In Vitro Cytotoxicity Evaluation of Nanosized Hydroxyapatite and Fluorapatite on Cell Lines and Their Relevance to the Alveolar Augmentation Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of nHAp and nFAp Tablets
2.2. Morphology Characterization
2.3. In Vitro Studies
2.3.1. Cell Line
2.3.2. Direct Contact
2.3.3. Indirect Method
MTT Assay
2.4. Fluoride Level Release Assessment
2.5. Statistical Analysis
3. Results
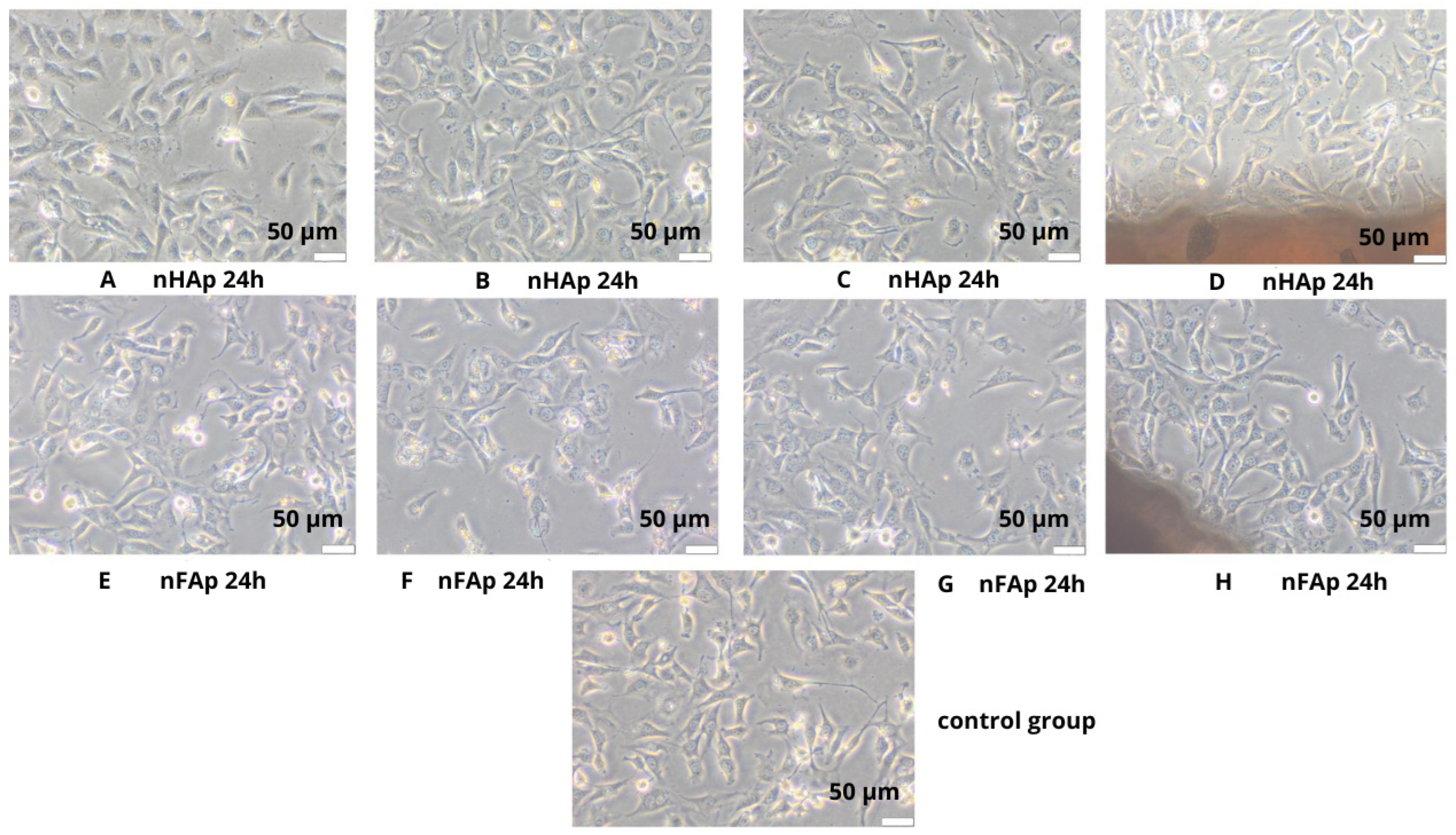
3.1. Morphology
3.2. Cytotoxicity Evaluation Results
3.3. Statistical Analysis Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deb, S.; Chana, S. Biomaterials in Relation to Dentistry; Frontiers of Oral Biology; Karger: Basel, Switzerland, 2015; Volume 17, pp. 1–12. [Google Scholar] [CrossRef]
- Iftikhar, S.; Jahanzeb, N.; Saleem, M.; ur Rehman, S.; Matinlinna, J.P.; Khan, A.S. The trends of dental biomaterials research and future directions: A mapping review. Saudi Dent. J. 2021, 33, 229. [Google Scholar] [CrossRef] [PubMed]
- Sharan, J.; Singh, S.; Lale, S.V.; Mishra, M.; Koul, V.; Kharbanda, O.P. Applications of nanomaterials in dental science: A review. J. Nanosci. Nanotechnol. 2017, 17, 2235–2255. [Google Scholar] [CrossRef]
- Bapat, R.A.; Joshi, C.P.; Bapat, P.; Chaubal, T.V.; Pandurangappa, R.; Jnanendrappa, N.; Gorain, B.; Khurana, S.; Kesharwani, P. The use of nanoparticles as biomaterials in dentistry. Drug Discov. Today 2019, 24, 85–98. [Google Scholar] [CrossRef]
- Sreenivasalu, P.K.P.; Dora, C.P.; Swami, R.; Jasthi, V.C.; Shiroorkar, P.N.; Nagaraja, S.; Asdaq, S.M.B.; Anwer, M.K. Nanomaterials in Dentistry: Current Applications and Future Scope. Nanomaterials 2022, 12, 1676. [Google Scholar] [CrossRef]
- Shahi, S.; Özcan, M.; Maleki Dizaj, S.; Sharifi, S.; Al-Haj Husain, N.; Eftekhari, A.; Ahmadian, E. A review on potential toxicity of dental material and screening their biocompatibility. Toxicol. Mech. Methods 2019, 29, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Steinle, H.; Golombek, S.; Hann, L.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Blood-Contacting Biomaterials: In vitro Evaluation of the Hemocompatibility. Front. Bioeng. Biotechnol. 2018, 6, 99. [Google Scholar] [CrossRef]
- Anderson, J.M. Future challenges in the in vitro and in vivo evaluation of biomaterial biocompatibility. Regen. Biomater. 2016, 3, 73. [Google Scholar] [CrossRef]
