Design and Synthesis of Luminescent Lanthanide-Based Bimodal Nanoprobes for Dual Magnetic Resonance (MR) and Optical Imaging
Abstract
1. Introduction
2. MR Imaging and Contrast Agents
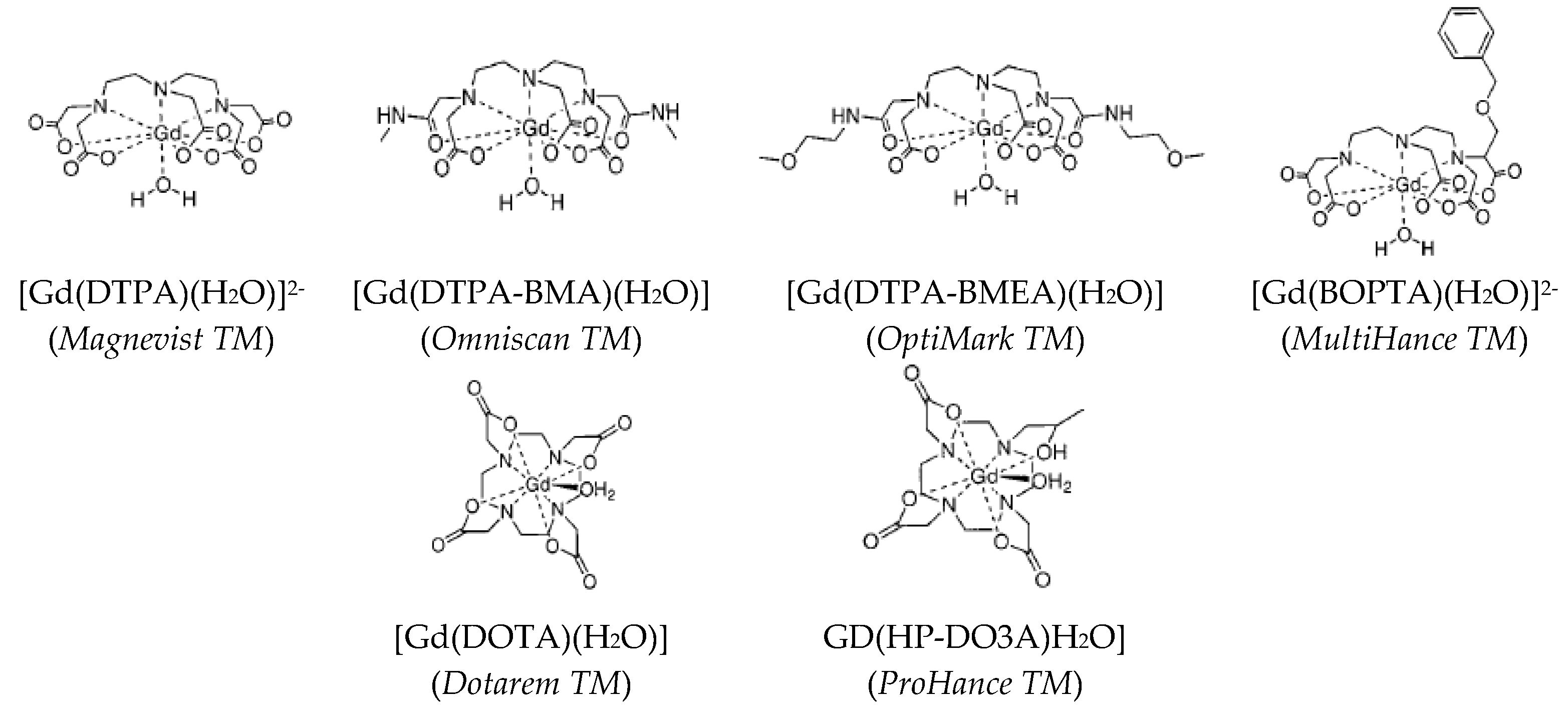
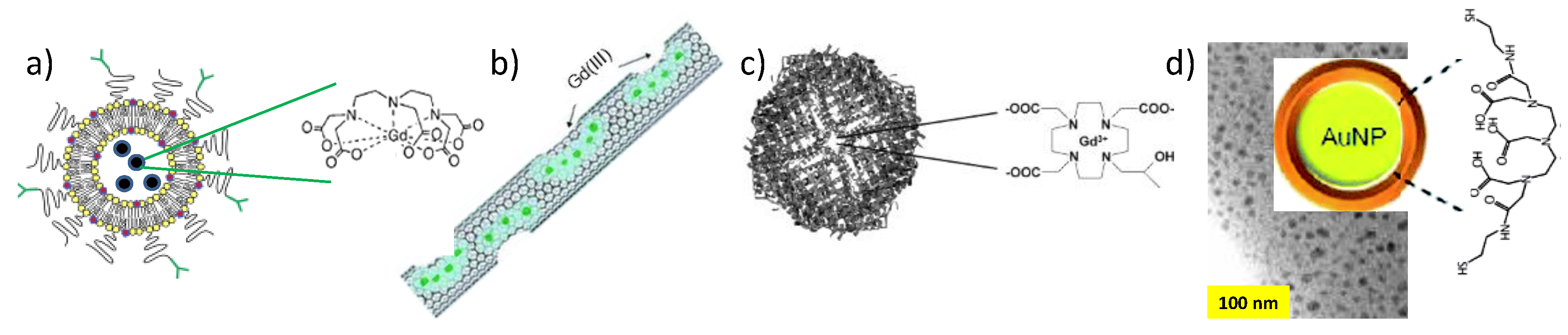
2.1. Positive Contrast Agents
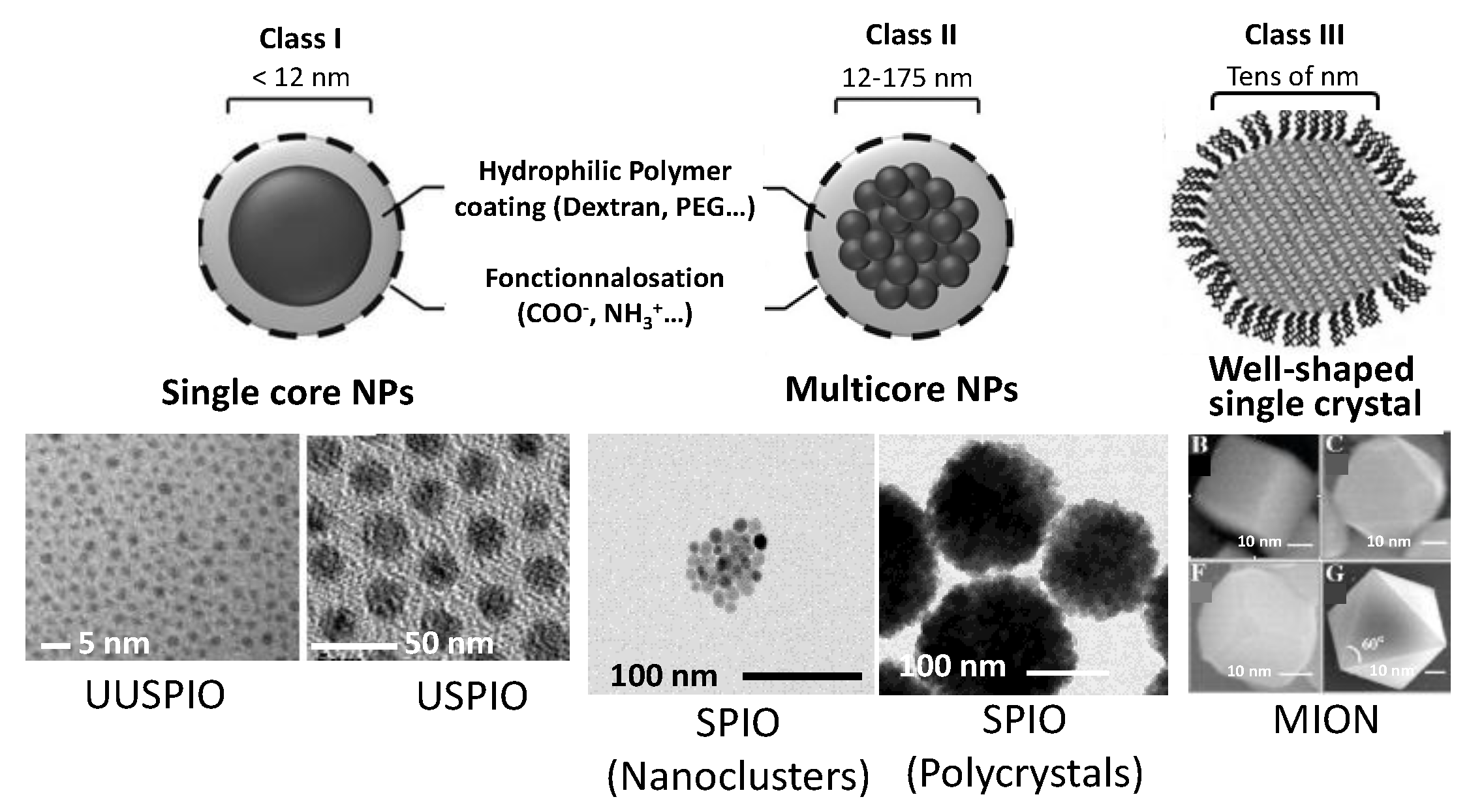
2.2. Negative Contrast Agents
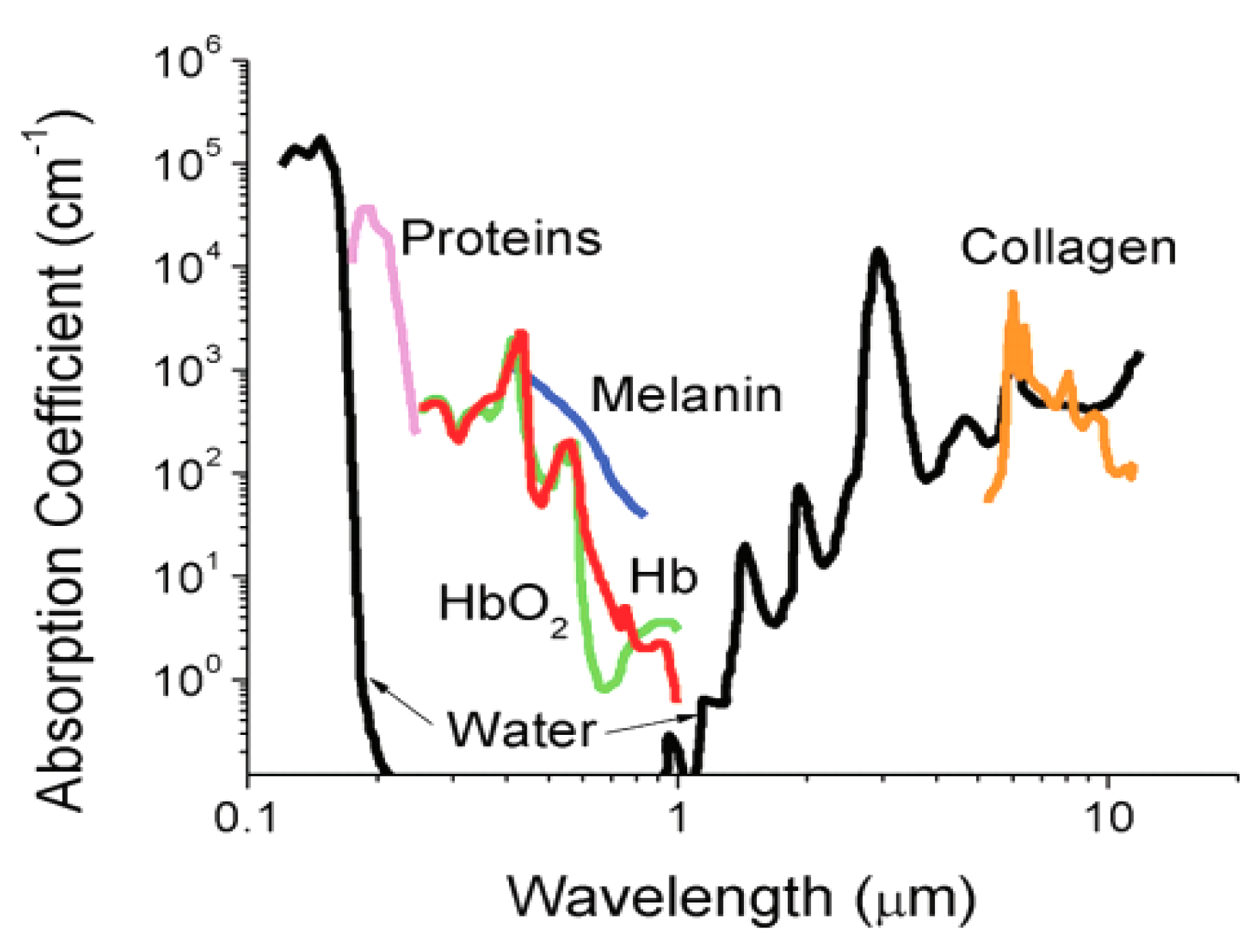
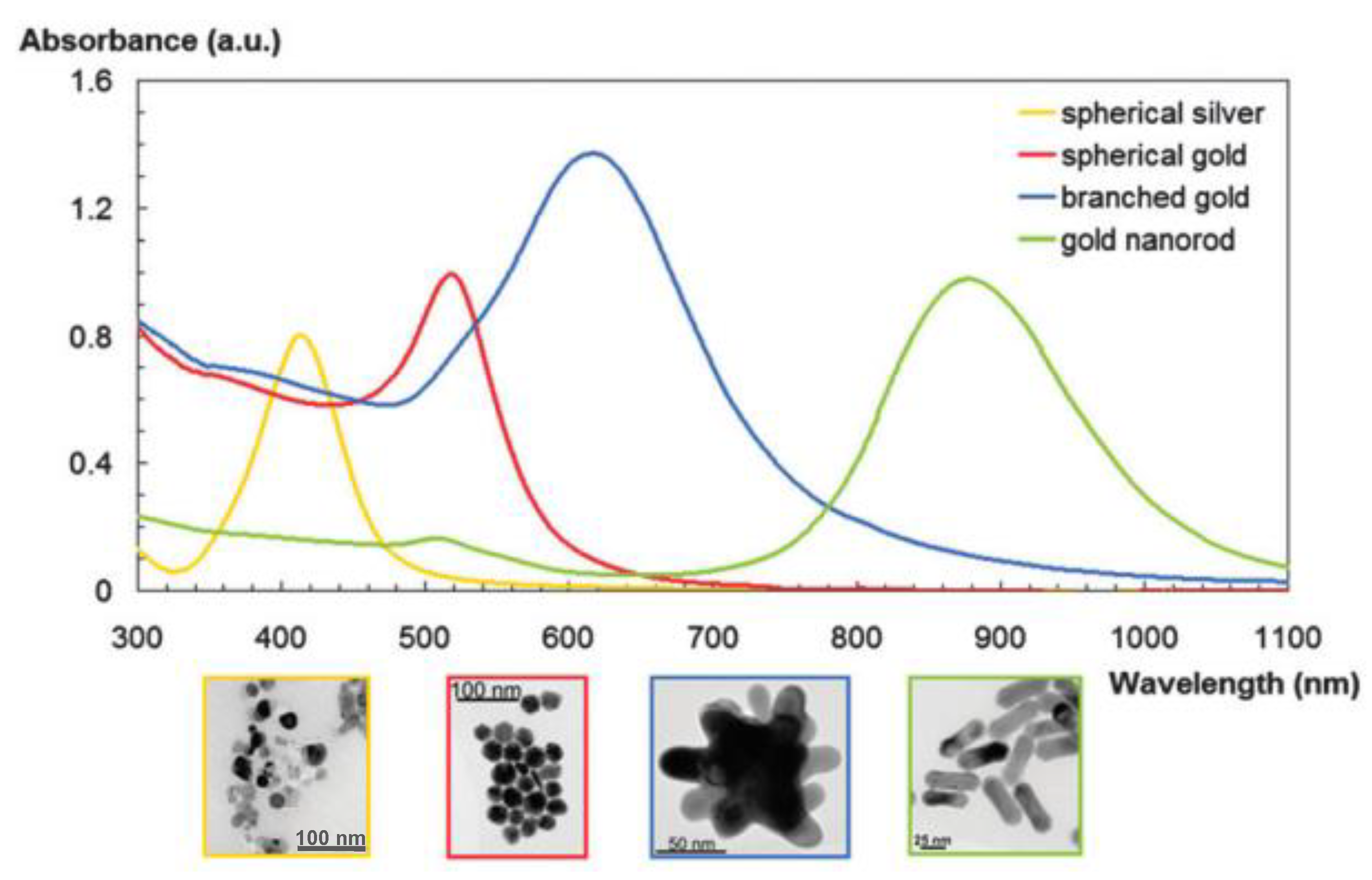
3. Optical Imaging Probes
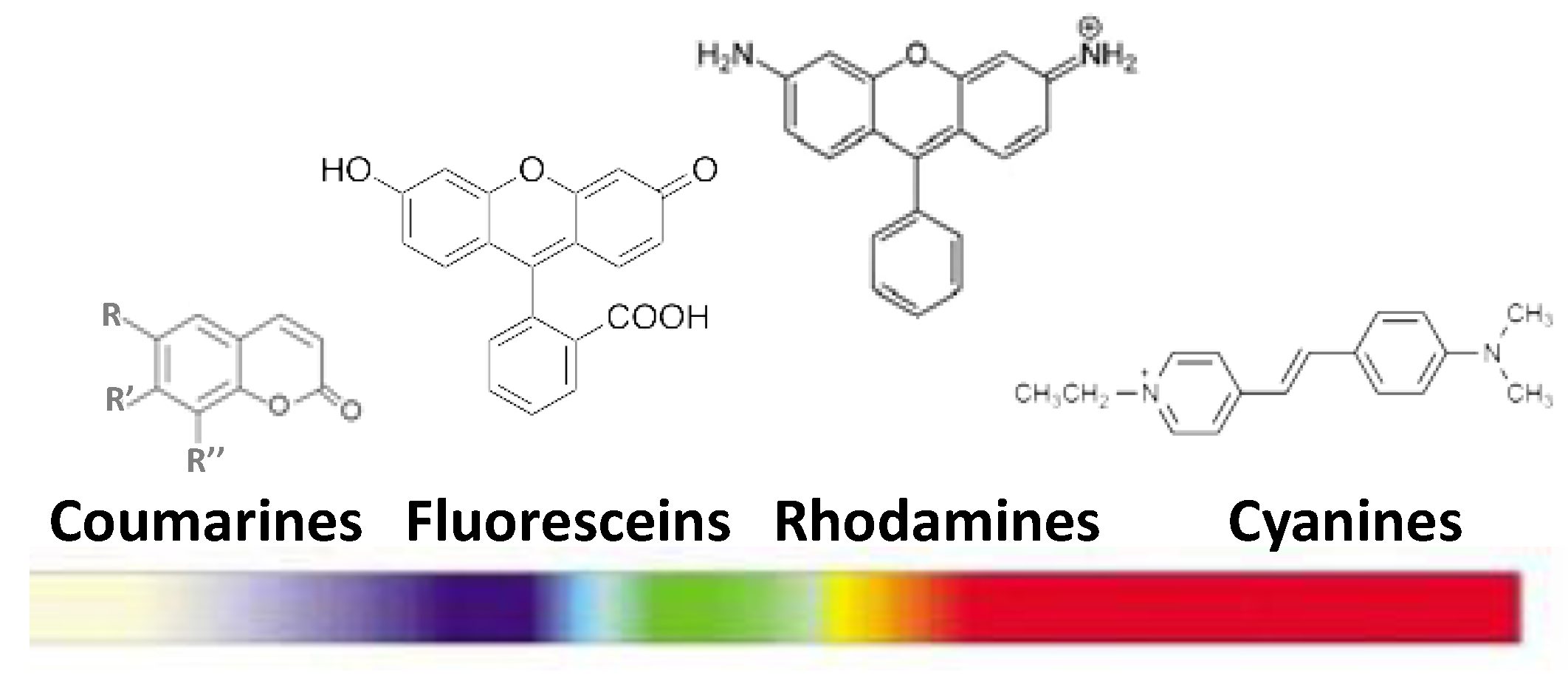
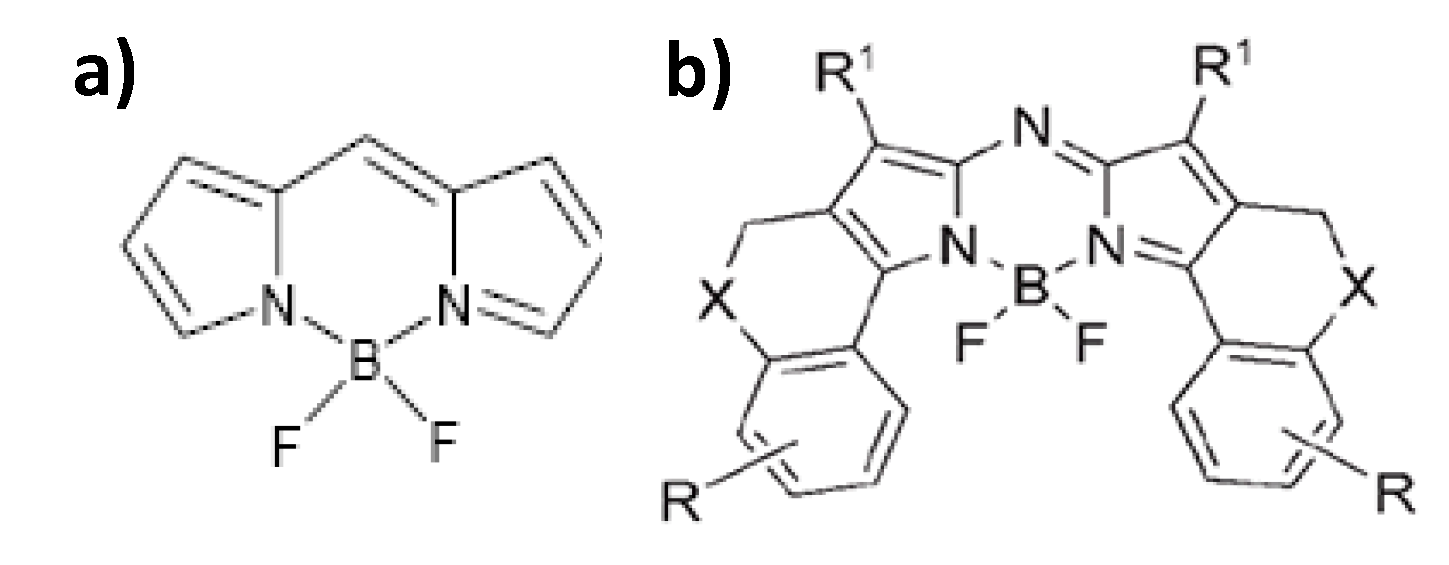
3.1. Ln-Free Dyes
3.2. Ln-Based Dyes
3.3. Molecular Dyes
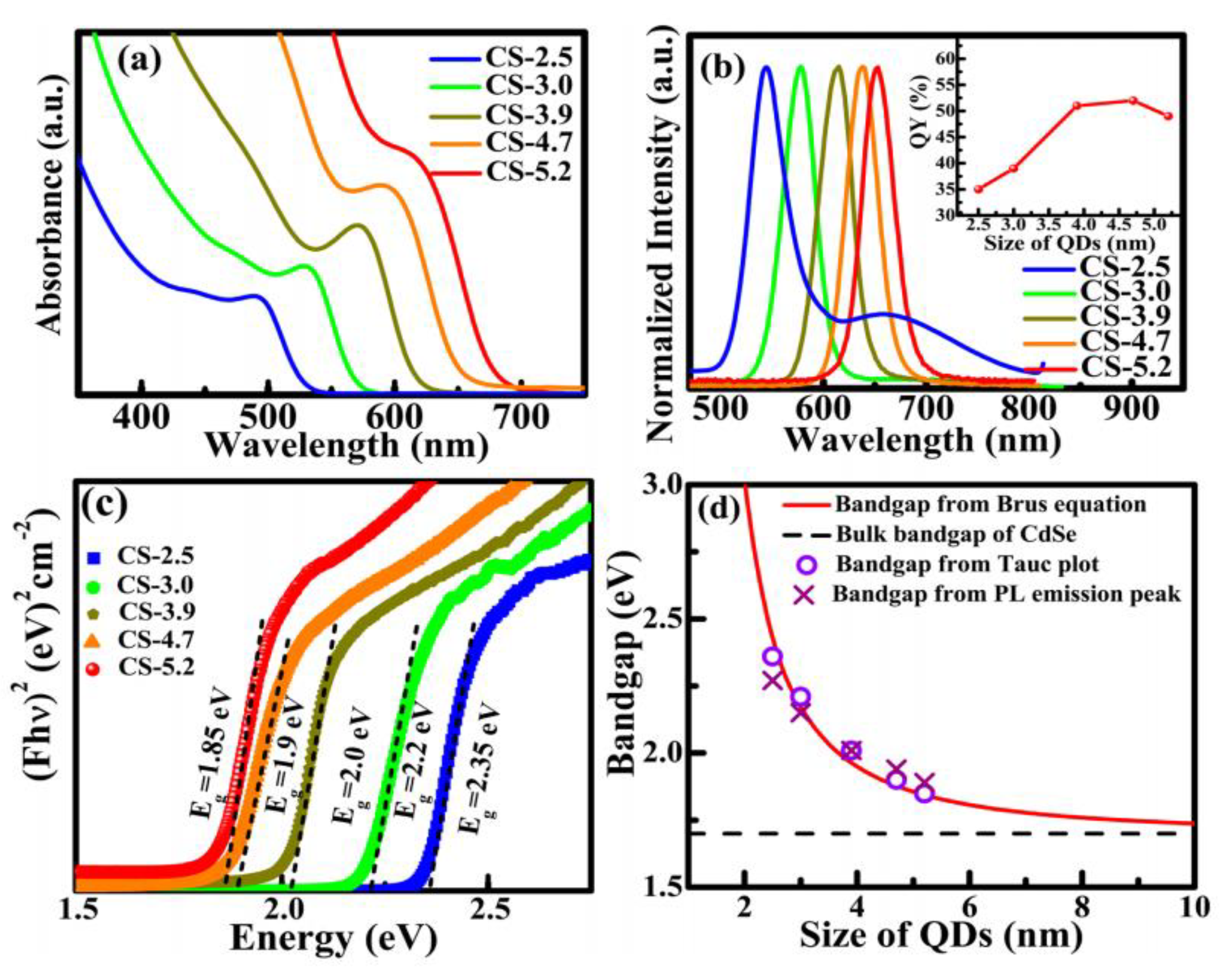
3.4. Solid Inorganic Dyes
4. Ln-Based Nanoprobes for Dual Magnetic and Optical Imaging
4.1. Molecular Dual Probes
4.2. Hybrid Probes
4.3. All-Inorganic Dual Probes
4.3.1. Single Nanocrystals
4.3.2. Core–Shell Crystalline Hetero-Nanostructures
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Edelman, R.R.; Hesselink, J.; Zlatkin, M. Clinical Magnetic Resonance Imaging: 3-Volume Set, 3rd ed.; Saunders: Philadelphia, PA, USA, 2005. [Google Scholar]
- Caravan, P.; Ellison, J.J.; McMurry, T.J.; Lauffer, R.B. Gadolinium(III) Chelates as MRI Contrast Agents: Structure, Dynamics, and Applications. Chem. Rev. 1999, 99, 2293–2352. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Cheon, J.; Lee, J.-H. Synergistically Integrated Nanoparticles as Multimodal Probes for Nanobiotechnology. Acc. Chem. Res. 2008, 41, 1630–1640. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Chen, H.; Lipowska, M.; Wang, L.; Yu, Q.; Yang, X.; Tiwari, D.; Yang, L.; Mao, H. A Dual-Modal Magnetic Nanoparticle Probe for Preoperative and Intraoperative Mapping of Sentinel Lymph Nodes by Magnetic Resonance and near Infrared Fluorescence Imaging. J. Biomater. Appl. 2013, 28, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Belkahla, H.; Haque, A.; Revzin, A.; Gharbi, T.; Constantinescu, A.A.; Micheau, O.; Hémadi, M.; Ammar, S. Coupling Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand to Iron Oxide Nanoparticles Increases Its Apoptotic Activity on HCT116 and HepG2 Malignant Cells: Effect of Magnetic Core Size. J. Interdiscip. Nanomed. 2019, 4, 34–50. [Google Scholar] [CrossRef]
- Hai, J.; Piraux, H.; Mazarío, E.; Volatron, J.; Ha-Duong, N.T.; Decorse, P.; Lomas, J.S.; Verbeke, P.; Ammar, S.; Wilhelm, C.; et al. Maghemite Nanoparticles Coated with Human Serum Albumin: Combining Targeting by the Iron-Acquisition Pathway and Potential in Photothermal Therapies. J. Mater. Chem. B 2017, 5, 3154–3162. [Google Scholar] [CrossRef] [PubMed]
- Andersson-Engels, S.; Wilson, B.C. In Vivo Fluorescence in Clinical Oncology: Fundamental and Practical Issues. J. Cell. Pharmacol. 1992, 3, 66–79. [Google Scholar]
- Weiss, J. Fluorescence of Organic Molecules. Nature 1943, 152, 176–178. [Google Scholar] [CrossRef]
- Ge, P.; Selvin, P.R. New 9- or 10-Dentate Luminescent Lanthanide Chelates. Bioconjug. Chem. 2008, 19, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Hemmilä, I. Luminescent Lanthanide Chelates—A Way to More Sensitive Diagnostic Methods. J. Alloy. Compd. 1995, 225, 480–485. [Google Scholar] [CrossRef]
- Khlebtsov, N.G.; Dykman, L.A. Optical Properties and Biomedical Applications of Plasmonic Nanoparticles. J. Quant. Spectrosc. Radiat. Transf. 2010, 111, 1–35. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Plasmonics in Biology and Plasmon-Controlled Fluorescence. Plasmonics 2006, 1, 5–33. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Yang, L.; Petros, J.A.; Marshall, F.F.; Simons, J.W.; Nie, S. In Vivo Molecular and Cellular Imaging with Quantum Dots. Curr. Opin. Biotechnol. 2005, 16, 63–72. [Google Scholar] [CrossRef]
- Walling, M.A.; Novak, J.A.; Shepard, J.R.E. Quantum Dots for Live Cell and In Vivo Imaging. Int. J. Mol. Sci. 2009, 10, 441–491. [Google Scholar] [CrossRef]
- Miao, H.; Wang, L.; Zhuo, Y.; Zhou, Z.; Yang, X. Label-Free Fluorimetric Detection of CEA Using Carbon Dots Derived from Tomato Juice. Biosens. Bioelectron. 2016, 86, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Zhao, Y.; Wang, S.; Liu, M.; Duan, Z.; Chen, Y.; Li, F.; Xu, F.; Lu, T. Recent Advances in Synthesis and Surface Modification of Lanthanide-Doped Upconversion Nanoparticles for Biomedical Applications. Biotechnol. Adv. 2012, 30, 1551–1561. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Rajabi, M.; Mousa, S.A. Multifunctional Nanomaterials and Their Applications in Drug Delivery and Cancer Therapy. Nanomaterials 2015, 5, 1690–1703. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Shuter, B.; Muhammad Idris, N. Hybrid Lanthanide Nanoparticles with Paramagnetic Shell Coated on Upconversion Fluorescent Nanocrystals. Langmuir 2009, 25, 12015–12018. [Google Scholar] [CrossRef]
- Que, E.L.; Chang, C.J. Responsive Magnetic Resonance Imaging Contrast Agents as Chemical Sensors for Metals in Biology and Medicine. Chem. Soc. Rev. 2009, 39, 51–60. [Google Scholar] [CrossRef]
- Bloembergen, N.; Morgan, L.O. Proton Relaxation Times in Paramagnetic Solutions. Effects of Electron Spin Relaxation. J. Chem. Phys. 1961, 34, 842–850. [Google Scholar] [CrossRef]
- Solomon, I. Relaxation Processes in a System of Two Spins. Phys. Rev. 1955, 99, 559–565. [Google Scholar] [CrossRef]
- Ayant, Y.; Belorizky, E.; Aluzon, J.; Gallice, J. Calcul des densités spectrales résultant d’un mouvement aléatoire de translation en relaxation par interaction dipolaire magnétique dans les liquides. J. Phys. 1975, 36, 991–1004. [Google Scholar] [CrossRef]
- Hwang, L.; Freed, J.H. Generalized Einstein Relations for Rotational and Translational Diffusion of Molecules Including Spin. J. Chem. Phys. 1975, 63, 118–130. [Google Scholar] [CrossRef]
- Gillis, P.; Roch, A.; Brooks, R.A. Corrected Equations for Susceptibility-Induced T2-Shortening. J. Magn. Reson. 1999, 137, 402–407. [Google Scholar] [CrossRef]
- Vuong, Q.L.; Berret, J.-F.; Fresnais, J.; Gossuin, Y.; Sandre, O. A Universal Scaling Law to Predict the Efficiency of Magnetic Nanoparticles as MRI T2-Contrast Agents. Adv. Healthc. Mater. 2012, 1, 502–512. [Google Scholar] [CrossRef]
- Naito, S.; Tazaki, H.; Okamoto, T.; Takeuchi, K.; Kan, S.; Takeuchi, Y.; Kamata, K. Comparison of Nephrotoxicity between Two Gadolinium-Contrasts, Gadodiamide and Gadopentetate in Patients with Mildly Diminished Renal Failure. J. Toxicol. Sci. 2017, 42, 379–384. [Google Scholar] [CrossRef][Green Version]
- Troughton, J.S.; Greenfield, M.T.; Greenwood, J.M.; Dumas, S.; Wiethoff, A.J.; Wang, J.; Spiller, M.; McMurry, T.J.; Caravan, P. Synthesis and Evaluation of a High Relaxivity Manganese(II)-Based MRI Contrast Agent. Inorg. Chem. 2004, 43, 6313–6323. [Google Scholar] [CrossRef]
- Nordhøy, W.; Anthonsen, H.W.; Bruvold, M.; Brurok, H.; Skarra, S.; Krane, J.; Jynge, P. Intracellular Manganese Ions Provide Strong T1 Relaxation in Rat Myocardium. Magn. Reson. Med. 2004, 52, 506–514. [Google Scholar] [CrossRef]
- Zheng, X.-Y.; Pellico, J.; Khrapitchev, A.A.; Sibson, N.R.; Davis, J.J. Dy-DOTA Integrated Mesoporous Silica Nanoparticles as Promising Ultrahigh Field Magnetic Resonance Imaging Contrast Agents. Nanoscale 2018, 10, 21041–21045. [Google Scholar] [CrossRef]
- Norek, M.; Peters, J.A. MRI Contrast Agents Based on Dysprosium or Holmium. Prog. Nucl. Magn. Reson. Spectrosc. 2011, 59, 64–82. [Google Scholar] [CrossRef]
- Yang, D.; Ma, P.; Hou, Z.; Cheng, Z.; Li, C.; Lin, J. Current Advances in Lanthanide Ion (Ln3+)-Based Upconversion Nanomaterials for Drug Delivery. Chem. Soc. Rev. 2015, 44, 1416–1448. [Google Scholar] [CrossRef] [PubMed]
