Micro- and Nanoplastics and the Oral Cavity: Implications for Oral and Systemic Health, Dental Practice, and the Environment—A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
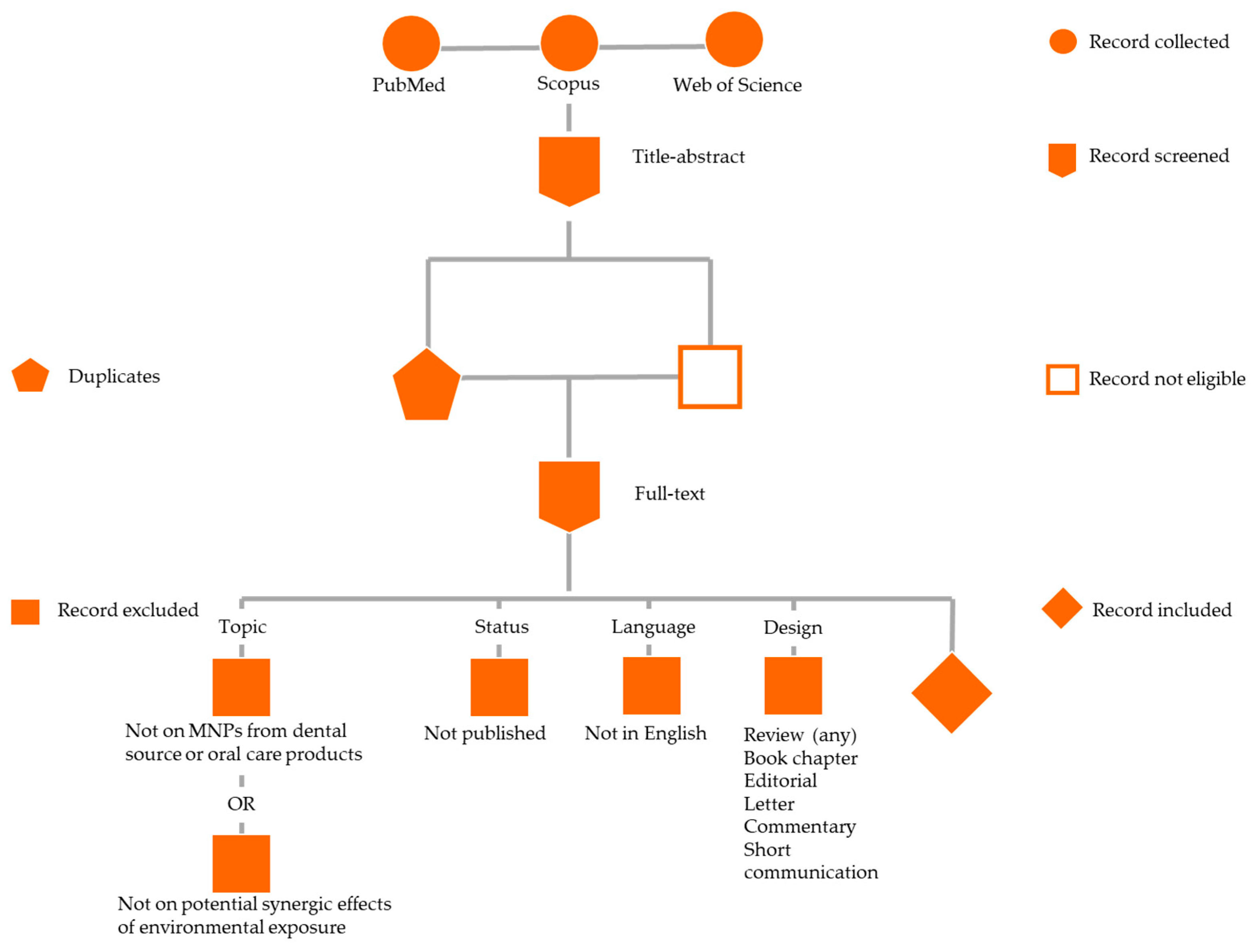
2.1. Search Strategy
2.2. Study Selection and Eligibility Criteria
2.3. Data Extraction, Collection, and Synthesis
3. Results
3.1. Human Epidemiological Evidence
3.2. Micro- and Nanoplastics and Human Health: Sources, Routes of Transmission, and Toxicity
3.2.1. Sources
3.2.2. Routes of Transmission
3.2.3. Toxicity
3.3. MNPs and Cancer
3.3.1. Accumulation and Bioavailability
3.3.2. Microbiota Dysbiosis and Cancer
3.3.3. Inflammation and Tumor Microenvironment
3.3.4. Genotoxicity and Epigenetic Alterations
3.3.5. Synergistic Effects with Environmental Contaminants
3.3.6. Cancer Promotion and Progression
3.4. MNPs and the Oral Cavity: Oral Health Status, Systemic Toxicity, and Cancerogenesis
3.4.1. Oral Cancer
3.4.2. Oral Health
3.4.3. Systemic Toxicity from MNPs Derived from the Oral Cavity and Dental Practice/Materials
3.4.4. Cancerogenesis Potentially Related to MNPs Derived from the Oral Cavity and Dental Practice/Materials
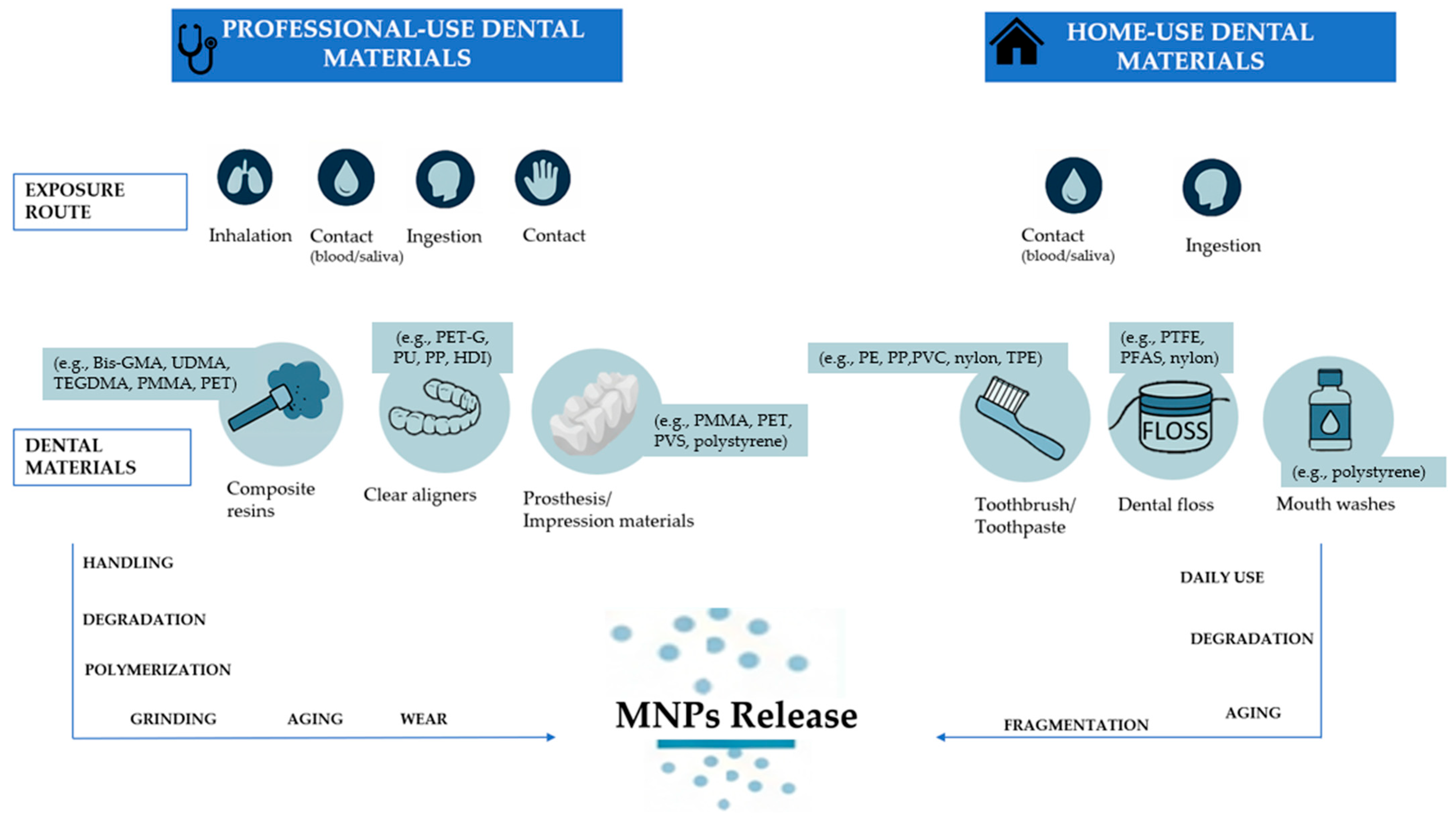
3.5. Dental Practice/Materials
3.5.1. Professional-Use Dental Materials
Resin-Based Composites
Clear Aligners
Dental Prosthesis and Impression Materials
3.5.2. Home-Use Dental Materials
Toothpaste, Toothbrushes, Dental Floss, and Mouthwashes
4. Discussion
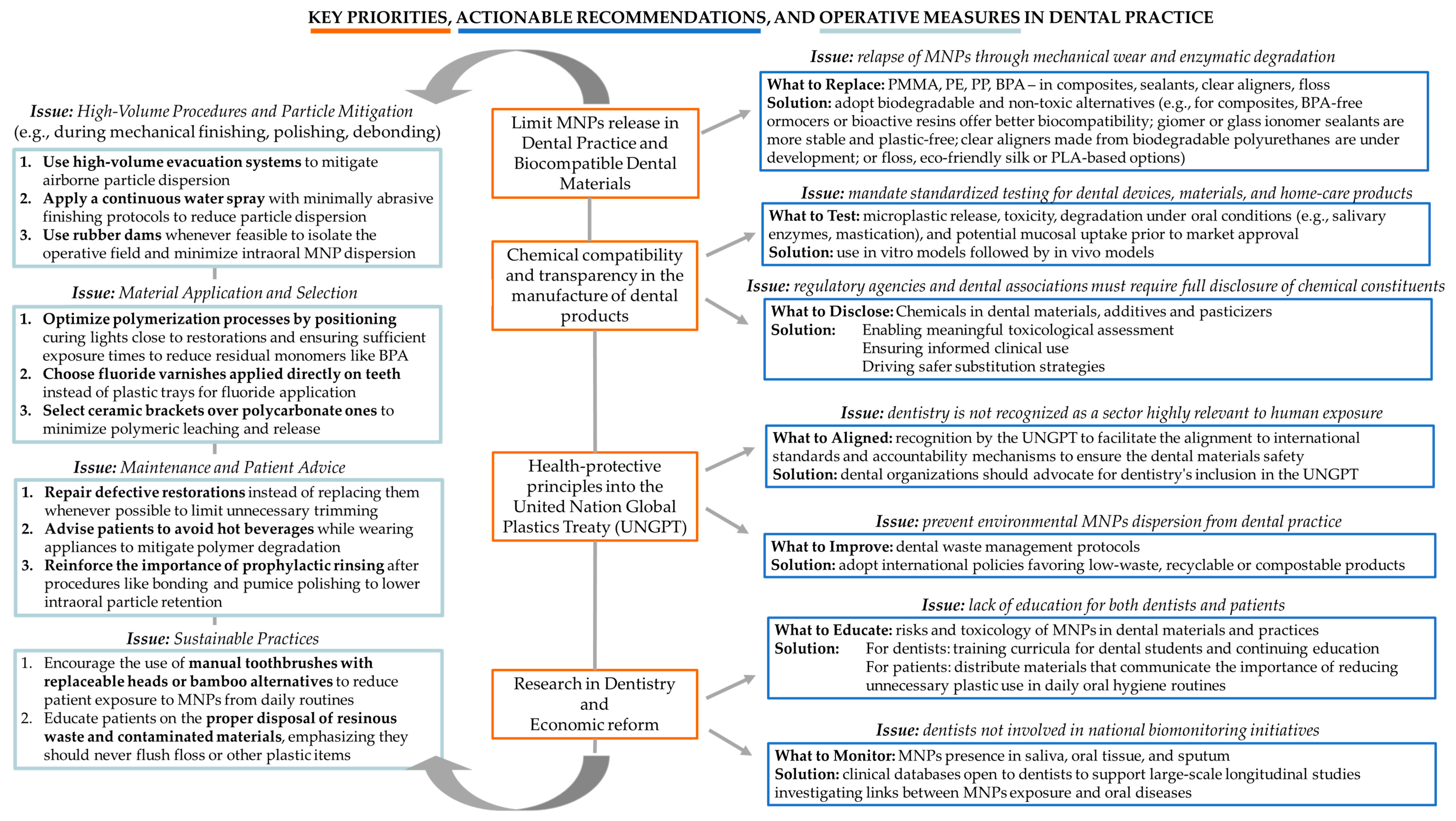
4.1. Policy and Recommendations for Dental Practice and Related Fields
4.1.1. System-Level Policy Recommendations
- MNPs-free materials: there is a need to establish a global cap on the production of virgin plastics, particularly those derived from fossil fuels [11]. Curbing upstream plastic production is viewed as essential not only to reduce environmental plastic burdens but also to minimize chemical exposures associated with extraction, polymerization, and manufacturing processes [11].
- Chemical compatibility and transparency: there is a necessity of eliminating hazardous substances from all stages of the plastic life cycle. This includes the targeted removal of persistent, bioaccumulative, and toxic chemicals, endocrine-disrupting chemicals, and other substances of very high concern [11]. Such a transition requires the development and enforcement of stringent regulatory frameworks that mandate full transparency and disclosure of all chemical constituents used in plastic materials [11]. Chemical transparency is considered a precondition for effective risk assessment, safer substitution, and innovation in green chemistry [11].
- Adoption of health-protective principles into United Nation Global Plastics Treaty: the integration of health-protective principles into the United Nations Global Plastics Treaty is currently under negotiation [11]. The treaty should prioritize public health as a core objective, impose binding obligations on countries to reduce harmful plastic production and use, and include mechanisms for supply chain accountability. Additionally, it should require the systematic monitoring and public reporting of plastic-related human exposures and health effects [11].
- Scientific research and economic reform: there is a need for sustained investment in scientific research [11]. This includes biomonitoring initiatives to track internal human exposure to micro- and NPs, longitudinal epidemiological studies to assess long-term health outcomes, and research in green chemistry to develop safe and sustainable alternatives to conventional plastics [11]. There is a need for public engagement and economic reform. Public awareness campaigns should be mobilized to inform consumers and stimulate demand for safer materials. Simultaneously, economic tools such as plastic taxation, extended producer responsibility schemes, and financial incentives for sustainable innovation should be deployed to reflect the true environmental and health costs of plastics and to drive systemic change [11].
4.1.2. Clinical Implications, Actionable Recommendations, and Operative Measures in Dental Practice
4.2. Limitations
4.3. Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Groh, K.J.; Backhaus, T.; Carney-Almroth, B.; Geueke, B.; Inostroza, P.A.; Lennquist, A.; Leslie, H.A.; Maffini, M.; Slunge, D.; Trasande, L.; et al. Overview of Known Plastic Packaging-Associated Chemicals and Their Hazards. Sci. Total Environ. 2019, 651, 3253–3268. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Andrady, A.L.; Duarte, A.C.; Rocha-Santos, T. A One Health Perspective of the Impacts of Microplastics on Animal, Human and Environmental Health. Sci. Total Environ. 2021, 777, 146094. [Google Scholar] [CrossRef]
- Prüst, M.; Meijer, J.; Westerink, R.H.S. The Plastic Brain: Neurotoxicity of Micro- and Nanoplastics. Part. Fibre Toxicol. 2020, 17, 24. [Google Scholar] [CrossRef]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and Quantification of Plastic Particle Pollution in Human Blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Deng, X.; Gui, Y.; Zhao, L. The Micro(Nano)Plastics Perspective: Exploring Cancer Development and Therapy. Mol. Cancer 2025, 24, 30. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Li, Y.; Powell, T.; Wang, X.; Wang, G.; Zhang, P. Microplastics as Contaminants in the Soil Environment: A Mini-Review. Sci. Total Environ. 2019, 691, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Wang, C.; Pan, Z.; Jin, C.; Fu, Z.; Jin, Y. Maternal Polystyrene Microplastic Exposure during Gestation and Lactation Altered Metabolic Homeostasis in the Dams and Their F1 and F2 Offspring. Environ. Sci. Technol. 2019, 53, 10978–10992. [Google Scholar] [CrossRef]
- Cannatà, D.; Giordano, F.; Bartolucci, M.L.; Galdi, M.; Bucci, R.; Martina, S. Attitude of Italian dental practitioners toward bruxism assessment and management: A survey-based study. Orthod. Craniofac. Res. 2024, 27, 228–236. [Google Scholar] [CrossRef]
- Su, L.; Deng, H.; Li, B.; Chen, Q.; Pettigrove, V.; Wu, C.; Shi, H. The Occurrence of Microplastic in Specific Organs in Commercially Caught Fishes from Coast and Estuary Area of East China. J. Hazard. Mater. 2019, 365, 716–724. [Google Scholar] [CrossRef]
- Rice, J.M.; Boffetta, P. 1,3-Butadiene, Isoprene and Chloroprene: Reviews by the IARC Monographs Programme, Outstanding Issues, and Research Priorities in Epidemiology. Chem. Biol. Interact. 2001, 135–136, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Landrigan, P.J.; Raps, H.; Cropper, M.; Bald, C.; Brunner, M.; Canonizado, E.M.; Charles, D.; Chiles, T.C.; Donohue, M.J.; Enck, J.; et al. The Minderoo-Monaco Commission on Plastics and Human Health. Ann. Glob. Health 2023, 89, 23, Erratum in Ann. Glob. Health 2023, 89, 71. [Google Scholar] [CrossRef]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human Consumption of Microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef]
