Biocompatibility and Antibacterial Properties of ZnO-Incorporated Anodic Oxide Coatings on TiZrNb Alloy
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis and Characteerization of ZnO Nanoparticles
2.3. Plasma Electrolytic Oxidation (PEO)
2.4. Surface Analysis
2.5. Antibacterial Assessment
2.6. Cell Culture
3. Results and Discussion
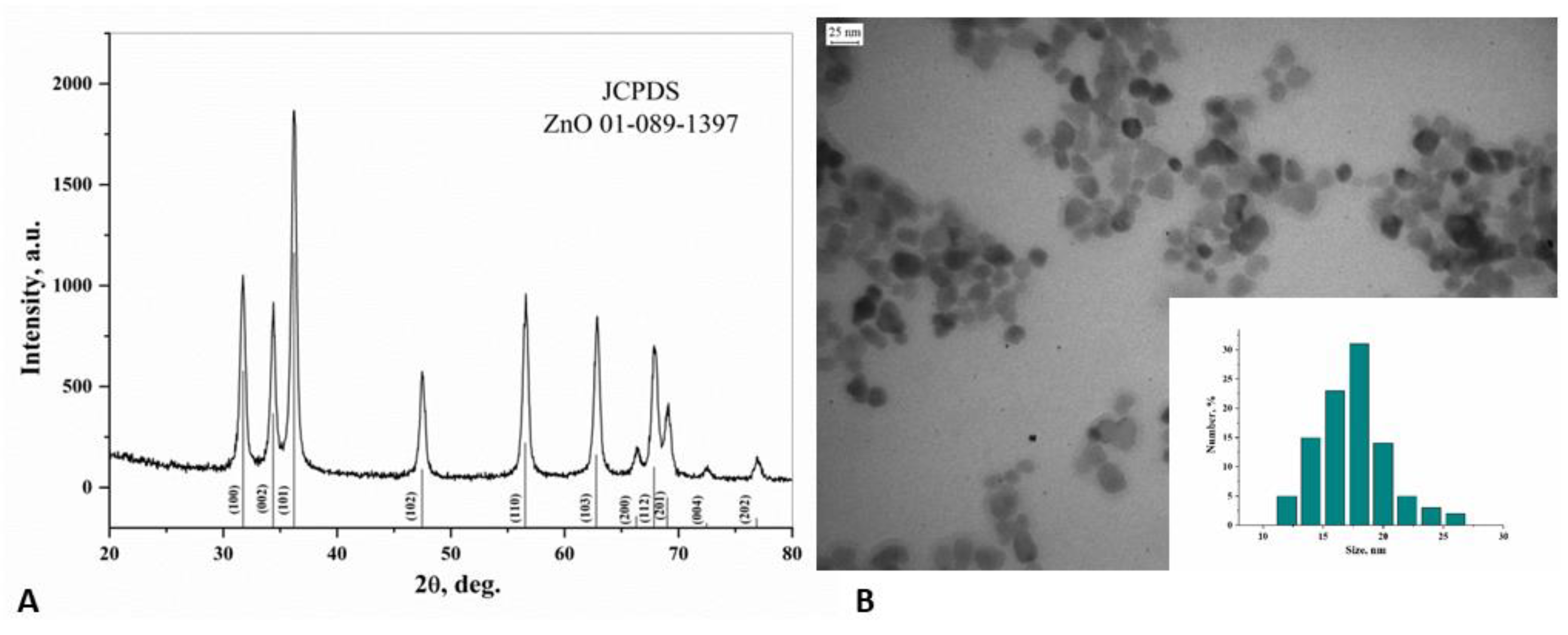
3.1. ZnO NPs Characterization
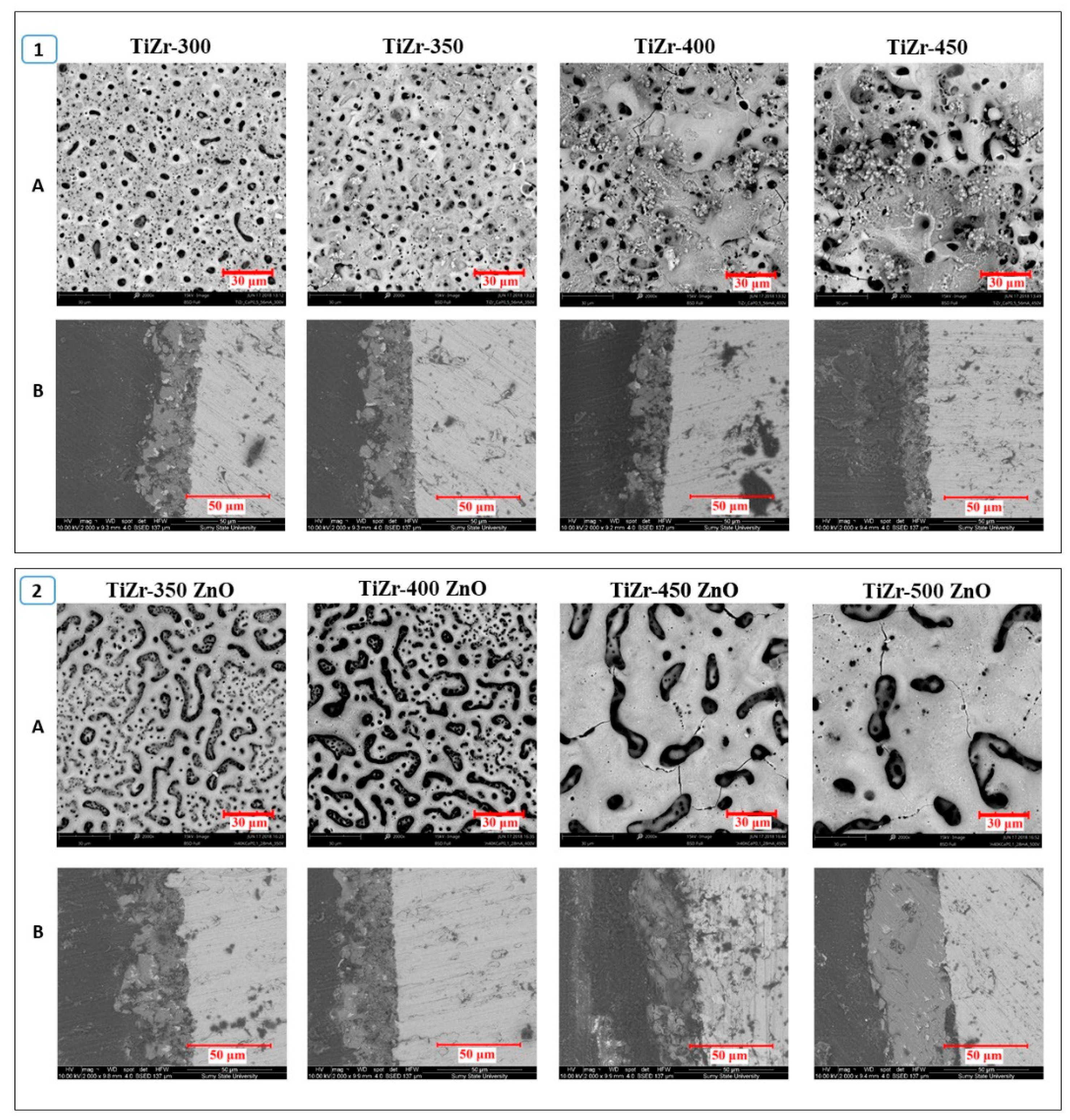
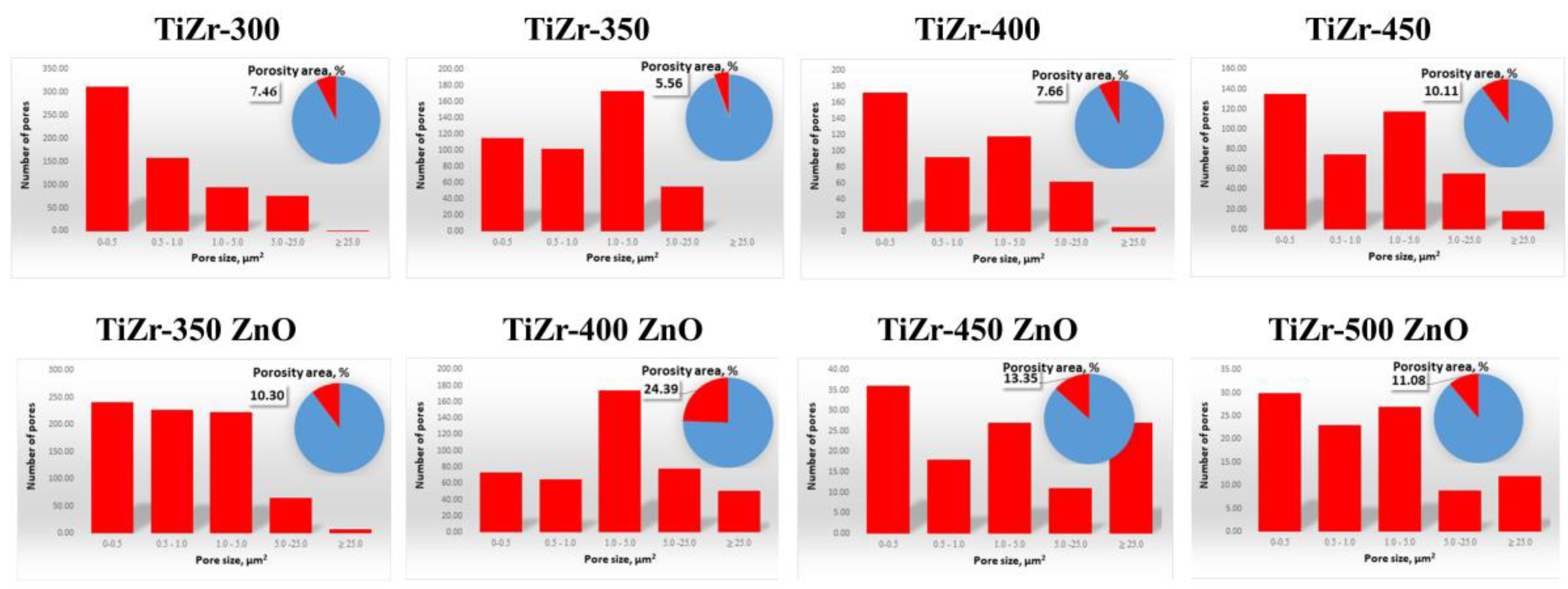
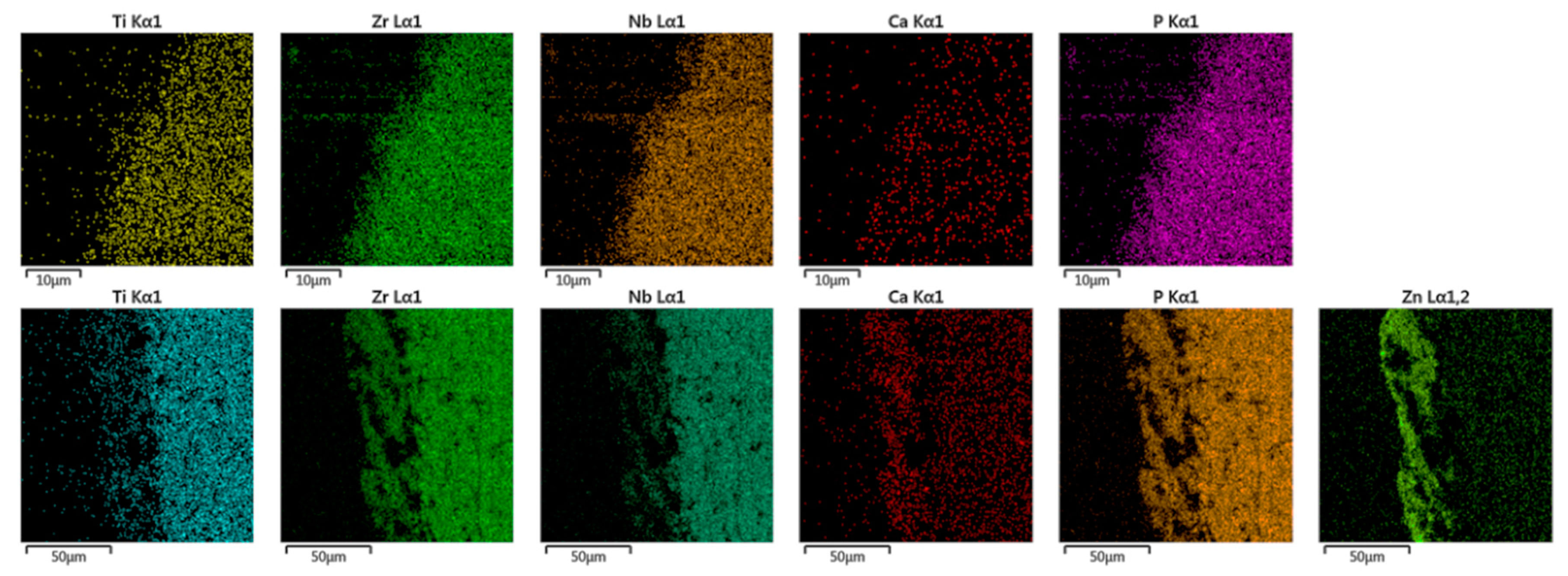
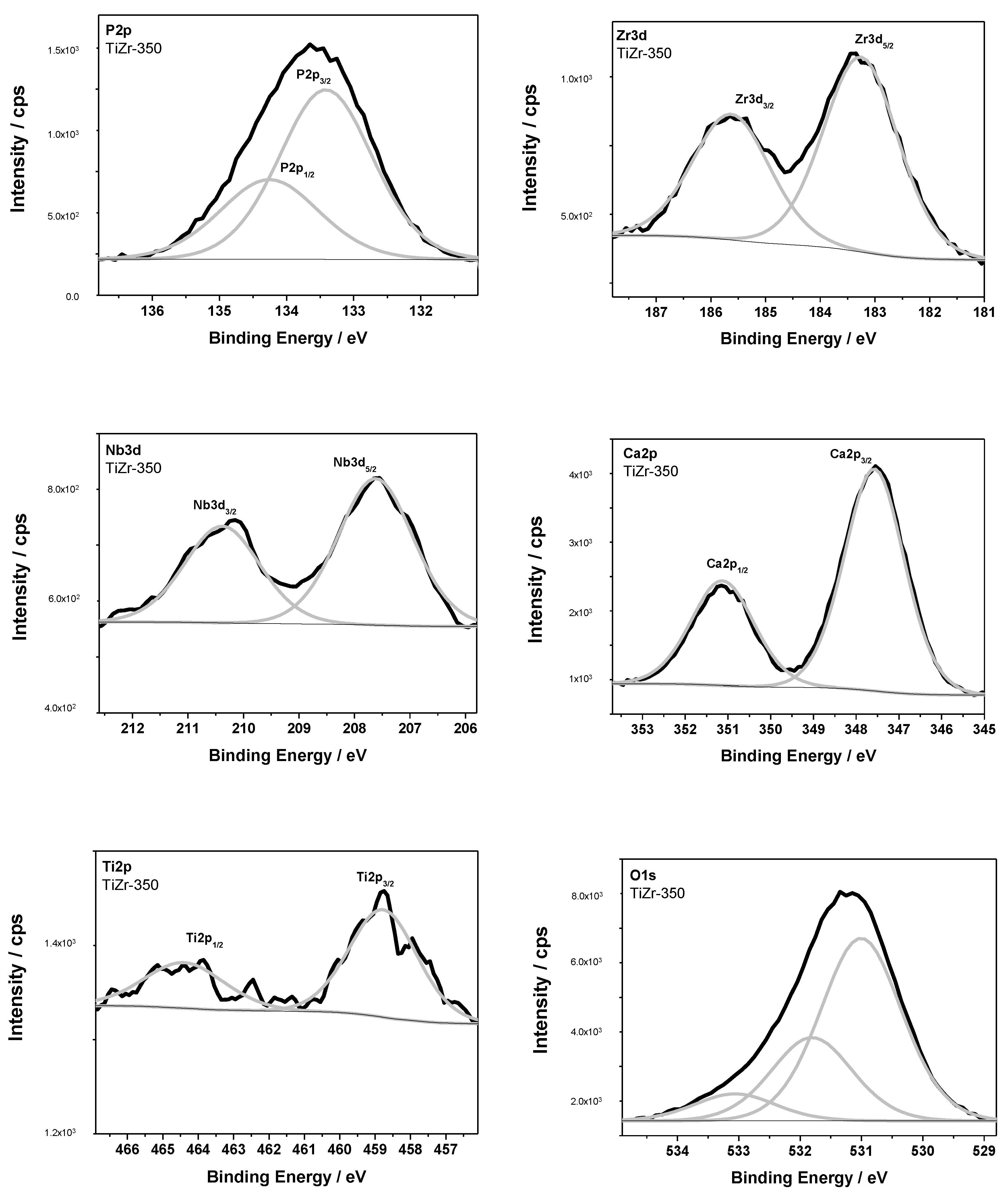
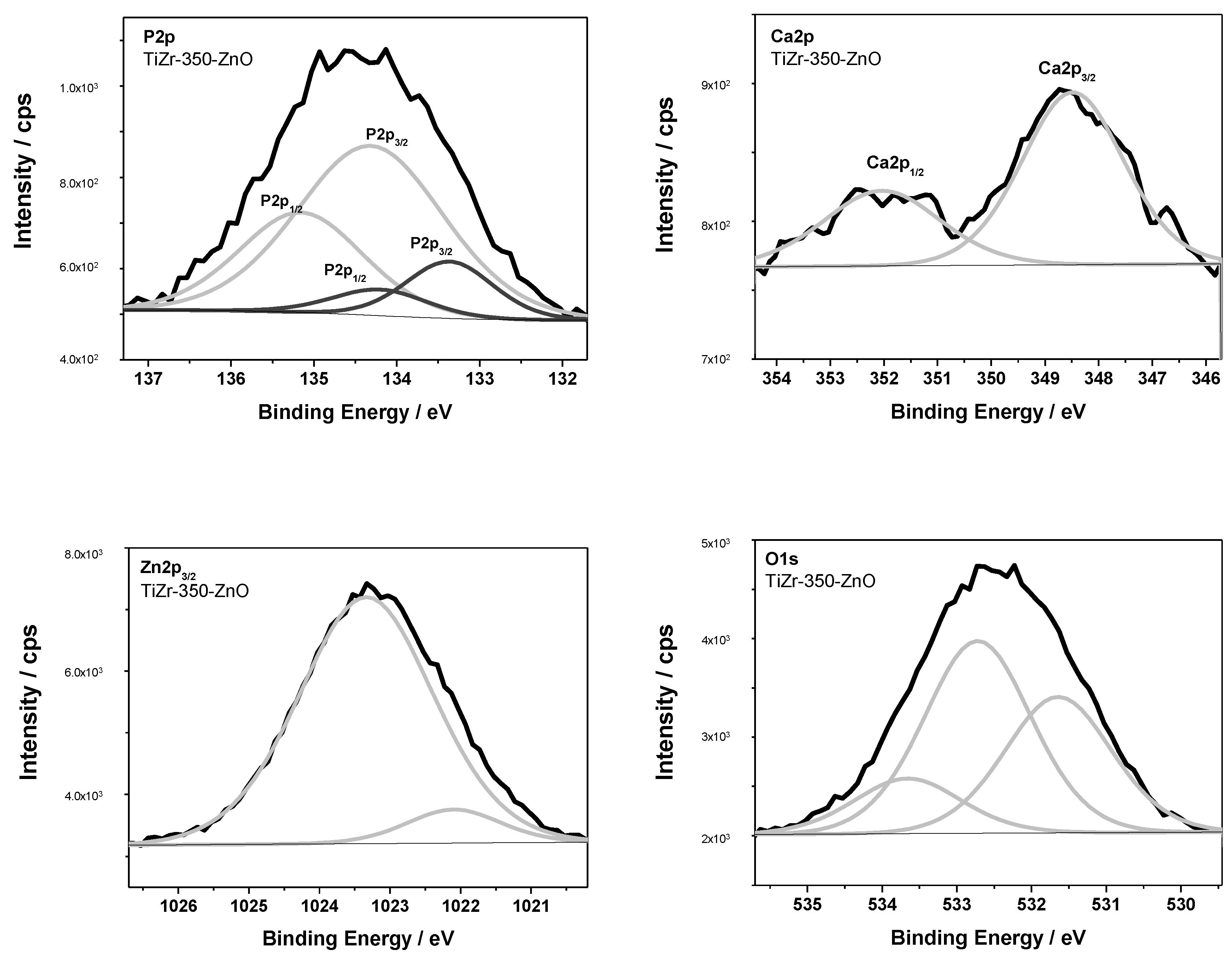
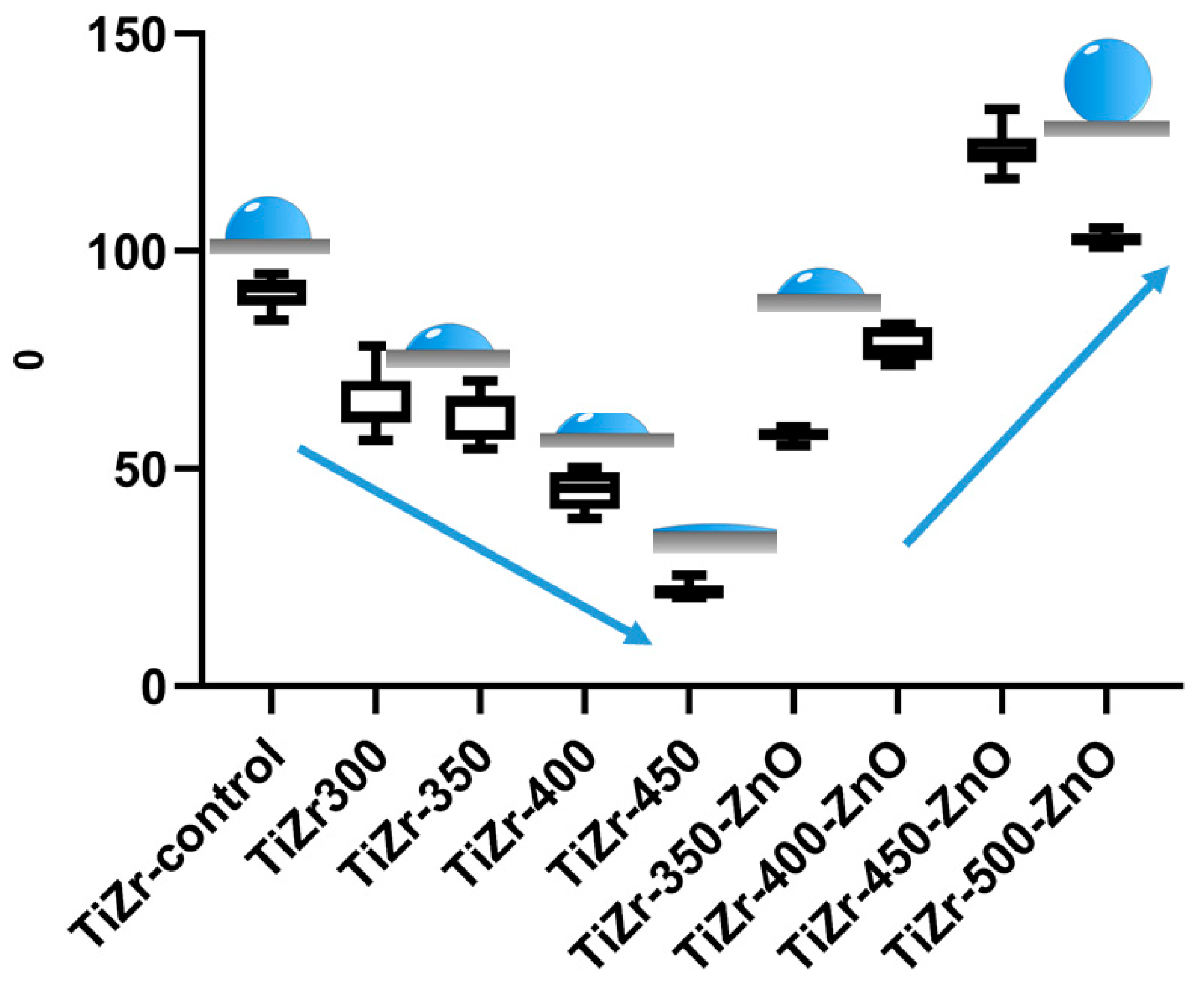
3.2. Surface Analysis of PEO Ceramic Coatings
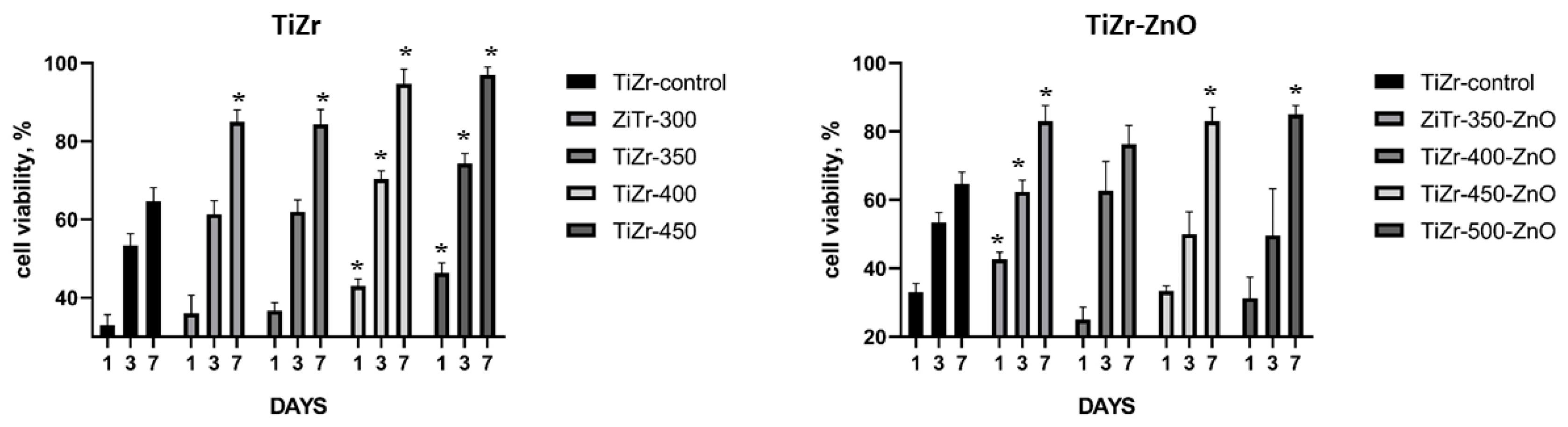
3.3. Cell Culture
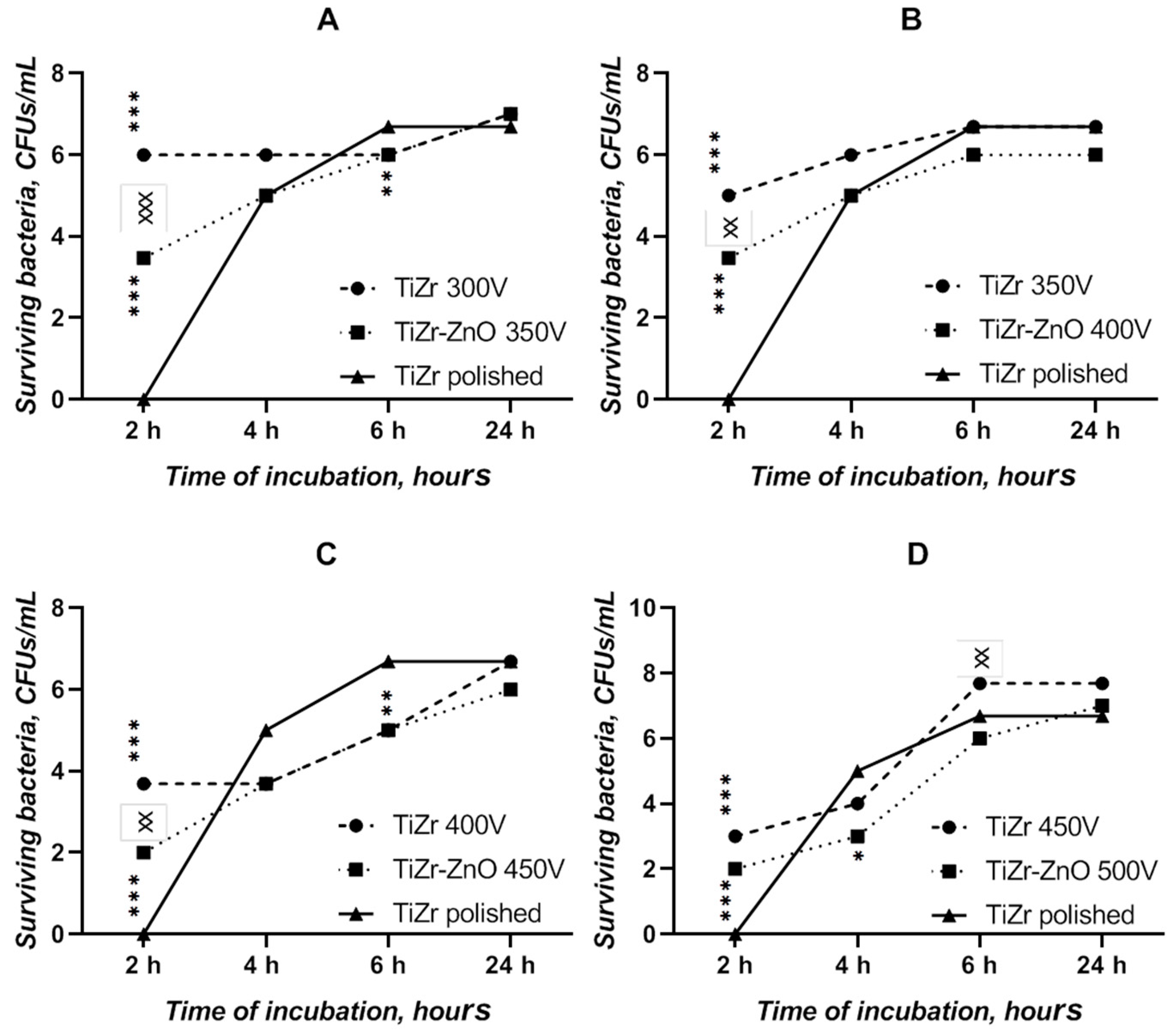
3.4. Bacterial Adhesion Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Piotrowski, B.; Baptista, A.A.; Patoor, E.; Bravetti, P.; Eberhardt, A.; Laheurte, P. Interaction of bone-dental implant with new ultra low modulus alloy using a numerical approach. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 1, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Isaksson, P.; Ferguson, S.J.; Persson, C. Young’s modulus of trabecular bone at the tissue level: A review. Acta Biomater. 2018, 78, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gepreel, M.A.-H.; Niinomi, M. Biocompatibility of Ti-alloys for long-term implantation. J. Mech. Behav. Biomed. Mater. 2013, 20, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, S.; Tsoi, J.K.H.; Matinlinna, J.P. Binary titanium alloys as dental implant materials-a review. Regen. Biomat. 