- Seo, M.H.; Kim, S.M. Ridge augmentation in implant dentistry. J. Korean Assoc. Oral Maxillofac. Surg. 2020, 46, 208–210. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.K.; Pan, Y.H.; Salamanca, E.; Lin, Y.T.; Chang, W.J. Prevention of Bone Resorption by HA/β-TCP + Collagen Composite after Tooth Extraction: A Case Series. Int. J. Environ. Res. Public Health 2019, 16, 4616. [Google Scholar] [CrossRef]
- Natto, Z.S.; Parashis, A.; Steffensen, B.; Ganguly, R.; Finkelman, M.D.; Jeong, Y.N. Efficacy of collagen matrix seal and collagen sponge on ridge preservation in combination with bone allograft: A randomized controlled clinical trial. J. Clin. Periodontol. 2017, 44, 649–659. [Google Scholar] [CrossRef]
- Rashid, M.; Roni, M.A.; Rahman, M. Clinical status of bioinspired and biomimetic materials. In Bioinspired and Biomimetic Materials for Drug Delivery; Woodhead Publishing: Cambridge, UK, 2021; pp. 277–294. [Google Scholar] [CrossRef]
- Sobczak, A.; Kowalski, Z.; Wzorek, Z. Preparation of Hydroxyapatite from Animal Bones. Acta Bioeng. Biomech. 2009, 11, 23–28. [Google Scholar] [PubMed]
- Sarsilmaz, F.; Orhan, N.; Unsaldi, E.; Durmus, A.S.; Colakoglu, N. A polyethylene-high proportion hydroxyapatite implant and its investigation in vivo. Acta Bioeng. Biomech. 2007, 9, 9. [Google Scholar]
- Pan, S.; Yu, H.; Yang, X.; Yang, X.; Wang, Y.; Liu, Q.; Jin, L.; Yang, Y. Application of Nanomaterials in Stem Cell Regenerative Medicine of Orthopedic Surgery. J. Nanomater. 2017, 2017, 1985942. [Google Scholar]
- Zakrzewski, W.; Dobrzynski, M.; Wiglusz, R.J.; Rybak, Z.; Szymonowicz, M. Selected nanomaterials’ application enhanced with the use of stem cells in acceleration of alveolar bone regeneration during augmentation process. Nanomaterials 2020, 10, 1216. [Google Scholar] [CrossRef]
- Alhilou, A.; Do, T.; Mizban, L.; Clarkson, B.H.; Wood, D.J.; Katsikogianni, M.G. Physicochemical and Antibacterial Characterization of a Novel Fluorapatite Coating. ACS Omega 2016, 1, 264–276. [Google Scholar] [CrossRef]
- Kosior, P.; Klimas, S.; Nikodem, A.; Wolicka, J.; Diakowska, D.; Watras, A.; Wiglusz, R.J.; Dobrzyński, M. An in vitro examination of fluoride ions release from selected materials—Resin-modified glass-ionomer cement (Vitremer) and nanohybrid composite material (TetricEvoCeram). Acta Bioeng. Biomech. 2023, 25, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Dobrzyński, W.; Nikodem, A.; Diakowska, D.; Wiglusz, R.J.; Watras, A.; Dobrzyński, M.; Mikulewicz, M. Comparison of the fluoride ion release from nanofluorapatite-modified orthodontic cement under different pH conditions—An in vitro study. Acta Bioeng. Biomech. 2023, 25, 159–176. [Google Scholar] [CrossRef]