- Lam, J.; Lanphear, B.P.; Bellinger, D.; Axelrad, D.A.; McPartland, J.; Sutton, P.; Davidson, L.; Daniels, N.; Sen, S.; Woodruff, T.J. Developmental PBDE Exposure and IQ/ADHD in Childhood: A Systematic Review and Meta-Analysis. Environ. Health Perspect. 2017, 125, 086001. [Google Scholar] [CrossRef]
- Deng, C.; Zhu, J.; Fang, Z.; Yang, Y.; Zhao, Q.; Zhang, Z.; Jin, Z.; Jiang, H. Identification and Analysis of Microplastics in Para-Tumor and Tumor of Human Prostate. eBioMedicine 2024, 108, 105360. [Google Scholar] [CrossRef]
- Protyusha, G.B.; Kavitha, B.; Robin, R.S.; Nithin, A.; Ineyathendral, T.R.; Shivani, S.S.; Anandavelu, I.; Sivasamy, S.; Deepak Samuel, V.; Purvaja, R. Microplastics in Oral Healthcare Products (OHPs) and Their Environmental Health Risks and Mitigation Measures. Environ. Pollut. 2024, 343, 123118. [Google Scholar] [CrossRef] [PubMed]
- Zha, H.; Lv, J.; Lou, Y.; Wo, W.; Xia, J.; Li, S.; Zhuge, A.; Tang, R.; Si, N.; Hu, Z.; et al. Alterations of Gut and Oral Microbiota in the Individuals Consuming Take-Away Food in Disposable Plastic Containers. J. Hazard. Mater. 2023, 441, 129903. [Google Scholar] [CrossRef] [PubMed]
- Motta, G.; Gualtieri, M.; Bengalli, R.; Saibene, M.; Belosi, F.; Nicosia, A.; Cabellos, J.; Mantecca, P. An Integrated New Approach Methodology for Inhalation Risk Assessment of Safe and Sustainable by Design Nanomaterials. Environ. Int. 2024, 183, 108420. [Google Scholar] [CrossRef]
- Horvatits, T.; Tamminga, M.; Liu, B.; Sebode, M.; Carambia, A.; Fischer, L.; Püschel, K.; Huber, S.; Fischer, E.K. Microplastics Detected in Cirrhotic Liver Tissue. eBioMedicine 2022, 82, 104147. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, Y.; Zhang, T.; Zhang, F.; Ren, H.; Zhang, Y. Analysis of Microplastics in Human Feces Reveals a Correlation between Fecal Microplastics and Inflammatory Bowel Disease Status. Environ. Sci. Technol. 2022, 56, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First Evidence of Microplastics in Human Placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef]
- Umeh, A.C.; Hassan, M.; Egbuatu, M.; Zeng, Z.; Al Amin, M.; Samarasinghe, C.; Naidu, R. Multicomponent PFAS Sorption and Desorption in Common Commercial Adsorbents: Kinetics, Isotherm, Adsorbent Dose, PH, and Index Ion and Ionic Strength Effects. Sci. Total Environ. 2023, 904, 166568. [Google Scholar] [CrossRef]
- Amato-Lourenço, L.F.; Carvalho-Oliveira, R.; Júnior, G.R.; dos Santos Galvão, L.; Ando, R.A.; Mauad, T. Presence of Airborne Microplastics in Human Lung Tissue. J. Hazard. Mater. 2021, 416, 126124. [Google Scholar] [CrossRef]
- Jenner, L.C.; Rotchell, J.M.; Bennett, R.T.; Cowen, M.; Tentzeris, V.; Sadofsky, L.R. Detection of Microplastics in Human Lung Tissue Using ΜFTIR Spectroscopy. Sci. Total Environ. 2022, 831, 154907. [Google Scholar] [CrossRef]
- Przekop, R.; Michalczuk, U.; Penconek, A.; Moskal, A. Effect of Microplastic Particles on the Rheological Properties of Human Saliva and Mucus. Int. J. Environ. Res. Public Health 2023, 20, 7037. [Google Scholar] [CrossRef]
- Prattichizzo, F.; Ceriello, A.; Pellegrini, V.; La Grotta, R.; Graciotti, L.; Olivieri, F.; Paolisso, P.; D’Agostino, B.; Iovino, P.; Balestrieri, M.L.; et al. Micro-Nanoplastics and Cardiovascular Diseases: Evidence and Perspectives. Eur. Heart J. 2024, 45, 4099–4110. [Google Scholar] [CrossRef]
- Revel, M.; Châtel, A.; Mouneyrac, C. Micro(Nano)Plastics: A Threat to Human Health? Curr. Opin. Environ. Sci. Health 2018, 1, 17–23. [Google Scholar] [CrossRef]
- Sangkham, S.; Faikhaw, O.; Munkong, N.; Sakunkoo, P.; Arunlertaree, C.; Chavali, M.; Mousazadeh, M.; Tiwari, A. A Review on Microplastics and Nanoplastics in the Environment: Their Occurrence, Exposure Routes, Toxic Studies, and Potential Effects on Human Health. Mar. Pollut. Bull. 2022, 181, 113832. [Google Scholar] [CrossRef] [PubMed]
- Winiarska, E.; Jutel, M.; Zemelka-Wiacek, M. The Potential Impact of Nano- and Microplastics on Human Health: Understanding Human Health Risks. Environ. Res. 2024, 251, 118535. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L. Microplastics in the Marine Environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Prata, J.C.; Castro, J.L.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T.; Cerqueira, M. The Importance of Contamination Control in Airborne Fibers and Microplastic Sampling: Experiences from Indoor and Outdoor Air Sampling in Aveiro, Portugal. Mar. Pollut. Bull. 2020, 159, 111522. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.C.; Park, B.J.; Palace, V.P. Microplastics in Aquatic Environments: Implications for Canadian Ecosystems. Environ. Pollut. 2016, 218, 269–280. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as Contaminants in the Marine Environment: A Review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Peng, L.; Fu, D.; Qi, H.; Lan, C.Q.; Yu, H.; Ge, C. Micro- and Nano-Plastics in Marine Environment: Source, Distribution and Threats—A Review. Sci. Total Environ. 2020, 698, 134254. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Kooi, M.; Law, K.L.; van Sebille, E. All Is Not Lost: Deriving a Top-down Mass Budget of Plastic at Sea. Environ. Res. Lett. 2017, 12, 114028. [Google Scholar] [CrossRef]
- Li, J.; Qu, X.; Su, L.; Zhang, W.; Yang, D.; Kolandhasamy, P.; Li, D.; Shi, H. Microplastics in Mussels along the Coastal Waters of China. Environ. Pollut. 2016, 214, 177–184. [Google Scholar] [CrossRef]
- Li, J.; Green, C.; Reynolds, A.; Shi, H.; Rotchell, J.M. Microplastics in Mussels Sampled from Coastal Waters and Supermarkets in the United Kingdom. Environ. Pollut. 2018, 241, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Iñiguez, M.E.; Conesa, J.A.; Fullana, A. Microplastics in Spanish Table Salt. Sci. Rep. 2017, 7, 8620. [Google Scholar] [CrossRef] [PubMed]
- Karami, A.; Golieskardi, A.; Keong Choo, C.; Larat, V.; Galloway, T.S.; Salamatinia, B. The Presence of Microplastics in Commercial Salts from Different Countries. Sci. Rep. 2017, 7, 46173. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Shi, H.; Li, L.; Li, J.; Jabeen, K.; Kolandhasamy, P. Microplastic Pollution in Table Salts from China. Environ. Sci. Technol. 2015, 49, 13622–13627. [Google Scholar] [CrossRef]
- Hernandez, L.M.; Xu, E.G.; Larsson, H.C.E.; Tahara, R.; Maisuria, V.B.; Tufenkji, N. Plastic Teabags Release Billions of Microparticles and Nanoparticles into Tea. Environ. Sci. Technol. 2019, 53, 12300–12310. [Google Scholar] [CrossRef]
- Kutralam-Muniasamy, G.; Pérez-Guevara, F.; Elizalde-Martínez, I.; Shruti, V.C. Branded Milks—Are They Immune from Microplastics Contamination? Sci. Total Environ. 2020, 714, 136823. [Google Scholar] [CrossRef]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Kosuth, M.; Mason, S.A.; Wattenberg, E. V Anthropogenic Contamination of Tap Water, Beer, and Sea Salt. PLoS ONE 2018, 13, e0194970. [Google Scholar] [CrossRef]
