2.3. Methods of Research
The determination of oxygen and nitrogen impurities was achieved through a reduction melting process in a graphite crucible within a helium-fueled pulse-resistance furnace, employing the TC-600 analyzer (LECO, 3000 Lakeview Avenue, St. Joseph, MI, USA). Nitrogen was detected using thermal conductivity, and oxygen was detected by the amount of released CO2 using the infrared absorption method.
To determine hydrogen impurities, we used reduced melting in a graphite crucible in an argon-fueled pulse-resistance furnace using the RHEN-602 analyzer (LECO, 3000 Lakeview Avenue, St. Joseph, MI, USA). Hydrogen was detected using thermal conductivity.
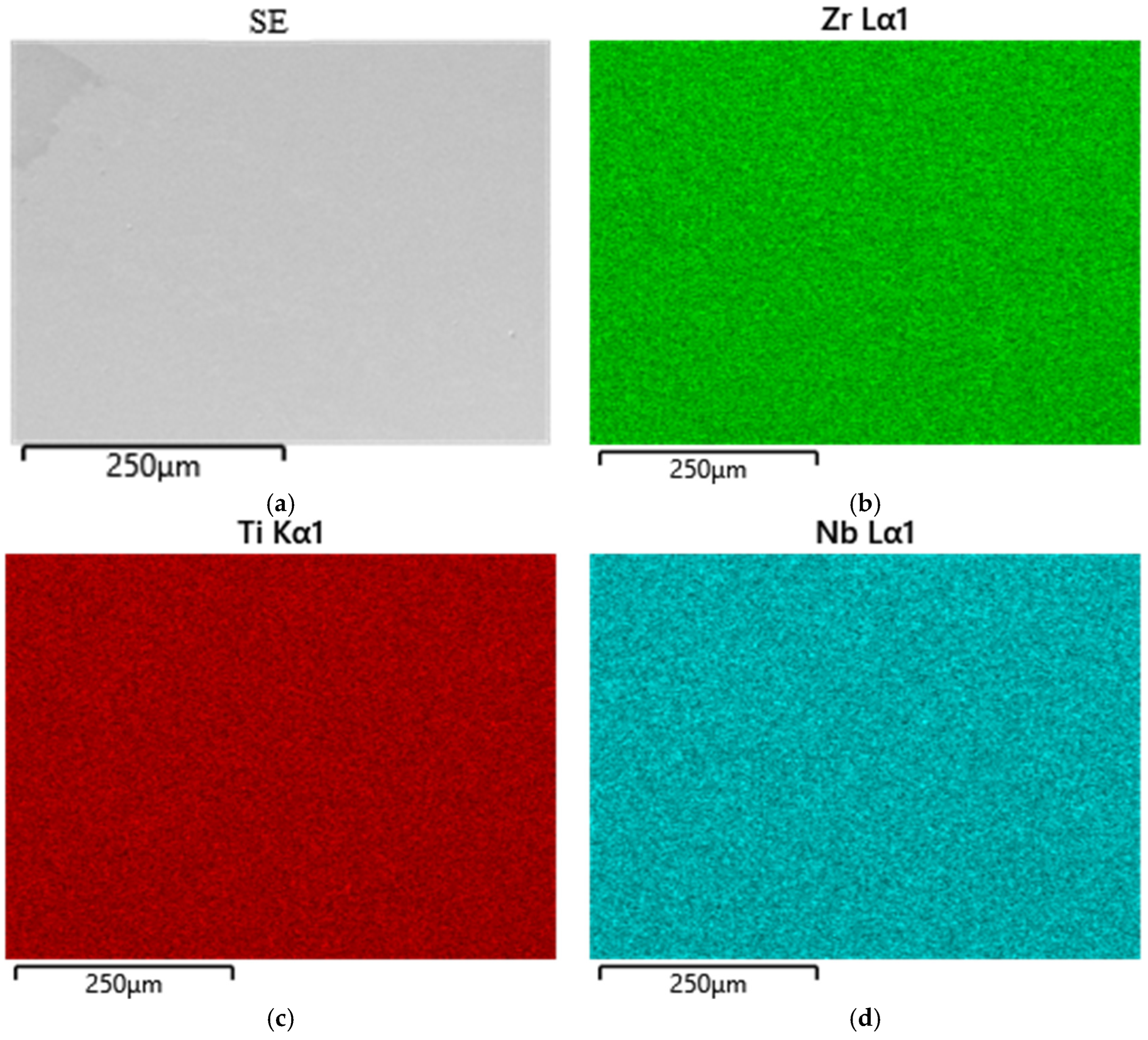
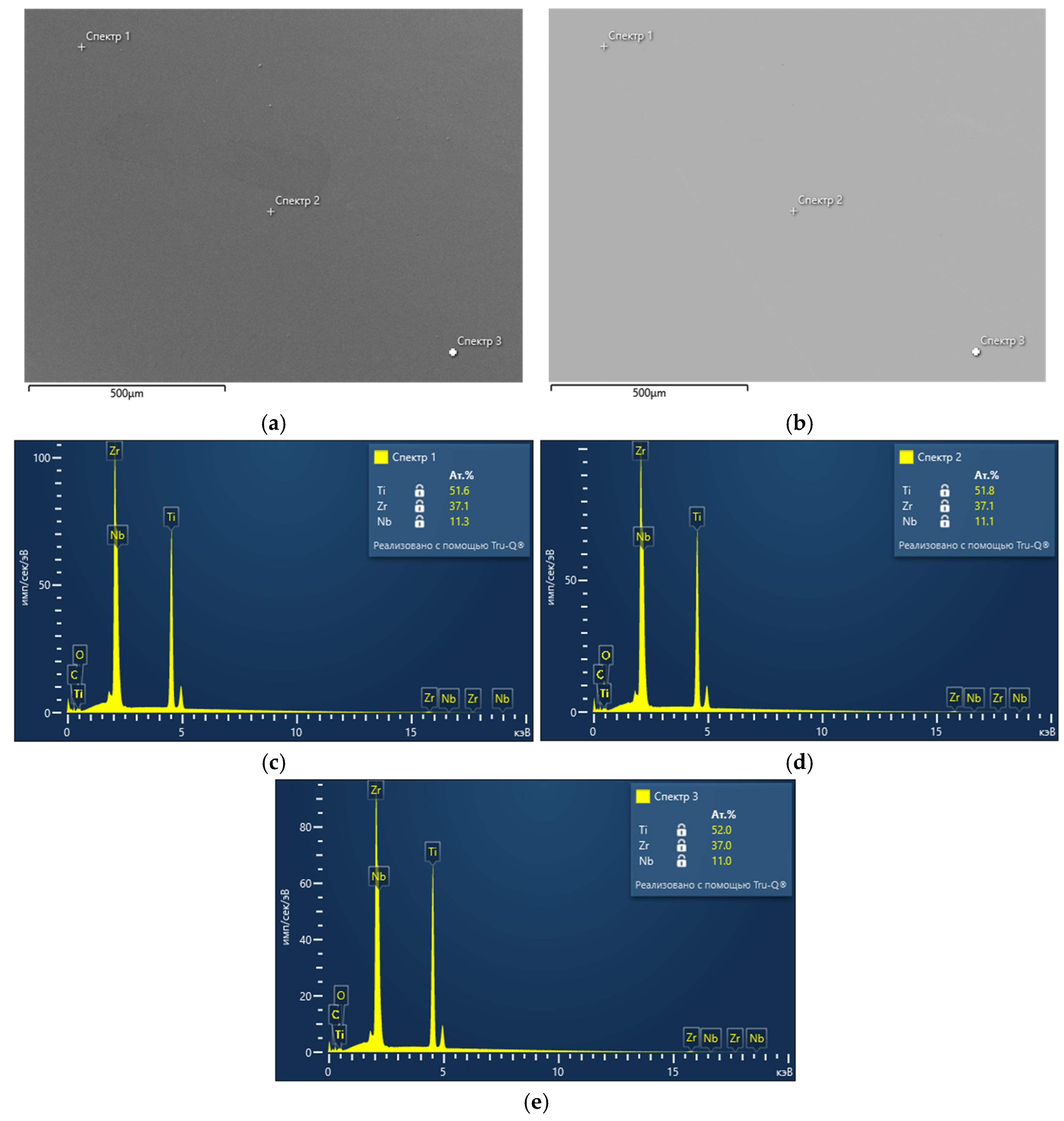
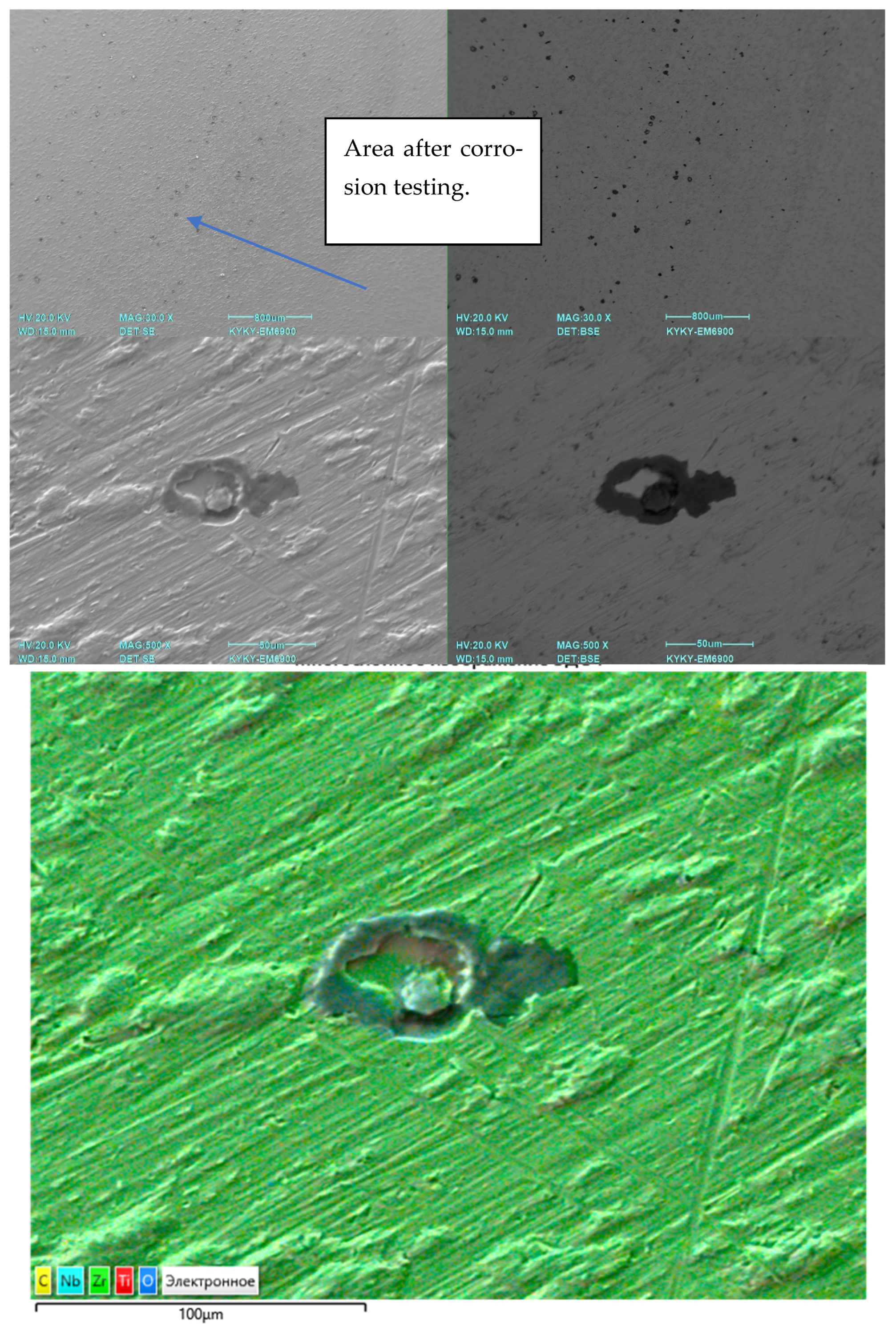
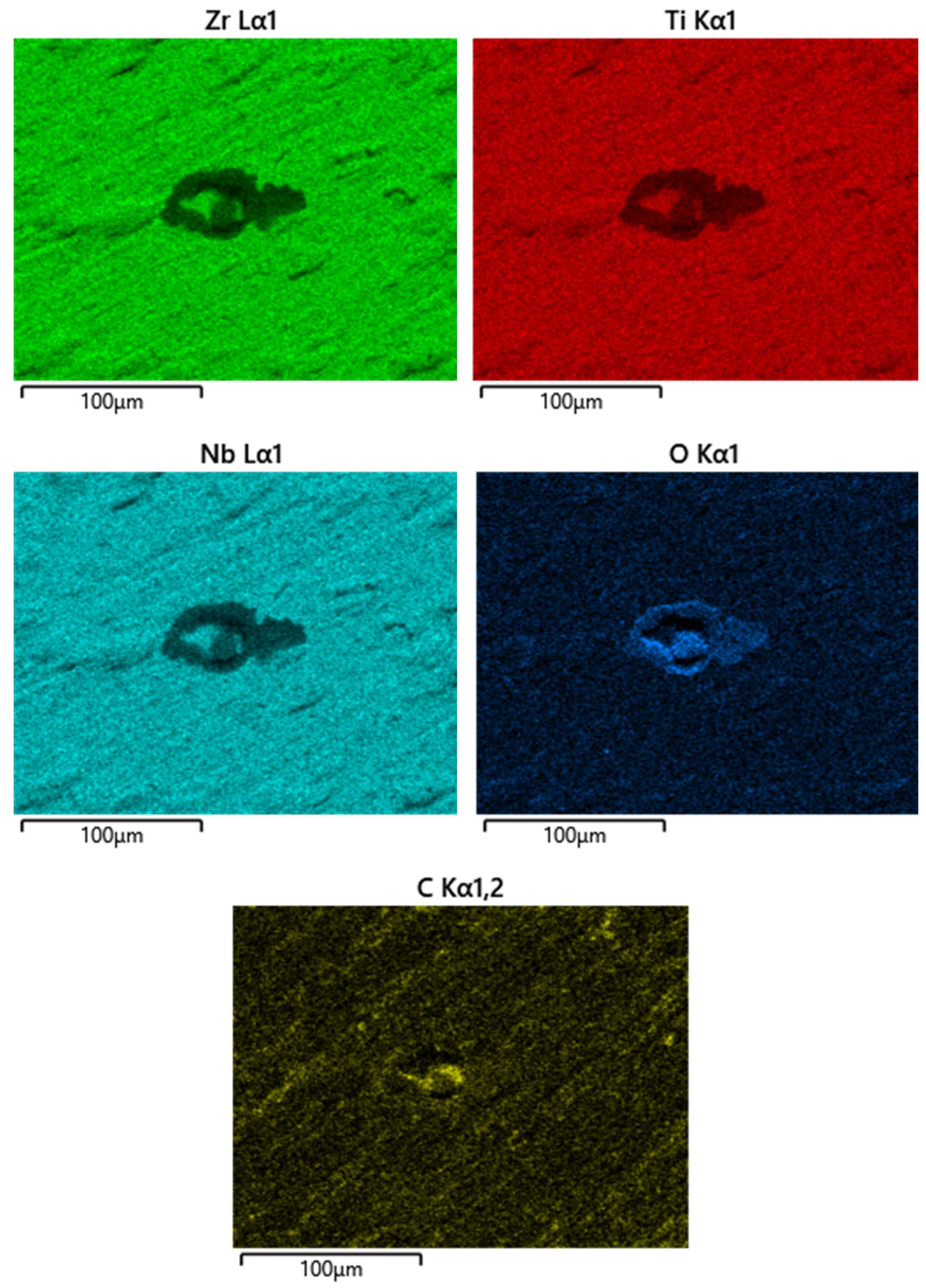
