Fabrication of Hollow Channels Surrounded by Gold Nanoparticles in Hydrogel by Femtosecond Laser Irradiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Hydrogel Preparation
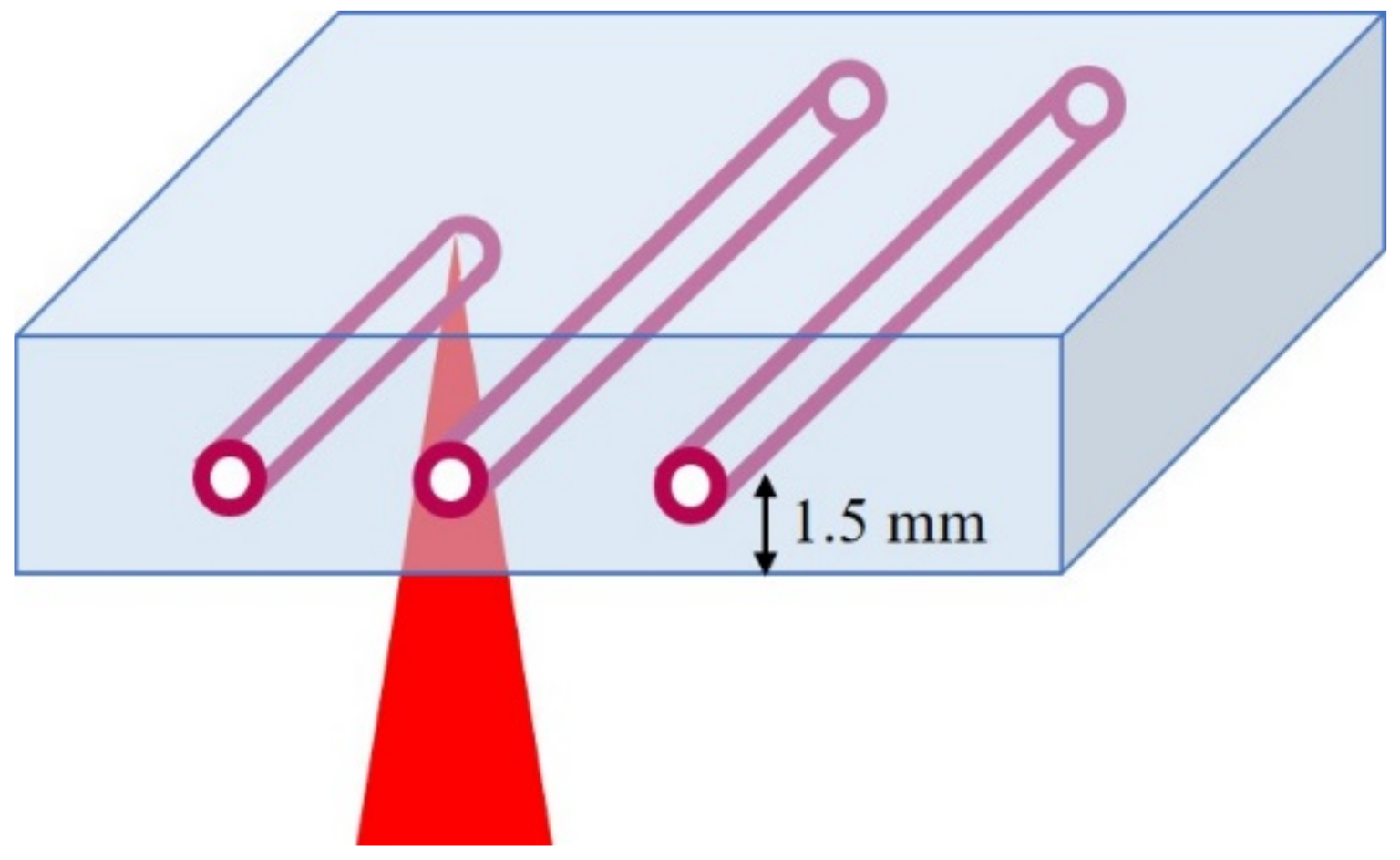
2.2. Laser Parameters
2.3. Absorption Spectrum Measurements
2.4. Digital Microscope
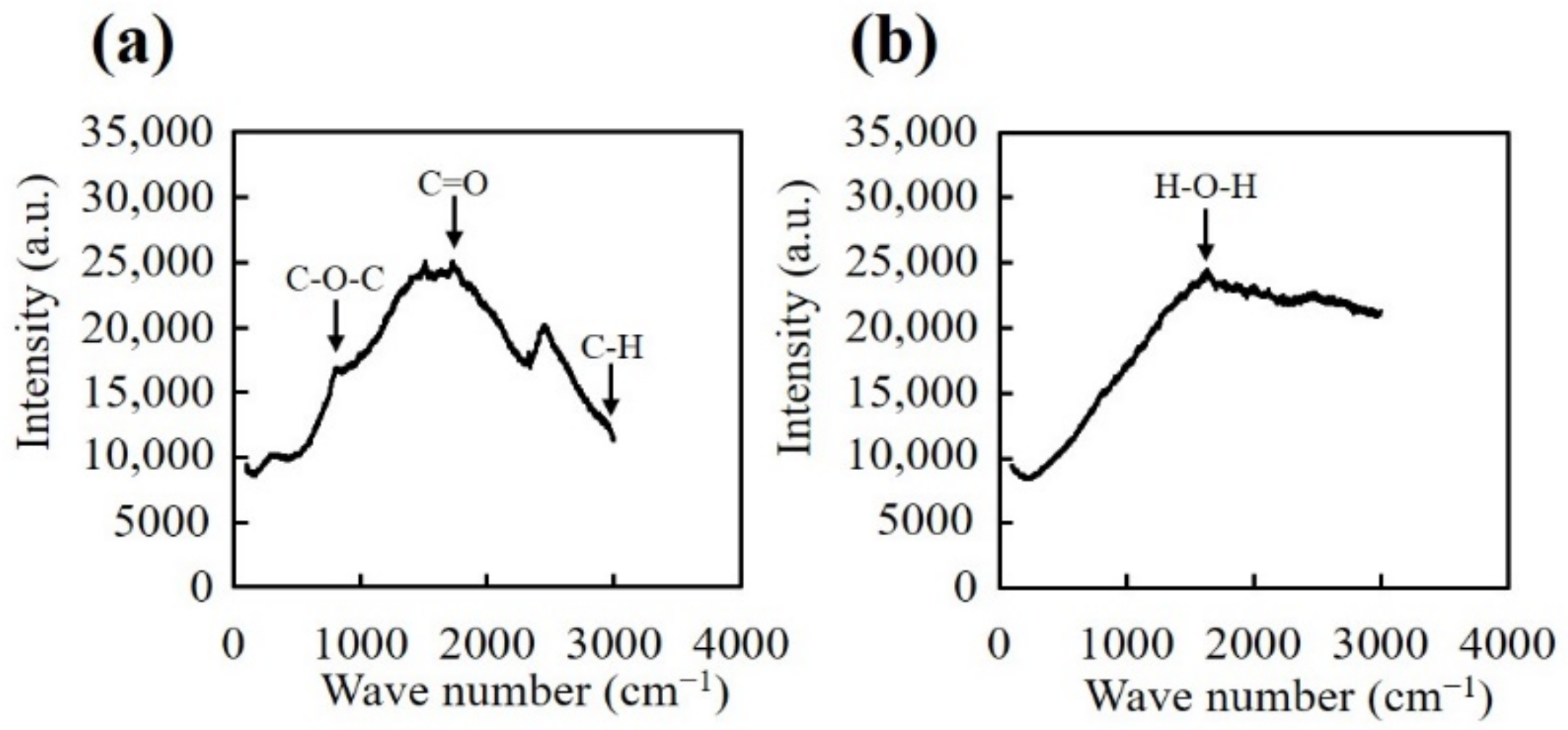
2.5. Raman Spectroscopy
3. Results and Discussion
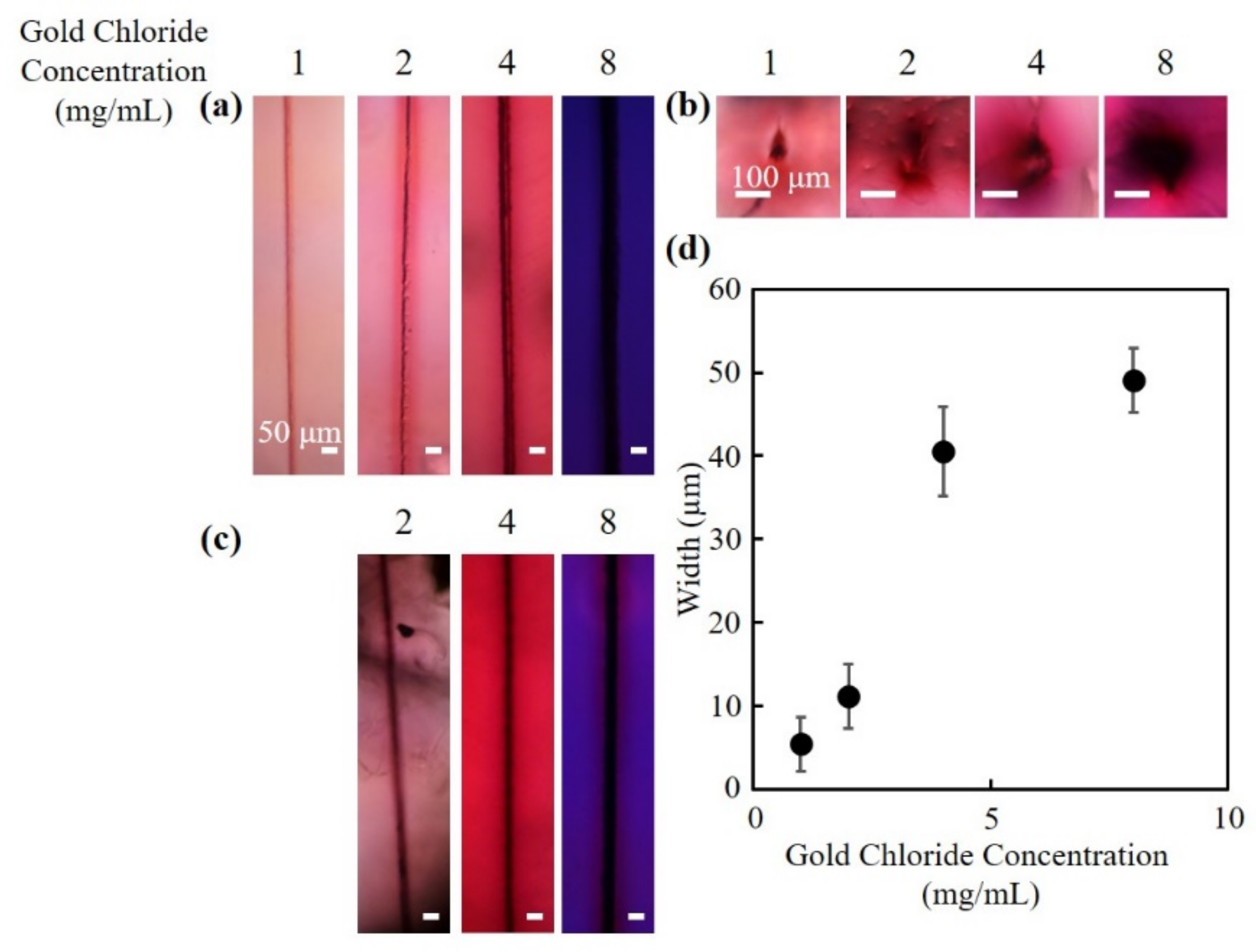
3.1. Fabrication of Hollow Channels Surrounded by Gold Nanoparticles
3.2. Absorption Spectra of the Fabricated Channels
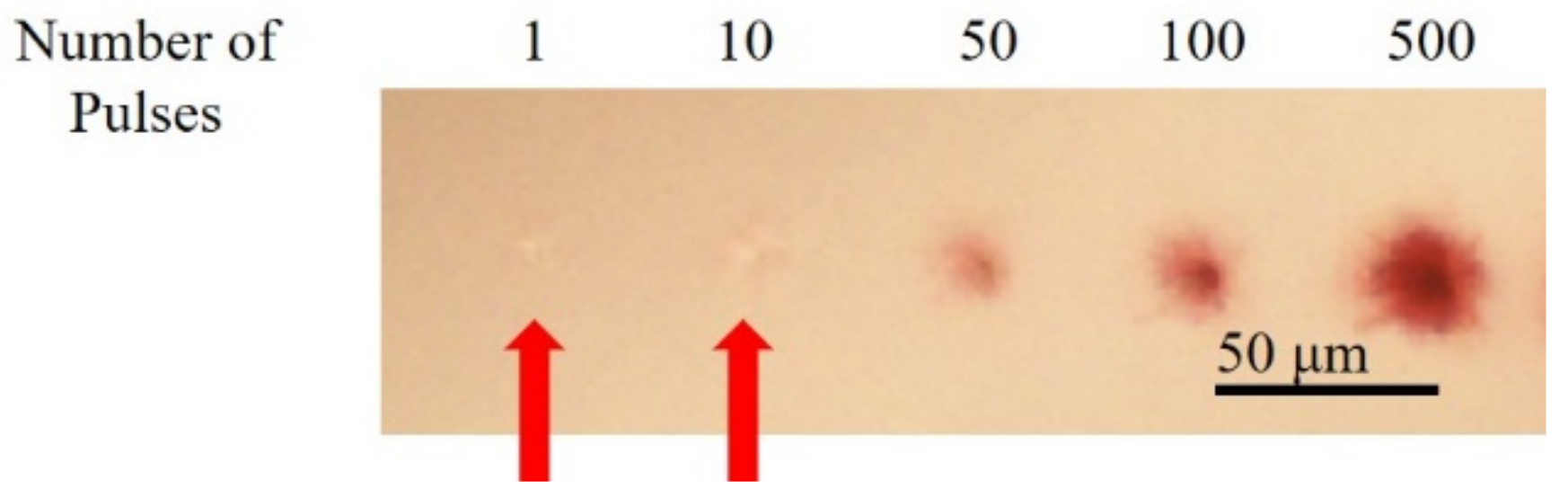
3.3. Fabrication of Structures with Different Number of Pulses
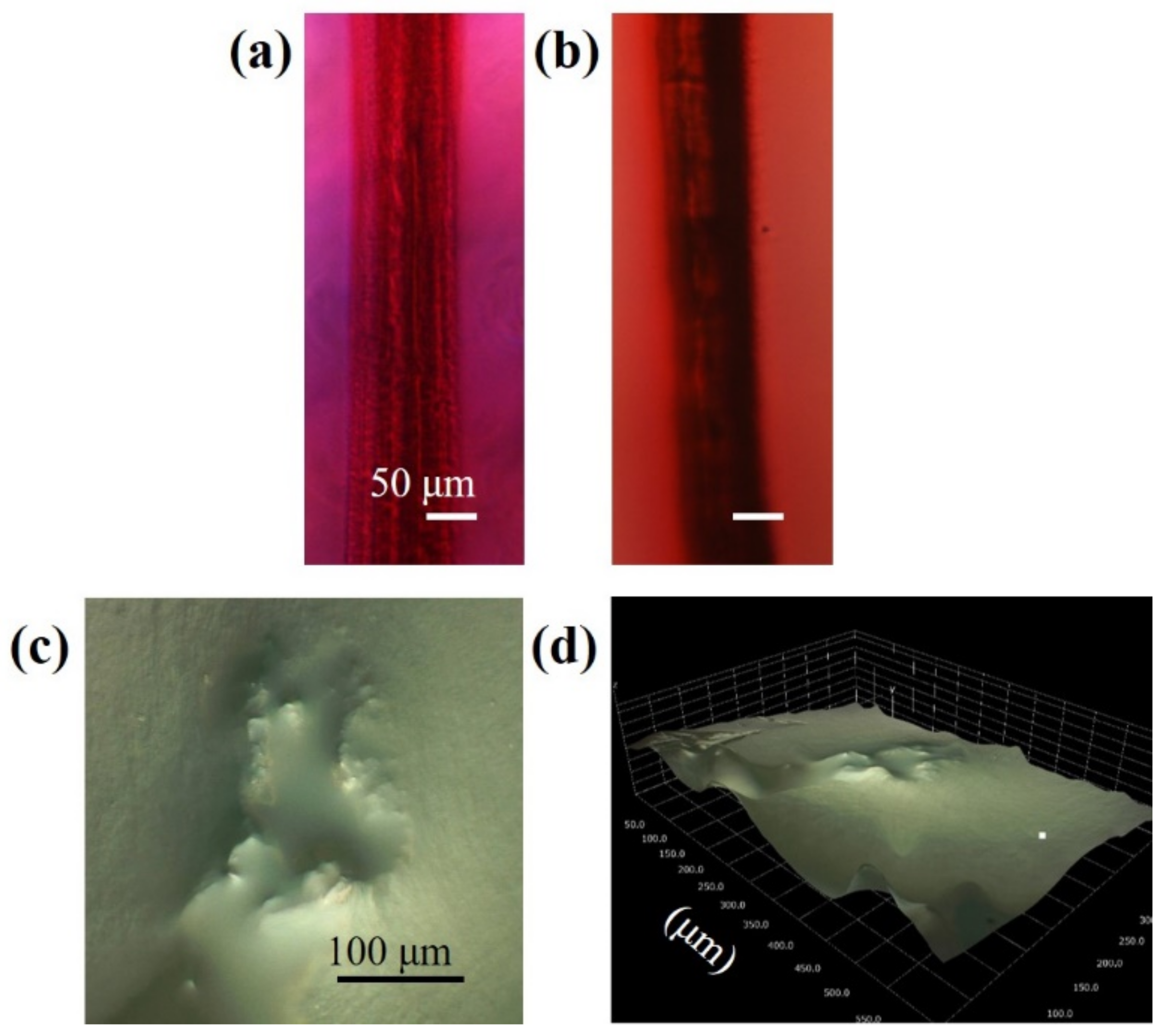
3.4. Anaylsis of Structures Consisting of Parallel Channels
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Butcher, D.T.; Alliston, T.; Weaver, V.M. A tense situation: Forcing tumour progression. Nat. Rev. Cancer 2009, 9, 108–122. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Cruise, G.M.; Scharp, D.S.; Hubbell, J.A. Characterization of permeability and network structure of interfacially photopolymerized poly(ethylene glycol) diacrylate hydrogels. Biomaterials 1998, 19, 1287–1294. [Google Scholar] [CrossRef]
