Enhancing Förster Resonance Energy Transfer (FRET) Efficiency of Titania–Lanthanide Hybrid Upconversion Nanomaterials by Shortening the Donor–Acceptor Distance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Characterizations
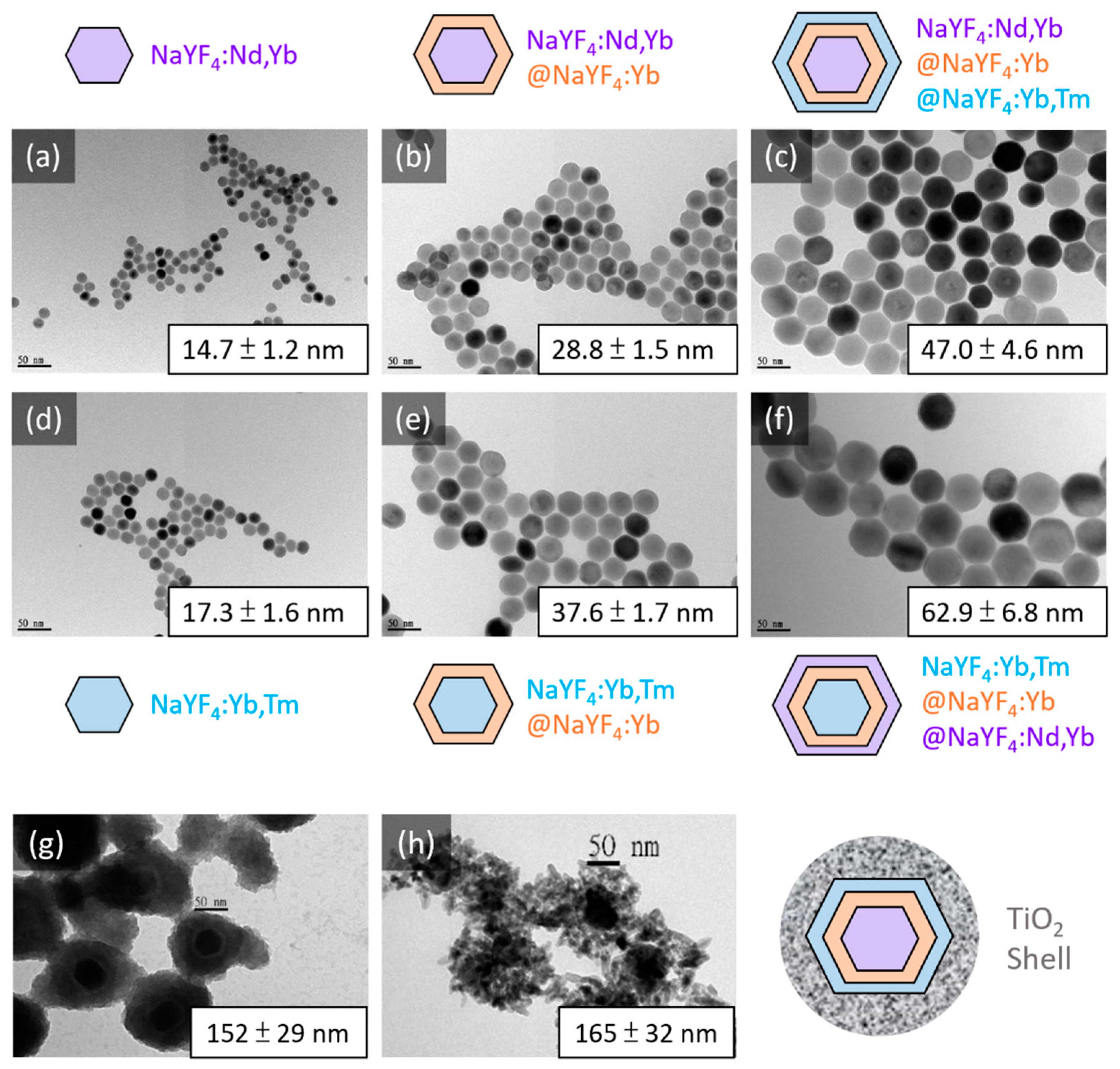
2.3. Synthesis of the Core NaYF4:Nd(15%),Yb(15%) and NaYF4:Yb(10%),Er(0.5%) NPs
2.4. Synthesis of the Core/Shell and Core/Shell/Shell NaYF4:Nd(15%),Yb(15%)@NaYF4:Yb(20%)@ NaYF4:Yb(20%), Tm (0.5%) and NaYF4:Yb(10%),Tm(0.5%)@NaYF4: Yb(20%)@NaYF4:Nd(20%),Yb(10%)
2.5. Coating TiO2 on the Core/Shell/Shell UCNPs
2.6. Preparation of UCNP@TiO2-PAH-PEG-FA
2.7. Reactive Oxygen Species Determination under 793 nm NIR Laser Irradiation
2.8. Cytotoxicity Assays
2.9. In Vitro NIR Induced PDT Effect
2.10. In Vitro Cellular Imaging
3. Results and Discussion
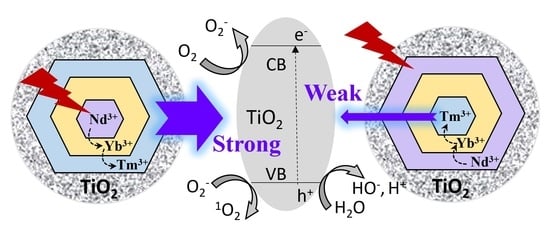
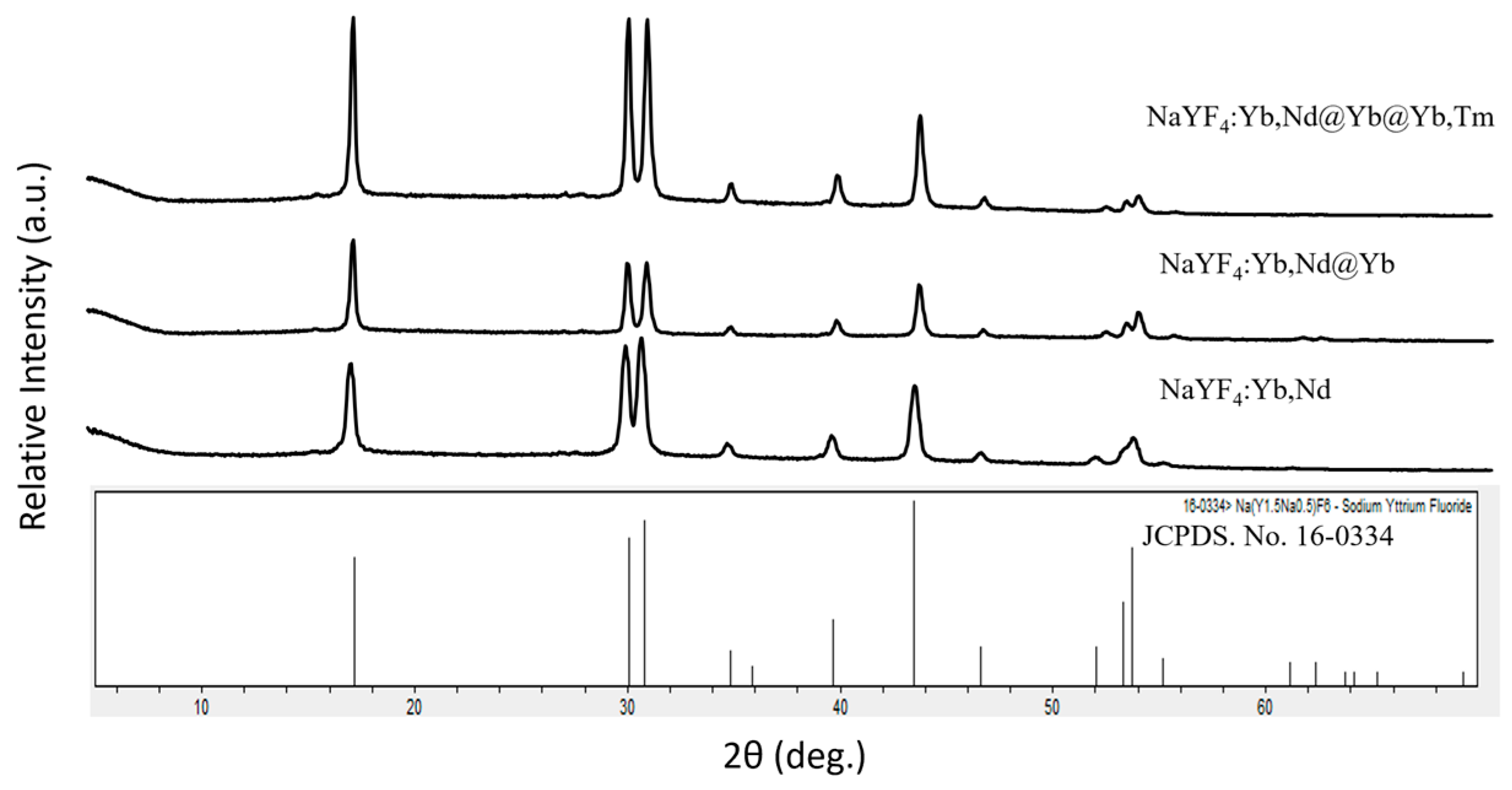
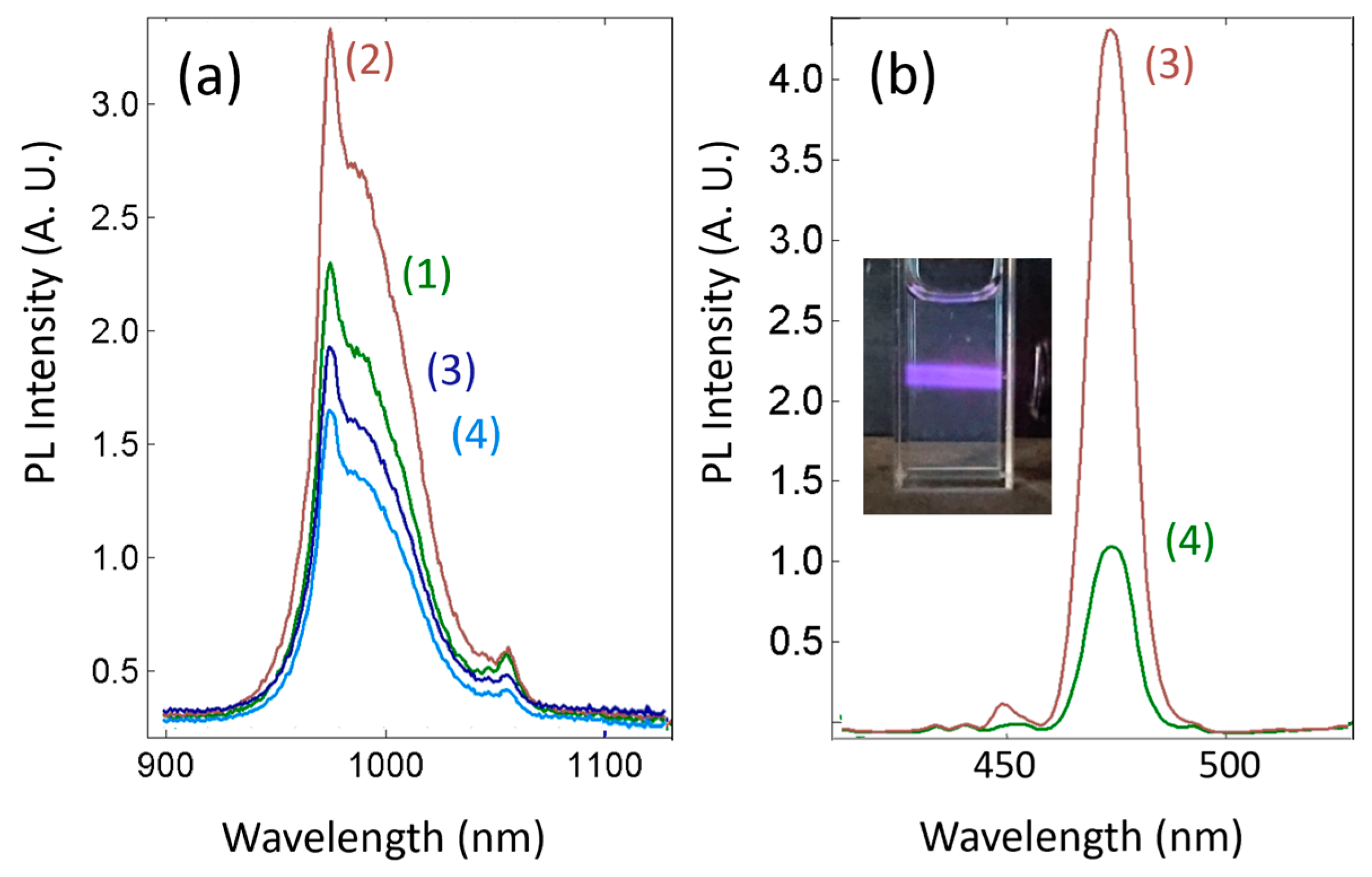
3.1. Design, Syntheses, Morphology and Luminescence Characterizations of the New Core/Shell/Shell UCNPs NaYF4:Nd3+(15%),Yb3+(15%)@NaYF4:Yb3+(20%)@NaYF4:Yb3+(20%),Tm3+(0.5%), i.e., Nd-Yb-Tm-UCNPs
3.2. TiO2 and PAH/PEG-FA Modifications and Zeta Potential Changes
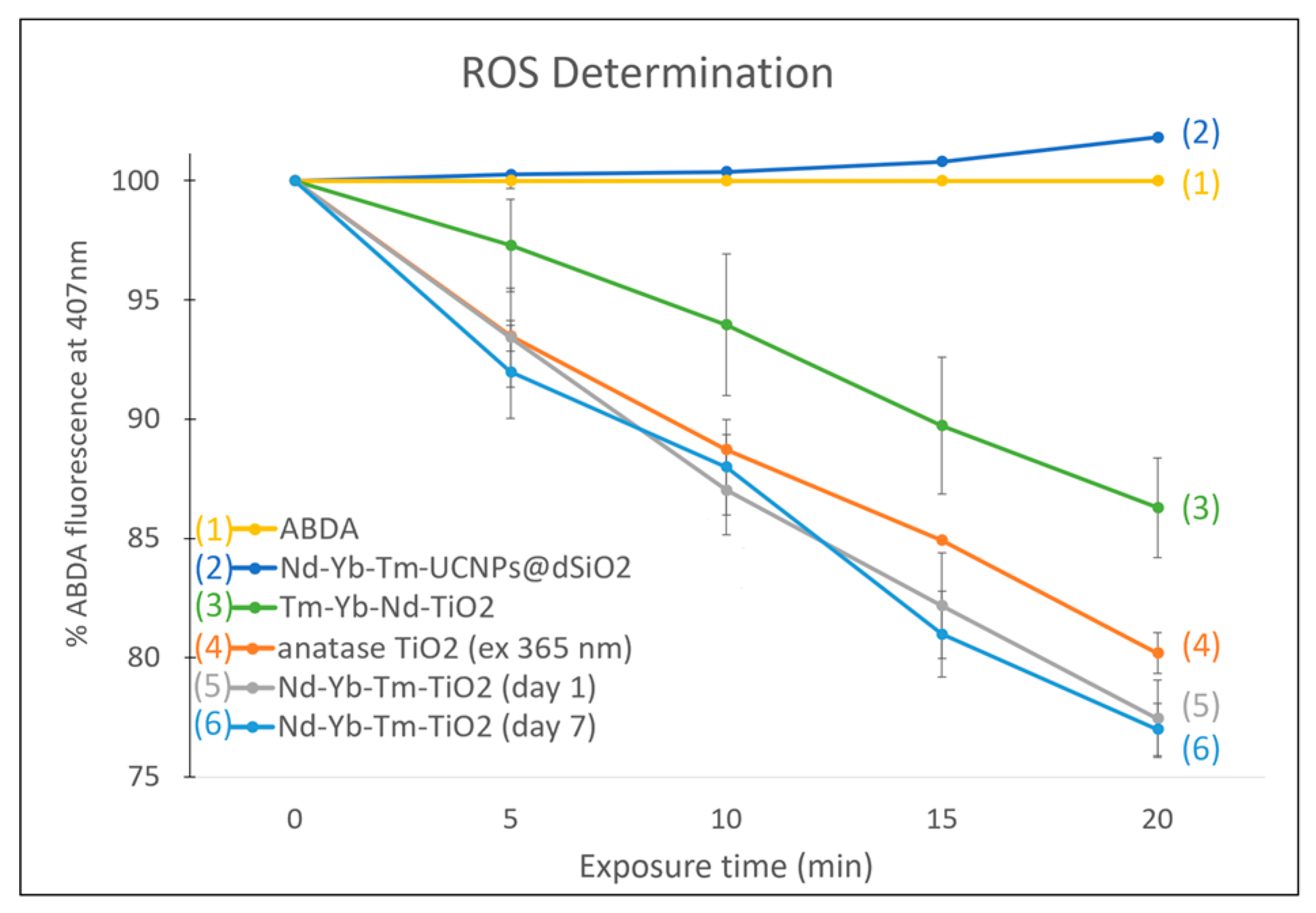
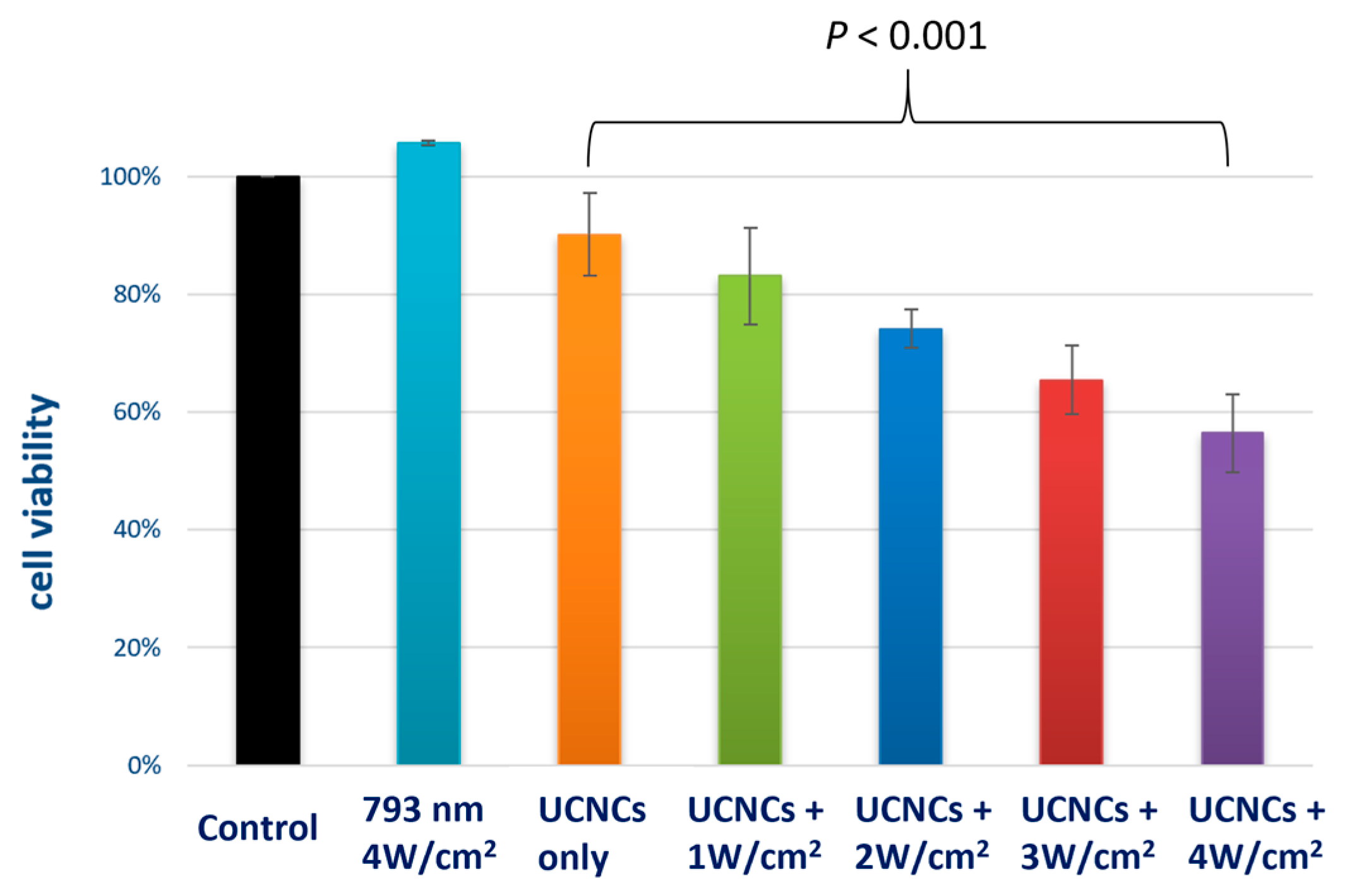
3.3. ROS Production, Stability, Imaging and PDT Cytotoxicity Studies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Couzin Frankel, J. Cancer Immunotherapy. Science 2013, 342, 1432–1433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhang, S.; Zhang, G.; Sun, X.; Lee, S.-T.; Liu, Z. Graphene in Mice: Ultrahigh in Vivo Tumor Uptake and Efficient Photothermal Therapy. Nano Lett. 2010, 10, 3318–3323. [Google Scholar] [CrossRef] [PubMed]
