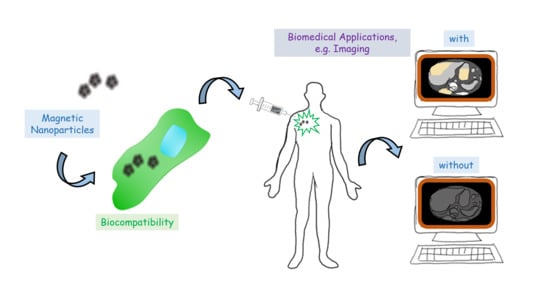
Magnetite-Arginine Nanoparticles as a Multifunctional Biomedical Tool
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanoparticle Synthesis and Characterization
2.1.1. Synthesis of polyR-Fe3O4 Nanoparticles
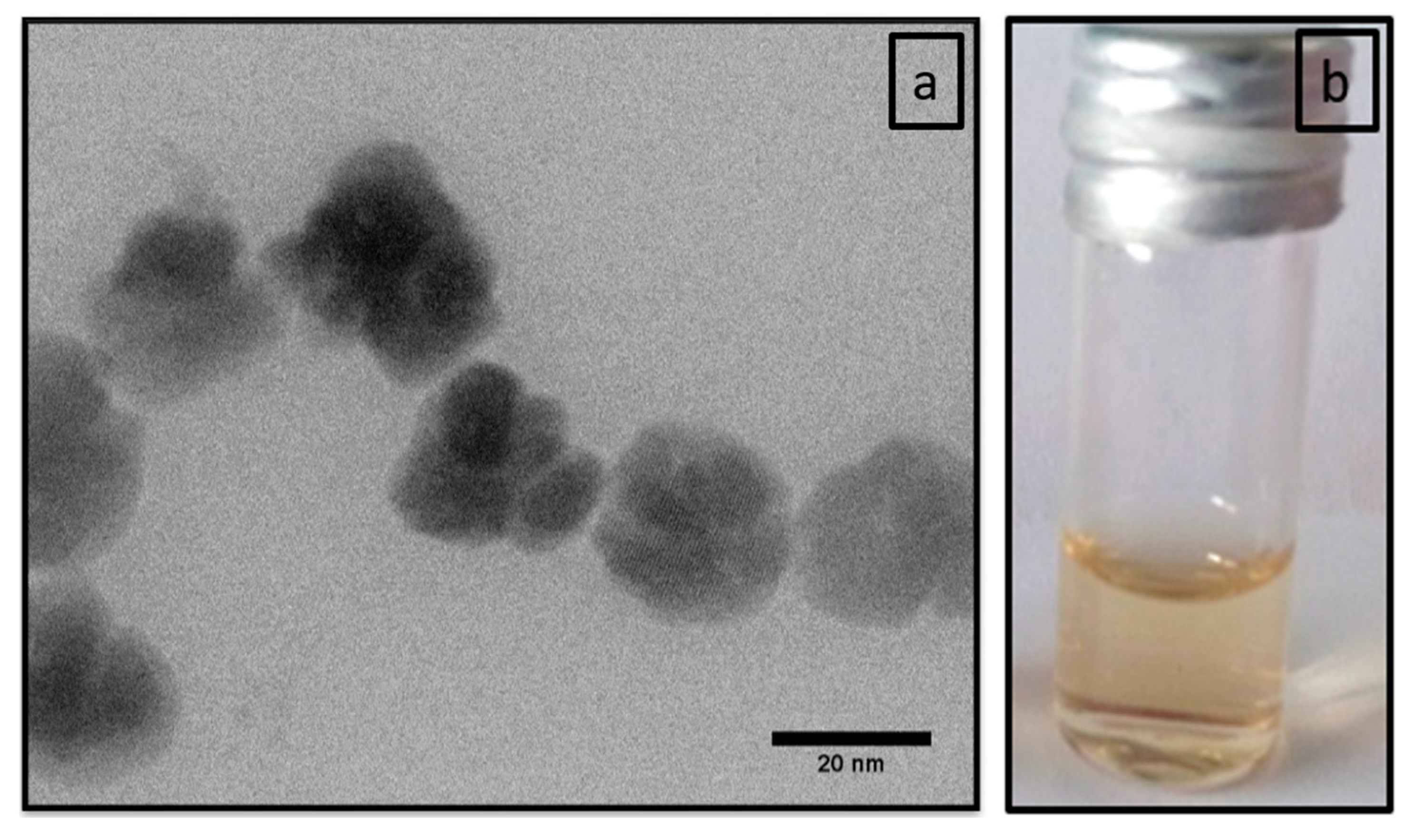
2.1.2. Transmission Electron Microscopy
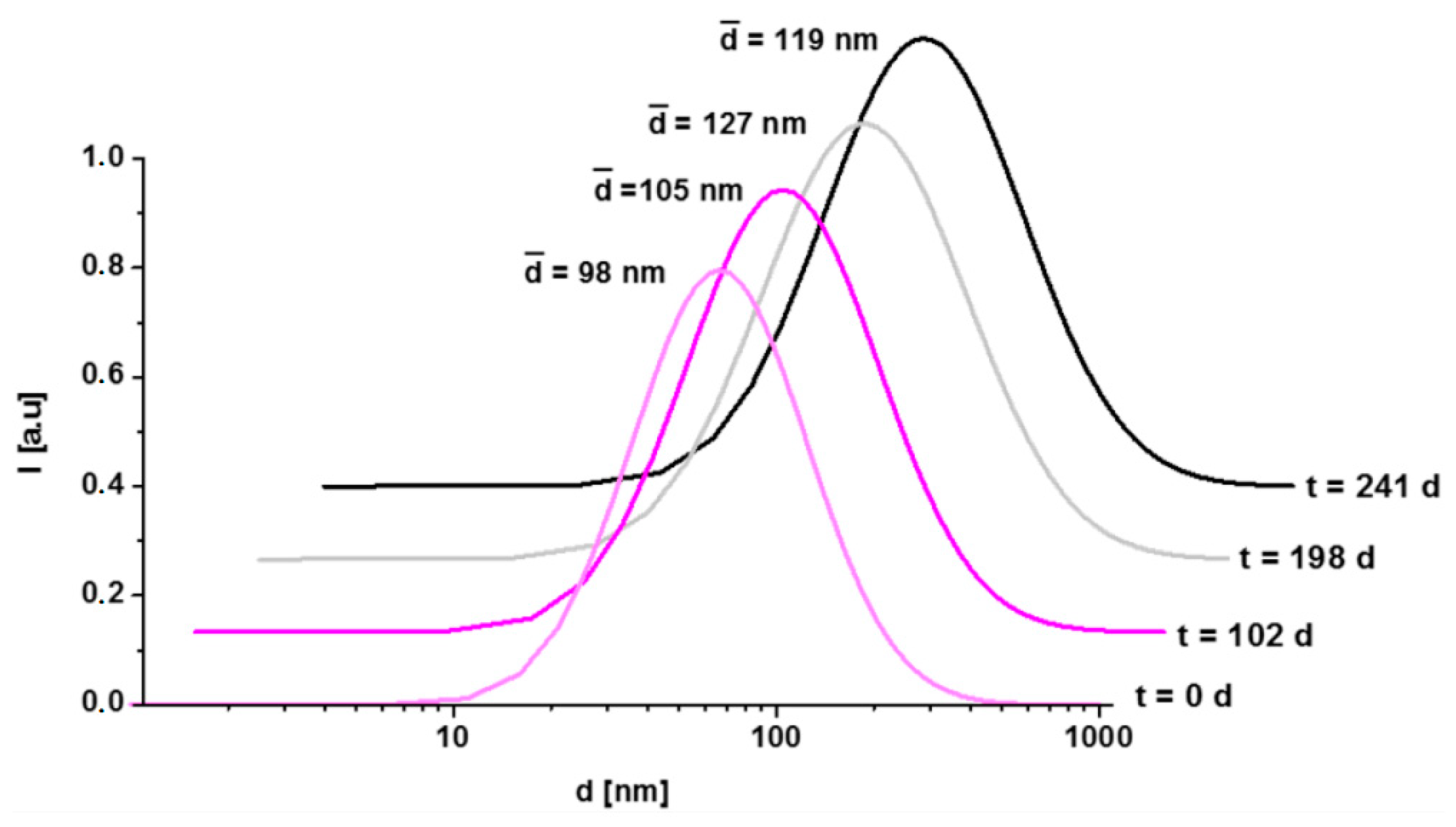
2.1.3. Dynamic Light Scattering and Zeta Potential
2.2. Biocompatibility
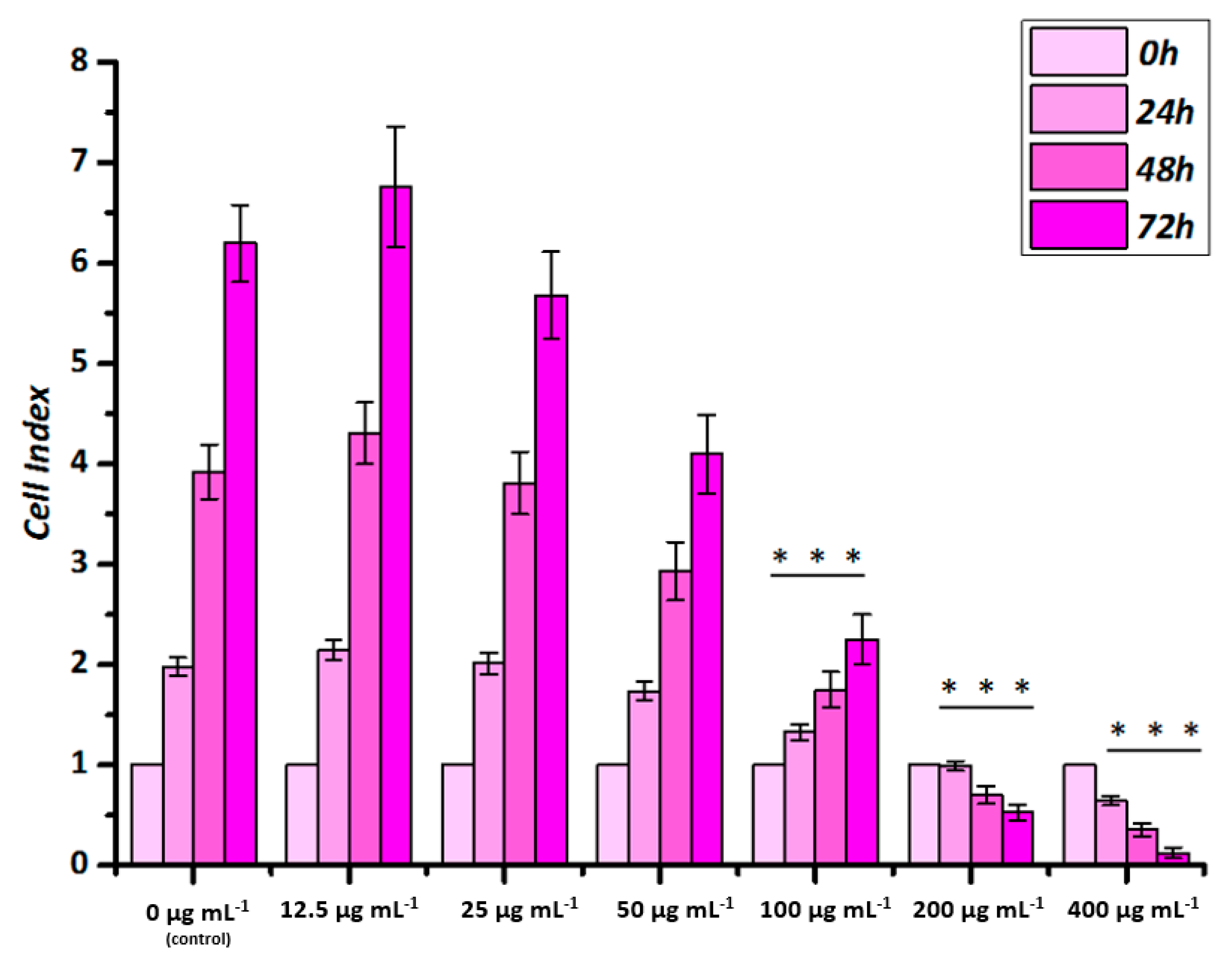
2.2.1. Real-Time Cell Analysis
2.2.2. Live-Cell Microscopy
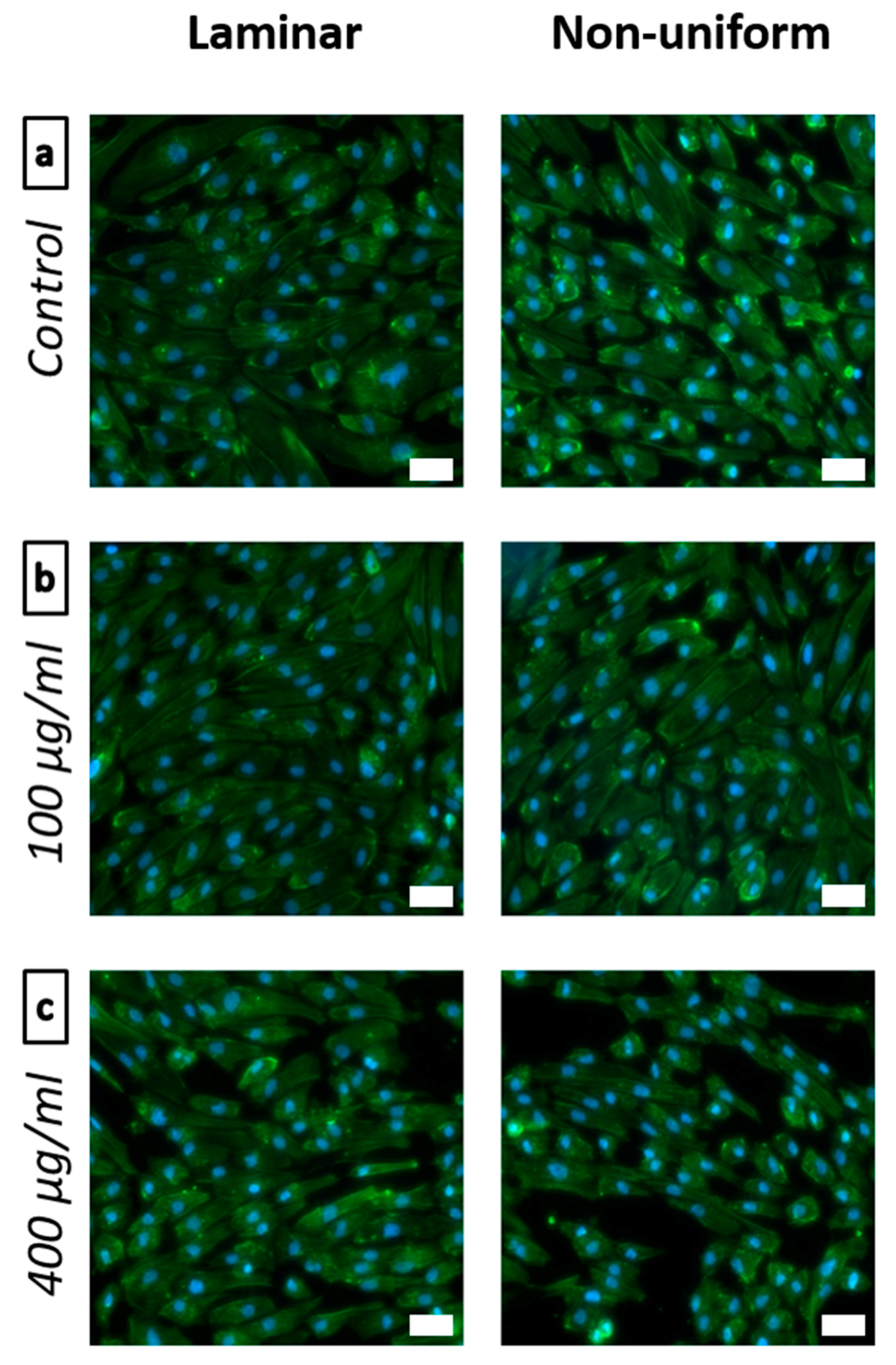
2.2.3. Flow Experiments with Bifurcation Model
2.3. Biomedical Applications
2.3.1. MRI
2.3.2. Magnetic Properties and Hyperthermia
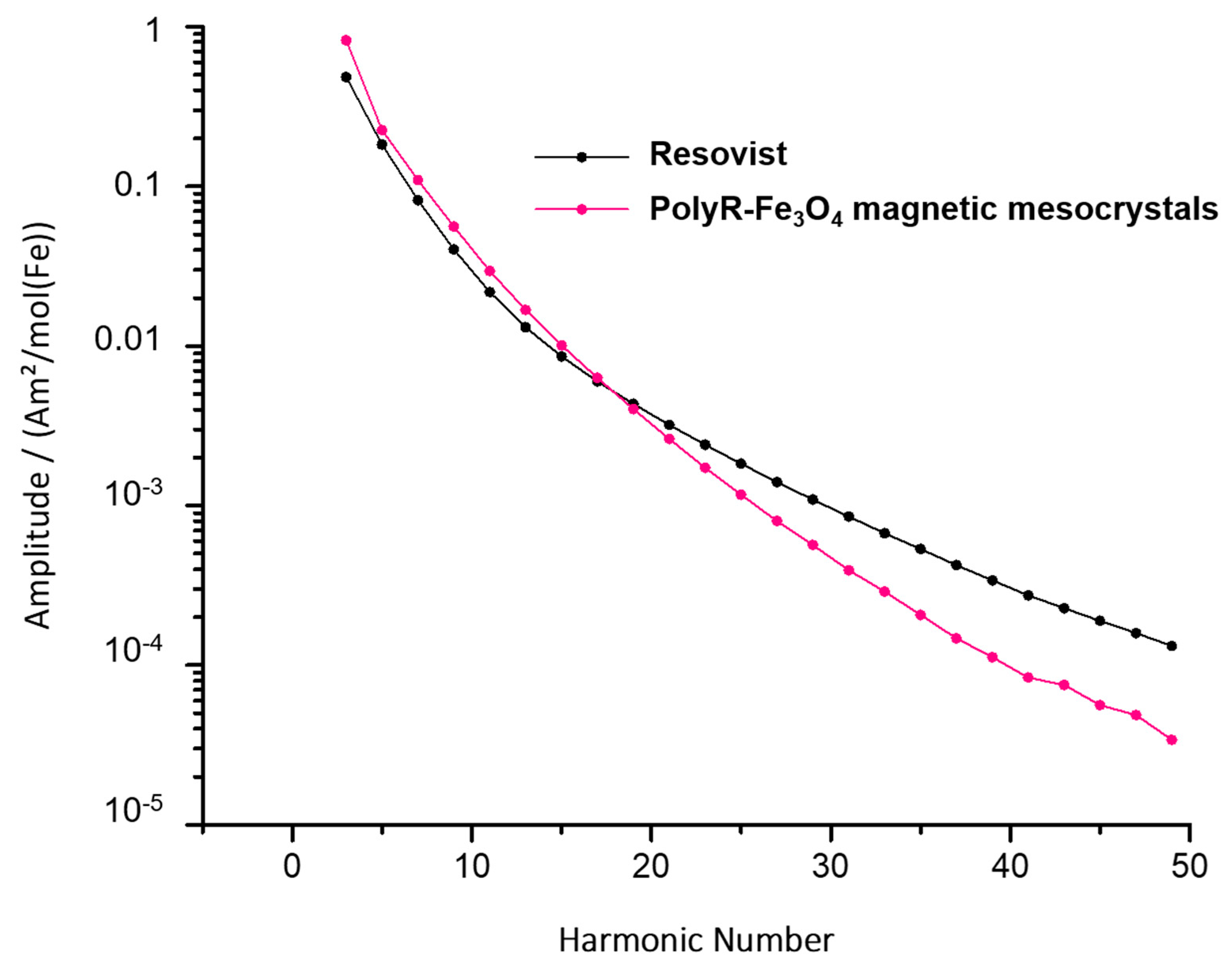
2.3.3. MPI
3. Results
3.1. Physicochemical Characterization of polyR-Magnetite Nanoparticles
3.2. Biocompatibility
3.2.1. Static Cell Viability Assays
3.2.2. Dynamic Cell Viability
3.3. Biomedical Applications
3.3.1. MRI Contrast Agents
3.3.2. Hyperthermia
3.3.3. MPI
4. Discussion
4.1. Biocompatibility
4.2. Biomedical Applications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Silva, A.K.; Espinosa, A.; Wilhelm, C.; Gazeau, F.; Kolosnjaj-Tabi, J. Medical Applications of Iron Oxide Nanoparticles. Iron Oxides 2016, 425–472. [Google Scholar] [CrossRef]
- Hergt, R.; Dutz, S. Magnetic particle hyperthermia—Biophysical limitations of a visionary tumour therapy. J. Magn. Magn. Mater. 2007, 311, 187–192. [Google Scholar] [CrossRef]
- Reichel, V.; Faivre, D. Magnetite Nucleation and Growth. In New Perspectives on Mineral Nucleation and Growth; Springer International Publishing: Cham, Switzerland, 2017; pp. 275–291. [Google Scholar] [CrossRef]