- Chen, M.B.; Srigunapalan, S.; Wheeler, A.R.; Simmons, C.A. A 3D microfluidic platform incorporating methacrylated gelatin hydrogels to study physiological cardiovascular cell-cell interactions. Lab Chip 2013, 13, 2591–2598. [Google Scholar] [CrossRef] [PubMed]
- Cuchiara, M.P.; Allen, A.C.B.; Chen, T.M.; Miller, J.S.; West, J.L. Multilayer microfluidic PEGDA hydrogels. Biomaterials 2010, 31, 5491–5497. [Google Scholar] [CrossRef]
- Moore, M.J.; Friedman, J.A.; Lewellyn, E.B.; Mantila, S.M.; Krych, A.J.; Ameenuddin, S.; Knight, A.M.; Lu, L.; Currier, B.L.; Spinner, R.J.; et al. Multiple-channel scaffolds to promote spinal cord axon regeneration. Biomaterials 2006, 27, 419–429. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, Y.; Li, X.; Xiao, Z.; Yao, Y.; Chu, Y.; Farkas, B.; Romano, I.; Brandi, F.; Dai, J. Functional Multichannel Poly(Propylene Fumarate)-Collagen Scaffold with Collagen-Binding Neurotrophic Factor 3 Promotes Neural Regeneration After Transected Spinal Cord Injury. Adv. Healthc. Mater. 2018, 7, 1800315. [Google Scholar] [CrossRef]
- Pawelec, K.M.; Hix, J.; Shapiro, E.M.; Sakamoto, J. The mechanics of scaling-up multichannel scaffold technology for clinical nerve repair. J. Mech. Behav. Biomed. Mater. 2019, 91, 247–254. [Google Scholar] [CrossRef]
