The Effects of a Varied Gold Shell Thickness on Iron Oxide Nanoparticle Cores in Magnetic Manipulation, T1 and T2 MRI Contrasting, and Magnetic Hyperthermia
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Multistage Magnetic-Plasmonic Nanoparticles
2.2. Magnetophoresis
2.3. NMR T1 and T2 Relaxation Measurements
2.4. Magnetic Hyperthermia
3. Results
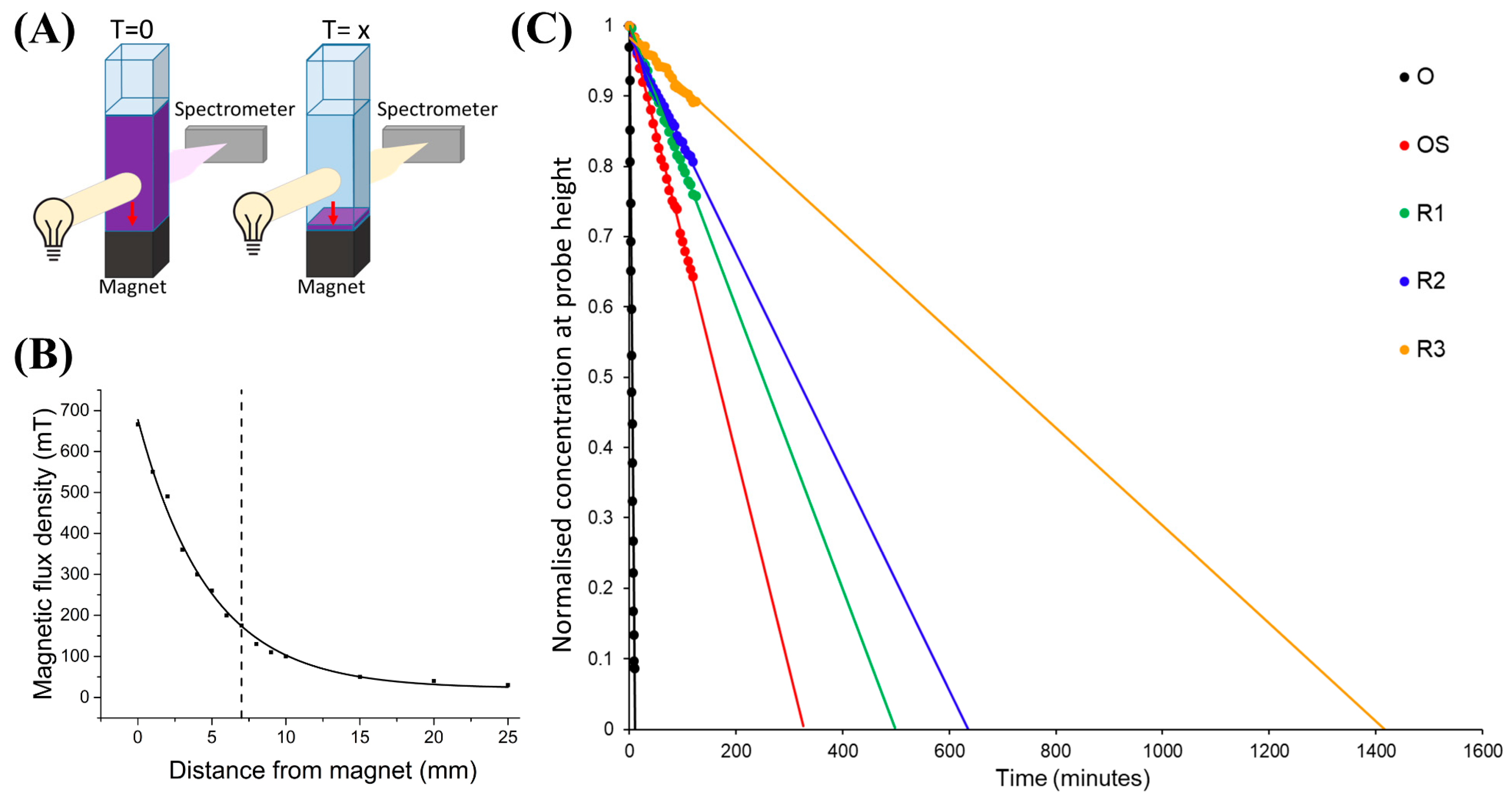
3.1. Magnetophoresis
3.2. NMR/MRI Contrast Agent
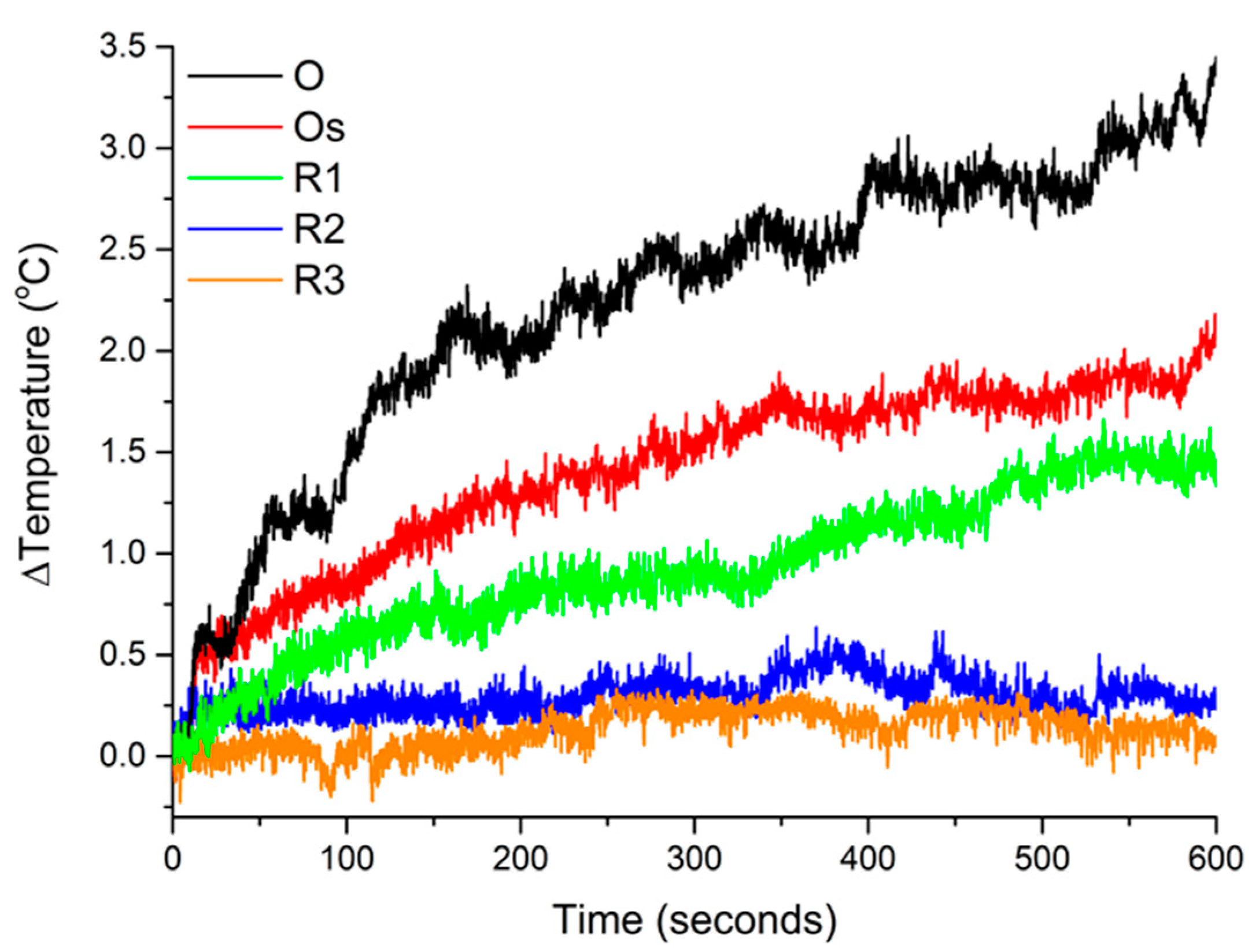
3.3. Magnetic Hyperthermia
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hu, X.; Tang, Y.; Hu, Y.; Lu, F.; Lu, X.; Wang, Y.; Li, J.; Li, Y.; Ji, Y.; Wang, W.; et al. Gadolinium-chelated conjugated polymer-based nanotheranostics for photoacoustic/magnetic resonance/NIR-II fluorescence imaging-guided cancer photothermal therapy. Theranostics 2019, 9, 4168–4181. [Google Scholar] [CrossRef] [PubMed]
- Dadfar, S.M.; Roemhild, K.; Drude, N.I.; von Stillfried, S.; Knüchel, R.; Kiessling, F.; Lammers, T. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 2019, 138, 302–325. [Google Scholar] [CrossRef] [PubMed]
- Knežević, N.; Kaluderović, G.N. Silicon-based nanotheranostics. Nanoscale 2017, 9, 12821–12829. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, P.; Vinhas, R.; Fernandes, A.; Baptista, P.V. Gold nanotheranostics: Proof-of-concept or clinical tool? Nanomaterials 2015, 5, 1853–1879. [Google Scholar] [CrossRef]
- Saini, A.; Maurer, T.; Lorenzo, I.I.; Santos, A.R.; Béal, J.; Goffard, J.; Gérard, D.; Vial, A.; Plain, J. Synthesis and SERS Application of SiO2@Au Nanoparticles. Plasmonics 2015, 10, 791–796. [Google Scholar] [CrossRef][Green Version]
- Rastinehad, A.R.; Anastos, H.; Wajswol, E.; Winoker, J.S.; Sfakianos, J.P.; Doppalapudi, S.K.; Carrick, M.R.; Knauer, C.J.; Taouli, B.; Lewis, S.C.; et al. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proc. Natl. Acad. Sci. USA 2019, 116, 18590–18596. [Google Scholar] [CrossRef]
- Imai, M.; Mine, K.; Tomonari, H.; Uchiyama, J.; Matuzaki, S.; Niko, Y.; Hadano, S.; Watanabe, S. Dark-Field Microscopic Detection of Bacteria using Bacteriophage-Immobilized SiO2@AuNP Core-Shell Nanoparticles. Anal. Chem. 2019, 91, 12352–12357. [Google Scholar] [CrossRef]
- Gossuin, Y.; Gillis, P.; Hocq, A.; Vuong, Q.L.; Roch, A. Magnetic resonance relaxation properties of superparamagnetic particles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 299–310. [Google Scholar] [CrossRef]
- Shao, H.; Yoon, T.-J.; Liong, M.; Weissleder, R.; Lee, H. Magnetic nanoparticles for biomedical NMR-based diagnostics. Beilstein J. Nanotechnol. 2010, 1, 142–154. [Google Scholar] [CrossRef]
- Price, P.M.; Mahmoud, W.E.; Al-Ghamdi, A.A.; Bronstein, L.M. Magnetic drug delivery: Where the field is going. Front. Chem. 2018, 6. [Google Scholar] [CrossRef]
- Dukenbayev, K.; Korolkov, I.V.; Tishkevich, D.I.; Kozlovskiy, A.L.; Trukhanov, S.V.; Gorin, Y.G.; Shumskaya, E.E.; Kaniukov, E.Y.; Vinnik, D.A.; Zdorovets, M.V.; et al. Fe3O4 nanoparticles for complex targeted delivery and boron neutron capture therapy. Nanomaterials 2019, 9, 494. [Google Scholar] [CrossRef]
