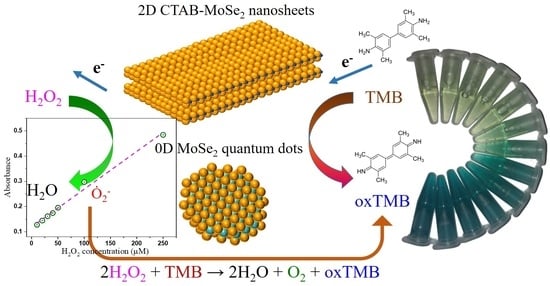
2D CTAB-MoSe2 Nanosheets and 0D MoSe2 Quantum Dots: Facile Top-Down Preparations and Their Peroxidase-Like Catalytic Activity for Colorimetric Detection of Hydrogen Peroxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Methods
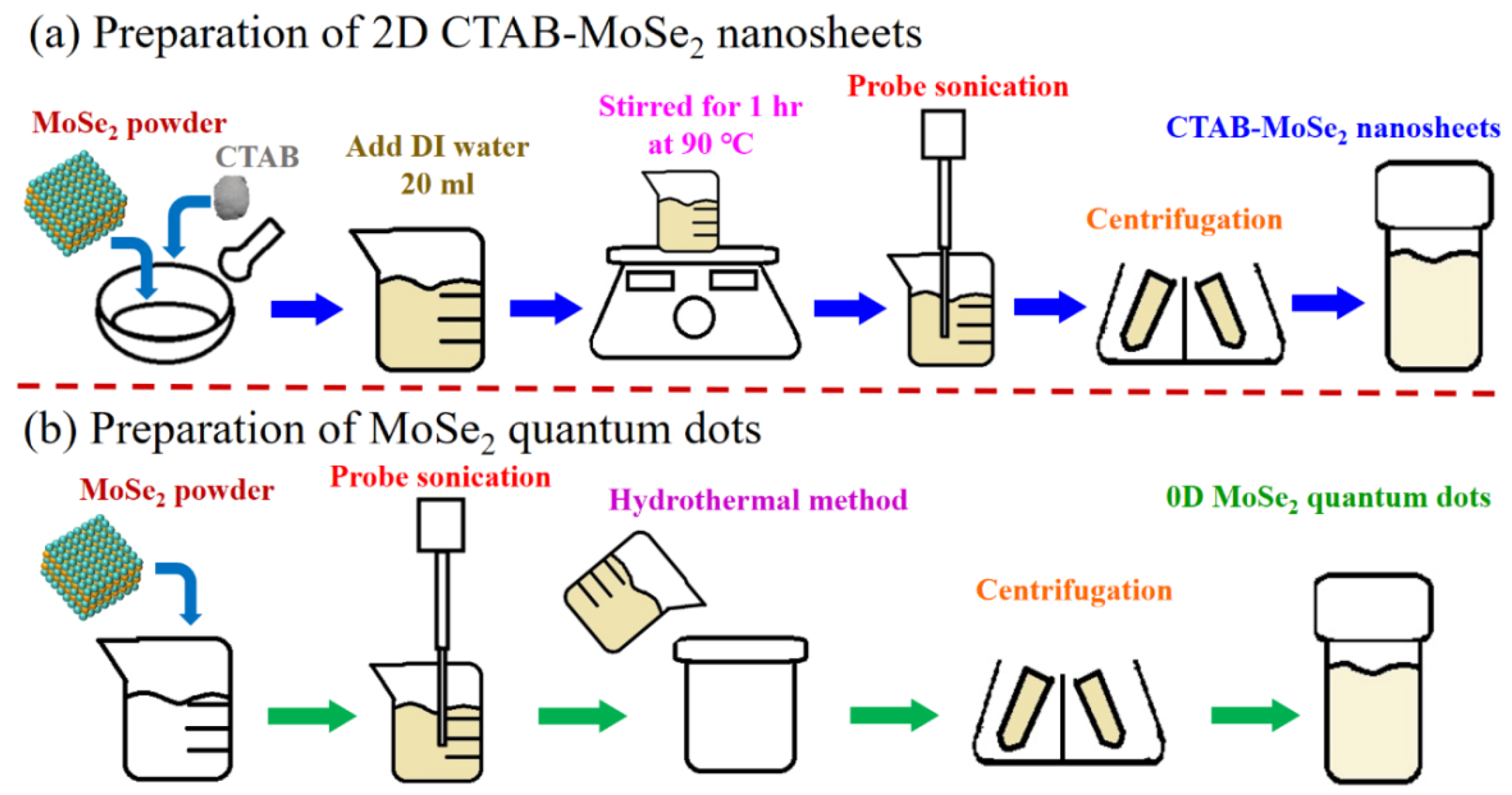
2.2.1. Preparation of Surfactant Modified Two-Dimensional (2D) MoSe2 Nanosheets (NSs)
2.2.2. Preparation of 0D MoSe2 QDs
2.3. Characterization
2.4. Peroxidase-Mimetic Activity of MoSe2 Quantum Dots (QDs) and 2D Cetyltrimethyl Ammonium Bromide (CTAB)-MoSe2 NSs
3. Results and Discussion
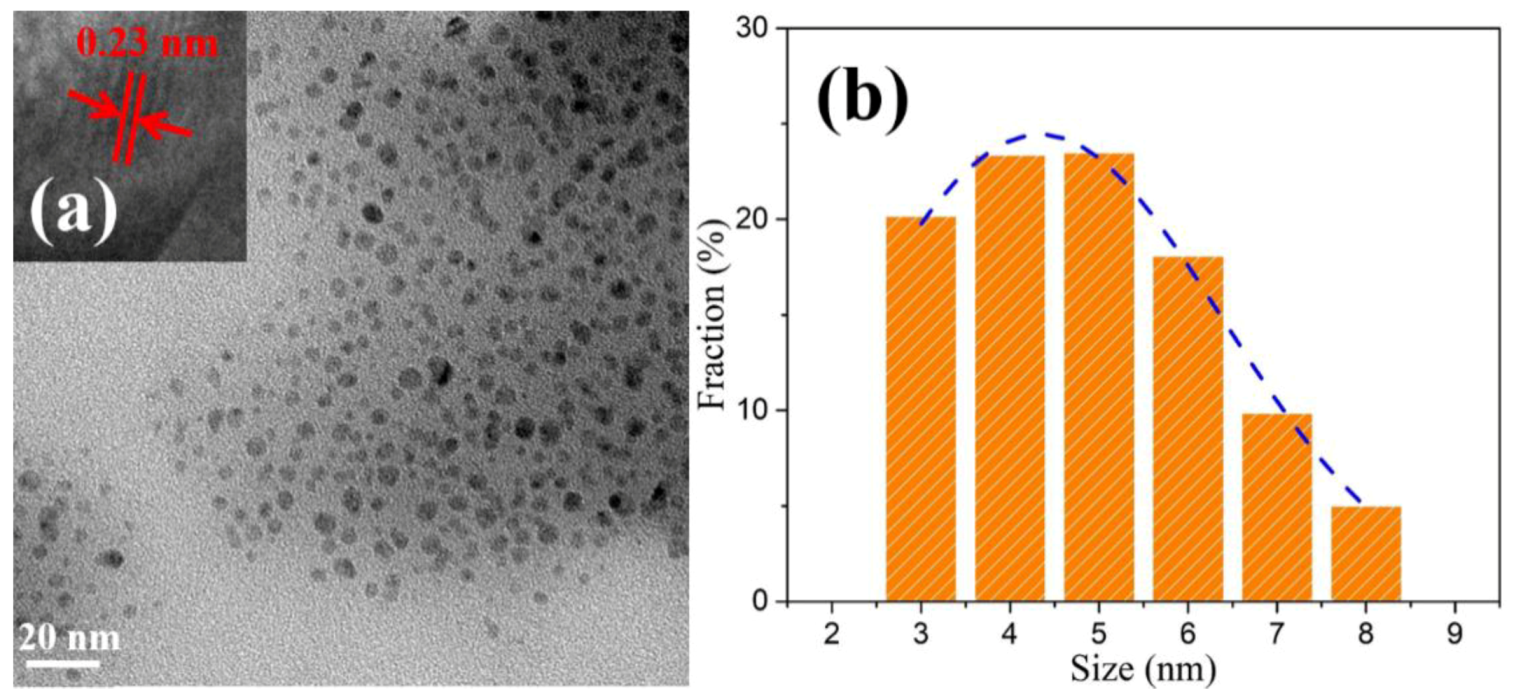
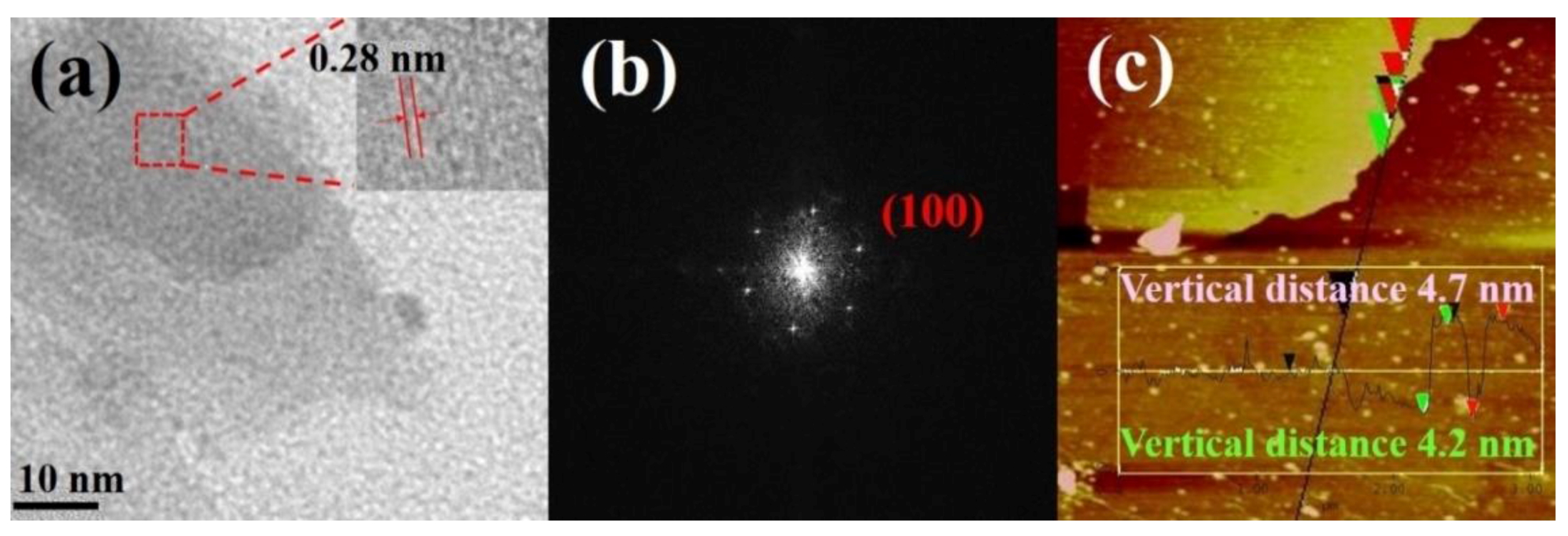
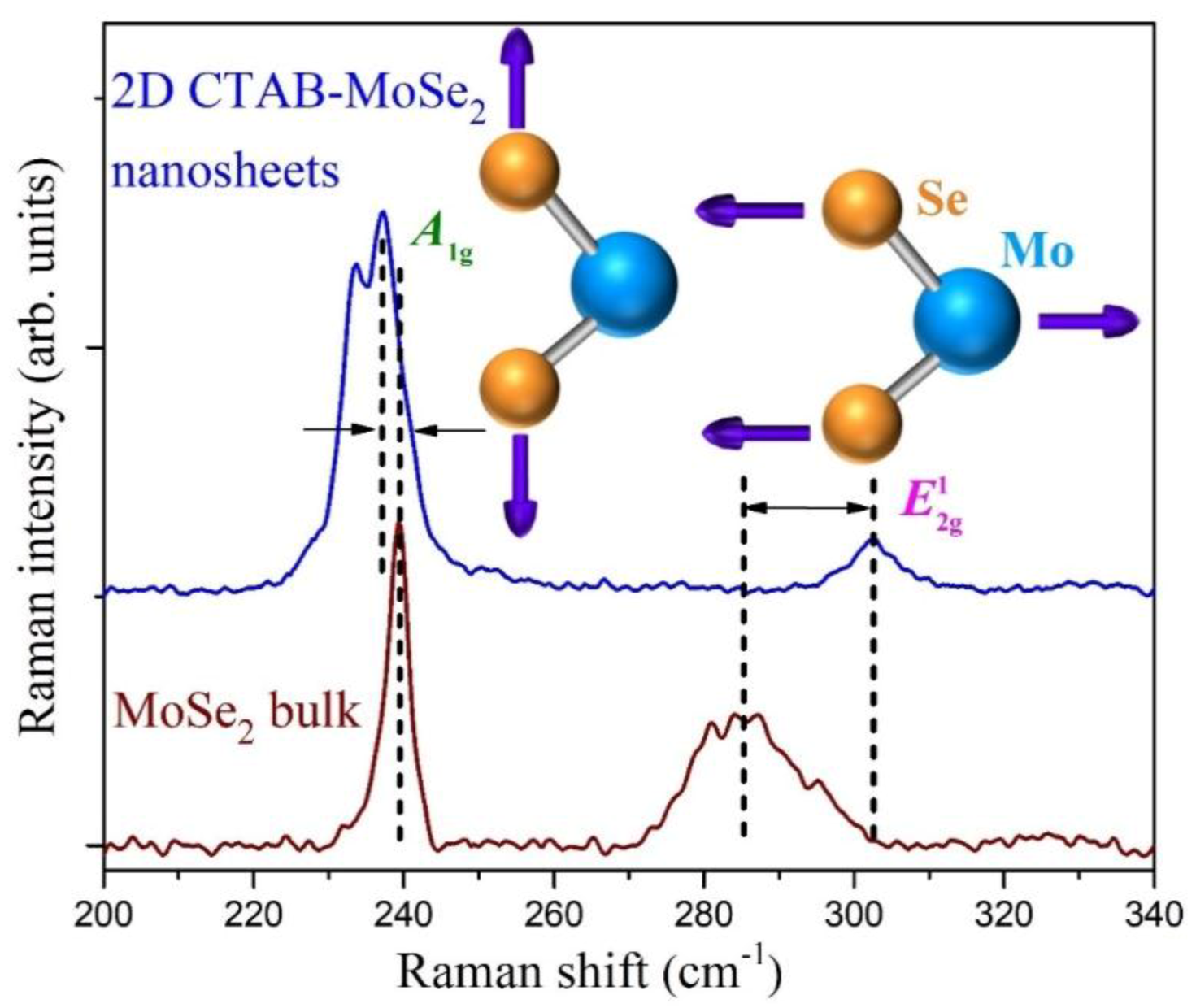
3.1. Structural Studies
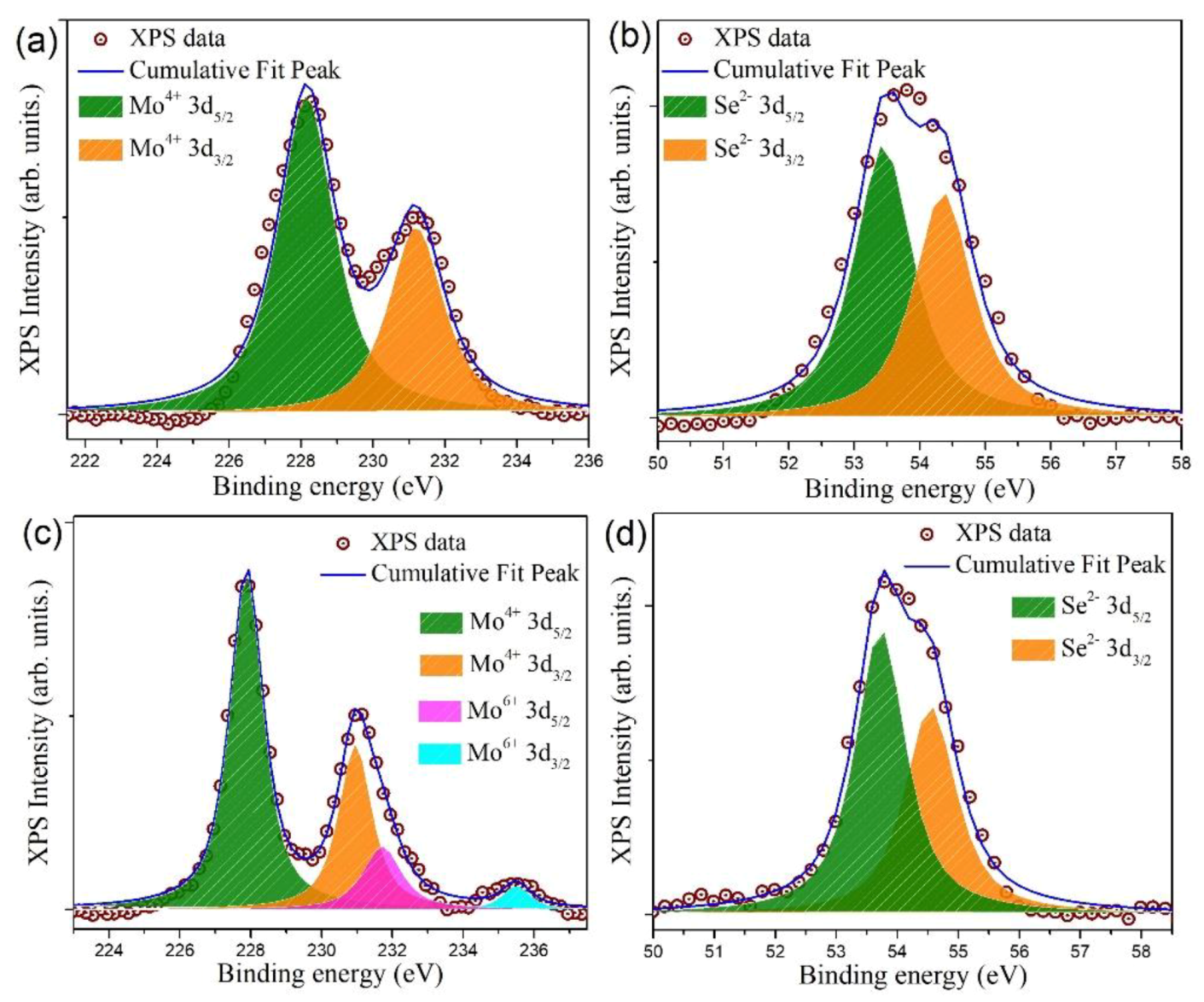
3.2. Surface Elemental and Valence State Analysis
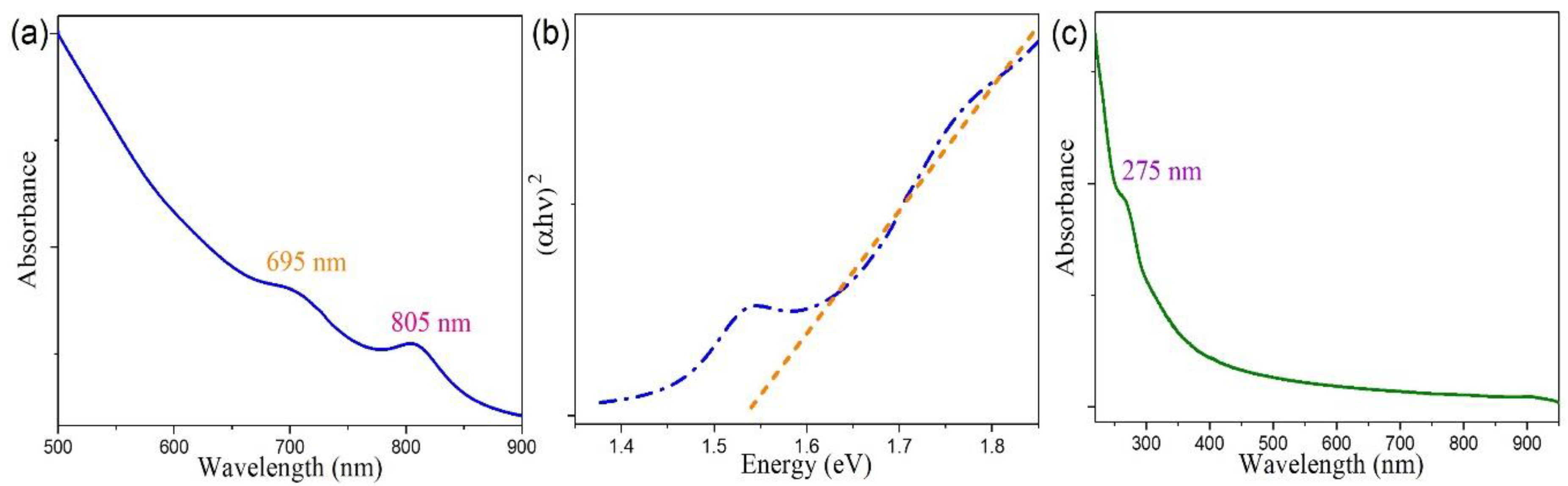
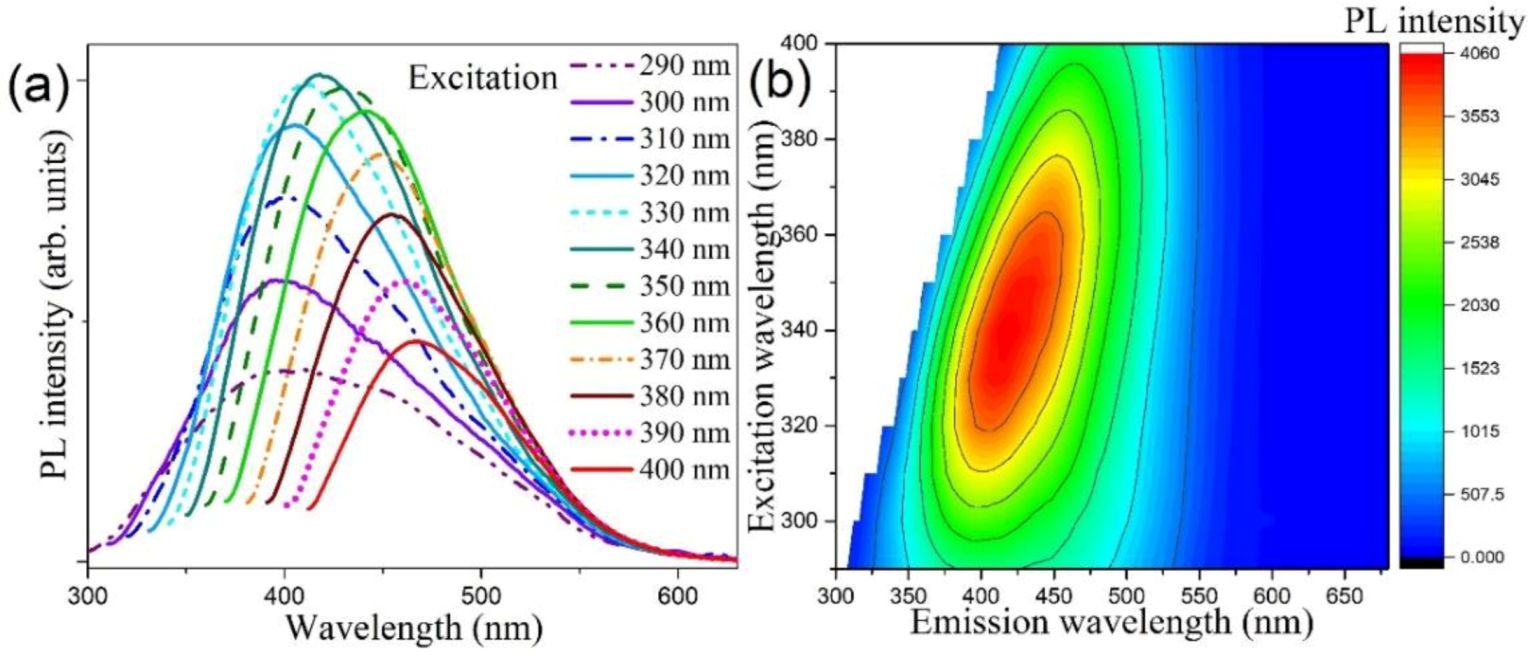
3.3. Optical Property Studies
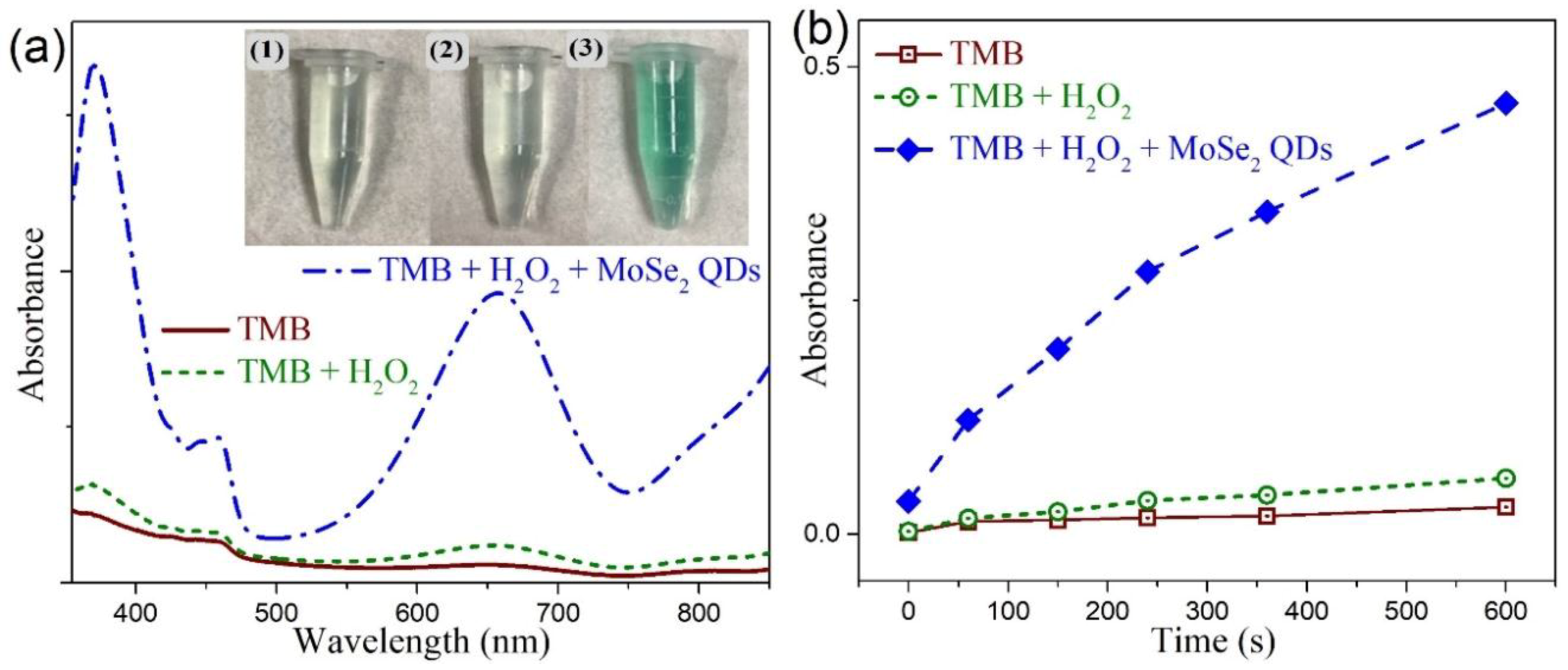
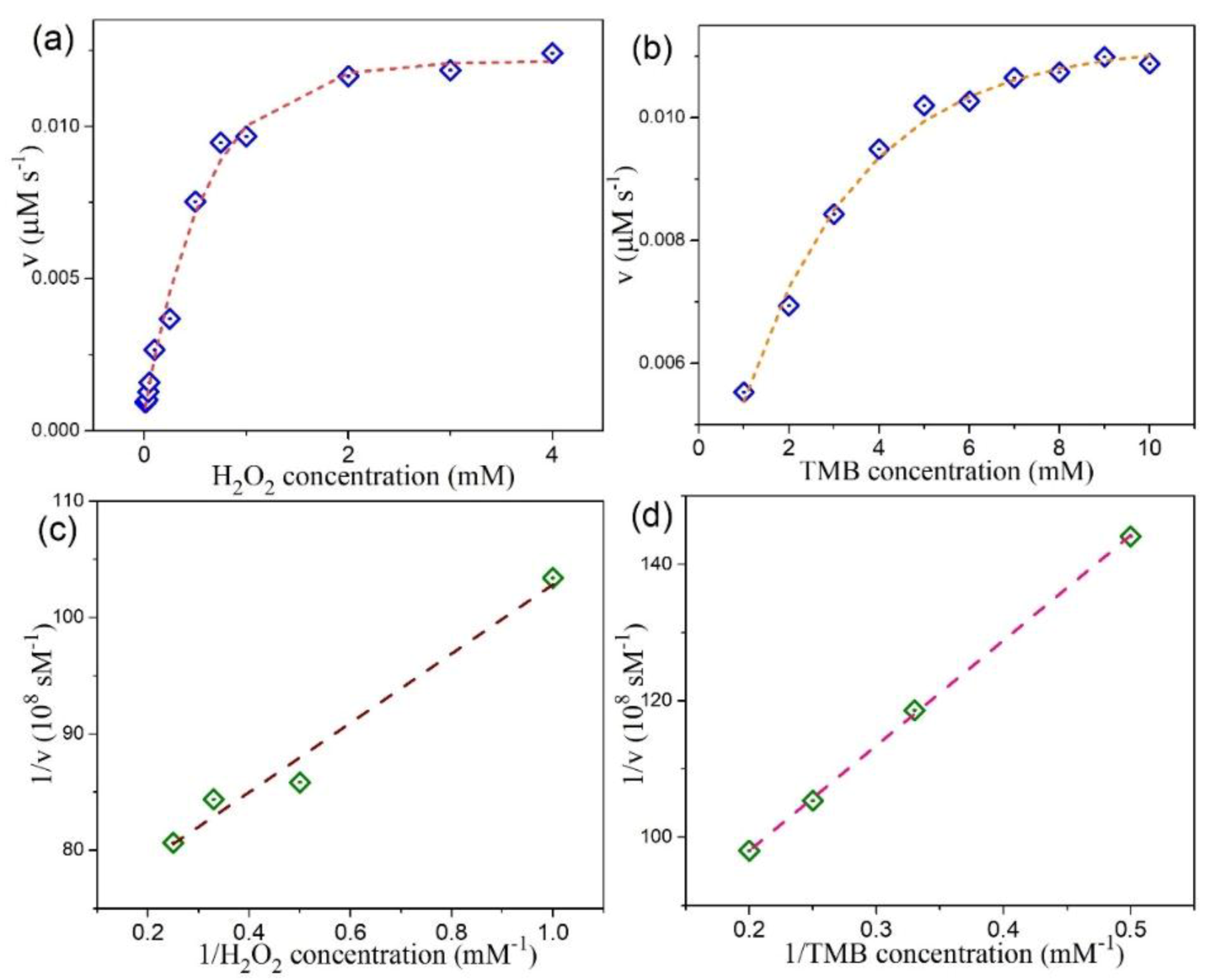
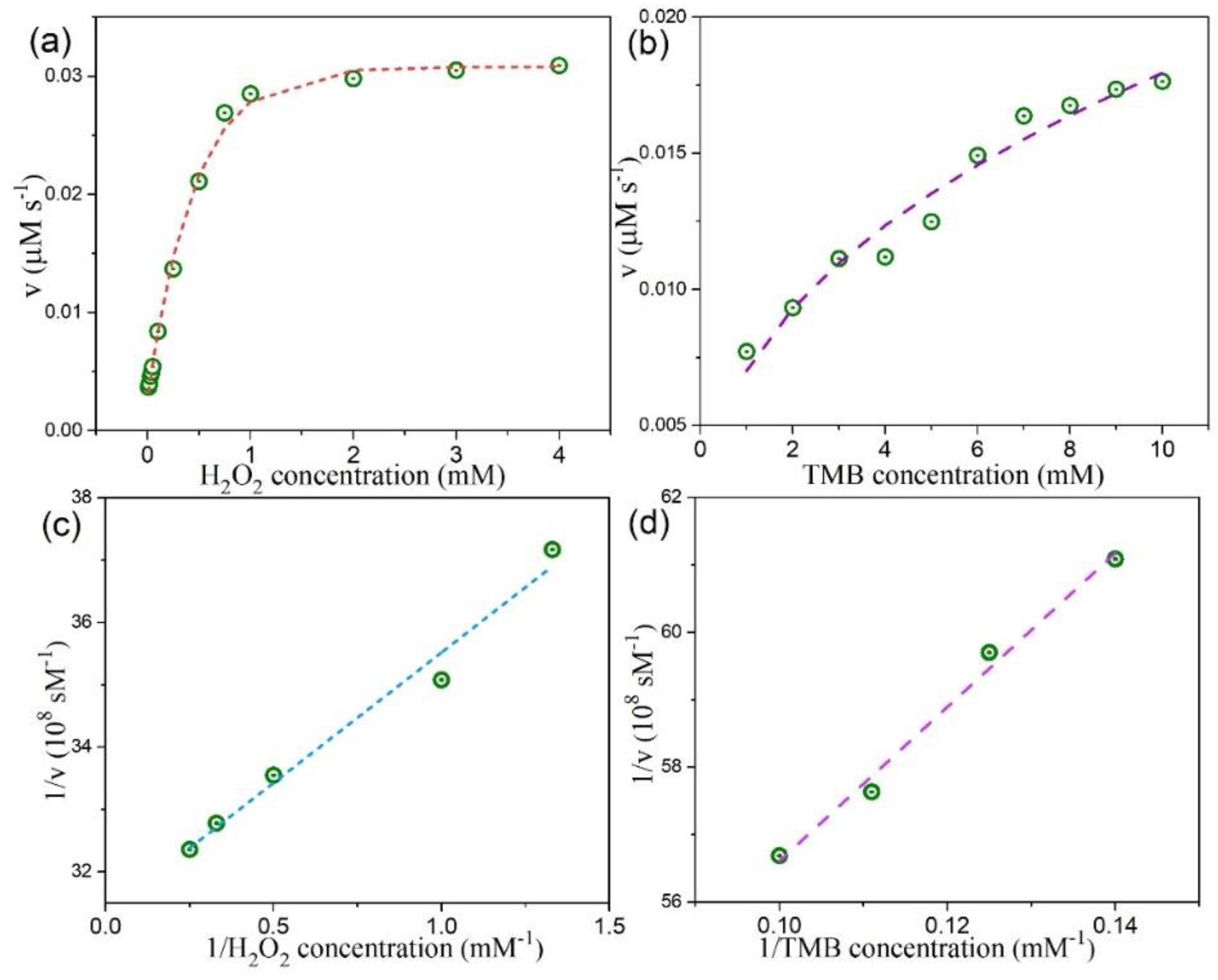
3.4. Peroxidase-Like Activities and Steady-State Kinetic Assay
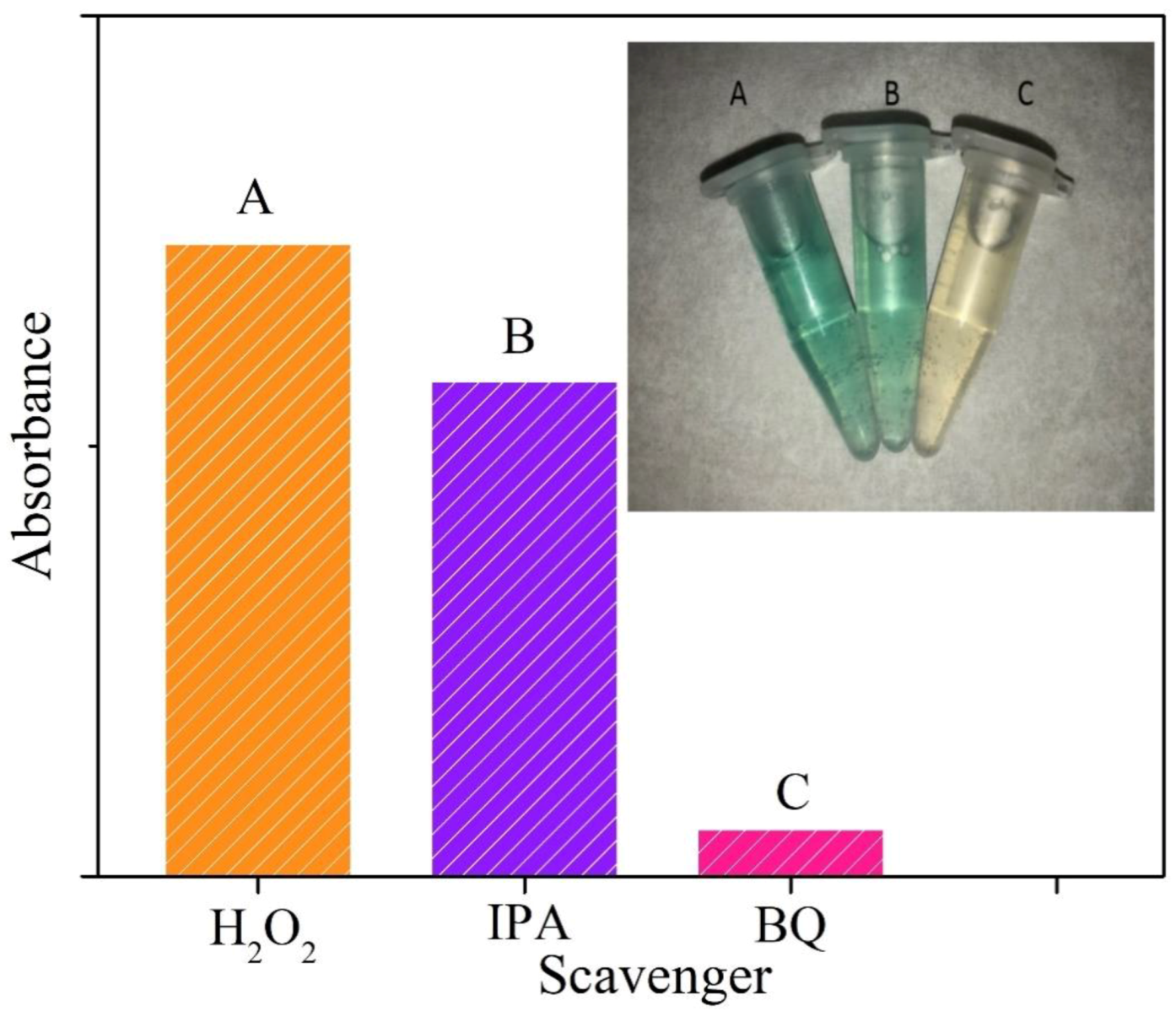
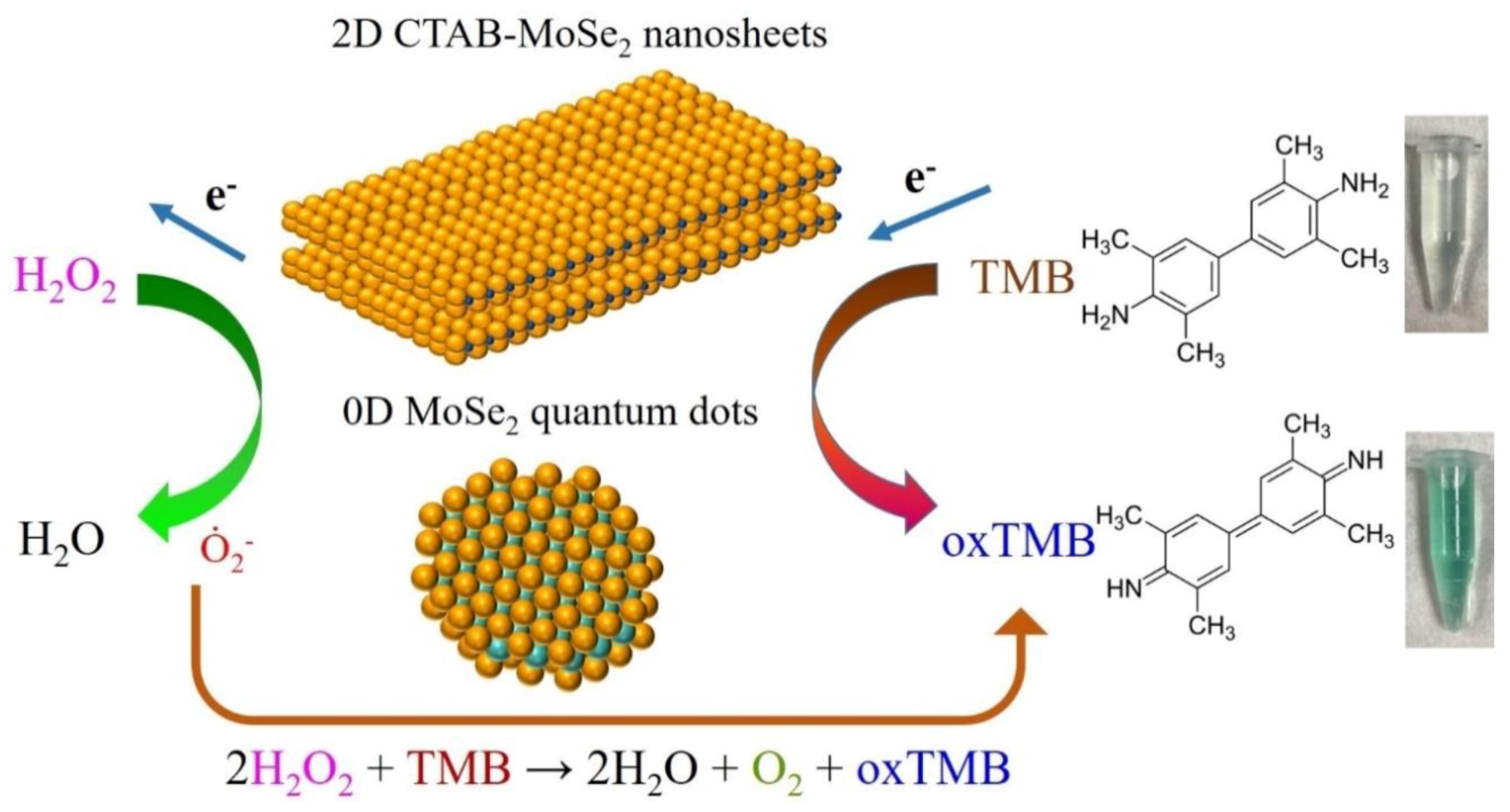
3.5. Actives Species Trapping Tests and Peroxidase-Like Catalytic Mechanism
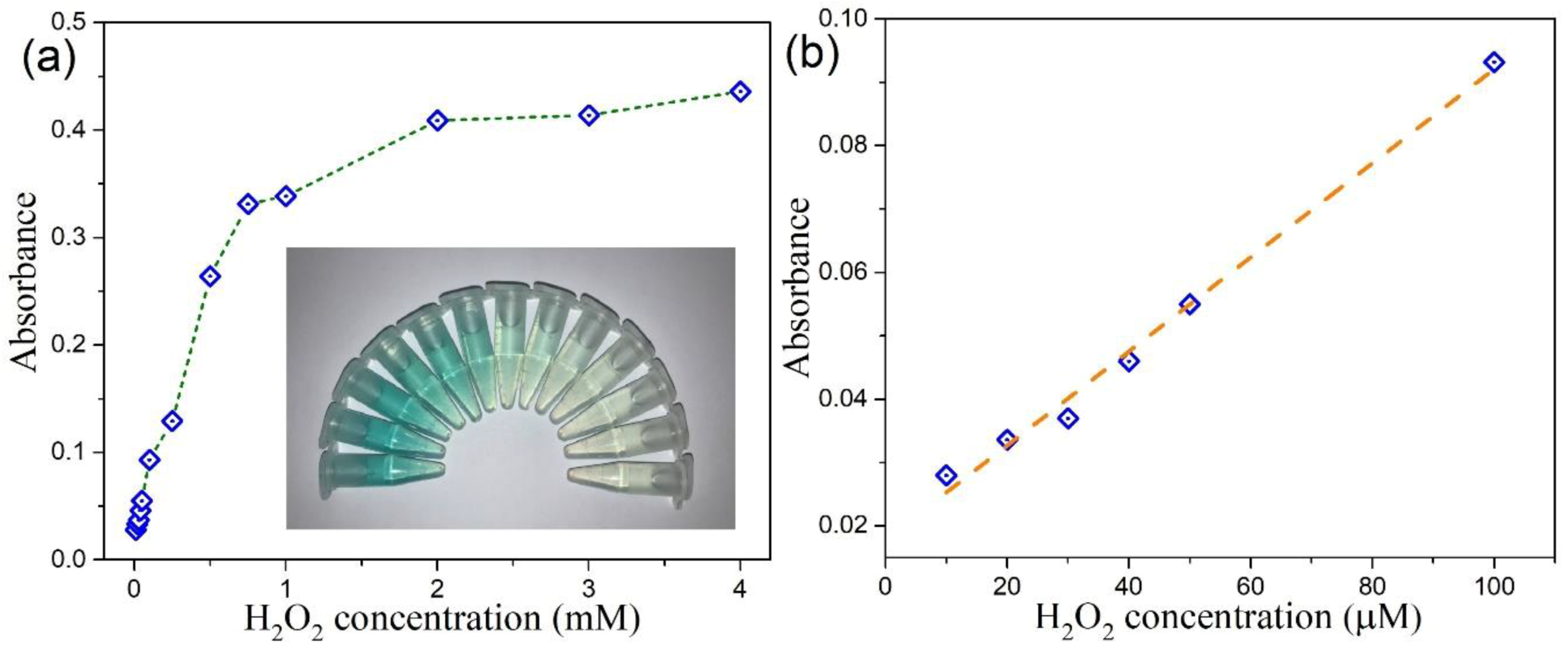
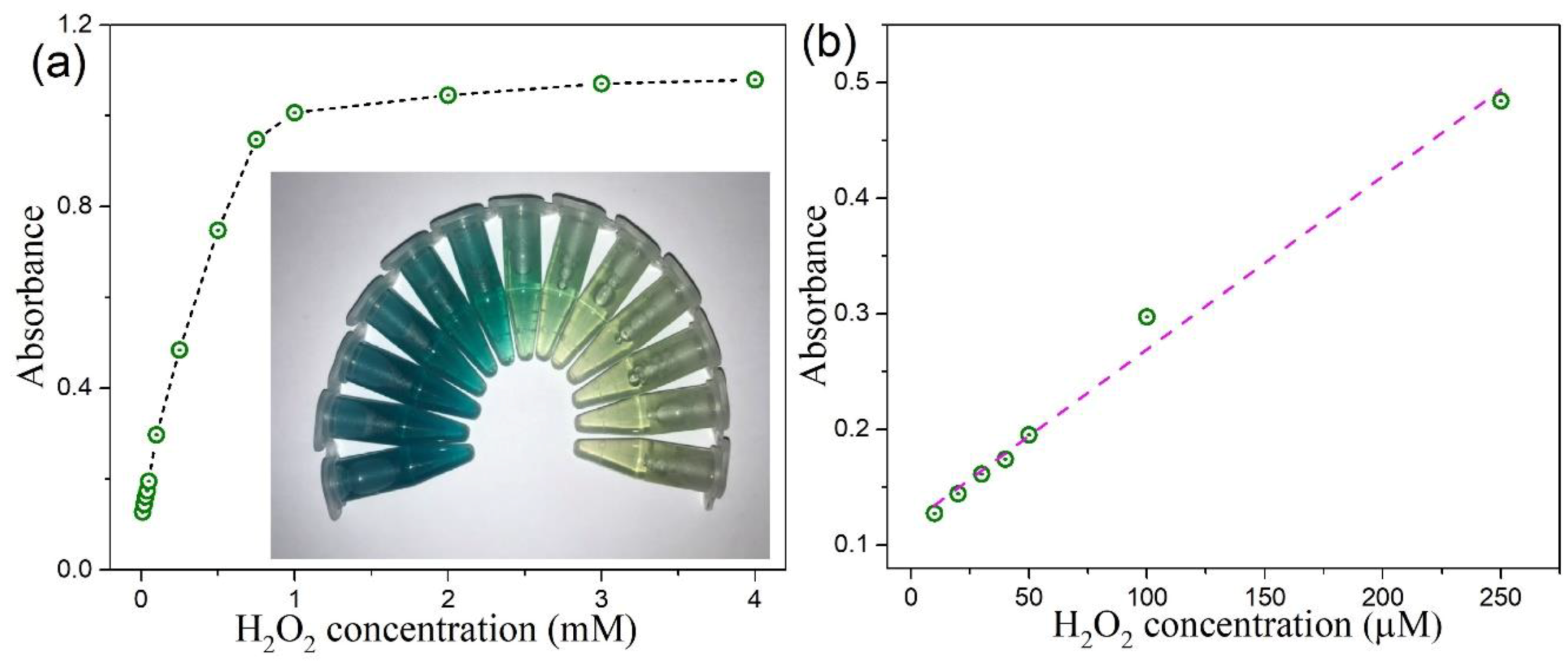
3.6. Colorimetric Detection of H2O2 by MoSe2-Based Assay System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhu, J.; Wang, S. In situ growth of copper oxide-graphite carbon nitride nanocomposites with peroxidase-mimicking activity for electrocatalytic and colorimetric detection of hydrogen peroxide. Carbon 2018, 129, 29–37. [Google Scholar] [CrossRef]
- Mao, L.; Osborne, P.G.; Yamamoto, K.; Kato, T. Continuous on-line measurement of cerebral hydrogen peroxide using enzyme-modified ring–disk plastic carbon film electrode. Anal Chem. 2002, 74, 3684–3689. [Google Scholar] [CrossRef] [PubMed]
- Belousov, V.V.; Fradkov, A.F.; Lukyanov, K.A.; Staroverov, D.B.; Shakhbazov, K.S.; Terskikh, A.V.; Lukyanov, S. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat. Methods 2006, 3, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Han, J.-M.; Zhang, Y.; Yang, X.; Zang, L. A selective fluorescence turn-on sensor for trace vapor detection of hydrogen peroxide. Chem. Commun. 2013, 49, 11779–11781. [Google Scholar] [CrossRef]
- Srikun, D.; Albers, A.E.; Nam, C.I.; Iavaron, A.T.; Chang, C.J.J. Organelle-targetable fluorescent probes for imaging hydrogen peroxide in living cells via SNAP-Tag protein labeling. J. Am. Chem. Soc. 2010, 132, 4455–4465. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Shang, F.J.; Luong, J.H.T.; Razeeb, K.M.; Glennon, J.D. Direct electrochemistry of horseradish peroxidase immobilized on a monolayer modified nanowire array electrode. Biosens. Bioelectron. 2010, 25, 1313–1318. [Google Scholar] [CrossRef] [Green Version]