- Papadimitriou, L.; Manganas, P.; Ranella, A.; Stratakis, E. Biofabrication for neural tissue engineering applications. Mater. Today Bio 2020, 6, 100043. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, S.; Singha, S.; Cho, M.R.; Gordon, R.J. 3D femtosecond laser patterning of collagen for directed cell attachment. Biomaterials 2004, 26, 4597–4605. [Google Scholar] [CrossRef]
- Sarig-Nadir, O.; Livnat, N.; Zajdman, R.; Shoham, S.; Seliktar, D. Laser Photoablation of Guidance Microchannels into Hydrogels Directs Cell Growth in Three Dimensions. Biophys. J. 2009, 96, 4743–4752. [Google Scholar] [CrossRef] [PubMed]
- De Paoli Lacerda, S.H.; Park, J.J.; Meuse, C.; Pristinski, D.; Becker, M.L.; Karim, A.; Douglas, J.F. Interaction of gold nanoparticles with common human blood proteins. ACS Nano 2010, 4, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Yesildag, C.; Zhang, Z.; Lensen, M. Surface Patterning of Gold Nanoparticles on PEG-Based Hydrogels to Control Cell Adhesion. Polymers 2017, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Tosa, N.; Bosson, J.; Vitrant, G.; Baldeck, P.; Stephan, O. Fabrication of metallic nanowires by two-photon absorption. Sci. Study Res. 2006, 7, 899–904. [Google Scholar]
