Electrospinning of Bovine Split Hide Collagen and Collagen/Glycosaminoglycan for a Study of Stem Cell Adhesion and Proliferation on the Mats: Influence of Composition and Structural Morphology
Abstract
1. Introduction
2. Experiment
2.1. Materials
2.2. Electrospinning Solutions
2.2.1. BSHC Solutions
- PBS and PBS20x solutions of BSHC
- PBS/ethanol and PBS20x/ethanol solutions of BSHC
- Acetic acid/ethanol solutions of BSHC
- HFP solutions BSHC
2.2.2. BSHC/GAGs Solutions
- Hyaluronic acid:BSHC/HA5 (5 wt.% HA), BSHC/HA10 (10 wt.%), BSHC/HA15 (15 wt.%).
- Chondroitin sulfate:BSHC/CS5 (5 wt.%), BSHC/CS10 (10 wt.%).
- Both hyaluronic acid and chondroitin sulfate:BSHC/HA5/CS5 (5 wt.% HA/ 5 wt.% CS);BSHC/HA10/CS5 (10 wt.% HA/ 5 wt. CS%);BSHC/HA10/CS10 (10 wt.% HA/10 wt.% CS).
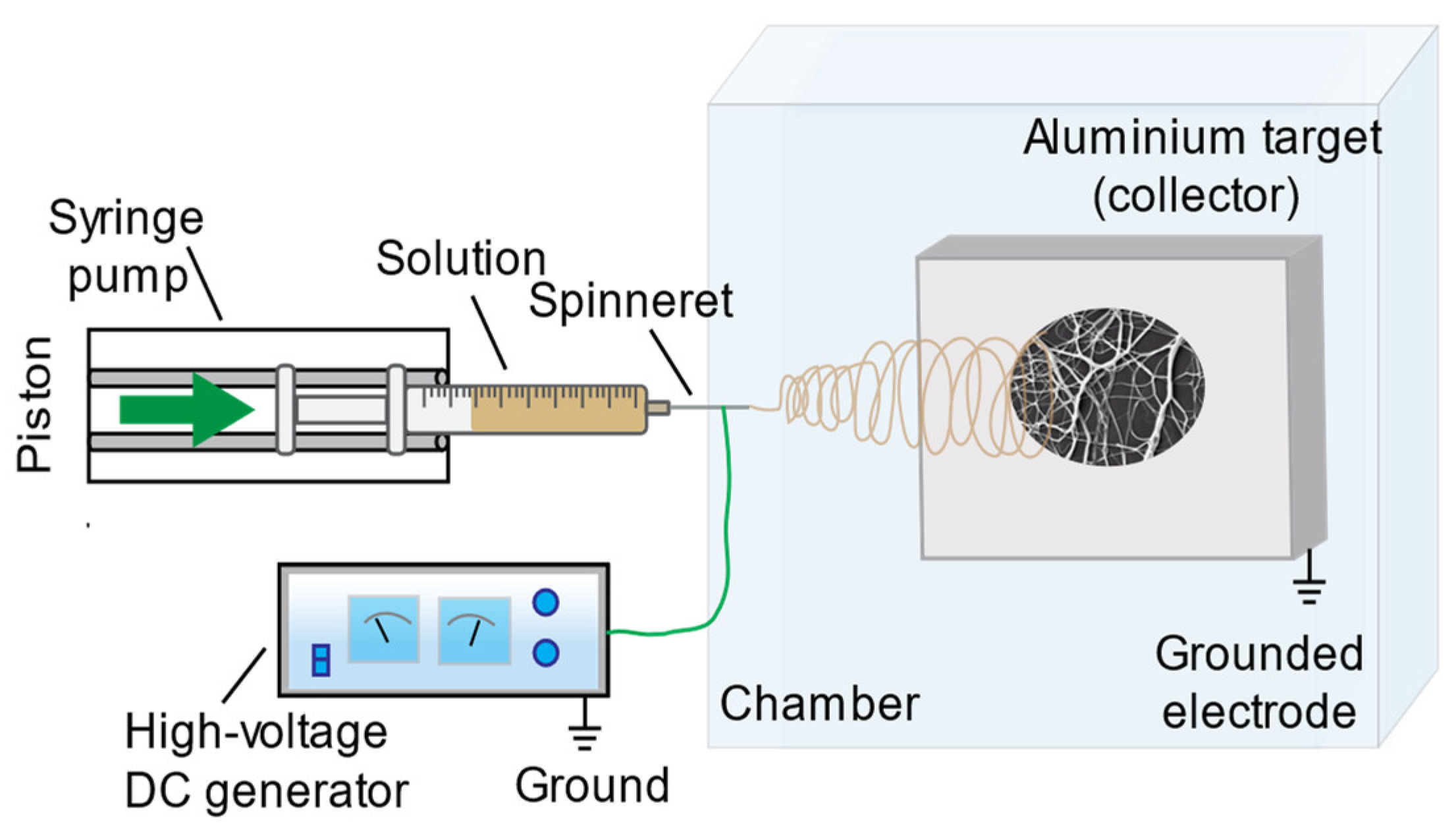
2.3. Electrospinning
2.4. Scanning Electron Microscopy