- Salunkhe, A.B.; Khot, V.M.; Pawar, S.H. Magnetic Hyperthermia with Magnetic Nanoparticles: A Status Review. Curr. Top. Med. Chem. 2014, 14, 572–594. [Google Scholar] [CrossRef] [PubMed]
- Thorat, N.D.; Tofail, S.A.M.; Von Rechenberg, B.; Townley, H.; Brennan, G.; Silien, C.; Yadav, H.M.; Steffen, T.; Bauer, J. Physically stimulated nanotheranostics for next generation cancer therapy: Focus on magnetic and light stimulations. Appl. Phys. Rev. 2019, 6, 041306. [Google Scholar] [CrossRef]
- Zborowski, M.; Chalmers, J.J. Rare cell separation and analysis by magnetic sorting. Anal. Chem. 2011, 83, 8050–8056. [Google Scholar] [CrossRef] [PubMed]
- Belyanina, I.; Kolovskaya, O.; Zamay, S.; Gargaun, A.; Zamay, T.; Kichkailo, A. Targeted magnetic nanotheranostics of cancer. Molecules 2017, 22, 975. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Jon, S. Gold nanoparticles in image-guided cancer therapy. Inorg. Chim. Acta 2012, 393, 154–164. [Google Scholar] [CrossRef]
- Alvarez-Puebla, R.A.; Liz-Marzán, L.M. SERS-based diagnosis and biodetection. Small 2010, 6, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Panchapakesan, B.; Book-Newell, B.; Sethu, P.; Rao, M.; Irudayaraj, J. Gold nanoprobes for theranostics. Nanomedicine 2011, 6, 1787–1811. [Google Scholar] [CrossRef]
- Artur, C.G.; Womack, T.; Zhao, F.; Eriksen, J.L.; Mayerich, D.; Shih, W.-C. Plasmonic nanoparticle-based expansion microscopy with surface-enhanced Raman and dark-field spectroscopic imaging. Biomed. Opt. Express 2018, 9, 603. [Google Scholar] [CrossRef]
- Sokolov, K.; Follen, M.; Aaron, J.; Pavlova, I.; Malpica, A.; Lotan, R.; Richards-Kortum, R. Real-time vital optical imaging of precancer using anti-epidermal growth factor receptor antibodies conjugated to gold nanoparticles. Cancer Res. 2003, 63, 1999–2004. [Google Scholar]
- Kuttner, C. Plasmonics in Sensing: From Colorimetry to SERS Analytics. In Plasmonics; IntechOpen: London, UK, 2018. [Google Scholar]
- Duncan, B.; Kim, C.; Rotello, V.M. Gold nanoparticle platforms as drug and biomacromolecule delivery systems. J. Control. Release 2010, 148, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med. Sci. 2008, 23, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Asharani, P.V.; Lianwu, Y.; Gong, Z.; Valiyaveettil, S. Comparison of the toxicity of silver, gold and platinum nanoparticles in developing zebrafish embryos. Nanotoxicology 2011, 5, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Li, Y.; Niu, D.; Ma, Z.; Gu, J.; Chen, Y.; Zhao, W.; Liu, X.; Liu, C.; Shi, J. Facile synthesis of monodisperse superparamagnetic Fe3O4 Core@hybrid@Au shell nanocomposite for bimodal imaging and photothermal therapy. Adv. Mater. 2011, 23, 5392–5397. [Google Scholar] [CrossRef]
- Esenturk, E.N.; Hight Walker, A.R. Gold nanostar @ iron oxide core–shell nanostructures: Synthesis, characterization, and demonstrated surface-enhanced Raman scattering properties. J. Nanoparticle Res. 2013, 15, 1364. [Google Scholar] [CrossRef]
- JitKang Lim, B.; Eggeman, A.; Lanni, F.; Tilton, R.D.; Majetich, S.A.; Majetich, S.A.; Eggeman, A.; Lim, J.K.; Tilton, R.D.; Lanni, F. Synthesis and Single-Particle Optical Detection of Low-Polydispersity Plasmonic-Superparamagnetic Nanoparticles. Adv. Mater 2008, 20, 1721–1726. [Google Scholar] [CrossRef]
