Modification of V2O5-WO3/TiO2 Catalyst by Loading of MnOx for Enhanced Low-Temperature NH3-SCR Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Catalyst Preparation
2.2.1. Precipitation Method (C)
2.2.2. Incipient-Wetness. Impregnation (I)
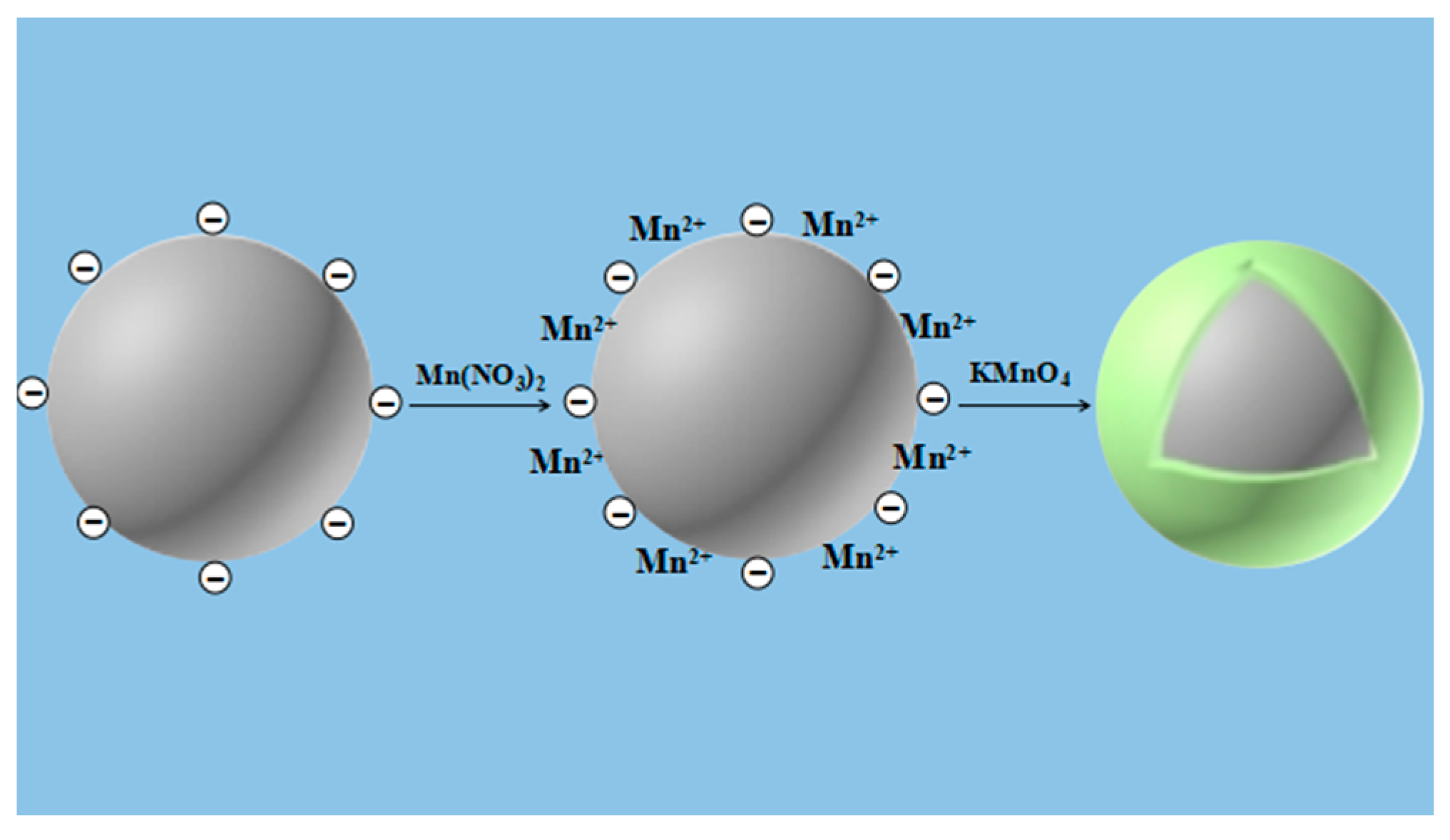
2.2.3. In-Situ Growth Method (S)
2.3. Catalyst Characterization
2.4. Catalytic Activity Measurement
3. Results
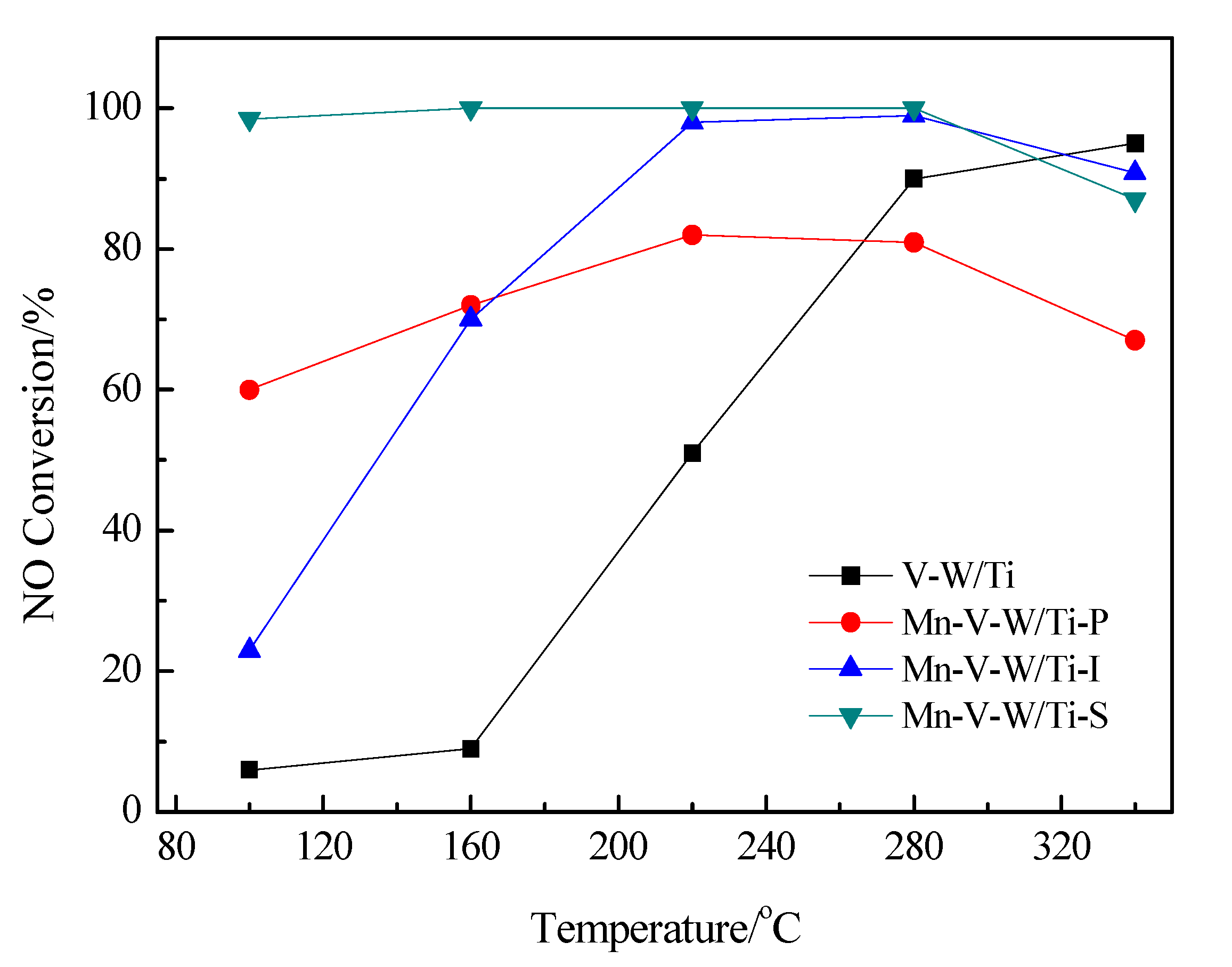
3.1. Catalytic Activity
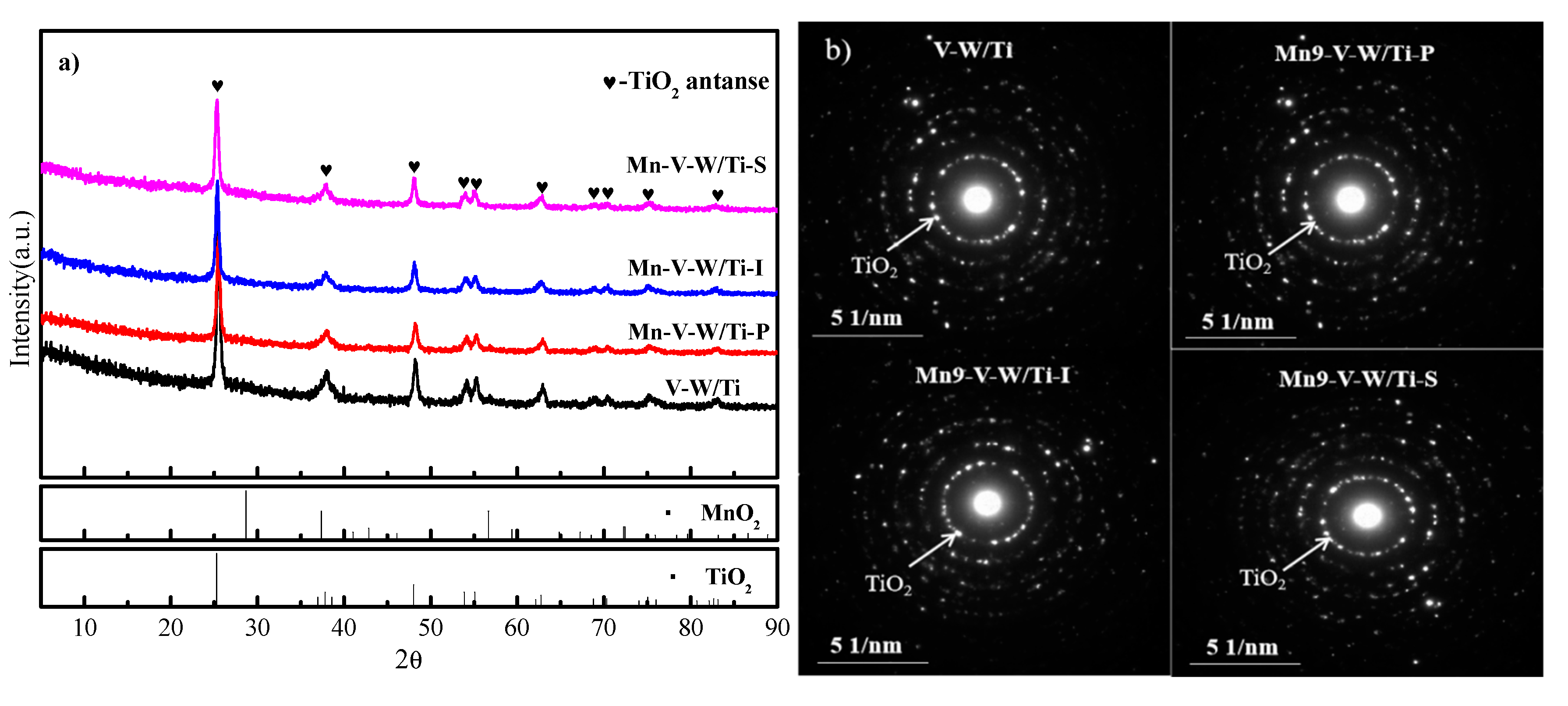
3.2. XRD and SAED Pattern Analysis
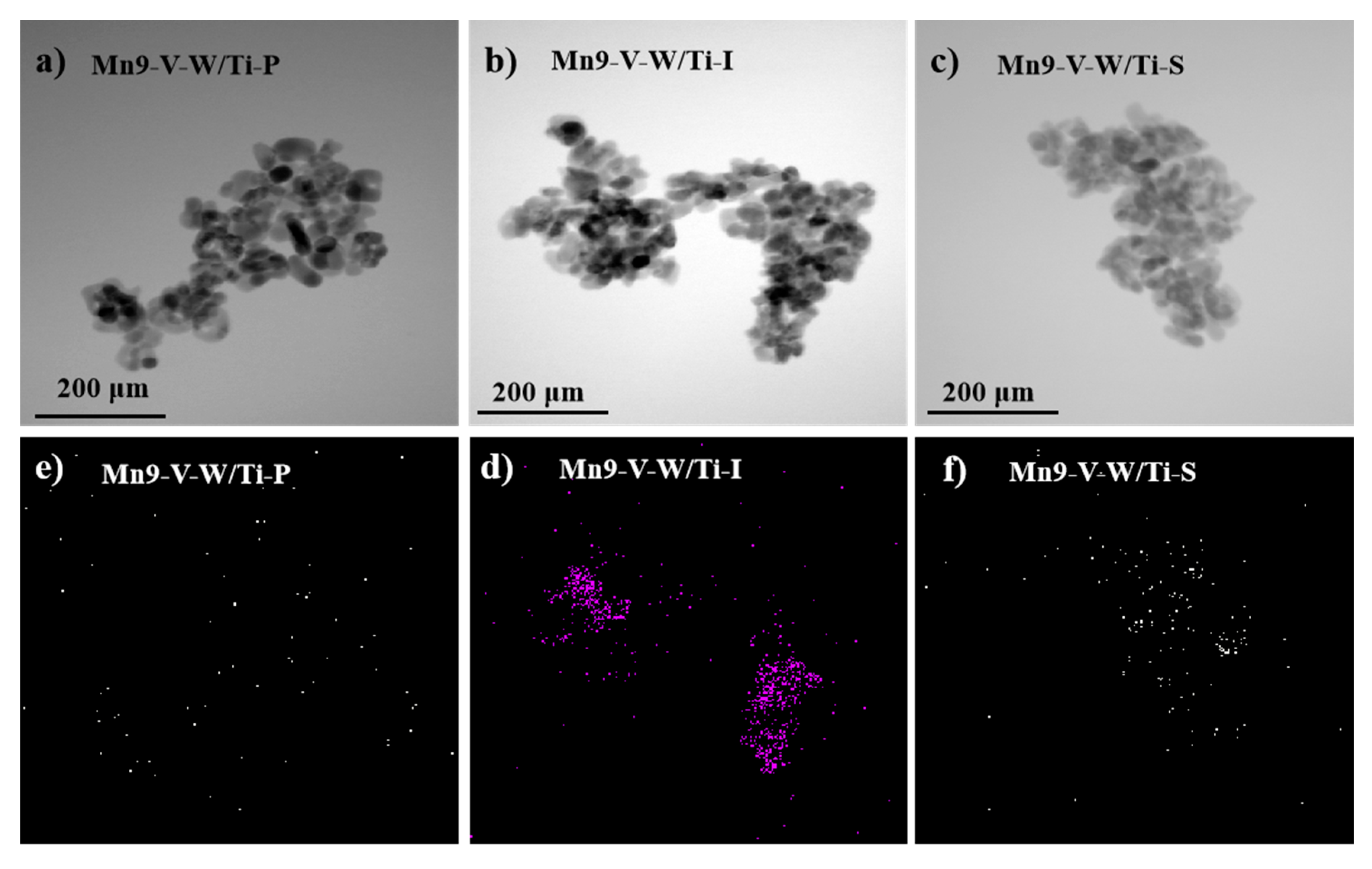
3.3. TEM and XRF Analysis
3.4. SEM and BET Analysis
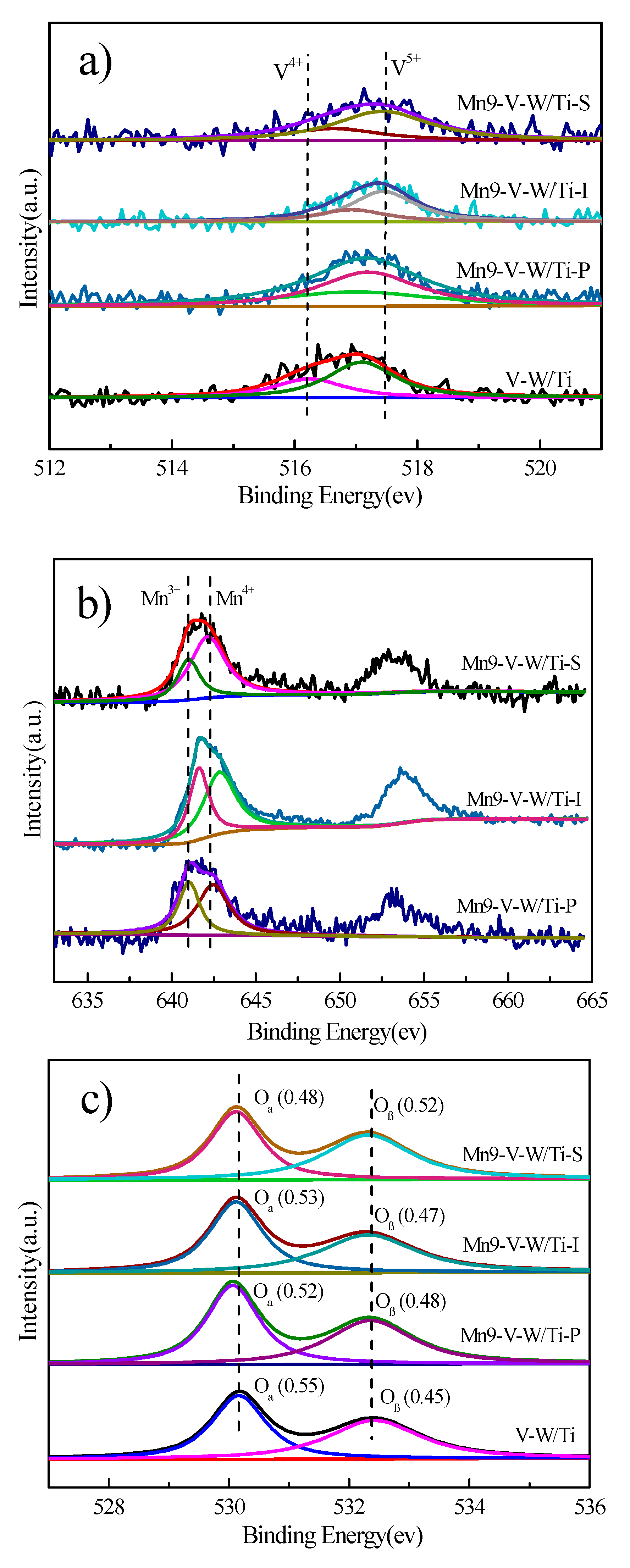
3.5. XPS Analysis
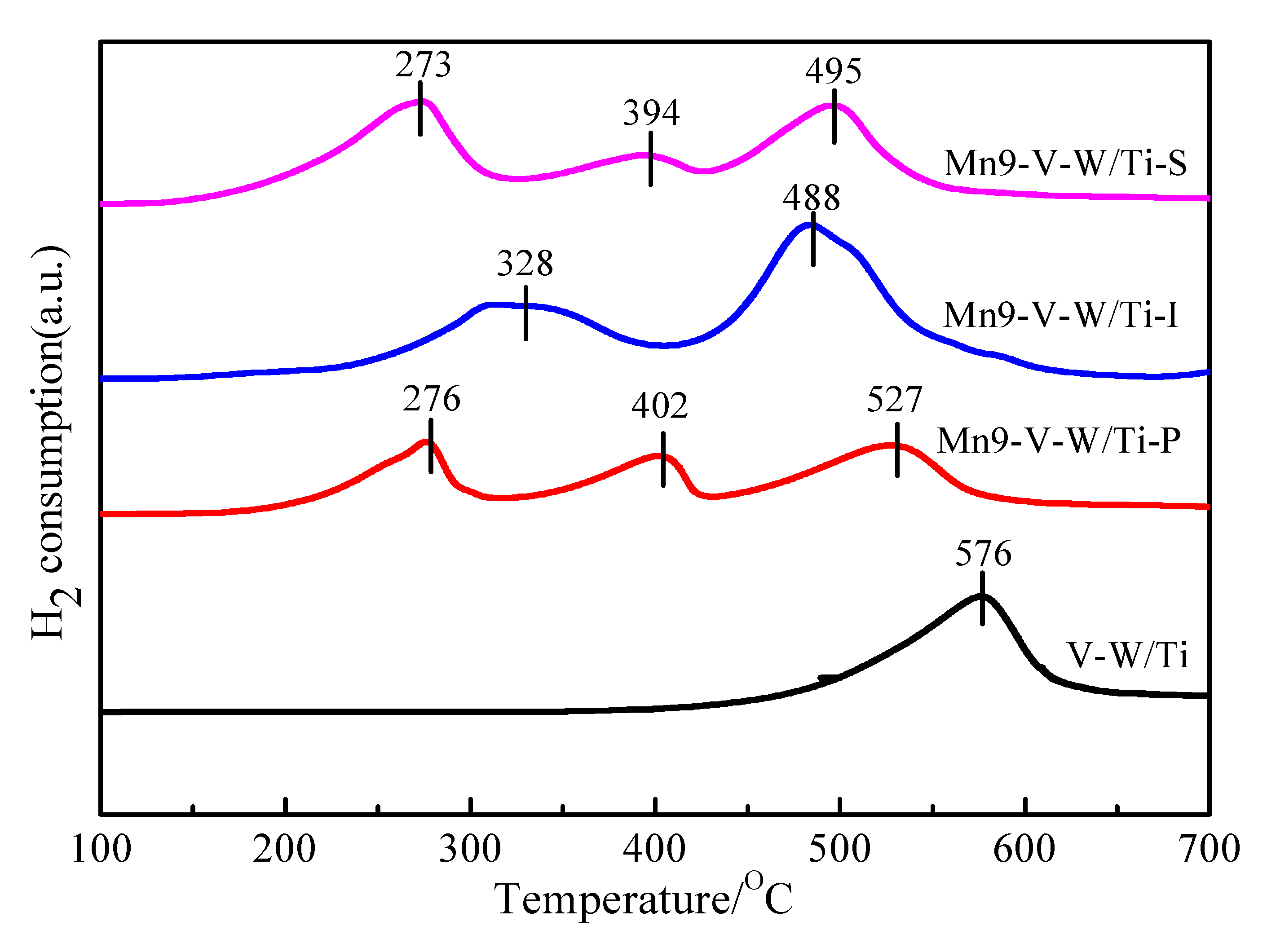
3.6. H2-TPR Analysis
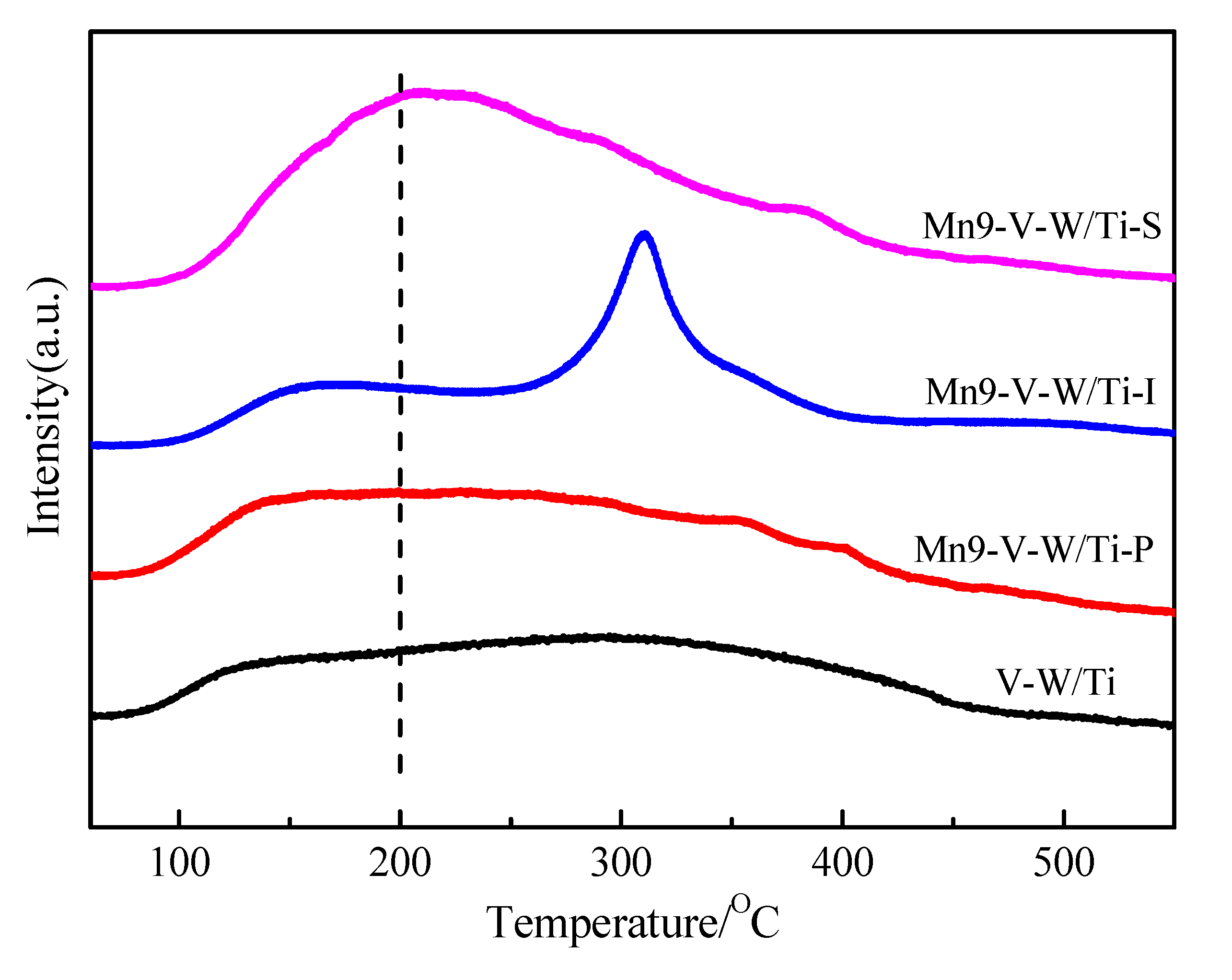
3.7. NH3-TPD Analysis
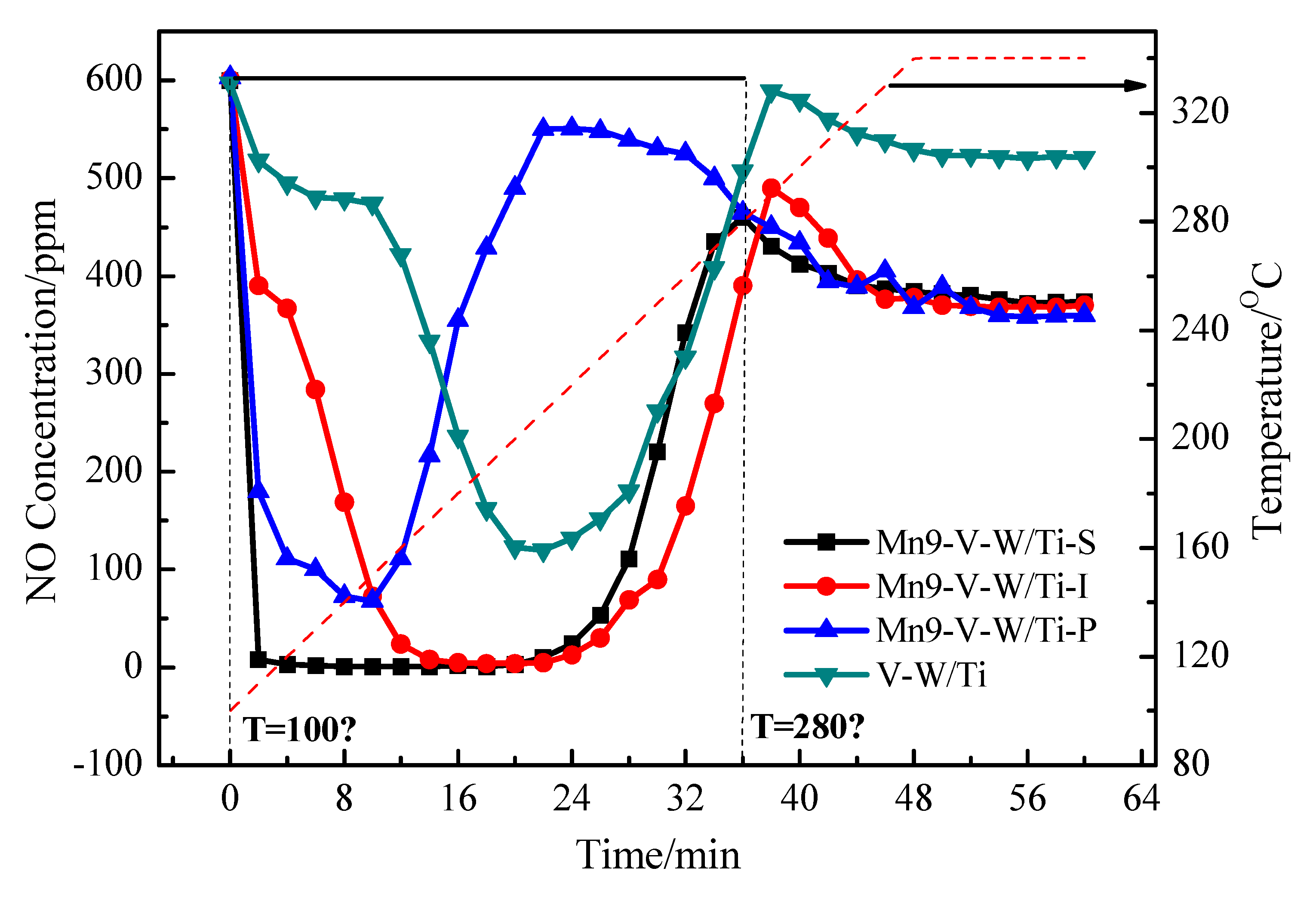
3.8. TPSR Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xu, W.J.; Zhang, G.X.; Chen, H.W.; Zhang, G.M.; Han, Y.; Chang, Y.C.; Gong, P. Mn/beta and Mn/ZSM-5 for the low-temperature selective catalytic reduction of NO with ammonia: Effect of manganese precursors. Chin. J. Catal. 2018, 39, 118–127. [Google Scholar] [CrossRef]