2017, 4, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Maity, T.; Balcı, Ö.; Gammer, C.; Ivanov, E.; Eckert, J.; Prashanth, K.G. High pressure torsion induced lowering of Young’s modulus in high strength TNZT alloy for bio-implant applications. J. Mech. Behav. Biomed. Mater. 2020, 103839. [Google Scholar] [CrossRef]
- Li, J.; Jansen, J.A.; Walboomers, X.F.; van den Beucken, J.J. Mechanical aspects of dental implants and osseointegration: A narrative review. J. Mech. Behav. Biomed. Mater. 2020, 103. [Google Scholar] [CrossRef]
- Nune, K.C.; Misra, R.D.K.; Li, S.J.; Hao, Y.L.; Yang, R. Osteoblast cellular activity on low elastic modulus Ti–24Nb–4Zr–8Sn alloy. Dent. Mater. 2017, 33, 152–165. [Google Scholar] [CrossRef]
- Mishchenko, O.; Ovchynnykov, O.; Kapustian, O.; Pogorielov, M. New Zr-Ti-Nb alloy for medical application: Development, chemical and mechanical properties, and biocompatibility. Materials 2020, 13, 1306. [Google Scholar] [CrossRef]
- Almas, K.; Smith, S.; Kutkut, A. What is the Best Micro and Macro Dental Implant Topography? Dent. Clin. N. Am. 2019, 63, 447–460. [Google Scholar] [CrossRef]
- Pierre, C.; Bertrand, G.; Rey, C.; Benhamou, O.; Combes, C. Calcium phosphate coatings elaborated by the soaking process on titanium dental implants: Surface preparation, processing and physical–chemical characterization. Dent. Mater. 2019, 35, e25–e35. [Google Scholar] [CrossRef]
- Füg, A.; Ulm, C.; Tangl, S.; Vasak, C.; Gruber, R.; Watzek, G. Long-term effects of magnetron-sputtered calcium phosphate coating on osseointegration of dental implants in non-human primates. Clin. Oral Implant. Res. 2009, 20, 183–188. [Google Scholar] [CrossRef]
