Cu2Se Nanoparticles Encapsulated by Nitrogen-Doped Carbon Nanofibers for Efficient Sodium Storage
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of Cu2Se-NC Nanofibers
2.2. Material Characterization
2.3. Electrochemical Measurements
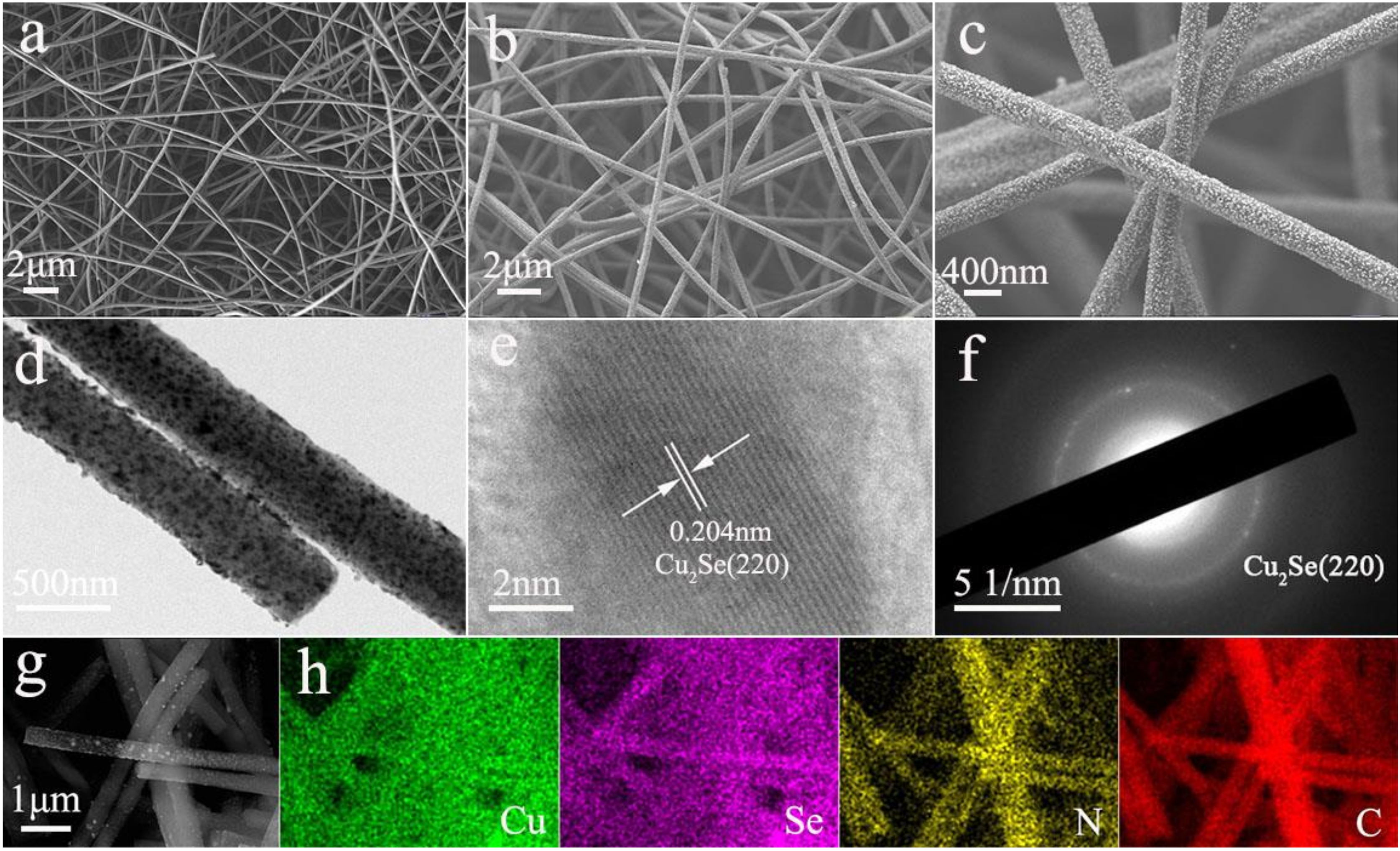
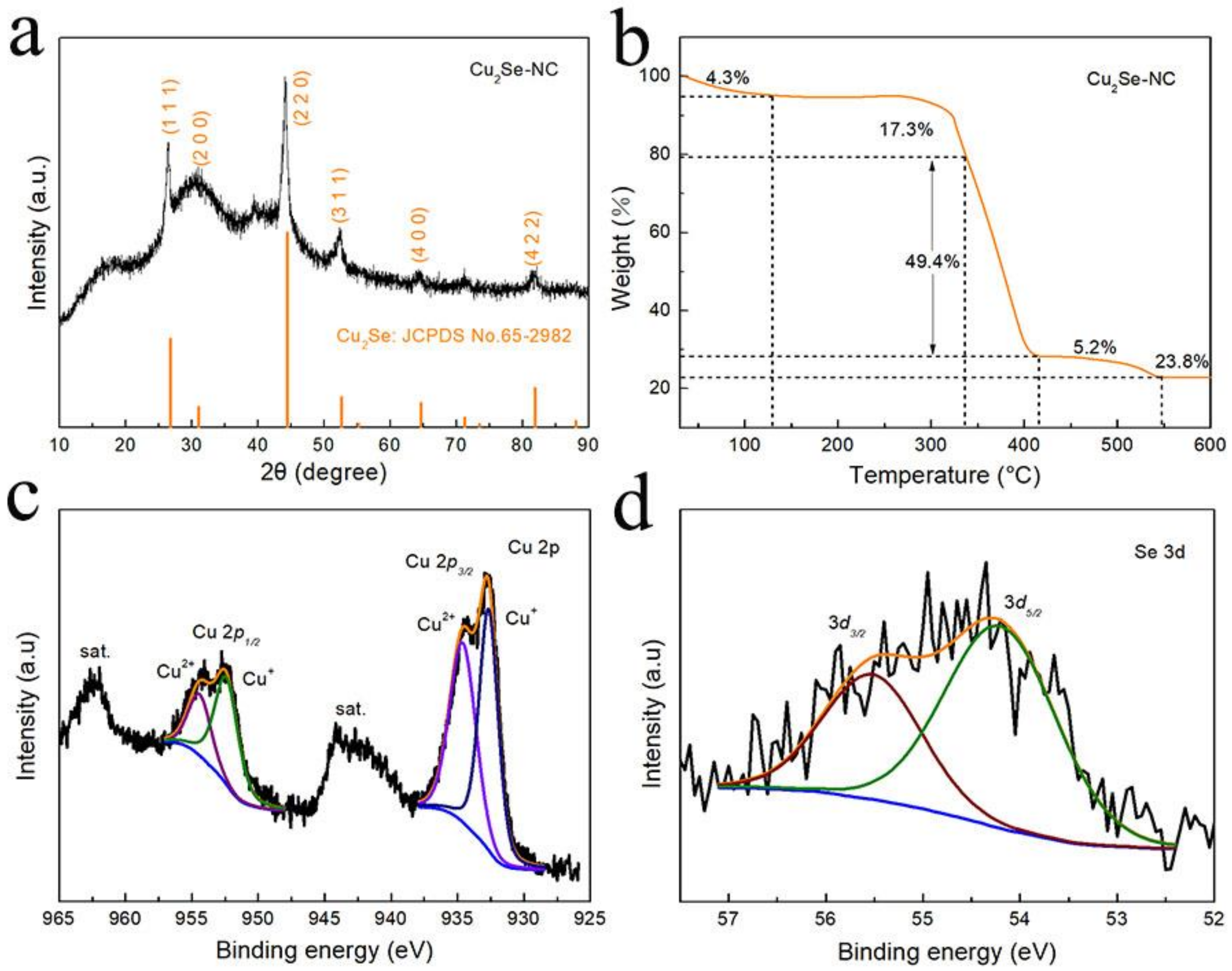
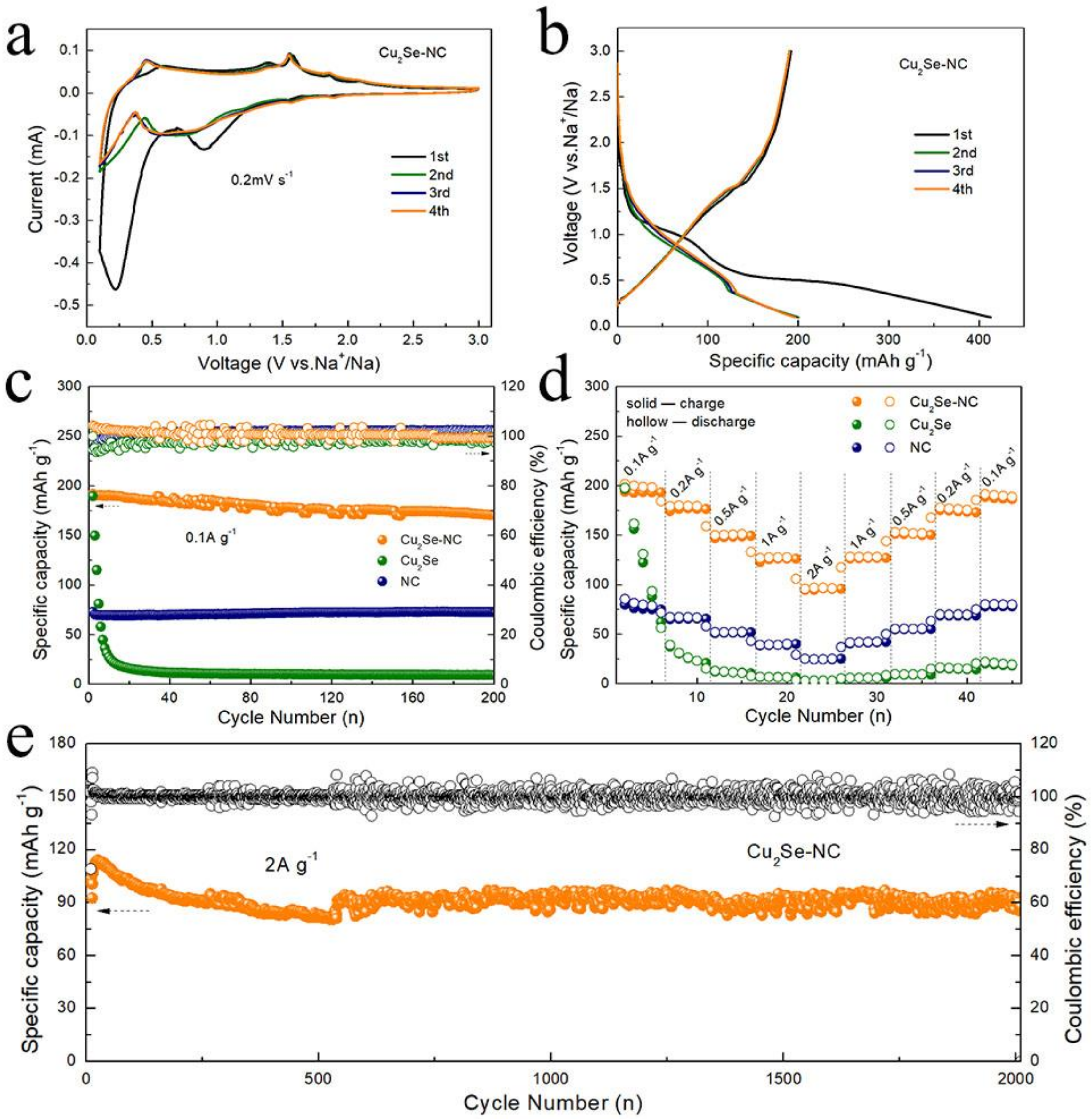
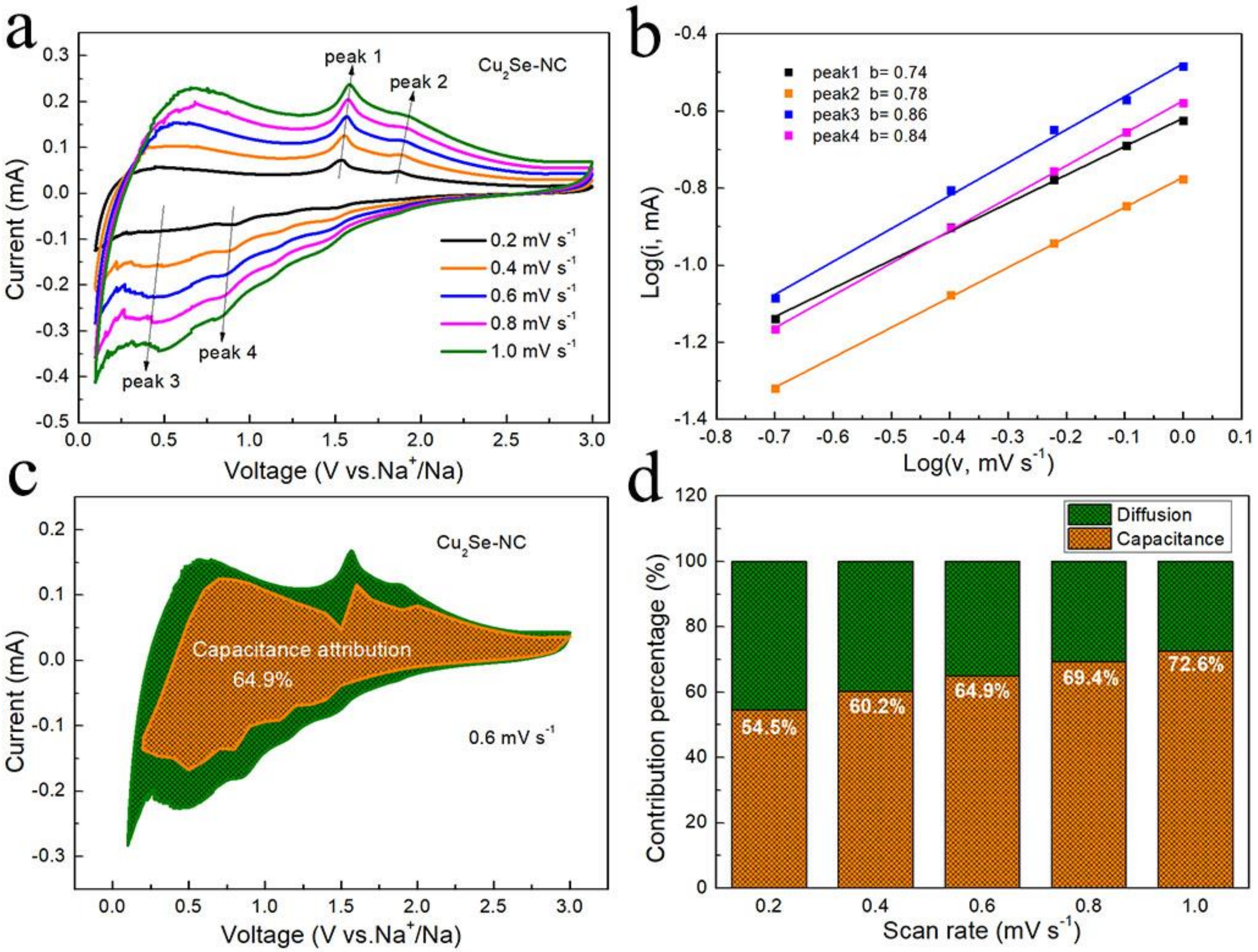
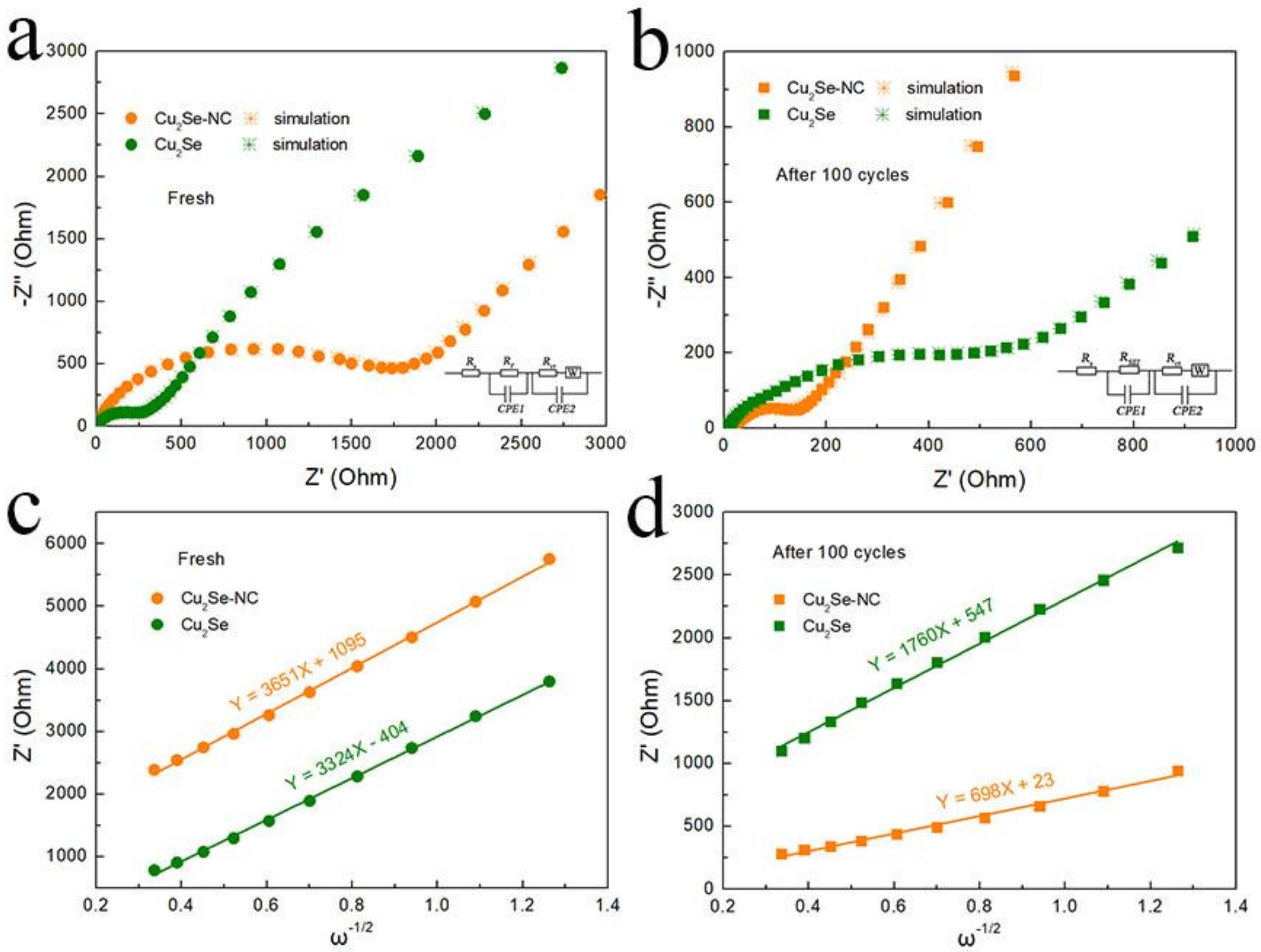
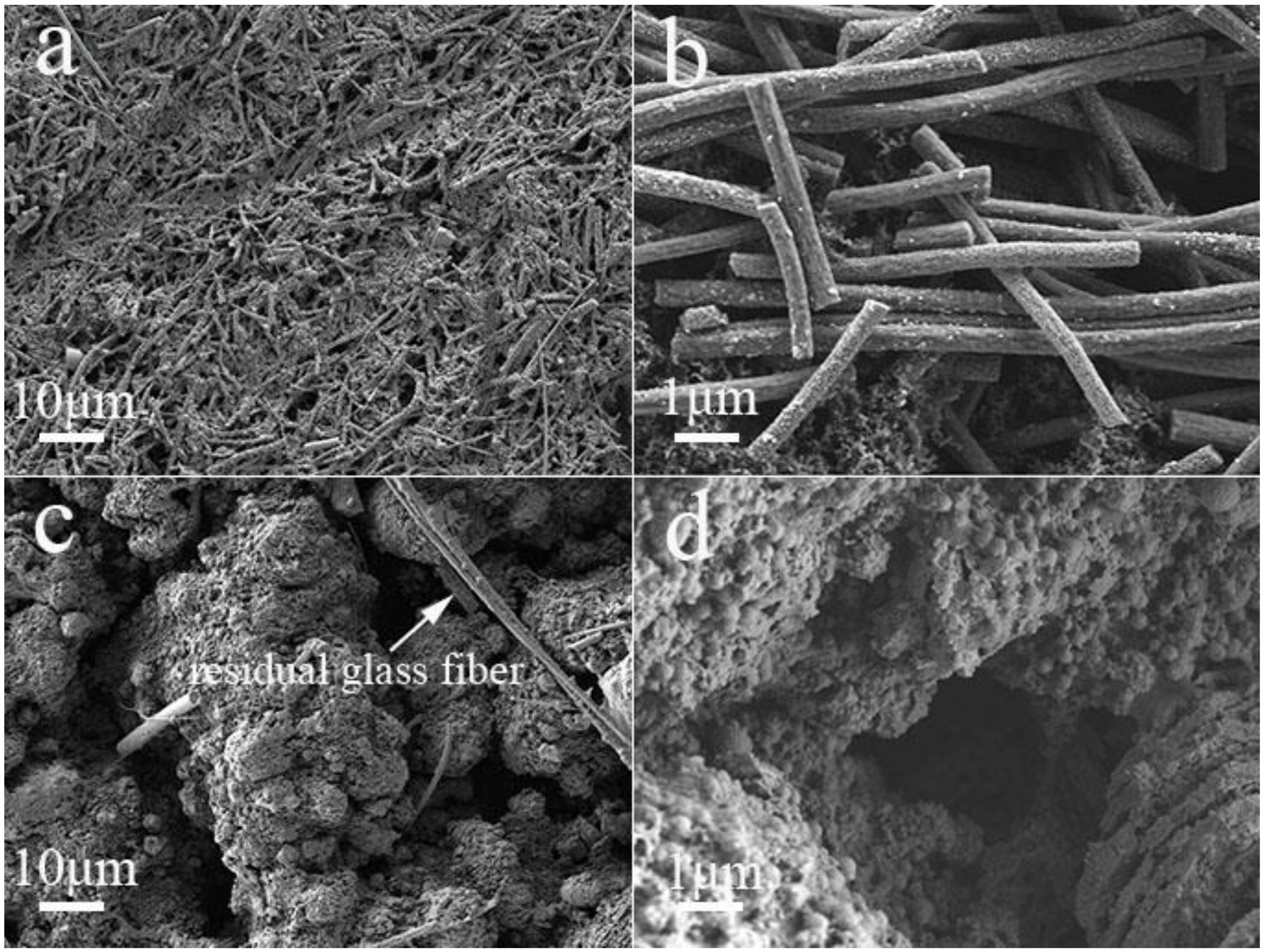
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fu, L.; Zhang, C.; Chen, B.; Zhang, Z.; Wang, X.; Zhao, J.; He, J.; Du, H.; Cui, G. Graphene boosted Cu2GeS3 for advanced lithium-ion batteries. Inorg. Chem. Front. 2017, 4, 541–546. [Google Scholar] [CrossRef]
- Shang, C.; Dong, S.; Zhang, S.; Hu, P.; Zhang, C.; Cui, G. A Ni3S2-PEDOT monolithic electrode for sodium batteries. Electrochem. Commun. 2015, 50, 24–27. [Google Scholar] [CrossRef]
- Fu, L.; Bi, Z.; Wei, B.; Huang, L.; Zhang, X.; Chen, Z.; Liao, H.; Li, M.; Shang, C.; Wang, X. Flower-like Cu2SnS3 Nanostructure Materials with High Crystallinity for Sodium Storage. Nanomaterials 2018, 8, 475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.; Tai, Z.; Liu, Q.; Wang, S.