To determine carbon and sulfur impurities, we used oxidizing melting in a ceramic crucible in an induction furnace with the flux using a CS-600 analyzer (LECO, 3000 Lakeview Avenue, St. Joseph, MI, USA). The elements were detected by the amount of released CO2 and SO2 using the infrared absorption method. The distribution of elements was studied using a scanning electron microscope (SEM) EM6900 (KYKY, Beijing, China) with an EDS detector from Oxford Instruments (Oxford Instruments, Abingdon, Oxfordshire, UK) using Aztec (version 6.0 SP2) software.
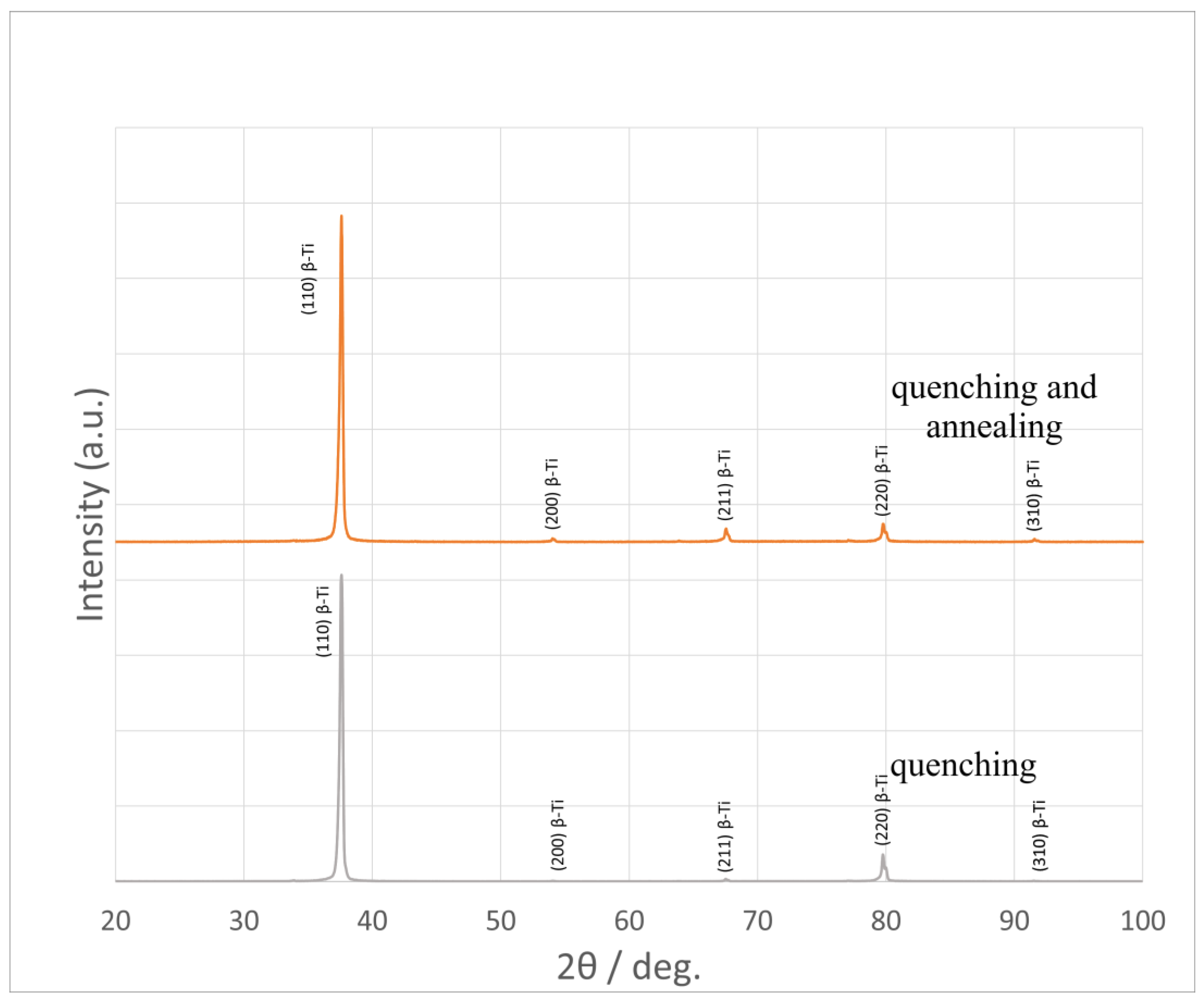
X-ray phase analysis was performed on the DX2700mini diffractometer (Dandong Haoyuan Instrument Co., Dandong, China). The studies were carried out in CuKα = 1.54178 Å radiation, 2θ = 20–100° range; the shooting step was 0.02°/s, the holding time per step was 0.2 s, the voltage on the tube was 40 kV, and the current was 13 mA. The analysis of the obtained diffraction patterns was carried out in the Match! 3 program.
