Low-Temperature Synthesis of Monolithic Titanium Carbide/Carbon Composite Aerogel
Abstract
1. Introduction
2. Experimental Setup
2.1. Materials
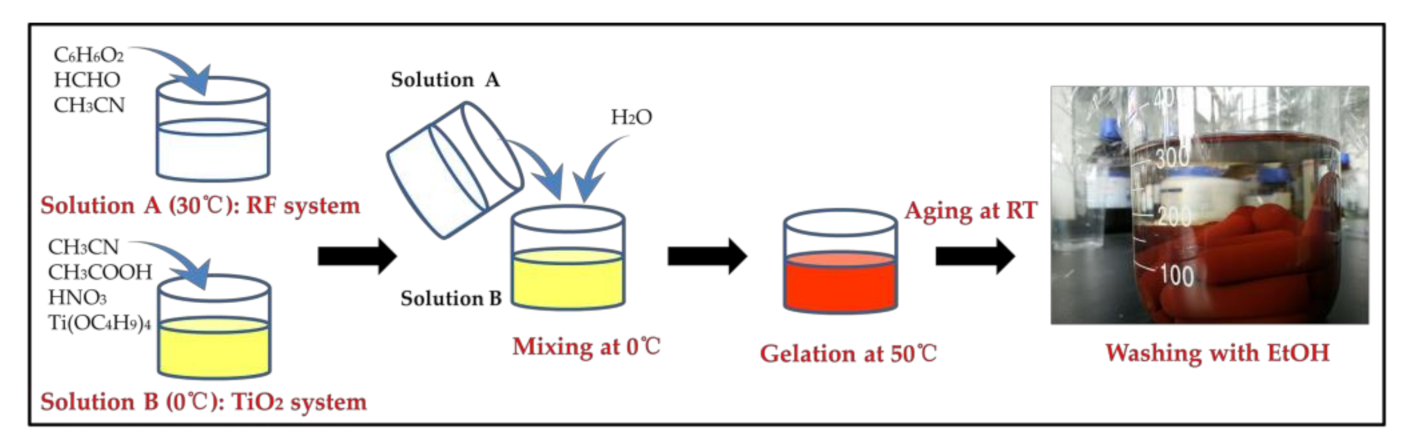
2.2. Preparation of RF/TiO2 Composite Gel and Aerogel
2.3. Conversion of RF/TiO2 Aerogel into TiC/C Composite Aerogel
2.4. Characterizations
3. Results and Discussion
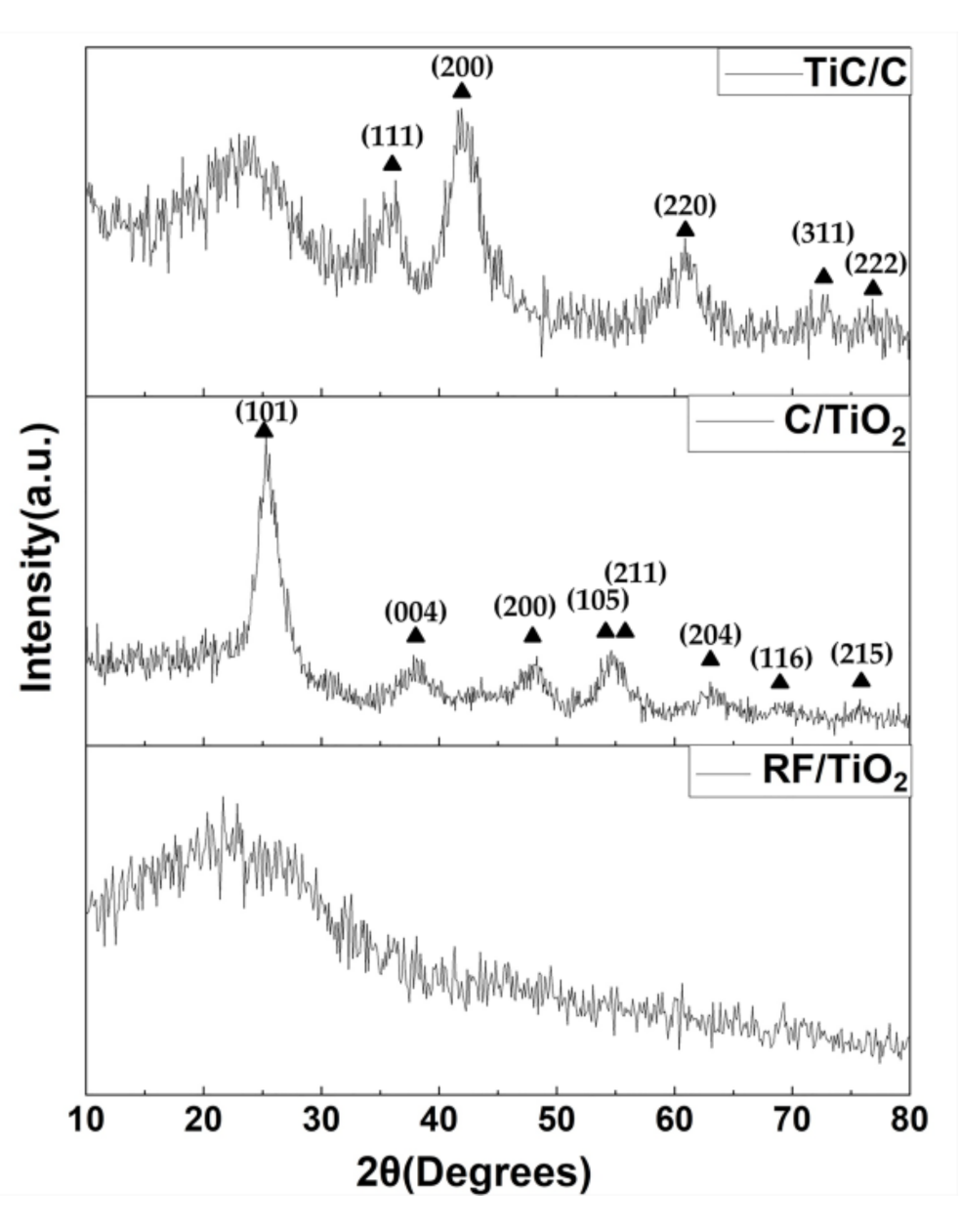
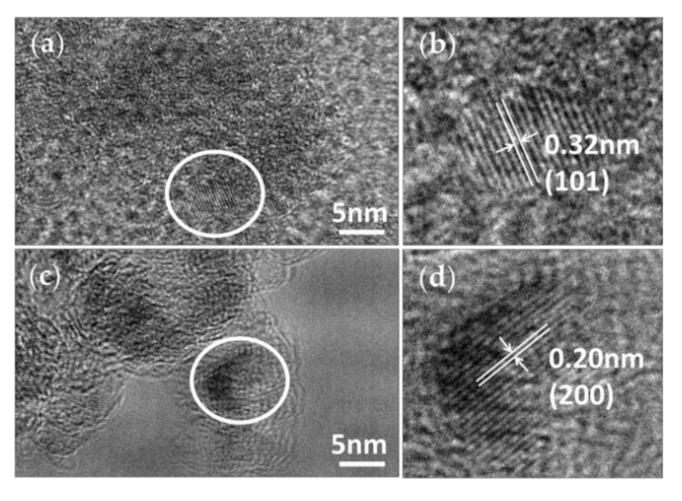
3.1. Appearance and Structural Characterization
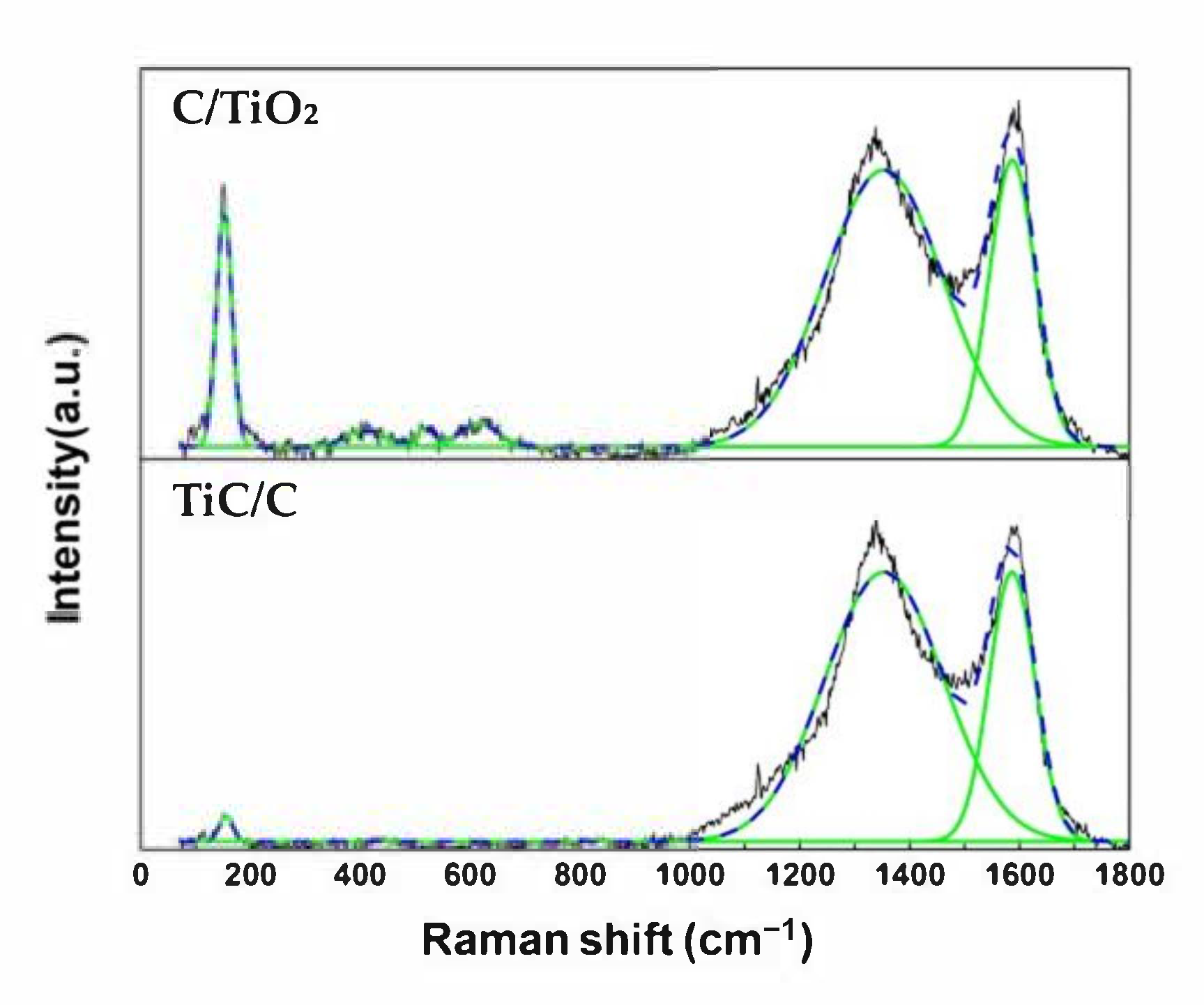
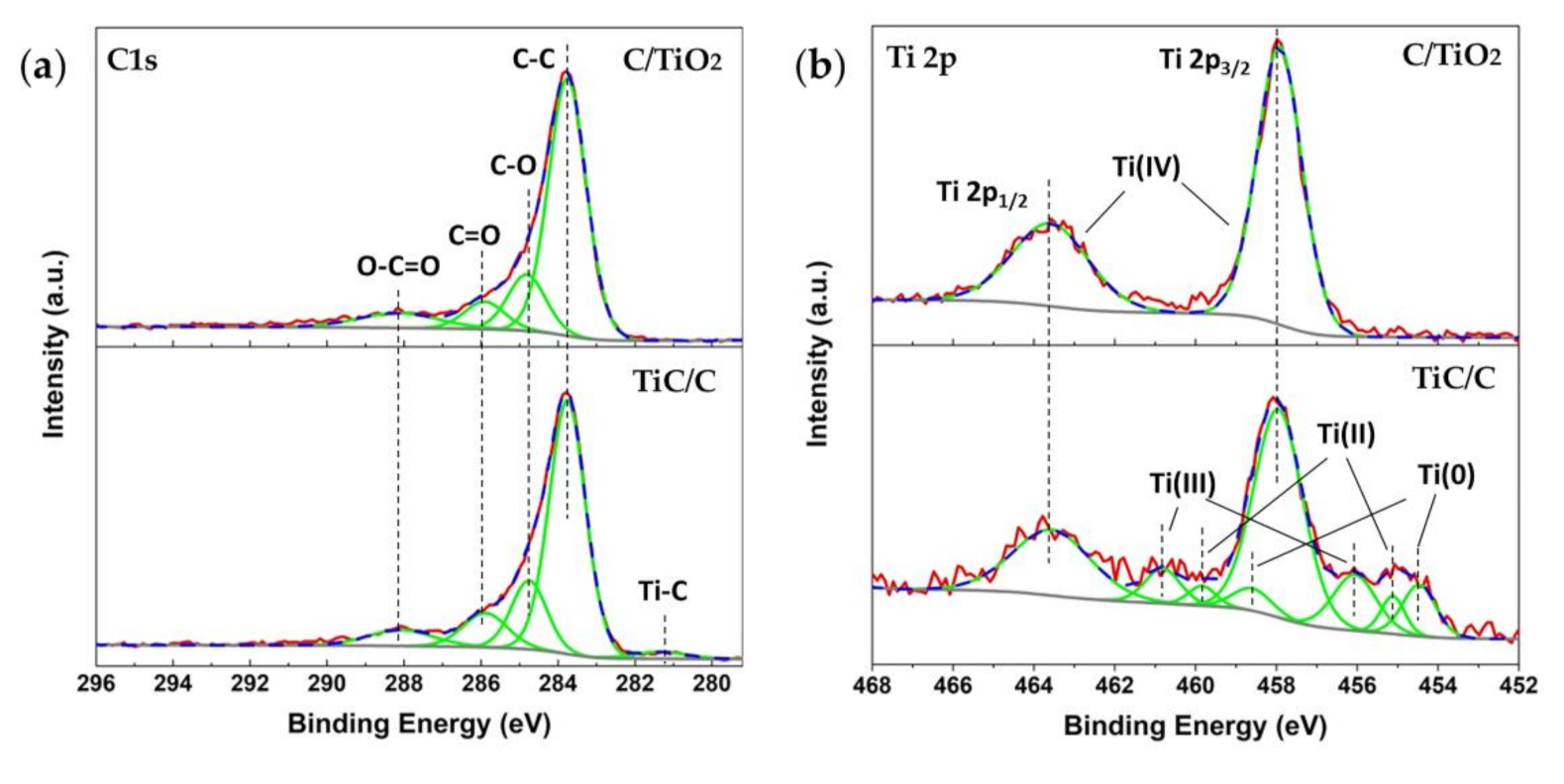
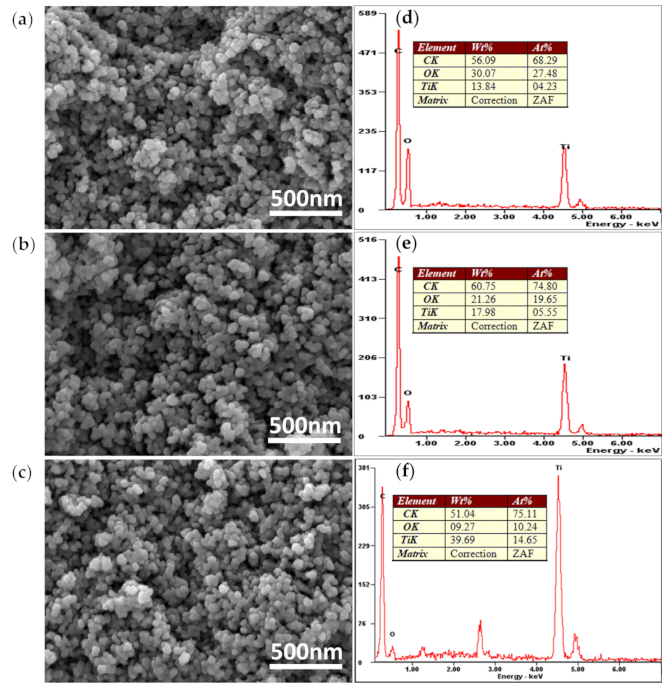
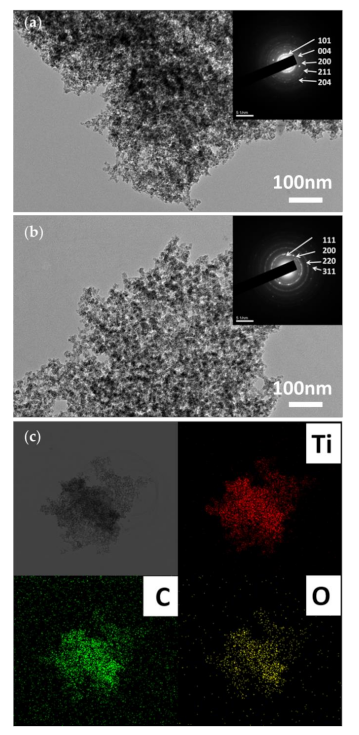
3.2. Morphology and Composition Analysis
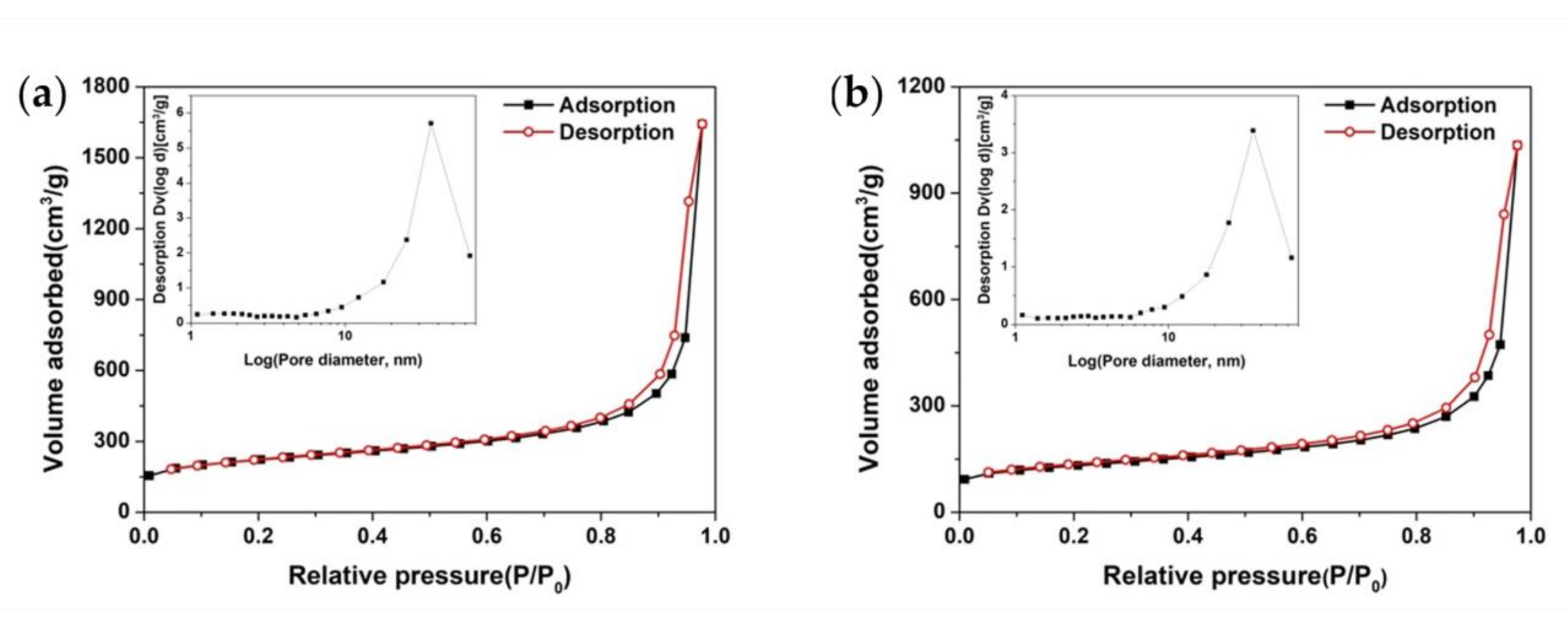
3.3. Specific Surface Area Analysis
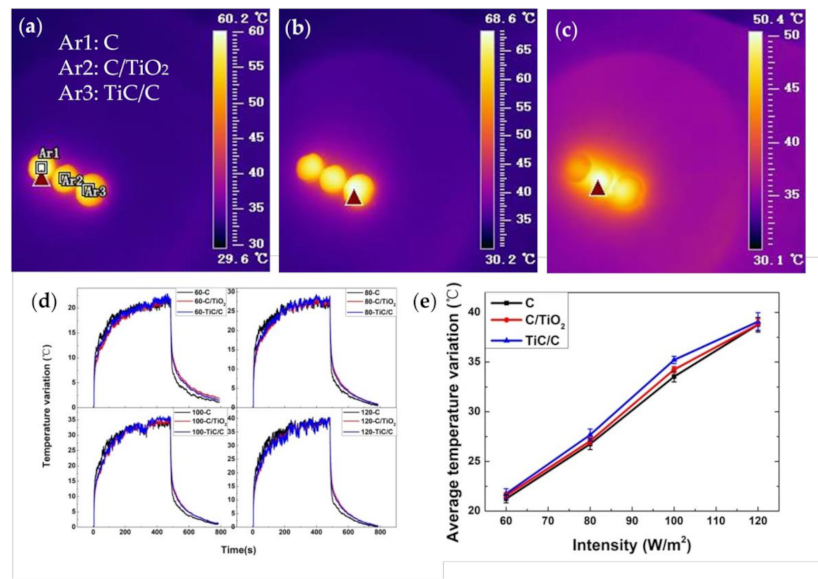
3.4. Photothermal Conversion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Chen, L.; Wang, L.; Fan, Q.; Pan, D.; Li, J.; Chi, F.; Xie, Y.; Yu, S.; Xiao, C.; et al. Synthesis of novel nanomaterials and their application in efficient removal of radionuclides. Sci. China Chem. 2019, 62, 933–967. [Google Scholar] [CrossRef]
- Duong-Viet, C.; Ba, H.; Liu, Y.; Truong-Phuoc, L.; Nhut, J.-M.; Pham-Huu, C. Nitrogen-doped carbon nanotubes on silicon carbide as a metal-free catalyst. Chin. J. Catal. 2014, 35, 906–913. [Google Scholar] [CrossRef]
- Gupta, S.; Yadav, A.; Singh, S.; Verma, N. Synthesis of Silicon Carbide-Derived Carbon as an Electrode of a Microbial Fuel Cell and an Adsorbent of Aqueous Cr(VI). Ind. Eng. Chem. Res. 2017, 56, 1233–1244. [Google Scholar] [CrossRef]