- Alimba, C.G.; Faggio, C.; Sivanesan, S.; Ogunkanmi, A.L.; Krishnamurthi, K. Micro(Nano)-Plastics in the Environment and Risk of Carcinogenesis: Insight into Possible Mechanisms. J. Hazard. Mater. 2021, 416, 126143. [Google Scholar] [CrossRef]
- Alimba, C.G.; Faggio, C. Microplastics in the Marine Environment: Current Trends in Environmental Pollution and Mechanisms of Toxicological Profile. Environ. Toxicol. Pharmacol. 2019, 68, 61–74. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Dick Vethaak, A.; Lavorante, B.R.B.O.; Lundebye, A.-K.; Guilhermino, L. Marine Microplastic Debris: An Emerging Issue for Food Security, Food Safety and Human Health. Mar. Pollut. Bull. 2018, 133, 336–348. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Cunha, S.C.; Monteiro, C.; Fernandes, J.O.; Guilhermino, L. Bisphenol A and Its Analogs in Muscle and Liver of Fish from the North East Atlantic Ocean in Relation to Microplastic Contamination. Exposure and Risk to Human Consumers. J. Hazard. Mater. 2020, 393, 122419. [Google Scholar] [CrossRef] [PubMed]
- Dris, R.; Gasperi, J.; Mirande, C.; Mandin, C.; Guerrouache, M.; Langlois, V.; Tassin, B. A First Overview of Textile Fibers, Including Microplastics, in Indoor and Outdoor Environments. Environ. Pollut. 2017, 221, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Wang, X.; Fang, T.; Xu, P.; Zhu, L.; Li, D. Source and Potential Risk Assessment of Suspended Atmospheric Microplastics in Shanghai. Sci. Total Environ. 2019, 675, 462–471. [Google Scholar] [CrossRef]
- Klein, M.; Fischer, E.K. Microplastic Abundance in Atmospheric Deposition within the Metropolitan Area of Hamburg, Germany. Sci. Total Environ. 2019, 685, 96–103. [Google Scholar] [CrossRef]
- Allen, S.; Allen, D.; Moss, K.; Le Roux, G.; Phoenix, V.R.; Sonke, J.E. Examination of the Ocean as a Source for Atmospheric Microplastics. PLoS ONE 2020, 15, e0232746. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.L.; Ng, C.T.; Zou, L.; Lu, Y.; Chen, J.; Bay, B.H.; Shen, H.-M.; Ong, C.N. Targeted Metabolomics Reveals Differential Biological Effects of Nanoplastics and NanoZnO in Human Lung Cells. Nanotoxicology 2019, 13, 1117–1132. [Google Scholar] [CrossRef]
- Chen, G.; Feng, Q.; Wang, J. Mini-Review of Microplastics in the Atmosphere and Their Risks to Humans. Sci. Total Environ. 2020, 703, 135504. [Google Scholar] [CrossRef]
- Fendall, L.S.; Sewell, M.A. Contributing to Marine Pollution by Washing Your Face: Microplastics in Facial Cleansers. Mar. Pollut. Bull. 2009, 58, 1225–1228. [Google Scholar] [CrossRef]
- Schirinzi, G.F.; Pérez-Pomeda, I.; Sanchís, J.; Rossini, C.; Farré, M.; Barceló, D. Cytotoxic Effects of Commonly Used Nanomaterials and Microplastics on Cerebral and Epithelial Human Cells. Environ. Res. 2017, 159, 579–587. [Google Scholar] [CrossRef]
- Dong, C.-D.; Chen, C.-W.; Chen, Y.-C.; Chen, H.-H.; Lee, J.-S.; Lin, C.-H. Polystyrene Microplastic Particles: In Vitro Pulmonary Toxicity Assessment. J. Hazard. Mater. 2020, 385, 121575. [Google Scholar] [CrossRef]
- Chen, Z.-H.; Wu, Y.-F.; Wang, P.-L.; Wu, Y.-P.; Li, Z.-Y.; Zhao, Y.; Zhou, J.-S.; Zhu, C.; Cao, C.; Mao, Y.-Y.; et al. Autophagy Is Essential for Ultrafine Particle-Induced Inflammation and Mucus Hyperproduction in Airway Epithelium. Autophagy 2016, 12, 297–311. [Google Scholar] [CrossRef]
- Xu, M.; Halimu, G.; Zhang, Q.; Song, Y.; Fu, X.; Li, Y.; Li, Y.; Zhang, H. Internalization and Toxicity: A Preliminary Study of Effects of Nanoplastic Particles on Human Lung Epithelial Cell. Sci. Total Environ. 2019, 694, 133794. [Google Scholar] [CrossRef]
- Lu, L.; Wan, Z.; Luo, T.; Fu, Z.; Jin, Y. Polystyrene Microplastics Induce Gut Microbiota Dysbiosis and Hepatic Lipid Metabolism Disorder in Mice. Sci. Total Environ. 2018, 631–632, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Shi, Y.; Yang, L.; Xiao, L.; Kehoe, D.K.; Gun’ko, Y.K.; Boland, J.J.; Wang, J.J. Microplastic Release from the Degradation of Polypropylene Feeding Bottles during Infant Formula Preparation. Nat. Food 2020, 1, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Gundlach, M.; Yang, S.; Jiang, J.; Velki, M.; Yin, D.; Hollert, H. Quantitative Investigation of the Mechanisms of Microplastics and Nanoplastics toward Zebrafish Larvae Locomotor Activity. Sci. Total Environ. 2017, 584–585, 1022–1031. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Y.; Lemos, B.; Ren, H. Tissue Accumulation of Microplastics in Mice and Biomarker Responses Suggest Widespread Health Risks of Exposure. Sci. Rep. 2017, 7, 46687. [Google Scholar] [CrossRef]
- Grafmueller, S.; Manser, P.; Diener, L.; Diener, P.-A.; Maeder-Althaus, X.; Maurizi, L.; Jochum, W.; Krug, H.F.; Buerki-Thurnherr, T.; von Mandach, U.; et al. Bidirectional Transfer Study of Polystyrene Nanoparticles across the Placental Barrier in an Ex Vivo Human Placental Perfusion Model. Environ. Health Perspect. 2015, 123, 1280–1286. [Google Scholar] [CrossRef]
- Sussarellu, R.; Suquet, M.; Thomas, Y.; Lambert, C.; Fabioux, C.; Pernet, M.E.J.; Le Goïc, N.; Quillien, V.; Mingant, C.; Epelboin, Y.; et al. Oyster Reproduction Is Affected by Exposure to Polystyrene Microplastics. Proc. Natl. Acad. Sci. USA 2016, 113, 2430–2435. [Google Scholar] [CrossRef]
- Forte, M.; Iachetta, G.; Tussellino, M.; Carotenuto, R.; Prisco, M.; De Falco, M.; Laforgia, V.; Valiante, S. Polystyrene Nanoparticles Internalization in Human Gastric Adenocarcinoma Cells. Toxicol. Vitr. 2016, 31, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xing, Y.; Lv, M.; Zhang, T.; Ya, H.; Jiang, B. Recent Advances on the Effects of Microplastics on Elements Cycling in the Environment. Sci. Total Environ. 2022, 849, 157884. [Google Scholar] [CrossRef]
- Wu, P.; Lin, S.; Cao, G.; Wu, J.; Jin, H.; Wang, C.; Wong, M.H.; Yang, Z.; Cai, Z. Absorption, Distribution, Metabolism, Excretion and Toxicity of Microplastics in the Human Body and Health Implications. J. Hazard. Mater. 2022, 437, 129361. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Li, P.; Zhu, X.; Chen, D.; Ommati, M.M.; Wang, H.; Han, L.; Xu, S.; Sun, P. Hepatotoxic of Polystyrene Microplastics in Aged Mice: Focus on the Role of Gastrointestinal Transformation and AMPK/FoxO Pathway. Sci. Total Environ. 2024, 917, 170471. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Rehati, P.; Yang, Z.; Cai, Z.; Guo, C.; Li, Y. The Potential Toxicity of Microplastics on Human Health. Sci. Total Environ. 2024, 912, 168946. [Google Scholar] [CrossRef]
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Distribution and Importance of Microplastics in the Marine Environment: A Review of the Sources, Fate, Effects, and Potential Solutions. Environ. Int. 2017, 102, 165–176. [Google Scholar] [CrossRef]
