Amphiphilic “Like-A-Brush” Oligonucleotide Conjugates with Three Dodecyl Chains: Self-Assembly Features of Novel Scaffold Compounds for Nucleic Acids Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Remarks
2.2. Materials
2.3. Oligonucleotides and Conjugates Synthesis
2.4. Critical Aggregation Concentration (CAC) Determination by Nile Red Encapsulation Assay
2.5. Characterization of Assembled DOCs Micellar Structures by DLS
2.6. Characterization of Assembled DOCs Micellar Structures by AFM
2.7. Characterization of Assembled DOCs Micellar Structures by TEM
2.8. EMSA and BSA Binding Experiments
2.9. Fluorescence Quenching Experiments
2.10. Cell Culture
2.11. Cells Transfection
2.12. Cellular Accumulation Assay
2.13. Confocal Fluorescence Microscopy
2.14. Statistical Analysis
3. Results
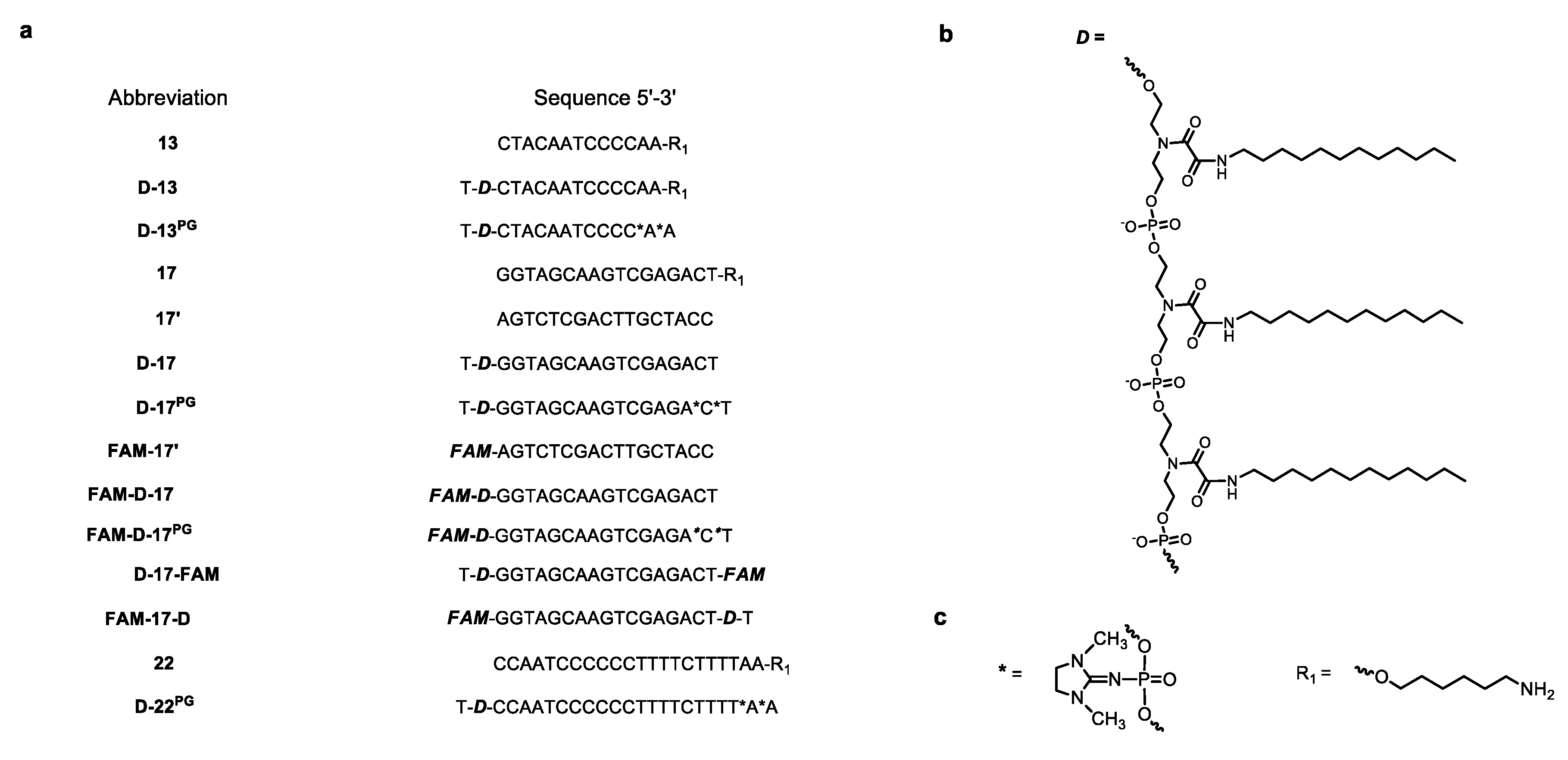
3.1. Oligonucleotides and DOCs in this Study
3.2. CAC Determination by Nile Red Encapsulation Assay
3.3. DLS Experiments
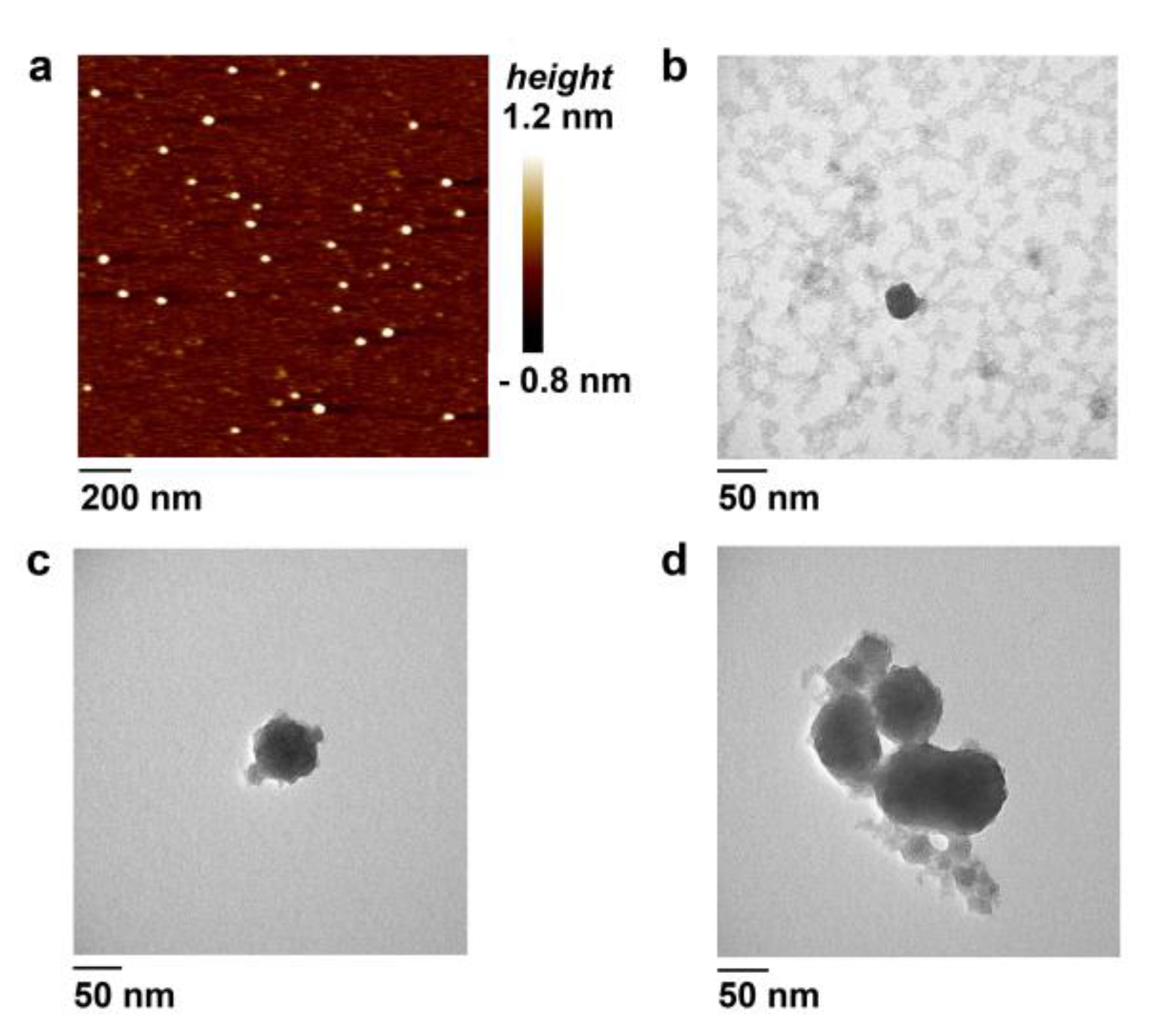
3.4. Atomic Force Microscopy
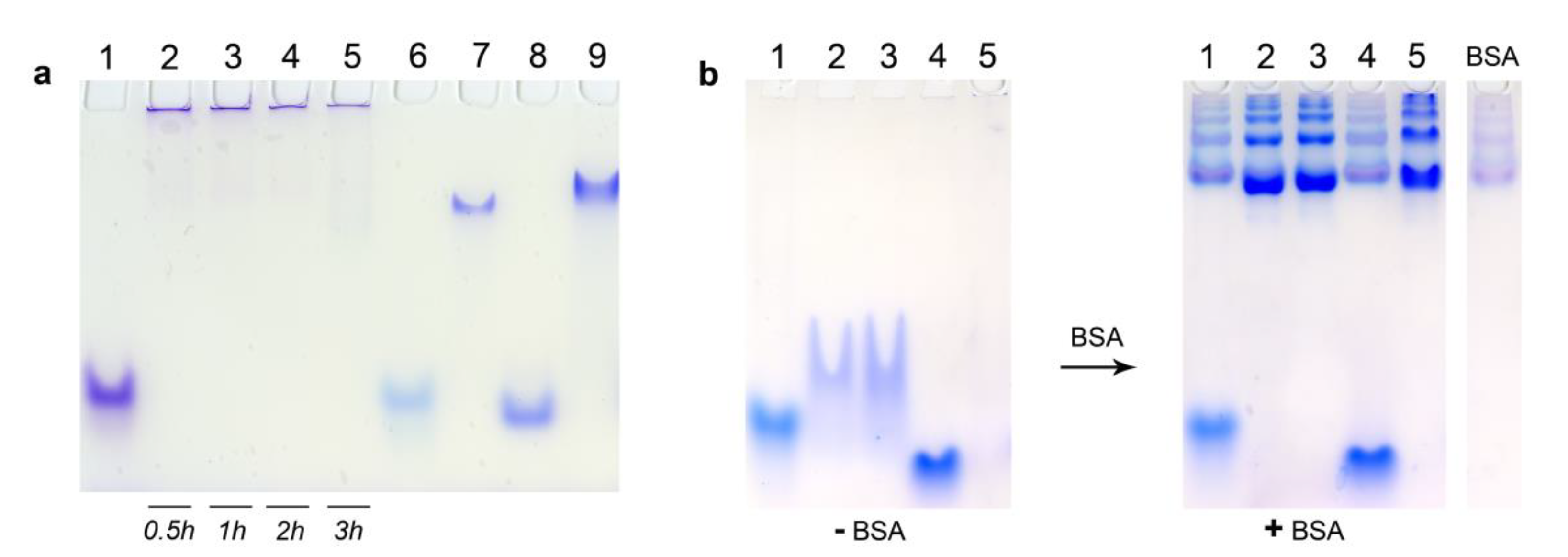
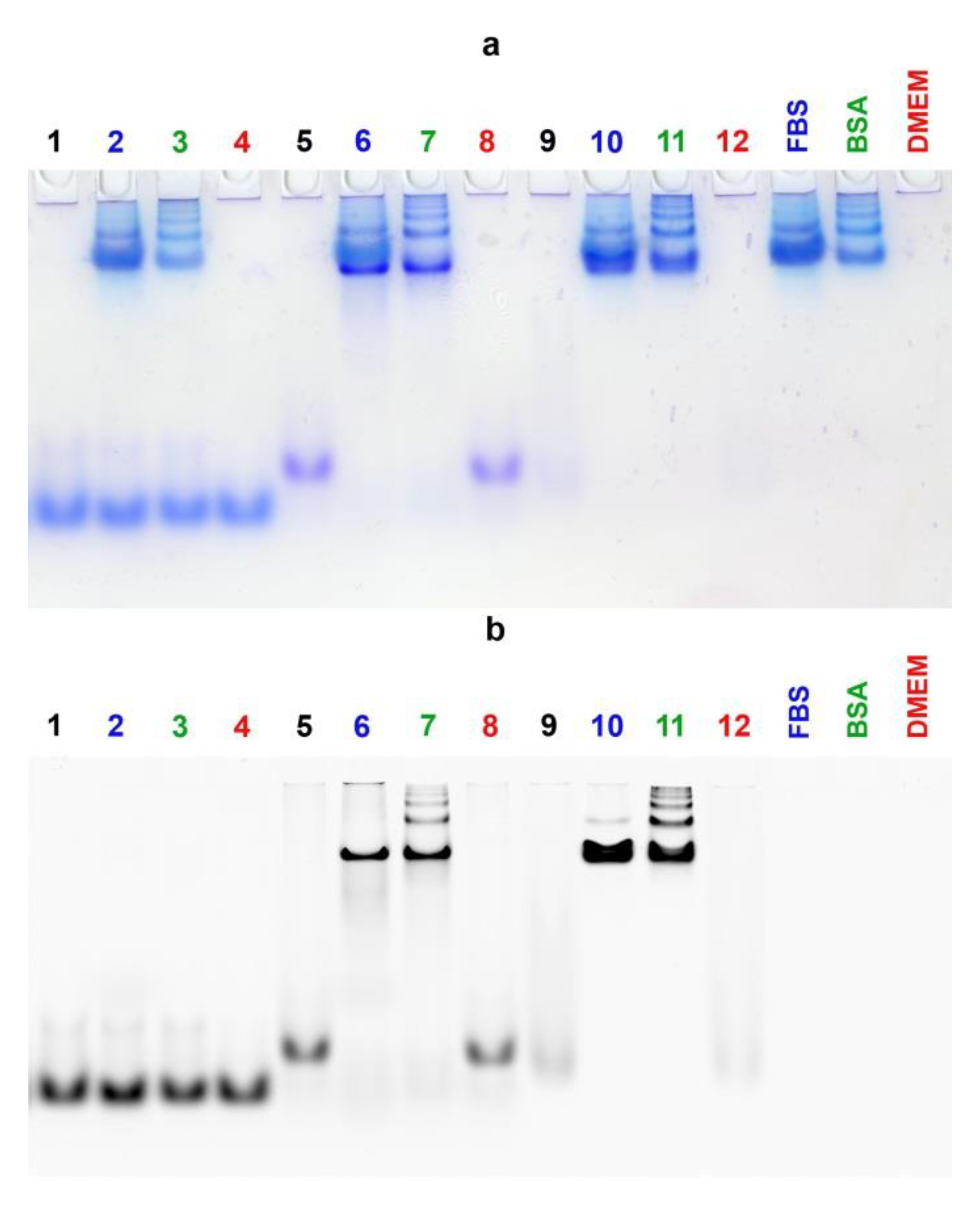
3.5. Electrophoretic Mobility Shift Assay
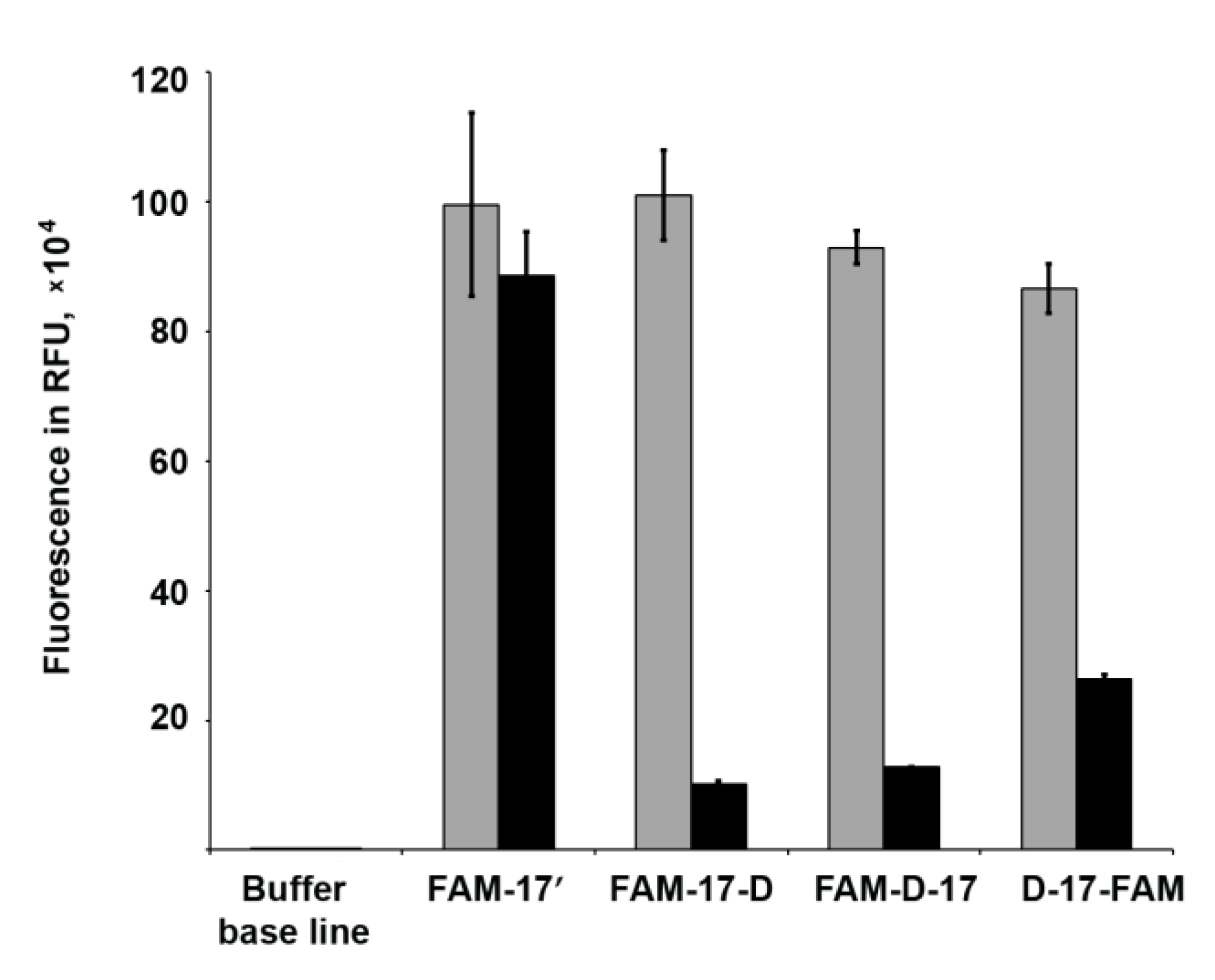
3.6. Fluorescence Quenching Experiments
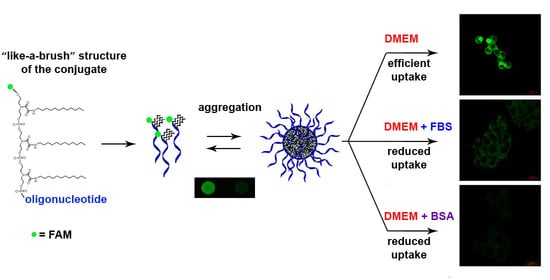
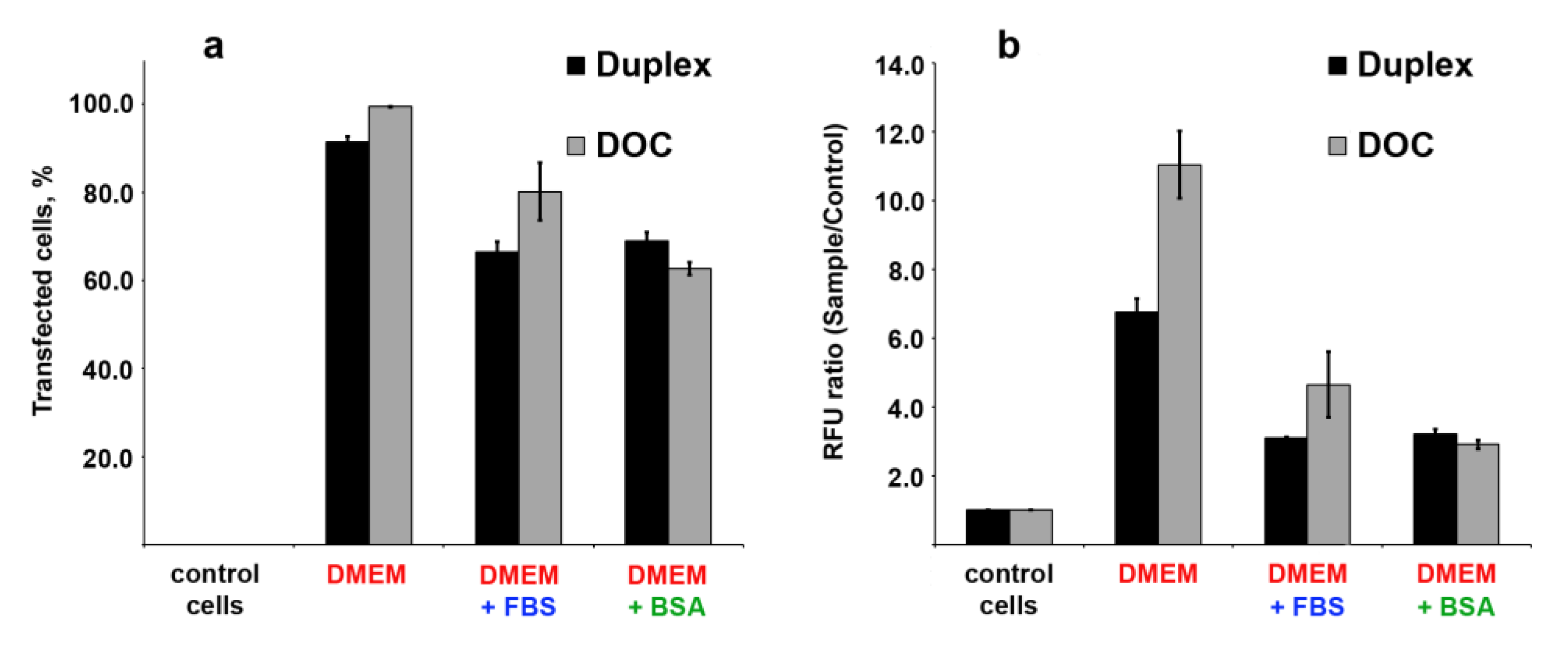
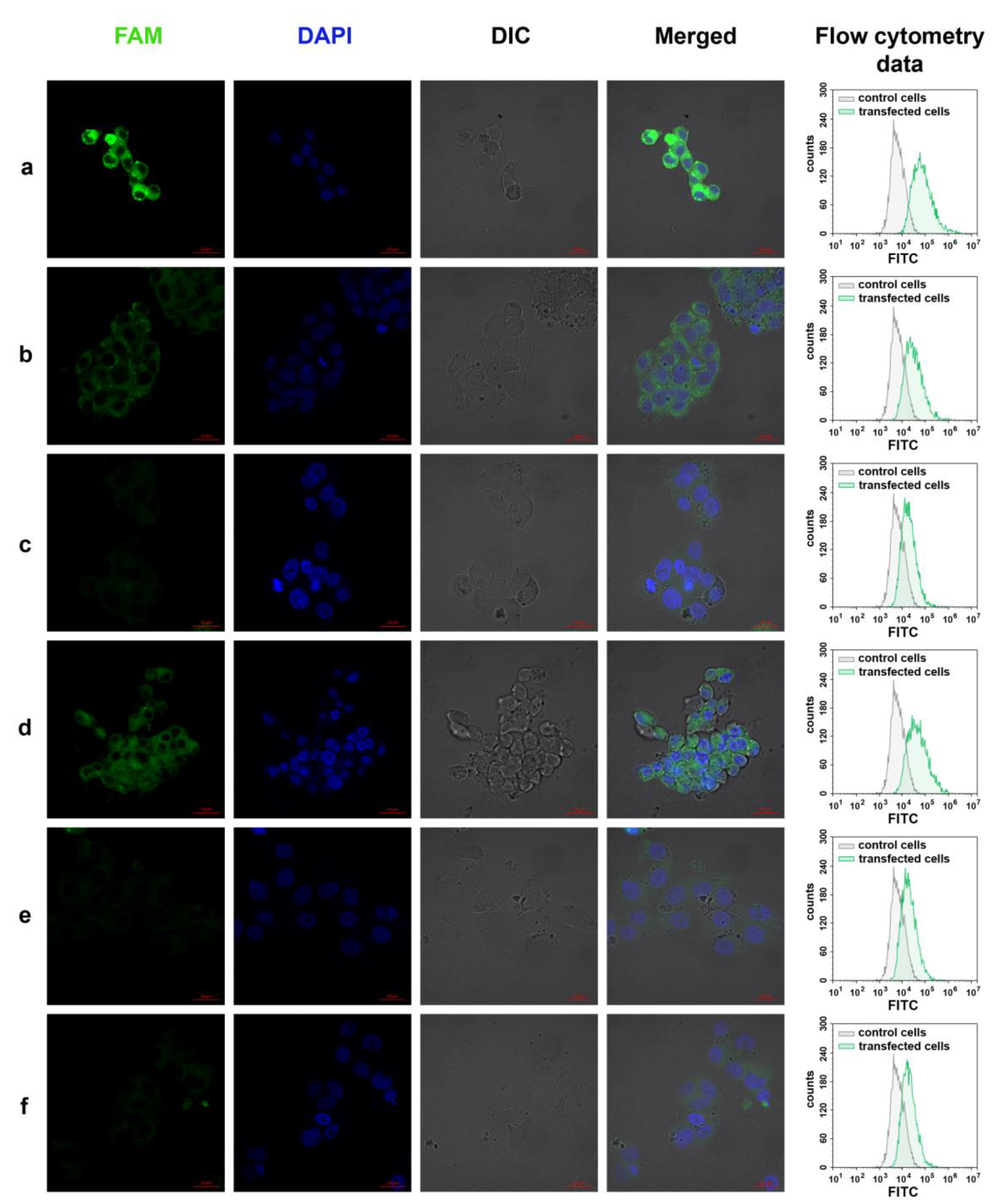
3.7. Cellular Uptake of Three Dodecyl-Containing Conjugates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gissot, A.; Camplo, M.; Grinstaff, M.W.; Barthélémy, P. Nucleoside, nucleotide and oligonucleotide based amphiphiles: A successful marriage of nucleic acids with lipids. Org. Biomol. Chem. 2008, 6, 1324–1333. [Google Scholar] [CrossRef]
- Patwa, A.; Gissot, A.; Bestel, I.; Barthélémy, P. Hybrid lipid oligonucleotide conjugates: Synthesis, self-assemblies and biomedical applications. Chem. Soc. Rev. 2011, 40, 5844–5854. [Google Scholar] [CrossRef]
- Raouane, M.; Desmaële, D.; Urbinati, G.; Massaad-Massade, L.; Couvreur, P. Lipid Conjugated Oligonucleotides: A Useful Strategy for Delivery. Bioconjugate Chem. 2012, 23, 1091–1104. [Google Scholar] [CrossRef]
- Craig, K.; Abrams, M.; Amiji, M. Recent preclinical and clinical advances in oligonucleotide conjugates. Expert Opin. Drug Deliv. 2018, 15, 629–640. [Google Scholar] [CrossRef]