- Yu, E.Y.; Bishop, M.; Zheng, B.; Ferguson, R.M.; Khandhar, A.P.; Kemp, S.J.; Krishnan, K.M.; Goodwill, P.W.; Conolly, S.M. Magnetic Particle Imaging: A Novel in Vivo Imaging Platform for Cancer Detection. Nano Lett. 2017, 17, 1648–1654. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, S.; Ashrafizadeh, M.; Zarrabi, A.; Roghanian, R.; Afshar, E.G.; Pardakhty, A.; Mohammadinejad, R.; Kumar, A.; Thakur, V.K. Multifunctional Polymeric Nanoplatforms for Brain Diseases Diagnosis, Therapy and Theranostics. Biomedicines 2020, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Ramírez-Acosta, C.M.; Cifuentes, J.; Cruz, J.C.; Reyes, L.H. Patchy Core/Shell, Magnetite/Silver Nanoparticles via Green and Facile Synthesis: Routes to Assure Biocompatibility. Nanomaterials 2020, 10, 1857. [Google Scholar] [CrossRef]
- Ateş, B.; Koytepe, S.; Ulu, A.; Gurses, C.; Thakur, V.K. Chemistry, Structures, and Advanced Applications of Nanocomposites from Biorenewable Resources. Chem. Rev. 2020, 120, 9304–9362. [Google Scholar] [CrossRef]
- Al-Kattan, A.; Ali, L.M.A.; Daurat, M.; Mattana, E.; Gary-Bobo, M. Biological Assessment of Laser-Synthesized Silicon Nanoparticles Effect in Two-Photon Photodynamic Therapy on Breast Cancer MCF-7 Cells. Nanomaterials 2020, 10, 1462. [Google Scholar] [CrossRef]
- Dutz, S.; Hergt, R. Magnetic nanoparticle heating and heat transfer on a microscale: Basic principles, realities and physical limitations of hyperthermia for tumour therapy. Int. J. Hyperth. 2013, 29, 790–800. [Google Scholar] [CrossRef]
- Bae, K.H.; Park, M.; Do, M.J.; Lee, N.; Ryu, J.H.; Kim, G.W.; Kim, C.; Park, T.G.; Hyeon, T.; Lee, J.S. Chitosan Oligosaccharide-Stabilized Ferrimagnetic Iron Oxide Nanocubes for Magnetically Modulated Cancer Hyperthermia. ACS Nano 2012, 6, 5266–5273. [Google Scholar] [CrossRef]