- Brennan, G.; Thorat, N.D.; Pescio, M.; Bergamino, S.; Bauer, J.; Liu, N.; Tofail, S.A.M.; Silien, C. Spectral drifts in surface textured Fe3O4-Au, core–shell nanoparticles enhance spectra-selective photothermal heating and scatter imaging. Nanoscale 2020, 12, 12632–12638. [Google Scholar] [CrossRef]
- Jain, P.K.; Xiao, Y.; Walsworth, R.; Cohen, A.E. Surface Plasmon Resonance Enhanced Magneto-Optics (SuPREMO): Faraday Rotation Enhancement in Gold-Coated Iron Oxide Nanocrystals. Nano Lett. 2009, 9, 1644–1650. [Google Scholar] [CrossRef]
- Chen, K.-L.; Yeh, Y.-W.; Chen, J.-M.; Hong, Y.-J.; Huang, T.-L.; Deng, Z.-Y.; Wu, C.-H.; Liao, S.-H.; Wang, L.-M. Influence of magnetoplasmonic γ-Fe2O3/Au core/shell nanoparticles on low-field nuclear magnetic resonance. Sci. Rep. 2016, 6, 35477. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, J.; Zhong, Y.; Zhang, J.; Wang, Z.; Wang, L.; An, Y.; Lin, M.; Gao, Z.; Zhang, D. Biocompatibility of Fe3O4@Au composite magnetic nanoparticles in vitro and in vivo. Int. J. Nanomed. 2011, 6, 2805–2819. [Google Scholar] [CrossRef] [PubMed]
- Karakoçak, B.B.; Raliya, R.; Davis, J.T.; Chavalmane, S.; Wang, W.N.; Ravi, N.; Biswas, P. Biocompatibility of gold nanoparticles in retinal pigment epithelial cell line. Toxicol. Vitr. 2016, 37, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.; Cole, B.; Ghelardini, M.; Powell, B.A.; Mefford, O.T. Quantitative Measurement of Ligand Exchange with Small-Molecule Ligands on Iron Oxide Nanoparticles via Radioanalytical Techniques. Langmuir 2016, 32, 13716–13727. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Cai, H.; Wang, X.; Cao, X.; Li, K.; Wang, S.H.; Guo, R.; Zheng, L.; Zhang, G.; Shi, X. Facile one-pot preparation, surface functionalization, and toxicity assay of APTS-coated iron oxide nanoparticles. Nanotechnology 2012, 23. [Google Scholar] [CrossRef] [PubMed]
- Duff, D.G.; Baiker, A.; Edwards, P.P. A new hydrosol of gold clusters. J. Chem. Soc. Chem. Commun. 1993, 9, 96–98. [Google Scholar] [CrossRef]
- Oldenburg, S.J.; Averitt, R.D.; Westcott, S.L.; Halas, N.J. Nanoengineering of optical resonances. Chem. Phys. Lett. 1998, 288, 243–247. [Google Scholar] [CrossRef]
- Herea, D.-D.; Danceanu, C.; Radu, E.; Labusca, L.; Lupu, N.; Chiriac, H. Comparative effects of magnetic and water-based hyperthermia treatments on human osteosarcoma cells. Int. J. Nanomed. 2018, 13, 5743–5751. [Google Scholar] [CrossRef]
- Lim, J.K.; Yeap, S.P.; Leow, C.H.; Toh, P.Y.; Low, S.C. Magnetophoresis of iron oxide nanoparticles at low field gradient: The role of shape anisotropy. J. Colloid Interface Sci. 2014, 421, 170–177. [Google Scholar] [CrossRef]
- Lyons, S.; Mc Kiernan, E.P.; Dee, G.; Brougham, D.F.; Morrin, A. Electrostatically modulated magnetophoretic transport of functionalised iron-oxide nanoparticles through hydrated networks. Nanoscale 2020, 12, 10550–10558. [Google Scholar] [CrossRef]
- Lim, J.; Lanni, C.; Evarts, E.R.; Lanni, F.; Tilton, R.D.; Majetich, S.A. Magnetophoresis of nanoparticles. ACS Nano 2011, 5, 217–226. [Google Scholar] [CrossRef]
- Lim, J.; Yeap, S.P.; Low, S.C. Challenges associated to magnetic separation of nanomaterials at low field gradient. Sep. Purif. Technol. 2014, 123, 171–174. [Google Scholar] [CrossRef]