- Okoye, C.O.; Addey, C.I.; Oderinde, O.; Okoro, J.O.; Uwamungu, J.Y.; Ikechukwu, C.K.; Okeke, E.S.; Ejeromedoghene, O.; Odii, E.C. Toxic Chemicals and Persistent Organic Pollutants Associated with Micro-and Nanoplastics Pollution. Chem. Eng. J. Adv. 2022, 11, 100310. [Google Scholar] [CrossRef]
- Qiao, J.; Chen, R.; Wang, M.; Bai, R.; Cui, X.; Liu, Y.; Wu, C.; Chen, C. Perturbation of Gut Microbiota Plays an Important Role in Micro/Nanoplastics-Induced Gut Barrier Dysfunction. Nanoscale 2021, 13, 8806–8816. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, M.; Wang, L.; Gu, W.; Li, X.; Han, Z.; Fu, X.; Wang, X.; Li, X.; Su, Z. Continuous Oral Exposure to Micro- and Nanoplastics Induced Gut Microbiota Dysbiosis, Intestinal Barrier and Immune Dysfunction in Adult Mice. Environ. Int. 2023, 182, 108353. [Google Scholar] [CrossRef]
- Su, Q.-L.; Wu, J.; Tan, S.-W.; Guo, X.-Y.; Zou, D.-Z.; Kang, K. The Impact of Microplastics Polystyrene on the Microscopic Structure of Mouse Intestine, Tight Junction Genes and Gut Microbiota. PLoS ONE 2024, 19, e0304686. [Google Scholar] [CrossRef]
- Jiang, J.; Shu, Z.; Qiu, L. Adverse Effects and Potential Mechanisms of Polystyrene Microplastics (PS-MPs) on the Blood-Testis Barrier. Environ. Geochem. Health 2024, 46, 238. [Google Scholar] [CrossRef]
- Hirt, N.; Body-Malapel, M. Immunotoxicity and Intestinal Effects of Nano- and Microplastics: A Review of the Literature. Part. Fibre Toxicol. 2020, 17, 57. [Google Scholar] [CrossRef]
- Denk, D.; Greten, F.R. Inflammation: The Incubator of the Tumor Microenvironment. Trends Cancer 2022, 8, 901–914. [Google Scholar] [CrossRef] [PubMed]
- de Visser, K.E.; Joyce, J.A. The Evolving Tumor Microenvironment: From Cancer Initiation to Metastatic Outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Xu, Q.; Wei, R.; Wang, W.; Ding, D.; Yang, Y.; Yao, J.; Zhang, L.; Hu, Y.-Q.; Wei, G.; et al. Cancer-Associated Dynamics and Potential Regulators of Intronic Polyadenylation Revealed by IPAFinder Using Standard RNA-Seq Data. Genome Res. 2021, 31, 2095–2106. [Google Scholar] [CrossRef] [PubMed]
- Akdis, M.; Aab, A.; Altunbulakli, C.; Azkur, K.; Costa, R.A.; Crameri, R.; Duan, S.; Eiwegger, T.; Eljaszewicz, A.; Ferstl, R.; et al. Interleukins (from IL-1 to IL-38), Interferons, Transforming Growth Factor β, and TNF-α: Receptors, Functions, and Roles in Diseases. J. Allergy Clin. Immunol. 2016, 138, 984–1010. [Google Scholar] [CrossRef]
- Peng, M.; Vercauteren, M.; Grootaert, C.; Rajkovic, A.; Boon, N.; Janssen, C.; Asselman, J. Cellular and Bioenergetic Effects of Polystyrene Microplastic in Function of Cell Type, Differentiation Status and Post-Exposure Time. Environ. Pollut. 2023, 337, 122550. [Google Scholar] [CrossRef]
- Wang, Z.; Altenburger, R.; Backhaus, T.; Covaci, A.; Diamond, M.L.; Grimalt, J.O.; Lohmann, R.; Schäffer, A.; Scheringer, M.; Selin, H.; et al. We Need a Global Science-Policy Body on Chemicals and Waste. Science 2021, 371, 774–776. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shen, J.; Lin, L.; Chen, J.; Wang, L.; Deng, X.; Wu, X.; Lin, Z.; Zhang, Y.; Yu, R.; et al. Exposure to Irregular Microplastic Shed from Baby Bottles Activates the ROS/NLRP3/Caspase-1 Signaling Pathway, Causing Intestinal Inflammation. Environ. Int. 2023, 181, 108296. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Meira, A.; Norfo, R.; Wen, S.; Chédeville, A.L.; Rahman, H.; O’Sullivan, J.; Wang, G.; Louka, E.; Kretzschmar, W.W.; Paterson, A.; et al. Single-Cell Multi-Omics Identifies Chronic Inflammation as a Driver of TP53-Mutant Leukemic Evolution. Nat. Genet. 2023, 55, 1531–1541. [Google Scholar] [CrossRef]
- Cassetta, L.; Pollard, J.W. A Timeline of Tumour-Associated Macrophage Biology. Nat. Rev. Cancer 2023, 23, 238–257. [Google Scholar] [CrossRef]
- Rhee, H.S.; Closser, M.; Guo, Y.; Bashkirova, E.V.; Tan, G.C.; Gifford, D.K.; Wichterle, H. Expression of Terminal Effector Genes in Mammalian Neurons Is Maintained by a Dynamic Relay of Transient Enhancers. Neuron 2016, 92, 1252–1265. [Google Scholar] [CrossRef]
- di Lauro, A.E.; Valletta, A.; Aliberti, A.; Cangiano, M.; Dolce, P.; Sammartino, G.; Gasparro, R. The Effectiveness of Autologous Platelet Concentrates in the Clinical and Radiographic Healing after Endodontic Surgery: A Systematic Review. Materials 2023, 16, 7187. [Google Scholar] [CrossRef]
- Bai, J.; Wang, Y.; Deng, S.; Yang, Y.; Chen, S.; Wu, Z. Microplastics Caused Embryonic Growth Retardation and Placental Dysfunction in Pregnant Mice by Activating GRP78/IRE1α/JNK Axis Induced Apoptosis and Endoplasmic Reticulum Stress. Part. Fibre Toxicol. 2024, 21, 36. [Google Scholar] [CrossRef]
- Casella, C.; Ballaz, S.J. Genotoxic and Neurotoxic Potential of Intracellular Nanoplastics: A Review. J. Appl. Toxicol. 2024, 44, 1657–1678. [Google Scholar] [CrossRef]
- Liu, L.; Liu, B.; Zhang, B.; Ye, Y.; Jiang, W. Polystyrene Micro(Nano)Plastics Damage the Organelles of RBL-2H3 Cells and Promote MOAP-1 to Induce Apoptosis. J. Hazard. Mater. 2022, 438, 129550. [Google Scholar] [CrossRef] [PubMed]
- Casella, C.; Vadivel, D.; Dondi, D. The Current Situation of the Legislative Gap on Microplastics (MPs) as New Pollutants for the Environment. Water Air Soil Pollut. 2024, 235, 778. [Google Scholar] [CrossRef]
- Menafra, R.; Brinkman, A.B.; Matarese, F.; Franci, G.; Bartels, S.J.J.; Nguyen, L.; Shimbo, T.; Wade, P.A.; Hubner, N.C.; Stunnenberg, H.G. Genome-Wide Binding of MBD2 Reveals Strong Preference for Highly Methylated Loci. PLoS ONE 2014, 9, e99603. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, G.; Sun, K.; Ren, J.; Zhou, J.; Liu, X.; Lin, F.; Yang, H.; Cao, J.; Nie, L.; et al. Association of Mixed Exposure to Microplastics with Sperm Dysfunction: A Multi-Site Study in China. eBioMedicine 2024, 108, 105369. [Google Scholar] [CrossRef] [PubMed]
- Brynzak-Schreiber, E.; Schögl, E.; Bapp, C.; Cseh, K.; Kopatz, V.; Jakupec, M.A.; Weber, A.; Lange, T.; Toca-Herrera, J.L.; del Favero, G.; et al. Microplastics Role in Cell Migration and Distribution during Cancer Cell Division. Chemosphere 2024, 353, 141463. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Qin, L.; Yang, Y.; Gao, J.; Zhang, Y.; Wang, H.; Wu, Q.; Xu, B.; Liu, H. Zinc-Based ROS Amplifiers Trigger Cancer Chemodynamic/Ion Interference Therapy Through Self-Cascade Catalysis. Small 2024, 20, 2402320. [Google Scholar] [CrossRef]
- Xu, H.; Dong, C.; Yu, Z.; Ozaki, Y.; Hu, Z.; Zhang, B.; Yao, W.; Yu, J.; Xie, Y. Detection and Analysis of Microplastics in Tissues and Blood of Human Cervical Cancer Patients. Environ. Res. 2024, 259, 119498. [Google Scholar] [CrossRef]
- Dayal, L.; Yadav, K.; Dey, U.; Das, K.; Kumari, P.; Raj, D.; Mandal, R.R. Recent Advancement in Microplastic Removal Process from Wastewater—A Critical Review. J. Hazard. Mater. Adv. 2024, 16, 100460. [Google Scholar] [CrossRef]