2.5. Cell Experiments
- Cell culture conditions
- Indirect Cytotoxicity Evaluation
- Overall cell morphology, cell number, and spreading area
- Immunostaining of actin cytoskeleton and focal adhesion contacts
- Cell proliferation assay
3. Results and Discussion
3.1. Electrospinning of BSHC from Benign Solvents
3.2. Electrospun BSHC Mats
3.3. Electrospun BSHC/GAG Mats
3.4. Behavior of Human Adipose-Derived Mesenchymal Stem Cells (hAD-MSCs)
3.4.1. Indirect Method
3.4.2. Direct Approach
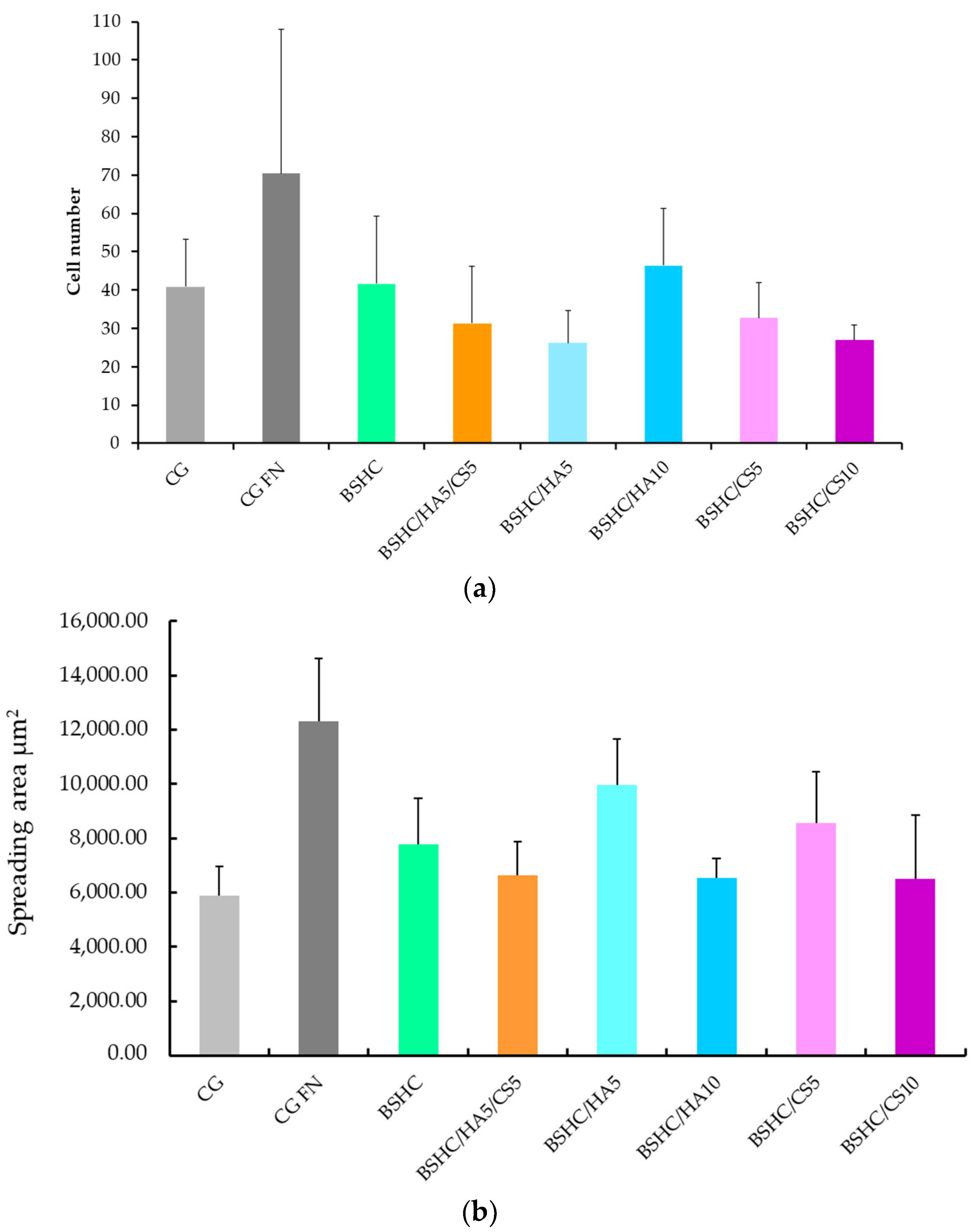
- Overall cell morphology, cell number, and spreading area
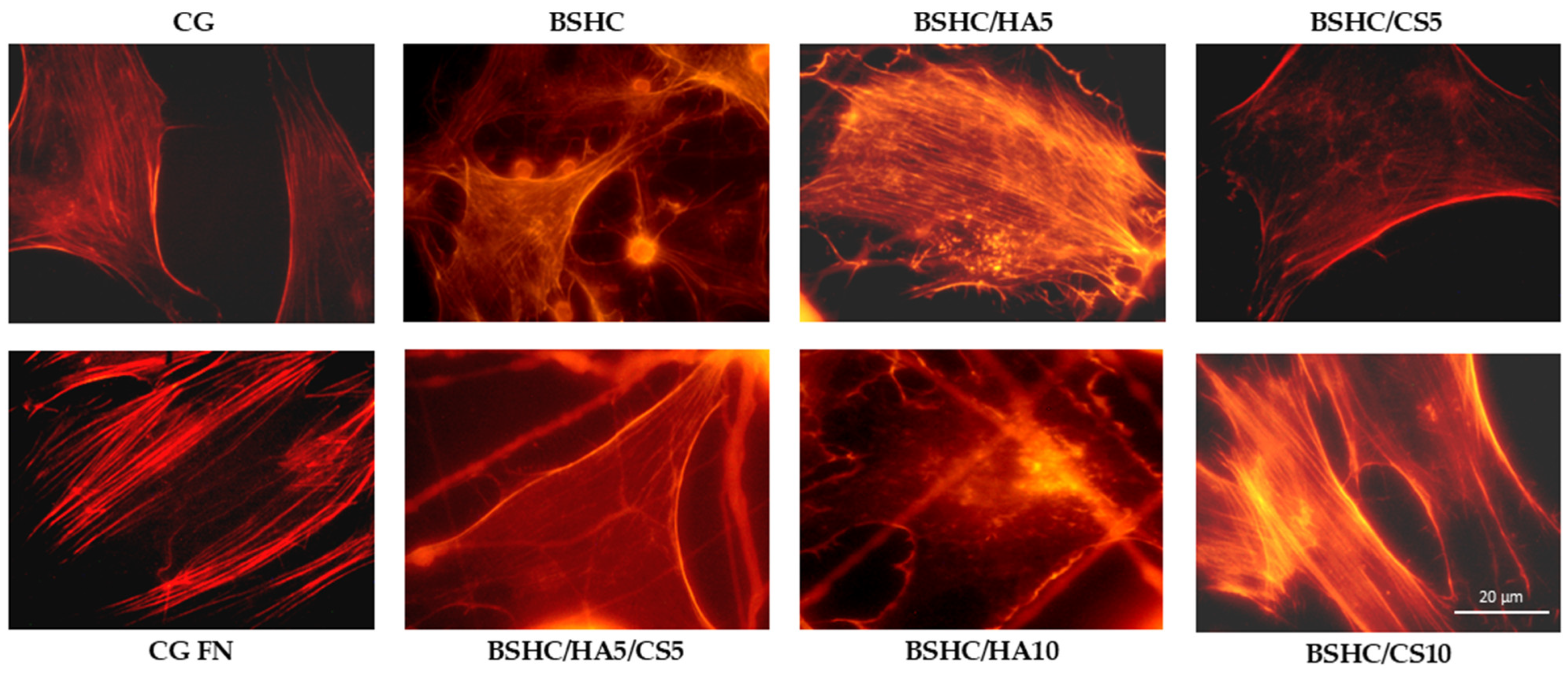
- Organization of actin cytoskeleton
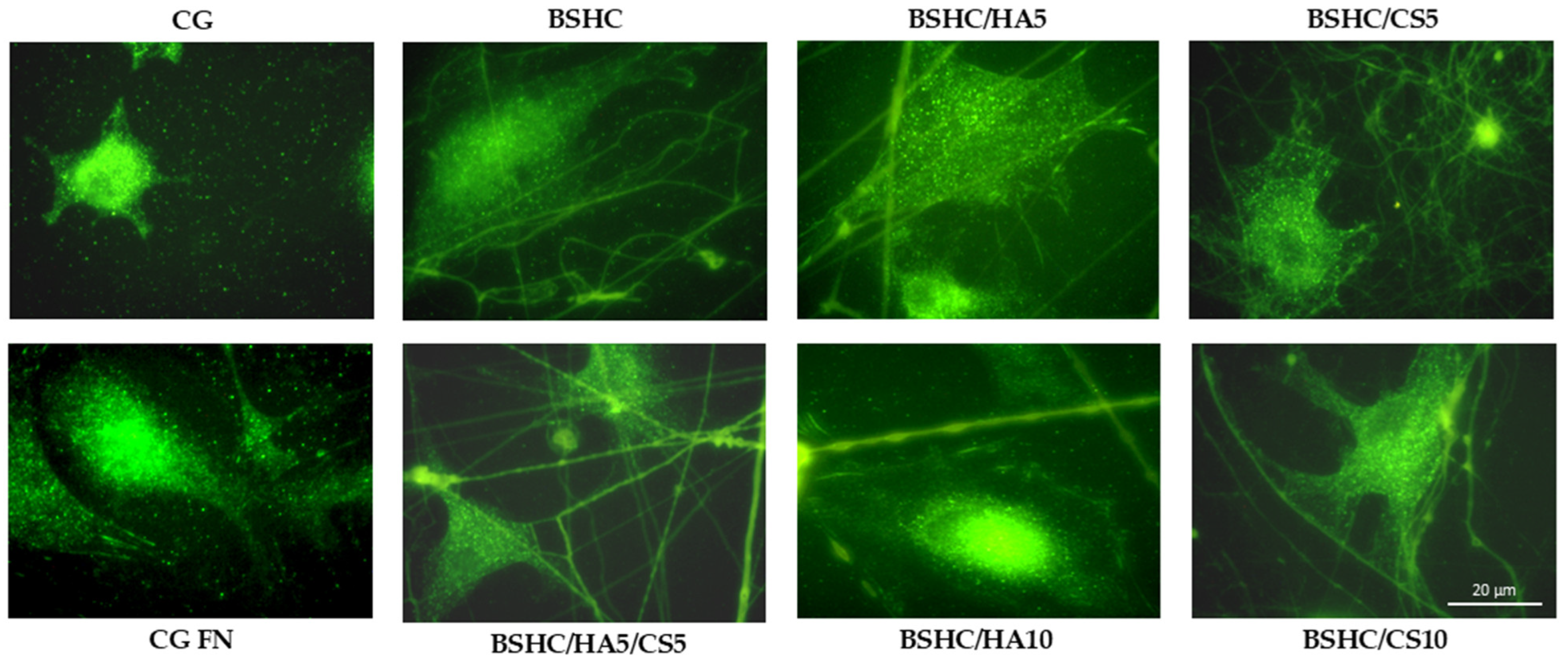
- Vinculin Expression and Focal Adhesion Formation on Electrospun Fibrous Mats
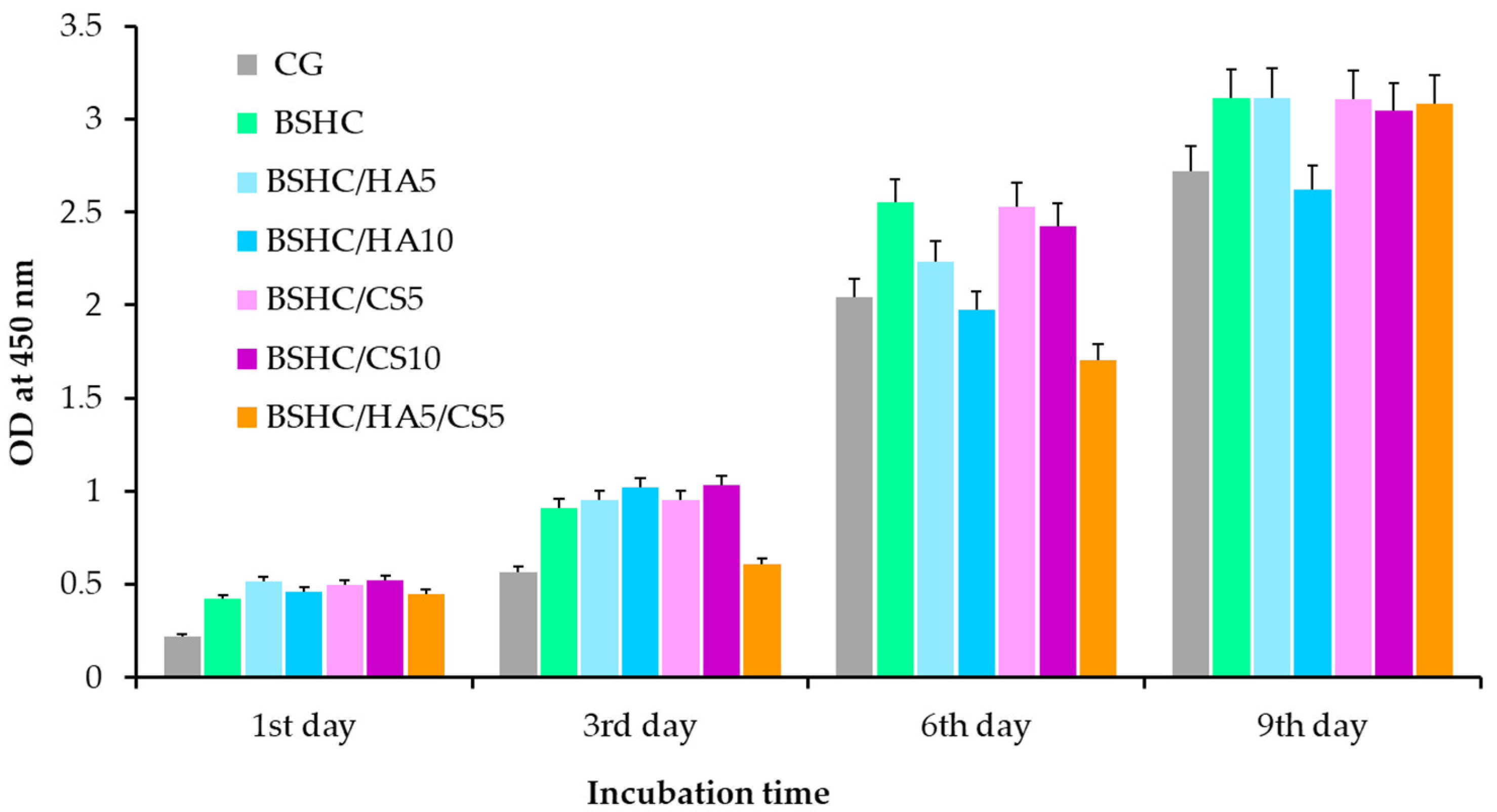
- Proliferation of hAD-MSCs on Electrospun Fibrous Mats
- The structural influence: Optimal cell spreading occurs on linear micro/submicron fiber mats (BSHC, BSHC/HA5, and BSHC/HA10); the fiber architecture (linear vs. branched) significantly impacts focal adhesion formation; mats with linear fibers provide superior mechanical support for cell attachment.
- Compositional effects: HA-containing mats (BSHC/HA5) show exceptional cell spreading, rivaling fibronectin controls; GAG incorporation influences initial adhesion but not long-term proliferation; all compositions maintain cytoskeletal integrity and actin expression.
- Adhesion–Proliferation Dynamics: While adhesion rates vary among mats, all support long-term proliferation (6-fold increase by day 9), an inverse relationship exists between the adhesion density and spreading area, and the composition/structure differentially regulates attachment versus spreading behavior.