- Yang, P.; Zhao, H.; Yang, Y.; Zhao, P.; Zhao, X.; Yang, L. Fabrication of N, P-Codoped Mo2C/Carbon Nanofibers Via Electrospinning as Electrocatalyst for Hydrogen Evolution Reaction. ES Mater. Manuf. 2020, 7, 34–39. [Google Scholar] [CrossRef]
- Han, D.; Mei, H.; Xiao, S.; Cheng, L. Macroscopic carbon nanotube assembly/silicon carbide matrix composites produced by gas phase route. Adv. Compos. Hybrid Mater. 2019, 2, 142–150. [Google Scholar] [CrossRef]
- Kumari, S.; Kumar, R.; Agrawal, P.R.; Prakash, S.; Mondal, D.P.; Dhakate, S.R. Fabrication of lightweight and porous silicon carbide foams as excellent microwave susceptor for heat generation. Mater. Chem. Phys. 2020, 253, 123211. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Q.; Qin, M.; Yin, R.; Zhang, Z.; Jia, B.; Qu, X. Synthesis of tungsten carbide nanopowders by direct carbonization of tungsten oxide and carbon: Effects of tungsten oxide source on phase structure and morphology evolution. Ceram. Int. 2020, 46, 8787–8795. [Google Scholar] [CrossRef]
- Yi, Z.; Shao, G.; Duan, X.; Sun, P.; Shi, X.; Xiong, Z.; Guo, J. Preparation of WC-Co powder by direct reduction and carbonization. China Particuology 2005, 3, 286–288. [Google Scholar] [CrossRef]
- Feng, L.; Fahrenholtz, W.G.; Hilmas, G.E.; Zhou, Y. Synthesis of single-phase high-entropy carbide powders. Scr. Mater. 2019, 162, 90–93. [Google Scholar] [CrossRef]
- Mojaki, S.C.; Mishra, S.B.; Mishra, A.K. Synthesis, characterization and material properties of titanium carbide nanocomposite derived from biochar. Mater. Lett. 2020, 264, 127317. [Google Scholar] [CrossRef]