- Li, N.; Yang, H.; Dong, Y.; Wei, B.; Liang, L.; Yun, X.; Tian, J.; Zheng, Y.; Duan, S.; Zhang, L. Prevalence and Implications of Microplastic Contaminants in General Human Seminal Fluid: A Raman Spectroscopic Study. Sci. Total Environ. 2024, 937, 173522. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, J.; Nishimura, N.; Shimada, Y. Toxicological Interactions of Microplastics/Nanoplastics and Environmental Contaminants: Current Knowledge and Future Perspectives. J. Hazard. Mater. 2021, 405, 123913. [Google Scholar] [CrossRef]
- Wang, T.; Yi, Z.; Liu, X.; Cai, Y.; Huang, X.; Fang, J.; Shen, R.; Lu, W.; Xiao, Y.; Zhuang, W.; et al. Multimodal Detection and Analysis of Microplastics in Human Thrombi from Multiple Anatomically Distinct Sites. eBioMedicine 2024, 103, 105118. [Google Scholar] [CrossRef]
- Xu, K.; Wang, Y.; Gao, X.; Wei, Z.; Han, Q.; Wang, S.; Du, W.; Wan, J.; Wan, C.; Chen, M. Polystyrene Microplastics and Di-2-Ethylhexyl Phthalate Co-Exposure: Implications for Female Reproductive Health. Environ. Sci. Ecotechnology 2024, 22, 100471. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Z.; Jin, Y.; Yang, M.; Zhang, Z.; Zhou, X.; Qiu, S.; Zou, X. Exploring the Relationships between Exposure Levels of Bisphenols and Phthalates and Prostate Cancer Occurrence. J. Hazard. Mater. 2024, 474, 134736. [Google Scholar] [CrossRef]
- Liu, D.; Guo, Z.-F.; Xu, Y.-Y.; Ka Shun Chan, F.; Xu, Y.-Y.; Johnson, M.; Zhu, Y.-G. Widespread Occurrence of Microplastics in Marine Bays with Diverse Drivers and Environmental Risk. Environ. Int. 2022, 168, 107483. [Google Scholar] [CrossRef]
- Li, L.; Zhou, H.; Zhang, C. Cuproptosis in Cancer: Biological Implications and Therapeutic Opportunities. Cell Mol. Biol. Lett. 2024, 29, 91. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Sun, R.; Yu, P.; Cheng, Y.; Wu, W.; Bao, J.; Alvarez, P.J.J. UV-Aging of Microplastics Increases Proximal ARG Donor-Recipient Adsorption and Leaching of Chemicals That Synergistically Enhance Antibiotic Resistance Propagation. J. Hazard. Mater. 2022, 427, 127895. [Google Scholar] [CrossRef] [PubMed]
- Tastet, V.; Le Vée, M.; Bruyère, A.; Fardel, O. Interactions of Human Drug Transporters with Chemical Additives Present in Plastics: Potential Consequences for Toxicokinetics and Health. Environ. Pollut. 2023, 331, 121882. [Google Scholar] [CrossRef] [PubMed]
- Vinod, L.A.; Rajendran, D.; Shivashankar, M.; Chandrasekaran, N. Surface Interaction of Vancomycin with Polystyrene Microplastics and Its Effect on Human Serum Albumin. Int. J. Biol. Macromol. 2024, 256, 128491. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Lin, Z. Exploring the Potential and Challenges of Developing Physiologically-Based Toxicokinetic Models to Support Human Health Risk Assessment of Microplastic and Nanoplastic Particles. Environ. Int. 2024, 186, 108617. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, H.; Shi, L.; Jia, Y.; Sheng, H. Detection and Quantification of Microplastics in Various Types of Human Tumor Tissues. Ecotoxicol. Environ. Saf. 2024, 283, 116818. [Google Scholar] [CrossRef]
- Hwang, J.; Choi, D.; Han, S.; Choi, J.; Hong, J. An Assessment of the Toxicity of Polypropylene Microplastics in Human Derived Cells. Sci. Total Environ. 2019, 684, 657–669. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Sun, K.; Wang, S.; Gong, D. Polystyrene Microplastics Induce Apoptosis and Necroptosis in Swine Testis Cells via ROS/MAPK/HIF1α Pathway. Environ. Toxicol. 2022, 37, 2483–2492. [Google Scholar] [CrossRef]
- Mu, Y.; Sun, J.; Li, Z.; Zhang, W.; Liu, Z.; Li, C.; Peng, C.; Cui, G.; Shao, H.; Du, Z. Activation of Pyroptosis and Ferroptosis Is Involved in the Hepatotoxicity Induced by Polystyrene Microplastics in Mice. Chemosphere 2022, 291, 132944. [Google Scholar] [CrossRef]
- Mierzejewski, K.; Kurzyńska, A.; Golubska, M.; Całka, J.; Gałęcka, I.; Szabelski, M.; Paukszto, Ł.; Andronowska, A.; Bogacka, I. New Insights into the Potential Effects of PET Microplastics on Organisms via Extracellular Vesicle-Mediated Communication. Sci. Total Environ. 2023, 904, 166967. [Google Scholar] [CrossRef]
- Elinav, E.; Nowarski, R.; Thaiss, C.A.; Hu, B.; Jin, C.; Flavell, R.A. Inflammation-Induced Cancer: Crosstalk between Tumours, Immune Cells and Microorganisms. Nat. Rev. Cancer 2013, 13, 759–771. [Google Scholar] [CrossRef]
- Das, A. The Emerging Role of Microplastics in Systemic Toxicity: Involvement of Reactive Oxygen Species (ROS). Sci. Total Environ. 2023, 895, 165076. [Google Scholar] [CrossRef]
- Kidane, D.; Chae, W.J.; Czochor, J.; Eckert, K.A.; Glazer, P.M.; Bothwell, A.L.M.; Sweasy, J.B. Interplay between DNA Repair and Inflammation, and the Link to Cancer. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 116–139. [Google Scholar] [CrossRef] [PubMed]
- Azzouz, D.; Khan, M.A.; Palaniyar, N. ROS Induces NETosis by Oxidizing DNA and Initiating DNA Repair. Cell Death Discov. 2021, 7, 113. [Google Scholar] [CrossRef]
- Jayavel, S.; Govindaraju, B.; Michael, J.R.; Viswanathan, B. Impacts of Micro and Nanoplastics on Human Health. Bull. Natl. Res. Cent. 2024, 48, 110. [Google Scholar] [CrossRef]
- Liang, B.; Zhong, Y.; Huang, Y.; Lin, X.; Liu, J.; Lin, L.; Hu, M.; Jiang, J.; Dai, M.; Wang, B.; et al. Underestimated Health Risks: Polystyrene Micro- and Nanoplastics Jointly Induce Intestinal Barrier Dysfunction by ROS-Mediated Epithelial Cell Apoptosis. Part. Fibre Toxicol. 2021, 18, 20. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Huang, X.; Bi, R.; Guo, Q.; Yu, X.; Zeng, Q.; Huang, Z.; Liu, T.; Wu, H.; Chen, Y.; et al. Detection and Analysis of Microplastics in Human Sputum. Environ. Sci. Technol. 2022, 56, 2476–2486. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Choudhury, H.; Ying, J.N.S.; Ling, J.F.S.; Ting, J.; Ting, J.S.S.; Zhia Hwen, I.K.; Suen, H.W.; Samsul Kamar, H.S.; Gorain, B.; et al. Mucoadhesive Nanocarriers as a Promising Strategy to Enhance Intracellular Delivery against Oral Cavity Carcinoma. Pharmaceutics 2022, 14, 795. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Jia, Z. Recent Insights into Uptake, Toxicity, and Molecular Targets of Microplastics and Nanoplastics Relevant to Human Health Impacts. iScience 2023, 26, 106061. [Google Scholar] [CrossRef]
- Du, J.; Wang, X.; Tao, T.; Zhang, X.; Jin, B.; Zhao, J.; Lv, Y.; Zhang, Q.; Hu, K.; Qv, W.; et al. Polystyrene Size-Dependent Impacts on Microbial Decomposers and Nutrient Cycling in Streams. Sci. Total Environ. 2023, 905, 167032. [Google Scholar] [CrossRef] [PubMed]
- Caputi, S.; Diomede, F.; Lanuti, P.; Marconi, G.D.; Di Carlo, P.; Sinjari, B.; Trubiani, O. Microplastics Affect the Inflammation Pathway in Human Gingival Fibroblasts: A Study in the Adriatic Sea. Int. J. Environ. Res. Public Health 2022, 19, 7782. [Google Scholar] [CrossRef] [PubMed]