-W.; Jin, H.; Wang, S.; Lai, W.; Chen, M.; Li, L.; Chen, L.; et al. Ultrathin 2D TiS2 Nanosheets for High Capacity and Long-Life Sodium Ion Batteries. Adv. Energy Mater. 2019, 9, 1803210. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, T.; Song, T.; Yu, X.-Y.; Lou, X.W.; Paik, U. Structure-designed synthesis of FeS2@C yolk–shell nanoboxes as a high-performance anode for sodium-ion batteries. Energy Environ. Sci. 2017, 10, 1576–1580. [Google Scholar] [CrossRef]
- Li, X.; Sun, M.; Ni, J.; Li, L. Template-Free Construction of Self-Supported Sb Prisms with Stable Sodium Storage. Adv. Energy Mater. 2019, 9, 1901096. [Google Scholar] [CrossRef]
- Zhao, Q.; Bi, R.; Cui, J.; Yang, X.; Zhang, L. TiO2–x Nanocages Anchored in N-Doped Carbon Fiber Films as a Flexible Anode for High-Energy Sodium-Ion Batteries. ACS Appl. Energy Mater. 2018, 1, 4459–4466. [Google Scholar] [CrossRef]
- Li, B.; Liu, Y.; Jin, X.; Jiao, S.; Wang, G.; Peng, B.; Zeng, S.; Shi, L.; Li, J.; Zhang, G. Designed Formation of Hybrid Nanobox Composed of Carbon Sheathed CoSe2 Anchored on Nitrogen-Doped Carbon Skeleton as Ultrastable Anode for Sodium-Ion Batteries. Small 2019, 15, 1902881. [Google Scholar] [CrossRef]