- Knorr, D.A.; Ni, Z.; Hermanson, D.; Hexum, M.K.; Bendzick, L.; Cooper, L.J.N.; Lee, D.A.; Kaufman, D.S. Clinical-Scale Derivation of Natural Killer Cells from Human Pluripotent Stem Cells for Cancer Therapy. Stem Cells Transl. Med. 2013, 2, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Bonnett, R. Photosensitizers of the porphyrin and phthalocyanine series for photodynamic therapy. Chem. Soc. Rev. 1995, 24, 19–33. [Google Scholar] [CrossRef]
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer 2006, 6, 535–545. [Google Scholar] [CrossRef] [Green Version]
- Lam, M.; Oleinick, N.L.; Nieminen, A.-L. Photodynamic therapy-induced apoptosis in epidermoid carcinoma cells reactive oxygen species and mitochondrial inner membrane permeabilization. J. Biol. Chem. 2001, 276, 47379–47386. [Google Scholar] [CrossRef] [Green Version]
- Shirata, C.; Kaneko, J.; Inagaki, Y.; Kokudo, T.; Sato, M.; Kiritani, S.; Akamatsu, N.; Arita, J.; Sakamoto, Y.; Hasegawa, K.; et al. Near-infrared photothermal/photodynamic therapy with indocyanine green induces apoptosis of hepatocellular carcinoma cells through oxidative stress. Sci. Rep. 2017, 7, 13958. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Lee, R.; Kim, E.; Lee, S.; Park, Y.I. Near-Infrared Light-Triggered Photodynamic Therapy and Apoptosis Using Upconversion Nanoparticles with Dual Photosensitizers. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef]
- Xie, X.; Gao, N.; Deng, R.; Sun, Q.; Xu, Q.-H.; Liu, X. Mechanistic Investigation of Photon Upconversion in Nd3+-Sensitized Core–Shell Nanoparticles. J. Am. Chem. Soc. 2013, 135, 12608–12611. [Google Scholar] [CrossRef]