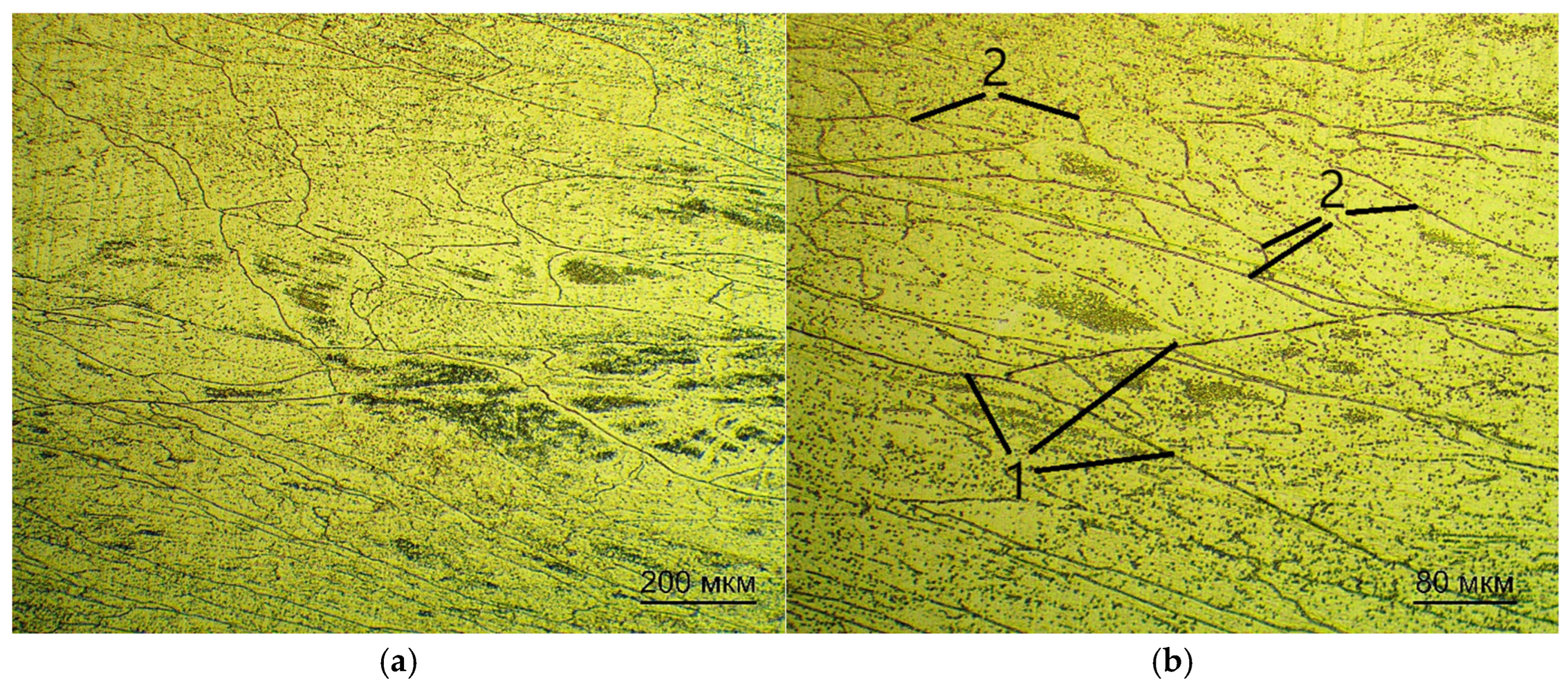
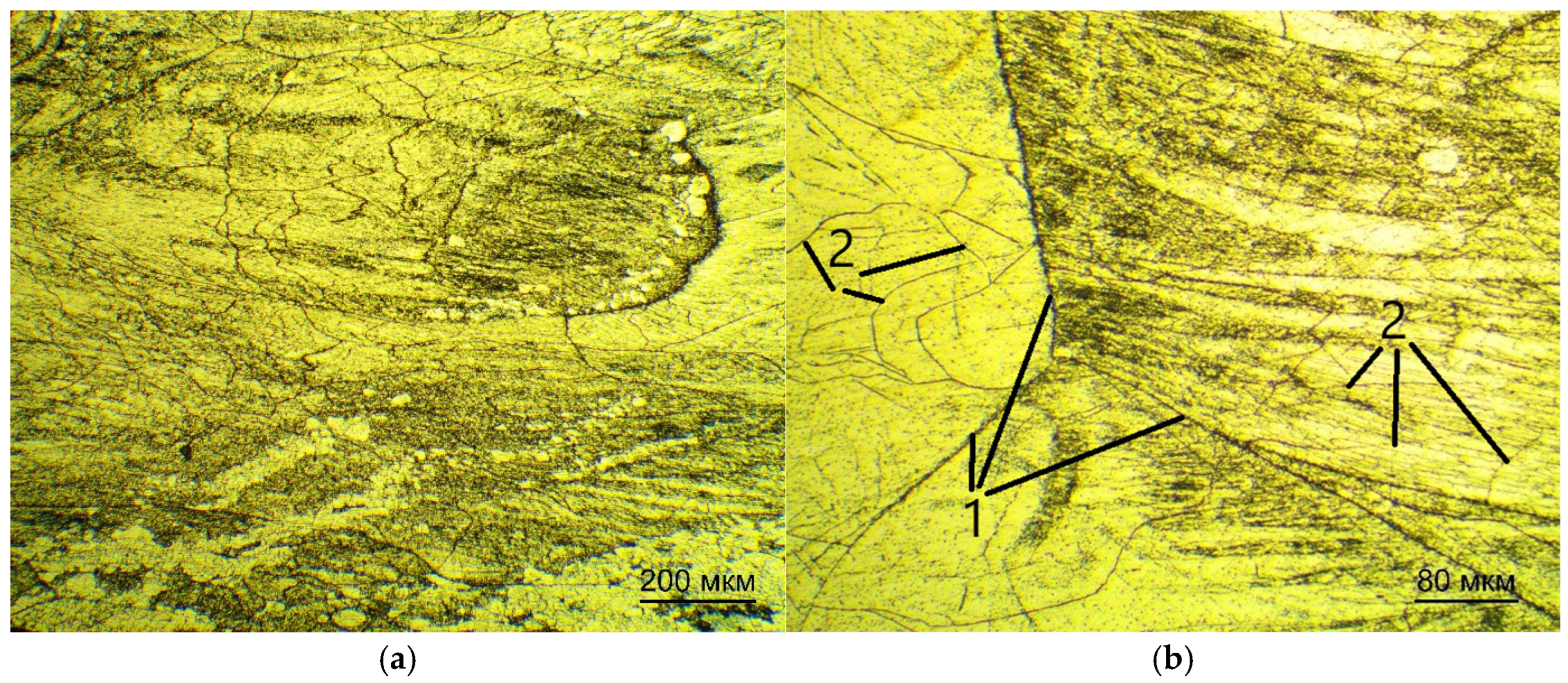
For optical examination of the microstructure, the samples were pre-pressed in a rigid mold into cylinders by means of one-sided pressing.
The samples were subjected to pressure within an IPA 40 air-hydraulic press (Remet, Bologna, Italy) using Aka-Resin Epoxy resin at temperatures ranging from 160 to 185 °C, with a recommended holding time of 20 min. This temperature range is recommended by the manufacturer of the epoxy resin. The sample was then placed into a mold, which was filled with granulated resin. Thereafter, the chamber was closed and the sample was compressed to a pressure of 6 bar. Subsequent to this, the device executed a predetermined program. Thereafter, the compressed sample was subjected to a grinding and polishing procedure. The grinding and polishing of samples for subsequent examination via optical microscopy was carried out in the following sequence.
On a Piatto diamond disk with the following grit:
P220 for 10 min;
P600 for 10 min;
P1000 for 5 min;
P1200 for 5 min;
P2500 for 5 min;
P4000 for 5 min.
The polishing was done on the Akasel NAPAL velvet polishing wheel with the DiaMaxx Poly diamond suspension. The surface of the samples was etched with a solution of nitric and hydrofluoric acids with distilled water in a ratio of HF:HNO3:15 H2O by volume. The sections were immersed in the etching solution for 5–30 s, then washed with running water.
The microstructure was examined on the microscope MET 5C (Altami, Saint Petersburg, Russia) [
34].
