Synthesizing CuO/CeO2/ZnO Ternary Nano-Photocatalyst with Highly Effective Utilization of Photo-Excited Carriers under Sunlight
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of CuO/CeO2/ZnO Nano-Composites
2.2. Characterization
2.3. Photocatalytic Experiments
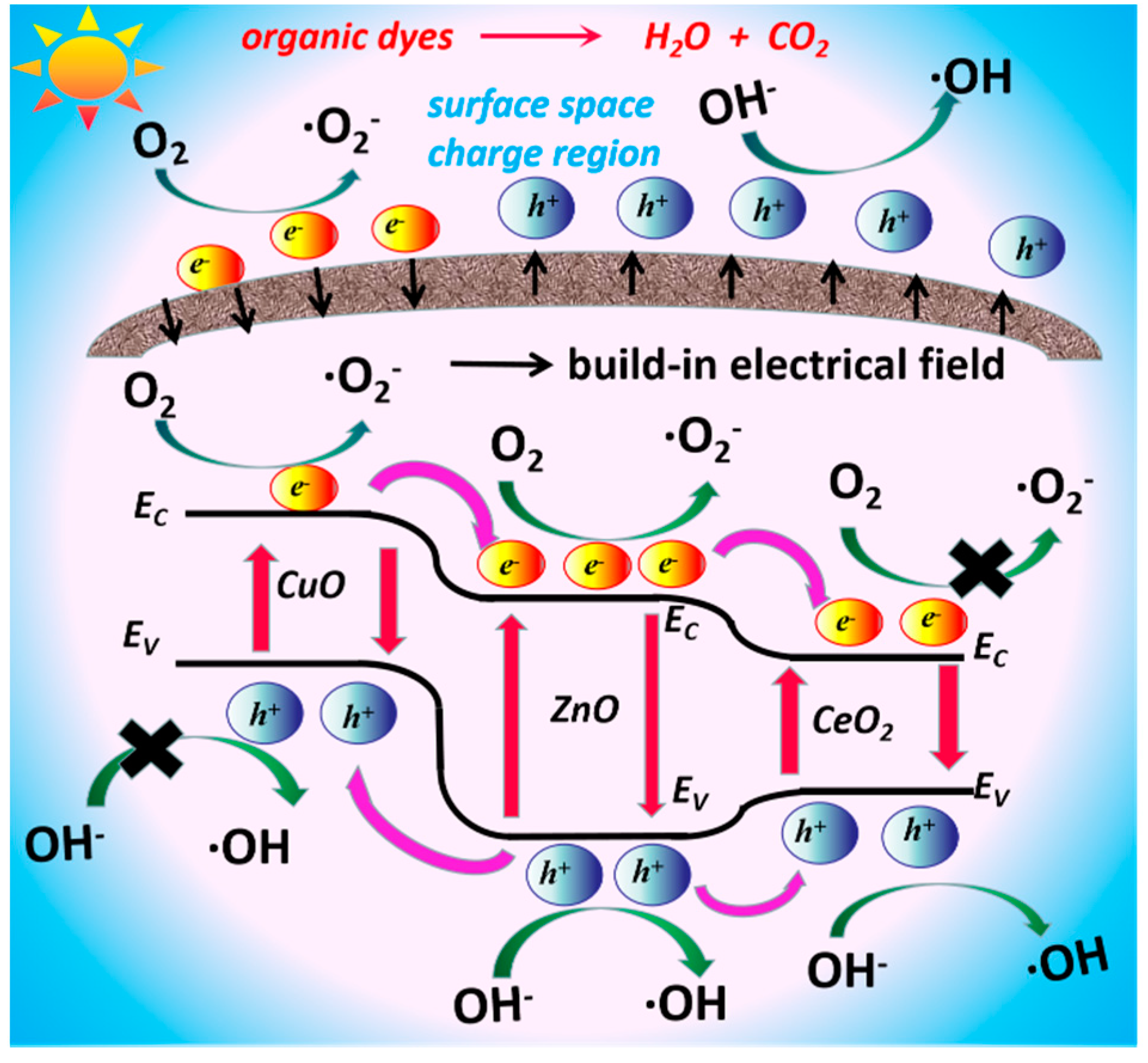
3. Results and Discussion
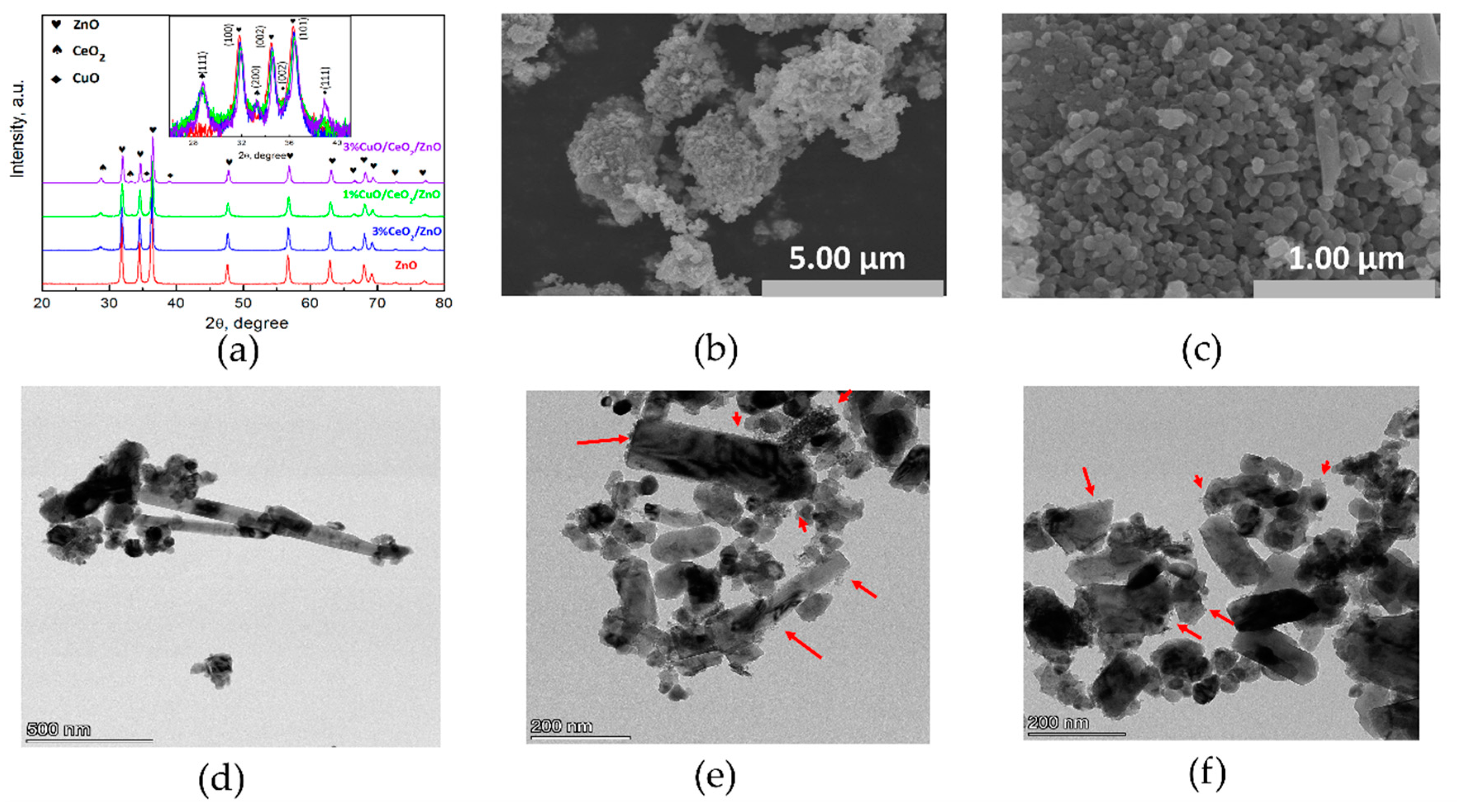
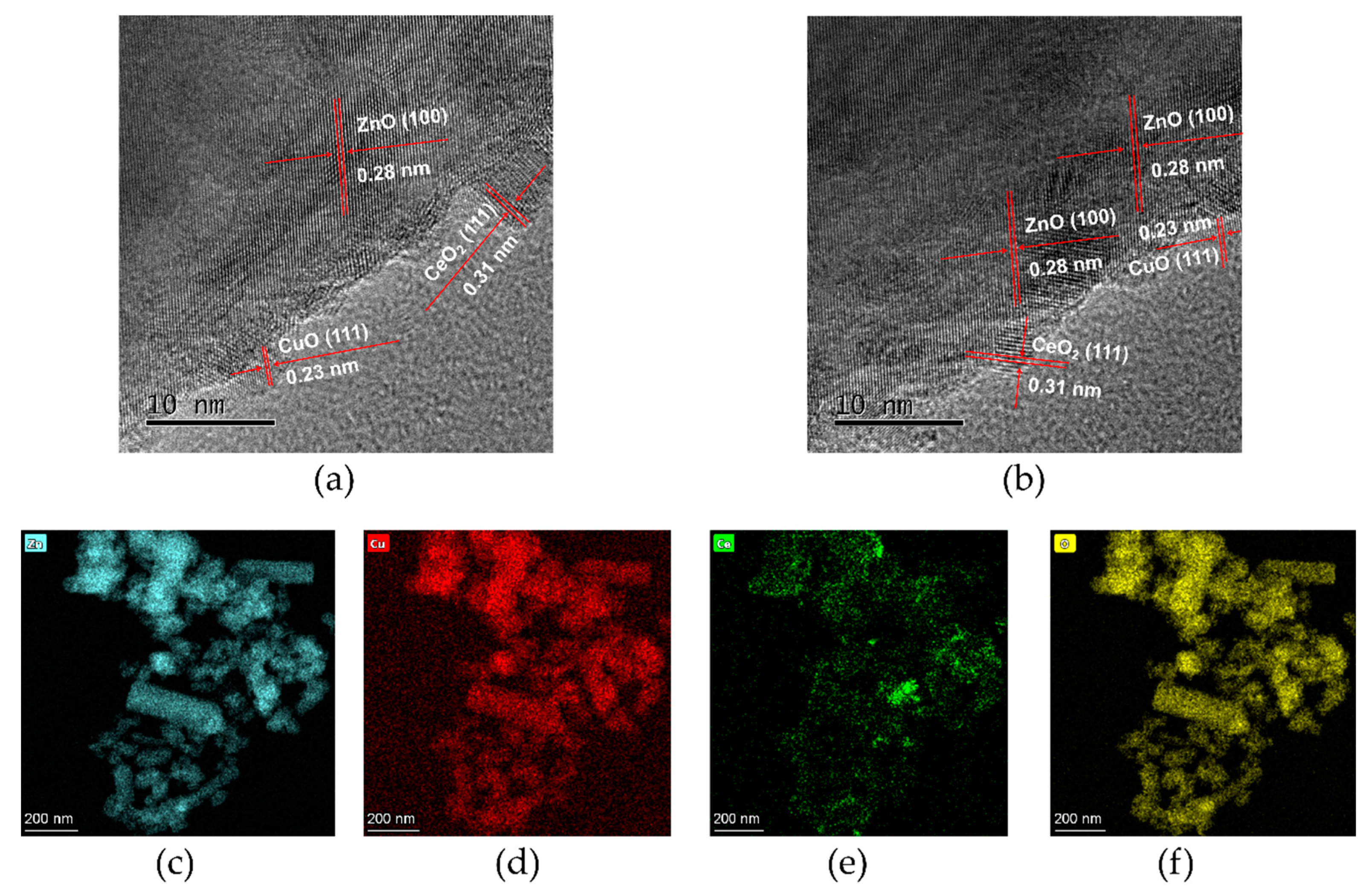
3.1. Structural and Morphological Study
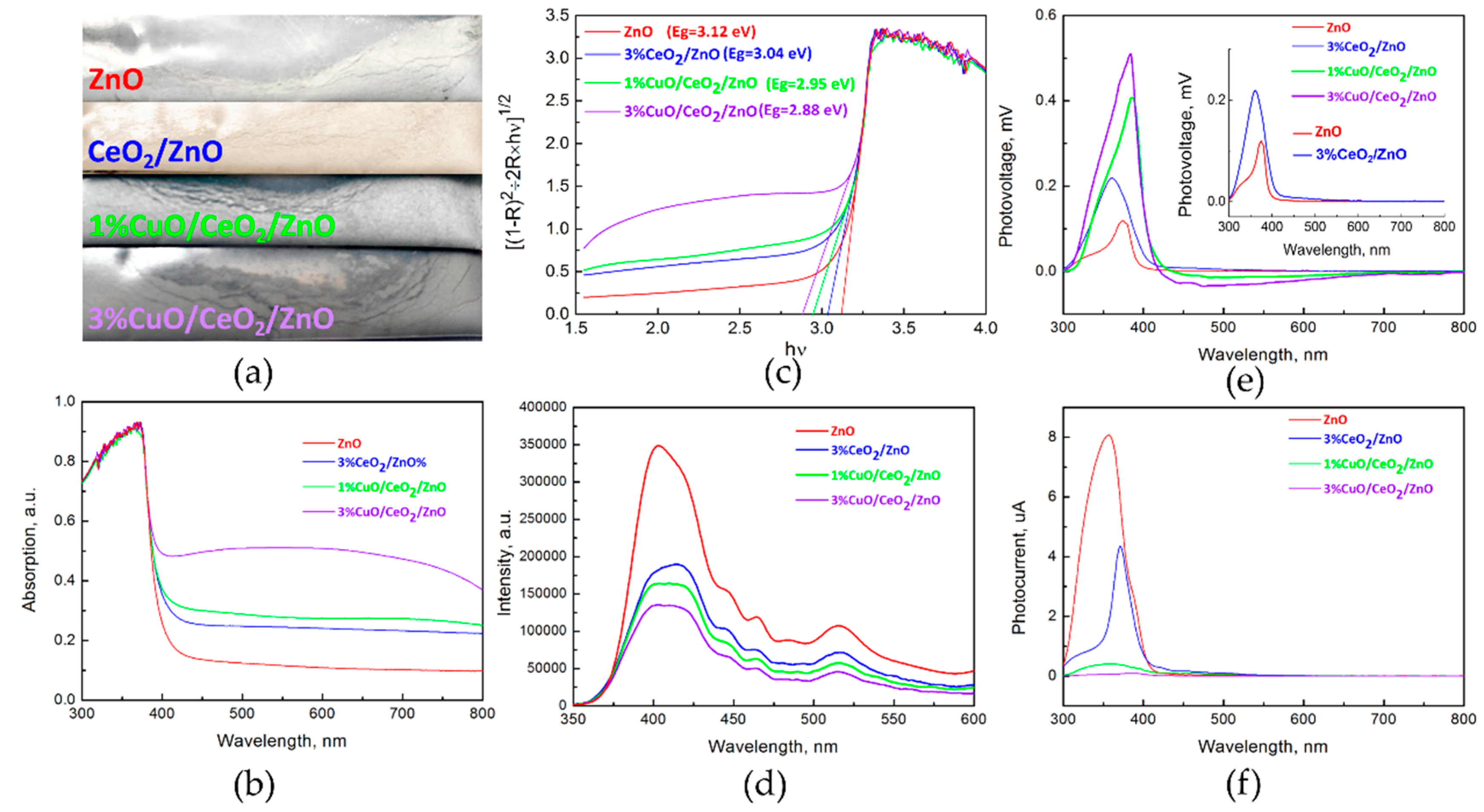
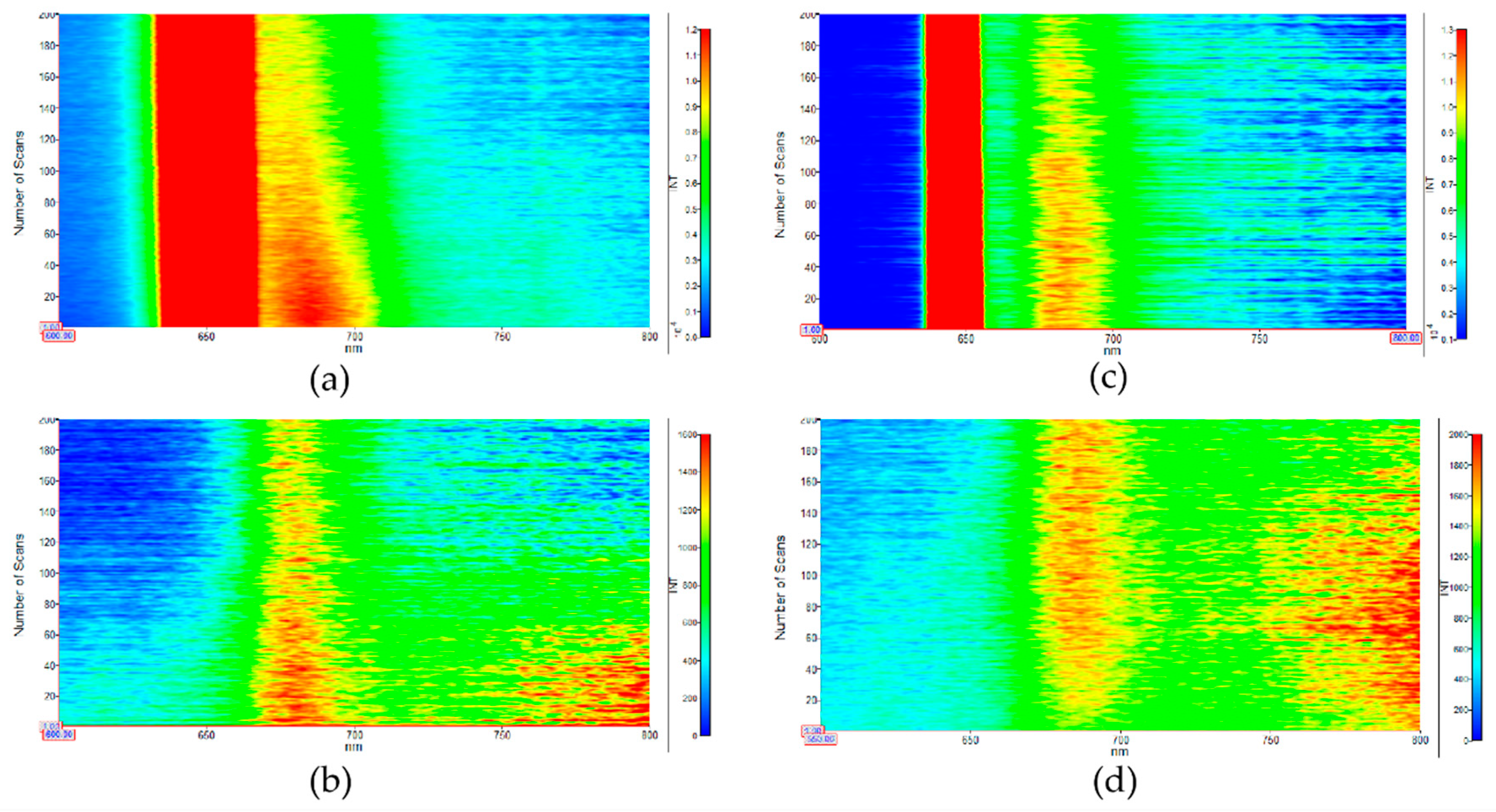
3.2. Optical Study
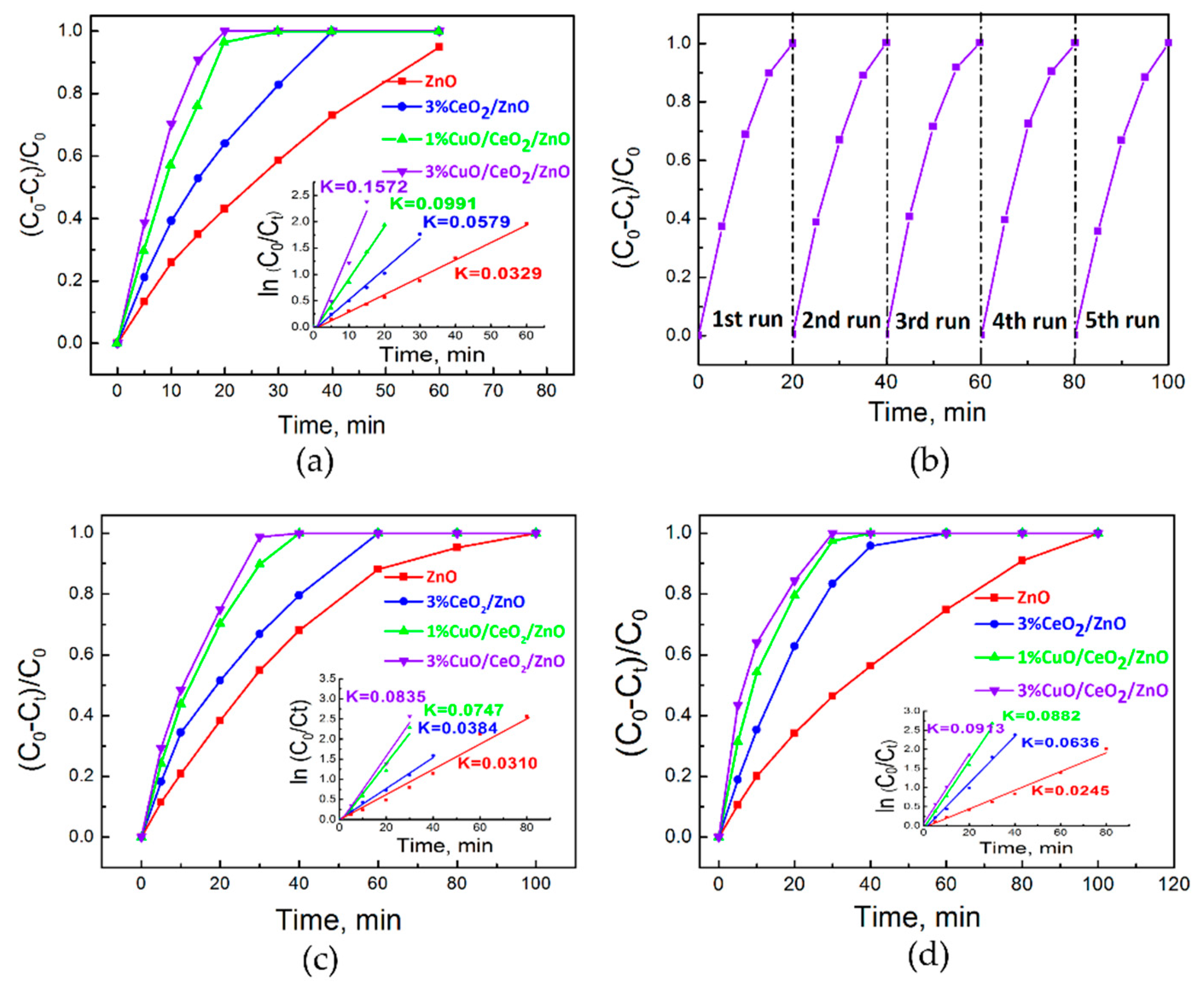
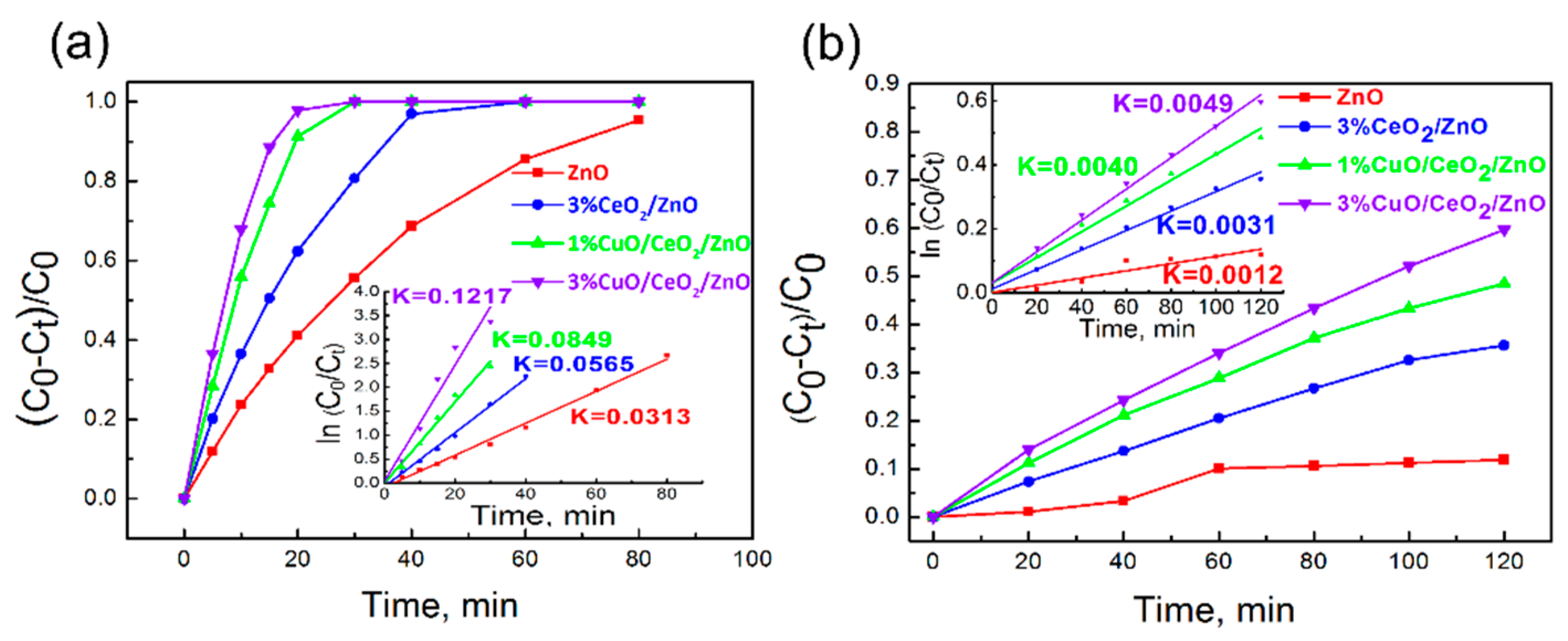
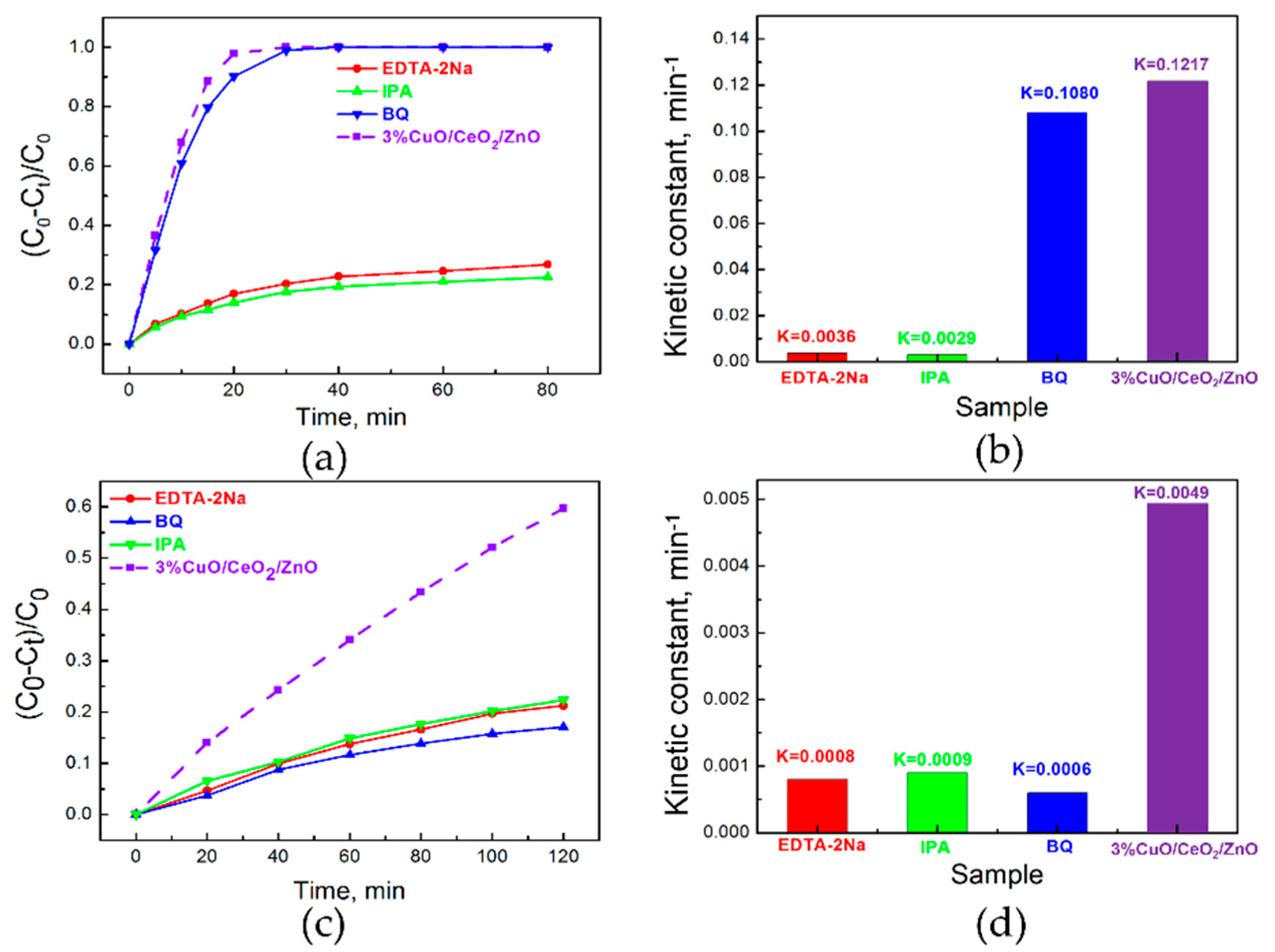
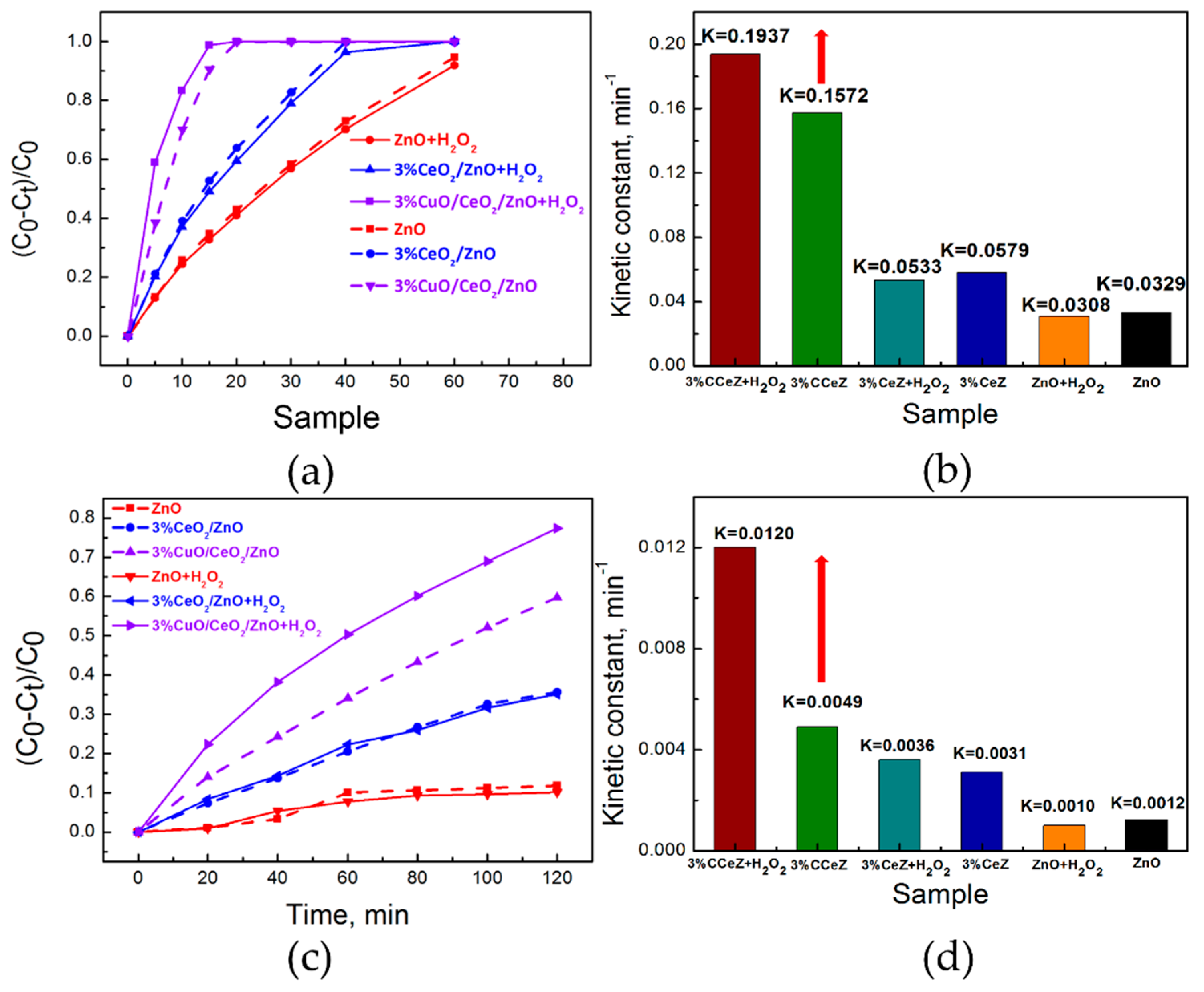
3.3. Photocatalytic Activity Study
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, X.B.; Liu, L.; Yu, P.Y.; Miao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.X.; Li, Y.P.; Pan, X.Y.; Cortie, D.; Huang, X.T.; Yi, Z.G. Photocatalytic oxidation of methane over silver decorated zinc oxide nanocatalysts. Nat. Commun. 2016, 7, 12273. [Google Scholar] [CrossRef] [PubMed]
- Schreier, M.; Héroguel, F.; Steier, L.; Ahmad, S.; Luterbacher, J.S.; Mayer, M.T.; Luo, J.S.; Grätzel, M. Solar conversion of CO2 to CO using Earth-abundant electrocatalysts prepared by atomic layer modification of CuO. Nat. Energy 2017, 2, 17087. [Google Scholar] [CrossRef]