- Yeap, S.P.; Ahmad, A.L.; Ooi, B.S.; Lim, J. Electrosteric stabilization and its role in cooperative magnetophoresis of colloidal magnetic nanoparticles. Langmuir 2012, 28, 14878–14891. [Google Scholar] [CrossRef] [PubMed]
- Bakhteeva, I.A.; Medvedeva, I.V.; Uimin, M.A.; Byzov, I.V.; Zhakov, S.V.; Yermakov, A.E.; Shchegoleva, N.N. Magnetic sedimentation and aggregation of Fe3O4@SiO2 nanoparticles in water medium. Sep. Purif. Technol. 2016, 159, 35–42. [Google Scholar] [CrossRef]
- Benelmekki, M.; Montras, A.; Martins, A.J.; Coutinho, P.J.G.; Martinez, L.M. Magnetophoresis behaviour at low gradient magnetic field and size control of nickel single core nanobeads. J. Magn. Magn. Mater. 2011, 323, 1945–1949. [Google Scholar] [CrossRef]
- Moeser, G.D.; Roach, K.A.; Green, W.H.; Hatton, T.A.; Laibinis, P.E. High-gradient magnetic separation of coated magnetic nanoparticles. AIChE J. 2004, 50, 2835–2848. [Google Scholar] [CrossRef]
- Gerbei, R.; Takayasu, M.; Friedlaender, F.J. Generalization of HGMS theory: The capture of ultra-fine particles. IEEE Trans. Magn. 1983, 19, 2115–2117. [Google Scholar] [CrossRef]
- Yavuz, C.T.; Mayo, J.T.; Yu, W.W.; Prakash, A.; Falkner, J.C.; Yean, S.; Cong, L.; Shipley, H.J.; Kan, A.; Tomson, M.; et al. Low-field magnetic separation of monodisperse Fe3O4 nanocrystals. Science 2006, 314, 964–967. [Google Scholar] [CrossRef]
- Faraudo, J.; Andreu, J.S.; Camacho, J. Understanding diluted dispersions of superparamagnetic particles under strong magnetic fields: A review of concepts, theory and simulations. Soft Matter 2013, 9, 6654–6664. [Google Scholar] [CrossRef]
- Zahn, K.; Lenke, R.; Maret, G. Friction coefficient of rod-like chains of spheres at very low Reynolds numbers. I. Experiment. J. Phys. II 1994, 4, 555–560. [Google Scholar] [CrossRef]
- Tamer, U.; Cetin, D.; Suludere, Z.; Boyaci, I.H.; Temiz, H.T.; Yegenoglu, H.; Daniel, P.; Dinçer, I.; Elerman, Y. Gold-coated iron composite nanospheres targeted the detection of Escherichia coli. Int. J. Mol. Sci. 2013, 14, 6223–6240. [Google Scholar] [CrossRef]
- Dizaji, A.N.; Yilmaz, M.; Piskin, E. Silver or gold deposition onto magnetite nanoparticles by using plant extracts as reducing and stabilizing agents. Artif. CellsNanomed. Biotechnol. 2016, 44, 1109–1115. [Google Scholar] [CrossRef]
- Xie, H.Y.; Zhen, R.; Wang, B.; Feng, Y.J.; Chen, P.; Hao, J. Fe3O4/Au core/shell nanoparticles modified with Ni2+-nitrilotriacetic acid specific to histidine-tagged proteins. J. Phys. Chem. C 2010, 114, 4825–4830. [Google Scholar] [CrossRef]
- Tomitaka, A.; Ota, S.; Nishimoto, K.; Arami, H.; Takemura, Y.; Nair, M. Dynamic magnetic characterization and magnetic particle imaging enhancement of magnetic-gold core–shell nanoparticles. Nanoscale 2019, 11, 6489–6496. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Lei, S.L.; Lu, J.H.; He, Y.; Chen, Z.W.; Ren, L.; Zhou, X. Potential use of SERS-assisted theranostic strategy based on Fe3O4/Au cluster/shell nanocomposites for bio-detection, MRI, and magnetic hyperthermia. Mater. Sci. Eng. C 2016, 64, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Montazerabadi, A.R.; Oghabian, M.A.; Irajirad, R.; Muhammadnejad, S.; Ahmadvand, D.; Delavari, H.H.; Mahdavi, S.R. Development of Gold-Coated Magnetic Nanoparticles as a Potential MRI Contrast Agent. Nano 2015, 10, 1550048. [Google Scholar] [CrossRef]