- Wang, K.-F.; Tang, X.-L.; Jiao, S.; Chou, K.-C.; Zhang, G.-H. A short and facile process to synthesize WC-Co cemented carbides. Int. J. Refract. Met. Hard Mater. 2020, 92, 105288. [Google Scholar] [CrossRef]
- Rambo, C.R.; Cao, J.; Rusina, O.; Sieber, H. Manufacturing of biomorphic (Si, Ti, Zr)-carbide ceramics by sol–gel processing. Carbon 2005, 43, 1174–1183. [Google Scholar] [CrossRef]
- Worsley, M.A.; Kuntz, J.D.; Pauzauskie, P.J.; Cervantes, O.; Zaug, J.M.; Gash, A.E.; Satcher, J.H.; Baumann, T.F. High surface area carbon nanotube-supported titanium carbonitride aerogels. J. Mater. Chem. 2009, 19, 5503–5506. [Google Scholar] [CrossRef]
- An, Z.; Zhang, R.; Fang, D. Synthesis of monolithic SiC aerogels with high mechanical strength and low thermal conductivity. Ceram. Int. 2019, 45, 11368–11374. [Google Scholar] [CrossRef]
- Chen, X.; Fan, J.; Lu, Q. Synthesis and characterization of TiC nanopowders via sol-gel and subsequent carbothermal reduction process. J. Solid State Chem. 2018, 262, 44–52. [Google Scholar] [CrossRef]
- Alsawat, M.; Altalhi, T.; Alotaibi, N.F.; Zaki, Z.I. Titanium carbide—Titanium boride composites by self propagating high temperature synthesis approach: Influence of zirconia additives on the mechanical properties. Results Phys. 2019, 13, 102292. [Google Scholar] [CrossRef]
- Jin, S.; Shen, P.; Lin, Q.; Zhan, L.; Jiang, Q. Growth Mechanism of TiCxduring Self-Propagating High-Temperature Synthesis in an Al−Ti−C System. Cryst. Growth Des. 2010, 10, 1590–1597. [Google Scholar] [CrossRef]