- Zhang, Q.P.; Xu, M.; You, B.; Zhang, Q.; Yuan, H.; Ostrikov, K. Oxygen Vacancy-Mediated ZnO Nanoparticle Photocatalyst for Degradation of Methylene Blue. Appl. Sci. 2018, 8, 353. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, N.; Gao, C.; Xiong, Y.J. Defect engineering in photocatalytic materials. Nano Energy 2018, 53, 296–336. [Google Scholar] [CrossRef]
- Tao, Q.; Huang, X.; Bi, J.; Wei, R.; Xie, C.; Zhou, Y.; Yu, L.; Hao, H.; Wang, J. Aerobic Oil-Phase Cyclic Magnetic Adsorption to Synthesize 1D Fe2O3@TiO2 Nanotube Composites for Enhanced Visible-Light Photocatalytic Degradation. Nanomaterials 2020, 10, 1345. [Google Scholar] [CrossRef]
- Šojić Merkulov, D.; Lazarević, M.; Djordjevic, A.; Náfrádi, M.; Alapi, T.; Putnik, P.; Rakočević, Z.; Novaković, M.; Miljević, B.; Bognár, S.; et al. Potential of TiO2 with Various Au Nanoparticles for Catalyzing Mesotrione Removal from Wastewaters under Sunlight. Nanomaterials 2020, 10, 1591. [Google Scholar] [CrossRef]
- Ren, C.L.; Yang, B.F.; Wu, M.; Xu, J.; Fu, Z.P.; Lv, Y.; Guo, T.; Zhao, Y.X.; Zhu, C.Q. Ag-loaded ZnO materials for photocatalytic water treatment. Chem. Eng. J. 2017, 315, 95–102. [Google Scholar]