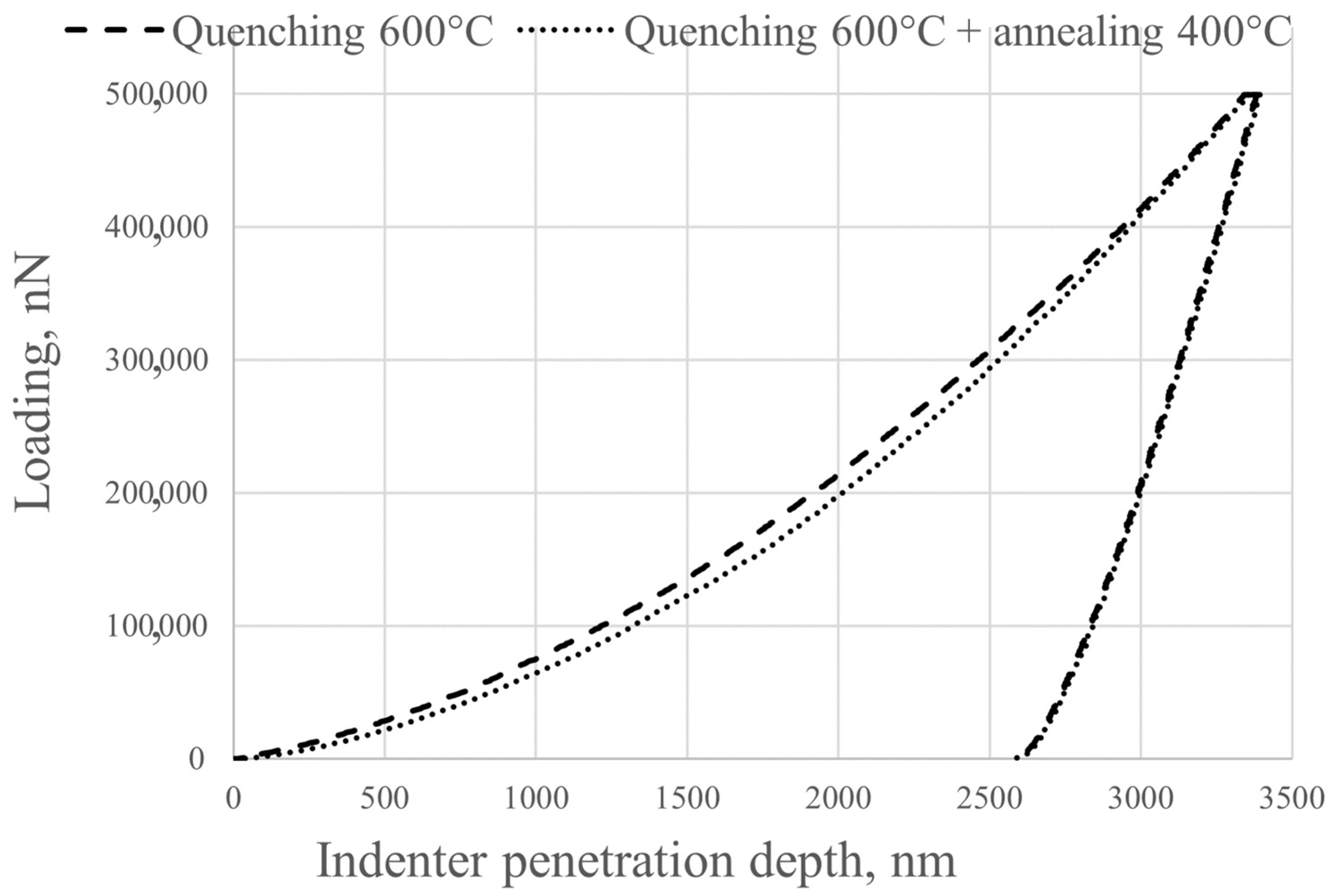
Nanohardness and Young’s modulus were determined using a NanoScan-4D nanohardness tester (NauchSpecPribor, Troitsk, Russia). Instrumental indentation was performed according to ISO 14577-1:2002 [
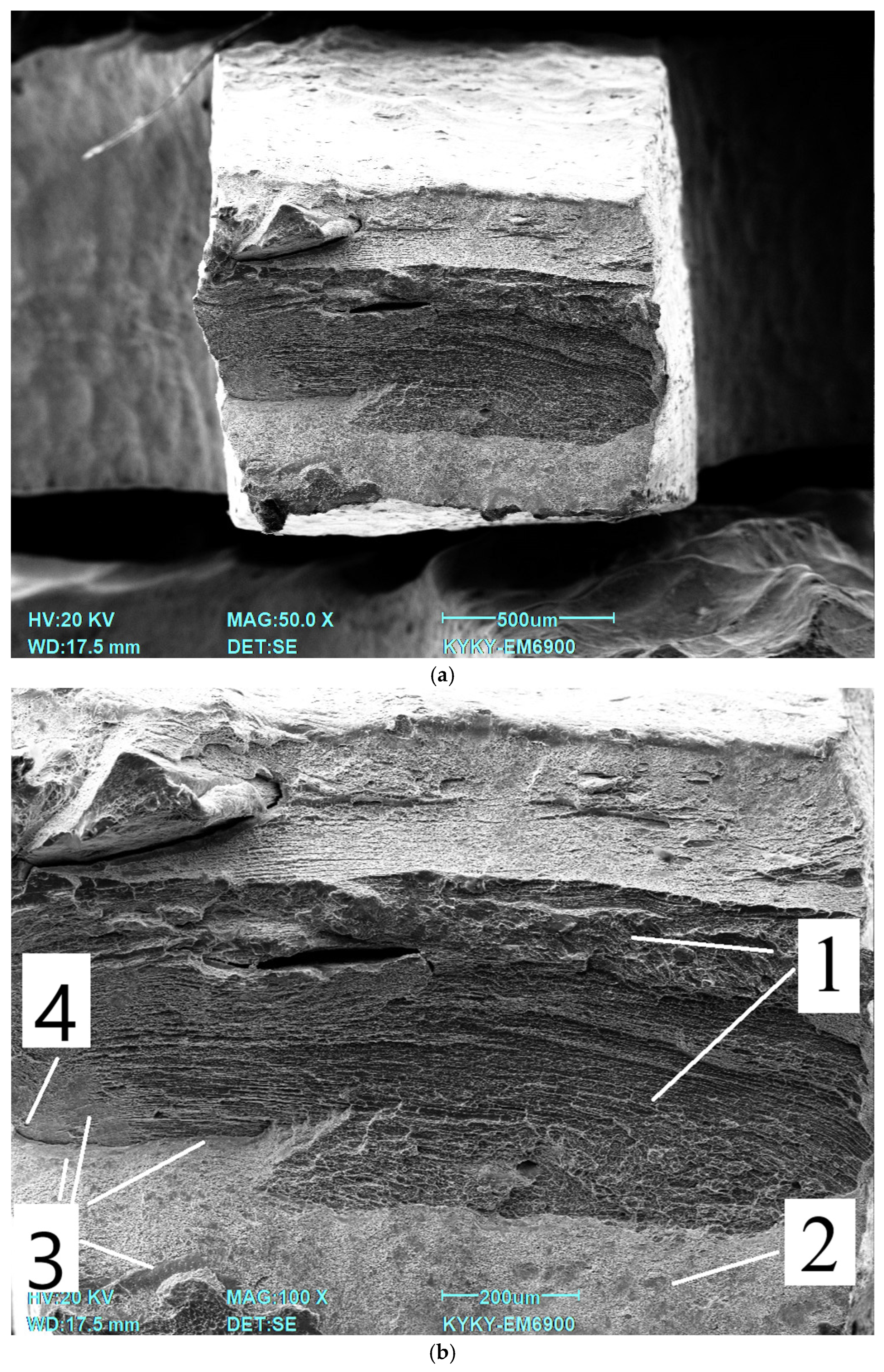
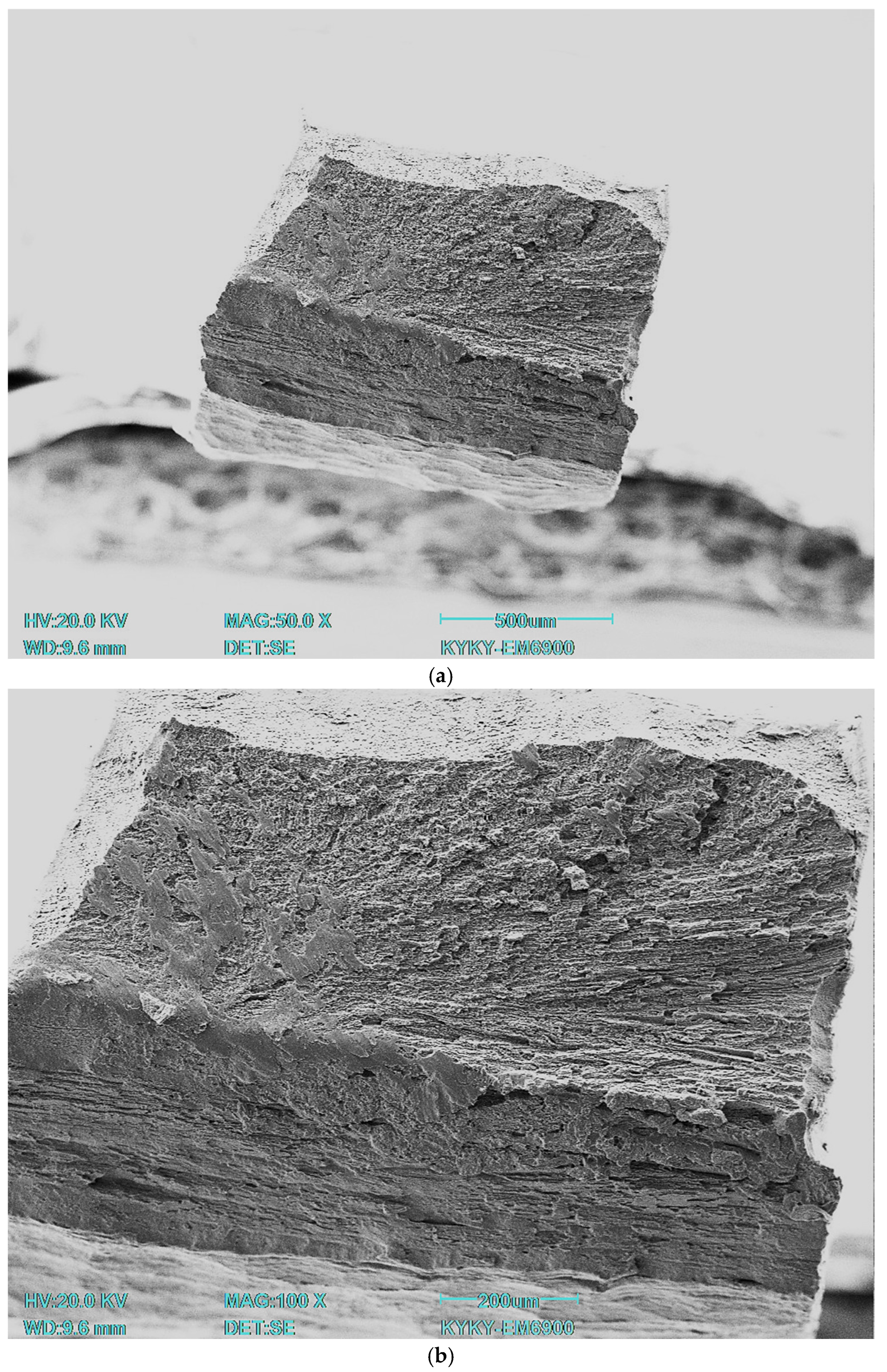
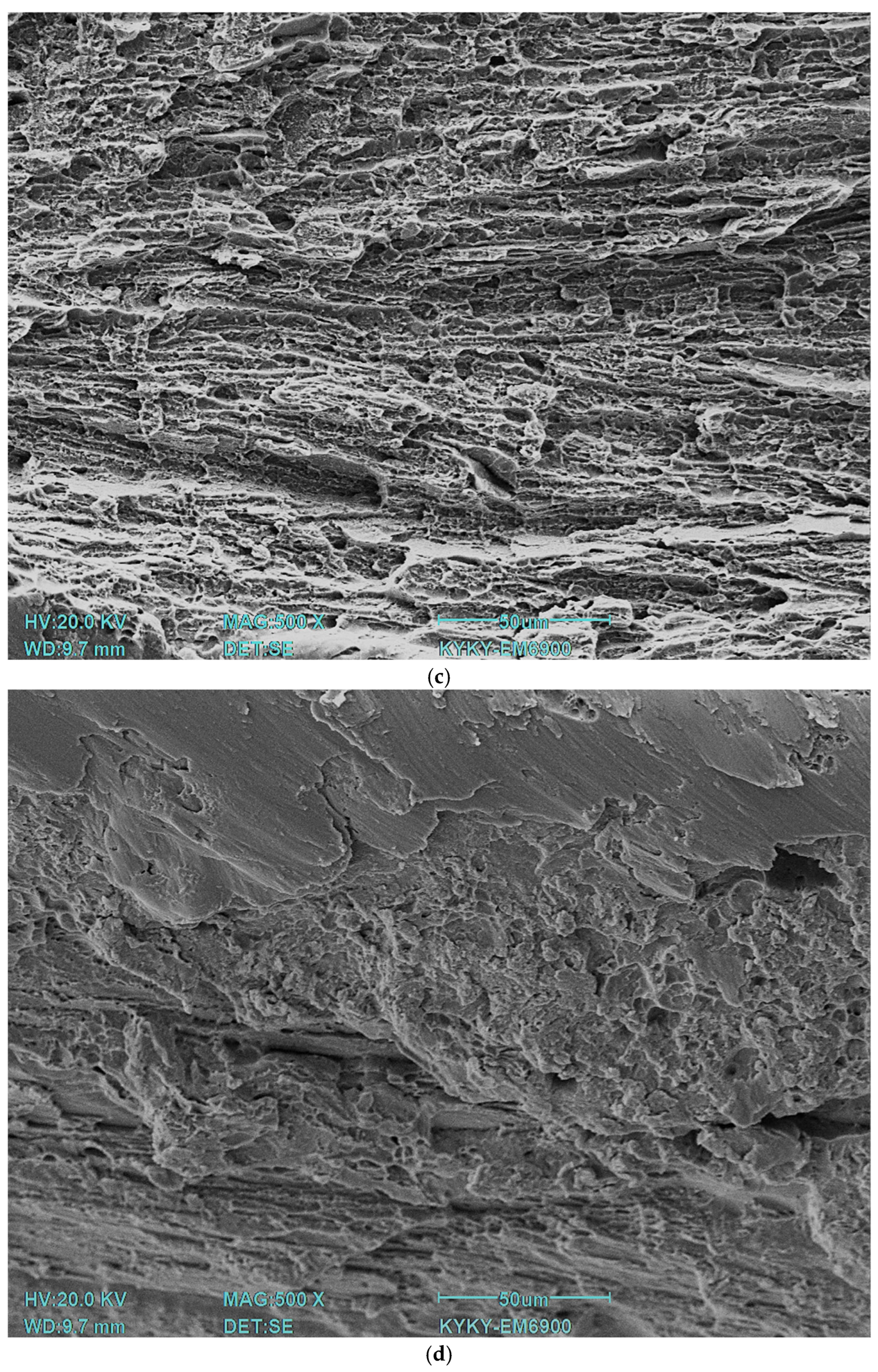
35]. All specimens were subjected to instrumental indentation into the matrix with a load of 500 mN using a Berkovich type diamond tip. Measurements were taken across the full width of the specimen. The load/unload rate was 20 mN/s, the contact time was 20 s, and the distance between indentations was 200 μm. The number of indentations for each treatment type was 27. The experimental curves were processed using NANOINDENTATION 3.0 software from CSM Instruments (Peseux, Switzerland) with a specified Poisson’s ratio (0.3) and were averaged over five experimental curves. Elastic recovery was defined as the ratio of elasticity to total indentation work. Fractographic studies were carried out on a KYKY EM6900 (KYKY, Beijing, China) scanning electron microscope using a secondary electron detector at an accelerating voltage of 20 kV.
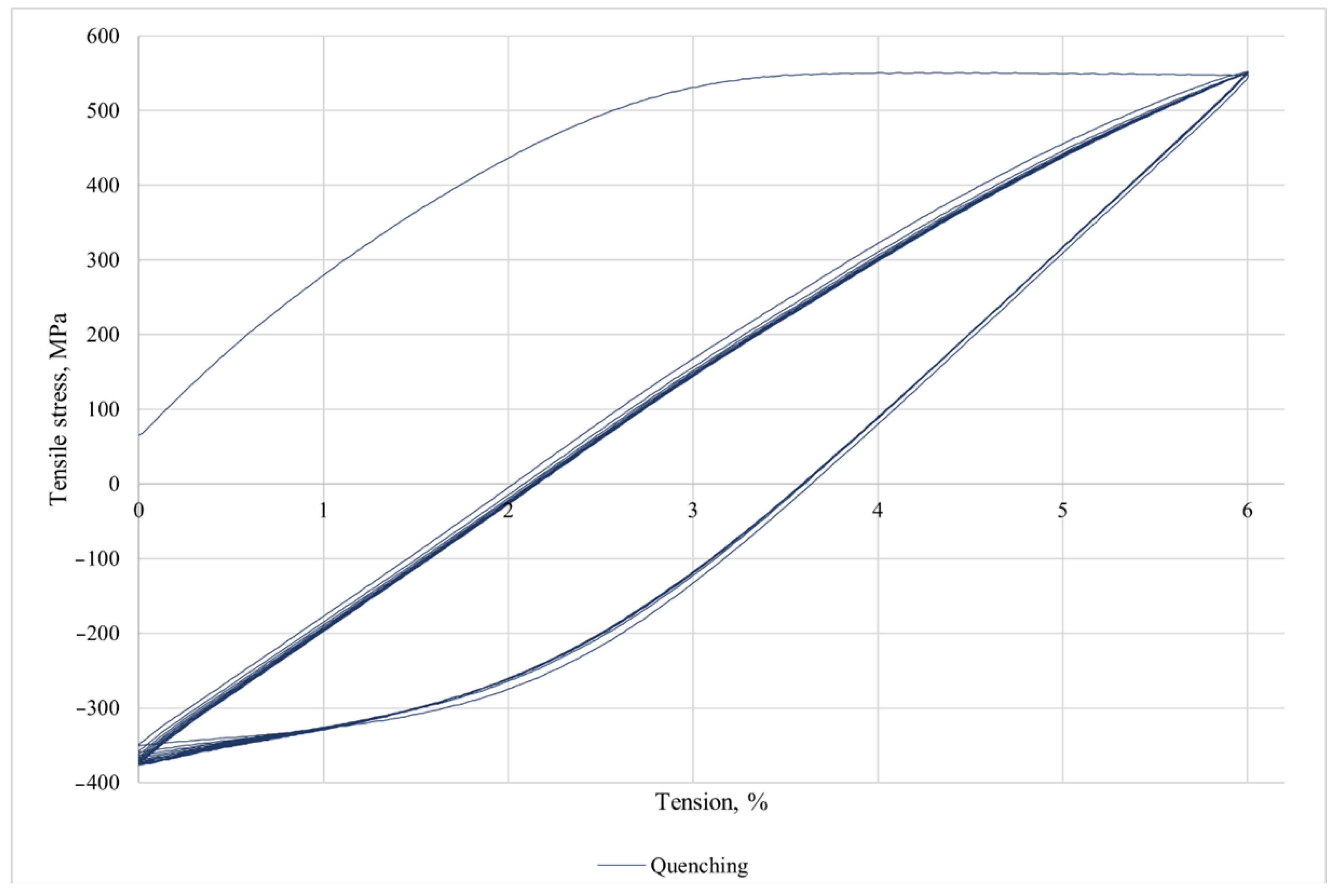
Mechanical studies were carried out on the Instron 3382 (Instron, Norwood, MA, USA) equipment. The data were processed in the INSTRON Bluehill 2.0 software program According to the standard GOST 1497-84 [
36]. The following parameters were determined: conditional yield strength σ
0.2, tensile strength σ
v, and relative elongation δ.