- Ding, J.W.; Zhang, K.; Wei, G.; Su, Z.Q. Fabrication of polypyrrole nanoplates decorated with silver and gold nanoparticles for sensor applications. RSC Adv. 2015, 5, 69745–69752. [Google Scholar] [CrossRef]
- Wei, H.; Wang, E.K. Fe3O4 magnetic nanoparticles as peroxidase mimetics and their applications in H2O2 and glucose detection. Anal. Chem. 2008, 80, 2250–2254. [Google Scholar] [CrossRef]
- Bao, Y.-W.; Hua, X.-W.; Ran, H.-H.; Zeng, J.; Wu, F.-G. Metal-doped carbon nanoparticles with intrinsic peroxidase-like activity for colorimetric detection of H2O2 and glucose. J. Mater. Chem. B 2019, 7, 296–304. [Google Scholar] [CrossRef]
- Nerthigan, Y.; Sharma, A.K.; Pandey, S.; Sharma, K.H.; Khan, M.S.; Hang, D.-R.; Wu, H.-F. Glucose oxidase assisted visual detection of glucose using oxygen deficient α-MoO3-x nanoflakes. Microchim. Acta 2018, 185, 65. [Google Scholar] [CrossRef]
- Sharma, A.K.; Pandey, S.; Sharma, K.H.; Nerthigan, Y.; Khan, M.S.; Hang, D.-R.; Wu, H.-F. Two dimensional α-MoO3-x nanoflakes as bare eye probe for hydrogen peroxide in biological fluids. Anal. Chim. Acta 2018, 1015, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.Q.; Zhang, Y.D.; Jiang, Z.W.; Wang, D.M.; Huang, C.Z.; Li, Y.F. Fe3O4 and metal–organic framework MIL-101(Fe) composites catalyze luminol chemiluminescence for sensitively sensing hydrogen peroxide and glucose. Talanta 2018, 179, 43–50. [Google Scholar] [CrossRef]
- Jin, L.; Meng, Z.; Zhang, Y.; Cai, S.; Zhang, Z.; Li, C.; Shang, L.; Shen, Y. Ultrasmall Pt nanoclusters as robust peroxidase mimics for colorimetric detection of glucose in human serum. ACS Appl. Mater. Interfaces 2017, 9, 10027–10033. [Google Scholar] [CrossRef]
- Wang, S.; Sun, J.; Jia, Y.; Yang, L.; Wang, N.; Xianyu, Y.; Chen, W.; Li, X.; Cha, R.; Jiang, X. Nanocrystalline cellulose-assisted generation of silver nanoparticles for nonenzymatic glucose detection and antibacterial agent. Biomacromolecules 2016, 17, 2472–2478. [Google Scholar] [CrossRef]
- Jv, Y.; Li, B.X.; Cao, R. Positively-charged gold nanoparticles as peroxidiase mimic and their application in hydrogen peroxide and glucose detection. Chem. Commun. 2010, 46, 8017–8019. [Google Scholar] [CrossRef] [PubMed]
- Teo, W.Z.; Chng, E.L.K.; Sofer, Z.; Pumera, M. Cytotoxicity of exfoliated transition-metal dichalcogenides (MoS2, WS2, and WSe2) is lower than that of graphene and its analogues. Chem. Eur. J. 2014, 20, 9627–9632. [Google Scholar] [CrossRef]