- Park, E.; Chin, S.; Jeong, J.; Jurng, J. Low-temperature NO oxidation over Mn/TiO2, nanocomposite synthesized by chemical vapor condensation: Effects of Mn precursor on the surface Mn species. Microporous Mesoporous Mater. 2012, 163, 96–101. [Google Scholar] [CrossRef]
- Ji, P.D.; Gao, X.; Du, X.S.; Zheng, C.H.; Luo, Z.Y.; Cen, K.F. Relationship between the molecular structure of V2O5/TiO2 catalysts and the reactivity of SO2 oxidation. Catal. Sci. Technol. 2016, 6, 1187–1194. [Google Scholar] [CrossRef]
- Zhao, X.; Hang, L.; Namuangruk, S.; Hu, H.; Hu, X.N.; Shi, L.Y.; Zhang, D.S. Morphology dependent performance of Zr-CeVO4/TiO2 for selective catalytic reduction of NO with NH3. Catal. Sci. Technol. 2016, 6, 5543–5553. [Google Scholar] [CrossRef]
- Ding, S.P.; Liu, F.D.; Shi, X.Y.; Liu, K.; Lian, Z.H.; Xie, L.J.; He, H. Significant Promotion Effect of Mo Additive on a Novel Ce-Zr Mixed Oxide Catalyst for the Selective Catalytic Reduction of NOx with NH3. ACS Appl. Mater. Interfaces 2015, 7, 9497. [Google Scholar] [CrossRef]
- Li, B.; Ren, Z.Y.; Ma, Z.X.; Huang, X.D.; Liu, F.; Zhang, X.B.; Yang, H.S. Selective catalytic reduction of NO by NH3 over CuO-CeO2 in the presence of SO2. Catal. Sci. Technol. 2016, 6, 1719–1725. [Google Scholar] [CrossRef]
- Huang, Z.G.; Zhu, Z.P.; Liu, Z.Y. Combined effect of H2O and SO2 on V2O5/AC catalysts for NO reduction with ammonia at lower temperatures. Appl. Catal. B Environ. 2002, 39, 361–368. [Google Scholar] [CrossRef]