- Kievit, F.M.; Zhang, M. Surface Engineering of Iron Oxide Nanoparticles for Targeted Cancer Therapy. Acc. Chem. Res. 2011, 44, 853–862. [Google Scholar] [CrossRef] [Green Version]
- Szlezak, M.; Nieciecka, D.; Joniec, A.; Pękała, M.; Gorecka, E.; Emo, M.; Stébé, M.J.; Krysiński, P.; Bilewicz, R. Monoolein Cubic Phase Gels and Cubosomes Doped with Magnetic Nanoparticles—Hybrid Materials for Controlled Drug Release. ACS Appl. Mater. Interfaces 2017, 9, 2796–2805. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.; Mallick, S.K.; Maiti, T.K.; Ghosh, S.K.; Pramanik, P. Synthesis of highly stable folic acid conjugated magnetite nanoparticles for targeting cancer cells. Nanotechnology 2007, 18, 385102. Available online: http://stacks.iop.org/0957-4484/18/i=38/a=385102 (accessed on 11 October 2020). [CrossRef]
- Taukulis, R.; Widdrat, M.; Kumari, M.; Heinke, D.; Rumpler, M.; Uebe, R.; Tompa, É.; Kraupner, A.; Cebers, A.; Schüler, D.; et al. Magnetic iron oxide nanoparticles as MRI contrast agents—A comprehensive physical and theoretical study. Magnetohydrodynamics 2015, 51, 721–748. Available online: http://real.mtak.hu/34358/ (accessed on 4 June 2018). [CrossRef] [Green Version]
- Busquets, M.A.; Estelrich, J.; Sánchez-Martín, M.J. Nanoparticles in magnetic resonance imaging: From simple to dual contrast agents. Int. J. Nanomed. 2015, 10, 1727. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.K.; Alipour, A.; Soran-Erdem, Z.; Aykut, Z.G.; Demir, H.V. Highly monodisperse low-magnetization magnetite nanocubes as simultaneous T1–T2 MRI contrast agents. Nanoscale 2015, 7, 10519–10526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutz, S.; Kettering, M.; Hilger, I.; Muller, R.; Zeisberger, M. Magnetic multicore nanoparticles for hyperthermia—Influence of particle immobilization in tumour tissue on magnetic properties. Nanotechnology 2011, 22, 265102. [Google Scholar] [CrossRef] [PubMed]
- Dutz, S. Are Magnetic Multicore Nanoparticles Promising Candidates for Biomedical Applications? IEEE Trans. Magn. 2016, 52, 1–3. [Google Scholar] [CrossRef]
- Prozorov, T.; Mallapragada, S.K.; Narasimhan, B.; Wang, L.; Palo, P.; Nilsen-Hamilton, M.; Williams, T.J.; Bazylinski, D.A.; Prozorov, R.; Canfield, P.C. Protein-Mediated Synthesis of Uniform Superparamagnetic Magnetite Nanocrystals. Adv. Funct. Mater. 2007, 17, 951–957. [Google Scholar] [CrossRef]
- Baumgartner, J.; Carillo, M.A.; Eckes, K.M.; Werner, P.; Faivre, D. Biomimetic Magnetite Formation: From Biocombinatorial Approaches to Mineralization Effects. Langmuir 2014, 30, 2129–2136. [Google Scholar] [CrossRef]
- Arakaki, A. A Novel Protein Tightly Bound to Bacterial Magnetic Particles in Magnetospirillum magneticum Strain AMB-1. J. Biol. Chem. 2003, 278, 8745–8750. [Google Scholar] [CrossRef] [Green Version]
- Bereczk-Tompa, É.; Pósfai, M.; Tóth, B.; Vonderviszt, F. Magnetite-Binding Flagellar Filaments Displaying the MamI Loop Motif. ChemBioChem 2016, 17, 2075–2082. [Google Scholar] [CrossRef] [PubMed]
- Jehle, F.; Valverde-Tercedor, C.; Reichel, V.; Carillo, M.A.; Bennet, M.; Günther, E.; Wirth, R.; Mickoleit, F.; Zarivach, R.; Schüler, D.; et al. Genetically Engineered Organization: Protein Template, Biological Recognition Sites, and Nanoparticles. Adv. Mater. Interfaces 2017, 4, 1600285. [Google Scholar] [CrossRef]