- Sorkin, V.; Pan, H.; Shi, H.; Quek, S.Y.; Zhang, Y.W. Nanoscale transition metal dichalcogenides: Structures, properties, and applications. Crit. Rev. Solid State Mater. Sci. 2014, 39, 319–367. [Google Scholar] [CrossRef]
- Singh, A.K.; Hwang, C.; Eom, J. Low-Voltage and high-performance multilayer MoS2 field-effect transistors with graphene electrodes. ACS Appl. Mater. Interfaces 2016, 8, 34699–34705. [Google Scholar] [CrossRef]
- Joseph, N.; Muhammed Shafi, P.; Chandra Bose, A. Recent advances in 2D-MoS2 and its composite nanostructures for supercapacitor electrode application. Energy Fuels 2020, 34, 6558–6597. [Google Scholar] [CrossRef]
- Lin, X.; Wang, X.; Zhou, Q.; Wen, C.; Su, S.; Xiang, J.; Cheng, P.; Hu, X.; Li, Y.; Wang, X.; et al. Magnetically recyclable MoS2/Fe3O4 hybrid composite as visible light responsive photocatalyst with enhanced photocatalytic performance. ACS Sustain. Chem. Eng. 2018, 7, 1673–1682. [Google Scholar] [CrossRef]
- Kalantar-zadeh, K.; Ou, J.Z. Biosensors based on two-dimensional MoS2. ACS Sens. 2016, 1, 5–16. [Google Scholar] [CrossRef]
- Gan, X.; Zhao, H.; Quan, X. Two-dimensional MoS2: A promising building block for biosensors. Biosens. Bioelectron. 2017, 89, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Huang, Y.; Wu, J.; Zhan, X.; Xie, Y.; Tang, D.; Cao, H.; Yun, W. Mixed-solvent liquid exfoliated MoS2 NPs as peroxidase mimetics for colorimetric detection of H2O2 and glucose. RSC Adv. 2018, 8, 7252–7259. [Google Scholar] [CrossRef] [Green Version]
- Zhao, K.; Gu, W.; Zheng, S.S.; Zhang, C.L.; Xian, Y.Z. SDS-MoS2 nanoparticles as highly-efficient peroxidase mimetics for colorimetric detection of H2O2 and glucose. Talanta 2015, 141, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Kim, Y.; Chung, H.S.; Kim, A.R.; Kwon, J.D.; Park, J.; Kim, Y.L.; Kwon, S.H.; Hahm, M.G.; Cho, B. Effect of Nb doping on chemical sensing performance of two-dimensional layered MoSe2. ACS Appl. Mater. Interfaces 2017, 9, 3817–3823. [Google Scholar] [CrossRef]
- Gholamvand, G.; McAteer, D.; Backes, C.; McEvoy, N.; Harvey, A.; Berner, N.C.; Hanlon, D.; Bradley, C.; Godwin, I.; Rovetta, A.; et al. Comparison of liquid exfoliated transition metal dichalcogenides reveals MoSe2 to be the most effective hydrogen evolution catalyst. Nanoscale 2016, 8, 5737–5749. [Google Scholar] [CrossRef]
- Wu, X.; Chen, T.; Wang, J.; Yang, G. Few-layered MoSe2 nanosheets as an efficient peroxidase nanozyme for highly sensitive colorimetric detection of H2O2 and xanthine. J. Mater. Chem. B 2018, 6, 105–111. [Google Scholar] [CrossRef]
- Pan, J.; Zhu, X.; Chen, X.; Zhao, Y.; Liu, J. Gd3+-doped MoSe2 nanosheets used as a theranostic agent for bimodal imaging and highly efficient photothermal cancer therapy. Biomater. Sci. 2018, 6, 372–387. [Google Scholar] [CrossRef]