- Qi, K.; Xie, J.L.; Fang, D.; Li, F.X.; He, F. Performance enhancement mechanism of Mn-based catalysts prepared under N2 for NOx removal: Evidence of the poor crystallization and oxidation of MnOx. Chin. J. Catal. 2017, 38, 845–851. [Google Scholar] [CrossRef]
- Zuo, J.L.; Chen, Z.G.; Wang, F.R.; Yu, Y.H.; Wang, L.F.; Li, X.H. Low-Temperature Selective Catalytic Reduction of NOx with NH3 over Novel Mn–Zr Mixed Oxide Catalysts. Ind. Eng. Chem. Res. 2014, 53, 2647–2655. [Google Scholar] [CrossRef]
- Wallin, M.; Forser, S.; Thormählen, P.; Skoglundh, M. Screening of TiO2-supported catalysts for selective NOx reduction with ammonia. Ind. Eng. Chem. Res. 2004, 43, 7723–7731. [Google Scholar] [CrossRef]
- Qi, G.; Yang, R.T. Performance and kinetics study for low-temperature SCR of NO with NH3 over MnOx-CeO2 catalyst. J. Catal. 2003, 217, 434–441. [Google Scholar] [CrossRef]
- Liu, Z.M.; Li, Y.; Zhu, T.L.; Su, H.; Zhu, J.Z. Selective catalytic reduction of NOx by NH3 over Mn-Promoted V2O5/TiO2 Catalyst. Ind. Eng. Chem. Res. 2014, 53, 12964–12970. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, L.; Li, H.L.; Gu, H.; Han, J.; Shi, L.Y. Highly dispersed V2O5/TiO2, modified with transition metals (Cu, Fe, Mn, Co) as efficient catalysts for the selective reduction of NO with NH3. Chin. J. Catal. 2015, 36, 1886–1899. [Google Scholar] [CrossRef]