- Rawlings, A.E.; Bramble, J.P.; Tang, A.; Somner, L.A.; Monnington, A.E.; Cooke, D.; McPherson, M.J.; Tomlinson, D.C.; Staniland, S.S. Phage display selected magnetite interacting Adhirons for shape controlled nanoparticle synthesis. Chem. Sci. 2015, 6, 5586–5594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenders, J.J.M.; Zope, H.R.; Yamagishi, A.; Bomans, P.H.; Arakaki, A.; Kros, A.; De With, G.; Sommerdijk, N.A.J.M. Bioinspired Magnetite Crystallization Directed by Random Copolypeptides. Adv. Funct. Mater. 2015, 25, 711–719. [Google Scholar] [CrossRef] [Green Version]
- Reichel, V.; Kovács, A.; Kumari, M.; Bereczk-Tompa, É.; Schneck, E.; Diehle, P.; Pósfai, M.; Hirt, A.M.; Duchamp, M.; Dunin-Borkowski, R.; et al. Single crystalline superstructured stable single domain magnetite nanoparticles. Sci. Rep. 2017, 7, 45484. [Google Scholar] [CrossRef] [Green Version]
- Faivre, D.; Godec, T.U. From Bacteria to Mollusks: The Principles Underlying the Biomineralization of Iron Oxide Materials. Angew. Chemie Int. Ed. 2015, 54, 4728–4747. [Google Scholar] [CrossRef]
- Mannucci, S.; Ghin, L.; Conti, G.; Tambalo, S.; Lascialfari, A.; Orlando, T.; Benati, N.; Bernardi, P.; Betterle, N.; Bassi, R.; et al. Magnetic Nanoparticles from Magnetospirillum gryphiswaldense Increase the Efficacy of Thermotherapy in a Model of Colon Carcinoma. PLoS ONE 2014, 9, e108959. [Google Scholar] [CrossRef]
- Alphandéry, E.; Faure, S.; Seksek, O.; Guyot, F.; Chebbi, I. Chains of Magnetosomes Extracted from AMB-1 Magnetotactic Bacteria for Application in Alternative Magnetic Field Cancer Therapy. ACS Nano 2011, 5, 6279–6296. [Google Scholar] [CrossRef]