- Osborn, M.F.; Khvorova, A. Improving Small Interfering RNA Delivery In Vivo Through Lipid Conjugation. Nucleic Acid Ther. 2018, 28, 128–136. [Google Scholar] [CrossRef] [Green Version]
- Benizri, S.; Gissot, A.; Martin, A.; Vialet, B.; Grinstaff, M.W.; Barthélémy, P. Bioconjugated Oligonucleotides: Recent Developments and Therapeutic Applications. Bioconjugate Chem. 2019, 30, 366–383. [Google Scholar] [CrossRef]
- Boutorin, A.S.; Gus’kova, L.V.; Ivanova, E.M.; Kobetz, N.D.; Zarytova, V.F.; Ryte, A.S.; Yurchenko, L.V.; Vlassov, V.V. Synthesis of alkylating oligonucleotide derivatives containing cholesterol or phenazinium residues at their 3′-terminus and their interaction with DNA within mammalian cells. FEBS Lett. 1989, 254, 129–132. [Google Scholar] [CrossRef] [Green Version]
- Dovydenko, I.; Tarassov, I.; Venyaminova, A.; Entelis, N. Method of carrier-free delivery of therapeutic RNA importable into human mitochondria: Lipophilic conjugates with cleavable bonds. Biomaterials 2016, 76, 408–417. [Google Scholar] [CrossRef] [Green Version]
- Borisenko, G.G.; Zaitseva, M.A.; Chuvilin, A.N.; Pozmogova, G.E. DNA modification of live cell surface. Nucleic Acids Res. 2009, 37, e28. [Google Scholar] [CrossRef] [Green Version]
- Karaki, S.; Benizri, S.; Mejías, R.; Baylot, V.; Branger, N.; Nguyen, T.; Vialet, B.; Oumzil, K.; Barthélémy, P.; Rocchi, P. Lipid-oligonucleotide conjugates improve cellular uptake and efficacy of TCTP-antisense in castration-resistant prostate cancer. J. Control. Release 2017, 258, 1–9. [Google Scholar] [CrossRef]
- Asahi, W.; Kurihara, R.; Takeyama, K.; Umehara, Y.; Kimura, Y.; Kondo, T.; Tanabe, K. Aggregate Formation of BODIPY-Tethered Oligonucleotides That Led to Efficient Intracellular Penetration and Gene Regulation. ACS Appl. Bio Mater. 2019, 2, 4456–4463. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, Z.; Kang, H.; Wu, Y.; Sefan, K.; Tan, W. DNA Based Micelles: Synthesis, Micellar Properties and Size-dependent Cell Permeability. Chemistry 2010, 16, 3791–3797. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wu, C.S.; Jimenez, E.; Zhu, Z.; Dajac, J.G.; You, M.; Han, D.; Zhang, X.; Tan, W. DNA Micelle Flares for Intracellular mRNA Imaging and Gene Therapy. Angew. Chem. Int. Ed. 2013, 52, 2012–2016. [Google Scholar] [CrossRef] [Green Version]