- Giordano, M.E.; Lionetto, F.; Lionetto, M.G. Impact of Polyethylene Terephthalate Nanoplastics (PET) on Fibroblasts: A Study on NIH-3T3 Cells. Front. Physiol. 2025, 16, 1580682. [Google Scholar] [CrossRef]
- Abbasi, S.; Turner, A. Human Exposure to Microplastics: A Study in Iran. J. Hazard. Mater. 2021, 403, 123799. [Google Scholar] [CrossRef]
- Dorsch, A.; Förschner, F.; Ravandeh, M.; da Silva Brito, W.A.; Saadati, F.; Delcea, M.; Wende, K.; Bekeschus, S. Nanoplastic Size and Surface Chemistry Dictate Decoration by Human Saliva Proteins. ACS Appl. Mater. Interfaces 2024, 16, 25977–25993. [Google Scholar] [CrossRef]
- Djouina, M.; Vignal, C.; Dehaut, A.; Caboche, S.; Hirt, N.; Waxin, C.; Himber, C.; Beury, D.; Hot, D.; Dubuquoy, L.; et al. Oral Exposure to Polyethylene Microplastics Alters Gut Morphology, Immune Response, and Microbiota Composition in Mice. Environ. Res. 2022, 212, 113230. [Google Scholar] [CrossRef]
- Tanna, D.; Bhandary, S. The Hidden Hazards: The Silent Invasion of Microplastics in Dentistry (Review). World Acad. Sci. J. 2025, 7, 35. [Google Scholar] [CrossRef]
- An, R.; Wang, X.; Yang, L.; Zhang, J.; Wang, N.; Xu, F.; Hou, Y.; Zhang, H.; Zhang, L. Polystyrene Microplastics Cause Granulosa Cells Apoptosis and Fibrosis in Ovary through Oxidative Stress in Rats. Toxicology 2021, 449, 152665. [Google Scholar] [CrossRef]
- Bora, S.S.; Gogoi, R.; Sharma, M.R.; Anshu; Borah, M.P.; Deka, P.; Bora, J.; Naorem, R.S.; Das, J.; Teli, A.B. Microplastics and Human Health: Unveiling the Gut Microbiome Disruption and Chronic Disease Risks. Front. Cell. Infect. Microbiol. 2024, 14, 1492759. [Google Scholar] [CrossRef]
- Luan, J.; Wen, L.; Bao, Y.; Bai, H.; Zhao, C.; Zhang, S.; Man, X.; Yin, T.; Feng, X. Systemic Toxicity of Biodegradable Polyglycolic Acid Microplastics on the Gut-Liver-Brain Axis in Zebrafish. Sci. Total Environ. 2024, 954, 176898. [Google Scholar] [CrossRef]
- Rajkumar, D.S.; Padmanaban, R. Impact of Bisphenol A and Analogues Eluted from Resin-based Dental Materials on Cellular and Molecular Processes: An Insight on Underlying Toxicity Mechanisms. J. Appl. Toxicol. 2025, 45, 4–22. [Google Scholar] [CrossRef]
- Sabour, A.; El Helou, M.; Roger-Leroi, V.; Bauer, C. Release and Toxicity of Bisphenol-A (BPA) Contained in Orthodontic Adhesives: A Systematic Review. Int. Orthod. 2021, 19, 1–14. [Google Scholar] [CrossRef]
- Hildebrandt, L.; Nack, F.L.; Zimmermann, T.; Pröfrock, D. Microplastics as a Trojan Horse for Trace Metals. J. Hazard. Mater. Lett. 2021, 2, 100035. [Google Scholar] [CrossRef]
- Yang, Y.-F.; Chen, C.-Y.; Lu, T.-H.; Liao, C.-M. Toxicity-Based Toxicokinetic/Toxicodynamic Assessment for Bioaccumulation of Polystyrene Microplastics in Mice. J. Hazard. Mater. 2019, 366, 703–713. [Google Scholar] [CrossRef]
- Guraka, A.; Souch, G.; Duff, R.; Brown, D.; Moritz, W.; Kermanizadeh, A. Microplastic-Induced Hepatic Adverse Effects Evaluated in Advanced Quadruple Cell Human Primary Models Following Three Weeks of Repeated Exposure. Chemosphere 2024, 364, 143032. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Hao, J.; Zhang, M.; Liu, H.; Tian, F.; Zhang, X.; Jiang, Z.; Chen, C.; Gao, M.; Zhang, H. Identification and Analysis of Microplastics in Peritumoral and Tumor Tissues of Colorectal Cancer. Sci. Rep. 2025, 15, 16130. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhao, Y.; Xu, H. Trojan Horse in the Intestine: A Review on the Biotoxicity of Microplastics Combined Environmental Contaminants. J. Hazard. Mater. 2022, 439, 129652. [Google Scholar] [CrossRef]
- Saha, U.; Jena, S.; Simnani, F.Z.; Singh, D.; Choudhury, A.; Naser, S.S.; Lenka, S.S.; Kirti, A.; Nandi, A.; Sinha, A.; et al. The Unseen Perils of Oral-Care Products Generated Micro/Nanoplastics on Human Health. Ecotoxicol. Environ. Saf. 2025, 290, 117526. [Google Scholar] [CrossRef]
- Van Landuyt, K.L.; Hellack, B.; Van Meerbeek, B.; Peumans, M.; Hoet, P.; Wiemann, M.; Kuhlbusch, T.A.J.; Asbach, C. Nanoparticle Release from Dental Composites. Acta Biomater. 2014, 10, 365–374. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J.; Guo, J.; Yao, M.; Liu, Y.; Qian, J.; Ma, Q. Release of Microplastics during Dental Procedures and Denture Wear: Impact on Dental Personnel and Patients. J. Hazard. Mater. 2025, 494, 138463. [Google Scholar] [CrossRef]
- Wang, X.; Ren, X.-M.; He, H.; Li, F.; Liu, K.; Zhao, F.; Hu, H.; Zhang, P.; Huang, B.; Pan, X. Cytotoxicity and Pro-Inflammatory Effect of Polystyrene Nano-Plastic and Micro-Plastic on RAW264.7 Cells. Toxicology 2023, 484, 153391. [Google Scholar] [CrossRef] [PubMed]
- Krifka, S.; Petzel, C.; Hiller, K.-A.; Frank, E.-M.; Bosl, C.; Spagnuolo, G.; Reichl, F.-X.; Schmalz, G.; Schweikl, H. Resin Monomer-Induced Differential Activation of MAP Kinases and Apoptosis in Mouse Macrophages and Human Pulp Cells. Biomaterials 2010, 31, 2964–2975. [Google Scholar] [CrossRef] [PubMed]
- Wulff, J.; Schweikl, H.; Rosentritt, M. Cytotoxicity of Printed Resin-Based Splint Materials. J. Dent. 2022, 120, 104097. [Google Scholar] [CrossRef] [PubMed]
- LoMauro, A.; Aliverti, A. Sex Differences in Respiratory Function. Breathe 2018, 14, 131–140. [Google Scholar] [CrossRef]
- Di Spirito, F.; Raimondo, A.; Di Palo, M.P.; Martina, S.; Fordellone, M.; Rosa, D.; Amato, M.; Lembo, S. Oral Lesions and Oral Health-Related Quality of Life in Adult Patients with Psoriasis: A Retrospective Chart Review. Life 2024, 14, 347. [Google Scholar] [CrossRef]
- Godallage, A.N.; Kolekar, S.; Olsen, K.E.; Bonnesen, B.; Petersen, J.K.; Clementsen, P.F.; Bodtger, U.; Sivapalan, P. Asymptomatic Lung Nodules in Dental Professionals: A Diagnostic Challenge. Respir. Med. Case Rep. 2022, 38, 101691. [Google Scholar] [CrossRef]
- Vasse, G.F.; Melgert, B.N. Microplastic and Plastic Pollution: Impact on Respiratory Disease and Health. Eur. Respir. Rev. 2024, 33, 230226. [Google Scholar] [CrossRef]
- Han, Q.; Gao, X.; Wang, S.; Wei, Z.; Wang, Y.; Xu, K.; Chen, M. Co-Exposure to Polystyrene Microplastics and Di-(2-Ethylhexyl) Phthalate Aggravates Allergic Asthma through the TRPA1-P38 MAPK Pathway. Toxicol. Lett. 2023, 384, 73–85. [Google Scholar] [CrossRef]
- Kubo, S. Longevity of Resin Composite Restorations. Jpn. Dent. Sci. Rev. 2011, 47, 43–55. [Google Scholar] [CrossRef]
- Pisano, M.; Iandolo, A.; Abdellatif, D.; Chiacchio, A.; Galdi, M.; Martina, S. Effects of Different Curing Methods on the Color Stability of Composite Resins. Restor. Dent. Endod. 2024, 49, e33. [Google Scholar] [CrossRef]
- You, H.-J.; Jo, Y.-J.; Kim, G.; Kwon, J.; Yoon, S.-B.; Youn, C.; Kim, Y.; Kang, M.-J.; Cho, W.-S.; Kim, J.-S. Disruption of Early Embryonic Development in Mice by Polymethylmethacrylate Nanoplastics in an Oxidative Stress Mechanism. Chemosphere 2024, 361, 142407. [Google Scholar] [CrossRef]