- Choi, S.-H.; Jang, Y.S.; Jang, J.H.; Bae, T.S.; Lee, S.J.; Lee, M.H. Enhanced antibacterial activity of titanium by surface modification with polydopamine and silver for dental implant application. J. Appl. Biomater. Funct. Mater. 2019, 17. [Google Scholar] [CrossRef] [PubMed]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wu, Z.; Leung, A.; Chen, X.; Landao-Bassonga, E.; Gao, J.; Chen, L.; Zheng, M.; Yao, F.; Yang, H.; et al. Fabrication of a silver nanoparticle-coated collagen membrane with anti-bacterial and anti-inflammatory activities for guided bone regeneration. Biomed. Mater. 2018, 13. [Google Scholar] [CrossRef]
- Mao, B.-H.; Tsai, J.C.; Chen, C.W.; Yan, S.J.; Wang, Y.J. Mechanisms of silver nanoparticle-induced toxicity and important role of autophagy. Nanotoxicology 2016, 10, 1021–1040. [Google Scholar] [CrossRef]
- Hadrup, N.; Sharma, A.K.; Loeschner, K. Toxicity of silver ions, metallic silver, and silver nanoparticle materials after in vivo dermal and mucosal surface exposure: A review. Regul. Toxicol. Pharmacol. 2018, 98, 257–267. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Li, Z.; Zhu, S.; Yuan, X.; Cui, Z.; Yang, X.; Chu, P.K.; Wu, S. Nano Ag/ZnO-Incorporated Hydroxyapatite Composite Coatings: Highly Effective Infection Prevention and Excellent Osteointegration. ACS Appl. Mater. Interfaces 2018, 10, 1266–1277. [Google Scholar] [CrossRef]
- Shen, X.; Hu, Y.; Xu, G.; Chen, W.; Xu, K.; Ran, Q.; Ma, P.; Zhang, Y.; Li, J.; Cai, K. Regulation of the biological functions of osteoblasts and bone formation by Zn-incorporated coating on microrough titanium. ACS Appl. Mater. Interfaces 2014, 6, 16426–16440. [Google Scholar] [CrossRef]