- Bellec, M.; Royon, A.; Bousquet, B.; Bourhis, K.; Treguer, M.; Cardinal, T.; Richardson, M.; Canioni, L. Beat the diffraction limit in 3D direct laser writing in photosensitive glass. Opt. Express 2009, 17, 10304–10318. [Google Scholar] [CrossRef]
- Machida, M.; Niidome, T.; Onoe, H.; Heisterkamp, A.; Terakawa, M. Spatially-targeted laser fabrication of multi-metal microstructures inside a hydrogel. Opt. Express 2019, 27, 14657–14666. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.H.; Sun, J.; Longtin, J.P. Breakdown threshold and localized electron density in water induced by ultrashort laser pulses. J. Appl. Phys. 2002, 91, 2530–2536. [Google Scholar] [CrossRef]
- Schaffer, C.B.; Nishimura, N.; Glezer, E.N.; Kim, A.M.-T.; Mazur, E. Dynamics of femtosecond laser-induced breakdown in water from femtoseconds to microseconds. Opt. Express 2002, 10, 169–203. [Google Scholar] [CrossRef]
- Noack, J.; Vogel, A. Laser-induced plasma formation in water at nanosecond to femtosecond time scales: Calculation of thresholds, absorption coefficients, and energy density. IEEE J. Quantum Electron. 1999, 35, 1156–1167. [Google Scholar] [CrossRef]