- Menyuk, N.; Dwight, K.; Pierce, J.W. NaYF4: Yb,Er—An efficient upconversion phosphor. Appl. Phys. Lett. 1972, 21, 159–161. [Google Scholar] [CrossRef]
- Guyot, Y.; Manaa, H.; Rivoire, J.Y.; Moncorgé, R.; Garnier, N.; Descroix, E.; Bon, M.; Laporte, P. Excited-state-absorption and upconversion studies of Nd3+-doped single crystals Y3Al5O12, YLiF4, and LaMgAl11O19. Phys. Rev. B 1995, 51, 784–799. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Tian, G.; Gu, Z.; Yang, Y.; Gu, L.; Zhao, Y.; Ma, Y.; Yao, J. Elimination of Photon Quenching by a Transition Layer to Fabricate a Quenching-Shield Sandwich Structure for 800 nm Excited Upconversion Luminescence of Nd3+-Sensitized Nanoparticles. Adv. Mater. 2014, 26, 2831–2837. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Shen, B.; Wei, H.-L.; Liu, Z.; Chen, Z.; Zhang, Y.; Su, Y.; Zhang, J.-Z.; Wang, H.; Su, Q. Comparative investigation of the optical spectroscopic and thermal effect in Nd3+-doped nanoparticles. Nanoscale 2019, 11, 10220–10228. [Google Scholar] [CrossRef]
- Van Dam, G.M.; Themelis, G.; Crane, L.M.A.; Harlaar, N.J.; Pleijhuis, R.G.; Kelder, W.; Sarantopoulos, A.; de Jong, J.S.; Arts, H.J.G.; van der Zee, A.G.J.; et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: First in-human results. Nat. Med. 2011, 17, 1315–1319. [Google Scholar] [CrossRef]
- Danhier, F.; Le Breton, A.; Préat, V. RGD-Based Strategies to Target Alpha(v) Beta(3) Integrin in Cancer Therapy and Diagnosis. Mol. Pharm. 2012, 9, 2961–2973. [Google Scholar] [CrossRef]