- Wu, Z.B.; Jiang, B.Q.; Liu, Y. Effect of transition metals addition on the catalyst of manganese/titania for low-temperature selective catalytic reduction of nitric oxide with ammonia. Appl. Catal. B Environ. 2008, 79, 347–355. [Google Scholar] [CrossRef]
- Li, J.H.; Chen, J.J.; Ke, R.; Luo, C.K.; Hao, J.M. Effects of precursors on the surface Mn species and the activities for NO reduction over MnOx/TiO2 catalysts. Catal. Commun. 2007, 8, 1896–1900. [Google Scholar] [CrossRef]
- Zuo, H.Q.; Xu, D.Y.; Liu, W.; Dan, H.J.; Liu, X.H.; Lin, S.; Hou, P. Heat-treated Dolomite-palygorskite clay supported MnOx catalysts prepared by various methods for low temperature selective catalytic reduction (SCR) with NH3. Appl. Clay Sci. 2018, 2, 276–283. [Google Scholar] [CrossRef]
- Qi, G.; Yang, R.T. Low-temperature selective catalytic reduction of NO with NH3 over iron and manganese oxides supported on titania. Appl. Catal B Environ. 2003, 44, 217–225. [Google Scholar] [CrossRef]
- Zhang, H.G.; Li, X.Z.; Su, H.; Chen, X.F.; Zuo, S.X.; Yan, X.Y.; Liu, W.J.; Yao, C. Sol–gel synthesis of upconversion perovskite/attapulgite heterostructures for photocatalytic fixation of nitrogen. J. Sol-Gel Sci. Technol. 2019, 92, 154–162. [Google Scholar] [CrossRef]
- Jo, S.; Shin, B.; Shin, M.-C.; Tyne, C.; Lee, H. Dispersion and valence state of MnO2/Ce(1-x)ZrxO2–TiO2 for low temperature NH3-SCR. Catal. Commun. 2014, 57, 134–137. [Google Scholar] [CrossRef]
- Xiong, Z.B.; Lu, C.M. Study on the modification of iron-cerium mixed oxide catalyst for selective catalytic reduction of NO. J. Fuel Chem. Technol. 2013, 41, 361–367. [Google Scholar]
- Kang, M.; Park, E.D.; Kim, J.M.; Yie, J.E. Manganese oxide catalysts for NOx reduction with NH3, at low temperatures. Appl. Catal. A Gen. 2007, 327, 261–269. [Google Scholar] [CrossRef]
- Tian, W.; Yang, H.S.; Fan, X.Y.; Zhang, X.B. Catalytic reduction of NOx with NH3 over different-shaped MnO2 at low temperature. J. Hazard. Mater. 2011, 188, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Shen, B.; Chi, G.; Zhang, X. Low-temperature SCR of NO over Fe and Co co-doped Mn-Ce/TiO2 catalyst. Chin. J. Environ. Eng. 2017, 11, 3633–3639. [Google Scholar]
- Chao, M.X.; Mao, D.S.; Li, G.H.; Li, G.; Yu, J.; Guo, X.M. Low-temperature selective catalytic reduction of NO with NH3 over Mn–Ce–Ox/TiO2: A comparison between catalyst preparation methods. J. Sol-Gel Sci. Technol. 2020, 1–12. [Google Scholar] [CrossRef]