- Yang, C.; Feng, J.; Lv, F.; Zhou, J.; Lin, C.; Wang, K.; Zhang, Y.; Yang, Y.; Wang, W.; Li, J.; et al. Metallic Graphene-Like VSe2 Ultrathin Nanosheets: Superior Potassium-Ion Storage and Their Working Mechanism. Adv. Mater. 2018, 30, 1800036. [Google Scholar] [CrossRef]
- Fang, Y.; Yu, X.Y.; Lou, X.W.D. Formation of Hierarchical Cu-Doped CoSe2 Microboxes via Sequential Ion Exchange for High-Performance Sodium-Ion Batteries. Adv. Mater. 2018, 30, 1706668. [Google Scholar] [CrossRef]
- Yousaf, M.; Wang, Y.; Chen, Y.; Wang, Z.; Firdous, A.; Ali, Z.; Mahmood, N.; Zou, R.; Guo, S.; Han, R.P.S. A 3D Trilayered CNT/MoSe2/C Heterostructure with an Expanded MoSe2 Interlayer Spacing for an Efficient Sodium Storage. Adv. Energy Mater. 2019, 9, 1900567. [Google Scholar] [CrossRef]
- Fu, L.; Wang, X.; Ma, J.; Zhang, C.; He, J.; Xu, H.; Chai, J.; Li, S.; Chai, F.; Cui, G. Graphene-Encapsulated Copper tin Sulfide Submicron Spheres as High-Capacity Binder-Free Anode for Lithium-Ion Batteries. ChemElectroChem 2017, 4, 1124–1129. [Google Scholar] [CrossRef]
- Shang, C.; Hu, L.; Fu, L.; Huang, L.; Xue, B.; Wang, X.; Shui, L.; Zhou, G. Improving lithium storage capability of ternary Sn-based sulfides by enhancing inactive/active element ratio. Solid State Ion. 2019, 337, 47–55. [Google Scholar] [CrossRef]