- Biocompatibility: Rapid confluence was achieved within 24 h across all mats; no indirect cytotoxicity was observed, and all mats supported sustained growth comparable to controls.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| hAD-MSCs | Human adipose-derived mesenchymal stem cells. |
| BSHC | Bovine split hide collagen. |
| DMEM | Dulbecco’s Modified Eagle Medium. |
| MSCs | Mesenchymal stem cells. |
| ECM | Extracellular matrix. |
| GAG | Glycosaminoglycan. |
| SEM | Scanning electron microscopy. |
| HFP | Hexafluoropropanol. |
| BPS | Standard phosphate buffer. |
| CG-FN | Cover glass fibronectin coated. |
| GA | Glutar aldehyde. |
| CS | Chondroitin sulfate. |
| CG | Cover glass. |
References
- Lu, W.P.; Guo, Y.; Lu, W.P.; Guo, Y. Electrospinning of Collagen and Its Derivatives for Biomedical Applications. In Novel Aspects of Nanofibers; IntechOpen: London, UK, 2018; ISBN 978-1-78923-075-8. [Google Scholar]
- Bianchi, S.; Bernardi, S.; Simeone, D.; Torge, D.; Macchiarelli, G.; Marchetti, E. Proliferation and Morphological Assessment of Human Periodontal Ligament Fibroblast towards Bovine Pericardium Membranes: An In Vitro Study. Materials 2022, 15, 8284. [Google Scholar] [CrossRef]
- Kai, D.; Jin, G.; Prabhakaran, M.P.; Ramakrishna, S. Electrospun Synthetic and Natural Nanofibers for Regenerative Medicine and Stem Cells. Biotechnol. J. 2013, 8, 59–72. [Google Scholar] [CrossRef]
- Blackstone, B.N.; Gallentine, S.C.; Powell, H.M. Collagen-Based Electrospun Materials for Tissue Engineering: A Systematic Review. Bioengineering 2021, 8, 39. [Google Scholar] [CrossRef]
- Hu, X.; Wu, Z.; Zhang, Z.; Yao, H.; Wang, D.-A. Type II Collagen Scaffolds for Tissue Engineering. Commun. Mater. 2024, 5, 149. [Google Scholar] [CrossRef]
- Kulkarni, M.A.; Gangal, A.R.; Khare, S.S.; Mundada, H.G.; Pujari, R.R. Electrospun Collagen Based Nanofibrous Mats for Wound Healing: An Integrative Review. Biosci. Biotechnol. Res. Asia 2022, 19, 515–528. [Google Scholar] [CrossRef]
- Drobota, M.; Gradinaru, L.M.; Vlad, S.; Bargan, A.; Butnaru, M.; Angheloiu, M.; Aflori, M. Preparation and Characterization of Electrospun Collagen Based Composites for Biomedical Applications. Materials 2020, 13, 3961. [Google Scholar] [CrossRef]
- Roche, S.; Ronzière, M.-C.; Herbage, D.; Freyria, A.-M. Native and DPPA Cross-Linked Collagen Sponges Seeded with Fetal Bovine Epiphyseal Chondrocytes Used for Cartilage Tissue Engineering. Biomaterials 2000, 22, 9–18. [Google Scholar] [CrossRef]
- Luo, X.; Guo, Z.; He, P.; Chen, T.; Li, L.; Ding, S.; Li, H. Study on Structure, Mechanical Property and Cell Cytocompatibility of Electrospun Collagen Nanofibers Crosslinked by Common Agents. Int. J. Biol. Macromol. 2018, 113, 476–486. [Google Scholar] [CrossRef]
- Zong, X.; Bien, H.; Chung, C.-Y.; Yin, L.; Fang, D.; Hsiao, B.S.; Chu, B.; Entcheva, E. Electrospun Fine-Textured Scaffolds for Heart Tissue Constructs. Biomaterials 2005, 26, 5330–5338. [Google Scholar] [CrossRef] [PubMed]
- Hapach, L.A.; VanderBurgh, J.A.; Miller, J.P.; Reinhart-King, C.A. Manipulation of in Vitro Collagen Matrix Architecture for Scaffolds of Improved Physiological Relevance. Phys. Biol. 2015, 12, 061002. [Google Scholar] [CrossRef]
- Zeugolis, D.I.; Khew, S.T.; Yew, E.S.Y.; Ekaputra, A.K.; Tong, Y.W.; Yung, L.-Y.L.; Hutmacher, D.W.; Sheppard, C.; Raghunath, M. Electro-Spinning of Pure Collagen Nano-Fibres—Just an Expensive Way to Make Gelatin? Biomaterials 2008, 29, 2293–2305. [Google Scholar] [CrossRef]
- Dong, B.; Arnoult, O.; Smith, M.E.; Wnek, G.E. Electrospinning of Collagen Nanofiber Scaffolds from Benign Solvents. Macromol. Rapid Commun. 2009, 30, 539–542. [Google Scholar] [CrossRef]
- Arnoult, O. A Novel Benign Solution for Collagen Processing. Ph.D. Thesis, Case Western Reserve University, Cleveland, OH, USA, 2010. [Google Scholar]
- Visser, D.; Rogg, K.; Fuhrmann, E.; Marzi, J.; Schenke-Layland, K.; Hartmann, H. Electrospinning of Collagen: Enzymatic and Spectroscopic Analyses Reveal Solvent-Independent Disruption of the Triple-Helical Structure. J. Mater. Chem. B 2023, 11, 2207–2218. [Google Scholar] [CrossRef]