- Gangopadhyay, P.; Gallet, S.; Franz, E.; Persoons, A.; Verbiest, T. Novel superparamagnetic core(shell) nanoparticles for magnetic targeted drug delivery and hyperthermia treatment. IEEE Trans. Magn. 2005, 41, 4194–4196. [Google Scholar] [CrossRef]
- Billen, A.; de Cattelle, A.; Jochum, J.K.; Van Bael, M.J.; Billen, J.; Seo, J.W.; Brullot, W.; Koeckelberghs, G.; Verbiest, T. Novel synthesis of superparamagnetic plasmonic core-shell iron oxide-gold nanoparticles. Phys. B Condens. Matter 2019, 560, 85–90. [Google Scholar] [CrossRef]
- Hao, L.; Leng, Y.; Zeng, L.; Chen, X.; Chen, J.; Duan, H.; Huang, X.; Xiong, Y.; Chen, X. Core–Shell-Heterostructured Magnetic–Plasmonic Nanoassemblies with Highly Retained Magnetic–Plasmonic Activities for Ultrasensitive Bioanalysis in Complex Matrix. Adv. Sci. 2020, 7. [Google Scholar] [CrossRef]
- Song, Y.; Ding, J.; Wang, Y. Shell-dependent evolution of optical and magnetic properties of Co@Au core-shell nanoparticles. J. Phys. Chem. C 2012, 116, 11343–11350. [Google Scholar] [CrossRef]
- Ammar, M.; Mazaleyrat, F.; Bonnet, J.P.; Audebert, P.; Brosseau, A.; Wang, G.; Champion, Y. Synthesis and characterization of core–shell structure silica-coated Fe29.5Ni70.5 nanoparticles. Nanotechnology 2007, 18, 285606. [Google Scholar] [CrossRef]
- Banerjee, S.; Raja, S.O.; Sardar, M.; Gayathri, N.; Ghosh, B.; Dasgupta, A. Iron oxide nanoparticles coated with gold: Enhanced magnetic moment due to interfacial effects. J. Appl. Phys. 2011, 109, 123902. [Google Scholar] [CrossRef]
- León-Félix, L.; Chaker, J.; Parise, M.; Coaquira, J.A.H.; De Los Santos Valladares, L.; Bustamante, A.; Garg, V.K.; Oliveira, A.C.; Morais, P.C. Synthesis and characterization of uncoated and gold-coated magnetite nanoparticles. Hyperfine Interact. 2014, 224, 179–188. [Google Scholar] [CrossRef]
- Runge, V.M. Safety of the Gadolinium-Based Contrast Agents for Magnetic Resonance Imaging, Focusing in Part on Their Accumulation in the Brain and Especially the Dentate Nucleus. Investig. Radiol. 2016, 51, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhao, Z.; Zhang, H.; Wang, Z.; Chen, X.; Wang, R.; Chen, Z.; Gao, J. Interplay between longitudinal and transverse contrasts in Fe3O4 nanoplates with (111) exposed surfaces. ACS Nano 2014, 8, 7976–7985. [Google Scholar] [CrossRef] [PubMed]
- Roch, A.; Gossuin, Y.; Muller, R.N.; Gillis, P. Superparamagnetic colloid suspensions: Water magnetic relaxation and clustering. J. Magn. Magn. Mater. 2005, 293, 532–539. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, L.; Chen, H.; Hu, K.; Delahunty, I.; Gao, S.; Xie, J. Surface impact on nanoparticle-based magnetic resonance imaging contrast agents. Theranostics 2018, 8, 2521–2548. [Google Scholar] [CrossRef]
- Marangoni, V.S.; Neumann, O.; Henderson, L.; Kaffes, C.C.; Zhang, H.; Zhang, R.; Bishnoi, S.; Ayala-Orozco, C.; Zucolotto, V.; Bankson, J.A.; et al. Enhancing T1 magnetic resonance imaging contrast with internalized gadolinium(III) in a multilayer nanoparticle. Proc. Natl. Acad. Sci. USA 2017, 114, 6960–6965. [Google Scholar] [CrossRef]