- Pokholenko, O.; Gissot, A.; Vialet, B.; Bathany, K.; Thiéry, A.; Barthélémy, P. Lipid oligonucleotide conjugates as responsive nanomaterials for drug delivery. J. Mater. Chem. B 2013, 1, 5329–5334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwardson, T.G.W.; Carneiro, K.M.M.; Serpel, C.J.; Sleiman, H.F. An Efficient and Modular Route to Sequence-Defined Polymers Appended to DNA. Angew. Chem. Int. Ed. 2014, 53, 1–6. [Google Scholar] [CrossRef]
- Trinh, T.; Chidchob, P.; Bazzi, H.S.; Sleiman, H.F. DNA micelles as nanoreactors: Efficient DNA functionalization with hydrophobic organic molecules. Chem. Commun. 2016, 52, 10914–10917. [Google Scholar] [CrossRef]
- Cozzoli, L.; Gjonaj, L.; Stuart, M.C.A.; Poolman, B.; Roelfes, G. Responsive DNA G-quadruplex micelles. Chem. Commun. 2018, 54, 260–263. [Google Scholar] [CrossRef] [Green Version]
- Kauss, T.; Arpin, C.; Bientz, L.; Nguyen, P.V.; Vialet, B.; Benizri, S.; Barthélémy, P. Lipid oligonucleotides as a new strategy for tackling the antibiotic resistance. Sci. Rep. 2020, 10, 1054. [Google Scholar] [CrossRef]
- Dentinger, P.M.; Simmons, B.A.; Cruz, E.; Sprague, M. DNA-Mediated Delivery of Lipophilic Molecules via Hybridization to DNA-Based Vesicular Aggregates. Langmuir 2006, 22, 2935–2937. [Google Scholar] [CrossRef]
- Thompson, M.P.; Chien, M.-P.; Ku, T.-H.; Rush, A.-M.; Gianneschi, N.C. Smart Lipids for Programmable Nanomaterials. Nano Lett. 2010, 10, 2690–2693. [Google Scholar] [CrossRef] [Green Version]
- Raouane, M.; Desmaele, D.; Gilbert-Sirieix, M.; Gueutin, C.; Zouhiri, F.; Bourgaux, C.; Lepeltier, E.; Gref, R.; Ben Salah, R.; Clayman, G.; et al. Synthesis, Characterization, and in Vivo Delivery of siRNA-Squalene Nanoparticles Targeting Fusion Oncogene in Papillary Thyroid Carcinoma. J. Med. Chem. 2011, 54, 4067–4076. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Liu, X.; Bai, H.; Wang, R.; Tan, J.; Peng, X.; Tan, W. Engineering Stability-Tunable DNA Micelles Using Photocontrollable Dissociation of an Intermolecular G-Quadruplex. ACS Nano 2017, 11, 12087–12093. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Yin, H.; Rajabi, M.; Li, H.; Vieweger, M.; Guo, S.; Shu, D.; Guo, P. RNA-based micelles: A novel platform for paclitaxel loading and delivery. J. Control. Release 2018, 276, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Herbert, B.-S.; Gellert, G.C.; Hochreiter, A.; Pongracz, K.; Wright, W.E.; Zielinska, D.; Chin, A.C.; Harley, C.B.; Shay, J.W.; Gryaznov, S.M. Lipid modification of GRN163, an N3′→P5′ thio-phosphoramidate oligonucleotide, enhances the potency of telomerase inhibition. Oncogene 2005, 24, 5262–5268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Allen, N.; Prakash, T.P.; Liang, X.; Crooke, S.T. Lipid Conjugates Enhance Endosomal Release of Antisense Oligonucleotides Into Cells. Nucleic Acids Ther. 2019, 29, 245–255. [Google Scholar] [CrossRef]
- Wolfrum, C.; Shi, S.; Jayaprakash, K.N.; Jayaraman, M.; Wang, G.; Pandey, R.K.; Rajeev, K.G.; Nakayama, T.; Charrise, K.; Ndungo, E.M.; et al. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat. Biotechnol. 2007, 25, 1149–1157. [Google Scholar] [CrossRef]
- Roloff, A.; Nelles, D.A.; Thompson, M.P.; Yeo, G.W.; Gianneschi, N.C. Self-Transfecting Micellar RNA: Modulating Nanoparticle Cell Interactions via High Density Display of Small Molecule Ligands on Micelle Coronas. Bioconjugate Chem. 2018, 29, 126–135. [Google Scholar] [CrossRef]
- Simonova, O.N.G.; Pishnyi, D.V.; Vlassov, V.V.; Zenkova, M.A. Modified Concatemeric Oligonucleotide Complexes: New System for Efficient Oligonucleotide Transfer into Mammalian Cells. Hum. Gene Ther. 2008, 19, 532–546. [Google Scholar] [CrossRef]
- Charbgoo, F.; Alibolandi, M.; Taghdisi, S.M.; Abnous, K.; Soltani, F.; Ramezani, M. MUC1 aptamer-targeted DNA micelles for dual tumor therapy using doxorubicin and KLA peptide. Nanomedicine 2018, 14, 685–697. [Google Scholar] [CrossRef]
- Banga, R.G.; Chernyak, N.; Narayan, S.P.; Nguyen, S.T.; Mirkin, C.A. Liposomal Spherical Nucleic Acids. J. Am. Chem. Soc. 2014, 136, 9866–9869. [Google Scholar] [CrossRef]
- Banga, R.G.; Meckes, B.; Narayan, S.P.; Sprangers, A.J.; Nguyen, S.T.; Mirkin, C.A. Cross-Linked Micellar Spherical Nucleic Acids from Thermoresponsive Templates. J. Am. Chem. Soc. 2017, 139, 4278–4281. [Google Scholar] [CrossRef] [PubMed]