- Brasch, R.C. Rationale and Applications for Macromolecular Gd-Based Contrast Agents. Magn. Reson. Med. 1991, 22, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Orang-Khadivi, K.; Pierce, B.L.; Ollom, C.M.; Floyd, L.J.; Siegle, R.L.; Williams, R.F. New Magnetic Resonance Imaging Techniques for the Detection of Breast Cancer. Breast Cancer Res. Treat. 1994, 32, 119–135. [Google Scholar] [CrossRef] [PubMed]
- Adam, G.; Neuerburg, J.; Spüntrup, E.; Mühler, A.; Surg, K.S.V.; Günther, R.W. Gd-DTPA-Cascade-Polymer: Potential Blood Pool Contrast Agent for MR Imaging. J. Magn. Reson. Imaging 1994, 4, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Schmiedl, U.; Ogan, M.; Paajanen, H.; Marotti, M.; Crooks, L.E.; Brito, A.C.; Brasch, R.C. Albumin Labeled with Gd-DTPA as an Intravascular, Blood Pool-Enhancing Agent for MR Imaging: Biodistribution and Imaging Studies. Radiology 1987, 162, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Brechbiel, M.W. Dendrimer-Based Macromolecular MRI Contrast Agents: Characteristics and Application. Mol. Imaging 2003, 2, 1–10. [Google Scholar] [CrossRef]
- Schwendener, R.A. Liposomes as Carriers for Paramagnetic Gadolinium Chelates as Organ Specific Contrast Agents for Magnetic Resonance Imaging (Mri). J. Liposome Res. 1994, 4, 837–855. [Google Scholar] [CrossRef]
- Avti, P.K.; Talukdar, Y.; Sirotkin, M.V.; Shroyer, K.R.; Sitharaman, B. Toward Single-Walled Carbon Nanotube–Gadolinium Complex as Advanced MRI Contrast Agents: Pharmacodynamics and Global Genomic Response in Small Animals. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101B, 1039–1049. [Google Scholar] [CrossRef]
- Gizzatov, A.; Hernández-Rivera, M.; Keshishian, V.; Mackeyev, Y.; Law, J.J.; Guven, A.; Sethi, R.; Qu, F.; Muthupillai, R.; Cabreira-Hansen, M.; et al. Surfactant-Free Gd3+-Ion-Containing Carbon Nanotube MRI Contrast Agents for Stem Cell Labeling. Nanoscale 2015, 7, 12085–12091. [Google Scholar] [CrossRef]
- Vittorio, O.; Duce, S.L.; Pietrabissa, A.; Cuschieri, A. Multiwall Carbon Nanotubes as MRI Contrast Agents for Tracking Stem Cells. Nanotechnology 2011, 22, 095706. [Google Scholar] [CrossRef]
- Aime, S.; Frullano, L.; Geninatti Crich, S. Compartmentalization of a Gadolinium Complex in the Apoferritin Cavity: A Route to Obtain High Relaxivity Contrast Agents for Magnetic Resonance Imaging. Angew. Chem. Int. Ed. 2002, 41, 1017–1019. [Google Scholar] [CrossRef]
- Makino, A.; Harada, H.; Okada, T.; Kimura, H.; Amano, H.; Saji, H.; Hiraoka, M.; Kimura, S. Effective Encapsulation of a New Cationic Gadolinium Chelate into Apoferritin and Its Evaluation as an MRI Contrast Agent. Nanomedicine 2011, 7, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-K.; Jung, H.-Y.; Park, J.-A.; Huh, M.-I.; Jung, J.-C.; Chang, Y.; Kim, T.-J. Gold Nanoparticles Coated with Gadolinium-DTPA-Bisamide Conjugate of Penicillamine (Au@GdL) as a T1-Weighted Blood Pool Contrast Agent. J. Mater. Chem. 2010, 20, 5411–5417. [Google Scholar] [CrossRef]
- Sieving, P.F.; Watson, A.D.; Rocklage, S.M. Preparation and Characterization of Paramagnetic Polychelates and Their Protein Conjugates. Bioconjug. Chem. 1990, 1, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Wiener, E.C.; Brechbiel, M.W.; Brothers, H.; Magin, R.L.; Gansow, O.A.; Tomalia, D.A.; Lauterbur, P.C. Dendrimer-Based Metal Chelates: A New Class of Magnetic Resonance Imaging Contrast Agents. Magn. Reson. Med. 1994, 31, 1–8. [Google Scholar] [CrossRef]
- Sitharaman, B.; Kissell, K.R.; Hartman, K.B.; Tran, L.A.; Baikalov, A.; Rusakova, I.; Sun, Y.; Khant, H.A.; Ludtke, S.J.; Chiu, W.; et al. Superparamagnetic Gadonanotubes Are High-Performance MRI Contrast Agents. Chem. Commun. 2005, 21, 3915–3917. [Google Scholar] [CrossRef]
- Huang, C.-C.; Khu, N.-H.; Yeh, C.-S. The Characteristics of Sub 10 nm Manganese Oxide T1 Contrast Agents of Different Nanostructured Morphologies. Biomaterials 2010, 31, 4073–4078. [Google Scholar] [CrossRef]
- Na, H.B.; Lee, J.H.; An, K.; Park, Y.I.; Park, M.; Lee, I.S.; Nam, D.-H.; Kim, S.T.; Kim, S.-H.; Kim, S.-W.; et al. Development of a T1 Contrast Agent for Magnetic Resonance Imaging Using MnO Nanoparticles. Angew. Chem. Int. Ed. 2007, 46, 5397–5401. [Google Scholar] [CrossRef]
- Hedlund, A.; Ahrén, M.; Gustafsson, H.; Abrikossova, N.; Warntjes, M.; Jönsson, J.-I.; Uvdal, K.; Engström, M. Gd2O3 Nanoparticles in Hematopoietic Cells for MRI Contrast Enhancement. Int. J. Nanomed. 2011, 6, 3233–3240. [Google Scholar]
- Ahrén, M.; Selegård, L.; Klasson, A.; Söderlind, F.; Abrikossova, N.; Skoglund, C.; Bengtsson, T.; Engström, M.; Käll, P.-O.; Uvdal, K. Synthesis and Characterization of PEGylated Gd2O3 Nanoparticles for MRI Contrast Enhancement. Langmuir 2010, 26, 5753–5762. [Google Scholar] [CrossRef]
- Klasson, A.; Ahrén, M.; Hellqvist, E.; Söderlind, F.; Rosén, A.; Käll, P.-O.; Uvdal, K.; Engström, M. Positive MRI Contrast Enhancement in THP-1 Cells with Gd2O3 Nanoparticles. Contrast Media Mol. Imaging 2008, 3, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Hemery, G.; Keyes, A.C.; Garaio, E.; Rodrigo, I.; Garcia, J.A.; Plazaola, F.; Garanger, E.; Sandre, O. Tuning Sizes, Morphologies, and Magnetic Properties of Monocore Versus Multicore Iron Oxide Nanoparticles through the Controlled Addition of Water in the Polyol Synthesis. Inorg. Chem. 2017, 56, 8232–8243. [Google Scholar] [CrossRef] [PubMed]
- Tromsdorf, U.I.; Bruns, O.T.; Salmen, S.C.; Beisiegel, U.; Weller, H. A Highly Effective, Nontoxic T1 MR Contrast Agent Based on Ultrasmall PEGylated Iron Oxide Nanoparticles. Nano Lett. 2009, 9, 4434–4440. [Google Scholar] [CrossRef] [PubMed]
- Ranga, A.; Agarwal, Y.; Garg, K.J. Gadolinium Based Contrast Agents in Current Practice: Risks of Accumulation and Toxicity in Patients with Normal Renal Function. Ind. J. Radiol. Imaging 2017, 27, 141. [Google Scholar]
- An, K.; Kwon, S.G.; Park, M.; Na, H.B.; Baik, S.-I.; Yu, J.H.; Kim, D.; Son, J.S.; Kim, Y.W.; Song, I.C.; et al. Synthesis of Uniform Hollow Oxide Nanoparticles through Nanoscale Acid Etching. Nano Lett. 2008, 8, 4252–4258. [Google Scholar] [CrossRef]
- Rohrer, M.; Bauer, H.; Mintorovitch, J.; Requardt, M.; Weinmann, H.-J. Comparison of Magnetic Properties of MRI Contrast Media Solutions at Different Magnetic Field Strengths. Investig. Radiol. 2005, 40, 715–724. [Google Scholar] [CrossRef]
- Wáng, Y.X.J.; Idée, J.-M. A Comprehensive Literatures Update of Clinical Researches of Superparamagnetic Resonance Iron Oxide Nanoparticles for Magnetic Resonance Imaging. Quant. Imaging Med. Surg. 2017, 7, 88–122. [Google Scholar] [CrossRef]
- Wang, Y.-X.J. Superparamagnetic Iron Oxide Based MRI Contrast Agents: Current Status of Clinical Application. Quant. Imaging Med. Surg. 2011, 1, 35–40. [Google Scholar]
- Constantinides, C.D.; Rogers, J.; Herzka, D.A.; Boada, F.E.; Bolar, D.; Kraitchman, D.; Gillen, J.; Bottomley, P.A. Superparamagnetic Iron Oxide MION as a Contrast Agent for Sodium MRI in Myocardial Infarction. Magn. Reson. Med. 2001, 46, 1164–1168. [Google Scholar] [CrossRef]
- Berkova, Z.; Jirak, D.; Zacharovova, K.; Lukes, I.; Kotkova, Z.; Kotek, J.; Kacenka, M.; Kaman, O.; Rehor, I.; Hajek, M.; et al. Gadolinium- and Manganite-Based Contrast Agents with Fluorescent Probes for Both Magnetic Resonance and Fluorescence Imaging of Pancreatic Islets: A Comparative Study. ChemMedChem 2013, 8, 614–621. [Google Scholar] [CrossRef]