- Wang, P.; Xie, T.F.; Li, H.Y.; Peng, L.; Zhang, Y.; Wu, T.S.; Pang, S.; Zhao, Y.F.; Wang, D.J. Synthesis and plasmon-induced charge-transfer properties of monodisperse gold-doped titania microspheres. Chem. Eur. J. 2009, 15, 4366–4372. [Google Scholar] [CrossRef]
- Liu, Y.T.; Zhang, Q.P.; Xu, M.; Yuan, H.; Chen, Y.; Zhang, J.X.; Luo, K.Y.; Zhang, J.Q.; You, B. Novel and efficient synthesis of Ag-ZnO nanoparticles for the sunlight-induced photocatalytic degradation. Appl. Surf. Sci. 2019, 476, 632–640. [Google Scholar] [CrossRef]
- Ren, S.T.; Wang, Y.Y.; Fan, G.H.; Gao, R.X.; Liu, W.J. Sandwiched ZnO@Au@CdS nanorod arrays with enhanced visible-light-driven photocatalytical performance. Nanotechnology 2017, 28, 465403. [Google Scholar] [CrossRef] [PubMed]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and Catalytic Applications of CeO2-Based Materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, A.D.; Perera, S.D.; Tan, K.; Chabal, Y.; Balkus, K.J. Synthesis, Characterization, and Photocatalytic Activity of Y-doped CeO2 nanorods. ACS Catal. 2014, 4, 577–584. [Google Scholar] [CrossRef]
