A Modular Biomimetic Preclinical Platform to Elucidate the Interaction Between Cancer Cells and the Bone Metastatic Niche
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Sr-Doped α-Tricalcium Phosphate Cements (SrCPCs)
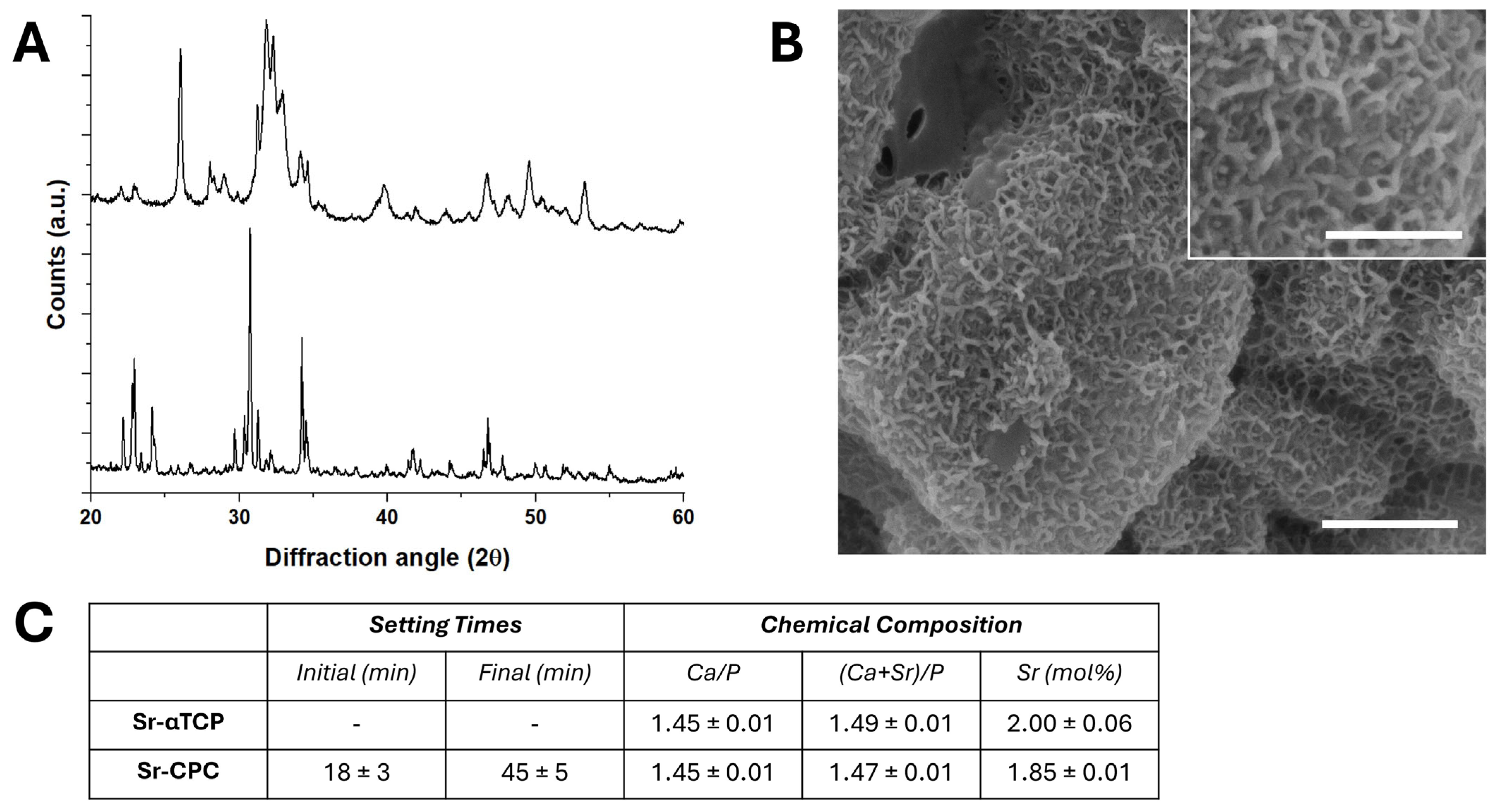
2.2. Characterization of SrCPCs
2.3. Sterilization and Preconditioning of SrCPCs
2.4. In Vitro Culturing of BC Cells
2.5. Osteoclasts Differentiation from Human Peripheral Blood Mononuclear Cells
2.6. Osteoblast Differentiation from Human Mesenchymal Stem Cells
2.7. Immunofluorescence Analysis
2.8. Cell Fluorescence Quantification
2.9. Culture of Ex Vivo Material from Bone Metastatic Patients on SrCPCs
2.10. Viability Assay of the Ex Vivo Models
2.11. Evaluation of Cellular Necrosis of Ex Vivo Models
2.12. Histology
2.13. Gene Expression Analyses
2.13.1. Procedure for Cell Culture Experiments
2.13.2. Procedure for Ex Vivo Models
2.14. Statistical Analyses
3. Results
3.1. Characterization of Strontium-Doped Apatitic Bone Cement (SrCPCs)
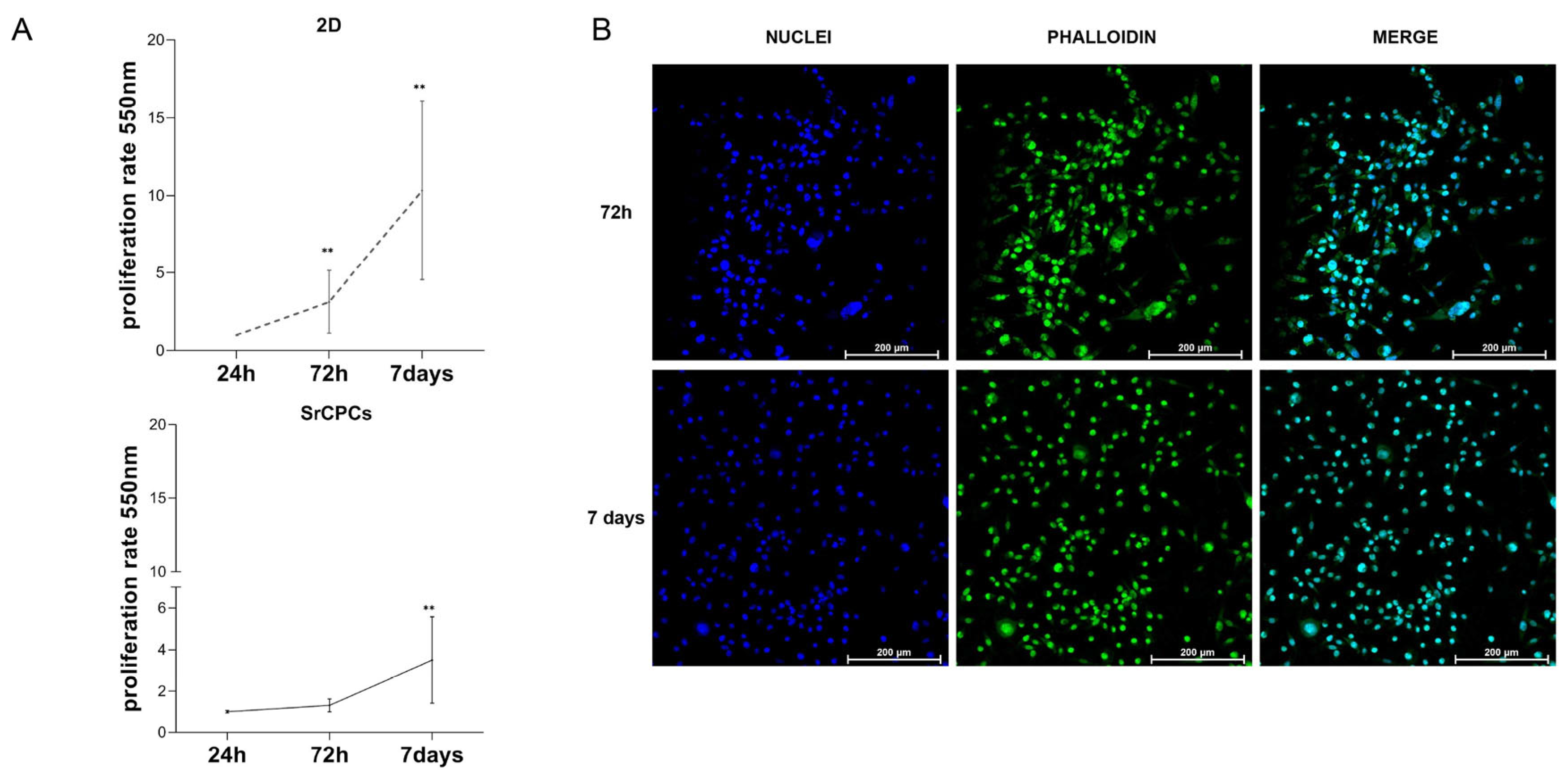
3.2. Culture of BC Cells on SrCPCs
3.3. OCs Differentiation on SrCPCs
3.4. OBs Differentiation on SrCPCs
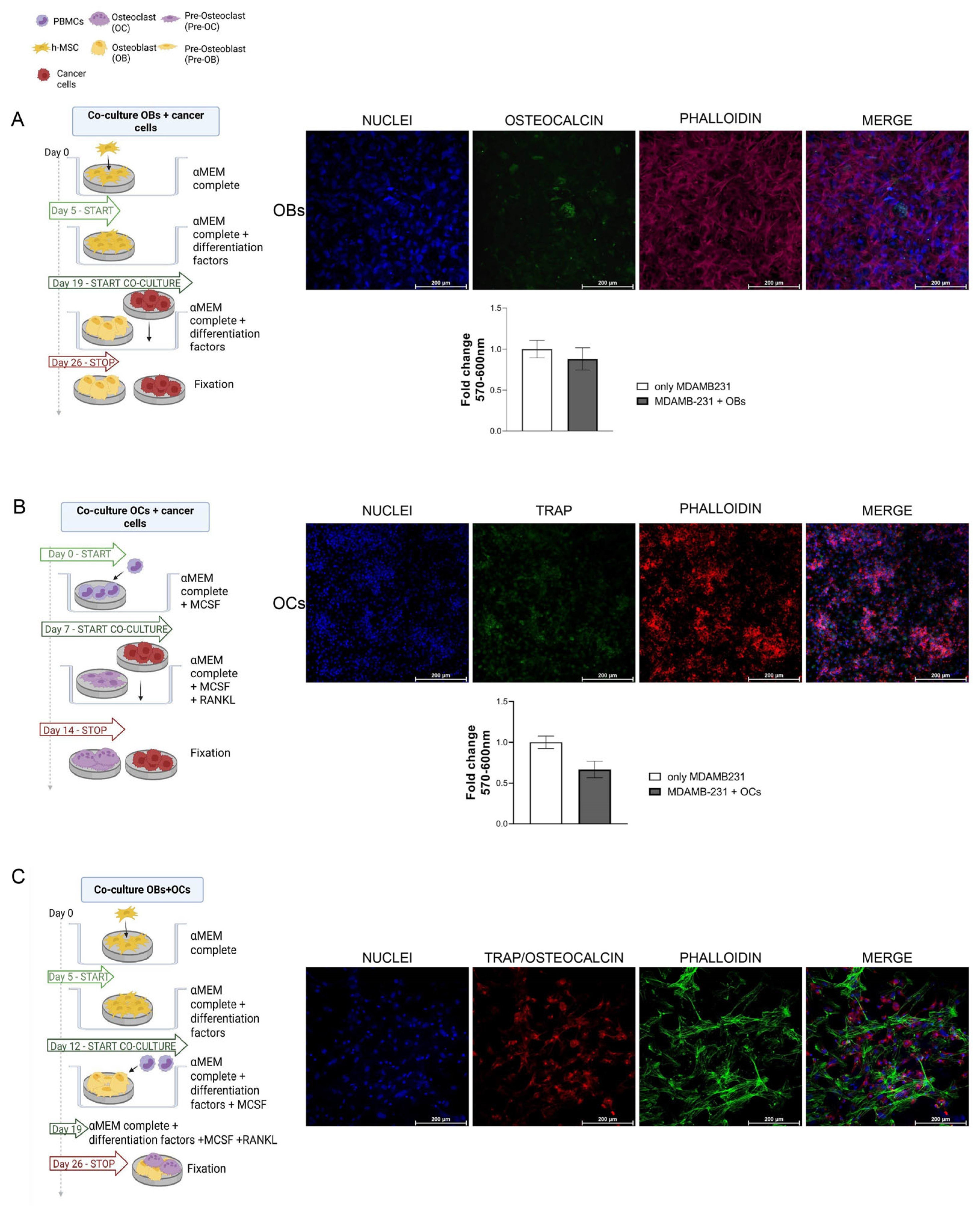
3.5. Cancer Cells and Bone Cells Co-Cultures
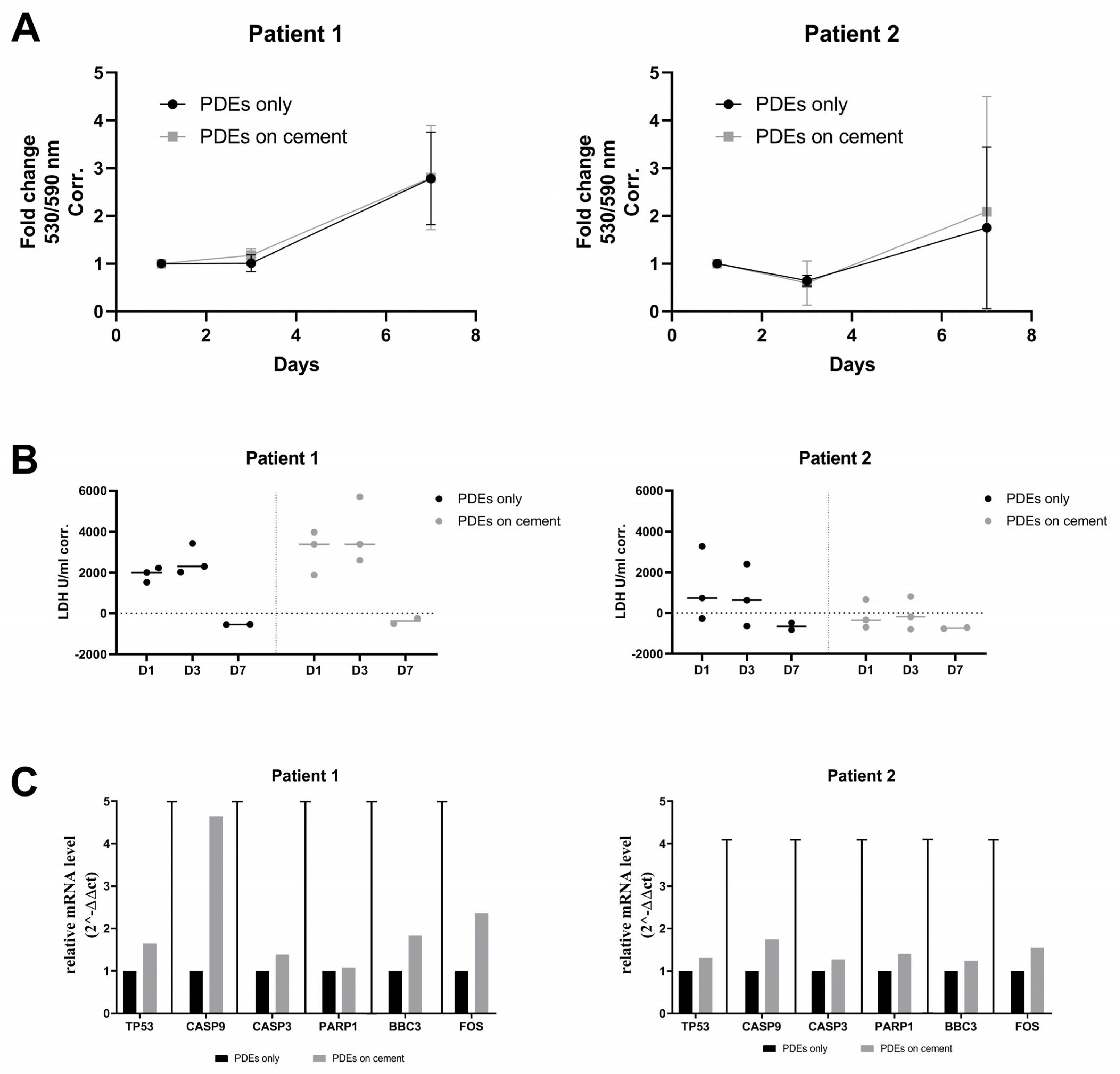
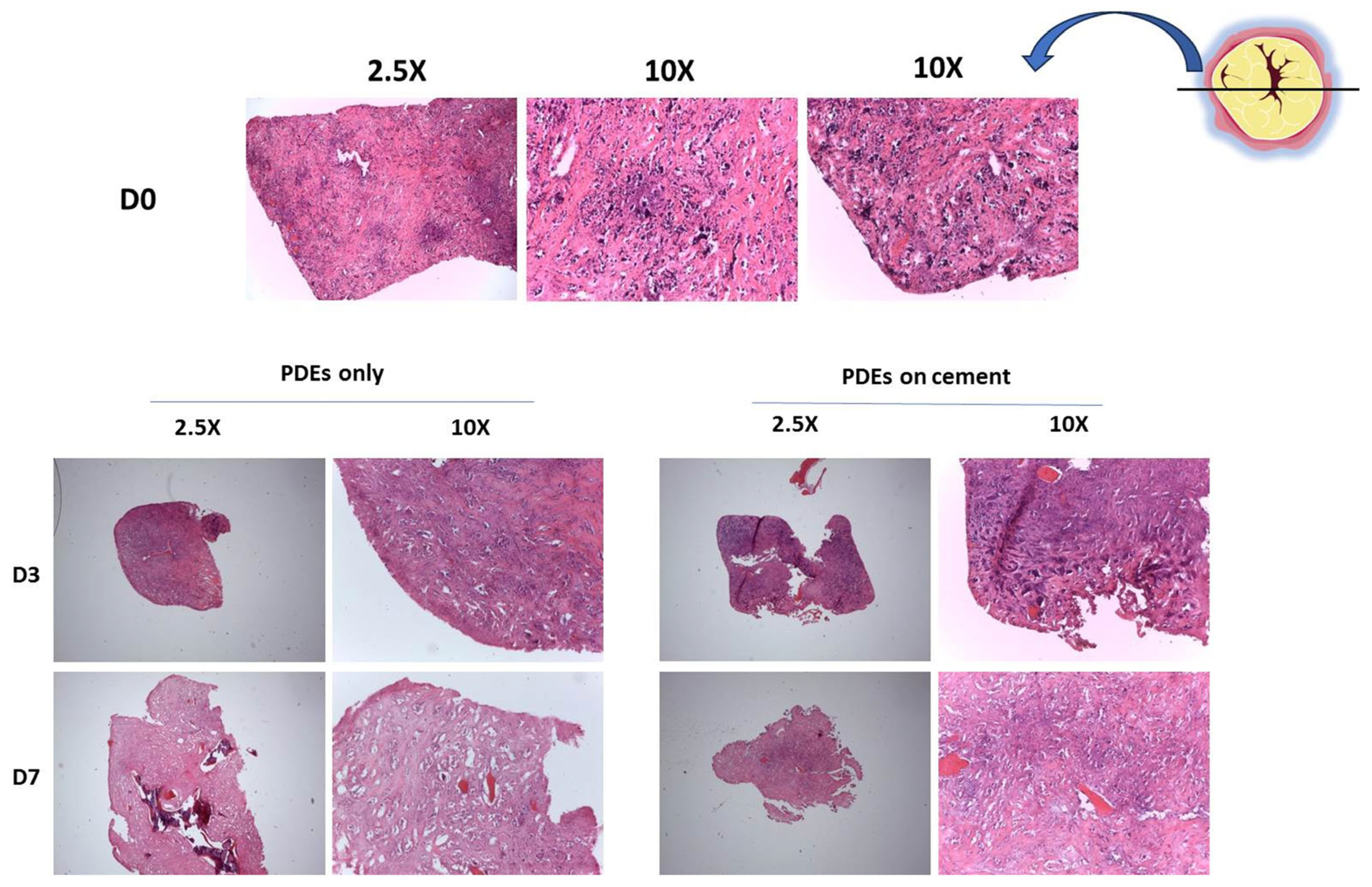
3.6. Ex Vivo Validation of the Biocompatibility of SrCPCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giaquinto, A.N.; Sung, H.; Newman, L.A.; Freedman, R.A.; Smith, R.A.; Star, J.; Jemal, A.; Siegel, R.L. Breast cancer statistics 2024. CA Cancer J. Clin. 2024, 74, 477–495. [Google Scholar] [CrossRef] [PubMed]
- Sui, L.; Wang, J.; Jiang, W.G.; Song, X.; Ye, L. Molecular mechanism of bone metastasis in breast cancer. Front. Oncol. 2024, 13, 1401113. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Zheng, L.W.; Ding, Y.; Chen, Y.F.; Cai, Y.W.; Wang, L.P.; Huang, L.; Liu, C.C.; Shao, Z.M.; Yu, K.D. Breast cancer: Pathogenesis and treatments. Signal Transduct. Target. Ther. 2025, 10, 49. [Google Scholar] [CrossRef]
- Coleman, R.E.; Croucher, P.I.; Padhani, A.R.; Clézardin, P.; Chow, E.; Fallon, M.; Guise, T.; Colangeli, S.; Capanna, R.; Costa, L. Bone metastases. Nat. Rev. Dis. Prim. 2020, 6, 83. [Google Scholar] [CrossRef]
- Kang, Y.; Siegel, P.M.; Shu, W.; Drobnjak, M.; Kakonen, S.M.; Cordón-Cardo, C.; Guise, T.A.; Massagué, J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 2003, 3, 537–549. [Google Scholar] [CrossRef]