- Melo, P.; Montalbano, G.; Boggio, E.; Gigliotti, C.L.; Dianzani, C.; Dianzani, U.; Vitale-Brovarone, C.; Fiorilli, S. Electrospun Collagen Scaffold Bio-Functionalized with Recombinant ICOS-Fc: An Advanced Approach to Promote Bone Remodelling. Polymers 2022, 14, 3780. [Google Scholar] [CrossRef]
- Caliari, S.R.; Ramirez, M.A.; Harley, B.A.C. The Development of Collagen-GAG Scaffold-Membrane Composites for Tendon Tissue Engineering. Biomaterials 2011, 32, 8990–8998. [Google Scholar] [CrossRef]
- Harley, B.A.C.; Gibson, L.J. In Vivo and in Vitro Applications of Collagen-GAG Scaffolds. Chem. Eng. J. 2008, 137, 102–121. [Google Scholar] [CrossRef]
- Yang, P.; Lu, Y.; Gou, W.; Qin, Y.; Tan, J.; Luo, G.; Zhang, Q. Glycosaminoglycans’ Ability to Promote Wound Healing: From Native Living Macromolecules to Artificial Biomaterials. Adv. Sci. 2024, 11, 2305918. [Google Scholar] [CrossRef]
- Tierney, C.M.; Haugh, M.G.; Liedl, J.; Mulcahy, F.; Hayes, B.; O’Brien, F.J. The Effects of Collagen Concentration and Crosslink Density on the Biological, Structural and Mechanical Properties of Collagen-GAG Scaffolds for Bone Tissue Engineering. J. Mech. Behav. Biomed. Mater. 2009, 2, 202–209. [Google Scholar] [CrossRef]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The Effect of Mean Pore Size on Cell Attachment, Proliferation and Migration in Collagen–Glycosaminoglycan Scaffolds for Bone Tissue Engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Lungu, A.; Albu, M.G.; Florea, N.M.; Stancu, I.C.; Vasile, E.; Iovu, H. The Influence of Glycosaminoglycan Type on the Collagen-Glycosaminoglycan Porous Scaffolds. Dig. J. Nanomater. Biostructures 2011, 6, 1867–1875. [Google Scholar]
- Law, J.X.; Liau, L.L.; Saim, A.; Yang, Y.; Idrus, R. Electrospun Collagen Nanofibers and Their Applications in Skin Tissue Engineering. Tissue Eng. Regen. Med. 2017, 14, 699–718. [Google Scholar] [CrossRef]
- Anderegg, U.; Halfter, N.; Schnabelrauch, M.; Hintze, V. Collagen/Glycosaminoglycan-Based Matrices for Controlling Skin Cell Responses. Biol. Chem. 2021, 402, 1325–1335. [Google Scholar] [CrossRef]
- Tören, E.; Mazari, A.A. Needless Electrospun Collagen/Hyaluronic Acid Nanofibers for Skin Moisturization: Research. Polym. Adv. Technol. 2024, 35, e6434. [Google Scholar] [CrossRef]
- Yannas, I.V.; Tzeranis, D.S.; So, P.T.C. Regeneration Mechanism for Skin and Peripheral Nerves Clarified at the Organ and Molecular Scales. Curr. Opin. Biomed. Eng. 2018, 6, 1–7. [Google Scholar] [CrossRef]
- Menard, C.; Mitchell, S.; Spector, M. Contractile Behavior of Smooth Muscle Actin-Containing Osteoblasts in Collagen-GAG Matrices in Vitro: Implant-Related Cell Contraction. Biomaterials 2000, 21, 1867–1877. [Google Scholar] [CrossRef]
- Pfeiffer, E.; Vickers, S.M.; Frank, E.; Grodzinsky, A.J.; Spector, M. The Effects of Glycosaminoglycan Content on the Compressive Modulus of Cartilage Engineered in Type II Collagen Scaffolds. Osteoarthr. Cartil. 2008, 16, 1237–1244. [Google Scholar] [CrossRef]
- Caliari, S.R.; Harley, B.A.C. The Effect of Anisotropic Collagen-GAG Scaffolds and Growth Factor Supplementation on Tendon Cell Recruitment, Alignment, and Metabolic Activity. Biomaterials 2011, 32, 5330–5340. [Google Scholar] [CrossRef]
- Todd, E.A.; Mirsky, N.A.; Silva, B.L.G.; Shinde, A.R.; Arakelians, A.R.L.; Nayak, V.V.; Marcantonio, R.A.C.; Gupta, N.; Witek, L.; Coelho, P.G. Functional Scaffolds for Bone Tissue Regeneration: A Comprehensive Review of Materials, Methods, and Future Directions. J. Funct. Biomater. 2024, 15, 280. [Google Scholar] [CrossRef]
- Dewey, M.J.; Kolliopoulos, V.; Ngo, M.T.; Harley, B.A.C. Glycosaminoglycan Content of a Mineralized Collagen Scaffold Promotes Mesenchymal Stem Cell Secretion of Factors to Modulate Angiogenesis and Monocyte Differentiation. Materialia 2021, 18, 101149. [Google Scholar] [CrossRef]
- Keogh, M.B.; O’Brien, F.J.; Daly, J.S. Substrate Stiffness and Contractile Behaviour Modulate the Functional Maturation of Osteoblasts on a Collagen–GAG Scaffold. Acta Biomater. 2010, 6, 4305–4313. [Google Scholar] [CrossRef]