- Meckes, B.; Banga, R.G.; Nguyen, S.T.; Mirkin, C.A. Enhancing the Stability and Immunomodulatory Activity of Liposomal Spherical Nucleic Acids through Lipid-Tail DNA Modifications. Small 2018, 14, 1702909. [Google Scholar] [CrossRef] [PubMed]
- Markov, O.V.; Filatov, A.V.; Kupryushkin, M.S.; Chernikov, I.V.; Patutina, O.A.; Strunov, A.A.; Chernolovskaya, E.L.; Vlassov, V.V.; Pyshnyi, D.V.; Zenkova, M.A. Transport Oligonucleotides—A Novel System for Intracellular Delivery of Antisense Therapeutics. Molecules 2020, 25, 3663. [Google Scholar] [CrossRef] [PubMed]
- Frei, E. Albumin binding ligands and albumin conjugate uptake by cancer cells. Diabetol. Metab. Syndr. 2011, 3, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarett, S.M.; Werfel, T.A.; Lee, L.; Jackson, M.A.; Kilchrist, K.V.; Brantley-Sieders, D.; Duvall, C.L. Lipophilic siRNA targets albumin in situ and promotes bioavailability, tumor penetration, and carrier-free gene silencing. Proc. Natl. Acad. Sci. USA 2017, 114, E6490–E6497. [Google Scholar] [CrossRef] [Green Version]
- Kupryushkin, M.S.; Nekrasov, M.D.; Stetsenko, D.A.; Pyshnyi, D.V. Efficient Functionalization of Oligonucleotides by New Achiral Nonnucleosidic Monomers. Org. Lett. 2014, 16, 2842–2845. [Google Scholar] [CrossRef]
- Kupryushkin, M.S.; Pyshnyi, D.V.; Stetsenko, D.A. Phosphoryl guanidines: A new type of nucleic acid analogues. Acta Nat. 2014, 6, 116–118. [Google Scholar] [CrossRef]
- Stetsenko, D.A.; Kupryushkin, M.S.; Pyshnyi, D.V. Modified Oligonucleotides and Methods for Their Synthesis. International Patent No. WO2,016,028,187A1, 22 June 2014. [Google Scholar]
- Lomzov, A.A.; Kupryushkin, M.S.; Shernyukov, A.V.; Nekrasov, M.D.; Dovydenko, I.S.; Stetsenko, D.A.; Pyshnyi, D.V. Diastereomers of a mono-substituted phosphoryl guanidine trideoxyribonucleotide: Isolation and properties. Biochem. Biophys. Res. Commun. 2019, 513, 807–811. [Google Scholar] [CrossRef]
- Poulsen, C.S.; Pedersen, E.B.; Nielsen, C. DNA conjugated phenoxyaniline intercalators synthesis of diethanolaminoacetamide-type linkers. Acta Chem. Scand. 1999, 53, 425–431. [Google Scholar] [CrossRef] [Green Version]
- Hébert, N.; Davis, P.W.; DeBaets, E.L.; Acevedo, O.L. Synthesis of N-substituted hydroxyprolinol phosphoramidites for preparation of combinatorial libraries. Tetrahedron Lett. 1994, 35, 9509–9512. [Google Scholar] [CrossRef]