- Fang, D.; He, F.; Xie, J.L. Characterization and performance of common alkali metals and alkaline earth metals loaded Mn/TiO2 catalysts for NOx removal with NH3. J. Energy Inst. 2019, 92, 319–331. [Google Scholar] [CrossRef]
- Shu, Y.; Sun, H.; Quan, X.; Chen, S. Enhancement of catalytic activity over the iron-modified Ce/TiO2 catalyst for selective catalytic reduction of NOx with ammonia. J. Phys. Chem. C 2012, 116, 25319–25327. [Google Scholar] [CrossRef]
- Lietti, L. Reactivity of V2O5–WO3/TiO2 de-NOx catalysts by transient methods. Appl. Catal. B. Environ. 1996, 10, 281–297. [Google Scholar] [CrossRef]
- Ramis, G.; Yi, L.; Busca, G.; Turco, M.; Kotur, E.; Willey, R.J. Adsorption, activation, and oxidation of ammonia over SCR catalysts. J. Catal. 1995, 157, 523–535. [Google Scholar] [CrossRef]
- Putluru, S.S.R.; Riisager, A.; Fehrmann, R. The Effect of acidic and redox properties of V2O5/CeO2-ZrO2 catalysts in selective catalytic reduction of NO by NH3. Catal. Lett. 2009, 133, 370–375. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Qiu, C.T.; Xu, H.D.; Lin, T.; Lin, Z.E.; Gong, M.C.; Chen, Y.Q. Low-temperature selective catalytic reduction of NO with NH3 over monolith catalyst of MnOx/CeO2–ZrO2–Al2O3. Catal. Today 2011, 175, 171–176. [Google Scholar] [CrossRef]