- Pinho, S.L.C.; Pereira, G.A.; Voisin, P.; Kassem, J.; Bouchaud, V.; Etienne, L.; Peters, J.A.; Carlos, L.; Mornet, S.; Geraldes, C.F.G.C.; et al. Fine Tuning of the Relaxometry of γ-Fe2O3@SiO2 Nanoparticles by Tweaking the Silica Coating Thickness. ACS Nano 2010, 4, 5339–5349. [Google Scholar] [CrossRef]
- Joshi, H.M.; De, M.; Richter, F.; He, J.; Prasad, P.V.; Dravid, V.P. Effect of silica shell thickness of Fe3O4–SiOx core–shell nanostructures on MRI contrast. J. Nanoparticle Res. 2013, 15, 1448. [Google Scholar] [CrossRef]
- Ye, F.; Laurent, S.; Fornara, A.; Astolfi, L.; Qin, J.; Roch, A.; Martini, A.; Toprak, M.S.; Muller, R.N.; Muhammed, M. Uniform mesoporous silica coated iron oxide nanoparticles as a highly efficient, nontoxic MRI T2 contrast agent with tunable proton relaxivities. Contrast Media Mol. Imaging 2012, 7, 460–468. [Google Scholar] [CrossRef]
- Park, J.A.; Lee, J.J.; Kim, I.S.; Park, B.H.; Lee, G.H.; Kim, T.J.; Ri, H.C.; Kim, H.J.; Chang, Y. Magnetic and MR relaxation properties of avidin-biotin conjugated superparamagnetic nanoparticles. Colloids Surf. A Phys. Eng. Asp. 2008, 313, 288–291. [Google Scholar] [CrossRef]
- Peiris, P.M.; Schmidt, E.; Calabrese, M.; Karathanasis, E. Assembly of Linear Nano-Chains from Iron Oxide Nanospheres with Asymmetric Surface Chemistry. PLoS ONE 2011, 6, e15927. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carvalho, A.; Domingues, I.; Gonçalves, M.C. Core-shell superparamagnetic nanoparticles with interesting properties as contrast agents for MRI. Mater. Chem. Phys. 2015, 168, 42–49. [Google Scholar] [CrossRef]
- Umut, E.; Pineider, F.; Arosio, P.; Sangregorio, C.; Corti, M.; Tabak, F.; Lascialfari, A.; Ghigna, P. Magnetic, optical and relaxometric properties of organically coated gold-magnetite (Au-Fe3O4) hybrid nanoparticles for potential use in biomedical applications. J. Magn. Magn. Mater. 2012, 324, 2373–2379. [Google Scholar] [CrossRef]
- Lin, L.-S.; Yang, X.; Zhou, Z.; Yang, Z.; Jacobson, O.; Liu, Y.; Yang, A.; Niu, G.; Song, J.; Yang, H.H.; et al. Yolk–Shell Nanostructure: An Ideal Architecture to Achieve Harmonious Integration of Magnetic–Plasmonic Hybrid Theranostic Platform. Adv. Mater. 2017, 29, 1606681. [Google Scholar] [CrossRef]
- Wust, P.; Hildebrandt, B.; Sreenivasa, G.; Rau, B.; Gellermann, J.; Riess, H.; Felix, R.; Schlag, P. Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002, 3, 487–497. [Google Scholar] [CrossRef]
- Saville, S.L.; Qi, B.; Baker, J.; Stone, R.; Camley, R.E.; Livesey, K.L.; Ye, L.; Crawford, T.M.; Thompson Mefford, O. The formation of linear aggregates in magnetic hyperthermia: Implications on specific absorption rate and magnetic anisotropy. J. Colloid Interface Sci. 2014, 424, 141–151. [Google Scholar] [CrossRef]
- Das, H.; Debnath, N.; Arai, T.; Kawaguchi, T.; Sakamoto, N.; Shinozaki, K.; Suzuki, H.; Wakiya, N. Superparamagnetic magnesium ferrite/silica core-shell nanospheres: A controllable SiO2 coating process for potential magnetic hyperthermia application. Adv. Powder Technol. 2019, 30, 3171–3181. [Google Scholar] [CrossRef]