For the long-term immersion corrosion tests of Ti-38Zr–11Nb alloy samples, two types of samples were used for immersion tests—polished and with a rough surface—to simulate the surface treatment of the implant and stimulate the release of metal ions, as well as reference samples (VT1-0 and VT6) with periodic determination of the metal release into an environment simulating physiological conditions. Since the material is supposed to be implanted in the hip area, where it will come into contact with synovial (articular) fluid—it is a viscous liquid that fills the joint cavity and plays a key role in maintaining joint health, and it is produced from blood serum, normally pH 7.34–7.43—the tests were carried out in Ringer’s solution (pH 7.34), a standard buffer medium based on 0.9 wt.% NaCl.
Double-distilled water was used in the work. Standard solutions of the elements were prepared from metals with a high degree of purity (99.9%) or from certified standard solutions from Merk. The background is 1 N hydrochloric acid.
Samples of each type were placed in 100 mL of the used medium contained in flat-bottomed thermolab glass flasks. The vials were tightly sealed and stored in the dark between sampling at 37 °C. After the selected period (7, 14, and 21 days) of exposure of samples in the medium, samples were taken from the flasks for analysis (volume—2 mL) and diluted 5 times with 1.25 M HCl medium, which ensures the stability of analytes in solution [
37]). The initial buffer solutions were used as reference solutions. The research was carried out using an ICP-OES EXPEC 6500 inductively coupled plasma atomic emission spectrometer (EXPEC Technology, Beijing, China).
The induction argon plasma is the most efficient source of atomic emission, which, in principle, can be used to determine all elements except argon. Induction plasma is suitable for the determination of a wide range of concentrations, from ultra-low to macro-concentrations. NPP with ICP is less susceptible to interference than any other comparable spectrometric method.
The atoms and ions of the sample in the plasma are in an excited state. The intensity of the radiation emitted as the atoms and ions transition to lower energy levels is measured. Each element emits a specific wavelength of radiation. Analysis of the resulting spectrum allows for qualitative and quantitative analysis. The analyte passes through the central channel into the plasma zone and is heated to a temperature of approximately 8000 K. At this temperature, almost complete atomization, a high degree of atomic excitation, and partial ionization are achieved. A zone above a brightly glowing plasma is used to obtain the spectrum. Here, the atomic radiation can be measured at a low background level.
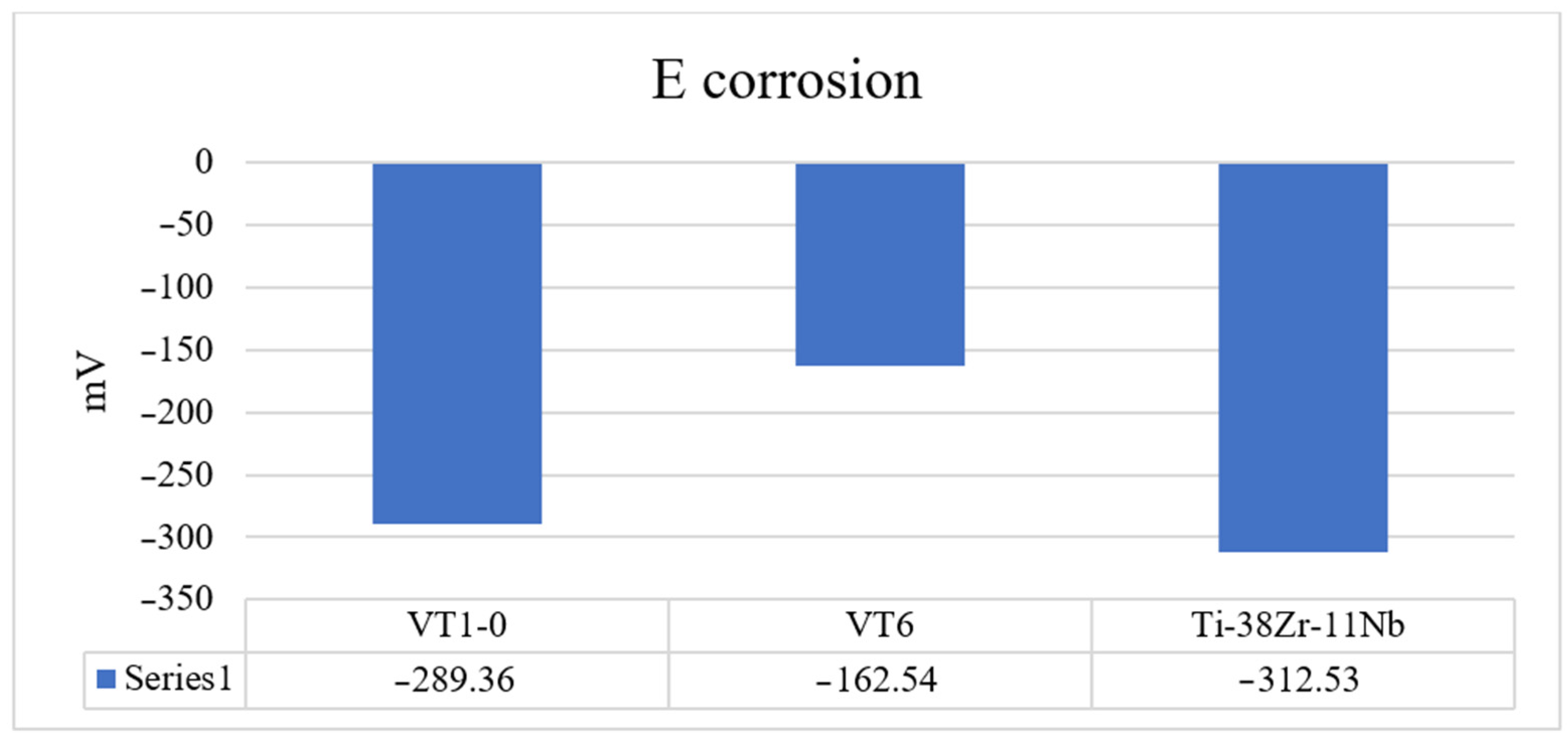
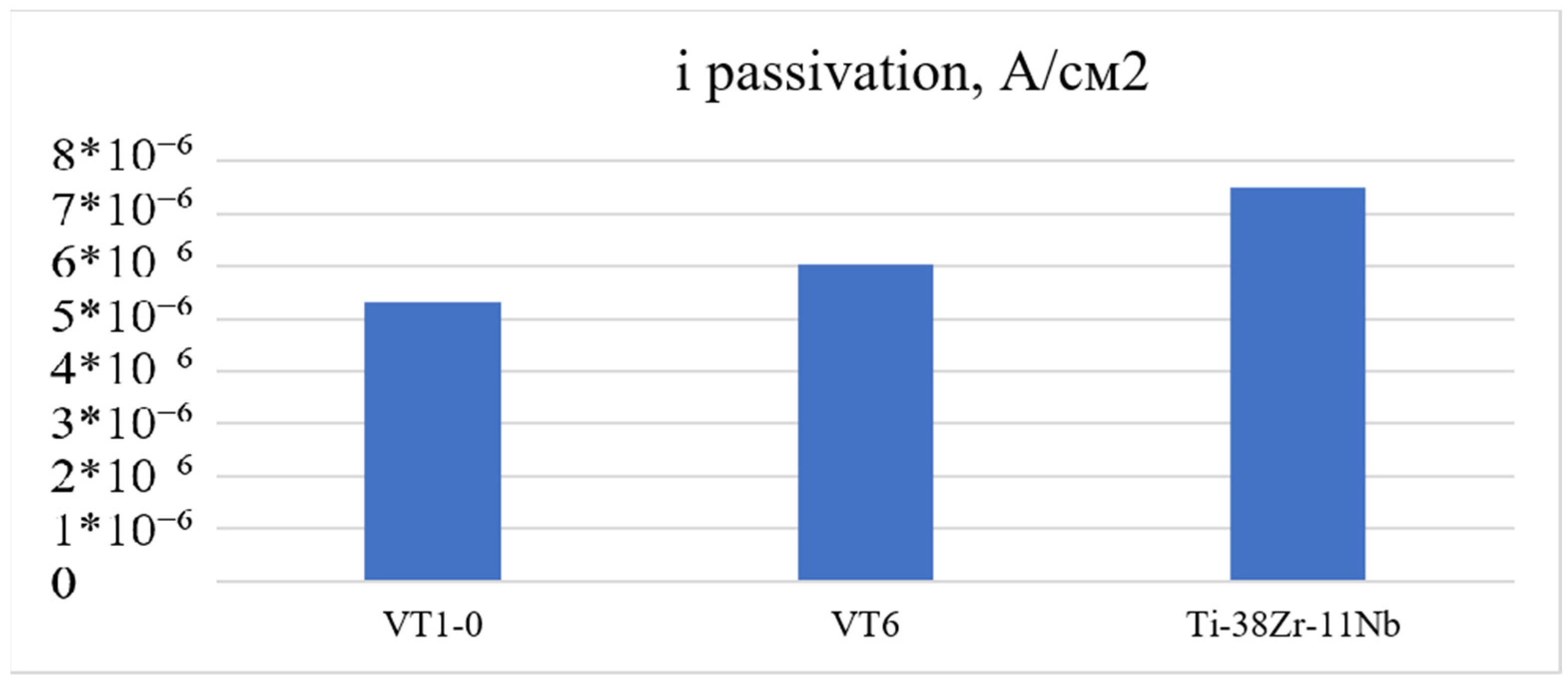
A 0.9% sodium chloride saline solution, widely used in medicine, was selected to evaluate corrosion resistance. The model solution used in the experiments is used to prepare dosage forms for injection, so the evaluation of this chloride concentration for corrosion potential, corrosion current density, and passivation current density is an urgent and practically important task.