- Li, B.L.; Setyawati, M.I.; Zou, H.L.; Dong, J.X.; Luo, H.Q.; Li, N.B.; Leong, D.T. Emerging 0D transition-metal dichalcogenides for sensors, biomedicine, and clean energy. Small 2017, 13, 1700527. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, X.; Zhang, W.L.; Lv, F.; Guo, S. Recent progress in two-dimensional inorganic quantum dots. Chem. Soc. Rev. 2018, 47, 586–625. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Q.; Jiang, K.; Wang, C.; Zhang, C. One-step synthesis of water-soluble and highly fluorescent MoS2 quantum dots for detection of hydrogen peroxide and glucose. Sens. Actuators B 2017, 252, 183–190. [Google Scholar] [CrossRef]
- Hang, D.-R.; Sun, D.-Y.; Chen, C.-H.; Wu, H.-F.; Chou, M.M.C.; Islam, S.E.; Sharma, K.H. Facile bottom-up preparation of WS2-based water-soluble quantum dots as luminescent probes for hydrogen peroxide and glucose. Nanoscale Res. Lett. 2019, 14, 271. [Google Scholar] [CrossRef] [PubMed]
- Gerislioglu, B.; Dong, L.; Ahmadivand, A.; Hu, H.; Nordlander, P.; Halas, N.J. Monolithic metal dimer-on-film structure: New plasmonic properties introduced by the underlying metal. Nano Lett. 2020, 20, 2087–2093. [Google Scholar] [CrossRef]
- Ahmadivand, A.; Gerislioglu, B. Tunable plexciton dynamics in electrically biased nanojunctions. J. Appl. Phys. 2020, 128, 063101. [Google Scholar] [CrossRef]
- Gerislioglu, B.; Ahmadivand, A. Theoretical study of photoluminescence spectroscopy of strong exciton-polariton coupling in dielectric nanodisks with anapole states. Mater. Today Chem. 2020, 16, 100254. [Google Scholar] [CrossRef]
- Backes, C.; Higgins, T.M.; Kelly, A.; Boland, C.; Harvey, A.; Hanlon, D.; Coleman, J.N. Guidelines for exfoliation, characterization and processing of layered materials produced by liquid exfoliation. Chem. Mater. 2017, 29, 243–255. [Google Scholar] [CrossRef]
- Hang, D.-R.; Sharma, K.H.; Chen, C.-H.; Islam, S.E. Enhanced photocatalytic performance of ZnO nanorods coupled by two-dimensional α-MoO3 nanoflakes under UV and visible light irradiation. Chem. Eur. J. 2016, 22, 12777–12784. [Google Scholar] [CrossRef]
- Chhetri, S.; Adak, N.C.; Samanta, P.; Murmu, N.C.; Kuila, T. Exploration of mechanical and thermal properties of CTAB-modified MoS2/LLDPE composites prepared by melt mixing. J. Compos. Sci. 2018, 2, 37. [Google Scholar] [CrossRef] [Green Version]
- Hang, D.-R.; Sharma, K.H.; Islam, S.E.; Chen, C.; Chou, M.M.C. Resonant Raman scattering and photoluminescent properties of nonpolar a-plane ZnO thin film on LiGaO2 substrate. Appl. Phys. Express 2014, 7, 041101. [Google Scholar] [CrossRef]