- Guo, R.T.; Wang, Q.S.; Pan, W.G.; Chen, Q.L.; Ding, H.L.; Yin, X.F.; Yang, N.Z.; Lu, C.Z.; Wang, S.X.; Yuan, Y.C. The poisoning effect of heavy metals doping on Mn/TiO2 catalyst for selective catalytic reduction of NO with NH3. J. Mol. Catal. A Chem. 2015, 407, 1–7. [Google Scholar] [CrossRef]
- Zhang, Q.M.; Song, C.L.; Lv, G.; Bin, F.; Pang, H.T.; Song, J.O. Effect of metal oxide partial substitution of V2O5, in V2O5–WO3/TiO2, on selective catalytic reduction of NO with NH3. Ind. Eng. Chem. 2015, 24, 79–86. [Google Scholar] [CrossRef]
- Shen, M.Q.; Li, C.X.; Wang, J.Q.; Xu, L.L.; Wang, W.L.; Wang, J. New insight into the promotion effect of Cu doped V2O5/WO3–TiO2 for low temperature NH3-SCR performance. RSC Adv. 2015, 5, 35155–35165. [Google Scholar] [CrossRef]

| Sample | Mn(%) |
|---|---|
| Mn-V-W/Ti-P | 4.42 |
| Mn-V-W/Ti-I | 7.09 |
| Mn-V-W/Ti-S | 6.45 |
| Sample | SBET (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|
| V-W/Ti | 90.5 | 0.441 | 15.6 |
| Mn-V-W/Ti-P | 58.9 | 0.153 | 11.4 |
| Mn-V-W/Ti-I | 38.9 | 0.218 | 11.2 |
| Mn-V-W/Ti-S | 91.3 | 0.266 | 11.1 |
| Sample | Binding Energy(ev) | Mn4+/Mn | V5+/V | |||
|---|---|---|---|---|---|---|
| Mn4+ | Mn3+ | V5+ | V4+ | |||