- Greenspan, P.; Mayer, E.P.; Fowler, S.D. Nile Red: A Selective Fluorescent Stain for Intracellular Lipid Droplets. J. Cell Biol. 1985, 100, 965–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bustamante, C.; Vesenka, J.; Tang, C.L.; Rees, W.; Guthold, M.; Keller, R. Circular DNA Molecules Imaged in Air by Scanning Force Microscopy. Biochemistry 1992, 31, 22–26. [Google Scholar] [CrossRef]
- Van der Vusse, G.J. Albumin as Fatty Acid Transporter. Drug Metab. Pharmacokinet. 2009, 24, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Sjöback, R.; Nygren, J.; Kubista, M. Absorption and fluorescence properties of fluorescein. Spectrochim. Acta A 1995, 51, L7–L21. [Google Scholar] [CrossRef]
- Zhegalova, N.G.; He, S.; Zhou, H.; Kim, D.M.; Berezin, M.Y. Minimization of self-quenching fluorescence on dyes conjugated to biomolecules with multiple labeling sites via asymmetrically charged NIR fluorophores. Contrast Media Mol. Imaging 2014, 9, 355–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuang, X.; Ha, T.; Kim, H.D.; Centner, T.; Labeit, S.; Chu, S. Fluorescence quenching: A tool for single-molecule protein-folding study. Proc. Natl. Acad. Sci. USA 2000, 97, 14241–14244. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.W.; Park, J.-H.; Hong, J.-I. Self-quenching Mechanism: The Influence of Quencher and Spacer on Quencher-fluorescein Probes. Bull. Korean Chem. Soc. 2007, 28, 1221–1223. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.F.; Knutson, J.R. Mechanism of Fluorescence Concentration Quenching of Carboxyfluorescein in Liposomes: Energy Transfer to Nonfluorescent Dimers. Anal. Biochem. 1988, 172, 61–77. [Google Scholar] [CrossRef]
- Rupich, N.; Chiuman, W.; Nutiu, R.; Mei, S.; Flora, K.K.; Li, Y.; Brennan, J.D. Quenching of Fluorophore-Labeled DNA Oligonucleotides by Divalent Metal Ions: Implications for Selection, Design, and Applications of Signaling Aptamers and Signaling Deoxyribozymes. J. Am. Chem. Soc. 2006, 128, 780–790. [Google Scholar] [CrossRef]
- Torimura, M.; Kurata, S.; Yamada, K.; Yokomaku, T.; Kamagata, Y.; Kanagawa, T.; Kurane, R. Fluorescence-Quenching Phenomenon by Photoinduced Electron Transfer between a Fluorescent Dye and a Nucleotide Base. Anal. Sci. 2001, 17, 155–160. [Google Scholar] [CrossRef] [Green Version]