- Park, J.K.; Kim, Y.-J.; Yeom, J.; Jeon, J.H.; Yi, G.-C.; Je, J.H.; Hahn, S.K. The topographic effect of zinc oxide nanoflowers on osteoblast growth and osseointegration. Adv. Mater. 2010, 22, 4857–4861. [Google Scholar] [CrossRef]
- He, W.; Kim, H.K.; Wamer, W.G.; Melka, D.; Callahan, J.H.; Yin, J.J. Photogenerated charge carriers and reactive oxygen species in ZnO/Au hybrid nanostructures with enhanced photocatalytic and antibacterial activity. J. Am. Chem. Soc. 2014, 136, 750–757. [Google Scholar] [CrossRef]
- Li, J.; Tan, L.; Liu, X.; Cui, Z.; Yang, X.; Yeung, K.W.K.; Chu, P.K.; Wu, S. Balancing Bacteria-Osteoblast Competition through Selective Physical Puncture and Biofunctionalization of ZnO/Polydopamine/Arginine-Glycine-Aspartic Acid-Cysteine Nanorods. ACS Nano 2017, 11, 11250–11263. [Google Scholar] [CrossRef] [PubMed]
- Sowa, M.; Parafiniuk, M.; Mouzêlo, C.; Kazek-Kęsika, A.; Zhidkov, I.S.; Kukharenko, A.I.; Cholakh, S.O.; Kurmaev, E.Z.; Simka, W. DC plasma electrolytic oxidation treatment of gum metal for dental implants. Electrochim. Acta 2019, 302, 10–20. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T.; Raaen, S.; Gaiaschi, S.; Chapon, P.; Matýsek, D.; Pietrzak, K.; Szymańska, M.; Dudek, Ł. Metal Ions Supported Porous Coatings by Using AC Plasma Electrolytic Oxidation Processing. Materials 2020, 13, 3838. [Google Scholar] [CrossRef] [PubMed]
- Rokosz, K.; Hryniewicz, T.; Dudek, Ł. Phosphate Porous Coatings Enriched with Selected Elements via PEO Treatment on Titanium and Its Alloys: A Review. Materials 2020, 13, 2468. [Google Scholar] [CrossRef] [PubMed]