- Zhu, C.; Huang, Y.; Xu, F.; Gao, P.; Ge, B.; Chen, J.; Zeng, H.; Sutter, E.; Sutter, P.; Sun, L. Defect-laden MoSe2 quantum dots made by turbulent shear mixing as enhanced electrocatalysts. Small 2017, 13, 1700565. [Google Scholar] [CrossRef]
- Islam, S.E.; Hang, D.-R.; Chen, C.-H.; Sharma, K.H. Facile and cost-efficient synthesis of quasi-0D/2D ZnO/MoS2 nanocomposites for highly enhanced visible-light-driven photocatalytic degradation of organic pollutants and antibiotics. Chem. Eur. J. 2018, 24, 9305–9315. [Google Scholar] [CrossRef]
- Qu, B.; Yu, X.; Chen, Y.; Zhu, C.; Li, C.; Yin, Z.; Zhang, X. Ultrathin MoSe2 nanosheets decorated on carbon fiber cloth as binder-free and high-performance electrocatalyst for hydrogen evolution. ACS Appl. Mater. Interfaces 2015, 7, 14170–14715. [Google Scholar] [CrossRef]
- Koroteev, V.; Bulusheva, L.; Asanov, I.; Shlyakhova, E.; Vyalikh, D.; Okotrub, A. Charge transfer in the MoS2/carbon nanotube composite. J. Phys. Chem. C 2011, 115, 21199–21204. [Google Scholar] [CrossRef]
- Guo, X.; Cao, G.; Ding, F.; Li, X.; Zhen, S.; Xue, Y.; Yan, Y.; Liu, T.; Sun, K. A bulky and flexible electrocatalyst for efficient hydrogen evolution based on the growth of MoS2 nanoparticles on carbon nanofiber foam. J. Mater. Chem. A 2015, 3, 5041–5046. [Google Scholar] [CrossRef]
- Smith, R.J.; King, P.J.; Lotya, M.; Wirtz, C.; Khan, U.; De, S.; O’Neill, A.; Duesberg, G.S.; Grunlan, J.C.; Moriarty, G. Large-scale exfoliation of inorganic layered compounds in aqueous surfactant solutions. Adv. Mater. 2011, 23, 3944–3948. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Feng, Y.; Chang, C.; Zhan, J.; Wang, C.; Zhao, Q.; Colemon, J.N.; Zhang, L.; Blau, W.J.; Wang, J. Broadband ultrafast nonlinear absorption and nonlinear refraction of layered molybdenum dichalcogenide semiconductors. Nanoscale 2014, 6, 10530–10535. [Google Scholar] [CrossRef] [Green Version]
- Dong, N.; Li, Y.; Feng, Y.; Zhang, S.; Zhang, X.; Chang, C.; Fan, J.; Zhang, L.; Wang, J. Optical limiting and theoretical modelling of layered transition metal dichalcogenide nanosheets. Sci. Rep. 2015, 5, 14646. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Gerber, I.C.; Bouet, L.; Lagarde, D.; Balocchi, A.; Vidal, M.; Amand, T.; Marie, X.; Urbaszek, B. 2015 Exciton states in monolayer MoSe2: Impact on interband transitions. 2D Mater. 2015, 2, 045005. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.; Neupane, G.P.; Dhakal, K.P.; Lee, J.; Yun, S.J.; Han, G.H.; Kim, J. Observation of charge transfer in heterostructures composed of MoSe2 quantum dots and a monolayer of MoS2 or WSe2. J. Phys. Chem. C 2017, 121, 1997–2004. [Google Scholar] [CrossRef]