- Lisy, M.R.; Hartung, A.; Lang, C.; Schüler, D.; Richter, W.; Reichenbach, J.R.; A Kaiser, W.; Hilger, I. Fluorescent Bacterial Magnetic Nanoparticles as Bimodal Contrast Agents. Investig. Radiol. 2007, 42, 235–241. [Google Scholar] [CrossRef]
- Kraupner, A.; Eberbeck, D.; Heinke, D.; Uebe, R.; Schüler, D.; Briel, A. Bacterial magnetosomes—Nature’s powerful contribution to MPI tracer research. Nanoscale 2017, 9, 5788–5793. [Google Scholar] [CrossRef]
- Lee, H.Y.; Mohammed, K.A.; Nasreen, N. L-Arginine-Incorporated Albumin Mesospheres: A Drug Delivery System for Cancer Therapy. In L-Arginine in Clinical Nutrition; Springer International Publishing: Cham, Switzerland, 2017; pp. 527–541. [Google Scholar] [CrossRef]
- Vazdar, M.; Heyda, J.; Mason, P.E.; Tesei, G.; Allolio, C.; Lund, M.; Jungwirth, P. Arginine “Magic”: Guanidinium Like-Charge Ion Pairing from Aqueous Salts to Cell Penetrating Peptides. Acc. Chem. Res. 2018, 51, 1455–1464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumgartner, J.; Bertinetti, L.; Widdrat, M.; Hirt, A.M.; Faivre, D. Formation of Magnetite Nanoparticles at Low Temperature: From Superparamagnetic to Stable Single Domain Particles. PLoS ONE 2013, 8, e57070. [Google Scholar] [CrossRef]
- Matuszak, J.; Baumgartner, J.; Zaloga, J.; Juenet, M.; Silva, A.E.; Franke, D.; Almer, G.; Texier, I.; Faivre, D.; Metselaar, J.M.; et al. Nanoparticles for intravascular applications: Physicochemical characterization and cytotoxicity testing. Nanomedicine 2016, 11, 597–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutz, S.; Hergt, R. Magnetic particle hyperthermia—A promising tumour therapy? Nanotechnology 2014, 25, 452001. [Google Scholar] [CrossRef] [PubMed]