- Lin, J.; Lim, J.M.; Youn, D.H.; Kawashima, K.; Kim, J.H.; Liu, Y.; Guo, H.; Henkelman, G.; Heller, A.; Mullins, C.B. Self-Assembled Cu-Sn-S Nanotubes with High (De)Lithiation Performance. ACS Nano 2017, 11, 10347–10356. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.L.; Sun, Q.; Fu, Z.W. Cu2Se with facile synthesis as a cathode material for rechargeable sodium batteries. Chem. Commun. 2013, 49, 5868–5870. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Y.; Fang, Y.; Zhang, J.; Gao, S.; Lou, X.W.D. Synthesis of Cobalt Sulfide Multi-shelled Nanoboxes with Precisely Controlled Two to Five Shells for Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2019, 58, 2675–2679. [Google Scholar] [CrossRef]
- Yang, H.; Xu, R.; Yao, Y.; Ye, S.; Zhou, X.; Yu, Y. Multicore-Shell Bi@N-doped Carbon Nanospheres for High Power Density and Long Cycle Life Sodium-and Potassium-Ion Anodes. Adv. Funct. Mater. 2019, 29, 1809195. [Google Scholar] [CrossRef]
- Pan, Q.; Zhang, Q.; Zheng, F.; Liu, Y.; Li, Y.; Ou, X.; Xiong, X.; Yang, C.; Liu, M. Construction of MoS2/C Hierarchical Tubular Heterostructures for High-Performance Sodium Ion Batteries. ACS Nano 2018, 12, 12578–12586. [Google Scholar] [CrossRef]