- Adams, G.P.; Weiner, L.M. Monoclonal antibody therapy of cancer. Nat. Biotechnol. 2005, 23, 1147–1157. [Google Scholar] [CrossRef]
- Hermanson, G.T. Bioconjugate Techniques, 3rd ed.; Academic Press: Boston, MA, USA, 2013. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, W.; Zhu, G.; Xie, J.; Chen, X. Rethinking cancer nanotheranostics. Nat. Rev. Mater. 2017, 2, 17024. [Google Scholar] [CrossRef]
- Cai, R.; Kubota, Y.; Shuin, T.; Sakai, H.; Hashimoto, K.; Fujishima, A. Induction of Cytotoxicity by Photoexcited TiO2 Particles. Cancer Res. 1992, 52, 2346–2348. [Google Scholar]
- Yamaguchi, S.; Kobayashi, H.; Narita, T.; Kanehira, K.; Sonezaki, S.; Kubota, Y.; Terasaka, S.; Iwasaki, Y. Novel Photodynamic Therapy Using Water-dispersed TiO2–Polyethylene Glycol Compound: Evaluation of Antitumor Effect on Glioma Cells and Spheroids in Vitro. Photochem. Photobiol. 2010, 86, 964–971. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Lucky, S.S.; Muhammad Idris, N.; Li, Z.; Huang, K.; Soo, K.C.; Zhang, Y. Titania Coated Upconversion Nanoparticles for Near-Infrared Light Triggered Photodynamic Therapy. ACS Nano 2015, 9, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Zhang, Y.; Deng, K.; Chen, Y.; Li, X.; Deng, X.; Cheng, Z.; Lian, H.; Li, C.; Lin, J. UV-Emitting Upconversion-Based TiO2 Photosensitizing Nanoplatform: Near-Infrared Light Mediated in Vivo Photodynamic Therapy via Mitochondria-Involved Apoptosis Pathway. ACS Nano 2015, 9, 2584–2599. [Google Scholar] [CrossRef]
- Hou, Z.; Deng, K.; Li, C.; Deng, X.; Lian, H.; Cheng, Z.; Jin, D.; Lin, J. 808 nm Light-triggered and hyaluronic acid-targeted dual-photosensitizers nanoplatform by fully utilizing Nd3+-sensitized upconversion emission with enhanced anti-tumor efficacy. Biomaterials 2016, 101, 32–46. [Google Scholar] [CrossRef]