- Nordling, S.; Nilsson, B.; Magnusson, P. A novel in vitro model for studying the interactions between human whole blood and endothelium. J. Vis. Exp. 2014, e52112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, N.; Yoo, D.; Ling, D.; Cho, M.H.; Hyeon, T.; Cheon, J. Iron Oxide Based Nanoparticles for Multimodal Imaging and Magnetoresponsive Therapy. Chem. Rev. 2015, 115, 10637–10689. [Google Scholar] [CrossRef]
- Pinkernelle, J.G.; Teichgräber, U.K.M.; Neumann, F.; Lehmkuhl, L.; Ricke, J.; Scholz, R.; Jordan, A.; Bruhn, H. Imaging of single human carcinoma cells in vitro using a clinical whole-body magnetic resonance scanner at 3.0 T. Magn. Reson. Med. Off. J. Int. Soc. Magn. Reason. Med. 2005, 53, 1187–1192. [Google Scholar] [CrossRef]
- Matuszak, J.; Dörfler, P.; Lyer, S.; Unterweger, H.; Juenet, M.; Chauvierre, C.; Alaarg, A.; Franke, D.; Almer, G.; Texier, I.; et al. Comparative analysis of nanosystems’ effects on human endothelial and monocytic cell functions. Nanotoxicology 2018, 12, 957–974. [Google Scholar] [CrossRef]
- Sun, J.; Guo, M.; Pang, H.; Qi, J.; Zhang, J.; Ge, Y. Treatment of malignant glioma using hyperthermia. Neural Regen. Res. 2013, 8, 2775. [Google Scholar]
- Chiu-Lam, A.; Rinaldi, C. Nanoscale Thermal Phenomena in the Vicinity of Magnetic Nanoparticles in Alternating Magnetic Fields. Adv. Funct. Mater. 2016, 26, 3933–3941. [Google Scholar] [CrossRef] [Green Version]
- Nemati, Z.; Das, R.; Alonso, J.; Clements, E.; Phan, M.H.; Srikanth, H. Iron Oxide Nanospheres and Nanocubes for Magnetic Hyperthermia Therapy: A Comparative Study. J. Electron. Mater. 2017, 46, 3764–3769. [Google Scholar] [CrossRef]