- Jin, S.; Shen, P.; Zhou, D.; Jiang, Q. Self-propagating high-temperature synthesis of nano-TiCx particles with different shapes by using carbon nano-tube as C source. Nanoscale Res. Lett. 2011, 6, 515. [Google Scholar] [CrossRef]
- Kurbatkina, V.V.; Patsera, E.I.; Levashov, E.A.; Timofeev, A.N. Self-propagating high-temperature synthesis of single-phase binary tantalum-hafnium carbide (Ta,Hf)C and its consolidation by hot pressing and spark plasma sintering. Ceram. Int. 2018, 44, 4320–4329. [Google Scholar] [CrossRef]
- Patsera, E.I.; Levashov, E.A.; Kurbatkina, V.V.; Kovalev, D.Y. Production of ultra-high temperature carbide (Ta,Zr)C by self-propagating high-temperature synthesis of mechanically activated mixtures. Ceram. Int. 2015, 41, 8885–8893. [Google Scholar] [CrossRef]
- Rahaei, M.B.; Yazdani rad, R.; Kazemzadeh, A.; Ebadzadeh, T. Mechanochemical synthesis of nano TiC powder by mechanical milling of titanium and graphite powders. Powder Technol. 2012, 217, 369–376. [Google Scholar] [CrossRef]
- Jia, H.; Zhang, Z.; Qi, Z.; Liu, G.; Bian, X. Formation of nanocrystalline TiC from titanium and different carbon sources by mechanical alloying. J. Alloys Compd. 2009, 472, 97–103. [Google Scholar] [CrossRef]
- Stanciu, V.I.; Vitry, V.; Delaunois, F. Study of the milling parameters optimization in the direct carburization of WO3 by mechanical alloying. Int. J. Refract. Met. Hard Mater. 2020, 87, 105160. [Google Scholar] [CrossRef]
- Zhao, Z.; Hu, W. Synthesis and characterization of chromium carbide nanopowders processed by mechanical alloying assisted microwave heating route. Int. J. Refract. Met. Hard Mater. 2016, 58, 206–210. [Google Scholar] [CrossRef]