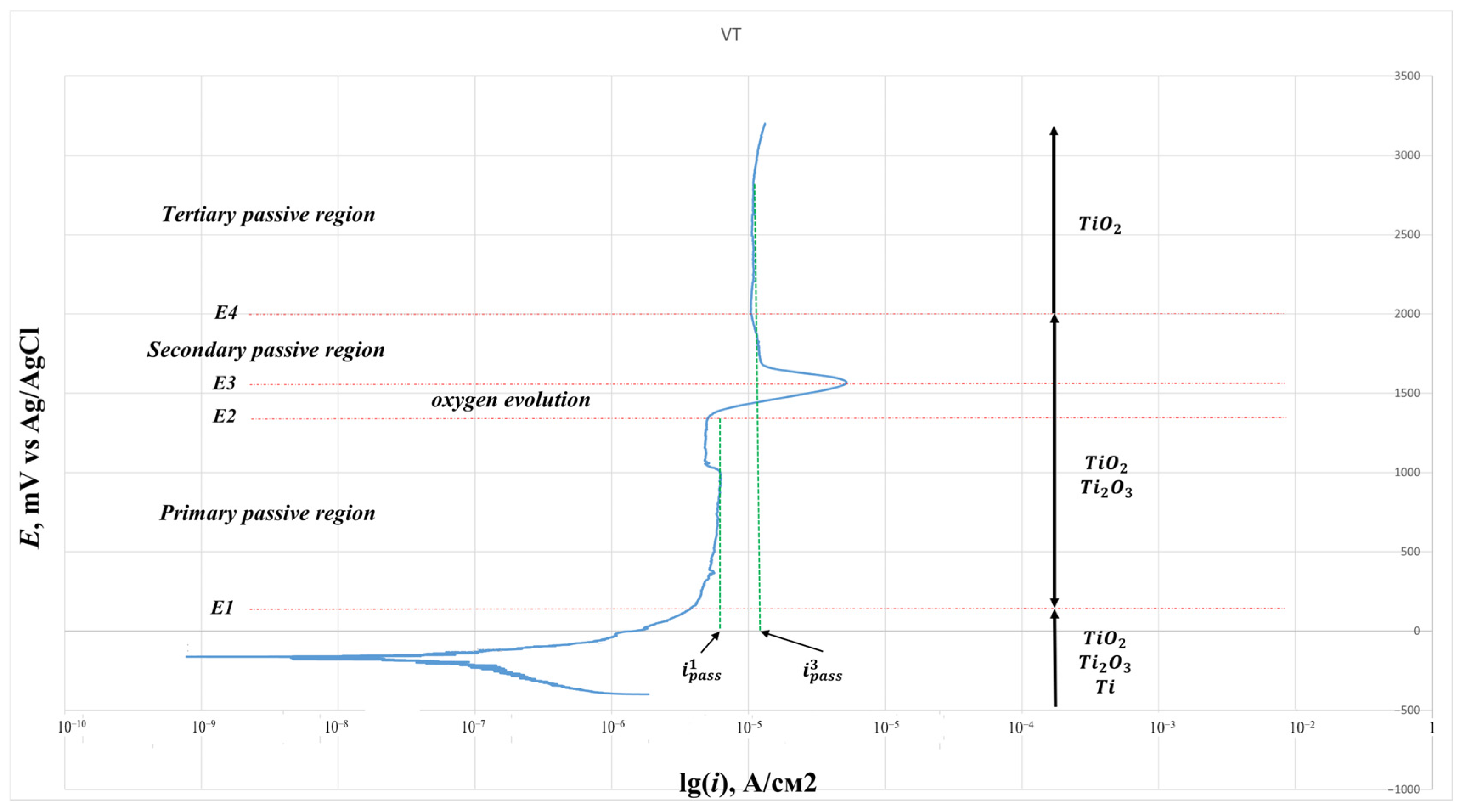
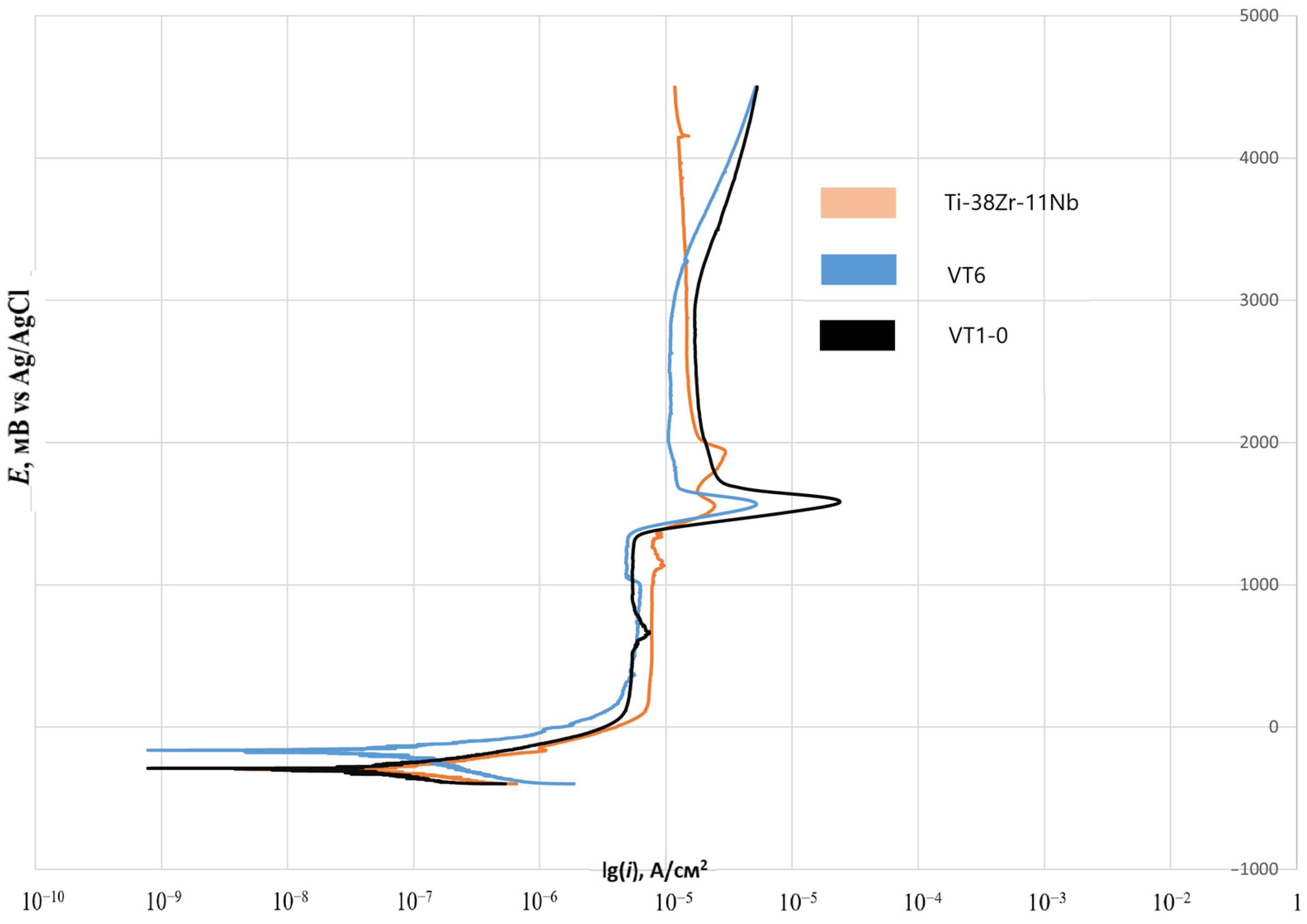
To assess the corrosion resistance of Ti-38Zr-11Nb alloy samples, as well as titanium alloys VT6 and VT1-0 commercially produced and used in medical applications, the potentiodynamic method according to GOST 9.912-89 was used [
38]. Accelerated testing methods for resistance to pitting corrosion were used. A silver chloride reference electrode was used for the study in a three-electrode cell. The auxiliary electrode was a platinum wire with a surface area 3 times larger than the area of the working electrode. The tests were carried out at room temperature. To prepare the studied titanium alloy plates, round samples with an area of 1 cm
2 were cut out on a wire erosion machine. Next, a wire was welded to each plate using spot welding to make an electrical contact. At the next stage, the plates were filled with epoxy resin. After curing the epoxy resin, the working surface of the plates was sanded and polished using 4000 grit sandpaper at the finishing stage. A potentiostat, the galvanostat P-30JM (Ellins, Chernogolovka, Russia) was used to construct the polarization curves. The scanning speed in the potentiodynamic mode is 1 mV/s. Considering that titanium alloys are passive, for a complete assessment of the passive zone, the potential sweep was carried out up to 4.5 V.
In vitro biocompatibility studies.
For in vitro testing, the samples were placed in the wells of a 96-well tablet. The samples were sterilized at 180 °C in a frying pan.
The direct contact method was used to study the adhesive characteristics of materials and determine their cytotoxicity for cells. In carrying out this study, we used a primary culture of human mesenchymal stem cells isolated from pulp, which was obtained from an institution licensed by the Ministry of Health of the Russian Federation operating within the framework of the legislation of the Russian Federation. Cell culture was performed in DMEM/F-12 medium (1:1, Life Technologies, Carlsbad, CA, USA), with 10% ETS, 2 mM L-glutamine, 100 U/mL penicillin, and 100 mcg/mL streptomycin vitamin solution (PanEco, Moscow, Russia) at 37 °C in an atmosphere of 5% OF CO2. As they grew and reached a subconfluent state, the cells were treated with 0.25% trypsin-EDTA solution and passed into new vials in a ratio of 1:2. Cells on passage 4 were used for research.