- Sethi, N.; Dai, X.; Winter, C.G.; Kang, Y. Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer Cell 2011, 19, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.J.; Guise, T.A.; Yin, J.J.; Elliott, J.; Horwood, N.J.; Martin, T.J.; Gillespie, M.T. Breast cancer cells interact with osteoblasts to support osteoclast formation. Endocrinology 1999, 140, 4451–4458. [Google Scholar] [CrossRef]
- Teicher, B.A.; Fricker, S.P. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin. Cancer Res. 2010, 16, 2927–2931. [Google Scholar] [CrossRef]
- Sethi, N.; Kang, Y. Unravelling the complexity of metastasis—Molecular understanding and targeted therapies. Nat. Rev. Cancer 2011, 11, 735–748. [Google Scholar] [CrossRef]
- Coleman, R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer Res. 2006, 2, 6243s–6249s. [Google Scholar] [CrossRef]
- Fizazi, K.; Carducci, M.; Smith, M.; Damião, R.; Brown, J.; Karsh, L.; Milecki, P.; Shore, N.; Rader, M.; Wang, H.; et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A randomised, double-blind study. Lancet 2011, 377, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Stopeck, A.T.; Lipton, A.; Body, J.J.; Steger, G.G.; Tonkin, K.; de Boer, R.H.; Lichinitser, M.; Fujiwara, Y.; Yardley, D.A.; Viniegra, M.; et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: A randomized, double-blind study. J. Clin. Oncol. 2010, 28, 5132–5139. [Google Scholar] [CrossRef] [PubMed]
- Henry, D.H.; Costa, L.; Goldwasser, F.; Hirsh, V.; Hungria, V.; Prausova, J.; Scagliotti, G.V.; Sleeboom, H.; Spencer, A.; Vadhan-Raj, S.; et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J. Clin. Oncol. 2011, 29, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Del Conte, A.; De Carlo, E.; Bertoli, E.; Stanzione, B.; Revelant, A.; Bertola, M.; Spina, M.; Bearz, A. Bone Metastasis and Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer (NSCLC): Microenvironment and Possible Clinical Implications. Int. J. Mol. Sci. 2022, 23, 6832. [Google Scholar] [CrossRef]
- Borciani, G.; Montalbano, G.; Baldini, N.; Cerqueni, G.; Vitale-Brovarone, C.; Ciapetti, G. Co-culture systems of osteoblasts and osteoclasts: Simulating In Vitro bone remodeling in regenerative approaches. Acta Biomater. 2020, 108, 22–45. [Google Scholar] [CrossRef]