| V-W/Ti | / | 517.09 | 516.24 | / | 0.70 | |
| Mn-V-W/Ti-P | 642.47 | 641.03 | 517.19 | 516.97 | 0.59 | 0.75 |
| Mn-V-W/Ti-I | 642.83 | 641.61 | 517.44 | 516.93 | 0.58 | 0.78 |
| Mn-V-W/Ti-S | 642.13 | 641.00 | 517.12 | 516.32 | 0.70 | 0.79 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Diao, Q.; Hu, X.; Wu, X.; Xiao, K.; Wang, J. Modification of V2O5-WO3/TiO2 Catalyst by Loading of MnOx for Enhanced Low-Temperature NH3-SCR Performance. Nanomaterials 2020, 10, 1900. https://doi.org/10.3390/nano10101900
Zhang X, Diao Q, Hu X, Wu X, Xiao K, Wang J. Modification of V2O5-WO3/TiO2 Catalyst by Loading of MnOx for Enhanced Low-Temperature NH3-SCR Performance. Nanomaterials. 2020; 10(10):1900. https://doi.org/10.3390/nano10101900
Chicago/Turabian StyleZhang, Xianlong, Qinchao Diao, Xiaorui Hu, Xueping Wu, Kesong Xiao, and Junwei Wang. 2020. "Modification of V2O5-WO3/TiO2 Catalyst by Loading of MnOx for Enhanced Low-Temperature NH3-SCR Performance" Nanomaterials 10, no. 10: 1900. https://doi.org/10.3390/nano10101900
APA StyleZhang, X., Diao, Q., Hu, X., Wu, X., Xiao, K., & Wang, J. (2020). Modification of V2O5-WO3/TiO2 Catalyst by Loading of MnOx for Enhanced Low-Temperature NH3-SCR Performance. Nanomaterials, 10(10), 1900. https://doi.org/10.3390/nano10101900