- Lee, C.C.; Kahar, S.M.; Voon, C.H. Microwave synthesis of silicon carbide nanowhiskers: Effect of molar ratio. Mater. Today Proc. 2020, 4, 571. [Google Scholar]
- Moshtaghioun, B.M.; Poyato, R.; Cumbrera, F.L.; de Bernardi-Martin, S.; Monshi, A.; Abbasi, M.H.; Karimzadeh, F.; Dominguez-Rodriguez, A. Rapid carbothermic synthesis of silicon carbide nano powders by using microwave heating. J. Eur. Ceram. Soc. 2012, 32, 1787–1794. [Google Scholar] [CrossRef]
- Ding, J.; Zhu, H.; Deng, C.; Li, G.; Wang, K.; Liu, J. Preparation and characterisation of porous biomorphic SiC/C ceramic from molten salt. Ceram. Int. 2015, 41, 11539–11545. [Google Scholar] [CrossRef]
- Kan, X.; Ding, J.; Yu, C.; Zhu, H.; Deng, C.; Li, G. Low-temperature fabrication of porous ZrC/C composite material from molten salts. Ceram. Int. 2017, 43, 6377–6384. [Google Scholar] [CrossRef]
- Nadimi, H.; Soltanieh, M.; Sarpoolaky, H. The formation mechanism of nanocrystalline TiC from KCl–LiCl molten salt medium. Ceram. Int. 2020, 46, 18725–18733. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Y.; Liu, R.; Liu, H.; Zhang, X.; Zeng, C.; Fu, C. In-situ synthesis of nanocrystalline TiC powders, nanorods, and nanosheets in molten salt by disproportionation reaction of Ti(II) species. J. Mater. Sci. Technol. 2020, 37, 173–180. [Google Scholar] [CrossRef]
- Tian, X.; Zhang, X.; Hu, Y.; Liu, B.; Yuan, Y.; Yang, L.; Chen, Q.; Liu, Z. Fabrication of iron carbide by plasma-enhanced atomic layer deposition. J. Mater. Res. 2019, 35, 813–821. [Google Scholar] [CrossRef]