- Ammar, N.; El-Tekeya, M.M.; Essa, S.; Essawy, M.M.; Talaat, D.M. Antibacterial effect and impact on caries activity of nanosilver fluoride and silver diamine fluoride in dentin caries of primary teeth: A randomized controlled clinical trial. BMC Oral Health 2022, 22, 657. [Google Scholar] [CrossRef]
- Sharma, N.; Bhatia, S.; Sodhi, A.S.; Batra, N. Oral microbiome and health. AIMS Microbiol. 2018, 4, 42. [Google Scholar] [CrossRef]
- Mostafalou, S.; Mohammadi, P. Fluoride. In Encyclopedia of Toxicology, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 4, pp. 735–742. [Google Scholar] [CrossRef]
- Epple, M.; Enax, J.; Meyer, F. Prevention of Caries and Dental Erosion by Fluorides—A Critical Discussion Based on Physico-Chemical Data and Principles. Dent. J. 2022, 10, 6. [Google Scholar] [CrossRef]
- Daima, H.K.; Bansal, V. Influence of Physicochemical Properties of Nanomaterials on Their Antibacterial Applications. In Nanotechnology in Diagnosis, Treatment and Prophylaxis of Infectious Diseases; Academic Press: Cambridge, MA, USA, 2015; pp. 151–166. [Google Scholar] [CrossRef]
- Lecloux, A.J. Discussion about the use of the volume-specific surface area (VSSA) as criteria to identify nanomaterials according to the EU definition: First part: Theoretical approach. J. Nanopart. Res. 2015, 17, 447. [Google Scholar] [CrossRef]
- Khurshid, Z.; Zafar, M.; Qasim, S.; Shahab, S.; Naseem, M.; AbuReqaiba, A. Advances in Nanotechnology for Restorative Dentistry. Materials 2015, 8, 717. [Google Scholar] [CrossRef] [PubMed]
- Joudeh, N.; Linke, D. Nanoparticle classification, physicochemical properties, characterization, and applications: A comprehensive review for biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef]
- Bonilla-Represa, V.; Abalos-Labruzzi, C.; Herrera-Martinez, M.; Guerrero-Pérez, M.O. Nanomaterials in Dentistry: State of the Art and Future Challenges. Nanomaterials 2020, 10, 1770. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. Regulatory biocompatibility requirements for biomaterials used in regenerative medicine. J. Mater. Sci. Mater. Med. 2015, 26, 89. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic Potential of Materials at the Nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef]