- Pradhan, S.; Keller, K.A.; Sperduto, J.L.; Slater, J.H. Fundamentals of Laser-Based Hydrogel Degradation and Applications in Cell and Tissue Engineering. Adv. Healthc. Mater. 2017, 6, 1700681–1700709. [Google Scholar] [CrossRef]
- Kang, S.; Vora, K.; Mazur, E. One-step direct-laser metal writing of sub100nm 3D silver nanostructures in a gelatin matrix. Nanotechnology 2015, 26, 121001–121007. [Google Scholar] [CrossRef] [PubMed]
- Terakawa, M.; Torres-Mapa, M.L.; Takami, A.; Heinemann, D.; Nedyalkov, N.N.; Nakajima, Y.; Hördt, A.; Ripken, T.; Heisterkamp, A. Femtosecond laser direct writing of metal microstructure in a stretchable poly(ethylene glycol) diacrylate (PEGDA) hydrogel. Opt. Lett. 2016, 41, 1392–1395. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Lorenzo, I.; Jradi, S.; Adam, P.M. Direct laser writing of random Au nanoparticle three-dimensional structures for highly reproducible micro-SERS measurements. Rsc Adv. 2014, 4, 4128–4133. [Google Scholar] [CrossRef]
- Tasche, D.; Weber, M.; Mrotzek, J.; Gerhard, C.; Wieneke, S.; Möbius, W.; Höfft, O.; Viöl, W. In situ investigation of the formation kinematics of plasma-generated silver nanoparticles. Nanomaterials 2020, 10, 555. [Google Scholar] [CrossRef]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G. Determination of size and concentration of gold nanoparticles from UV-Vis spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takayama, I.; Katayama, A.; Terakawa, M. Fabrication of Hollow Channels Surrounded by Gold Nanoparticles in Hydrogel by Femtosecond Laser Irradiation. Nanomaterials 2020, 10, 2529. https://doi.org/10.3390/nano10122529
Takayama I, Katayama A, Terakawa M. Fabrication of Hollow Channels Surrounded by Gold Nanoparticles in Hydrogel by Femtosecond Laser Irradiation. Nanomaterials. 2020; 10(12):2529. https://doi.org/10.3390/nano10122529
Chicago/Turabian StyleTakayama, Izumi, Akito Katayama, and Mitsuhiro Terakawa. 2020. "Fabrication of Hollow Channels Surrounded by Gold Nanoparticles in Hydrogel by Femtosecond Laser Irradiation" Nanomaterials 10, no. 12: 2529. https://doi.org/10.3390/nano10122529
APA StyleTakayama, I., Katayama, A., & Terakawa, M. (2020). Fabrication of Hollow Channels Surrounded by Gold Nanoparticles in Hydrogel by Femtosecond Laser Irradiation. Nanomaterials, 10(12), 2529. https://doi.org/10.3390/nano10122529