- Yin, H.; Li, Q.; Cao, M.; Zhang, W.; Zhao, H.; Li, C.; Huo, K.; Zhu, M. Nanosized-bismuth-embedded 1D carbon nanofibers as high-performance anodes for lithium-ion and sodium-ion batteries. Nano Res. 2017, 10, 2156–2167. [Google Scholar] [CrossRef]
- Li, Z.; Fang, Y.; Zhang, J.; Lou, X.W.D. Necklace-Like Structures Composed of Fe3N@C Yolk-Shell Particles as an Advanced Anode for Sodium-Ion Batteries. Adv. Mater. 2018, 30, 1800525. [Google Scholar] [CrossRef]
- Ma, D.; Li, Y.; Mi, H.; Luo, S.; Zhang, P.; Lin, Z.; Li, J.; Zhang, H. Robust SnO2-x Nanoparticle-Impregnated Carbon with Outstanding Electrochemical Performance for Advanced Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2018, 57, 8901–8905. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.S.; Lee, S.Y.; Kang, Y.C. First Introduction of NiSe2 to Anode Material for Sodium-Ion Batteries: A Hybrid of Graphene-Wrapped NiSe2/C Porous Nanofiber. Sci. Rep. 2016, 6, 23338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.; Li, M.; Yang, X.; Yu, D.; Zeng, Y.; Wang, C.; Chen, G.; Du, F. Nanosheets-Assembled CuSe Crystal Pillar as a Stable and High-Power Anode for Sodium-Ion and Potassium-Ion Batteries. Adv. Energy Mater. 2019, 9, 1900323. [Google Scholar] [CrossRef]