- Jenkins, T.L.; Little, D. Synthetic scaffolds for musculoskeletal tissue engineering: Cellular responses to fiber parameters. NPJ Regen. Med. 2019, 4, 15. [Google Scholar] [CrossRef]
- Pathi, S.P.; Kowalczewski, C.; Tadipatri, R.; Fischbach, C. A novel 3-D mineralized tumor model to study breast cancer bone metastasis. PLoS ONE 2010, 5, e8849. [Google Scholar] [CrossRef]
- Talukdar, S.; Mandal, M.; Hutmacher, D.W.; Russell, P.J.; Soekmadji, C.; Kundu, S.C. Engineered silk fibroin protein 3D matrices for in vitro tumor model. Biomaterials 2011, 32, 2149–2159. [Google Scholar] [CrossRef]
- Marlow, R.; Honeth, G.; Lombardi, S.; Cariati, M.; Hessey, S.; Pipili, A.; Mariotti, V.; Buchupalli, B.; Foster, K.; Bonnet, D.; et al. A novel model of dormancy for bone metastatic breast cancer cells. Cancer Res. 2013, 73, 6886–6899. [Google Scholar] [CrossRef]
- Cox, R.F.; Hernandez-Santana, A.; Ramdass, S.; McMahon, G.; Harmey, J.H.; Morgan, M.P. Microcalcifications in breast cancer: Novel insights into the molecular mechanism and functional consequence of mammary mineralisation. Br. J. Cancer 2012, 106, 525–537. [Google Scholar] [CrossRef]
- Sohn, H.S.; Oh, J.K. Review of bone graft and bone substitutes with an emphasis on fracture surgeries. Biomater. Res. 2019, 23, 9. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R. A broader strategy for osteoporosis interventions. Nat. Rev. Endocrinol. 2020, 16, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Xie, K.; Guo, Y.; Tan, J.; Wu, J.; Yang, Y.; Fu, P.; Wang, L.; Jiang, W.; Hao, Y. Fabrication and Biological Activity of 3D-Printed Polycaprolactone/Magnesium Porous Scaffolds for Critical Size Bone Defect Repair. ACS Biomater. Sci. Eng. 2020, 6, 5120–5131. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef]
- Lodoso-Torrecilla, I.; van den Beucken, J.J.J.P.; Jansen, J.A. Calcium phosphate cements: Optimization toward biodegradability. Acta Biomater. 2021, 119, 1–12. [Google Scholar] [CrossRef]
- Sprio, S.; Dapporto, M.; Montesi, M.; Panseri, S.; Lattanzi, W.; Pola, E.; Logroscino, G.; Tampieri, A. Novel Osteointegrative Sr-Substituted Apatitic Cements Enriched with Alginate. Materials 2016, 9, 763. [Google Scholar] [CrossRef]
- Montesi, M.; Panseri, S.; Dapporto, M.; Tampieri, A.; Sprio, S. Sr-substituted bone cements direct mesenchymal stem cells, osteoblasts and osteoclasts fate. PLoS ONE 2017, 12, e0172100. [Google Scholar] [CrossRef]