- Qamar, M.T.; Aslam, M.; Ismail, I.M.I.; Salah, N.; Hameed, A. Synthesis, Characterization, and Sunlight Mediated Photocatalytic Activity of CuO Coated ZnO for the Removal of Nitrophenols. ACS. Appl. Mater. Interfaces 2015, 7, 8757–8769. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Chen, Y.; Hu, W.Y.; Liu, Y.T.; Zhang, Q.P.; Yuan, H.; Wang, X.Y.; Zhang, J.X.; Luo, K.Y.; Li, J. Designed synthesis of microstructure and defect-controlled Cu-doped ZnO–Ag nanoparticles: Exploring high-efficiency sunlight-driven photocatalysts. J. Phys. D Appl. Phys. 2019, 53, 025106. [Google Scholar] [CrossRef]
- Zhou, G.; Xu, X.Y.; Ding, T.; Feng, B.; Bao, Z.J.; Hu, J.G. Well-steered charge-carrier transfer in 3D branched CuxO/ZnO@Au heterostructures for efficient photocatalytic hydrogen evolution. ACS Appl. Mater. Interfaces 2015, 7, 26819–26827. [Google Scholar] [CrossRef]
- Subhan, M.A.; Uddin, N.; Sarker, P.; Azad, A.K.; Begum, K. Photoluminescence, photocatalytic and antibacterial activities of CeO2·CuO·ZnO nanocomposite fabricated by co-precipitation method. Spectrochim. Acta A 2015, 149, 839–850. [Google Scholar] [CrossRef]
- Shi, Z.S.; Tan, Q.Q.; Wu, D.F. Enhanced CO2 hydrogenation to methanol over TiO2 nanotubes-supported CuO-ZnO-CeO2 catalyst. Appl. Catal. A Gen. 2019, 581, 58–66. [Google Scholar] [CrossRef]