- Kazek-Kęsik, A.; Krok-Borkowicz, M.; Dercz, G.; Donesz-Sikorska, A.; Pamuła, E.; Simka, W. Multilayer coatings formed on titanium alloy surfaces by plasma electrolytic oxidation-electrophoretic deposition methods. Electrochim. Acta 2016, 204, 294–306. [Google Scholar] [CrossRef]
- Korniienko, V.; Oleshko, O.; Husak, Y.; Deineka, V.; Holubnycha, V.; Mishchenko, O.; Kazek-Kęsik, A.; Jakóbik-Kolon, A.; Pshenychnyi, R.; Leśniak-Ziółkowska, K.; et al. Formation of a Bacteriostatic Surface on ZrNb Alloy via Anodization in a Solution Containing Cu Nanoparticles. Materials 2020, 13, e3913. [Google Scholar] [CrossRef]
- Oleshko, O.; Liubchak, I.; Husak, Y.; Korniienko, V.; Yusupova, A.; Oleshko, T.; Banasiuk, R.; Szkodo, M.; Matros-Taranets, I.; Kazek-Kęsik, A.; et al. In Vitro Biological Characterization of Silver-Doped Anodic Oxide Coating on Titanium. Materials 2020, 13, 4359. [Google Scholar] [CrossRef]
- Oleshko, O.; Deineka, V.; Husak, Y.; Korniienko, V.; Mishchenko, O.; Holubnycha, V.; Pisarek, M.; Michalska, J.; Kazek-Kęsik, A.; Jakóbik-Kolon, A.; et al. Ag Nanoparticle-Decorated Oxide Coatings Formed via Plasma Electrolytic Oxidation on ZrNb Alloy. Materials 2019, 12, 3742. [Google Scholar] [CrossRef]
- Jian, Y.T.; Yang, Y.; Tian, T.; Stanford, C.; Zhang, X.P.; Zhao, K. Effect of pore size and porosity on the biomechanical properties and cytocompatibility of porous NiTi alloys. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Kujala, S.; Ryhänen, J.; Danilov, A.; Tuukkanen, J. Effect of porosity on the osteointegration and bone ingrowth of a weight-bearing nickel-titanium bone graft substitute. Biomaterials 2003, 24, 4691–4697. [Google Scholar] [CrossRef]