- Kačenka, M.; Kaman, O.; Kotek, J.; Falteisek, L.; Černý, J.; Jirák, D.; Herynek, V.; Zacharovová, K.; Berková, Z.; Jendelová, P.; et al. Dual Imaging Probes for Magnetic Resonance Imaging and Fluorescence Microscopy Based on Perovskite Manganite Nanoparticles. J. Mater. Chem. 2010, 21, 157–164. [Google Scholar] [CrossRef]
- Haghniaz, R.; Bhayani, K.R.; Umrani, R.D.; Paknikar, K.M. Dextran Stabilized Lanthanum Strontium Manganese Oxide Nanoparticles for Magnetic Resonance Imaging. RSC Adv. 2013, 3, 18489–18497. [Google Scholar] [CrossRef]
- Bárcena, C.; Sra, A.K.; Chaubey, G.S.; Khemtong, C.; Liu, J.P.; Gao, J. Zinc Ferrite Nanoparticles as MRI Contrast Agents. Chem. Commun. 2008, 2224–2226. [Google Scholar] [CrossRef]
- Lu, J.; Ma, S.; Sun, J.; Xia, C.; Liu, C.; Wang, Z.; Zhao, X.; Gao, F.; Gong, Q.; Song, B.; et al. Manganese Ferrite Nanoparticle Micellar Nanocomposites as MRI Contrast Agent for Liver Imaging. Biomaterials 2009, 30, 2919–2928. [Google Scholar] [CrossRef] [PubMed]
- Joshi, H.M.; Lin, Y.P.; Aslam, M.; Prasad, P.V.; Schultz-Sikma, E.A.; Edelman, R.; Meade, T.; Dravid, V.P. Effects of Shape and Size of Cobalt Ferrite Nanostructures on Their MRI Contrast and Thermal Activation. J. Phys. Chem. C 2009, 113, 17761–17767. [Google Scholar] [CrossRef]
- Ghasemian, Z.; Shahbazi-Gahrouei, D.; Manouchehri, S. Cobalt Zinc Ferrite Nanoparticles as a Potential Magnetic Resonance Imaging Agent: An In Vitro Study. Avicenna J. Med. Biotechnol. 2015, 7, 64–68. [Google Scholar] [PubMed]
- Sattarahmady, N.; Heidari, M.; Zare, T.; Lotfi, M.; Heli, H. Zinc–Nickel Ferrite Nanoparticles as a Contrast Agent in Magnetic Resonance Imaging. Appl. Magn. Reson. 2016, 47, 925–935. [Google Scholar] [CrossRef]
- Zahraei, M.; Monshi, A.; Shahbazi-Gahrouei, D.; Amirnasr, M.; Behdadfar, B.; Rostami, M. Synthesis and Characterization of Chitosan Coated Manganese Zinc Ferrite Nanoparticles as MRI Contrast Agents. J. Nanostruct. 2015, 5, 77–86. [Google Scholar]
- Shultz, M.D.; Calvin, S.; Fatouros, P.P.; Morrison, S.A.; Carpenter, E. Enhanced Ferrite Nanoparticles as MRI Contrast Agents. J. Magn. Magn. Mater. 2007, 311, 464–468. [Google Scholar] [CrossRef]
- Lacroix, L.-M.; Lachaize, S.; Falqui Blon, T.; Carrey, J.; Dumestre, M. Ultrasmall iron nanoparticles: Effect of size reduction on anisotropy and magnetization. J. Appl. Phys. 2008, 103, 07D521. [Google Scholar] [CrossRef]
- Mornet, S.; Vasseur, S.; Grasset, F.; Duguet, E. Magnetic Nanoparticle Design for Medical Diagnosis and Therapy. J. Mater. Chem. 2004, 14, 2161–2175. [Google Scholar] [CrossRef]
- Na, H.B.; Song, I.C.; Hyeon, T. Inorganic Nanoparticles for MRI Contrast Agents. Adv. Mater. 2009, 21, 2133–2148. [Google Scholar] [CrossRef]
- Dumestre, F.; Chaudret, B.; Amiens, C.; Renaud, P.; Fejes, P. Superlattices of Iron Nanocubes Synthesized from Fe[N(SiMe3)2]2. Science 2004, 303, 821–823. [Google Scholar] [CrossRef] [PubMed]
- Basti, H.; Ben Tahar, L.; Smiri, L.; Herbst, F.; Nowak, S.; Mangeney, C.; Merah, S. Surface Modification of γ-Fe2O3 Nanoparticles by Grafting from Poly-(Hydroxyethylmethacrylate) and Poly-(Methacrylic Acid): Qualitative and Quantitative Analysis of the Polymeric Coating. Colloids Surf. A Physicochem. Eng. Asp. 2016, 490, 222–231. [Google Scholar] [CrossRef]
- Ohno, K.; Mori, C.; Akashi, T.; Yoshida, S.; Tago, Y.; Tsujii, Y.; Tabata, Y. Fabrication of Contrast Agents for Magnetic Resonance Imaging from Polymer-Brush-Afforded Iron Oxide Magnetic Nanoparticles Prepared by Surface-Initiated Living Radical Polymerization. Biomacromolecules 2013, 14, 3453–3462. [Google Scholar] [CrossRef] [PubMed]
- Boal, A.K.; Frankamp, B.L.; Uzun, O.; Tuominen, M.T.; Rotello, V.M. Modulation of Spacing and Magnetic Properties of Iron Oxide Nanoparticles through Polymer-Mediated “Bricks and Mortar” Self-Assembly. Chem. Mater. 2004, 16, 3252–3256. [Google Scholar] [CrossRef]
- Ulbrich, K.; Holá, K.; Šubr, V.; Bakandritsos, A.; Tuček, J.; Zbořil, R. Targeted Drug Delivery with Polymers and Magnetic Nanoparticles: Covalent and Noncovalent Approaches, Release Control, and Clinical Studies. Chem. Rev. 2016, 116, 5338–5431. [Google Scholar] [CrossRef]
- Arsalani, N.; Fattahi, H.; Laurent, S.; Burtea, C.; Elst, L.V.; Muller, R.N. Polyglycerol-Grafted Superparamagnetic Iron Oxide Nanoparticles: Highly Efficient MRI Contrast Agent for Liver and Kidney Imaging and Potential Scaffold for Cellular and Molecular Imaging. Contrast Media Mol. Imaging 2012, 7, 185–194. [Google Scholar] [CrossRef]
- Lee, S.J.; Muthiah, M.; Lee, H.J.; Lee, H.-J.; Moon, M.-J.; Che, H.-L.; Heo, S.U.; Lee, H.-C.; Jeong, Y.Y.; Park, I.-K. Synthesis and Characterization of Magnetic Nanoparticle-Embedded Multi-Functional Polymeric Micelles for MRI-Guided Gene Delivery. Macromol. Res. 2012, 20, 188–196. [Google Scholar] [CrossRef]
- Ebert, S.; Bannwarth, M.B.; Musyanovych, A.; Landfester, K.; Münnemann, K. How Morphology Influences Relaxivity—Comparative Study of Superparamagnetic Iron Oxide–Polymer Hybrid Nanostructures. Contrast Media Mol. Imaging 2015, 10, 456–464. [Google Scholar] [CrossRef]
- Basti, H.; Ben Tahar, L.; Smiri, L.S.; Herbst, F.; Vaulay, M.-J.; Chau, F.; Ammar, S.; Benderbous, S. Catechol Derivatives-Coated Fe3O4 and γ-Fe2O3 Nanoparticles as Potential MRI Contrast Agents. J. Colloid Interface Sci. 2010, 341, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Bonvin, D.; Bastiaansen, J.A.M.; Stuber, M.; Hofmann, H.; Ebersold, M.M. Folic Acid on Iron Oxide Nanoparticles: Platform with High Potential for Simultaneous Targeting, MRI Detection and Hyperthermia Treatment of Lymph Node Metastases of Prostate Cancer. Dalton Trans. 2017, 46, 12692–12704. [Google Scholar] [CrossRef] [PubMed]
- Piraux, H.; Hai, J.; Gaudisson, T.; Ammar, S.; Gazeau, F.; El Hage Chahine, J.M.; Hémadi, M. Transferrin-Bearing Maghemite Nano-Constructs for Biomedical Applications. J. Appl. Phys. 2015, 117, 17A336. [Google Scholar] [CrossRef]
- Yang, J.; Luo, Y.; Xu, Y.; Li, J.; Zhang, Z.; Wang, H.; Shen, M.; Shi, X.; Zhang, G. Conjugation of Iron Oxide Nanoparticles with RGD-Modified Dendrimers for Targeted Tumor MR Imaging. ACS Appl. Mater. Interfaces 2015, 7, 5420–5428. [Google Scholar] [CrossRef]
- Zhang, C.; Jugold, M.; Woenne, E.C.; Lammers, T.; Morgenstern, B.; Mueller, M.M.; Zentgraf, H.; Bock, M.; Eisenhut, M.; Semmler, W.; et al. Specific Targeting of Tumor Angiogenesis by RGD-Conjugated Ultrasmall Superparamagnetic Iron Oxide Particles Using a Clinical 1.5-T Magnetic Resonance Scanner. Cancer Res. 2007, 67, 1555–1562. [Google Scholar] [CrossRef][Green Version]
- Lewin, M.; Carlesso, N.; Tung, C.-H.; Tang, X.-W.; Cory, D.; Scadden, D.T.; Weissleder, R. Tat Peptide-Derivatized Magnetic Nanoparticles Allow in Vivo Tracking and Recovery of Progenitor Cells. Nat. Biotechnol. 2000, 18, 410–414. [Google Scholar] [CrossRef]
- Ding, C.; Wu, K.; Wang, W.; Guan, Z.; Wang, L.; Wang, X.; Wang, R.; Liu, L.; Fan, J. Synthesis of a Cell Penetrating Peptide Modified Superparamagnetic Iron Oxide and MRI Detection of Bladder Cancer. Oncotarget 2016, 8, 4718–4729. [Google Scholar] [CrossRef]
- Alwi, R.; Telenkov, S.; Mandelis, A.; Leshuk, T.; Gu, F.; Oladepo, S.; Michaelian, K. Silica-Coated Super Paramagnetic Iron Oxide Nanoparticles (SPION) as Biocompatible Contrast Agent in Biomedical Photoacoustics. Biomed. Opt. Express (BOE) 2012, 3, 2500–2509. [Google Scholar] [CrossRef]
- Khosroshahi, M.E.; Ghazanfari, L. Preparation and Characterization of Silica-Coated Iron-Oxide Bionanoparticles under N2 Gas. Phys. E Low-Dimens. Syst. Nanostruct. 2010, 42, 1824–1829. [Google Scholar] [CrossRef]