- Tierney, C.M.; Jaasma, M.J.; O’Brien, F.J. Osteoblast Activity on Collagen-GAG Scaffolds Is Affected by Collagen and GAG Concentrations. J. Biomed. Mater. Res. Part A 2009, 91A, 92–101. [Google Scholar] [CrossRef]
- Murphy, C.M.; Duffy, G.P.; Schindeler, A.; O’brien, F.J. Effect of Collagen-Glycosaminoglycan Scaffold Pore Size on Matrix Mineralization and Cellular Behavior in Different Cell Types. J. Biomed. Mater. Res. Part A 2016, 104, 291–304. [Google Scholar] [CrossRef]
- Osmond, M.J.; Krebs, M.D.; Pantcheva, M.B. Human Trabecular Meshwork Cell Behavior Is Influenced by Collagen Scaffold Pore Architecture and Glycosaminoglycan Composition. Biotechnol. Bioeng. 2020, 117, 3150–3159. [Google Scholar] [CrossRef]
- O’Brien, F.J.; Harley, B.A.; Yannas, I.V.; Gibson, L.J. The Effect of Pore Size on Cell Adhesion in Collagen-GAG Scaffolds. Biomaterials 2005, 26, 433–441. [Google Scholar] [CrossRef]
- Li, Z.; Hu, X.; Zhong, J.F. Mesenchymal Stem Cells: Characteristics, Function, and Application. Stem Cells Int. 2019, 2019, 8106818. [Google Scholar] [CrossRef]
- Tanabe, S. Role of Mesenchymal Stem Cells in Cell Life and Their Signaling. World J. Stem Cells 2014, 6, 24–32. [Google Scholar] [CrossRef]
- Koczkowska, M.; Kostecka, A.; Zawrzykraj, M.; Myszczyński, K.; Skoniecka, A.; Deptuła, M.; Tymińska, A.; Czerwiec, K.; Jąkalski, M.; Zieliński, J.; et al. Identifying Differentiation Markers Between Dermal Fibroblasts and Adipose-derived Mesenchymal Stromal Cells (AD-MSCs) in Human Visceral and Subcutaneous Tissues Using Single-cell Transcriptomics. Stem Cell Res. Ther. 2025, 16, 64–73. [Google Scholar] [CrossRef]
- Alcayaga-Miranda, F.; Cuenca, J.; Khoury, M. Antimicrobial Activity of Mesenchymal Stem Cells: Current Status and New Perspectives of Antimicrobial Peptide-Based Therapies. Front. Immunol. 2017, 8, 339. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Available online: https://www.liebertpub.com/doi/10.1089/107632701300062859 (accessed on 7 April 2025).
- Kern, S.; Eichler, H.; Stoeve, J.; Klüter, H.; Bieback, K. Comparative Analysis of Mesenchymal Stem Cells from Bone Marrow, Umbilical Cord Blood, or Adipose Tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kwon, B.; Lee, K.; Son, Y.H.; Chung, S.G. Therapeutic Mechanisms of Human Adipose-Derived Mesenchymal Stem Cells in a Rat Tendon Injury Model. Am. J. Sports Med. 2017, 45, 1429–1439. [Google Scholar] [CrossRef] [PubMed]
- Melief, S.M.; Zwaginga, J.J.; Fibbe, W.E.; Roelofs, H. Adipose Tissue-Derived Multipotent Stromal Cells Have a Higher Immunomodulatory Capacity Than Their Bone Marrow-Derived Counterparts. Stem Cells Transl. Med. 2013, 2, 455–463. [Google Scholar] [CrossRef]
- Doshi, J.; Reneker, D.H. Electrospinning Process and Applications of Electrospun Fibers. J. Electrost. 1995, 35, 151–160. [Google Scholar] [CrossRef]
- Castilla-Casadiego, D.A.; Rivera-Martínez, C.A.; Quiñones-Colón, B.A.; Almodóvar, J. Electrospun Collagen Scaffolds. In Electrospun Biomaterials and Related Technologies; Almodovar, J., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 21–55. ISBN 978-3-319-70049-6. [Google Scholar]
- Huang, G.P.; Shanmugasundaram, S.; Masih, P.; Pandya, D.; Amara, S.; Collins, G.; Arinzeh, T.L. An Investigation of Common Crosslinking Agents on the Stability of Electrospun Collagen Scaffolds. J. Biomed. Mater. Res. Part A 2015, 103, 762–771. [Google Scholar] [CrossRef]
- Campiglio, C.E.; Marcolin, C.; Draghi, L.; Campiglio, C.E.; Marcolin, C.; Draghi, L. Electrospun ECM Macromolecules as Biomimetic Scaffold for Regenerative Medicine: Challenges for Preserving Conformation and Bioactivity. AIMS Mater. Sci. 2017, 4, 638–669. [Google Scholar] [CrossRef][Green Version]
- Yannas, I.V.; Lee, E.; Orgill, D.P.; Skrabut, E.M.; Murphy, G.F. Synthesis and Characterization of a Model Extracellular Matrix That Induces Partial Regeneration of Adult Mammalian Skin. Proc. Natl. Acad. Sci. USA 1989, 86, 933–937. [Google Scholar] [CrossRef]
- Zhong, S.; Teo, W.E.; Zhu, X.; Beuerman, R.; Ramakrishna, S.; Yung, L.Y.L. Formation of Collagen−Glycosaminoglycan Blended Nanofibrous Scaffolds and Their Biological Properties. Biomacromolecules 2005, 6, 2998–3004. [Google Scholar] [CrossRef]