- Yu, X.; Zhu, Y. Preparation of magnetic mesoporous silica nanoparticles as a multifunctional platform for potential drug delivery and hyperthermia. Sci. Technol. Adv. Mater. 2016, 17, 229–238. [Google Scholar] [CrossRef]
- Hu, Y.; Meng, L.; Niu, L.; Lu, Q. Facile synthesis of superparamagnetic Fe3O4@polyphosphazene@Au shells for magnetic resonance imaging and photothermal therapy. Acs Appl. Mater. Interfaces 2013, 5, 4586–4591. [Google Scholar] [CrossRef]
- Lanier, O.L.; Korotych, O.I.; Monsalve, A.G.; Wable, D.; Savliwala, S.; Grooms, N.W.F.; Nacea, C.; Tuitt, O.R.; Dobson, J. Evaluation of magnetic nanoparticles for magnetic fluid hyperthermia. Int. J. Hyperth. 2019, 36, 687–701. [Google Scholar] [CrossRef] [PubMed]
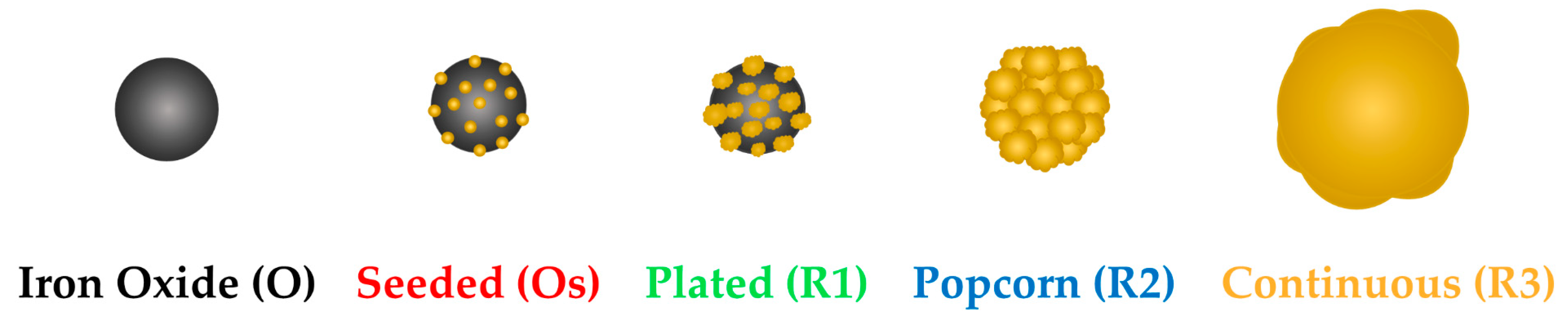


| Application | Fe3O4 (O) | Fe3O4 + Au Seeds (Os) | Fe3O4 + Thin Au Shell (R1) | Fe3O4 + Medium Au Shell (R2) | Fe3O4 + Thick Au Shell (R3) |
|---|---|---|---|---|---|
| Nanoparticle total diameter (nm) [28] | 20.5 ± 1.3 | O + ~3 nm seeds | 28.5 ± 2.2 | 42.1 ± 10.3 | 103.7 ± 16.9 |
| Shell thickness (nm) [28] | - | - | ≈4 | ≈10.8 | ≈41.6 |
| Zeta Potential (mV) [28] | 0.0 ± 4.1 | −10.0 ± 6.8 | −27.3± 2.8 | −22.4 ± 2.1 | −22.1 ± 3.2 |
| Magnetophoresis time (min) | 12 | 326 | 498 | 636 | 1421 |
| Magnetophoresis speed, vmag (μm/s) | 9.7 | 0.4 (4.1%) | 0.2 (2.1%) | 0.2 (2.1%) | 0.1(1%) |
| T1 relaxivity r1 (mM/s) | 0.9 | 0.7 (77.8%) | 0.7 (77.8%) | 0.3 (33.3%) | 0.2 (22.2%) |
| T2 relaxivity r2 (mM/s) | 32.7 | 21.9 (67%) | 15.4 (47.1%) | 15.1 (46.2%) | 13.6 (41.6%) |
| Relaxivity ratio r2/r1 | 35.6 | 30.6 (86%) | 23.3 (65.4%) | 51.5 (144.7%) | 55.5 (155.9%) |
| Max. temperature | 3.4 | 2.1 (61.8%) | 1.5 (44.1%) | 0.3 (8.8%) | 0.1 (2.9%) |
| Initial 60 s ΔT/Δt (m °C/s) | 18.8 | 11.7 (62.2%) | 6.2 (33%) | 1.7 (9%) | 1.3 (6.9%) |
| Specific power absorption (SPA) (W/g) | 93.7 | 58.3 (62.2%) | 30.9 (33%) | 8.5 (9.1%) | 6.5 (6.9%) |
| Intrinsic loss power parameter (ILP) (H m2/kg) | 0.7 | 0.5 (71.4%) | 0.2 (28.6%) | 0.1 (14.3%) | 0.1 (14.3%) |
| Sample | r1 (mM−1 s−1) | r2 (mM−1 s−1) | r2/r1 | Frequency (MHz) | Field (T) | Ref. |
|---|---|---|---|---|---|---|
| Fe3O4 (20 nm) @Au (5 nm) | - | 181.35 | - 1 | 3 | [56] | |
| Fe3O4 (20 nm) @Au (5 nm) + PEG 2 | 162.3 | - | 3 | [56] | ||
| Fe3O4 9 nm + 1 nm SiO2 shell | 94 | - | 3 | [70] | ||
| Fe3O4 9 nm + 5 nm SiO2 shell | 68 | - | 3 | [70] | ||
| Fe3O4 9 nm + 10 nm SiO2 shell | 47 | - | 3 | [70] | ||
| Fe3O4 9 nm + 13 nm SiO2 shell | 32 | - | 3 | [70] | ||
| Fe2O3 ≈ 10 nm | 32 | ≈228 | ≈7.1 | r1 20r2 ≈500 | r1 0.4r2 11.7 | [69] |
| Fe2O3 ≈10 nm + ≈2 nm SiO2 shell | 11.2 | ≈100 | ≈8.9 | “ 3 | “ | [69] |
| Fe2O3 ≈10 nm + ≈8 nm SiO2 shell | <2 | ≈64 | ≈32.0 | “ | “ | [69] |
| Fe2O3 ≈10 nm + ≈15 nm SiO2 shell | <2 | ≈47 | ≈23.5 | “ | “ | [69] |
| Fe2O3 ≈10 nm + ≈20 nm SiO2 shell | <2 | ≈38 | ≈19.0 | “ | “ | [69] |
| Fe2O3 ≈10 nm + ≈28 nm SiO2 shell | <2 | ≈23 | ≈11.5 | “ | “ | [69] |
| Fe2O3 ≈10 nm + ≈52 nm SiO2 shell | <2 | ≈15 | ≈7.5 | “ | “ | [69] |
| Fe2O3 ≈10 nm + ≈67 nm SiO2 shell | <2 | ≈13 | ≈6.5 | “ | “ | [69] |
| Cluster core–shell Fe3O4 6 nm–APTES (≈96.6 nm total) | 0.006 | 40.6 | 6766.7 | 300 | 7 | [74] |