- Brun, A.; Moignot, N.; Colombier, M.L.; Dursun, E. Emerging Nanotechnology in Non-Surgical Periodontal Therapy in Animal Models: A Systematic Review. Nanomaterials 2020, 10, 1414. [Google Scholar] [CrossRef]
- Bohner, M.; Santoni, B.L.G.; Döbelin, N. β-tricalcium phosphate for bone substitution: Synthesis and properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef]
- Välimäki, V.V.; Aro, H.T. Molecular basis for action of bioactive glasses as bone graft substitute. Scand. J. Surg. 2006, 95, 95–102. [Google Scholar] [CrossRef]
- Babuska, V.; Kasi, P.B.; Chocholata, P.; Wiesnerova, L.; Dvorakova, J.; Vrzakova, R.; Nekleionova, A.; Landsmann, L.; Kulda, V. Nanomaterials in Bone Regeneration. Appl. Sci. 2022, 12, 6793. [Google Scholar] [CrossRef]
- Pina, S.; Oliveira, J.M.; Reis, R.L. Natural-based nanocomposites for bone tissue engineering and regenerative medicine: A review. Adv. Mater. 2015, 27, 1143–1169. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Rybak, Z.; Pajączkowska, M.; Nowicka, J.; Szymonowicz, M.; Rusak, A.; Wiglusz, R.J.; Szyszka, K.; Chmielowiec, J.; Chodaczek, G.; et al. Antimicrobial Properties and Cytotoxic Effect Evaluation of Nanosized Hydroxyapatite and Fluorapatite Dedicated for Alveolar Bone Regeneration. Appl. Sci. 2024, 14, 7845. [Google Scholar] [CrossRef]
- Szymonowicz, M.; Rybak, Z.; Fraczek-Szczypta, A.; Paluch, D.; Rusak, A.; Nowicka, K.; Blazewicz, M. Haemocompatibility and cytotoxic studies of non-metallic composite materials modified with magnetic nano and microparticles. Acta Bioeng. Biomech. 2015, 17, 49–58. [Google Scholar] [CrossRef]
- Tomanik, M.; Kobielarz, M.; Filipiak, J.; Szymonowicz, M.; Rusak, A.; Mroczkowska, K.; Antończak, A.; Pezowicz, C. Laser Texturing as a Way of Influencing the Micromechanical and Biological Properties of the Poly(L-Lactide) Surface. Materials 2020, 13, 3786. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009. Available online: https://www.iso.org/standard/36406.html (accessed on 2 September 2024).
- ISO 10993-12:2021; Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials. ISO: Geneva, Switzerland, 2021. Available online: https://www.iso.org/standard/75769.html (accessed on 2 February 2025).