- Tian, H.; Chen, Y.; Ding, C.; Li, G. Interaction Study in Homogeneous Collagen-Chondroitin sulfate Blends by Twodimensional Infrared Spectroscopy. Carbohydr. Polym. 2012, 89, 542–550. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; International Standard: Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Zhang, L.; Webster, T.J. Nanotechnology and Nanomaterials: Promises for Improved Tissue Regeneration. Nano Today 2009, 4, 66–80. [Google Scholar] [CrossRef]
- Fröhlich, E.; Salar-Behzadi, S. Toxicological Assessment of Inhaled Nanoparticles: Role of in Vivo, Ex Vivo, in Vitro, and in Silico Studies. Int. J. Mol. Sci. 2014, 15, 4795–4822. [Google Scholar] [CrossRef]
- Clift, M.J.D.; Rothen-Rutishauser, B.; Brown, D.M.; Duffin, R.; Donaldson, K.; Proudfoot, L.; Guy, K.; Stone, V. The Impact of Different Nanoparticle Surface Chemistry and Size on Uptake and Toxicity in a Murine Macrophage Cell Line. Toxicol. Appl. Pharmacol. 2008, 232, 418–427. [Google Scholar] [CrossRef]
- Armour, A.D.; Powell, H.M.; Boyce, S.T. Fluorescein Diacetate for Determination of Cell Viability in Tissue-Engineered Skin. Tissue Eng. Part C Methods 2008, 14, 89–96. [Google Scholar] [CrossRef]
- Dalton, C.J.; Lemmon, C.A. Fibronectin: Molecular Structure, Fibrillar Structure and Mechanochemical Signaling. Cells 2021, 10, 2443. [Google Scholar] [CrossRef]
- Pankov, R.; Yamada, K.M. Fibronectin at a Glance. J. Cell Sci. 2002, 115, 3861–3863. [Google Scholar] [CrossRef]
- Aota, S.; Yamada, K.M. Fibronectin and Cell Adhesion: Specificity of Integrin–Ligand Interaction. In Advances in Enzymology and Related Areas of Molecular Biology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1995; pp. 1–21. ISBN 978-0-470-12316-4. [Google Scholar]
- Baer, P.C.; Geiger, H. Adipose-Derived Mesenchymal Stromal/Stem Cells: Tissue Localization, Characterization, and Heterogeneity. Stem Cells Int. 2012, 2012, 812693. [Google Scholar] [CrossRef]
- Geiger, B.; Bershadsky, A.; Pankov, R.; Yamada, K.M. Transmembrane Crosstalk between the Extracellular Matrix and the Cytoskeleton. Nat. Rev. Mol. Cell Biol. 2001, 2, 793–805. [Google Scholar] [CrossRef]
- Barnes, C.P.; Sell, S.A.; Boland, E.D.; Simpson, D.G.; Bowlin, G.L. Nanofiber Technology: Designing the next Generation of Tissue Engineering Scaffolds. Adv. Drug Deliv. Rev. 2007, 59, 1413–1433. [Google Scholar] [CrossRef]
- Matthews, J.A.; Wnek, G.E.; Simpson, D.G.; Bowlin, G.L. Electrospinning of Collagen Nanofibers. Biomacromolecules 2002, 3, 232–238. [Google Scholar] [CrossRef]
- Denchai, A.; Tartarini, D.; Mele, E. Cellular Response to Surface Morphology: Electrospinning and Computational Modeling. Front. Bioeng. Biotechnol. 2018, 24, 155. [Google Scholar] [CrossRef]
- Schnell, E.; Klinkhammer, K.; Balzer, S.; Brook, G.; Klee, D.; Dalton, P.; Mey, J. Guidance of Glial Cell Migration and Axonal Growth on Electrospun Nanofibers of Poly-ε-Caprolactone and a Collagen/Poly-ε-Caprolactone Blend. Biomaterials 2007, 28, 3012–3025. [Google Scholar] [CrossRef]
- Gourlay, C.W.; Ayscough, K.R. The Actin Cytoskeleton: A Key Regulator of Apoptosis and Ageing? Nat. Rev. Mol. Cell Biol. 2005, 6, 583–589. [Google Scholar] [CrossRef]
- Paim, Á.; Tessaro, I.C.; Cardozo, N.S.M.; Pranke, P. Mesenchymal Stem Cell Cultivation in Electrospun Scaffolds: Mechanistic Modeling for Tissue Engineering. J. Biol. Phys. 2018, 44, 245–271. [Google Scholar] [CrossRef]
- Bershadsky, A.D.; Balaban, N.Q.; Geiger, B. Adhesion-Dependent Cell Mechanosensitivity. Annu. Rev. Cell Dev. Biol. 2003, 19, 677–695. [Google Scholar] [CrossRef]
- Parsons, J.T.; Horwitz, A.R.; Schwartz, M.A. Cell Adhesion: Integrating Cytoskeletal Dynamics and Cellular Tension. Nat. Rev. Mol. Cell Biol. 2010, 11, 633–643. [Google Scholar] [CrossRef]
- Huang, Z.-M.; Zhang, Y.-Z.; Kotaki, M.; Ramakrishna, S. A Review on Polymer Nanofibers by Electrospinning and Their Applications in Nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, Q.; Huo, B. Focal Adhesion Regulates Osteogenic Differentiation of Mesenchymal Stem Cells and Osteoblasts. Biomater. Transl. 2021, 2, 312–322. [Google Scholar] [CrossRef]