- Patnaik, S.; Swaina, G.; Parida, K.M. Highly efficient charge transfer through a double Z-scheme mechanism by a Cu-promoted MoO3/g-C3N4 hybrid nanocomposite with superior electrochemical and photocatalytic performance. Nanoscale 2018, 10, 5950–5964. [Google Scholar] [CrossRef]
- Shang, Y.Y.; Chen, X.; Liu, W.W.; Tan, P.F.; Chen, H.Y.; Wu, L.D.; Ma, C.; Xiong, X.; Pan, J. Photocorrosion inhibition and high-efficiency photoactivity of porous g-C3N4/Ag2CrO4 composites by simple microemulsion-assisted co-precipitation method. Appl. Catal. B Environ. 2017, 204, 78–88. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Yang, X.Y.; Zhang, P.; Liu, D.; Wang, Y.L.; Jin, Z.Z.; Mamba, B.B.; Kuvarega, A.T.; Gui, J.Z. One-step hydrothermal fabrication of SrMoO4/MoS2 composites with strong interfacial contacts for efficient photoreduction removal of Cr(vi). CrystEngComm 2020, 22, 4489–4499. [Google Scholar] [CrossRef]
- Wu, Y.P.; Zhu, J.H.; Huang, L. A review of three-dimensional graphene-based materials: Synthesis and applications to energy conversion/storage and environment. Carbon 2019, 143, 610–640. [Google Scholar] [CrossRef]