- Kosior, P.; Dobrzynski, M.; Zakrzewska, A.; Diakowska, D.; Nienartowicz, J.; Blicharski, T.; Nagel, S.; Sikora, M.; Wiglusz, K.; Watras, A.; et al. Comparison of the Fluoride Ion Release from Composite and Compomer Materials under Varying pH Conditions—Preliminary In vitro Study. Appl. Sci. 2022, 12, 12540. [Google Scholar] [CrossRef]
- Kumar, B.; Kashyap, N.; Avinash, A.; Chevvuri, R.; Sagar, M.K.; Shrikant, K. The composition, function and role of saliva in maintaining oral health: A review. Int. J. Contemp. Dent. Med. Rev. 2017, 6, 11217. [Google Scholar]
- Farooq, I.; Bugshan, A. The role of salivary contents and modern technologies in the remineralization of dental enamel: A narrative review. F1000Research 2020, 9, 171. [Google Scholar] [CrossRef]
- Soleimani, B.; Goli, H.; Naranjian, M.; Mousavi, S.J.; Nahvi, A. Comparison of Antimicrobial Activity of Fluoride Varnishes Against Streptococcus mutans and Lactobacillus acidophilus: An In vitro Study. Iran. J. Pediatr. 2021, 31, 111422. [Google Scholar] [CrossRef]
- Van Loveren, C. Antimicrobial Activity of Fluoride and Its in vivo Importance: Identification of Research Questions. Caries Res. 2001, 35, 65–70. [Google Scholar] [CrossRef]
- Erlangga, M.; Charlena, C.; Suparto, I.H. Synthesis and Characterization of Fluorapatite-Copper(II) Oxide with Sol-Gel Method as an Antibacterial Biomaterial. J. Kim. Sains dan Apl. 2024, 27, 174–181. [Google Scholar] [CrossRef]
- Vogel, G.L.; Zhang, Z.; Carey, C.M.; Ly, A.; Chow, L.C.; Proskin, H.M. Composition of plaque and saliva following a sucrose challenge and use of an alpha-tricalcium-phosphate-containing chewing gum. J. Dent. Res. 1998, 77, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Wells, P.M.; Sprockett, D.D.; Bowyer, R.C.E.; Kurushima, Y.; Relman, D.A.; Williams, F.M.K.; Steves, C.J. Influential factors of saliva microbiota composition. Sci. Rep. 2022, 12, 18894. [Google Scholar] [CrossRef]
- Gal, J.Y.; Fovet, Y.; Adib-Yadzi, M. About a synthetic saliva for in vitro studies. Talanta 2001, 53, 1103–1115. [Google Scholar] [CrossRef]
- Moreau, J.L.; Xu, H.H.K. Fluoride releasing restorative materials: Effects of pH on mechanical properties and ion release. Dent. Mater. 2010, 26, e227–e235. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J. Fluoride-releasing dental restorative materials: An update. Balk. J. Dent. Med. 2014, 18, 60–69. [Google Scholar] [CrossRef]
- Dhumal, R.S.; Chauhan, R.S.; Patil, V.; Rathi, N.; Nene, K.; Tirupathi, S.P.; Patil, L.; Nankar, M.Y.; Khandelwal, A.P. Comparative Evaluation of Fluoride Release from Four Commercially Available Pediatric Dental Restorative Materials. Int. J. Clin. Pediatr. Dent. 2023, 16, S6–S12. [Google Scholar] [CrossRef]
- Wongphattarakul, S.; Kuson, R.; Sastraruji, T.; Suttiat, K. Fluoride Release and Rechargeability of Poly(lactic acid) Composites with Glass Ionomer Cement. Polymer 2023, 15, 4041. [Google Scholar] [CrossRef]
- Gul, H.; Zahid, S.; Zahid, S.; Kaleem, M.; Khan, A.S.; Shah, A.T. Sol-gel derived fluoride-doped bioactive glass powders: Structural and long-term fluoride release/pH analysis. J. Non. Cryst. Solids 2018, 498, 216–222. [Google Scholar] [CrossRef]
- AMBRA. Fluoride Release Profile for Commercial Fissure Sealant (Flow-Color (Red), Arkona) in Various pH. 2024. Available online: https://ambra.pwr.edu.pl/publication/piszko-2024/?utm_source=chatgpt.com (accessed on 2 February 2025).