- Benson, R.S. Use of radiation in biomaterials science. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2002, 191, 752–757. [Google Scholar] [CrossRef]
- Górny, R.L.; Gołofit-Szymczak, M.; Pawlak, A.; Ławniczek-Wałczyk, A.; Cyprowski, M.; Stobnicka, A.; Płocińska, M.; Kowalska, J. Effectiveness of UV-C radiation in inactivation of microorganisms on materials with different surface structures. Ann. Agric. Environ. Environ. Med. 2024, 31, 27287–27293. [Google Scholar] [CrossRef]
- Tapia-Guerrero, Y.S.; Del Prado-Audelo, M.L.; Borbolla-Jiménez, F.V.; Gomez, D.M.G.; García-Aguirre, I.; Colín-Castro, C.A.; Morales-González, J.A.; Leyva-Gómez, G.; Magaña, J.J. Effect of UV and Gamma Irradiation Sterilization Processes in the Properties of Different Polymeric Nanoparticles for Biomedical Applications. Materials 2020, 13, 1090. [Google Scholar] [CrossRef]
- Mercatali, L.; Spadazzi, C.; Miserocchi, G.; Liverani, C.; De Vita, A.; Bongiovanni, A.; Recine, F.; Amadori, D.; Ibrahim, T. The Effect of Everolimus in an In Vitro Model of Triple Negative Breast Cancer and Osteoclasts. Int. J. Mol. Sci. 2016, 17, 1827. [Google Scholar] [CrossRef] [PubMed]
- McCloy, R.A.; Rogers, S.; Caldon, C.E.; Lorca, T.; Castro, A.; Burgess, A. Partial inhibition of Cdk1 in G 2 phase overrides the SAC and decouples mitotic events. Cell Cycle 2014, 13, 1400–1412. [Google Scholar] [CrossRef] [PubMed]
- De Vita, A.; Recine, F.; Miserocchi, G.; Pieri, F.; Spadazzi, C.; Cocchi, C.; Vanni, S.; Liverani, C.; Farnedi, A.; Fabbri, F.; et al. The potential role of the extracellular matrix in the activity of trabectedin in UPS and L-sarcoma: Evidences from a patient-derived primary culture case series in tridimensional and zebrafish models. J. Exp. Clin. Cancer Res. 2021, 40, 165. [Google Scholar] [CrossRef] [PubMed]
- Guerrieri, A.N.; Bellotti, C.; Penzo, M.; Columbaro, M.; Pannella, M.; De Vita, A.; Gambarotti, M.; Mercatali, L.; Laranga, R.; Dozza, B.; et al. A novel patient-derived immortalised cell line of myxofibrosarcoma: A tool for preclinical drugs testing and the generation of near-patient models. BMC Cancer 2023, 23, 1194. [Google Scholar] [CrossRef]
- Dussault, A.A.; Pouliot, M. Rapid and simple comparison of messenger RNA levels using real-time PCR. Biol. Proced. Online 2006, 8, 1–10. [Google Scholar] [CrossRef]
