390/nano10020318
Chicago/Turabian StyleFujieda, Shun, Thomas Gaudisson, Jean-Marc Grenèche, Michel François, and Souad Ammar. 2020. "Synthesis of Magnetic Wires from Polyol-Derived Fe-Glycolate Wires" Nanomaterials 10, no. 2: 318. https://doi.org/10.3390/nano10020318
APA StyleFujieda, S., Gaudisson, T., Grenèche, J.-M., François, M., & Ammar, S. (2020). Synthesis of Magnetic Wires from Polyol-Derived Fe-Glycolate Wires. Nanomaterials, 10(2), 318. https://doi.org/10.3390/nano10020318