- Liu, T.; Li, Y.; Zhao, L.; Zheng, F.; Guo, Y.; Li, Y.; Pan, Q.; Liu, Y.; Hu, J.; Yang, C. In Situ Fabrication of Carbon-Encapsulated Fe7X8 (X = S, Se) for Enhanced Sodium Storage. ACS Appl. Mater. Interfaces 2019, 11, 19040–19047. [Google Scholar] [CrossRef]
- Yuan, C.; Li, J.; Hou, L.; Zhang, X.; Shen, L.; Lou, X.W.D. Ultrathin Mesoporous NiCo2O4 Nanosheets Supported on Ni Foam as Advanced Electrodes for Supercapacitors. Adv. Funct. Mater 2012, 22, 4592–4597. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, Q.; Xu, W.; Cao, G.; Wang, Y.; Pan, A.; Liang, S. A Confined Replacement Synthesis of Bismuth Nanodots in MOF Derived Carbon Arrays as Binder-Free Anodes for Sodium-Ion Batteries. Adv. Sci. 2019, 6, 1900162. [Google Scholar] [CrossRef] [Green Version]
- Shang, C.; Hu, L.; Lin, Q.; Fu, X.; Wang, X.; Zhou, G. Integration of NaV6O15·nH2O nanowires and rGO as cathode materials for efficient sodium storage. Appl. Surf. Sci. 2019, 494, 458–464. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, X.; Mi, L.; Liu, C.; Zhang, J.; Cui, S.; Feng, X.; Cao, Y.; Shen, C. High-Performance Flexible Freestanding Anode with Hierarchical 3D Carbon-Networks/Fe7S8/Graphene for Applicable Sodium-Ion Batteries. Adv. Mater. 2019, 31, 1806664. [Google Scholar] [CrossRef]