- Zeng, L.; Pan, Y.; Tian, Y.; Wang, X.; Ren, W.; Wang, S.; Lu, G.; Wu, A. Doxorubicin-loaded NaYF4:Yb/Tm–TiO2 inorganic photosensitizers for NIR-triggered photodynamic therapy and enhanced chemotherapy in drug-resistant breast cancers. Biomaterials 2015, 57, 93–106. [Google Scholar] [CrossRef]
- Yang, G.; Yang, D.; Yang, P.; Lv, R.; Li, C.; Zhong, C.; He, F.; Gai, S.; Lin, J. A Single 808 nm Near-Infrared Light-Mediated Multiple Imaging and Photodynamic Therapy Based on Titania Coupled Upconversion Nanoparticles. Chem. Mater. 2015, 27, 7957–7968. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, K.; Liu, X.; Dohnalová, K.; Gregorkiewicz, T.; Kong, X.; Aalders, M.C.G.; Buma, W.J.; Zhang, H. Critical Shell Thickness of Core/Shell Upconversion Luminescence Nanoplatform for FRET Application. J. Phys. Chem. Lett. 2011, 2, 2083–2088. [Google Scholar] [CrossRef]
- Ding, Y.; Wu, F.; Zhang, Y.; Liu, X.; de Jong, E.M.L.D.; Gregorkiewicz, T.; Hong, X.; Liu, Y.; Aalders, M.C.G.; Buma, W.J.; et al. Interplay between Static and Dynamic Energy Transfer in Biofunctional Upconversion Nanoplatforms. J. Phys. Chem. Lett. 2015, 6, 2518–2523. [Google Scholar] [CrossRef]
- Muhr, V.; Würth, C.; Kraft, M.; Buchner, M.; Baeumner, A.J.; Resch-Genger, U.; Hirsch, T. Particle-Size-Dependent Förster Resonance Energy Transfer from Upconversion Nanoparticles to Organic Dyes. Anal. Chem. 2017, 89, 4868–4874. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xue, B.; Kong, X.; Tu, L.; Liu, X.; Zhang, Y.; Chang, Y.; Luo, Y.; Zhao, H.; Zhang, H. 808 nm driven Nd3+-sensitized upconversion nanostructures for photodynamic therapy and simultaneous fluorescence imaging. Nanoscale 2015, 7, 190–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, K.; Liu, H.; Kraft, M.; Shikha, S.; Zheng, X.; Ågren, H.; Würth, C.; Resch-Genger, U.; Zhang, Y. A protected excitation-energy reservoir for efficient upconversion luminescence. Nanoscale 2018, 10, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Siefe, C.; Mehlenbacher, R.D.; Peng, C.S.; Zhang, Y.; Fischer, S.; Lay, A.; McLellan, C.A.; Alivisatos, A.P.; Chu, S.; Dionne, J.A. Sub-20 nm Core–Shell–Shell Nanoparticles for Bright Upconversion and Enhanced Förster Resonant Energy Transfer. J. Am. Chem. Soc. 2019, 141, 16997–17005. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.A.; Lin, S.-L.; Hsu, C.-C. Lanthanide-Doped Fluoride Nanocomposites, Production Method and Applications. U.S. Patent Application Publication US 2019/0210886 A1, 11 July 2019. [Google Scholar]
- Lin, S.-L.; Chen, Z.-R.; Chang, C.A. Nd3+ sensitized core-shell-shell nanocomposites loaded with IR806 dye for photothermal therapy and up-conversion luminescence imaging by a single wavelength NIR light irradiation. Nanotheranostics 2018, 2, 243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Shen, D.; Yang, J.; Yao, C.; Che, R.; Zhang, F.; Zhao, D. Successive Layer-by-Layer Strategy for Multi-Shell Epitaxial Growth: Shell Thickness and Doping Position Dependence in Upconverting Optical Properties. Chem. Mater. 2013, 25, 106–112. [Google Scholar] [CrossRef]