- Santra, S.; Tapec, R.; Theodoropoulou, N.; Dobson, J.; Hebard, A.F.; Tan, W. Synthesis and Characterization of Silica-Coated Iron Oxide Nanoparticles in Microemulsion: The Effect of Nonionic Surfactants. Langmuir 2001, 17, 2900–2906. [Google Scholar] [CrossRef]
- Donadel, K.; Felisberto, M.D.V.; Laranjeira, M.C.M. Preparation and Characterization of Hydroxyapatite-Coated Iron Oxide Particles by Spray-Drying Technique. Acad. Bras. Ciênc. 2009, 81, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Mordon, S.; Devoisselle, J.M.; Maunoury, V. In Vivo PH Measurement and Imaging of Tumor Tissue Using a PH-Sensitive Fluorescent Probe (5,6-Carboxyfluorescein): Instrumental and Experimental Studies. Photochem. Photobiol. 1994, 60, 274–279. [Google Scholar] [CrossRef]
- Maréchal, X.; Mordon, S.; Devoisselle, J.M.; Bégu, S.; Guery, B.; Neviére, R.; Buys, B.; Dhelin, G.; Lesage, J.C.; Mathieu, D.; et al. In Vivo Application of Intestinal PH Measurement Using 2,7‘-Bis(Carboxyethyl)-5,6-Carboxyfluorescein (BCECF) Fluorescence Imaging. Photochem. Photobiol. 1999, 70, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Girven, K.S.; Sparta, D.R. Probing Deep Brain Circuitry: New Advances in in Vivo Calcium Measurement Strategies. ACS Chem. Neurosci. 2017, 8, 243–251. [Google Scholar] [CrossRef]
- Pozzan, T.; Rudolf, R. Measurements of Mitochondrial Calcium in Vivo. Biochim. Biophys. Acta (BBA) Bioenerg. 2009, 1787, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Pansare, V.; Hejazi, S.; Faenza, W.; Prud’homme, R.K. Review of Long-Wavelength Optical and NIR Imaging Materials: Contrast Agents, Fluorophores and Multifunctional Nano Carriers. Chem. Mater. 2012, 24, 812–827. [Google Scholar] [CrossRef]
- Gibbs-Strauss, S.L.; Rosenberg, M.; Clough, B.L.; Troyan, S.L.; Frangioni, J.V. First-in-Human Clinical Trials of Imaging Devices: An Example from Optical Imaging. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2009, 1, 2001–2004. [Google Scholar]
- Sensarn, S.; Zavaleta, C.L.; Segal, E.; Rogalla, S.; Lee, W.; Gambhir, S.S.; Bogyo, M.; Contag, C.H. A Clinical Wide-Field Fluorescence Endoscopic Device for Molecular Imaging Demonstrating Cathepsin Protease Activity in Colon Cancer. Mol. Imaging Biol. 2016, 18, 820–829. [Google Scholar] [CrossRef]
- Sharman, M.J.; Mansfield, C.S.; Whittem, T. The Exogenous Fluorophore, Fluorescein, Enables in Vivo Assessment of the Gastrointestinal Mucosa via Confocal Endomicroscopy: Optimization of Intravenous Dosing in the Dog Model. J. Vet. Pharmacol. Therap. 2013, 36, 450–455. [Google Scholar] [CrossRef]
- Benninger, R.K.P.; Piston, D.W. Two-Photon Excitation Microscopy for the Study of Living Cells and Tissues. Curr. Protoc. Cell Biol. 2013, 59, 3.41.1–4.27.15. [Google Scholar] [CrossRef]
- Becker, W. Fluorescence Lifetime Imaging-Techniques and Applications. J. Microsc. 2012, 247, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Cubeddu, R.; Canti, G.; Pifferi, A.; Taroni, P.; Valentini, G. Fluorescence Lifetime Imaging of Experimental Tumors in Hematoporphyrin Derivative-Sensitized Mice. Photochem. Photobiol. 1997, 66, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Wünsch, U.J.; Murphy, K.R.; Stedmon, C.A. Fluorescence Quantum Yields of Natural Organic Matter and Organic Compounds: Implications for the Fluorescence-Based Interpretation of Organic Matter Composition. Front. Mar. Sci. 2015, 2, 98. [Google Scholar] [CrossRef]
- Würth, C.; Grabolle, M.; Pauli, J.; Spieles, M.; Resch-Genger, U. Relative and Absolute Determination of Fluorescence Quantum Yields of Transparent Samples. Nat. Protoc. 2013, 8, 1535–1550. [Google Scholar] [CrossRef]
- Zhao, W.; Carreira, E.M. Conformationally Restricted Aza-BODIPY: Highly Fluorescent, Stable near-Infrared Absorbing Dyes. Chemistry 2006, 12, 7254–7263. [Google Scholar] [CrossRef]
- Borwankar, A.U.; Willsey, B.W.; Twu, A.; Hung, J.J.; Stover, R.J.; Wang, T.W.; Feldman, M.D.; Milner, T.E.; Truskett, T.M.; Johnston, K.P. Gold Nanoparticles with High Densities of Small Protuberances on Nanocluster Cores with Strong NIR Extinction. RSC Adv. 2015, 5, 104674–104687. [Google Scholar] [CrossRef]
- Xiao, L.; Yeung, E.S. Optical Imaging of Individual Plasmonic Nanoparticles in Biological Samples. Annu. Rev. Anal. Chem. 2014, 7, 89–111. [Google Scholar] [CrossRef]
- Wang, Z. Plasmon—Resonant Gold Nanoparticles for Cancer Optical Imaging. Sci. China Phys. Mech. Astron. 2013, 56, 506–513. [Google Scholar] [CrossRef]
- Sun, Y.; Xia, Y. Gold and Silver Nanoparticles: A Class of Chromophores with Colors Tunable in the Range from 400 to 750 Nm. Analyst 2003, 128, 686–691. [Google Scholar] [CrossRef]
- Jans, H.; Huo, Q. Gold Nanoparticle-Enabled Biological and Chemical Detection and Analysis. Chem. Soc. Rev. 2012, 41, 2849–2866. [Google Scholar] [CrossRef]
- Sheng, Y.; Xue, J. Synthesis and Properties of Au-Fe3O4 Heterostructured Nanoparticles. J. Colloid Interface Sci. 2012, 374, 96–101. [Google Scholar] [CrossRef] [PubMed]
- León Félix, L.; Coaquira, J.A.H.; Martínez, M.A.R.; Goya, G.F.; Mantilla, J.; Sousa, M.H.; de los Santos Valladares, L.; Barnes, C.H.W.; Morais, P.C. Structural and Magnetic Properties of Core-Shell Au/Fe3O4 Nanoparticles. Sci. Rep. 2017, 7, 41732. [Google Scholar] [CrossRef] [PubMed]
- Pastoriza-Santos, I.; Liz-Marzán, L.M. Formation of PVP-Protected Metal Nanoparticles in DMF. Langmuir 2002, 18, 2888–2894. [Google Scholar] [CrossRef]
- Kubo, S.; Diaz, A.; Tang, Y.; Mayer, T.S.; Khoo, I.C.; Mallouk, T.E. Tunability of the Refractive Index of Gold Nanoparticle Dispersions. Nano Lett. 2007, 7, 3418–3423. [Google Scholar] [CrossRef] [PubMed]
- Brus, L. Electronic Wave Functions in Semiconductor Clusters: Experiment and Theory. J. Phys. Chem. 1986, 90, 2555–2560. [Google Scholar] [CrossRef]
- Das, T.K.; Ilaiyaraja, P.; Sudakar, C. Whispering Gallery Mode Enabled Efficiency Enhancement: Defect and Size Controlled CdSe Quantum Dot Sensitized Whisperonic Solar Cells. Sci. Rep. 2018, 8, 9709. [Google Scholar] [CrossRef]
- Yu, W.W.; Peng, X. Formation of High-Quality CdS and Other II–VI Semiconductor Nanocrystals in Noncoordinating Solvents: Tunable Reactivity of Monomers. Angew. Chem. Int. Ed. 2002, 41, 2368–2371. [Google Scholar] [CrossRef]
- Kuçur, E.; Boldt, F.M.; Cavaliere-Jaricot, S.; Ziegler, J.; Nann, T. Quantitative Analysis of Cadmium Selenide Nanocrystal Concentration by Comparative Techniques. Anal. Chem. 2007, 79, 8987–8993. [Google Scholar] [CrossRef]
- Hinds, S.; Myrskog, S.; Levina, L.; Koleilat, G.; Yang, J.; Kelley, S.O.; Sargent, E.H. NIR-Emitting Colloidal Quantum Dots Having 26% Luminescence Quantum Yield in Buffer Solution. J. Am. Chem. Soc. 2007, 129, 7218–7219. [Google Scholar] [CrossRef]
- Shavel, A.; Gaponik, N.; Eychmüller, A. Factors Governing the Quality of Aqueous CdTe Nanocrystals: Calculations and Experiment. J. Phys. Chem. B 2006, 110, 19280–19284. [Google Scholar] [CrossRef]
- Voznyy, O.; Levina, L.; Fan, F.; Walters, G.; Fan, J.Z.; Kiani, A.; Ip, A.H.; Thon, S.M.; Proppe, A.H.; Liu, M.; et al. Origins of Stokes Shift in PbS Nanocrystals. Nano Lett. 2017, 17, 7191–7195. [Google Scholar] [CrossRef] [PubMed]
- Greben, M.; Fucikova, A.; Valenta, J. Photoluminescence Quantum Yield of PbS Nanocrystals in Colloidal Suspensions. J. Appl. Phys. 2015, 117, 144306. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, K.; Zhang, J.; Yang, K.; He, C.; Dong, F.; Yang, B. Synthesis of Size and Shape Controlled PbS Nanocrystals and Their Self-Assembly. Colloids Surf. A Physicochem. Eng. Asp. 2010, 355, 114–120. [Google Scholar] [CrossRef]