- Mclaren, A.; Valdes-Solis, T.; Li, G.Q.; Tsang, S.C. Shape and size effects of ZnO nanocrystals on photocatalytic activity. J. Am. Chem. Soc. 2009, 131, 12540–12541. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Das, P.P.; Maity, S.; Ghosh, P.; Devia, P.S. Solution grown ZnO rods: Synthesis, characterization and defect mediated photocatalytic activity. Appl. Catal. B Environ. 2015, 165, 128–138. [Google Scholar] [CrossRef]
- Kukovecz, A.; Kordas, K.; Kiss, J.; Konya, Z. Atomic scale characterization and surface chemistry of metal modified titanate nanotubes and nanowires. Surf. Sci. Rep. 2016, 71, 473–546. [Google Scholar] [CrossRef]
- Issarapanacheewin, S.; Wetchakun, K.; Phanichphant, S.; Kangwansupamonkon, W.; Wetchakun, N. A novel CeO2/Bi2WO6 composite with highly enhanced photocatalytic activity. Mater. Lett. 2015, 156, 28–31. [Google Scholar] [CrossRef]
- Jiang, T.F.; Xie, T.F.; Zhang, Y.; Chen, L.P.; Peng, L.L.; Li, H.Y.; Wang, D.J. Photoinduced charge transfer in ZnO/Cu2O heterostructure films studied by surface photovoltage technique. Phys. Chem. Chem. Phys. 2010, 12, 15476–15481. [Google Scholar] [CrossRef]
- Wetchakun, N.; Chaiwichain, S.; Inceesungvorn, B.; Pingmuang, K.; Phanichphant, S.; Minett, A.I.; Chen, J. BiVO4/CeO2 Nanocomposites with High Visible-Light-Induced Photocatalytic Activity. ACS Appl. Mater. Interfaces 2012, 4, 3718–3723. [Google Scholar] [CrossRef]