- Lee, D.J.; Kwon, J.; Kim, Y.-I.; Wang, X.; Wu, T.-J.; Lee, Y.-T.; Kim, S.; Miguez, P.; Ko, C.-C. Effect of pore size in bone regeneration using polydopamine-laced hydroxyapatite collagen calcium silicate scaffolds fabricated by 3D mould printing technology. Orthod. Craniofacl. Res. 2019, 22, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Rey, C.; Combes, C.; Drouet, C.; Grossin, D. 1.111—Bioactive Ceramics: Physical Chemistry. Elsevier 2011, 1, 187–281. [Google Scholar] [CrossRef]
- Nada, A.A.; Rouby, W.M.A.E.; Bekheet, M.F.; Antuch, M.; Weber, M.; Miele, P.; Viter, R.; Roualdes, S.; Millet, P.; Bechelany, M. Highly textured boron/nitrogen co-doped TiO2 with honeycomb structure showing enhanced visible-light photoelectrocatalytic activity. Appl. Surf. Sci. 2020, 505. [Google Scholar] [CrossRef]
- Viter, R.; Tereshchenko, A.; Smyntyna, V.; Ogorodniichuk, J.; Starodub, N.; Yakimova, R.; Khranovskyy, V.; Ramanavicius, A. Toward development of optical biosensors based on photoluminescence of TiO2 nanoparticles for the detection of Salmonella. Sens. Actuators B Chem. 2017, 252, 95–102. [Google Scholar] [CrossRef]
- Fedorenko, V.; Viter, R.; Mrówczyński, R.; Damberga, D.; Coy, E.; Iatsunskyi, I. Synthesis and photoluminescence properties of hybrid 1D core–shell structured nanocomposites based on ZnO/polydopamine. RSC Adv. 2020, 10, 29751–29758. [Google Scholar] [CrossRef]
- Damberga, D.; Viter, R.; Fedorenko, V.; Iatsunskyi, I.; Coy, E.; Graniel, O. Photoluminescence Study of Defects in ZnO-Coated Polyacrylonitrile Nanofibers. J. Phys. Chem. C 2020, 124, 9434–9441. [Google Scholar] [CrossRef]
- Menzies, K.L.; Jones, L. The impact of contact angle on the biocompatibility of biomaterials. Optom. Vis. Sci. 2010, 87, 387–399. [Google Scholar] [CrossRef]