- Du, H.; Chen, C.; Krishnan, R.; Krauss, T.D.; Harbold, J.M.; Wise, F.W.; Thomas, M.G.; Silcox, J. Optical Properties of Colloidal PbSe Nanocrystals. Nano Lett. 2002, 2, 1321–1324. [Google Scholar] [CrossRef]
- Lifshitz, E.; Brumer, M.; Kigel, A.; Sashchiuk, A.; Bashouti, M.; Sirota, M.; Galun, E.; Burshtein, Z.; Le Quang, A.Q.; Ledoux-Rak, I.; et al. Air-Stable PbSe/PbS and PbSe/PbSexS1-x Core-Shell Nanocrystal Quantum Dots and Their Applications. J. Phys. Chem. B 2006, 110, 25356–25365. [Google Scholar] [CrossRef]
- Xu, S.; Kumar, S.; Nann, T. Rapid Synthesis of High-Quality InP Nanocrystals. J. Am. Chem. Soc. 2006, 128, 1054–1055. [Google Scholar] [CrossRef]
- Lucey, D.W.; MacRae, D.J.; Furis, M.; Sahoo, Y.; Cartwright, A.N.; Prasad, P.N. Monodispersed InP Quantum Dots Prepared by Colloidal Chemistry in a Noncoordinating Solvent. Chem. Mater. 2005, 17, 3754–3762. [Google Scholar] [CrossRef]
- Reiss, P.; Protière, M.; Li, L. Core/Shell Semiconductor Nanocrystals. Small 2009, 5, 154–168. [Google Scholar] [CrossRef]
- Jiang, W.; Mardyani, S.; Fischer, H.; Chan, W.C.W. Design and Characterization of Lysine Cross-Linked Mercapto-Acid Biocompatible Quantum Dots. Chem. Mater. 2006, 18, 872–878. [Google Scholar] [CrossRef]
- Li, J.J.; Wang, Y.A.; Guo, W.; Keay, J.C.; Mishima, T.D.; Johnson, M.B.; Peng, X. Large-Scale Synthesis of Nearly Monodisperse CdSe/CdS Core/Shell Nanocrystals Using Air-Stable Reagents via Successive Ion Layer Adsorption and Reaction. J. Am. Chem. Soc. 2003, 125, 12567–12575. [Google Scholar] [CrossRef]
- Iyer, G.; Pinaud, F.; Tsay, J.; Weiss, S. Solubilization of Quantum Dots with a Recombinant Peptide from Escherichia Coli. Small 2007, 3, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Yuwen, L.; Bao, B.; Liu, G.; Tian, J.; Lu, H.; Luo, Z.; Zhu, X.; Boey, F.; Zhang, H.; Wang, L. One-Pot Encapsulation of Luminescent Quantum Dots Synthesized in Aqueous Solution by Amphiphilic Polymers. Small 2011, 7, 1456–1463. [Google Scholar] [CrossRef] [PubMed]
- Resch-Genger, U.; Grabolle, M.; Cavaliere-Jaricot, S.; Nitschke, R.; Nann, T. Quantum Dots versus Organic Dyes as Fluorescent Labels. Nat. Methods 2008, 5, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Gorelikov, I.; Martin, A.L.; Seo, M.; Matsuura, N. Silica-Coated Quantum Dots for Optical Evaluation of Perfluorocarbon Droplet Interactions with Cells. Langmuir 2011, 27, 15024–15033. [Google Scholar] [CrossRef]
- Zhang, B.; Gong, X.; Hao, L.; Cheng, J.; Han, Y.; Chang, J. A Novel Method to Enhance Quantum Yield of Silica-Coated Quantum Dots for Biodetection. Nanotechnology 2008, 19, 465604. [Google Scholar] [CrossRef] [PubMed]
- Hardman, R. A Toxicologic Review of Quantum Dots: Toxicity Depends on Physicochemical and Environmental Factors. Environ. Health Perspect. 2006, 114, 165–172. [Google Scholar] [CrossRef]
- Geys, J.; Nemmar, A.; Verbeken, E.; Smolders, E.; Ratoi, M.; Hoylaerts, M.F.; Nemery, B.; Hoet, P.H.M. Acute Toxicity and Prothrombotic Effects of Quantum Dots: Impact of Surface Charge. Environ. Health Perspect. 2008, 116, 1607–1613. [Google Scholar] [CrossRef]
- Aillon, K.L.; Xie, Y.; El-Gendy, N.; Berkland, C.J.; Forrest, M.L. Effects of Nanomaterial Physicochemical Properties on in Vivo Toxicity. Adv. Drug Deliv. Rev. 2009, 61, 457–466. [Google Scholar] [CrossRef]
- Tsoi, K.M.; Dai, Q.; Alman, B.A.; Chan, W.C.W. Are Quantum Dots Toxic? Exploring the Discrepancy between Cell Culture and Animal Studies. Acc. Chem. Res. 2013, 46, 662–671. [Google Scholar] [CrossRef]
- Choi, H.S.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Ipe, B.I.; Bawendi, M.G.; Frangioni, J.V. Renal Clearance of Nanoparticles. Nat. Biotechnol. 2007, 25, 1165–1170. [Google Scholar] [CrossRef]
- Yang, S.-T.; Cao, L.; Luo, P.G.; Lu, F.; Wang, X.; Wang, H.; Meziani, M.J.; Liu, Y.; Qi, G.; Sun, Y.-P. Carbon Dots for Optical Imaging in Vivo. J. Am. Chem. Soc. 2009, 131, 11308–11309. [Google Scholar] [CrossRef] [PubMed]
- Bourlinos, A.B.; Stassinopoulos, A.; Anglos, D.; Zboril, R.; Karakassides, M.; Giannelis, E.P. Surface Functionalized Carbogenic Quantum Dots. Small 2008, 4, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Badger, P.D.; Geib, S.J.; Petoud, S. Sensitization of Near-Infrared-Emitting Lanthanide Cations in Solution by Tropolonate Ligands. Angew. Chem. Int. Ed. 2005, 44, 2508–2512. [Google Scholar] [CrossRef] [PubMed]
- Mathis, G. HTRF(R) Technology. J. Biomol. Screen. 1999, 4, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Hemmilá, I.; Mukkala, V.-M. Time-Resolution in Fluorometry Technologies, Labels, and Applications in Bioanalytical Assays. Crit. Rev. Clin. Lab. Sci. 2001, 38, 441–519. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G.; Piguet, C. Taking Advantage of Luminescent Lanthanide Ions. Chem. Soc. Rev. 2005, 34, 1048–1077. [Google Scholar] [CrossRef] [PubMed]
- Petoud, S.; Cohen, S.M.; Bünzli, J.-C.G.; Raymond, K.N. Stable Lanthanide Luminescence Agents Highly Emissive in Aqueous Solution: Multidentate 2-Hydroxyisophthalamide Complexes of Sm3+, Eu3+, Tb3+, Dy3+. J. Am. Chem. Soc. 2003, 125, 13324–13325. [Google Scholar] [CrossRef]
- Sy, M.; Nonat, A.; Hildebrandt, N.; Charbonnière, L.J. Lanthanide-Based Luminescence Biolabelling. Chem. Commun. 2016, 52, 5080–5095. [Google Scholar] [CrossRef]
- Moore, E.G.; Samuel, A.P.S.; Raymond, K.N. From Antenna to Assay. Acc. Chem. Res. 2009, 42, 542–552. [Google Scholar] [CrossRef]
- Bonnet, C.S.; Buron, F.; Caillé, F.; Shade, C.M.; Drahoš, B.; Pellegatti, L.; Zhang, J.; Villette, S.; Helm, L.; Pichon, C.; et al. Pyridine-Based Lanthanide Complexes Combining MRI and NIR Luminescence Activities. Chem. Eur. J. 2012, 18, 1419–1431. [Google Scholar] [CrossRef]
- Nocton, G.; Nonat, A.; Gateau, C.; Mazzanti, M. Water Stability and Luminescence of Lanthanide Complexes of Tripodal Ligands Derived from 1,4,7-Triazacyclononane: Pyridinecarboxamide versus Pyridinecarboxylate Donors. Helv. Chim. Acta. 2009, 92, 2257–2273. [Google Scholar] [CrossRef]
- Walton, J.W.; Bourdolle, A.; Butler, S.J.; Soulie, M.; Delbianco, M.; McMahon, B.K.; Pal, R.; Puschmann, H.; Zwier, J.M.; Lamarque, L.; et al. Very Bright Europium Complexes That Stain Cellular Mitochondria. Chem. Commun. 2013, 49, 1600–1602. [Google Scholar] [CrossRef] [PubMed]
- Lukeš, I.; Kotek, J.; Vojtíšek, P.; Hermann, P. Complexes of Tetraazacycles Bearing Methylphosphinic/Phosphonic Acid Pendant Arms with Copper(II), Zinc(II) and Lanthanides(III). A Comparison with Their Acetic Acid Analogues. Coord. Chem. Rev. 2001, 216–217, 287–312. [Google Scholar] [CrossRef]
- Charpentier, C.; Salaam, J.; Lecointre, A.; Jeannin, O.; Nonat, A.; Charbonnière, L.J. Phosphonated Podand Type Ligand for the Complexation of Lanthanide CationsPhosphonated Podand Type Ligand for the Complexation of Lanthanide Cations. Eur. J. Inorg. Chem. 2019, 2019, 2168–2174. [Google Scholar] [CrossRef]
- Wang, F.; Liu, X. Recent Advances in the Chemistry of Lanthanide-Doped Upconversion Nanocrystals. Chem. Soc. Rev. 2009, 38, 976–989. [Google Scholar] [CrossRef]
- Ong, L.C.; Gnanasammandhan, M.K.; Nagarajan, S.; Zhang, Y. Upconversion: Road to El Dorado of the Fluorescence World. Luminescence 2010, 25, 290–293. [Google Scholar] [CrossRef]
- Liang, S.; Liu, Y.; Tang, Y.; Xie, Y.; Sun, H.; Zhang, H.; Yang, B. A User-Friendly Method for Synthesizing High-Quality NaYF4:Yb,Er(Tm) Nanocrystals in Liquid Paraffin. J. Nanomater. 2011, 302364. [Google Scholar] [CrossRef]
- Sudheendra, L.; Ortalan, V.; Dey, S.; Browning, N.D.; Kennedy, I.M. Plasmonic Enhanced Emissions from Cubic NaYF4:Yb:Er/Tm Nanophosphors. Chem. Mater. 2011, 23, 2987–2993. [Google Scholar] [CrossRef]