- Yin, J.J.; Selander, K.; Chirgwin, J.M.; Dallas, M.; Grubbs, B.G.; Wieser, R.; Massagué, J.; Mundy, G.R.; Guise, T.A. TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J. Clin. Investig. 1999, 103, 197–206. [Google Scholar] [CrossRef]
- Vasudevan, J.; Jiang, K.; Fernandez, J.G.; Lim, C.T. Extracellular matrix mechanobiology in cancer cell migration. Acta Biomater. 2023, 163, 351–364. [Google Scholar] [CrossRef]
- Cui, H.; Esworthy, T.; Zhou, X.; Hann, S.Y.; Glazer, R.I.; Li, R.; Zhang, L.G. Engineering a Novel 3D Printed Vascularized Tissue Model for Investigating Breast Cancer Metastasis to Bone. Adv. Healthc. Mater. 2020, 9, e1900924. [Google Scholar] [CrossRef]
- Holen, I.; Nutter, F.; Wilkinson, J.M.; Evans, C.A.; Avgoustou, P.; Ottewell, P.D. Human breast cancer bone metastasis In Vitro and In Vivo: A novel 3D model system for studies of tumour cell-bone cell interactions. Clin. Exp. Metastasis 2015, 32, 689–702. [Google Scholar] [CrossRef]
- Bersini, S.; Jeon, J.S.; Dubini, G.; Arrigoni, C.; Chung, S.; Charest, J.L.; Moretti, M.; Kamm, R.D. A microfluidic 3D In Vitro model for specificity of breast cancer metastasis to bone. Biomaterials 2014, 35, 2454–2461. [Google Scholar] [CrossRef]
- Marinkovic, M.; Block, T.J.; Rakian, R.; Li, Q.; Wang, E.; Reilly, M.A.; Dean, D.D.; Chen, X.D. One size does not fit all: Developing a cell-specific niche for In Vitro study of cell behavior. Matrix Biol. 2016, 52, 426–441. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Tang, M.; Duan, W.; Xia, S.; Liu, W.; Wang, Q. 3D Bioprinting for Tumor Metastasis Research. ACS Biomater. Sci. Eng. 2023, 9, 3116–3133. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Castro, N.J.; Cui, H.; Zhou, X.; Boualam, B.; McGrane, R.; Glazer, R.I.; Zhang, L.G. A 3D printed nano bone matrix forcharacterization of breast cancer cell and osteoblast interactions. Nanotechnology 2016, 27, 315103. [Google Scholar] [CrossRef]
- Glaser, D.E.; Curtis, M.B.; Sariano, P.A.; Rollins, Z.A.; Shergill, B.S.; Anand, A.; Deely, A.M.; Shirure, V.S.; Anderson, L.; Lowen, J.M.; et al. Organ-on-a-chip model of vascularized human bone marrow niches. Biomaterials 2022, 280, 121245. [Google Scholar] [CrossRef]
- Tanzawa, Y.; Tsuchiya, H.; Shirai, T.; Nishida, H.; Hayashi, K.; Takeuchi, A.; Kawahara, M.; Tomita, K. Potentiation of the antitumor effect of calcium phosphate cement containing anticancer drug and caffeine on rat osteosarcoma. J. Orthop. Sci. 2011, 16, 77–84. [Google Scholar] [CrossRef]