- Ou, X.; Cao, L.; Liang, X.; Zheng, F.; Zheng, H.S.; Yang, X.; Wang, J.H.; Yang, C.; Liu, M. Fabrication of SnS2/Mn2SnS4/Carbon Heterostructures for Sodium-Ion Batteries with High Initial Coulombic Efficiency and Cycling Stability. ACS Nano 2019, 13, 3666–3676. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Wang, Y.X.; Feng, X.; Chen, W.; Qian, J.; Ai, X.; Yang, H.; Cao, Y. In Situ Formation of Co9S8 Nanoclusters in Sulfur-Doped Carbon Foam as a Sustainable and High-Rate Sodium-Ion Anode. ACS Appl. Mater. Interfaces 2019, 11, 19218–19226. [Google Scholar] [CrossRef]
- Ni, Q.; Bai, Y.; Guo, S.; Ren, H.; Chen, G.; Wang, Z.; Wu, F.; Wu, C. Carbon Nanofiber Elastically Confined Nanoflowers: A Highly Efficient Design for Molybdenum Disulfide-Based Flexible Anodes Toward Fast Sodium Storage. ACS Appl. Mater. Interfaces 2019, 11, 5183–5192. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Song, D.; Wu, J.; Wang, Z.; Huang, S.; Xu, Y.; Chen, Z.; Zhao, B.; Zhang, J. Sandwich-like SnS2/Graphene/SnS2 with Expanded Interlayer Distance as High-Rate Lithium/Sodium-Ion Battery Anode Materials. ACS Nano 2019, 13, 9100–9111. [Google Scholar] [CrossRef] [PubMed]
- Gan, Q.; He, H.; Zhu, Y.; Wang, Z.; Qin, N.; Gu, S.; Li, Z.; Luo, W.; Lu, Z. Defect-Assisted Selective Surface Phosphorus Doping to Enhance Rate Capability of Titanium Dioxide for Sodium Ion Batteries. ACS Nano 2019, 13, 9247–9258. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Qi, S.; Yu, M.; Feng, X.; Cui, S.; Zhang, J.; Mi, L. Design of FeS2@rGO composite with enhanced rate and cyclic performances for sodium ion batteries. Electrochim. Acta 2017, 230, 1–9. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, L.; Shang, C.; Akinoglu, E.M.; Wang, X.; Zhou, G. Cu2Se Nanoparticles Encapsulated by Nitrogen-Doped Carbon Nanofibers for Efficient Sodium Storage. Nanomaterials 2020, 10, 302. https://doi.org/10.3390/nano10020302
Hu L, Shang C, Akinoglu EM, Wang X, Zhou G. Cu2Se Nanoparticles Encapsulated by Nitrogen-Doped Carbon Nanofibers for Efficient Sodium Storage. Nanomaterials. 2020; 10(2):302. https://doi.org/10.3390/nano10020302
Chicago/Turabian StyleHu, Le, Chaoqun Shang, Eser Metin Akinoglu, Xin Wang, and Guofu Zhou. 2020. "Cu2Se Nanoparticles Encapsulated by Nitrogen-Doped Carbon Nanofibers for Efficient Sodium Storage" Nanomaterials 10, no. 2: 302. https://doi.org/10.3390/nano10020302
APA StyleHu, L., Shang, C., Akinoglu, E. M., Wang, X., & Zhou, G. (2020). Cu2Se Nanoparticles Encapsulated by Nitrogen-Doped Carbon Nanofibers for Efficient Sodium Storage. Nanomaterials, 10(2), 302. https://doi.org/10.3390/nano10020302