- Yu, J.Y.; Zhuang, S.D.; Xu, X.Y.; Zhu, W.C.; Fenga, B.; Hu, J.G. Photogenerated electron reservoir in hetero-p–n CuO–ZnO nanocomposite device for visible-light-driven photocatalytic reduction of aqueous Cr(vi). J. Mater. Chem. A 2015, 3, 1199–1207. [Google Scholar] [CrossRef]
- Toe, C.Y.; Zheng, Z.K.; Wu, H.; Scott, J.; Amal, R.; Ng, Y.H. Photocorrosion of Cuprous Oxide in Hydrogen Production: Rationalising Self-Oxidation or Self-Reduction. Angew. Chem. Int. Ed. Engl. 2018, 57, 13613–13617. [Google Scholar] [CrossRef]
| Samples | Zn(CH3COO)2·2H2O/g | Ce(NO3)3·6H2O/g | Cu(CH3COO)2·H2O/g |
|---|---|---|---|
| ZnO | 4.4343 | 0.0000 | 0.0000 |
| 3%CeO2/ZnO | 4.4343 | 0.2667 | 0.0000 |
| 1%CuO/CeO2/ZnO | 4.4343 | 0.2667 | 0.0403 |
| 3%CuO/CeO2/ZnO | 4.4343 | 0.2667 | 0.1210 |
| Samples | 2θ (100)/° | d(100)/nm | d(002)/nm | d(101)/nm | a/nm | c/nm | D/nm | Cell Vol/Å3 |
|---|---|---|---|---|---|---|---|---|
| ZnO | 31.84 | 0.2808 | 0.2598 | 0.2473 | 0.3243 | 0.5448 | 36.4 | 49.6 |
| 3%CeO2/ZnO | 31.87 | 0.2805 | 0.2595 | 0.2471 | 0.3239 | 0.5439 | 32.7 | 49.4 |
| 1%CuO/CeO2/ZnO | 31.92 | 0.2797 | 0.2590 | 0.2465 | 0.3230 | 0.5429 | 30.3 | 49.0 |