- Xu, S.; Li, D.; Wu, P. One-pot, facile, and versatile synthesis of monolayer MoS2/WS2 quantum dots as bioimaging probes and efficient electrocatalysts for hydrogen evolution reaction. Adv. Funct. Mater. 2015, 25, 1127–1136. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, C.; Gu, W.; Ding, C.; Li, X.; Xian, Y. Facile synthesis of water-soluble WS2 quantum dots for turn-on fluorescent measurement of lipoic acid. J. Phys. Chem. C 2016, 120, 12170–12177. [Google Scholar] [CrossRef]
- Hang, D.-R.; Islam, S.E.; Sharma, K.H.; Chen, C.; Liang, C.-T.; Chou, M.M.C. Optical characteristics of nonpolar a-plane ZnO thin film on (010) LiGaO2 substrate. Semicond. Sci. Technol. 2014, 29, 085004. [Google Scholar] [CrossRef]
- Hang, D.-R.; Islam, S.E.; Chen, C.-H.; Sharma, K.H. Full solution-processed synthesis and mechanisms of a recyclable and bifunctional Au/ZnO plasmonic platform for enhanced UV/Vis photocatalysis and optical properties. Chem. Eur. J. 2016, 22, 14950–14961. [Google Scholar] [CrossRef]
- Gan, Z.X.; Liu, L.Z.; Wu, H.Y.; Hao, Y.L.; Shan, Y.; Wu, X.L.; Chu, P.K. Quantum confinement effects across two-dimensional planes in MoS2 quantum dots. Appl. Phys. Lett. 2015, 106, 233113. [Google Scholar] [CrossRef]
- Roy, A.; Movva, H.C.P.; Satpati, B.; Kim, K.; Dey, R.; Rai, A.; Pramanik, T.; Guchhait, S.; Tutuc, E.; Banerjee, S.K. Structural and electrical properties of MoTe2 and MoSe2 grown by molecular beam epitaxy. ACS Appl. Mater. Interfaces 2016, 8, 7396–7402. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Yan, H.; Brus, L.E.; Heinz, T.F.; Hone, J.; Ryu, S. Anomalous lattice vibrations of single- and few-layer MoS2. ACS Nano 2010, 4, 2695–2700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonndorf, P.; Schmidt, R.; Böttger, P.; Zhang, X.; Börner, J.; Liebig, A.; Albrecht, M.; Kloc, C.; Gordan, O.; Zahn, D.R.T.; et al. Photoluminescence emission and Raman response of monolayer MoS2, MoSe2, and WSe2. Opt. Express 2013, 21, 4908. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Zheng, C.; Fuhrer, M.S.; Yan, J. Helicity-resolved Raman scattering of MoS2, MoSe2, WS2, and WSe2 atomic layers. Nano Lett. 2015, 15, 2526–2532. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Zhao, Y.; Zhang, J.; Xiong, Q.; Quek, S.Y. Anomalous frequency trends in MoS2 thin films attributed to surface effects. Phys. Rev. B 2013, 88, 075320. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Lee, J.U.; Nam, D.Y.; Cheong, H. Davydov splitting and excitonic resonance effects in Raman spectra of few-layer MoSe2. ACS Nano 2016, 10, 8113–8120. [Google Scholar] [CrossRef] [Green Version]
- Luan, C.-Y.; Xie, S.; Ma, C.; Wang, S.; Kong, Y.; Xu, M. Elucidation of luminescent mechanisms of size-controllable MoSe2 quantum dots. Appl. Phys. Lett. 2017, 111, 073105. [Google Scholar] [CrossRef]