- Scandurra, R.; Scotto d′Abusco, A.; Longo, G. A Review of the Effect of a Nanostructured Thin Film Formed by Titanium Carbide and Titanium Oxides Clustered around Carbon in Graphitic Form on Osseointegration. Nanomaterials 2020, 10, 1233. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Bao, Z.; Du, A.; Zhu, X.; Wu, G.; Shen, J.; Zhou, B. Synthesis of resorcinol–formaldehyde/silica composite aerogels and their low-temperature conversion to mesoporous silicon carbide. Microporous Mesoporous Mater. 2012, 149, 16–24. [Google Scholar] [CrossRef]
- Chen, K.; Huang, X.; Zhang, Z.; Du, A.; Zhou, B.; Xu, Y.; Zhou, Z.; Wang, Y. Low temperature pseudomorphic synthesis of nanocrystalline carbide aerogels for electrocatalysis. J. Mater. Chem. A 2015, 3, 11745–11749. [Google Scholar] [CrossRef]
- Kong, Y.; Shen, X.; Cui, S.; Fan, M. Preparation of monolith SiC aerogel with high surface area and large pore volume and the structural evolution during the preparation. Ceram. Int. 2014, 40, 8265–8271. [Google Scholar] [CrossRef]
- Schuchardt, A.; Braniste, T.; Mishra, Y.K.; Deng, M.; Mecklenburg, M.; Stevens-Kalceff, M.A.; Raevschi, S.; Schulte, K.; Kienle, L.; Adelung, R.; et al. Three-dimensional Aerographite-GaN hybrid networks: Single step fabrication of porous and mechanically flexible materials for multifunctional applications. Sci. Rep. 2015, 5, 8839. [Google Scholar] [CrossRef]
- Yuksel, R.; Buyukcakir, O.; Panda, P.K.; Lee, S.H.; Jiang, Y.; Singh, D.; Hansen, S.; Adelung, R.; Mishra, Y.K.; Ahuja, R.; et al. Necklace-like Nitrogen-Doped Tubular Carbon 3D Frameworks for Electrochemical Energy Storage. Adv. Funct. Mater. 2020, 30, 1909725. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Su, T.; Chen, J.; Zhang, C.; Lai, X.; Jiang, D.; Wu, Z.; Sun, C.; Li, B.; et al. Self-Healing Polymer Composites Based on Hydrogen Bond Reinforced with Graphene Oxide. ES Mater. Manuf. 2019, 4, 31–37. [Google Scholar] [CrossRef]
- Liu, C.; Fang, Q.; Wang, D.; Yan, C.; Liu, F.; Wang, N.; Guo, Z.; Jiang, Q. Carbon and Boron Nitride Nanotubes: Structure, Property and Fabrication. ES Mater. Manuf. 2019, 3, 2–15. [Google Scholar] [CrossRef]
- Prehal, C.; Fitzek, H.; Kothleitner, G.; Presser, V.; Gollas, B.; Freunberger, S.A.; Abbas, Q. Persistent and reversible solid iodine electrodeposition in nanoporous carbons. Nat. Commun. 2020, 11, 4838. [Google Scholar] [CrossRef]
- Ran, F.; Yang, X.; Shao, L. Recent progress in carbon-based nanoarchitectures for advanced supercapacitors. Adv. Compos. Hybrid Mater. 2018, 1, 32–55. [Google Scholar] [CrossRef]
- Lewin, E.; Persson, P.O.Å.; Lattemann, M.; Stüber, M.; Gorgoi, M.; Sandell, A.; Ziebert, C.; Schäfers, F.; Braun, W.; Halbritter, J.; et al. On the origin of a third spectral component of C1s XPS-spectra for nc-TiC/a-C nanocomposite thin films. Surf. Coat. Technol. 2008, 202, 3563–3570. [Google Scholar] [CrossRef]
- Samuelsson, M.; Sarakinos, K.; Högberg, H.; Lewin, E.; Jansson, U.; Wälivaara, B.; Ljungcrantz, H.; Helmersson, U. Growth of Ti-C nanocomposite films by reactive high power impulse magnetron sputtering under industrial conditions. Surf. Coat. Technol. 2012, 206, 2396–2402. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Godfroid, T.; Gouttebaron, R.; Dauchot, J.P.; Leclère, P.; Lazzaroni, R.; Hecq, M. Growth of ultrathin Ti films deposited on SnO2 by magnetron sputtering. Thin Solid Films 2003, 437, 57–62. [Google Scholar] [CrossRef]