- Li, X.; Huang, Y.; Zu, D.; Liu, H.; He, H.; Bao, Q.; He, Y.; Liang, C.; Luo, G.; Teng, Y.; et al. PMMA Nanoplastics Induce Gastric Epithelial Cellular Senescence and CGAS-STING-Mediated Inflammation via ROS Overproduction and NHEJ Suppression. Ecotoxicol. Environ. Saf. 2024, 287, 117284. [Google Scholar] [CrossRef] [PubMed]
- Rathee, M.; Malik, P.; Singh, J. Bisphenol A in Dental Sealants and Its Estrogen like Effect. Indian J. Endocrinol. Metab. 2012, 16, 339. [Google Scholar] [CrossRef]
- Bontempo, P.; Mita, L.; Doto, A.; Miceli, M.; Nebbioso, A.; Lepore, I.; Franci, G.; Menafra, R.; Carafa, V.; Conte, M.; et al. Molecular Analysis of the Apoptotic Effects of BPA in Acute Myeloid Leukemia Cells. J. Transl. Med. 2009, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Putrino, A.; Barbato, E.; Galluccio, G. Clear Aligners: Between Evolution and Efficiency—A Scoping Review. Int. J. Environ. Res. Public Health 2021, 18, 2870. [Google Scholar] [CrossRef]
- D’Antò, V.; Valletta, R.; De Simone, V.; Pisano, M.; Martina, S. Clear Aligners Treatment of Class III Subdivision with an Extraction of a Lower Bicuspid. Int. J. Environ. Res. Public Health 2023, 20, 3550. [Google Scholar] [CrossRef]
- Gold, B.P.; Siva, S.; Duraisamy, S.; Idaayath, A.; Kannan, R. Properties of Orthodontic Clear Aligner Materials—A Review. J. Evol. Med. Dent. Sci. 2021, 10, 3288–3294. [Google Scholar] [CrossRef]
- Bucci, R.; Rongo, R.; Levatè, C.; Michelotti, A.; Barone, S.; Razionale, A.V.; D’Antò, V. Thickness of Orthodontic Clear Aligners after Thermoforming and after 10 Days of Intraoral Exposure: A Prospective Clinical Study. Prog. Orthod. 2019, 20, 36. [Google Scholar] [CrossRef]
- De Stefano, A.A.; Horodynski, M.; Galluccio, G. Can Clear Aligners Release Microplastics That Impact the Patient’s Overall Health? A Systematic Review. Materials 2025, 18, 2564. [Google Scholar] [CrossRef] [PubMed]
- Quinzi, V.; Orilisi, G.; Vitiello, F.; Notarstefano, V.; Marzo, G.; Orsini, G. A Spectroscopic Study on Orthodontic Aligners: First Evidence of Secondary Microplastic Detachment after Seven Days of Artificial Saliva Exposure. Sci. Total Environ. 2023, 866, 161356. [Google Scholar] [CrossRef]
- Eliades, T.; Eliades, G. Intraoral Ageing of Aligners and Attachments: Adverse Effects on Clinical Efficiency and Release of Biologically-Active Compounds. Korean J. Orthod. 2024, 54, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Martina, S.; Rongo, R.; Bucci, R.; Razionale, A.V.; Valletta, R.; D’Antò, V. In Vitro Cytotoxicity of Different Thermoplastic Materials for Clear Aligners. Angle Orthod. 2019, 89, 942–945. [Google Scholar] [CrossRef] [PubMed]
- Premaraj, T.; Simet, S.; Beatty, M.; Premaraj, S. Oral Epithelial Cell Reaction after Exposure to Invisalign Plastic Material. Am. J. Orthod. Dentofac. Orthop. 2014, 145, 64–71. [Google Scholar] [CrossRef]
- Ferreira, M.; Costa, H.; Veiga, N.; Correia, M.J.; Gomes, A.T.P.C.; Lopes, P.C. Do Clear Aligners Release Toxic Chemicals?—A Systematic Review. J. Funct. Biomater. 2025, 16, 173. [Google Scholar] [CrossRef]
- Di Spirito, F.; D’Ambrosio, F.; Cannatà, D.; D’Antò, V.; Giordano, F.; Martina, S. Impact of Clear Aligners versus Fixed Appliances on Periodontal Status of Patients Undergoing Orthodontic Treatment: A Systematic Review of Systematic Reviews. Healthcare 2023, 11, 1340. [Google Scholar] [CrossRef]
- Amato, A.; Martina, S.; De Benedetto, G.; Michelotti, A.; Amato, M.; Di Spirito, F. Hypersensitivity in Orthodontics: A Systematic Review of Oral and Extra-Oral Reactions. J. Clin. Med. 2025, 14, 4766. [Google Scholar] [CrossRef]
- Yazdi, M.; Daryanavard, H.; Ashtiani, A.H.; Moradinejad, M.; Rakhshan, V. A Systematic Review of Biocompatibility and Safety of Orthodontic Clear Aligners and Transparent Vacuum-Formed Thermoplastic Retainers: Bisphenol-A Release, Adverse Effects, Cytotoxicity, and Estrogenic Effects. Dent. Res. J. 2023, 20, 41. [Google Scholar] [CrossRef]
- Alwafi, A.; Bichu, Y.M.; Avanessian, A.; Adel, S.M.; Vaid, N.R.; Zou, B. Overview of Systematic Reviews and Meta-Analyses Assessing the Predictability and Clinical Effectiveness of Clear Aligner Therapy. Dent. Rev. 2023, 3, 100074. [Google Scholar] [CrossRef]
- Bakdach, W.M.M.; Haiba, M.; Hadad, R. Changes in Surface Morphology, Chemical and Mechanical Properties of Clear Aligners during Intraoral Usage: A Systematic Review and Meta-Analysis. Int. Orthod. 2022, 20, 100610. [Google Scholar] [CrossRef]
- da Costa Valente, M.L.; da Silva, G.G.; de Castro, D.T.; Marinho, V.T.; Bachmann, L.; Agnelli, J.A.M.; dos Reis, A.C. Analysis of the Physical, Mechanical and Morphological Properties of Polyethylene Terephthalate Polymer in the Manufacture of Dentistry Prosthetic Components. Polym. Bull. 2023, 80, 11883–11898. [Google Scholar] [CrossRef]
- Galo Silva, G.; da Costa Valente, M.L.; Bachmann, L.; dos Reis, A.C. Use of Polyethylene Terephthalate as a Prosthetic Component in the Prosthesis on an Overdenture Implant. Mater. Sci. Eng. C 2019, 99, 1341–1349. [Google Scholar] [CrossRef]
- Saiyed, F.; Aga, M.; Subasree, S. Microplastics in Dentistry: A Narrative Review on Sources, Risks, and Sustainable Practices. J. Chem. Health Risks 2025, 15, 221–233. [Google Scholar]
- Anadioti, E.; Musharbash, L.; Blatz, M.B.; Papavasiliou, G.; Kamposiora, P. 3D Printed Complete Removable Dental Prostheses: A Narrative Review. BMC Oral Health 2020, 20, 343. [Google Scholar] [CrossRef]
- Di Spirito, F.; Giordano, F.; Di Palo, M.P.; Ferraro, C.; Cecere, L.; Frucci, E.; Caggiano, M.; Lo Giudice, R. Customized 3D-Printed Mesh, Membrane, Bone Substitute, and Dental Implant Applied to Guided Bone Regeneration in Oral Implantology: A Narrative Review. Dent. J. 2024, 12, 303. [Google Scholar] [CrossRef]
- Battista, E.; Gasparro, R.; Cacciola, M.; Sammartino, G.; Marenzi, G. Dynamic Navigation System for Immediate Implant Placement in the Maxillary Aesthetic Region. Appl. Sci. 2022, 12, 5510. [Google Scholar] [CrossRef]
- Jorge, J.H.; Giampaolo, E.T.; Machado, A.L.; Vergani, C.E. Cytotoxicity of Denture Base Acrylic Resins: A Literature Review. J. Prosthet. Dent. 2003, 90, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Madhumitha, C.T.; Karmegam, N.; Biruntha, M.; Arun, A.; Al Kheraif, A.A.; Kim, W.; Kumar, P. Extraction, Identification, and Environmental Risk Assessment of Microplastics in Commercial Toothpaste. Chemosphere 2022, 296, 133976. [Google Scholar] [CrossRef] [PubMed]
- Ustabasi, G.S.; Baysal, A. Bacterial Interactions of Microplastics Extracted from Toothpaste under Controlled Conditions and the Influence of Seawater. Sci. Total Environ. 2020, 703, 135024. [Google Scholar] [CrossRef]
- Teubl, B.J.; Stojkovic, B.; Docter, D.; Pritz, E.; Leitinger, G.; Poberaj, I.; Prassl, R.; Stauber, R.H.; Fröhlich, E.; Khinast, J.G.; et al. The Effect of Saliva on the Fate of Nanoparticles. Clin. Oral Investig. 2018, 22, 929–940. [Google Scholar] [CrossRef] [PubMed]