- Yin, M.; Ju, E.; Chen, Z.; Li, Z.; Ren, J.; Qu, X. Upconverting Nanoparticles with a Mesoporous TiO2 Shell for Near-Infrared-Triggered Drug Delivery and Synergistic Targeted Cancer Therapy. Chem. Eur. J. 2014, 20, 14012–14017. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Lin, S.-L.; Chang, C.A. Lanthanide-Doped Core–Shell–Shell Nanocomposite for Dual Photodynamic Therapy and Luminescence Imaging by a Single X-ray Excitation Source. ACS Appl. Mater. Interfaces 2018, 10, 7859–7870. [Google Scholar] [CrossRef]
- Yin, A.; Zhang, Y.; Sun, L.; Yan, C. Colloidal synthesis and blue based multicolor upconversion emissions of size and composition controlled monodisperse hexagonal NaYF4: Yb,Tm nanocrystals. Nanoscale 2010, 2, 953–959. [Google Scholar] [CrossRef]
- Shen, J.; Chen, G.; Ohulchanskyy, T.Y.; Kesseli, S.J.; Buchholz, S.; Li, Z.; Prasad, P.N.; Han, G. Tunable Near Infrared to Ultraviolet Upconversion Luminescence Enhancement in (α-NaYF4:Yb,Tm)/CaF2 Core/Shell Nanoparticles for In situ Real-time Recorded Biocompatible Photoactivation. Small 2013, 9, 3213–3217. [Google Scholar] [CrossRef]
- Huang, W.; Ding, M.; Huang, H.; Jiang, C.; Song, Y.; Ni, Y.; Lu, C.; Xu, Z. Uniform NaYF4:Yb, Tm hexagonal submicroplates: Controlled synthesis and enhanced UV and blue upconversion luminescence. Mater. Res. Bull. 2013, 48, 300–304. [Google Scholar] [CrossRef]
- Li, M.; Liu, X.; Liu, L.; Ma, B.; Li, B.; Zhao, X.; Tong, W.; Wang, X. β-NaYF4:Yb,Tm: Upconversion properties by controlling the transition probabilities at the same energy level. Inorg. Chem. Front. 2016, 3, 1082–1090. [Google Scholar] [CrossRef]