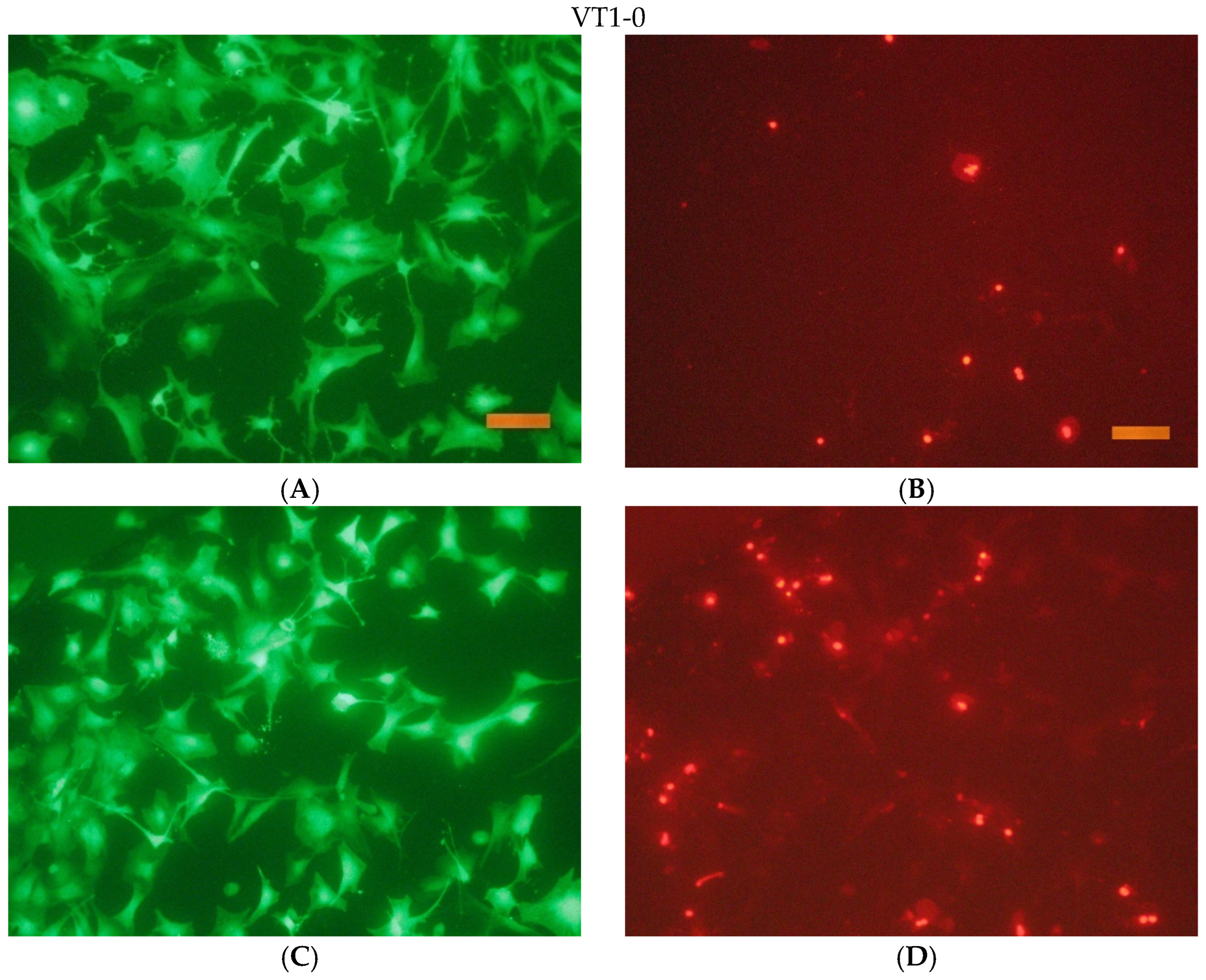
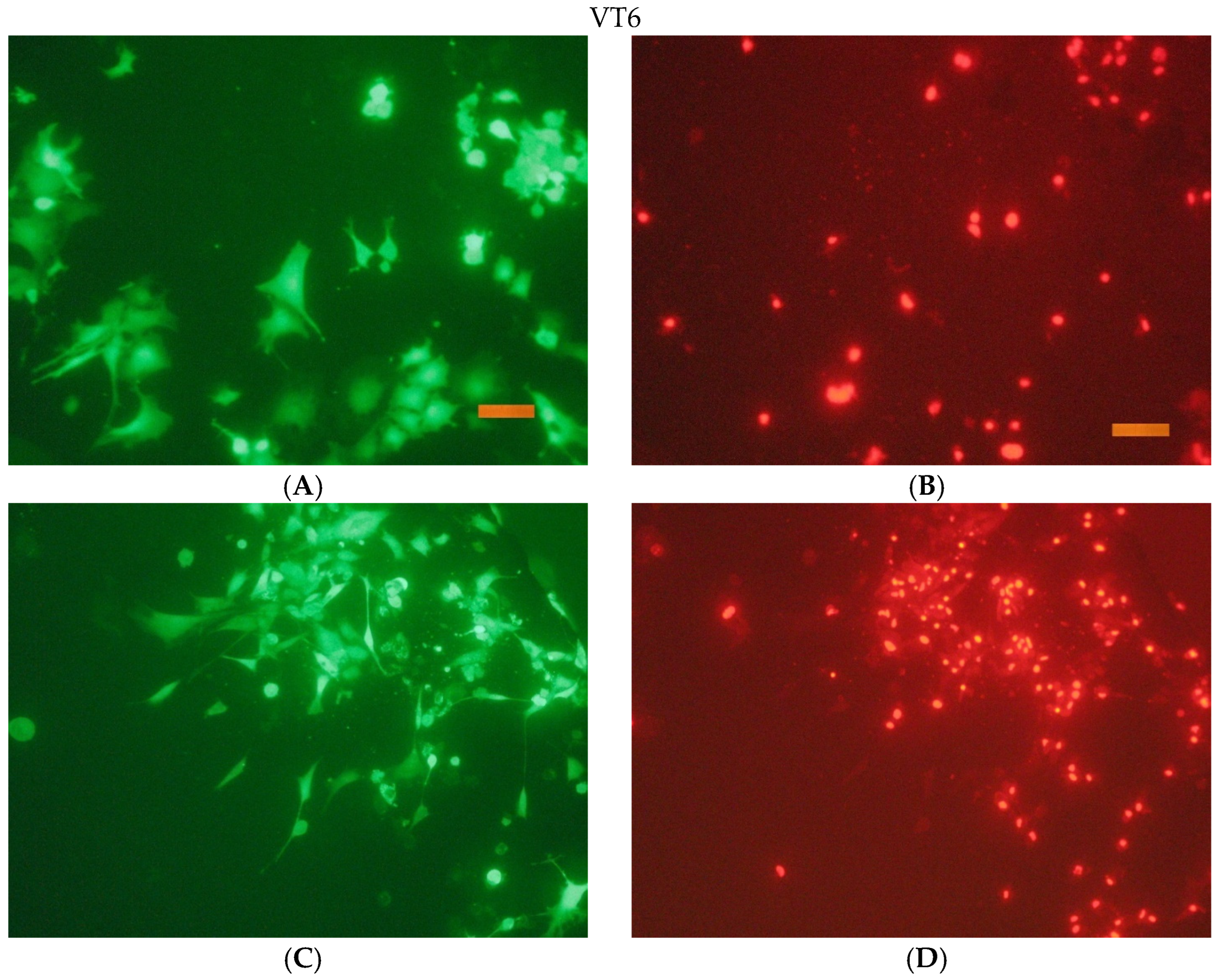
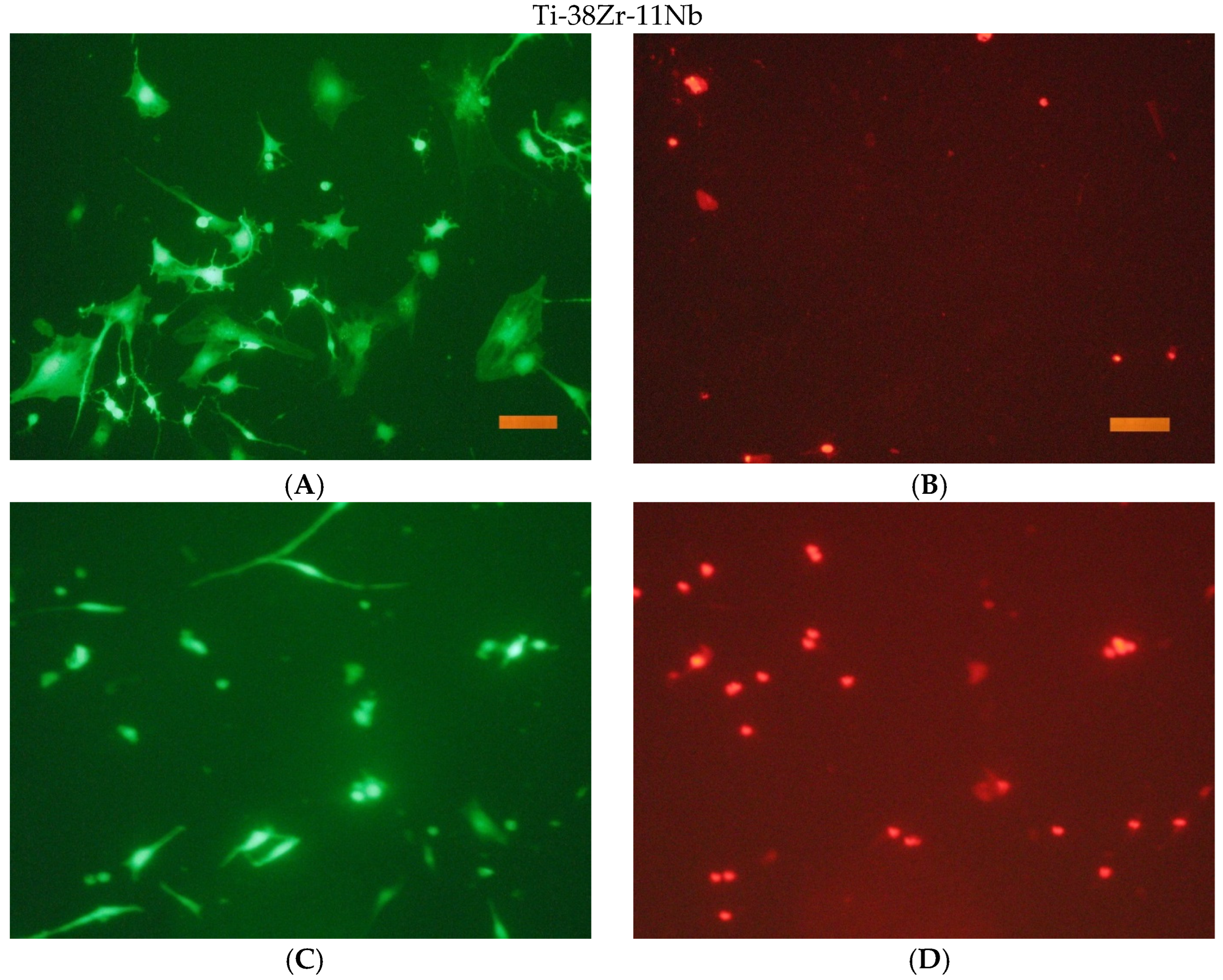
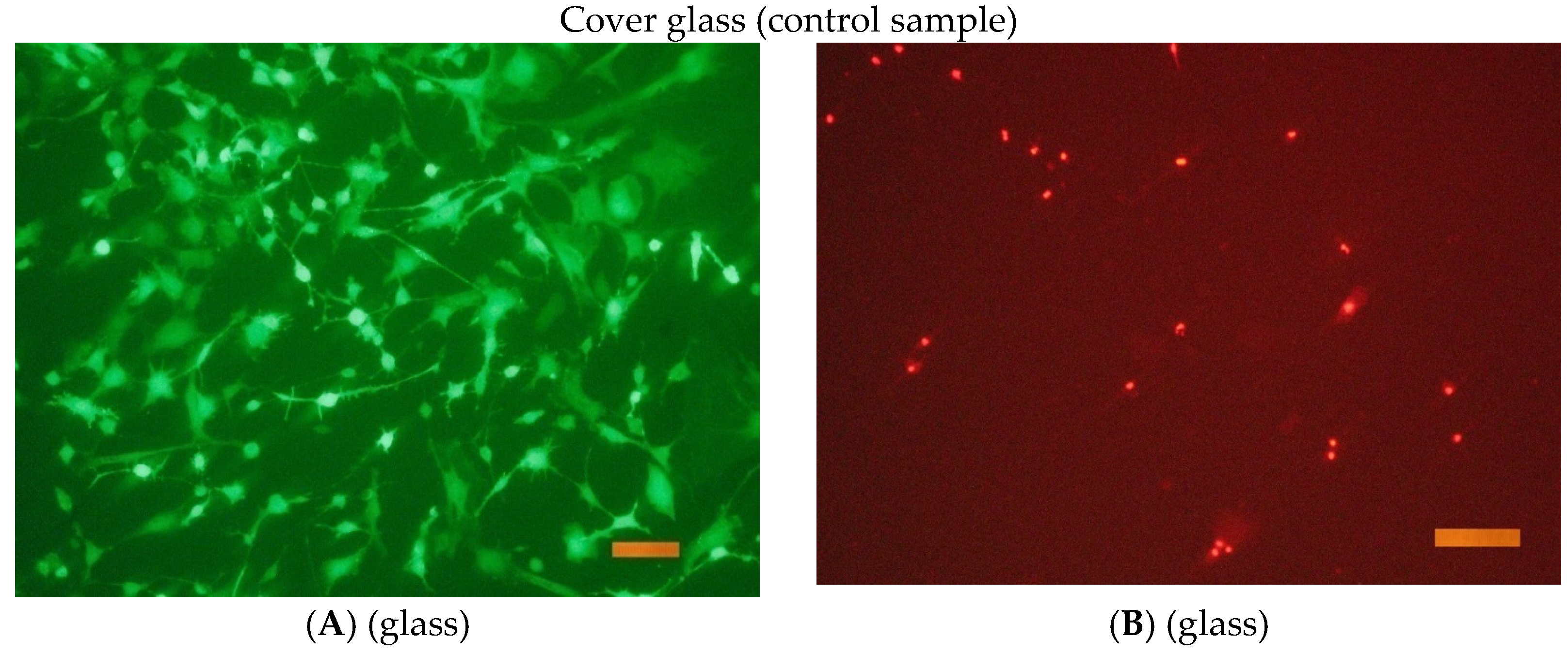
The cells were seeded onto the surface in cells of a 96-well tablet at a concentration of 30 thousand cells/cm2 (DMEM/F12 + 10% FBS medium). Cover glasses of the same size were used as a control.