- Lopez-Heredia, M.A.; Kamphuis, G.J.; Thüne, P.C.; Öner, F.C.; Jansen, J.A.; Walboomers, X.F. An injectable calcium phosphate cement for the local delivery of paclitaxel to bone. Biomaterials 2011, 32, 5411–5416. [Google Scholar] [CrossRef] [PubMed]
- Vechasilp, J.; Tangtrakulwanich, B.; Oungbho, K.; Yuenyongsawad, S. The efficacy of methotrexate-impregnated hydroxyapatite composites on human mammary carcinoma cells. J. Orthop. Surg. 2007, 15, 56–61. [Google Scholar] [CrossRef]
- Miki, Y.; Ono, K.; Hata, S.; Suzuki, T.; Kumamoto, H.; Sasano, H. The advantages of co-culture over mono cell culture in simulating in vivo environment. J. Steroid Biochem. Mol. Biol. 2012, 131, 68–75. [Google Scholar] [CrossRef]
- Calejo, I.; Costa-Almeida, R.; Gonçalves, A.I.; Berdecka, D.; Reis, R.L.; Gomes, M.E. Bi-directional modulation of cellular interactions in an In Vitro co-culture model of tendon-to-bone interface. Cell Prolif. 2018, 51, e12493. [Google Scholar] [CrossRef]
- Dapporto, M.; Tavoni, M.; Restivo, E.; Carella, F.; Bruni, G.; Mercatali, L.; Visai, L.; Tampieri, A.; Iafisco, M.; Sprio, S. Strontium-doped apatitic bone cements with tunable antibacterial and antibiofilm ability. Front. Bioeng. Biotechnol. 2022, 10, 969641. [Google Scholar] [CrossRef]
- Sheng, X.; Li, C.; Wang, Z.; Xu, Y.; Sun, Y.; Zhang, W.; Liu, H.; Wang, J. Advanced applications of strontium-containing biomaterials in bone tissue engineering. Mater. Today Bio 2023, 20, 100636. [Google Scholar] [CrossRef] [PubMed]
- Liverani, C.; De Vita, A.; Minardi, S.; Kang, Y.; Mercatali, L.; Amadori, D.; Bongiovanni, A.; La Manna, F.; Ibrahim, T.; Tasciotti, E. A biomimetic 3D model of hypoxia-driven cancer progression. Sci. Rep. 2019, 9, 12263. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Whitman, M.A.; Shimpi, A.A.; Sempertegui, N.D.; Chiou, A.E.; Druso, J.E.; Verma, A.; Lux, S.C.; Cheng, Z.; Paszek, M.; et al. Bone-matrix mineralization dampens integrin-mediated mechanosignalling and metastatic progression in breast cancer. Nat. Biomed. Eng. 2023, 7, 1455–1472. [Google Scholar] [CrossRef]
- Pylostomou, A.; Demir, Ö.; Loca, D. Calcium phosphate bone cements as local drug delivery systems for bone cancer treatment. Biomater. Adv. 2023, 148, 213367. [Google Scholar] [CrossRef] [PubMed]