| 3%CuO/CeO2/ZnO | 31.95 | 0.2795 | 0.2585 | 0.2459 | 0.3227 | 0.5416 | 28.6 | 48.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, K.; Li, J.; Hu, W.; Li, H.; Zhang, Q.; Yuan, H.; Yu, F.; Xu, M.; Xu, S. Synthesizing CuO/CeO2/ZnO Ternary Nano-Photocatalyst with Highly Effective Utilization of Photo-Excited Carriers under Sunlight. Nanomaterials 2020, 10, 1946. https://doi.org/10.3390/nano10101946
Luo K, Li J, Hu W, Li H, Zhang Q, Yuan H, Yu F, Xu M, Xu S. Synthesizing CuO/CeO2/ZnO Ternary Nano-Photocatalyst with Highly Effective Utilization of Photo-Excited Carriers under Sunlight. Nanomaterials. 2020; 10(10):1946. https://doi.org/10.3390/nano10101946
Chicago/Turabian StyleLuo, Kaiyi, Jing Li, Wenyu Hu, Han Li, Qiuping Zhang, Huan Yuan, Fei Yu, Ming Xu, and Shuyan Xu. 2020. "Synthesizing CuO/CeO2/ZnO Ternary Nano-Photocatalyst with Highly Effective Utilization of Photo-Excited Carriers under Sunlight" Nanomaterials 10, no. 10: 1946. https://doi.org/10.3390/nano10101946
APA StyleLuo, K., Li, J., Hu, W., Li, H., Zhang, Q., Yuan, H., Yu, F., Xu, M., & Xu, S. (2020). Synthesizing CuO/CeO2/ZnO Ternary Nano-Photocatalyst with Highly Effective Utilization of Photo-Excited Carriers under Sunlight. Nanomaterials, 10(10), 1946. https://doi.org/10.3390/nano10101946