- Yu, X.; Li, M.; Xie, M.; Chen, L.; Li, Y.; Wang, Q. Dopant-Controlled Synthesis of Water-Soluble Hexagonal NaYF4Nanorods with Efficient Upconversion Fluorescence for Multicolor Bioimaging. Nano Res. 2010, 3, 51–60. [Google Scholar] [CrossRef]
- Kamimura, M.; Miyamoto, D.; Saito, Y.; Soga, K.; Nagasaki, Y. Design of Poly(Ethylene Glycol)/Streptavidin Coimmobilized Upconversion Nanophosphors and Their Application to Fluorescence Biolabeling. Langmuir 2008, 24, 8864–8870. [Google Scholar] [CrossRef]
- Singh, S.K.; Singh, A.K.; Kumar, D.; Prakash, O.; Rai, S.B. Efficient UV–Visible up-Conversion Emission in Er3+,Yb3+ Co-Doped La2O3 Nano-Crystalline Phosphor. Appl. Phys. B 2010, 98, 173–179. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, C.; Peng, C.; Li, C.; Wang, L.; Chai, R.; Lin, J. Controllable Red, Green, Blue (RGB) and Bright White Upconversion Luminescence of Lu2O3:Yb3+/Er3+/Tm3+ Nanocrystals through Single Laser Excitation at 980 Nm. Chemistry 2009, 15, 4649–4655. [Google Scholar] [CrossRef] [PubMed]
- Hizhnyi, Y.; Chornii, V.; Nedilko, S.; Slobodyanik, M.; Terebilenko, K.; Boyko, V.; Gomenyuk, O.; Sheludko, V. Luminescence Spectroscopy of Ln-Doped Bi-Containing Phosphates and Molybdates. Radiat. Meas. 2016, 90, 314–318. [Google Scholar] [CrossRef]
- Yue, D.; Lu, W.; Li, C.; Zhang, X.; Liu, C.; Wang, Z. Controllable Synthesis of Ln3+ (Ln = Tb, Eu) Doped Zinc Phosphate Nano-/Micro-Structured Materials: Phase, Morphology and Luminescence Properties. Nanoscale 2014, 6, 2137–2145. [Google Scholar] [CrossRef]
- Heer, S.; Lehmann, O.; Haase, M.; Güdel, H.-U. Blue, Green, and Red Upconversion Emission from Lanthanide-Doped LuPO4 and YbPO4 Nanocrystals in a Transparent Colloidal Solution. Angew. Chem. Int. Ed. 2003, 42, 3179–3182. [Google Scholar] [CrossRef]
- Boyer, J.-C.; Cuccia, L.A.; Capobianco, J.A. Synthesis of Colloidal Upconverting NaYF4:Er3+/Yb3+ and Tm3+/Yb3+ Monodisperse Nanocrystals. Nano Lett. 2007, 7, 847–852. [Google Scholar] [CrossRef]
- Heer, S.; Kömpe, K.; Güdel, H.-U.; Haase, M. Highly Efficient Multicolour Upconversion Emission in Transparent Colloids of Lanthanide-Doped NaYF4 Nanocrystals. Adv. Mater. 2004, 16, 2102–2105. [Google Scholar] [CrossRef]
- Yi, G.S.; Chow, G.M. Synthesis of Hexagonal-Phase NaYF4:Yb,Er and NaYF4:Yb,Tm Nanocrystals with Efficient Up-Conversion Fluorescence. Adv. Funct. Mater. 2006, 16, 2324–2329. [Google Scholar] [CrossRef]
- Yin, A.; Zhang, Y.; Sun, L.; Yan, C. Colloidal Synthesis and Blue Based Multicolor Upconversion Emissions of Size and Composition Controlled Monodisperse Hexagonal NaYF4:Yb,Tm Nanocrystals. Nanoscale 2010, 2, 953–959. [Google Scholar] [CrossRef]
- Vetrone, F.; Naccache, R.; Mahalingam, V.; Morgan, C.G.; Capobianco, J.A. The Active-Core/Active-Shell Approach: A Strategy to Enhance the Upconversion Luminescence in Lanthanide-Doped Nanoparticles. Adv. Funct. Mater. 2009, 19, 2924–2929. [Google Scholar] [CrossRef]
- Dong, H.; Sun, L.-D.; Yan, C.-H. Energy Transfer in Lanthanide Upconversion Studies for Extended Optical Applications. Chem. Soc. Rev. 2015, 44, 1608–1634. [Google Scholar] [CrossRef] [PubMed]
- Auzel, F. Upconversion and Anti-Stokes Processes with f and d Ions in Solids. Chem. Rev. 2004, 104, 139–174. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Jiang, S. Multicolor Core/Shell-Structured Upconversion Fluorescent Nanoparticles. Adv. Mater. 2008, 20, 4765–4769. [Google Scholar] [CrossRef]
- Ehlert, O.; Thomann, R.; Darbandi, M.; Nann, T. A Four-Color Colloidal Multiplexing Nanoparticle System. ACS Nano 2008, 2, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Qin, X.; Yao, N.; Ju, Y. Synthesis of Monodisperse Hexagonal NaYF4:Yb, Ln (Ln = Er, Ho and Tm) Upconversion Nanocrystals in TOPO. Nanotechnology 2007, 18, 445607. [Google Scholar] [CrossRef]
- Liu, C.; Chen, D. Controlled Synthesis of Hexagon Shaped Lanthanide-Doped LaF3 Nanoplates with Multicolor Upconversion Fluorescence. J. Mater. Chem. 2007, 17, 3875–3880. [Google Scholar] [CrossRef]
- Wang, G.; Peng, Q.; Li, Y. Up-Conversion Luminescence of Monodisperse CaF2:Yb3+/Er3+ Nanocrystals. J. Am. Chem. Soc. 2009, 131, 14200–14201. [Google Scholar] [CrossRef]
- Qin, X.; Yokomori, T.; Ju, Y. Flame Synthesis and Characterization of Rare-Earth (Er3+, Ho3+, and Tm3+) Doped Upconversion Nanophosphors. Appl. Phys. Lett. 2007, 90, 073104. [Google Scholar] [CrossRef]
- Chatterjee, D.K.; Rufaihah, A.J.; Zhang, Y. Upconversion Fluorescence Imaging of Cells and Small Animals Using Lanthanide Doped Nanocrystals. Biomaterials 2008, 29, 937–943. [Google Scholar] [CrossRef]
- Bogdan, N.; Rodríguez, E.M.; Sanz-Rodríguez, F.; de la Cruz, M.C.I.; Juarranz, Á.; Jaque, D.; Solé, J.G.; Capobianco, J.A. Bio-Functionalization of Ligand-Free Upconverting Lanthanide Doped Nanoparticles for Bio-Imaging and Cell Targeting. Nanoscale 2012, 4, 3647–3650. [Google Scholar] [CrossRef]
- Surender, E.M.; Comby, S.; Martyn, S.; Cavanagh, B.; Lee, T.C.; Brougham, D.F.; Gunnlaugsson, T. Cyclen Lanthanide-Based Micellar Structures for Application as Luminescent [Eu(III)] and Magnetic [Gd(III)] Resonance Imaging (MRI) Contrast Agents. Chem. Commun. 2016, 52, 10858–10861. [Google Scholar] [CrossRef] [PubMed]
- Kamaly, N.; Kalber, T.; Ahmad, A.; Oliver, M.H.; So, P.-W.; Herlihy, A.H.; Bell, J.D.; Jorgensen, M.R.; Miller, A.D. Bimodal Paramagnetic and Fluorescent Liposomes for Cellular and Tumor Magnetic Resonance Imaging. Bioconjug. Chem. 2008, 19, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Tsotsalas, M.; Busby, M.; Gianolio, E.; Aime, S.; De Cola, L. Functionalized Nanocontainers as Dual Magnetic and Optical Probes for Molecular Imaging Applications. Chem. Mater. 2008, 20, 5888–5893. [Google Scholar] [CrossRef]
- Hwang, Y.; Park, S.-H.; Lee, J.W. Applications of Functionalized Carbon Nanotubes for the Therapy and Diagnosis of Cancer. Polymers 2017, 9, 13. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Y.; Yang, W.; Li, Q. Dual-Lanthanide-Chelated Silica Nanoparticles as Labels for Highly Sensitive Time-Resolved Fluorometry. Chem. Mater. 2007, 19, 5875–5881. [Google Scholar] [CrossRef]
- Nassar, E.J.; Ciuffi, K.J.; Ribeiro, S.J.L.; Messaddeq, Y. Europium Incorporated in Silica Matrix Obtained by Sol-Gel: Luminescent Materials. Mater. Res. 2003, 6, 557–562. [Google Scholar] [CrossRef]
- Pinho, S.L.C.; Faneca, H.; Geraldes, C.F.G.C.; Delville, M.-H.; Carlos, L.D.; Rocha, J. Lanthanide-DTPA Grafted Silica Nanoparticles as Bimodal-Imaging Contrast Agents. Biomaterials 2012, 33, 925–935. [Google Scholar]
- Rieter, W.J.; Kim, J.S.; Taylor, K.M.L.; An, H.; Lin, W.; Tarrant, T.; Lin, W. Hybrid Silica Nanoparticles for Multimodal Imaging. Angew. Chem. Int. Ed. 2007, 46, 3680–3682. [Google Scholar] [CrossRef]
- Bloemen, M.; Vandendriessche, S.; Goovaerts, V.; Brullot, W.; Vanbel, M.; Carron, S.; Geukens, N.; Parac-Vogt, T.; Verbiest, T. Synthesis and Characterization of Holmium-Doped Iron Oxide Nanoparticles. Materials 2014, 7, 1155–1164. [Google Scholar] [CrossRef]
- Groman, E.V.; Bouchard, J.C.; Reinhardt, C.P.; Vaccaro, D.E. Ultrasmall Mixed Ferrite Colloids as Multidimensional Magnetic Resonance Imaging, Cell Labeling, and Cell Sorting Agents. Bioconjug. Chem. 2007, 18, 1763–1771. [Google Scholar] [CrossRef]
- Maalej, N.M.; Qurashi, A.; Assadi, A.A.; Maalej, R.; Shaikh, M.N.; Ilyas, M.; Gondal, M.A. Synthesis of Gd2O3:Eu Nanoplatelets for MRI and Fluorescence Imaging. Nanoscale Res. Lett. 2015, 10, 215. [Google Scholar] [CrossRef] [PubMed]