- Villard, P.-H.; Barlesi, F.; Armand, M.; Dao, T.-M.-A.; Pascussi, G.-M.; Fouchier, F.; Champion, S.; Dufour, C.; Giniès, C.; Khalil, A.; et al. CYP1A1 Induction in the Colon by Serum: Involvement of the PPARα Pathway and Evidence for a New Specific Human PPREα Site. PLoS ONE 2011, 6, e14629. [Google Scholar] [CrossRef] [PubMed]
- Vialet, B.; Gissot, A.; Delzor, R.; Barthélémy, P. Controlling G-quadruplex formation via lipid modification of oligonucleotide sequences. Chem. Commun. 2017, 53, 11560–11563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Moynihan, K.D.; Zheng, Y.; Szeto, G.L.; Li, A.V.; Huang, B.; Van Egeren, D.S.; Park, C.; Irvine, D.G. Structure-based Programming of Lymph Node Targeting in Molecular Vaccines. Nature 2014, 507, 519–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.; Chen, T.; Han, D.; You, M.; Peng, L.; Cansiz, S.; Zhu, G.; Li, C.; Xiong, X.; Jimenez, E.; et al. Engineering of Switchable Aptamer Micelle Flares for Molecular Imaging in Living Cells. ACS Nano 2013, 7, 5724–5731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilner, S.E.; Sparks, S.E.; Cowburn, D.; Girvin, M.E.; Levy, M. Controlling lipid micelle stability using oligonucleotide headgroups. J. Am. Chem. Soc. 2015, 137, 2171–2174. [Google Scholar] [CrossRef] [Green Version]
- Golombek, S.K.; May, J.-N.; Theek, B.; Appold, L.; Drude, N.; Kiessling, F.; Lammers, T. Tumor Targeting via EPR: Strategies to Enhance Patient Responses. Adv. Drug Deliv. Rev. 2018, 130, 17–38. [Google Scholar] [CrossRef]
- Danhier, F. To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? J. Control. Release 2016, 244, 108–121. [Google Scholar] [CrossRef]
- Crooke, S.T.; Vickers, T.A.; Liang, X.-H. Phosphorothioate modified oligonucleotide—protein interactions. Nucleic Acids Res. 2020, 48, 5235–5253. [Google Scholar] [CrossRef]
- Crooke, S.T.; Seth, P.P.; Vickers, T.A.; Liang, X.-H. The Interaction of Phosphorothioate-Containing RNA Targeted Drugs with Proteins Is a Critical Determinant of the Therapeutic Effects of These Agents. J. Am. Chem. Soc. 2020, 142, 14754–14771. [Google Scholar] [CrossRef]