- Iacovita, C.; Florea, A.; Dudric, R.; Páll, E.; Moldovan, A.; Tetean, R.; Stiufiuc, R.; Lucaciu, C.M. Small versus Large Iron Oxide Magnetic Nanoparticles: Hyperthermia and Cell Uptake Properties. Molecules 2016, 21, 1357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yen, H.J.; Young, Y.A.; Tsai, T.N.; Cheng, K.M.; Chen, X.A.; Chen, Y.C.; Chen, C.C.; Young, J.J.; Hong, P.D. Positively charged gold nanoparticles capped with folate quaternary chitosan: Synthesis, cytotoxicity, and uptake by cancer cells. Carbohydr. Polym. 2018, 183, 140–150. [Google Scholar] [CrossRef] [PubMed]
- A Pankhurst, Q.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R167–R181. [Google Scholar] [CrossRef] [Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reichel, V.E.; Matuszak, J.; Bente, K.; Heil, T.; Kraupner, A.; Dutz, S.; Cicha, I.; Faivre, D. Magnetite-Arginine Nanoparticles as a Multifunctional Biomedical Tool. Nanomaterials 2020, 10, 2014. https://doi.org/10.3390/nano10102014
Reichel VE, Matuszak J, Bente K, Heil T, Kraupner A, Dutz S, Cicha I, Faivre D. Magnetite-Arginine Nanoparticles as a Multifunctional Biomedical Tool. Nanomaterials. 2020; 10(10):2014. https://doi.org/10.3390/nano10102014
Chicago/Turabian StyleReichel, Victoria E., Jasmin Matuszak, Klaas Bente, Tobias Heil, Alexander Kraupner, Silvio Dutz, Iwona Cicha, and Damien Faivre. 2020. "Magnetite-Arginine Nanoparticles as a Multifunctional Biomedical Tool" Nanomaterials 10, no. 10: 2014. https://doi.org/10.3390/nano10102014
APA StyleReichel, V. E., Matuszak, J., Bente, K., Heil, T., Kraupner, A., Dutz, S., Cicha, I., & Faivre, D. (2020). Magnetite-Arginine Nanoparticles as a Multifunctional Biomedical Tool. Nanomaterials, 10(10), 2014. https://doi.org/10.3390/nano10102014