| Cluster core–shell Fe3O4 6 nm–GPTMS 4 (≈22.0 nm total) | 0.026 | 14.4 | 553.8 | 300 | 7 | [74] |
| Cluster core–shell Fe3O4 6 nm–TEOS 5 (≈66.6 nm total) | 0.016 | 13.8 | 862.5 | 300 | 7 | [74] |
| Fe3O4 (30 nm) with asymmetric surface chemistry (amine and thiol) | - | 44.87 | - | 1.4 | [73] | |
| Nanochains of Fe3O4 (30 nm) with amine and thiol surface | - | 101.05 | - | 1.4 | [73] | |
| Fe3O4 (11 nm)–CTAB 6 | 31.25 (13.69) | 81.37 (82.18) | 2.6 (6.0) | 20 (60) | 0.47 (1.41) | [71] |
| Fe3O4 (12 nm) @mSiO2 shell (50 nm total) | 3.65 (1.31) | 84.26 (92.13) | 23.1 (70.3) | “ | “ | [71] |
| Fe3O4 (12 nm) @mSiO2 shell (75 nm total) | 2.13 (0.97) | 79.93 (87.54) | 37.5 (90.3) | “ | “ | [71] |
| Fe3O4 (12 nm) @mSiO2 shell (95 nm total) | 0.61 (0.31) | 50.13 (55.44) | 82.2 (178.8) | “ | “ | [71] |
| Fe3O4 (≈7.48 nm) | ≈37 | ≈48 | ≈1.3 | ≈10 | - | [75] |
| Fe3O4 (≈7.48 nm)–Au (5–8 nm) dimer | ≈5 | ≈62 | ≈12.4 | “ | - | [75] |
| Au core (5–8 nm)–Fe3O4 shell (≈15.92 nm total) | ≈27 | ≈41 | ≈1.5 | “ | - | [75] |
| Fe3O4 cluster (200 nm) | - | 230.7 | - | - | [55] | |
| Fe3O4 cluster (200 nm) + 5 nm Au seeds | - | 147.7 | - | - | [55] | |
| Fe3O4 cluster (200 nm) + 20 nm Au seeds | - | 163.1 | - | - | [55] | |
| Fe3O4 cluster (200 nm) + 25 nm Au shell | - | 158.2 | - | - | [55] | |
| Fe3O4 | - | 167 | - | - | [76] | |
| Fe3O4–Au (core–shell) | - | 61.9 | - | - | [76] | |
| Fe3O4–Au (yolk–shell) | - | 149.4 | - | - | [76] | |
| γ-Fe2O3 core–Au Shell ≈28.38 nm | ≈8.82 (≈10.35) | ≈4.04 (≈3.99) | - (532 nm light) | 100 μT | [30] | |
| 20.5 ± 1.3 nm Fe3O4 (O) | 0.92 | 32.70 | 35.55 | 60 | 1.5 | This work |
| O + ≈3 nm Au seeds (Os) | 0.72 | 21.94 | 30.56 | “ | “ | “ |
| O + ≈4 nm thick Au shell (R1) | 0.66 | 15.43 | 23.27 | “ | “ | “ |
| O + ≈10.8 nm thick Au shell (R2) | 0.29 | 15.06 | 51.48 | “ | “ | “ |
| O + ≈41.6 nm thick Au shell (R3) | 0.24 | 13.58 | 55.48 | “ | “ | “ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brennan, G.; Bergamino, S.; Pescio, M.; Tofail, S.A.M.; Silien, C. The Effects of a Varied Gold Shell Thickness on Iron Oxide Nanoparticle Cores in Magnetic Manipulation, T1 and T2 MRI Contrasting, and Magnetic Hyperthermia. Nanomaterials 2020, 10, 2424. https://doi.org/10.3390/nano10122424
Brennan G, Bergamino S, Pescio M, Tofail SAM, Silien C. The Effects of a Varied Gold Shell Thickness on Iron Oxide Nanoparticle Cores in Magnetic Manipulation, T1 and T2 MRI Contrasting, and Magnetic Hyperthermia. Nanomaterials. 2020; 10(12):2424. https://doi.org/10.3390/nano10122424
Chicago/Turabian StyleBrennan, Grace, Silvia Bergamino, Martina Pescio, Syed A. M. Tofail, and Christophe Silien. 2020. "The Effects of a Varied Gold Shell Thickness on Iron Oxide Nanoparticle Cores in Magnetic Manipulation, T1 and T2 MRI Contrasting, and Magnetic Hyperthermia" Nanomaterials 10, no. 12: 2424. https://doi.org/10.3390/nano10122424
APA StyleBrennan, G., Bergamino, S., Pescio, M., Tofail, S. A. M., & Silien, C. (2020). The Effects of a Varied Gold Shell Thickness on Iron Oxide Nanoparticle Cores in Magnetic Manipulation, T1 and T2 MRI Contrasting, and Magnetic Hyperthermia. Nanomaterials, 10(12), 2424. https://doi.org/10.3390/nano10122424