- Goldys, E.M.; Drozdowicz-Tomsia, K.; Jinjun, S.; Dosev, D.; Kennedy, I.M.; Yatsunenko, S.; Godlewski, M. Optical Characterization of Eu-Doped and Undoped Gd2O3 Nanoparticles Synthesized by the Hydrogen Flame Pyrolysis Method. J. Am. Chem. Soc. 2006, 128, 14498–14505. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Xie, M.Y.; Ding, Y.; Yu, X.F. Synthesis of Fe3O4@LaF3:Ce,Tb Nanocomposites with Bright Fluorescence and Strong Magnetism. Appl. Surf. Sci. 2009, 255, 4623–4626. [Google Scholar] [CrossRef]
- Lu, H.; Yi, G.; Zhao, S.; Chen, D.; Guo, L.-H.; Cheng, J. Synthesis and Characterization of Multi-Functional Nanoparticles Possessing Magnetic, up-Conversion Fluorescence and Bio-Affinity Properties. J. Mater. Chem. 2004, 14, 1336–1341. [Google Scholar] [CrossRef]
- Wang, Y.; Song, S.; Liu, J.; Liu, D.; Zhang, H. ZnO-Functionalized Upconverting Nanotheranostic Agent: Multi-Modality Imaging-Guided Chemotherapy with On-Demand Drug Release Triggered by PH. Angew. Chem. Int. Ed. 2015, 54, 536–540. [Google Scholar] [CrossRef]
- Du, X.; Wang, X.; Meng, L.; Bu, Y.; Yan, X. Enhance the Er3+ Upconversion Luminescence by Constructing NaGdF4:Er3+@NaGdF4:Er3+ Active-Core/Active-Shell Nanocrystals. Nanoscale Res. Lett. 2017, 12, 163. [Google Scholar] [CrossRef]
- Shen, J.; Sun, L.-D.; Zhang, Y.-W.; Yan, C.-H. Superparamagnetic and Upconversion Emitting Fe3O4/NaYF4: Yb,Er Hetero-Nanoparticles via a Crosslinker Anchoring Strategy. Chem. Commun. 2010, 46, 5731–5733. [Google Scholar] [CrossRef]
- Jańczewski, D.; Zhang, Y.; Das, G.K.; Yi, D.K.; Padmanabhan, P.; Bhakoo, K.K.; Tan, T.T.Y.; Selvan, S.T. Bimodal Magnetic–Fluorescent Probes for Bioimaging. Microsc. Res. Tech. 2011, 74, 563–576. [Google Scholar] [CrossRef]
- Bonnet, C.S.; Tóth, É. Towards Highly Efficient, Intelligent and Bimodal Imaging Probes: Novel Approaches Provided by Lanthanide Coordination Chemistry. Comptes Rendus Chim. 2010, 13, 700–714. [Google Scholar] [CrossRef]
- Pellegatti, L.; Zhang, J.; Drahos, B.; Villette, S.; Suzenet, F.; Guillaumet, G.; Petoud, S.; Tóth, É. Pyridine-Based Lanthanidecomplexes: Towards Bimodal Agents Operating as near Infrared Luminescent and MRI Reporters. Chem. Commun. 2008, 6591–6593. [Google Scholar] [CrossRef]
- Caillé, F.; Bonnet, C.S.; Buron, F.; Villette, S.; Helm, L.; Petoud, S.; Suzenet, F.; Tóth, É. Isoquinoline-Based Lanthanide Complexes: Bright NIR Optical Probes and Efficient MRI Agents. Inorg. Chem. 2012, 51, 2522–2532. [Google Scholar] [CrossRef] [PubMed]
- Tallec, G.; Fries, P.H.; Imbert, D.; Mazzanti, M. High Relaxivity and Stability of a Hydroxyquinolinate-Based Tripodal Monoaquagadolinium Complex for Use as a Bimodal MRI/Optical Imaging Agent. Inorg. Chem. 2011, 50, 7943–7945. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, S.; Pope, S.J.A. Lanthanide-Sensitized Lanthanide Luminescence: Terbium-Sensitized Ytterbium Luminescence in a Trinuclear Complex. J. Am. Chem. Soc. 2003, 125, 10526–10527. [Google Scholar] [CrossRef] [PubMed]
- Natrajan, L.S.; Villaraza, A.J.L.; Kenwright, A.M.; Faulkner, S. Controlled Preparation of a Heterometallic Lanthanide Complex Containing Different Lanthanides in Symmetrical Binding Pockets. Chem. Commun. 2009, 6020–6022. [Google Scholar] [CrossRef]
- Tei, L.; Gugliotta, G.; Avedano, S.; Giovenzana, G.B.; Botta, M. Application of the Ugi Four-Component Reaction to the Synthesis of Ditopic Bifunctional Chelating Agents. Org. Biomol. Chem. 2009, 7, 4406–4414. [Google Scholar] [CrossRef]
- Jauregui, M.; Perry, W.S.; Allain, C.; Vidler, L.R.; Willis, M.C.; Kenwright, A.M.; Snaith, J.S.; Stasiuk, G.J.; Lowe, M.P.; Faulkner, S. Changing the Local Coordination Environment in Mono- and Bi- Nuclear Lanthanide Complexes through “Click” Chemistry. Dalton Trans. 2009, 32, 6283–6285. [Google Scholar] [CrossRef]
- Placidi, M.P.; Villaraza, A.J.L.; Natrajan, L.S.; Sykes, D.; Kenwright, A.M.; Faulkner, S. Synthesis and Spectroscopic Studies on Azo-Dye Derivatives of Polymetallic Lanthanide Complexes: Using Diazotization to Link Metal Complexes Together. J. Am. Chem. Soc. 2009, 131, 9916–9917. [Google Scholar] [CrossRef]
- Debroye, E.; Parac-Vogt, T.N. Towards Polymetallic Lanthanide Complexes as Dual Contrast Agents for Magnetic Resonance and Optical Imaging. Chem. Soc. Rev. 2014, 43, 8178–8192. [Google Scholar] [CrossRef]
- Dehaen, G.; Eliseeva, S.V.; Kimpe, K.; Laurent, S.; Vander Elst, L.; Muller, R.N.; Dehaen, W.; Binnemans, K.; Parac-Vogt, T.N. A Self-Assembled Complex with a Titanium(IV) Catecholate Core as a Potential Bimodal Contrast Agent. Chem.-Eur. J. 2012, 18, 293–302. [Google Scholar] [CrossRef]
- Dehaen, G.; Verwilst, P.; Eliseeva, S.V.; Laurent, S.; Vander Elst, L.; Muller, R.N.; De Borggraeve, W.M.; Binnemans, K.; Parac-Vogt, T.N. A Heterobimetallic Ruthenium–Gadolinium Complex as a Potential Agent for Bimodal Imaging. Inorg. Chem. 2011, 50, 10005–10014. [Google Scholar] [CrossRef]
- Debroye, E.; Ceulemans, M.; Vander Elst, L.; Laurent, S.; Muller, R.N.; Parac-Vogt, T.N. Controlled Synthesis of a Novel Heteropolymetallic Complex with Selectively Incorporated Lanthanide(III) Ions. Inorg. Chem. 2014, 53, 1257–1259. [Google Scholar] [CrossRef] [PubMed]
- Andolina, C.M.; Klemm, P.J.; Floyd, W.C.; Fréchet, J.M.J.; Raymond, K.N. Analysis of Lanthanide Complex Dendrimer Conjugates for Bimodal NIR and MRI Imaging. Macromolecules 2012, 45, 8982–8990. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lemonnier, J.-F.; Guénée, L.; Beuchat, C.; Wesolowski, T.A.; Mukherjee, P.; Waldeck, D.H.; Gogick, K.A.; Petoud, S.; Piguet, C. Optimizing Sensitization Processes in Dinuclear Luminescent Lanthanide Oligomers: Selection of Rigid Aromatic Spacers. J. Am. Chem. Soc. 2011, 133, 16219–16234. [Google Scholar] [CrossRef] [PubMed]
- Wild, A.; Winter, A.; Schlütter, F.; Schubert, U.S. Advances in the Field of π-Conjugated 2,2′:6′,2″-Terpyridines. Chem. Soc. Rev. 2011, 40, 1459–1511. [Google Scholar] [CrossRef]
- Chen, X.-Y.; Yang, X.; Holliday, B.J. Photoluminescent Europium-Containing Inner Sphere Conducting Metallopolymer. J. Am. Chem. Soc. 2008, 130, 1546–1547. [Google Scholar] [CrossRef]
- Carron, S.; Li, Q.Y.; Elst, L.V.; Muller, R.N.; Parac-Vogt, T.N.; Capobianco, J.A. Assembly of near Infra-Red Emitting Upconverting Nanoparticles and Multiple Gd(III)-Chelates as a Potential Bimodal Contrast Agent for MRI and Optical Imaging. Dalton Trans. 2015, 44, 11331–11339. [Google Scholar] [CrossRef]
- Carron, S.; Bloemen, M.; Vander Elst, L.; Laurent, S.; Verbiest, T.; Parac-Vogt, T.N. Ultrasmall Superparamagnetic Iron Oxide Nanoparticles with Europium(III) DO3A as a Bimodal Imaging Probe. Chem. Eur. J. 2016, 22, 4521–4527. [Google Scholar] [CrossRef]
- Feng, J.; Song, S.-Y.; Deng, R.-P.; Fan, W.-Q.; Zhang, H.-J. Novel Multifunctional Nanocomposites: Magnetic Mesoporous Silica Nanospheres Covalently Bonded with near-Infrared Luminescent Lanthanide Complexes. Langmuir 2010, 26, 3596–3600. [Google Scholar] [CrossRef]
- Pinheiro, P.C.; Daniel-da-Silva, A.L.; Tavares, D.S.; Calatayud, M.P.; Goya, G.F.; Trindade, T. Fluorescent Magnetic Bioprobes by Surface Modification of Magnetite Nanoparticles. Materials 2013, 6, 3213–3225. [Google Scholar] [CrossRef]
- Zhang, Y.; Das, G.K.; Xu, R.; Tan, T.T.Y. Tb-Doped Iron Oxide: Bifunctional Fluorescent and Magnetic Nanocrystals. J. Mater. Chem. 2009, 19, 3696–3703. [Google Scholar] [CrossRef]
- Das, G.K.; Heng, B.C.; Ng, S.-C.; White, T.; Loo, J.S.C.; D’Silva, L.; Padmanabhan, P.; Bhakoo, K.K.; Selvan, S.T.; Tan, T.T.Y. Gadolinium Oxide Ultranarrow Nanorods as Multimodal Contrast Agents for Optical and Magnetic Resonance Imaging. Langmuir 2010, 26, 8959–8965. [Google Scholar] [CrossRef] [PubMed]