- Akinc, A.; Zumbuehl, A.; Goldberg, M.; Leshchiner, E.S.; Busini, V.; Hossain, N.; Bacallado, S.A.; Nguyen, D.N.; Fuller, J.; Alvarez, R.; et al. A Combinatorial Library of Lipid-Like Materials for Delivery of RNAi Therapeutics. Nat. Biotechnol. 2008, 26, 561–569. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, T.; Jiang, C. Biomacromolecules as carriers in drug delivery and tissue engineering. Acta Pharm. Sin. B 2017, 8, 34–50. [Google Scholar] [CrossRef] [PubMed]
- Kratz, F. A clinical update of using albumin as a drug vehicle—A commentary. J. Control. Release 2014, 190, 331–336. [Google Scholar] [CrossRef] [PubMed]
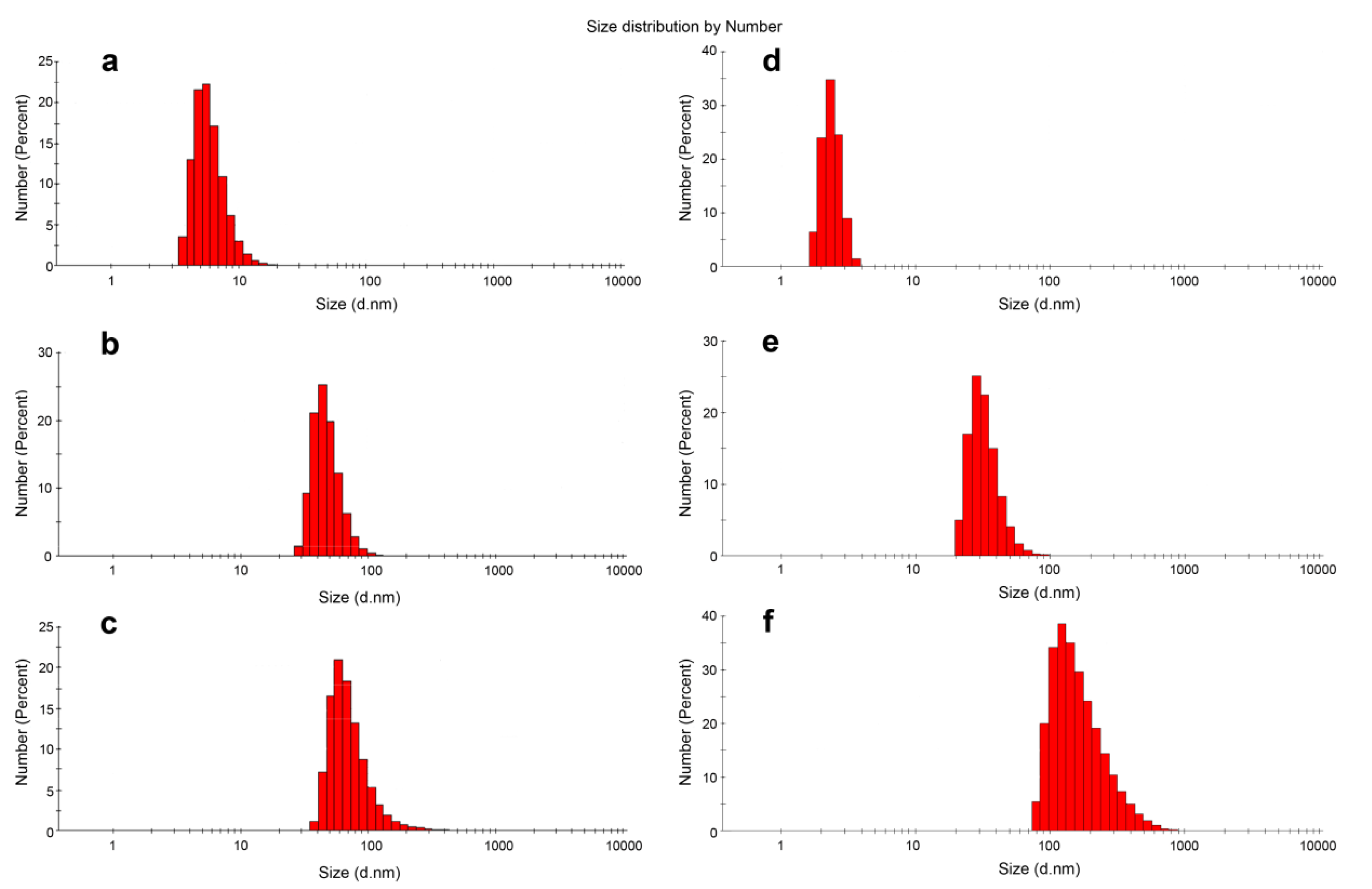
| DOC Type | Dh 1, nm | PDI |
|---|---|---|
| D-13 | 45.77 ± 13.03 | 0.109 |
| D-13PG | 65.30 ± 33.66 | 0.241 |
| D-17 | 32.67 ± 9.07 | 0.214 |
| D-17PG | 169.63 ± 96.40 | 0.225 |
| D-22PG | 80.76 ± 31.33 | 0.148 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlova, A.S.; Dovydenko, I.S.; Kupryushkin, M.S.; Grigor’eva, A.E.; Pyshnaya, I.A.; Pyshnyi, D.V. Amphiphilic “Like-A-Brush” Oligonucleotide Conjugates with Three Dodecyl Chains: Self-Assembly Features of Novel Scaffold Compounds for Nucleic Acids Delivery. Nanomaterials 2020, 10, 1948. https://doi.org/10.3390/nano10101948
Pavlova AS, Dovydenko IS, Kupryushkin MS, Grigor’eva AE, Pyshnaya IA, Pyshnyi DV. Amphiphilic “Like-A-Brush” Oligonucleotide Conjugates with Three Dodecyl Chains: Self-Assembly Features of Novel Scaffold Compounds for Nucleic Acids Delivery. Nanomaterials. 2020; 10(10):1948. https://doi.org/10.3390/nano10101948
Chicago/Turabian StylePavlova, Anna S., Ilya S. Dovydenko, Maxim S. Kupryushkin, Alina E. Grigor’eva, Inna A. Pyshnaya, and Dmitrii V. Pyshnyi. 2020. "Amphiphilic “Like-A-Brush” Oligonucleotide Conjugates with Three Dodecyl Chains: Self-Assembly Features of Novel Scaffold Compounds for Nucleic Acids Delivery" Nanomaterials 10, no. 10: 1948. https://doi.org/10.3390/nano10101948
APA StylePavlova, A. S., Dovydenko, I. S., Kupryushkin, M. S., Grigor’eva, A. E., Pyshnaya, I. A., & Pyshnyi, D. V. (2020). Amphiphilic “Like-A-Brush” Oligonucleotide Conjugates with Three Dodecyl Chains: Self-Assembly Features of Novel Scaffold Compounds for Nucleic Acids Delivery. Nanomaterials, 10(10), 1948. https://doi.org/10.3390/nano10101948