- Noimark, S.; Weiner, J.; Noor, N.; Allan, E.; Williams, C.K.; Shaffer, M.S.P.; Parkin, I.P. Dual-Mechanism Antimicrobial Polymer−ZnO Nanoparticle and Crystal Violet-Encapsulated Silicone. Adv. Funct. Mater. 2015, 25, 1367–1373. [Google Scholar] [CrossRef]
- Forson, A.M.; van der Mei, H.C.; Sjollema, J. Impact of solid surface hydrophobicity and micrococcal nuclease production on Staphylococcus aureus Newman biofilms. Sci. Rep. 2020, 10, 12093. [Google Scholar] [CrossRef]
- Chino, T.; Santer, D.M.; Giordano, D.; Chen, C.; Li, C.; Chen, C.H.; Darveau, R.P.; Clark, E.A. Effects of oral commensal and pathogenic bacteria on human dendritic cells. Oral Microbiol. Immunol. 2009, 24, 96–103. [Google Scholar] [CrossRef]
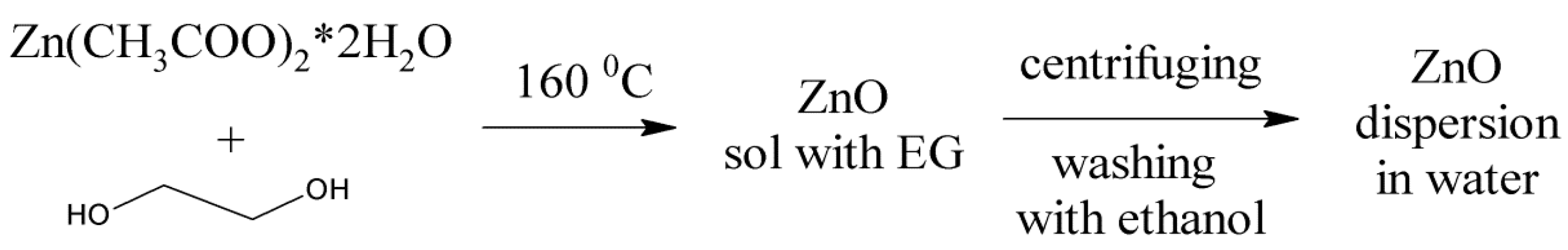

| Atomic Concentration, % | Ca/P Ratio | ||||||
|---|---|---|---|---|---|---|---|
| O | Ca | P | Ti | Zr | Zn | ||
| TiZr-300 | 68.6 | 14.7 | 13.3 | 1.8 | 1.6 | - | 1.1 |
| TiZr-350 | 68.7 | 14.8 | 13.6 | 1.5 | 1.5 | - | 1.1 |
| TiZr-400 | 70.0 | 14.2 | 13.2 | 1.2 | 1.4 | - | 1.1 |
| TiZr-450 | 71.7 | 13.3 | 10.4 | 1.2 | 3.5 | - | 1.3 |
| TiZr-350 ZnO | 68.6 | 4.3 | 9.9 | 3.7 | 7.1 | 6.5 | 0.4 |
| TiZr-400 ZnO | 70.1 | 3.9 | 9.5 | 3.2 | 6.8 | 6.5 | 0.4 |
| TiZr-450 ZnO | 67.6 | 5.4 | 9.4 | 2.1 | 5.8 | 9.7 | 0.6 |
| TiZr-500 ZnO | 68.2 | 5.2 | 9.8 | 2.1 | 4.7 | 9.9 | 0.5 |
| Samples | Binding Energy (BE)/eV | Chemical Bonds | ||||||
|---|---|---|---|---|---|---|---|---|
| Ti2p3/2 | Zr3d5/2 | Nb3d5/2 | Zn2p3/2 | P2p3/2 | Ca2p3/2 | O1s | ||
| TiZr-350 | 458.8 | 531.1 | Ti-O (TiO2) | |||||
| 183.3 | 531.1 | Zr-O (ZrO2) | ||||||
| 207.6 | 531.1 | Nb-O (Nb2O5) | ||||||
| 133.4 | 347.6 | 531.9 | calcium phophates | |||||