| Exposure Route | Primary Sources | Target Systems | Health Effects | Description | Common Sources | Potential Health Impact |
|---|---|---|---|---|---|---|
| Ingestion [4,7,14,20,34,35,36,37,38,39,40,41,42,43,44,45,46,47,59,65] | Contaminated food and water, seafood, salt, tea, dairy | Gastrointestinal tract, liver, kidneys | Gut barrier disruption, inflammation, microbial dysbiosis, tissue accumulation | Entry via consumption of contaminated food, water, seafood, salt, tea, dairy, and bottled water | Seafood, drinking water, table salt, tea bags, dairy, bottled water | Intestinal inflammation, disruption of microbiota, systemic distribution via M cells |
| Inhalation [2,48,49,50,51,52,53,57,58] | Airborne particles from textiles, industrial emissions, and sea spray | Respiratory tract, brain | Pulmonary inflammation, oxidative stress, DNA damage, potential neurotoxicity | Entry via breathing airborne MPs/NPs from textiles, industrial emissions, indoor dust, and sea spray | Synthetic textiles, industrial processes, sea breeze, atmospheric transport | Respiratory tract irritation, inflammation, potential neurotoxicity via blood–brain barrier |
| Dermal Contact [26,27,54,55] | Cosmetics, personal care products, airborne fallout | Skin, immune system | Allergic reactions, oxidative stress in epithelial cells, and potential for deeper tissue exposure with NPs | Entry via skin contact with cosmetic products or environmental fallout; more plausible with NPs | Cosmetics (e.g., exfoliants, face washes), personal care products, airborne particles | Skin irritation, immune activation, potential oxidative stress in epithelial cells |
| Mechanism | Description |
|---|---|
| Accumulation and Bioavailability | MNPs can enter the body through ingestion, inhalation, and dermal exposure. Once internalized, they are distributed to organs such as the liver, kidneys, spleen, lungs, and reproductive tissues. Their bioavailability is influenced by size, surface chemistry, and the ‘Trojan horse’ effect—co-transport of pollutants [22,23,66,67,68,69,70]. |
| Cellular and Molecular Toxicity | MNPs cause mitochondrial dysfunction, oxidative phosphorylation impairment, lysosomal permeabilization, ER stress, and apoptosis, contributing to tumor aggressiveness and therapy resistance [14,88,90,96]. They disrupt autophagy and induce necroptosis and pyroptosis, affecting stem and proliferative cells the most [81,88,90,110,111,112,113]. |
| Microbiota Dysbiosis | MNP exposure alters gut microbiota composition, reducing commensals and promoting pro-inflammatory bacteria. It impairs the gut barrier and stem cell regulation, enhances genotoxin bioavailability, and affects immune and metabolic pathways linked to cancer [72,73,74,75,77,78]. |
| Oxidative and Inflammatory Tumor Microenvironment | MNPs induce oxidative stress and chronic inflammation, leading to ROS overproduction and activation of cytokines (IL-1β, TNF-α, TGF-β) and inflammasomes (NLRP3). These microenvironments promote DNA damage immunosuppressive cell recruitment, the activation of pro-oncogenic pathways (NF-κB, HIF1α), and remodeling of the TME, EMT, angiogenesis, and tumor immune evasion [77,80,83,85,86,114,115,116,117]. |
| Genotoxicity and Epigenetic Alterations | MNPs cause DNA strand breaks, chromosomal instability, and micronuclei. They modulate epigenetic marks like DNA methylation, histone acetylation, and miRNA expression, affecting cancer-related genes and DNA repair [88,89,93,94]. |
| Synergistic Effects with Environmental Contaminants | MNPs adsorb and co-deliver heavy metals, POPs, EDCs, enhancing their bioavailability and toxicity. Co-exposure disrupts endocrine, reproductive, and immune systems, aggravating oxidative and genotoxic effects [71,99,102,110]. |
| Topic | Main Findings |
|---|---|
| General Health Hazards | MNPs from oral products/dental materials cause oral mucosal damage, dysbiosis, systemic inflammation, genotoxicity, and potential carcinogenesis [15,129]. |
| Systemic Dissemination | Dental-derived MNPs disseminate systemically, necessitating reassessment of dental material safety [62,129]. |
| Oral Cancer Pathogenesis | Chronic oral exposure to MNPs causes oxidative stress, mitochondrial dysfunction, DNA damage, promoting carcinogenesis [74,119,128]. |
| Real-World Exposure | MNPs (PE, PET, PS) found in human saliva and sputum; persistent contact risks inflammation and dysplasia [24,120]. |
| Oral Health Effects | MNPs reduce gingival cell viability, disrupt redox homeostasis, and impair oral barrier function [124,125]. |
| Oral Microbiota and Inflammation | MNPs alter oral microbiota, trigger cytokine release (IL-1β, IL-6, TNF-α), leading to periodontal damage [128]. |
| Systemic Toxicity | Oral-derived MNPs translocate to organs, induce oxidative stress, immune and endocrine disruption. |
| Endocrine Disruption | BPA, phthalates from dental plastics mimic hormones, affecting reproductive and thyroid systems [102,133,134]. |
| Trojan Horse Effect | MNPs carry toxicants like metals and antibiotics, enhancing systemic toxicity [135,139]. |
| Cancerogenesis | MNPs promote carcinogenesis via DNA damage, immune modulation, and microbiota dysbiosis [5,62,136]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Spirito, F.; Folliero, V.; Di Palo, M.P.; De Benedetto, G.; Aulisio, L.; Martina, S.; Rinaldi, L.; Franci, G. Micro- and Nanoplastics and the Oral Cavity: Implications for Oral and Systemic Health, Dental Practice, and the Environment—A Narrative Review. J. Funct. Biomater. 2025, 16, 332. https://doi.org/10.3390/jfb16090332
Di Spirito F, Folliero V, Di Palo MP, De Benedetto G, Aulisio L, Martina S, Rinaldi L, Franci G. Micro- and Nanoplastics and the Oral Cavity: Implications for Oral and Systemic Health, Dental Practice, and the Environment—A Narrative Review. Journal of Functional Biomaterials. 2025; 16(9):332. https://doi.org/10.3390/jfb16090332
Chicago/Turabian StyleDi Spirito, Federica, Veronica Folliero, Maria Pia Di Palo, Giuseppina De Benedetto, Leonardo Aulisio, Stefano Martina, Luca Rinaldi, and Gianluigi Franci. 2025. "Micro- and Nanoplastics and the Oral Cavity: Implications for Oral and Systemic Health, Dental Practice, and the Environment—A Narrative Review" Journal of Functional Biomaterials 16, no. 9: 332. https://doi.org/10.3390/jfb16090332
APA StyleDi Spirito, F., Folliero, V., Di Palo, M. P., De Benedetto, G., Aulisio, L., Martina, S., Rinaldi, L., & Franci, G. (2025). Micro- and Nanoplastics and the Oral Cavity: Implications for Oral and Systemic Health, Dental Practice, and the Environment—A Narrative Review. Journal of Functional Biomaterials, 16(9), 332. https://doi.org/10.3390/jfb16090332