- Qiao, F.M.; Qi, Q.Q.; Wang, Z.Z.; Xu, K.; Ai, S.Y. MnSe-loaded g-C3N4 nanocomposite with synergistic peroxidase-like catalysis: Synthesis and application toward colorimetric biosensing of H2O2 and glucose. Sensor. Actuator. B Chem. 2016, 229, 379–386. [Google Scholar] [CrossRef]
- Chen, L.J.; Sun, B.; Wang, X.; Qiao, F.M.; Ai, S.Y. 2D ultrathin nanosheets of Co-Al layered double hydroxides prepared in L-asparagine solution enhanced peroxidase-like activity and colorimetric detection of glucose. J. Mater. Chem. B 2013, 1, 2268–2274. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Qiao, F.M.; Chen, L.J.; Li, X.; Li, L.; Ai, S.Y. Peroxidase-like activity of manganese selenide nanoparticles and its analytical application for visual detection of hydrogen peroxide and glucose. Sensors Actuator B Chem. 2014, 193, 255–262. [Google Scholar] [CrossRef]
- Peng, J.; Weng, J. Enhanced peroxidase-like activity of MoS2/graphene oxide hybrid with light irradiation for glucose detection. Biosens. Bioelectron. 2017, 89, 652–658. [Google Scholar] [CrossRef]
- Vinita, N.R.N.; Prakash, R. One step synthesis of AuNPs@MoS2-QDs composite as a robust peroxidase-mimetic for instant unaided eye detection of glucose in serum, saliva and tear. Sensors Actuator B Chem. 2018, 263, 109–119. [Google Scholar] [CrossRef]
- Ju, P.; Xiang, Y.H.; Xiang, Z.B.; Wang, M.; Zhao, Y.; Zhang, D.; Yu, J.Q.; Han, X.X. BiOI hierarchical nanoflowers as novel robust peroxidase mimetics for colorimetric detection of H2O2. RSC Adv. 2016, 6, 17483–17493. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, Y.F. 2D/2D h-BN/N-doped MoS2 heterostructure catalyst with enhanced peroxidase-like performance for visual colorimetric determination of H2O2. Chem. Asian J. 2020, 15, 1315–1323. [Google Scholar] [CrossRef]
- Lin, T.R.; Zhong, L.S.; Wang, J.; Guo, L.Q.; Wu, H.Y.; Guo, Q.Q.; Fu, F.F.; Chen, G.N. Graphite-like carbon nitrides as peroxidase mimetics and their applications to glucose detection. Biosens. Bioelectron. 2014, 59, 89–93. [Google Scholar] [CrossRef]
- Ju, P.; He, Y.; Wang, M.; Han, X.; Jiang, F.; Sun, C.; Wu, C. Enhanced Peroxidase-like activity of MoS2 quantum dots functionalized g-C3N4 nanosheets towards colorimetric detection of H2O2. Nanomaterials 2018, 8, 976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Chen, J.; Gao, C.J.; Zhang, M.L.; Chen, J.Y.; Qiu, H.D. Hemin-functionalized WS2 nanosheets as highly active peroxidase mimetics for label-free colorimetric detection of H2O2 and glucose. Analyst 2015, 140, 2857–2863. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yu, H.; Jiang, X.; Luo, Z.; Zheng, Y. High sensitivity label-free detection of Fe3+ ion in aqueous solution using fluorescent MoS2 quantum dots. Sens. Actuators B 2019, 281, 989–997. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Lv, K.; Zhong, W.; Wang, H.; Wu, Z.; Yi, P.; Jiang, J. Cyclam-functionalized carbon dots sensor for sensitive and selective detection of copper(II) ion and sulfide anion in aqueous media and its imaging in live cells. Sens. Actuators B 2016, 224, 298–306. [Google Scholar] [CrossRef]
- Chhetri, R.K.; Kaarsholm, K.M.S.; Andersen, H.R. Colorimetric quantification methods for peracetic acid together with hydrogen peroxide for water disinfection process control. Int. J. Environ. Res. Public Health 2020, 17, 4656. [Google Scholar] [CrossRef] [PubMed]
| Catalyst | Linear Range (μM) | Detection Limit (μM) | Ref. |
|---|---|---|---|
| Positively-charged Au nanoparticles (NPs) | 2–200 | 0.5 | [15] |
| h-BN/N-MoS2 | 1–1000 | 0.4 | [69] |
| Few-layered MoSe2 nanosheets (NSs) | 10–160 | 0.4 | [27] |
| MoS2 NPs | 3–120 | 1.25 | [23] |
| SDS–MoS2 NPs | 2–100 | 0.32 | [24] |
| g-C3N4 | 5–100 | 1 | [70] |
| MoS2 QDs/g-C3N4 NSs | 2–50 | 0.155 | [71] |
| WS2 Nanosheets | 5–200 | 1.5 | [72] |
| 2D CTAB-MoSe2 | 10–250 | 4 | This work |
| 0D MoSe2 QDs quantum dots (QDs) | 10–100 | 4 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hang, D.-R.; Pan, Y.-Q.; Sharma, K.H.; Chou, M.M.C.; Islam, S.E.; Wu, H.-F.; Liang, C.-T. 2D CTAB-MoSe2 Nanosheets and 0D MoSe2 Quantum Dots: Facile Top-Down Preparations and Their Peroxidase-Like Catalytic Activity for Colorimetric Detection of Hydrogen Peroxide. Nanomaterials 2020, 10, 2045. https://doi.org/10.3390/nano10102045
Hang D-R, Pan Y-Q, Sharma KH, Chou MMC, Islam SE, Wu H-F, Liang C-T. 2D CTAB-MoSe2 Nanosheets and 0D MoSe2 Quantum Dots: Facile Top-Down Preparations and Their Peroxidase-Like Catalytic Activity for Colorimetric Detection of Hydrogen Peroxide. Nanomaterials. 2020; 10(10):2045. https://doi.org/10.3390/nano10102045
Chicago/Turabian StyleHang, Da-Ren, Ya-Qi Pan, Krishna Hari Sharma, Mitch M. C. Chou, Sk Emdadul Islam, Hui-Fen Wu, and Chi-Te Liang. 2020. "2D CTAB-MoSe2 Nanosheets and 0D MoSe2 Quantum Dots: Facile Top-Down Preparations and Their Peroxidase-Like Catalytic Activity for Colorimetric Detection of Hydrogen Peroxide" Nanomaterials 10, no. 10: 2045. https://doi.org/10.3390/nano10102045
APA StyleHang, D.-R., Pan, Y.-Q., Sharma, K. H., Chou, M. M. C., Islam, S. E., Wu, H.-F., & Liang, C.-T. (2020). 2D CTAB-MoSe2 Nanosheets and 0D MoSe2 Quantum Dots: Facile Top-Down Preparations and Their Peroxidase-Like Catalytic Activity for Colorimetric Detection of Hydrogen Peroxide. Nanomaterials, 10(10), 2045. https://doi.org/10.3390/nano10102045