- Petoral, R.M.; Söderlind, F.; Klasson, A.; Suska, A.; Fortin, M.A.; Abrikossova, N.; Selegård, L.; Käll, P.-O.; Engström, M.; Uvdal, K. Synthesis and Characterization of Tb3+-Doped Gd2O3 Nanocrystals: A Bifunctional Material with Combined Fluorescent Labeling and MRI Contrast Agent Properties. J. Phys. Chem. C 2009, 113, 6913–6920. [Google Scholar] [CrossRef]
- Das, G.K.; Zhang, Y.; D’Silva, L.; Padmanabhan, P.; Heng, B.C.; Chye Loo, J.S.; Selvan, S.T.; Bhakoo, K.K.; Yang Tan, T.T. Single-Phase Dy2O3:Tb3+ Nanocrystals as Dual-Modal Contrast Agent for High Field Magnetic Resonance and Optical Imaging. Chem. Mater. 2011, 23, 2439–2446. [Google Scholar] [CrossRef]
- Xu, W.; Bony, B.A.; Kim, C.R.; Baeck, J.S.; Chang, Y.; Bae, J.E.; Chae, K.S.; Kim, T.J.; Lee, G.H. Mixed Lanthanide Oxide Nanoparticles as Dual Imaging Agent in Biomedicine. Sci. Rep. 2013, 3, 3210. [Google Scholar] [CrossRef]
- Setua, S.; Menon, D.; Asok, A.; Nair, S.; Koyakutty, M. Folate Receptor Targeted, Rare-Earth Oxide Nanocrystals for Bi-Modal Fluorescence and Magnetic Imaging of Cancer Cells. Biomaterials 2010, 31, 714–729. [Google Scholar] [CrossRef] [PubMed]
- Debasu, M.L.; Ananias, D.; Pinho, S.L.C.; Geraldes, C.F.G.C.; Carlos, L.D.; Rocha, J. (Gd,Yb,Tb)PO4 Up-Conversion Nanocrystals for Bimodal Luminescence–MR Imaging. Nanoscale 2012, 4, 5154–5162. [Google Scholar] [CrossRef]
- Liu, J.; Huang, L.; Tian, X.; Chen, X.; Shao, Y.; Xie, F.; Chen, D.; Li, L. Magnetic and Fluorescent Gd2O3:Yb3+/Ln3+ Nanoparticles for Simultaneous Upconversion Luminescence/MR Dual Modal Imaging and NIR-Induced Photodynamic Therapy. Int. J. Nanomed. 2016, 12, 1–14. [Google Scholar] [CrossRef][Green Version]
- Syamchand, S.S.; George, S. The Upconversion Luminescence and Magnetism in Yb3+-Ho3+ Co-Doped LaF3 Nanocrystals for Potential Bimodal Imaging. J. Nanopart Res. 2016, 18, 385. [Google Scholar] [CrossRef]
- Cui, X.; Mathe, D.; Kovács, N.; Horváth, I.; Jauregui-Osoro, M.; Torres Martin de Rosales, R.; Mullen, G.E.D.; Wong, W.; Yan, Y.; Krüger, D.; et al. Synthesis, Characterization, and Application of Core–Shell Co0.16Fe2.84O4@NaYF4(Yb, Er) and Fe3O4@NaYF4(Yb, Tm) Nanoparticle as Trimodal (MRI, PET/SPECT, and Optical) Imaging Agents. Bioconjug. Chem. 2016, 27, 319–328. [Google Scholar] [CrossRef]
- Mnasri, W.; Ben Tahar, L.; Beaunier, P.; Abi Haidar, D.; Boissière, M.; Sandre, O.; Ammar, S. Polyol-Made Luminescent and Superparamagnetic β-NaY0.8Eu0.2F4@γ-Fe2O3 Core-Satellites Nanoparticles for Dual Magnetic Resonance and Optical Imaging. Nanomaterials 2020, 10, 393. [Google Scholar] [CrossRef]
- Baziulyte-Paulaviciene, D.; Karabanovas, V.; Stasys, M.; Jarockyte, G.; Poderys, V.; Sakirzanovas, S.; Rotomskis, R. Synthesis and Functionalization of NaGdF4:Yb,Er@NaGdF4 Core–Shell Nanoparticles for Possible Application as Multimodal Contrast Agents. Beilstein J. Nanotechnol. 2017, 8, 1815–1824. [Google Scholar] [CrossRef] [PubMed]

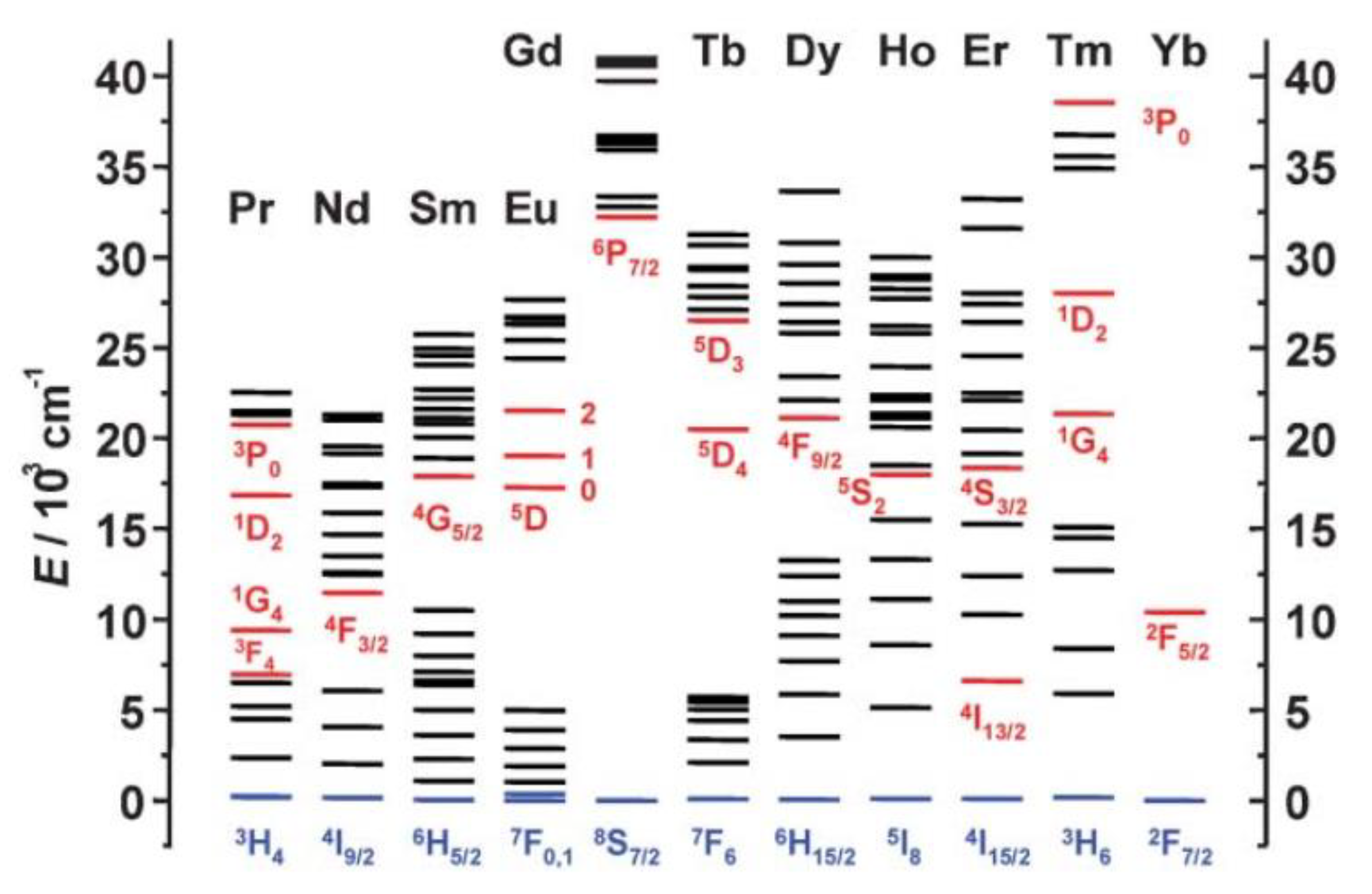
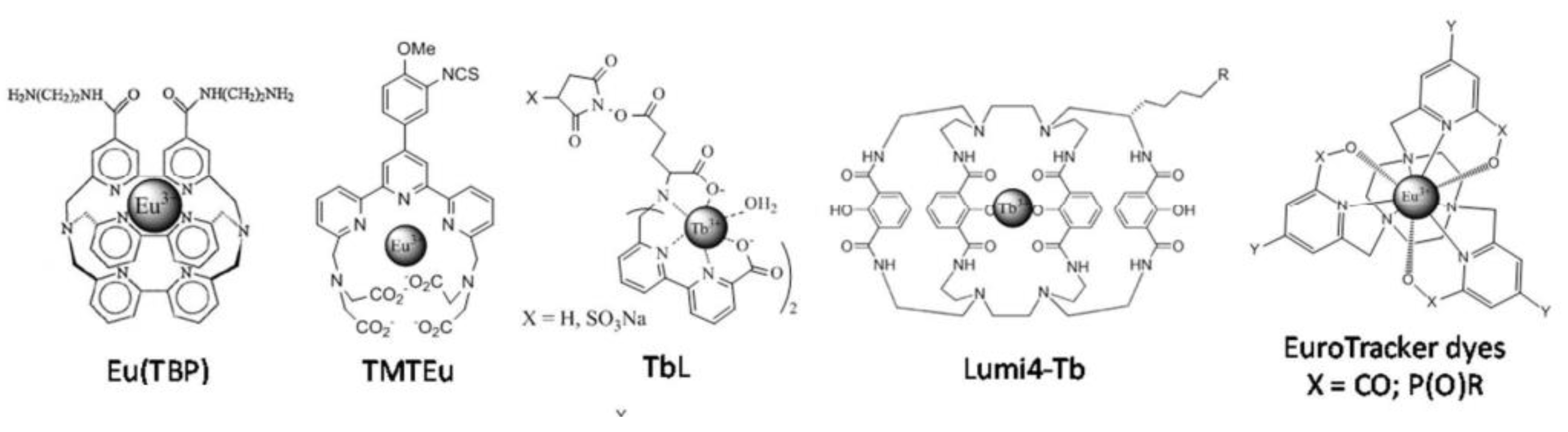

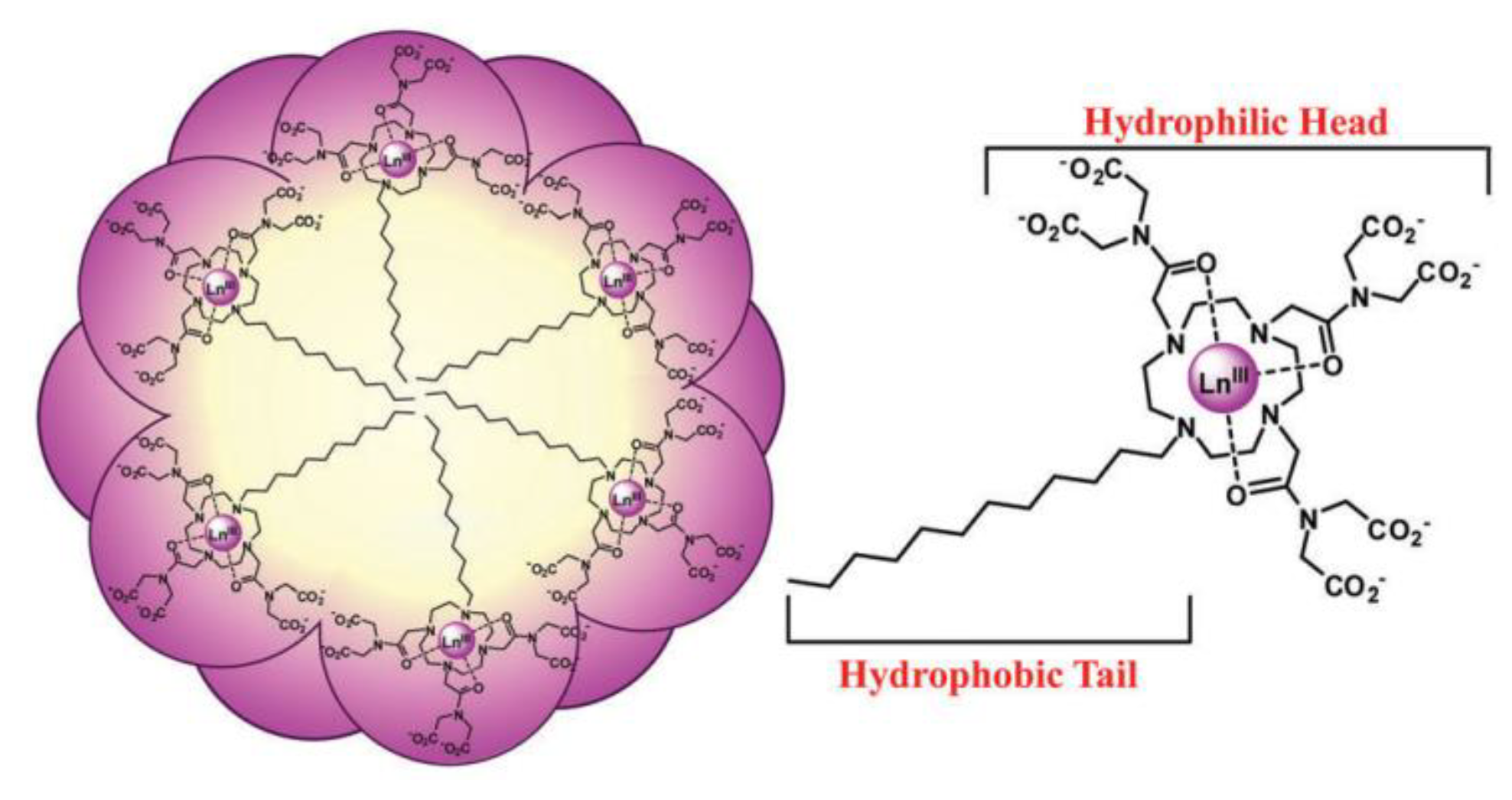
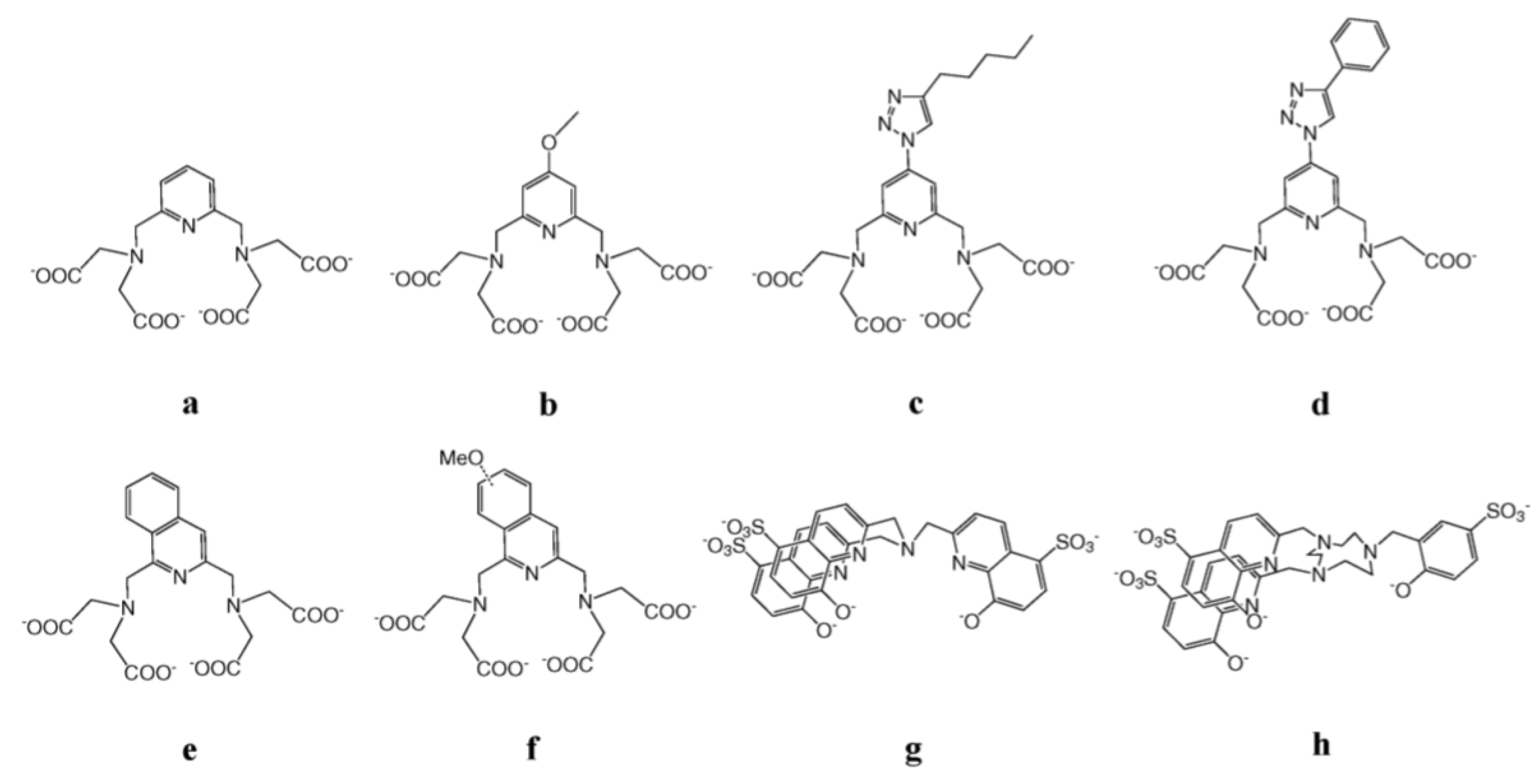
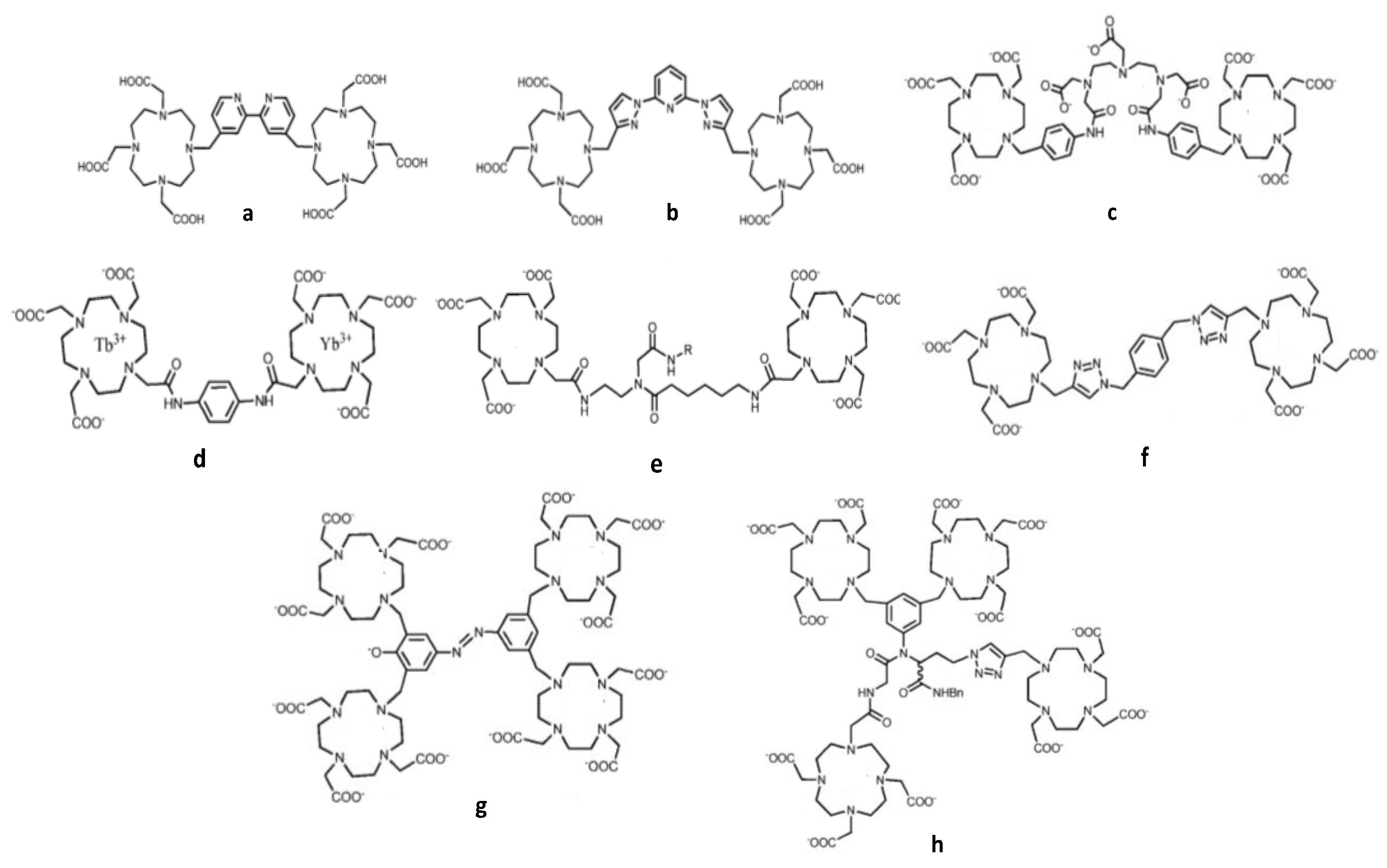
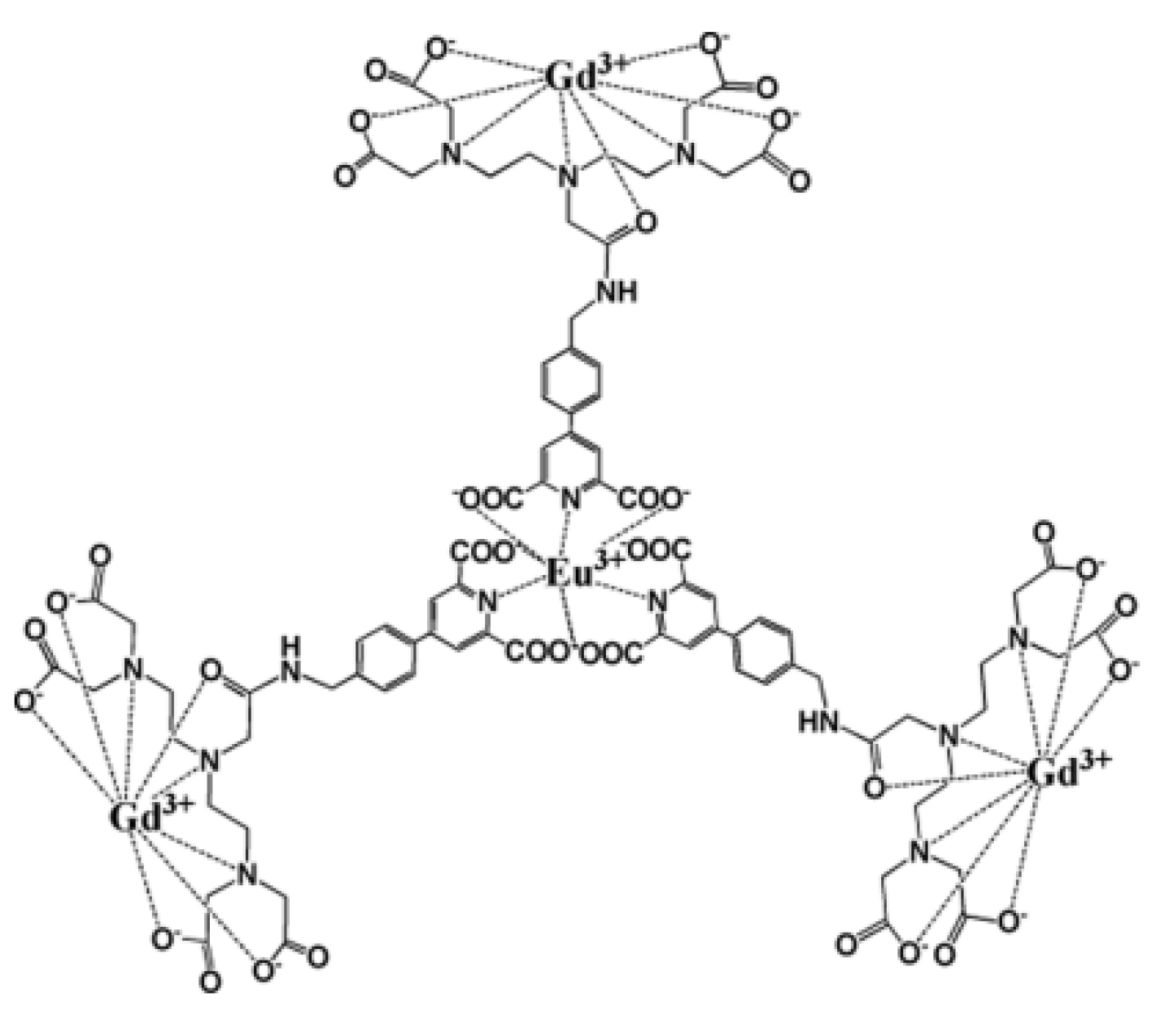
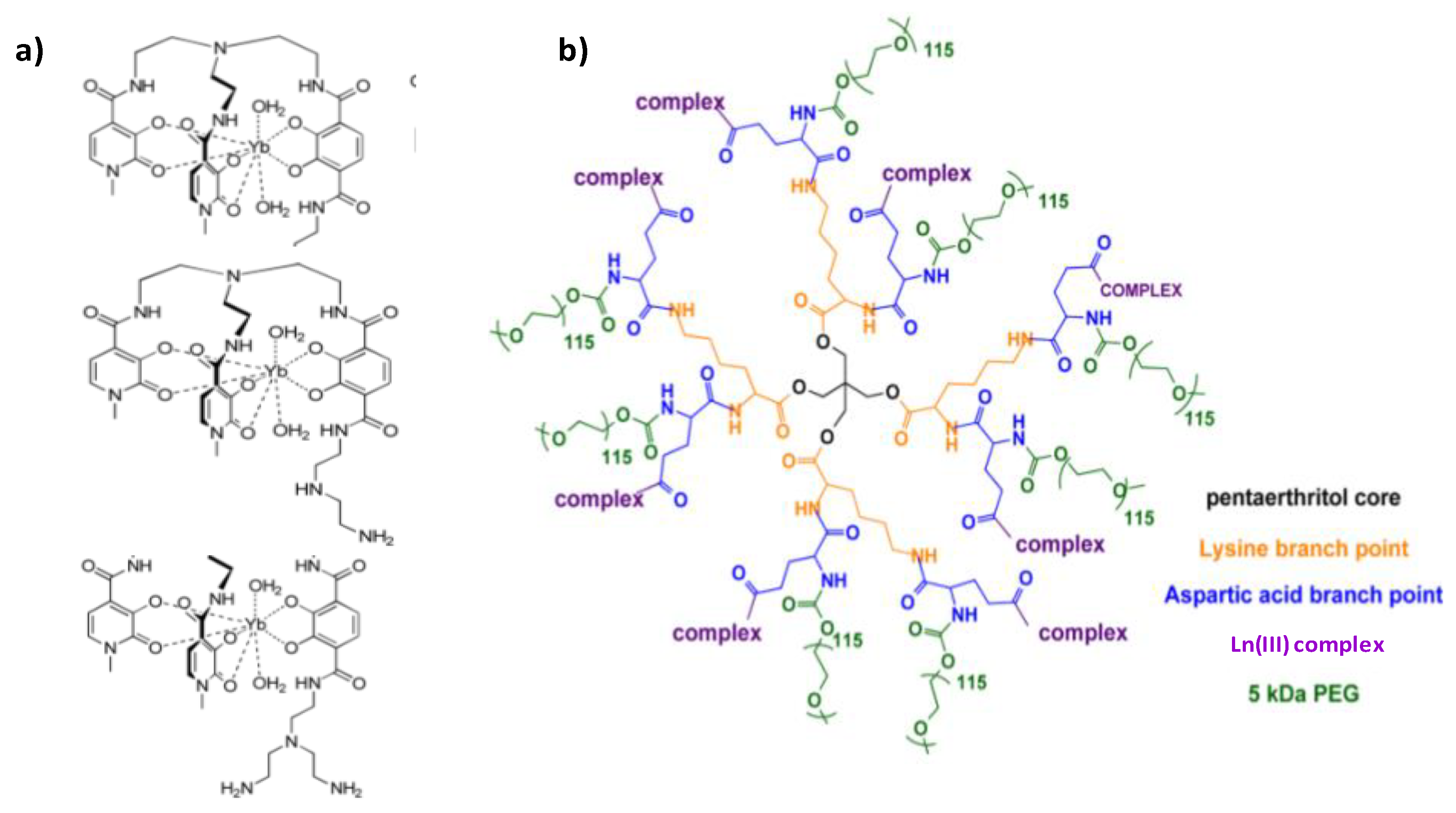



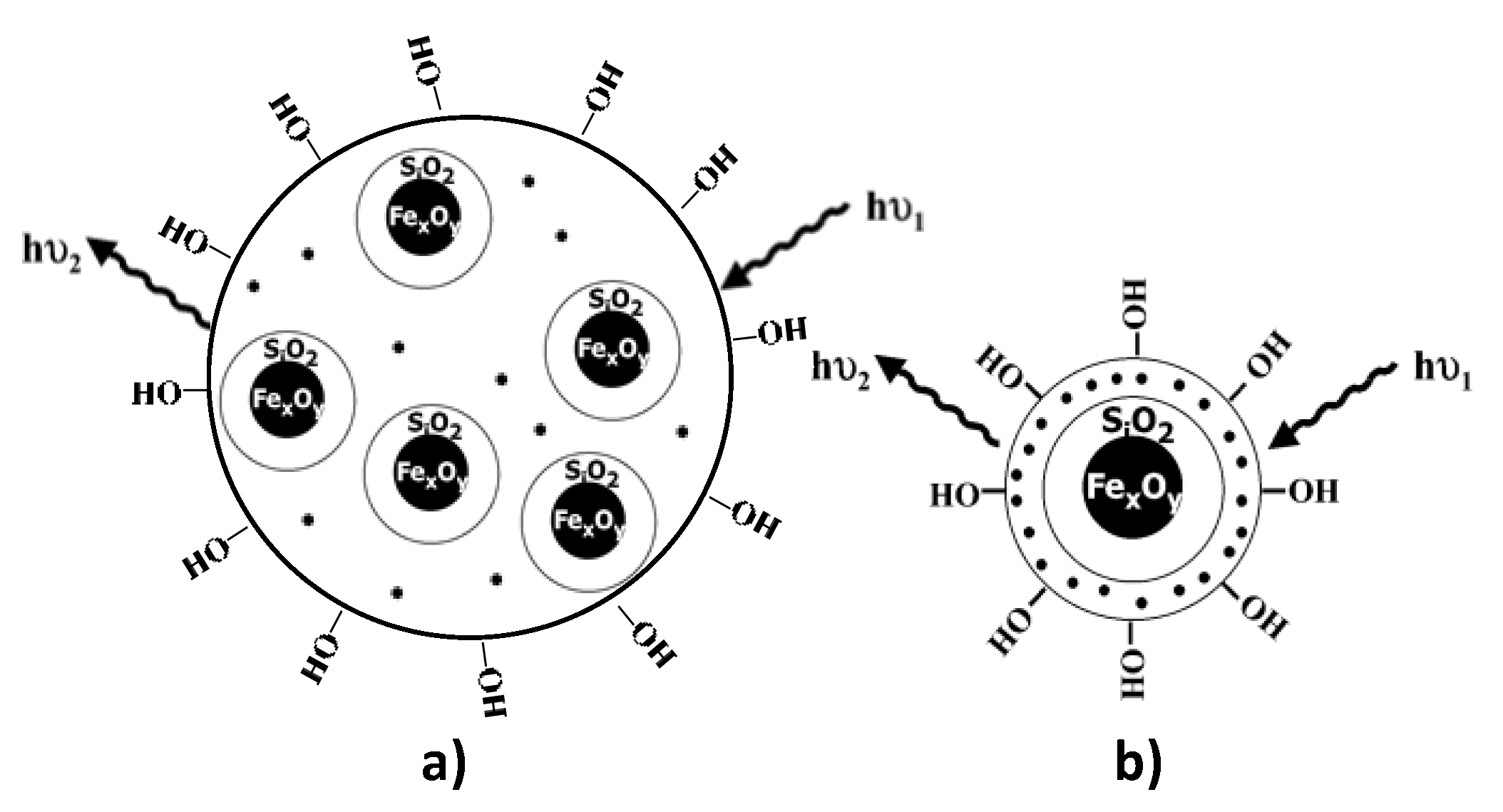

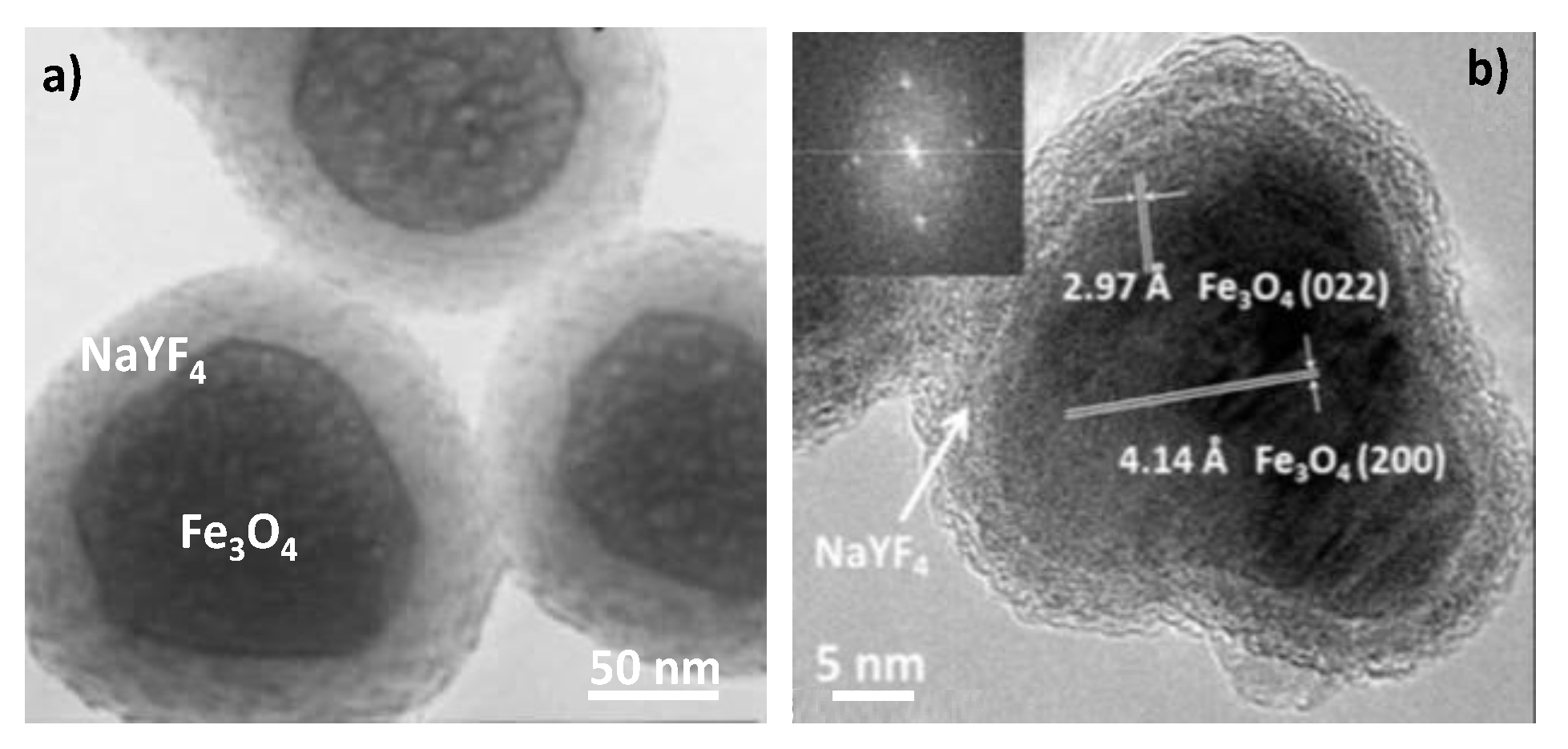

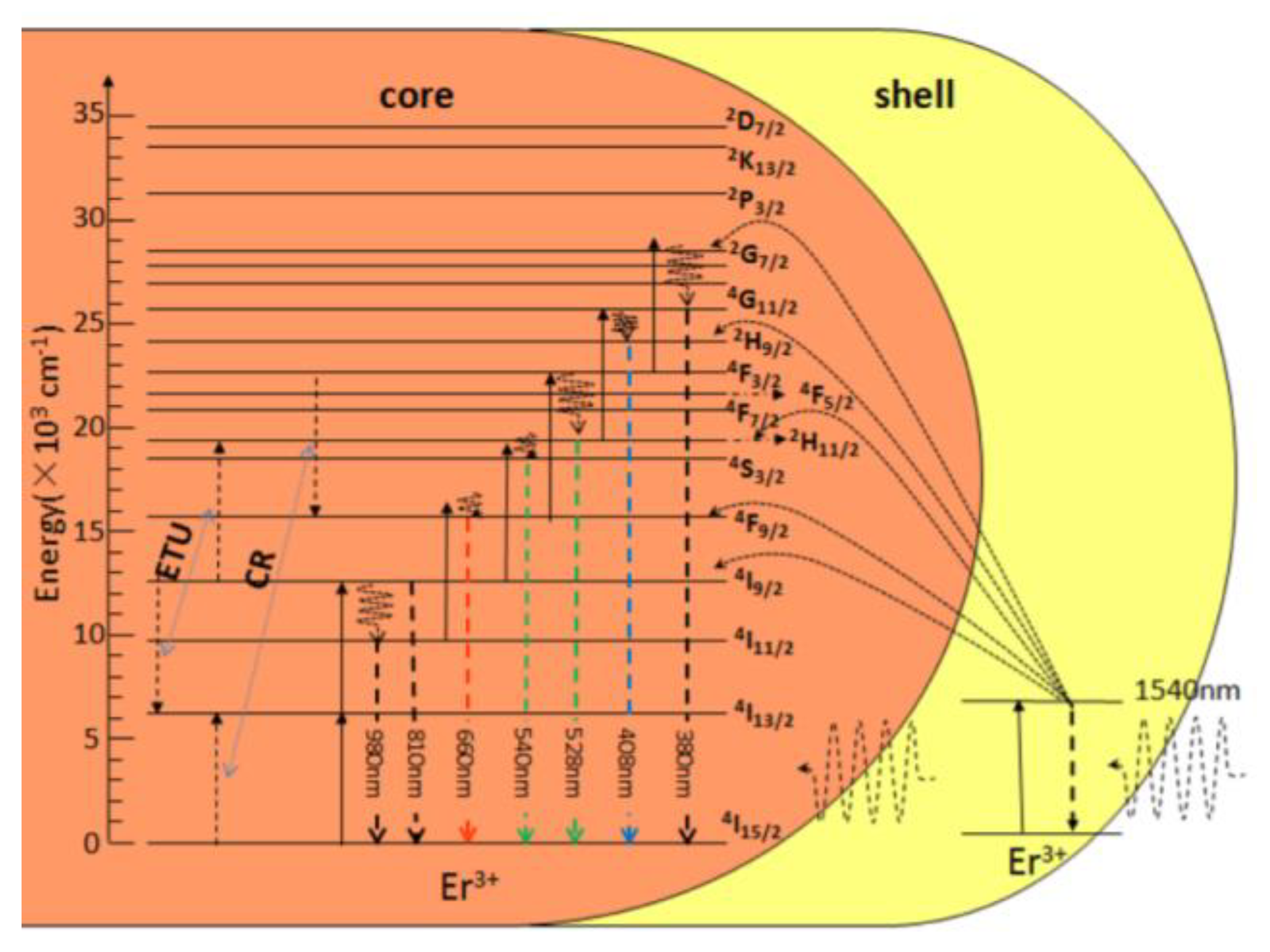
| Number Gd3+/CA | CA weight kD | r1/CA mM−1.s−1 | r1/Gd3+ mM−1.s−1 | T °C | fRF MHz | Ref. | |
|---|---|---|---|---|---|---|---|
| Gd-DTPA | 1 | 0.6 | 3.7 | 3.7 | 37 | 20 | [2] |
| Gd-DOTA | 1 | − | 4,2 | 4.2 | 25 | 20 | [2] |
| Dextran-Gd-DTPA | 15 | 75 | 57 | 11.0 | 37 | 20 | [32] |
| Polylysine-Gd-DTPA | 60 | 48.7 | 850 | 13.1 | 39 | 20 | [35] |
| Albumin-Gd-DTPA | 90 | 90 | 420 | 14.0 | 25 | 10 | [45] |
| 6-dendrimer-Gd-DTPA | 170 | 139 | 5800 | 34.0 | 37 | − | [46] |
| Gd-NTCs | − | 0.044–0.049 | 173–164 | 40 | 60 | − | [47] |
| Gd-DOTA-Apoferritin | 6 | − | 3.9 | 25 | 64 | − | [43] |
| Gd-Me2DO2A-Apoferritin | 36 | − | 35.9 | 25 | 64 | − | [43] |
| Size nm | r1/Mn+ mM−1.s−1 | T °C | B0 T | Ref. | |
|---|---|---|---|---|---|
| MnO spheres | 7 20 | 0.37 0.13 | 25 | 3.0 | [49] |
| MnO hollow spheres | 20 | 1.15 | 25 | 1.5 | [56] |
| Mn3O4 spheres | 9.8 | 1.31 | 25 | 3.0 | [48] |
| Mn3O4 platelets | 10 | 2.06 | 25 | 3.0 | [48] |
| Gd2O3 particles | 5 | 9.2 | 21–23 | 1.5 | [52] |
| Composition | Size nm | r2 mM−1.s−1 | r2/r1 | T °C | B0 T | Ref. | |
|---|---|---|---|---|---|---|---|
| SPIO | Fe2O3 NPs in Dextran | 50–100 | 160 | 4.0 | 37 | 0.47 | [72] |
| Fe2O3 NPs in carboxylate Dextran | 30–50 | 190 | 7.9 | 37 | 0.47 | ||
| USPIO | Fe2O3 NPs in Dextran | 17–20 | 53 | 2.2 | 37 | 0.47 | |
| MION | Fe2O3 NPs in Dextran | 18–24 | 35 | 2.2 | 37 | 0.47 |
| Composition | Size nm | r2 mM−1.s−1 | T °C | B0 T | Ref | |
|---|---|---|---|---|---|---|
| Manganite | 57 nm sized La0.75Sr0.25MnO3 NPs coated with a silica layer of 80–100 nm in thickness | 150 | 580 a 540 a 520 a | 20 20 20 | 0.5 1.5 3.0 | [62] |
| Ferrite | 4 nm sized CoFe2O4 NPs in carboxylate PEG | 30 | 185 b | 25 | 1.5 | [73] |
| 8 nm sized MnFe2O4 NPs in PEG-PCL | 80 | 66 b | 25 | 1.5 | ||
| Fe | Less than 10 nm sized Fe NPs coated by PEG | 10 | 129 c | 25 | 1.5 | [74] |
| EFNPs | Fe@NixFe3-xO4 NPs coated by PEG | 15 | 9.96 d | 25 | 2.4 | [70] |
| QD | λabsorption (nm) | λemission (nm) | FWHM (nm) | ε (M−1 cm−1) | Фf (%) | Ref. |
|---|---|---|---|---|---|---|
| CdS | 350–470 | 370–500 | ~30 | 1.0 × 105 (at 350 nm) 9.5 × 105 (at 450 nm) | ≤60 a | [118] |
| CdSe | 450–640 | 470–660 | ~30 | 1.0 × 105 (at 500 nm) 7.0 × 105 (at 630 nm) | 65 a 8 a | [119] |
| CdTe | 500–700 | 520–750 | 35–45 | 1.3 × 105 (at 570) 6.0 × 105 (at 700 nm) | 30 a 75 a | [120,121] |
| PbS | 800–3000 | >900 | 80–90 | − | 26 b 70 c | [122,123,124] |
| PbSe | 900–4000 | >1000 | 80–90 | 1.23 × 105 | 45 d | [125,126] |
| InP | 550–650 | 620–720 | 50–90 | − | 10–60 | [127,128] |
| Host Lattice | Sensitizer | Activator | Major Emission (nm) | Ref. |
|---|---|---|---|---|
| NaYF4 | Yb | Er | 518, 537 and 652 | [169] |
| Er | 540 and 660 | [168] | ||
| Er | 521, 539 and 651 | [174] | ||
| Tm | 450, 475 and 647 | [168] | ||
| Ho | 540 | [175] | ||
| Ho | 542 and 645, 658 | [176] | ||
| LaF3 | Yb | Er | 520, 545 and 659 | [177] |
| Tm | 475 | [177] | ||
| Ho | 542 and 645, 658 | [177] | ||
| CaF2 | Yb | Er | 524 and 654 | [178] |
| Y2O3 | Yb | Er | 550 and 660 | [161] |
| Ho | 543 and 665 | [179] | ||
| LuPO4 | Yb | Tm | 475 and 649 | [166] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mnasri, W.; Parvizian, M.; Ammar-Merah, S. Design and Synthesis of Luminescent Lanthanide-Based Bimodal Nanoprobes for Dual Magnetic Resonance (MR) and Optical Imaging. Nanomaterials 2021, 11, 354. https://doi.org/10.3390/nano11020354
Mnasri W, Parvizian M, Ammar-Merah S. Design and Synthesis of Luminescent Lanthanide-Based Bimodal Nanoprobes for Dual Magnetic Resonance (MR) and Optical Imaging. Nanomaterials. 2021; 11(2):354. https://doi.org/10.3390/nano11020354
Chicago/Turabian StyleMnasri, Walid, Mahsa Parvizian, and Souad Ammar-Merah. 2021. "Design and Synthesis of Luminescent Lanthanide-Based Bimodal Nanoprobes for Dual Magnetic Resonance (MR) and Optical Imaging" Nanomaterials 11, no. 2: 354. https://doi.org/10.3390/nano11020354
APA StyleMnasri, W., Parvizian, M., & Ammar-Merah, S. (2021). Design and Synthesis of Luminescent Lanthanide-Based Bimodal Nanoprobes for Dual Magnetic Resonance (MR) and Optical Imaging. Nanomaterials, 11(2), 354. https://doi.org/10.3390/nano11020354