| Samples | Diameter (cm) | Linear Shrinkage Ratio (%) | |
|---|---|---|---|
| RF/TiO2 | 234.8 ± 7.6 | 0.723 | -- |
| C/TiO2 | 355.1 ± 10.4 | 0.582 | 19.5% |
| TiC/C | 339.5 ± 7.9 | 0.569 | 2.2% |
| Sample | Specific Surface Area (m2/g) | Average Pore Diameter (nm) | Pore Size of Maximum Distribution (nm) | Total Pore Volume (cm3/g) |
|---|---|---|---|---|
| C/TiO2 | 780.6 | 13.05 | 36.1 | 2.547 |
| TiC/C | 459.5 | 13.97 | 35.7 | 1.605 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, T.; Zhou, B.; Zhang, Z.; Ji, X.; Yang, J.; Xie, Y.; Wang, H.; Du, A. Low-Temperature Synthesis of Monolithic Titanium Carbide/Carbon Composite Aerogel. Nanomaterials 2020, 10, 2527. https://doi.org/10.3390/nano10122527
Niu T, Zhou B, Zhang Z, Ji X, Yang J, Xie Y, Wang H, Du A. Low-Temperature Synthesis of Monolithic Titanium Carbide/Carbon Composite Aerogel. Nanomaterials. 2020; 10(12):2527. https://doi.org/10.3390/nano10122527
Chicago/Turabian StyleNiu, Tingting, Bin Zhou, Zehui Zhang, Xiujie Ji, Jianming Yang, Yuhan Xie, Hongqiang Wang, and Ai Du. 2020. "Low-Temperature Synthesis of Monolithic Titanium Carbide/Carbon Composite Aerogel" Nanomaterials 10, no. 12: 2527. https://doi.org/10.3390/nano10122527
APA StyleNiu, T., Zhou, B., Zhang, Z., Ji, X., Yang, J., Xie, Y., Wang, H., & Du, A. (2020). Low-Temperature Synthesis of Monolithic Titanium Carbide/Carbon Composite Aerogel. Nanomaterials, 10(12), 2527. https://doi.org/10.3390/nano10122527