- Dewhurst, R.M.; Scalzone, A.; Buckley, J.; Mattu, C.; Rankin, K.S.; Gentile, P.; Ferreira, A.M. Development of Natural-Based Bone Cement for a Controlled Doxorubicin-Drug Release. Front. Bioeng. Biotechnol. 2020, 8, 754. [Google Scholar] [CrossRef]
- Dolci, L.S.; Panzavolta, S.; Torricelli, P.; Albertini, B.; Sicuro, L.; Fini, M.; Bigi, A.; Passerini, N. Modulation of Alendronate release from a calcium phosphate bone cement: An In Vitro osteoblast-osteoclast co-culture study. Int. J. Pharm. 2019, 554, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Ben Ghedalia Peled, N.; Hoffman, D.K.; Barsky, L.; Zer, N.S.; Amar, K.; Rapaport, H.; Gheber, L.A.; Zhang, X.H.; Vago, R. Bone Endosteal Mimics Regulates Breast Cancer Development and Phenotype. Biomacromolecules 2024, 25, 2338–2347. [Google Scholar] [CrossRef]
- Bassi, G.; Panseri, S.; Dozio, S.M.; Sandri, M.; Campodoni, E.; Dapporto, M.; Sprio, S.; Tampieri, A.; Montesi, M. Scaffold-based 3D cellular models mimicking the heterogeneity of osteosarcoma stem cell niche. Sci. Rep. 2020, 10, 22294. [Google Scholar] [CrossRef]
- Vescio, R.A.; Redfern, C.H.; Nelson, T.J.; Ugoretz, S.; Stern, P.H.; Hoffman, R.M. In vivo-like drug responses of human tumors growing in three-dimensional gel-supported primary culture. Proc. Natl. Acad. Sci. USA 1987, 84, 5029–5033. [Google Scholar] [CrossRef]
- Tanahashi, M.; Yamada, T.; Moriyama, S.; Suzuki, E.; Niwa, H. The effect of the histoculture drug response assay (HDRA) based perioperative chemotherapy for non-small cell lung cancer. Kyobu Geka 2008, 61, 26–30. [Google Scholar]
- Dean, J.L.; McClendon, A.K.; Hickey, T.E.; Butler, L.M.; Tilley, W.D.; Witkiewicz, A.K.; Knudsen, E.S. Therapeutic response to CDK4/6 inhibition in breast cancer defined by Ex Vivo analyses of human tumors. Cell Cycle 2012, 11, 2756–2761. [Google Scholar] [CrossRef] [PubMed]
- Centenera, M.M.; Hickey, T.E.; Jindal, S.; Ryan, N.K.; Ravindranathan, P.; Mohammed, H.; Robinson, J.L.; Schiewer, M.J.; Ma, S.; Kapur, P.; et al. A patient-derived explant (PDE) model of hormone-dependent cancer. Mol. Oncol. 2018, 12, 1608–1622. [Google Scholar] [CrossRef] [PubMed]
- Bellido, T.; Delgado-Calle, J. Ex Vivo Organ Cultures as Models to Study Bone Biology. JBMR Plus 2020, 4, e10345. [Google Scholar] [CrossRef] [PubMed]