| TiZr-350-ZnO | 1022.1 | Zn-O (ZnO) | ||||||
| 1023.3 | ZnO precursor | |||||||
| 133.4 | 531.6 | phosphate [PO4]3−, Zn-O | ||||||
| 134.3 | 532.7 | metaphosphate [PO3]− | ||||||
| 348.5 | Ca-Cl (CaCl2) | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oleshko, O.; Husak, Y.; Korniienko, V.; Pshenychnyi, R.; Varava, Y.; Kalinkevich, O.; Pisarek, M.; Grundsteins, K.; Pogorielova, O.; Mishchenko, O.; et al. Biocompatibility and Antibacterial Properties of ZnO-Incorporated Anodic Oxide Coatings on TiZrNb Alloy. Nanomaterials 2020, 10, 2401. https://doi.org/10.3390/nano10122401
Oleshko O, Husak Y, Korniienko V, Pshenychnyi R, Varava Y, Kalinkevich O, Pisarek M, Grundsteins K, Pogorielova O, Mishchenko O, et al. Biocompatibility and Antibacterial Properties of ZnO-Incorporated Anodic Oxide Coatings on TiZrNb Alloy. Nanomaterials. 2020; 10(12):2401. https://doi.org/10.3390/nano10122401
Chicago/Turabian StyleOleshko, Oleksandr, Yevheniia Husak, Viktoriia Korniienko, Roman Pshenychnyi, Yuliia Varava, Oksana Kalinkevich, Marcin Pisarek, Karlis Grundsteins, Oksana Pogorielova, Oleg Mishchenko, and et al. 2020. "Biocompatibility and Antibacterial Properties of ZnO-Incorporated Anodic Oxide Coatings on TiZrNb Alloy" Nanomaterials 10, no. 12: 2401. https://doi.org/10.3390/nano10122401
APA StyleOleshko, O., Husak, Y., Korniienko, V., Pshenychnyi, R., Varava, Y., Kalinkevich, O., Pisarek, M., Grundsteins, K., Pogorielova, O., Mishchenko, O., Simka, W., Viter, R., & Pogorielov, M. (2020). Biocompatibility and Antibacterial Properties of ZnO-Incorporated Anodic Oxide Coatings on TiZrNb Alloy. Nanomaterials, 10(12), 2401. https://doi.org/10.3390/nano10122401