- Zaidel-Bar, R.; Itzkovitz, S.; Ma’ayan, A.; Iyengar, R.; Geiger, B. Functional Atlas of the Integrin Adhesome. Nat. Cell Biol. 2007, 9, 858–867. [Google Scholar] [CrossRef]
- Chung, E.; Yoon, G.; (Matthew) Suh, H. A Collagen-Hyaluronic Acid Matrix for Stem Cell Culture. In Biological, Physical and Technical Basics of Cell Engineering; Artmann, G., Artmann, A., Zhubanova, A., Digel, I., Eds.; Springer: Singapore, 2018. [Google Scholar] [CrossRef]
- Murphy, C.M.; Matsiko, A.; Haugh, M.G.; Gleeson, J.P.; O’Brien, F.J. Mesenchymal Stem Cell Fate is Regulated by the Composition and Mechanical Properties of Collagen-Glycosaminoglycan Scaffolds. J. Mech. Behav. Biomed. Mater. 2012, 11, 53–62. [Google Scholar] [CrossRef]
- Amann, E.; Wolff, P.; Breel, E.; Van Griensven, M.; Balmayor, E.R. Hyaluronic Acid Facilitates chondrogenesis and Matrix Deposition of Human Adipose Derived Mesenchymal Stem Cells and Human Chondrocytes Co-cultures. Acta Biomater. 2017, 52, 130–144. [Google Scholar] [CrossRef]
- He, X.; Wang, S.; Yu, X.; Zhou, X. Bone Marrow Mesenchymal Stem Cells Response on Collagen/hyaluronan/chondroitin Scaffold Enriched with Gentamicin-loaded Gelatin Microparticles for Skin Ttissue Engineering. J. Biomater. Appl. 2023, 38, 134–145. [Google Scholar] [CrossRef]
- Somaiah, C.; Kumar, A.; Mawrie, D.; Sharma, A.; Patil, S.D.; Bhattacharyya, J.; Swaminathan, R.; Jaganathan, B.G. Collagen Promotes Higher Adhesion, Survival and Proliferation of Mesenchymal Stem Cells. PLoS ONE 2015, 10, e0145068. [Google Scholar] [CrossRef]
- Kennedy, K.M.; Bhaw-Luximon, A.; Jhurry, D. Cell-matrix mechanical interaction in electrospun polymeric scaffolds for tissue engineering: Implications for scaffold design and performance. Acta Biomater. 2017, 50, 41–55. [Google Scholar] [CrossRef] [PubMed]
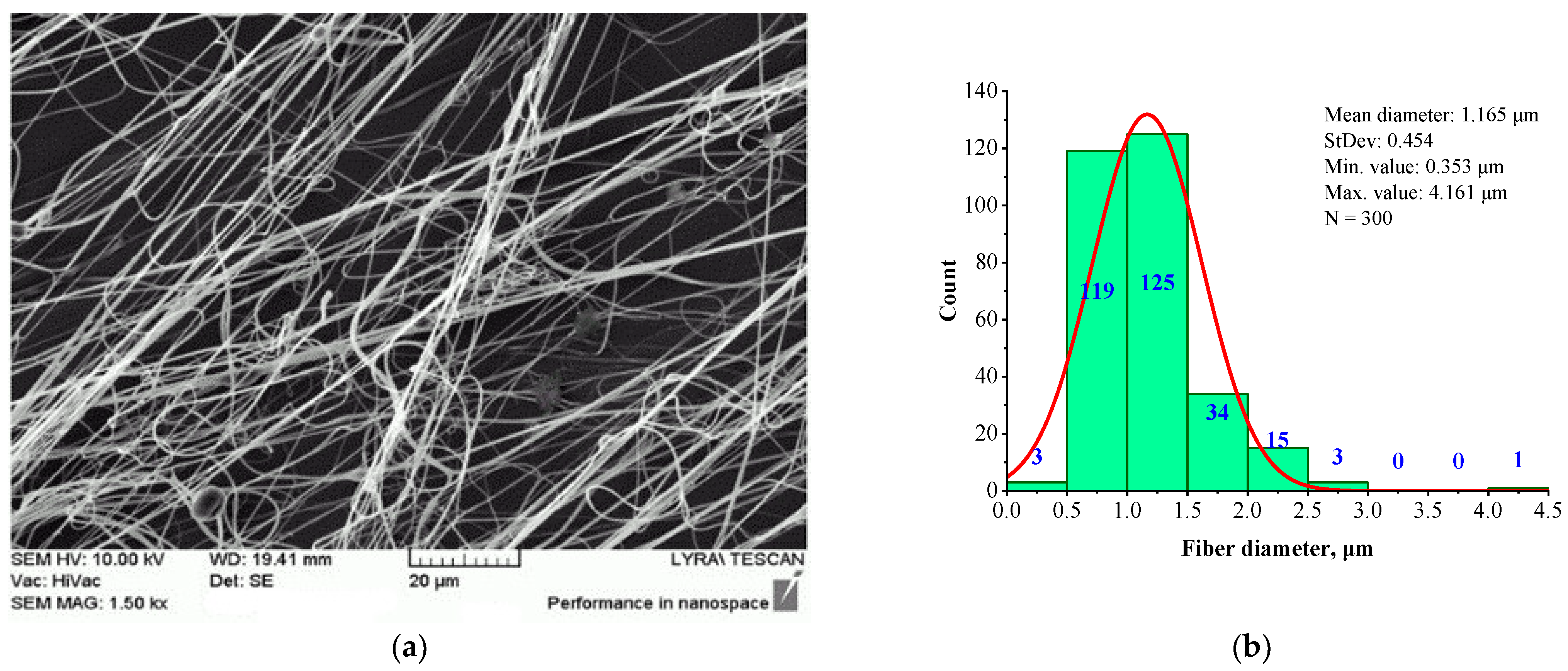
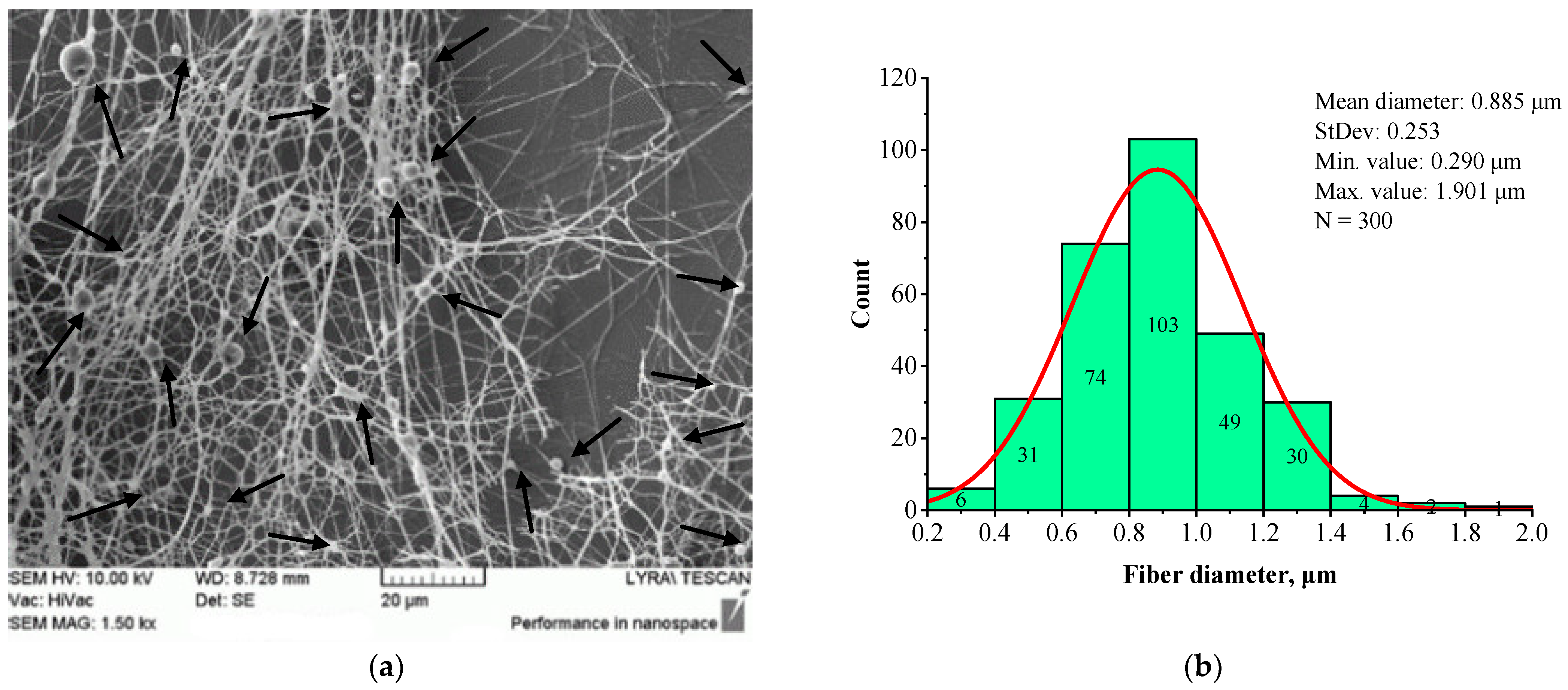
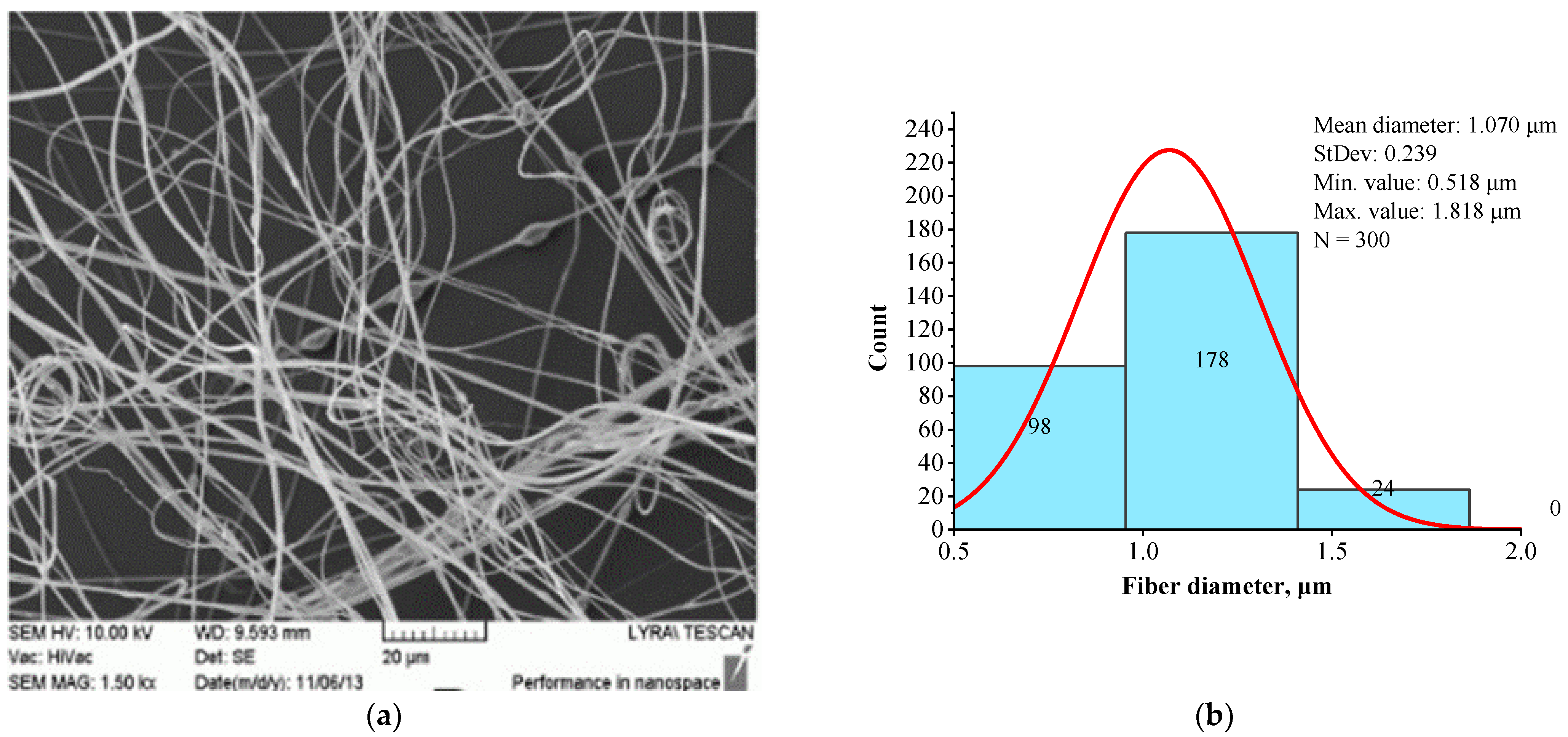
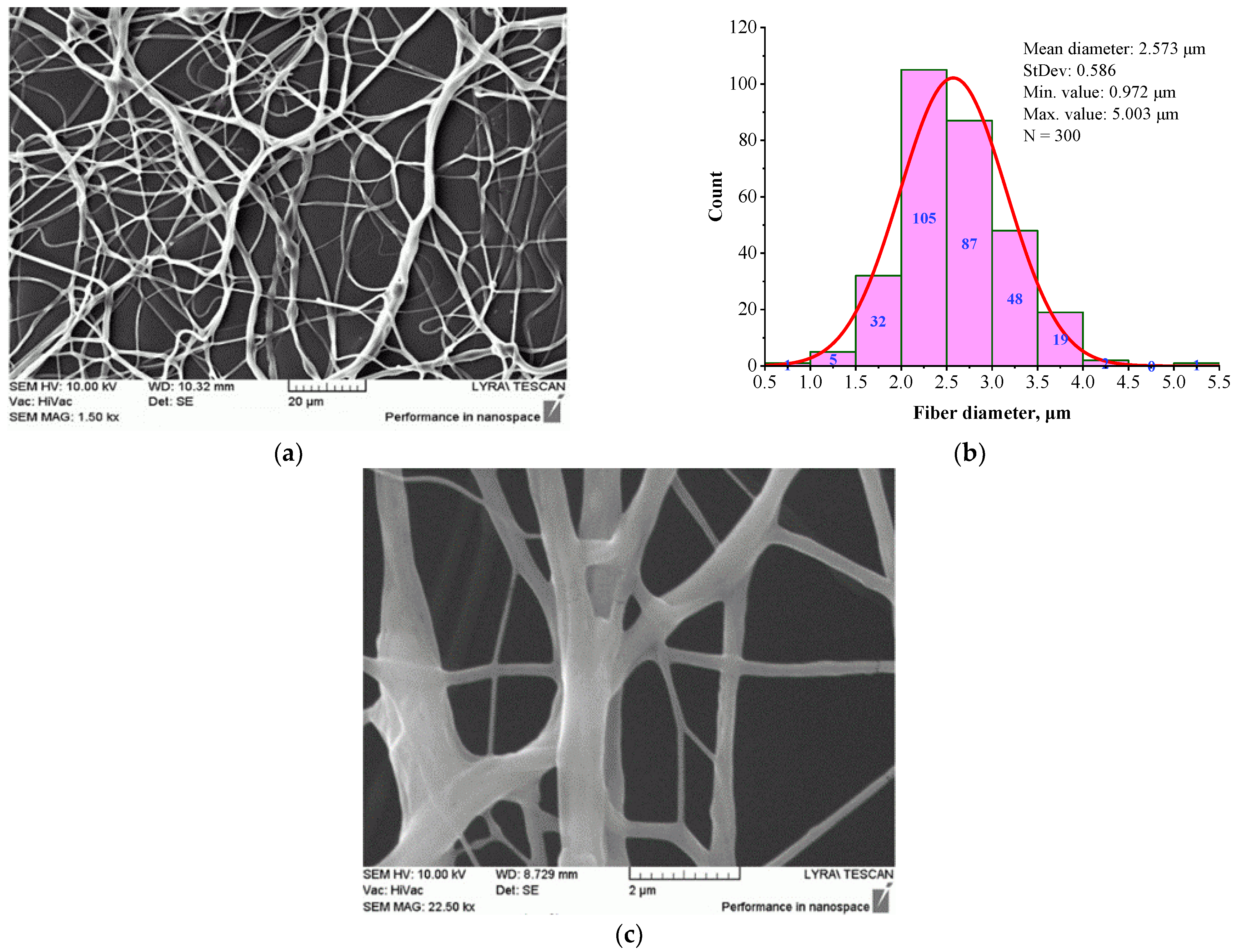



| Sample | Structure | Mean Fiber Diameter (µm) | Min Diameter (µm) | Max Diameter (µm) |
|---|---|---|---|---|
| BHSC | Linear | 1.165 ± 0.454 | 0.353 | 3.003 |
| BSHC/HA5 | Linear | 1.070 ± 0.518 | 0.518 | 1.818 |
| BSHC/HA10 | Linear | 0.832 ± 0.270 | 0.129 | 1.707 |
| BSHC/CS5 | Branched | 2.573 ± 0.586 | 0.972 | 5.003 |
| BSHC/CS10 | Branched | 2.196 ±0.838 | 0.705 | 5.193 |
| BSHC/HA5/CS5 | High degree of entanglement | 0.960 ± 0.233 | 0.397 | 1.741 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vladkova, T.G.; Gospodinova, D.N.; Dineff, P.D.; Keremidarska-Markova, M.; Hristova-Panusheva, K.; Krasteva, N. Electrospinning of Bovine Split Hide Collagen and Collagen/Glycosaminoglycan for a Study of Stem Cell Adhesion and Proliferation on the Mats: Influence of Composition and Structural Morphology. J. Funct. Biomater. 2025, 16, 219. https://doi.org/10.3390/jfb16060219
Vladkova TG, Gospodinova DN, Dineff PD, Keremidarska-Markova M, Hristova-Panusheva K, Krasteva N. Electrospinning of Bovine Split Hide Collagen and Collagen/Glycosaminoglycan for a Study of Stem Cell Adhesion and Proliferation on the Mats: Influence of Composition and Structural Morphology. Journal of Functional Biomaterials. 2025; 16(6):219. https://doi.org/10.3390/jfb16060219
Chicago/Turabian StyleVladkova, Todorka G., Dilyana N. Gospodinova, Peter D. Dineff, Milena Keremidarska-Markova, Kamelia Hristova-Panusheva, and Natalia Krasteva. 2025. "Electrospinning of Bovine Split Hide Collagen and Collagen/Glycosaminoglycan for a Study of Stem Cell Adhesion and Proliferation on the Mats: Influence of Composition and Structural Morphology" Journal of Functional Biomaterials 16, no. 6: 219. https://doi.org/10.3390/jfb16060219
APA StyleVladkova, T. G., Gospodinova, D. N., Dineff, P. D., Keremidarska-Markova, M., Hristova-Panusheva, K., & Krasteva, N. (2025). Electrospinning of Bovine Split Hide Collagen and Collagen/Glycosaminoglycan for a Study of Stem Cell Adhesion and Proliferation on the Mats: Influence of Composition and Structural Morphology. Journal of Functional Biomaterials, 16(6), 219. https://doi.org/10.3390/jfb16060219