After the end of cultivation, the viability of cells cultured on the surface of the studied materials was evaluated using an Axiovert 200 fluorescence microscope. The method of fluorescent staining cells with SYTO 9 and propidium iodide was used for the analysis. The fluorescent dye SYTO 9 in the study mode λvozb = 450–490 nm and λemiss = 515–565 nm stains the DNA and RNA of living and dead cells green. The intercalating reagent propidium iodide in the study mode λvozb = 546 nm and λemiss = 575–640 nm stains the nuclei of dead cells red.
To study the cytotoxicity of the samples, they were studied by direct contact.
The studies were carried out using hoods and the materials themselves in accordance with the requirements of GOST R ISO 10993.5-99, ‘Medical devices. Assessment of the biological effect of medical products. Part 5. Cytotoxicity testing: in vitro methods.’ [
39], and GOST R ISO 10993.12-99, ‘Medical devices. Assessment of the biological effect of medical products. Part 12. Sample preparation and standard samples.’ [
40].
The study of cell adhesion and growth on the surface of materials was carried out using NCTC clone L929 cells and primary culture of mouse fibroblasts obtained from the skin and muscle tissue of 13-day-old embryos of C57BL/6-Tg(ACTbEGFP)1Osb/J mice. The cell culture in passage 4 was used to test the materials. The cells were cultured at 37 °C in an atmosphere of 5% CO2 in DMEM/F12 (1:1) medium with the addition of 5% embryonic veal serum (ETS) and 100 U/mL penicillin/streptomycin.
DMEM/F12 culture medium with the addition of 5% fetal veal serum (ETS) and 100 mg/mL of penicillin/streptomycin was used as a model medium for the preparation of extracts. The extracts were prepared in sterile foam flakes with asepsis for 4 days at 37 °C. The ratio of the surface area of the material in square centimeters and the volume of the model medium in milliliters for the materials allowed us to determine the amount of medium required for incubation (600–800 µL for each type of alloy using all of the samples presented). Three takes were used for sampling and control.
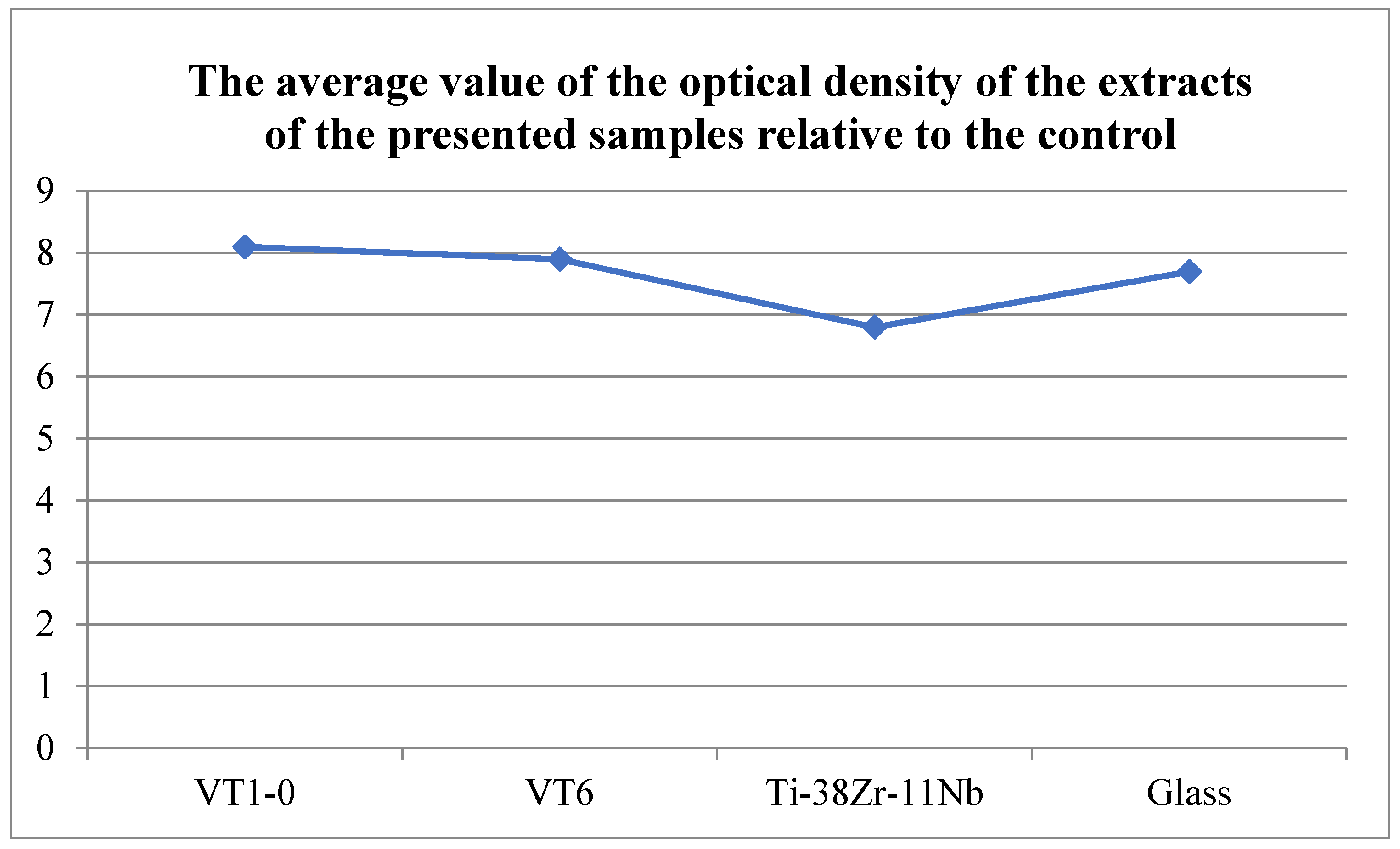
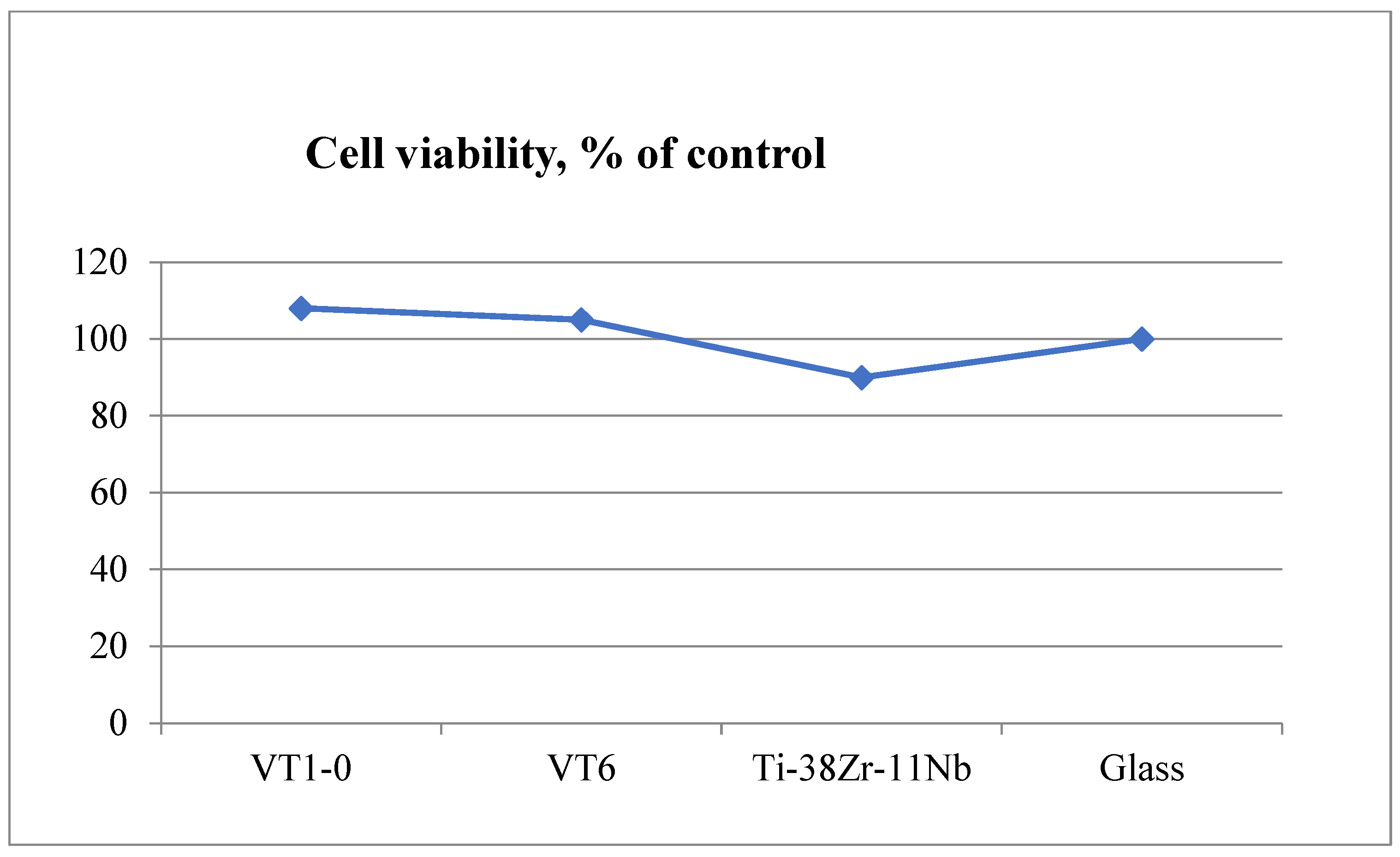
The cytotoxicity of extracts was assessed by adding them to the cell culture in the wells of a 96-well plate (6–8 wells per variety), followed by cultivation at 37 °C for 1 and 3 days. The inoculation concentration of cells was 10,000/cm2, the concentration of embryonic veal serum in the culture medium was 5%. As a control, appropriate dilutions of DMEM/F12 medium of 5% ETS were used, aged for 1 and 6 days at 37 °C. The cytotoxicity of the extracts was determined by cell viability relative to the control using the MTT test. An amount of 10% DMSO was used as a positive control, and a culture medium exposed to the conditions and procedures of the study was used as a background control. A day after the start of cultivation, 10 mL of MTT solution was added to each well (5 mg/mL in a PBS solution [150 mM sodium phosphate buffer, pH 7.2, 150 mM NaCl] filtered through filters with a pore diameter of 0.2 microns). After holding for 3 h at 37 °C in a humidified atmosphere of 5% CO2, the liquid was removed, 100 µL of dimethyl sulfoxide (DMSO) was added, and the formed formazan salts were dissolved by shaking the plates at room temperature for 5 min. The color development was recorded by measuring the optical density at a wavelength of 540 nm in the wells of a 96-well plate using a photometer (model 680 BIO-RAD, Hercules, CA, USA). The number of parallel experiments was at least three.
Upon completion of cultivation, samples were prepared for examination using scanning electron microscopy (SEM). The samples were washed in a 0.1 M phosphate–salt buffer (pH 7.4) and fixed for 12 h at a temperature of 5 °C in 2.5% buffered glutaraldehyde solution. After fixation, the samples were washed with water and dehydrated at 4 °C sequentially in a battery of an aqueous ethanol solution of increasing concentrations of 50%, 75%, 80%, and 90% and in absolute ethanol at the final stage. At each stage, the samples were immersed twice for 5 min in an appropriate solution of ethyl alcohol. To remove the alcohol, the samples were transferred to hexamethyldisilazane (HMDS) for 30 min, after which they were dried in air. The morphology of cells on the surface of the samples was studied using a KYKY-SEM 6900 scanning electron microscope (KYKY, Beijing, China) in secondary electrons (SE type detector).