| Time (h) | Deionized H2O (µg/mm2/h) | AS pH of 4.5 (µg/mm2/h) | AS pH of 7.0 (µg/mm2/h) | AS pH of 7.5 (µg/mm2/h) | p-Value (ANOVA for Independent Groups) |
|---|---|---|---|---|---|
| 3 | 1.906 ± 0.485 | 0.356 ± 0.034 | 0.056 ± 0.049 | 2.806 ± 0.531 | <0.0001 * |
| 24 | 0.244 ± 0.016 | 0.051 ± 0.005 | 0.006 ± 0.005 | 0.018 ± 0.001 | <0.0001 * |
| 48 | 0.209 ± 0.021 | 0.048 ± 0.011 | 0.009 ± 0.001 | 0.008 ± 0.000 | <0.0001 * |
| 72 | 0.196 ± 0.026 | 0.027 ± 0.014 | 0.014 ± 0.008 | 0.015 ± 0.005 | <0.0001 * |
| 96 | 0.251 ± 0.025 | 0.016 ± 0.003 | 0.019 ± 0.014 | 0.013 ± 0.005 | <0.0001 * |
| 168 | 0.067 ± 0.002 | 0.005 ± 0.002 | 0.004 ± 0.003 | 0.003 ± 0.000 | <0.0001 * |
| Mean + SD | 0.479 ± 0.096 | 0.084 ± 0.012 | 0.018 ± 0.013 | 0.477 ± 0.090 | - |
| p-value (ANOVA for dependent samples) | <0.0001 * | <0.0001 * | 0.096 | <0.0001 * | - |
| post hoc Tukey test | p < 0.0001 * for 3 h vs. all time subgroups | p < 0.0001 * for 3 h vs. all time subgroups p = 0.041 * for 24 h vs. 168 h | - | p < 0.0001 * for 3 h vs. all time subgroups | - |
| Time (h) | Deionized H2O (µg/mm2) | AS pH of 4.5 (µg/mm2) | AS pH of 7.0 (µg/mm2) | AS pH of 7.5 (µg/mm2) |
|---|---|---|---|---|
| 3 | 5.718 ± 1.457 | 1.068 ± 0.104 | 0.169 ± 0.148 | 8.418 ± 1.594 |
| 24 | 10.852 ± 1.794 | 2.154 ± 0.226 | 0.311 ± 0.271 | 8.800 ± 1.613 |
| 48 | 15.881 ± 2.315 | 3.326 ± 0.513 | 0.530 ± 0.283 | 9.012 ± 1.613 |
| 72 | 20.587 ± 2.958 | 3.977 ± 0.869 | 0.866 ± 0.497 | 9.387 ± 1.751 |
| 96 | 26.631 ± 3.577 | 4.359 ± 0.963 | 1.341 ± 0.855 | 9.699 ± 1.880 |
| 168 | 31.503 ± 3.780 | 4.723 ± 1.123 | 1.680 ± 1.075 | 9.936 ± 1.907 |
| Correlation (Pearson test) | r = 0.898 p = 0.015 * | r = 0.818 p = 0.047 * | r = 0.926 p = 0.008 * | r = 0.947 p = 0.004 * |
| Time (h) | Deionized H2O (µg/mm2/h) | AS pH of 4.5 (µg/mm2/h) | AS pH of 7.0 (µg/mm2/h) | AS pH of 7.5 (µg/mm2/h) | p-Value (ANOVA for Independent Groups) |
|---|---|---|---|---|---|
| 3 | 0.178 ± 0.058 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.104 ± 0.012 | <0.0001 * |
| 24 | 0.009 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.010 ± 0.000 | <0.0001 * |
| 48 | 0.015 ± 0.004 | 0.007 ± 0.012 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.074 |
| 72 | 0.026 ± 0.012 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.021 ± 0.006 | <0.002 * |
| 96 | 0.028 ± 0.010 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.008 ± 0.000 | <0.0004 * |
| 168 | 0.005 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.003 ± 0.000 | <0.0001 * |
| Mean + SD | 0.043 ± 0.014 | 0.001 ± 0.002 | 0.000 ± 0.000 | 0.024 ± 0.003 | - |