- Shafi, A.A.; Schiewer, M.J.; de Leeuw, R.; Dylgjeri, E.; McCue, P.A.; Shah, N.; Gomella, L.G.; Lallas, C.D.; Trabulsi, E.J.; Centenera, M.M.; et al. Patient-derived Models Reveal Impact of the Tumor Microenvironment on Therapeutic Response. Eur. Urol. Oncol. 2018, 1, 325–337. [Google Scholar] [CrossRef]
- Edwards, J.R.; Mundy, G.R. Advances in osteoclast biology: Old findings and new insights from mouse models. Nat. Rev. Rheumatol. 2011, 7, 235–243. [Google Scholar] [CrossRef]

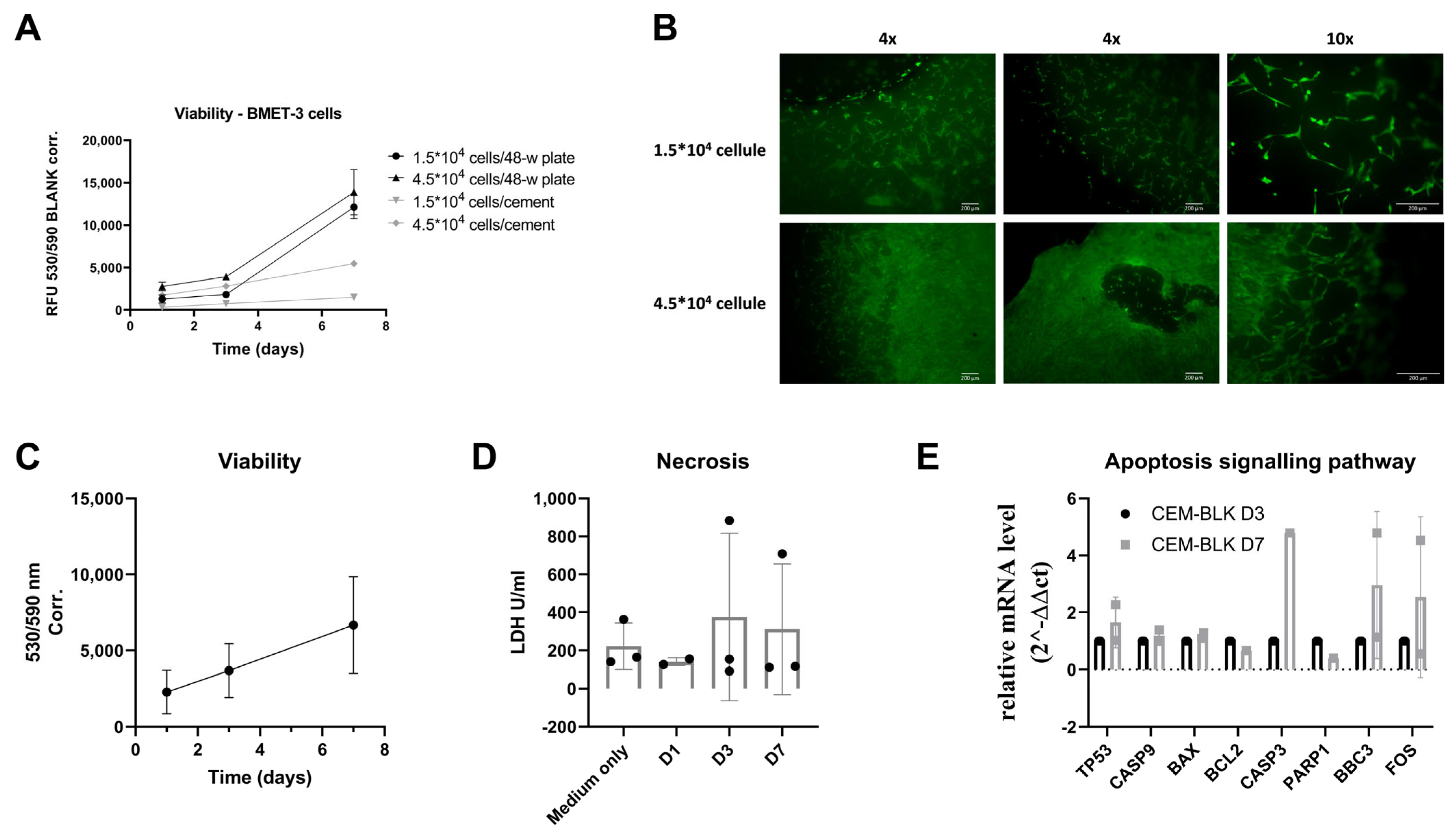
| Gene Symbol | Assay Catalog (Qiagen) |
|---|---|
| TP53 | PPH00213A |
| CASP9 | PPH00353A |
| FOS | PPH00094A |
| BAX | PPH00078A |
| BBC3 | PPH02204A |
| BCL2 | PPH00079A |
| PARP1 | PPH00686A |
| CASP3 | PPH00107A |
| GAPDH | PPH00150A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cocchi, C.; Dapporto, M.; Guerrieri, A.N.; Liverani, C.; Tavoni, M.; Bellotti, C.; Spadazzi, C.; Tampieri, A.; Gambarotti, M.; Miserocchi, G.; et al. A Modular Biomimetic Preclinical Platform to Elucidate the Interaction Between Cancer Cells and the Bone Metastatic Niche. J. Funct. Biomater. 2025, 16, 220. https://doi.org/10.3390/jfb16060220
Cocchi C, Dapporto M, Guerrieri AN, Liverani C, Tavoni M, Bellotti C, Spadazzi C, Tampieri A, Gambarotti M, Miserocchi G, et al. A Modular Biomimetic Preclinical Platform to Elucidate the Interaction Between Cancer Cells and the Bone Metastatic Niche. Journal of Functional Biomaterials. 2025; 16(6):220. https://doi.org/10.3390/jfb16060220
Chicago/Turabian StyleCocchi, Claudia, Massimiliano Dapporto, Ania Naila Guerrieri, Chiara Liverani, Marta Tavoni, Chiara Bellotti, Chiara Spadazzi, Anna Tampieri, Marco Gambarotti, Giacomo Miserocchi, and et al. 2025. "A Modular Biomimetic Preclinical Platform to Elucidate the Interaction Between Cancer Cells and the Bone Metastatic Niche" Journal of Functional Biomaterials 16, no. 6: 220. https://doi.org/10.3390/jfb16060220
APA StyleCocchi, C., Dapporto, M., Guerrieri, A. N., Liverani, C., Tavoni, M., Bellotti, C., Spadazzi, C., Tampieri, A., Gambarotti, M., Miserocchi, G., Sprio, S., Lucarelli, E., Iafisco, M., Ibrahim, T., De Vita, A., & Mercatali, L. (2025). A Modular Biomimetic Preclinical Platform to Elucidate the Interaction Between Cancer Cells and the Bone Metastatic Niche. Journal of Functional Biomaterials, 16(6), 220. https://doi.org/10.3390/jfb16060220