- Wang, F.; Wang, J.; Liu, X. Direct Evidence of a Surface Quenching Effect on Size-Dependent Luminescence of Upconversion Nanoparticles. Angew. Chem. Int. Ed. 2010, 49, 7456–7460. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-L.; Chang, C.A. Optimising FRET-Efficiency of Nd3+-Sensitised Upconversion Nanocomposites by Shortening the Emitter–Photosensitizer Distance. Nanoscale 2020, 12, 8742–8749. [Google Scholar] [CrossRef] [PubMed]
- Wyld, L.; Smith, O.; Lawry, J.; Reed, M.; Brown, N. Cell cycle phase influences tumour cell sensitivity to aminolaevulinic acid-induced photodynamic therapy in vitro. Br. J. Cancer 1998, 78, 50–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hepel, M.; Stobiecka, M.; Peachey, J.; Miller, J. Intervention of glutathione in pre-mutagenic catechol-mediated DNA damage in the presence of copper (II) ions. Mutat. Res. 2012, 735, 1–11. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.-L.; Chen, H.-C.; Chang, C.A. Enhancing Förster Resonance Energy Transfer (FRET) Efficiency of Titania–Lanthanide Hybrid Upconversion Nanomaterials by Shortening the Donor–Acceptor Distance. Nanomaterials 2020, 10, 2035. https://doi.org/10.3390/nano10102035
Lin S-L, Chen H-C, Chang CA. Enhancing Förster Resonance Energy Transfer (FRET) Efficiency of Titania–Lanthanide Hybrid Upconversion Nanomaterials by Shortening the Donor–Acceptor Distance. Nanomaterials. 2020; 10(10):2035. https://doi.org/10.3390/nano10102035
Chicago/Turabian StyleLin, Syue-Liang, Han-Chun Chen, and Cheng Allen Chang. 2020. "Enhancing Förster Resonance Energy Transfer (FRET) Efficiency of Titania–Lanthanide Hybrid Upconversion Nanomaterials by Shortening the Donor–Acceptor Distance" Nanomaterials 10, no. 10: 2035. https://doi.org/10.3390/nano10102035