| p-value (ANOVA for dependent samples) | <0.0001 * | 0.458 | - | <0.0001 * | - |
| post hoc Tukey test | p < 0.0001 * for 3 h vs. all time subgroups | - | - | p < 0.0001 * for 3 h vs. all time subgroups p = 0.006 for 48 h vs. 72 h p = 0.018 for 72 h vs. 168 h | - |
| Time (h) | Deionized H2O (µg/mm2) | AS pH of 4.5 (µg/mm2) | AS pH of 7.0 (µg/mm2) | AS pH of 7.5 (µg/mm2) |
|---|---|---|---|---|
| 3 | 0.534 ± 0.175 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.314 ± 0.037 |
| 24 | 0.743 ± 0.181 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.527 ± 0.037 |
| 48 | 1.105 ± 0.294 | 1.176 ± 0.306 | 0.000 ± 0.000 | 0.527 ± 0.037 |
| 72 | 1.746 ± 0.599 | 1.176 ± 0.306 | 0.000 ± 0.000 | 1.033 ± 0.182 |
| 96 | 2.425 ± 0.846 | 1.176 ± 0.306 | 0.000 ± 0.000 | 1.245 ± 0.182 |
| 168 | 2.797 ± 0.874 | 1.176 ± 0.306 | 0.000 ± 0.000 | 1.457 ± 0.182 |
| Correlation (Pearson test) | r = 0.927 p = 0.008 * | r = −0.723 p = 0.104 | - | r = 0.910 p = 0.012 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakrzewski, W.; Szymonowicz, M.; Nikodem, A.; Rusak, A.; Rybak, Z.; Szyszka, K.; Diakowska, D.; Wiatrak, B.; Wiglusz, R.J.; Dobrzyński, M. In Vitro Cytotoxicity Evaluation of Nanosized Hydroxyapatite and Fluorapatite on Cell Lines and Their Relevance to the Alveolar Augmentation Process. J. Funct. Biomater. 2025, 16, 125. https://doi.org/10.3390/jfb16040125
Zakrzewski W, Szymonowicz M, Nikodem A, Rusak A, Rybak Z, Szyszka K, Diakowska D, Wiatrak B, Wiglusz RJ, Dobrzyński M. In Vitro Cytotoxicity Evaluation of Nanosized Hydroxyapatite and Fluorapatite on Cell Lines and Their Relevance to the Alveolar Augmentation Process. Journal of Functional Biomaterials. 2025; 16(4):125. https://doi.org/10.3390/jfb16040125
Chicago/Turabian StyleZakrzewski, Wojciech, Maria Szymonowicz, Anna Nikodem, Agnieszka Rusak, Zbigniew Rybak, Katarzyna Szyszka, Dorota Diakowska, Benita Wiatrak, Rafal J. Wiglusz, and Maciej Dobrzyński. 2025. "In Vitro Cytotoxicity Evaluation of Nanosized Hydroxyapatite and Fluorapatite on Cell Lines and Their Relevance to the Alveolar Augmentation Process" Journal of Functional Biomaterials 16, no. 4: 125. https://doi.org/10.3390/jfb16040125
APA StyleZakrzewski, W., Szymonowicz, M., Nikodem, A., Rusak, A., Rybak, Z., Szyszka, K., Diakowska, D., Wiatrak, B., Wiglusz, R. J., & Dobrzyński, M. (2025). In Vitro Cytotoxicity Evaluation of Nanosized Hydroxyapatite and Fluorapatite on Cell Lines and Their Relevance to the Alveolar Augmentation Process. Journal of Functional Biomaterials, 16(4), 125. https://doi.org/10.3390/jfb16040125












