Simulation of the Periodontal Ligament in Dental Materials Research: A CAD/CAM-Based Method for PDL Modeling
Abstract
1. Introduction
2. Materials and Methods
2.1. CAD/CAM-Based Method for the PDL Simulation
- A central socket for the coronal part of the replica, ensuring fixed orientation and maintaining the root at a specific distance from the socket base;
- A 2 mm vertical clearance around the socket for excess polyether material during placement;
- An outer flange matching the block’s contour with a 0.05 mm offset, providing alignment and ensuring the root axis was parallel to the socket’s long axis. The 10 mm-high flange acted as a guide for reproducible root placement within the polyether mass.
2.2. Statistical Analysis
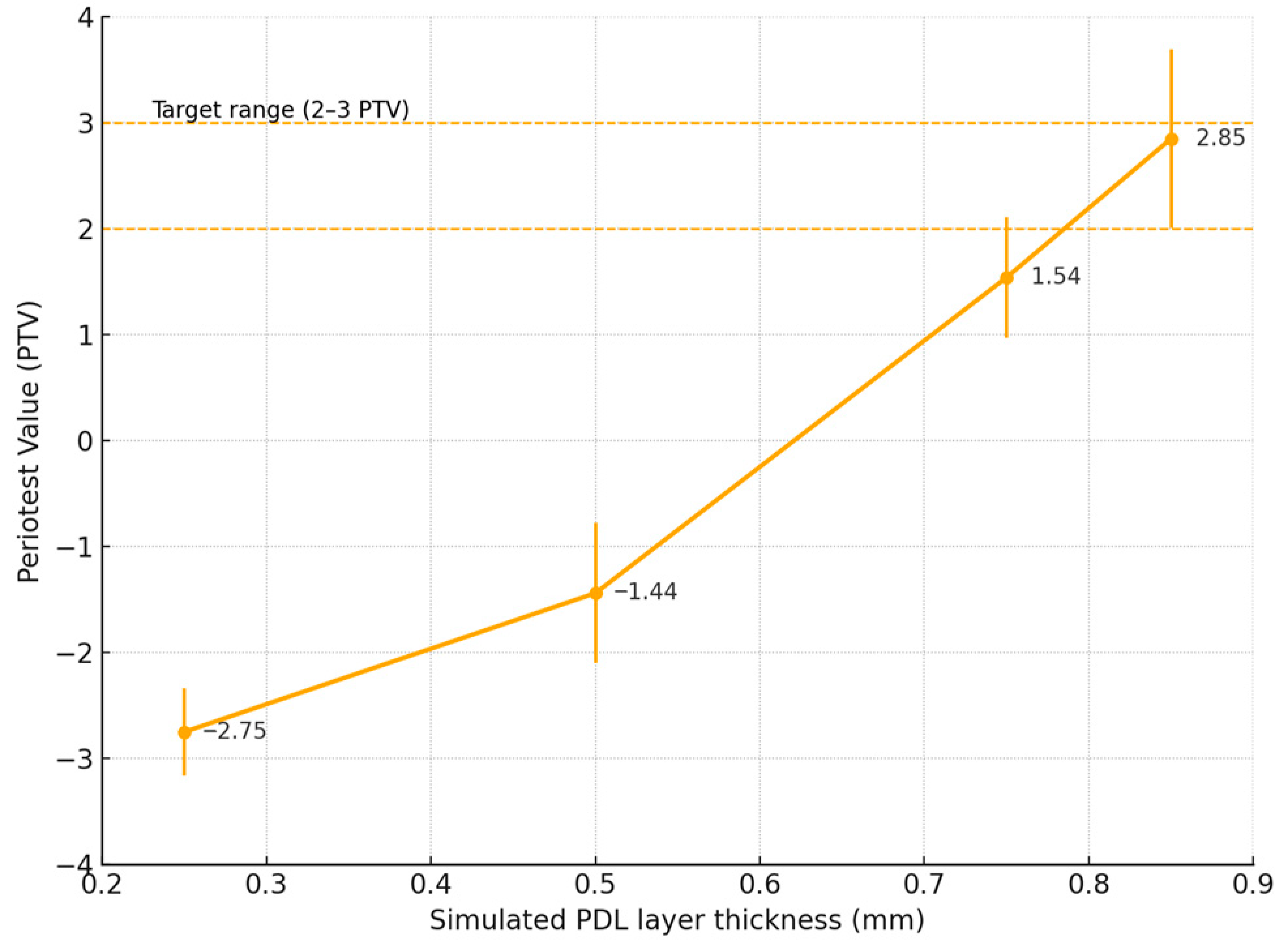
3. Results
Experimental Model Measurements
4. Discussion
4.1. The Influence of PDL Simulation on Mechanical Testing Outcomes
4.2. Methods for Simulating the PDL
4.3. Materials Used for PDL Simulation, PDL Layer Thickness, and Model Validation
4.4. Reporting Checklist for Studies Including PDL Simulation
- PDL fabrication method: method of creating the PDL space and applying the analog (e.g., lost-wax technique, spacer foil, direct coating, or CAD/CAM-based digital offset).
- PDL analog: material type, brand, viscosity
- PDL layer thickness: target value, tolerance, and method of verification (e.g., µCT, optical measurement, or radiograph).
- Functional validation: validation method (e.g., Periotest, static deflection, or mobility assessment) and target range representing physiological tooth mobility.
- Positioning and alignment: description of root-axis alignment procedure, use of positioning devices or jigs.
- Tooth and substrate: type of tooth, number of roots, arch, and substrate material (e.g., natural tooth, PMMA, resin, gypsum).
- Socket material: composition, manufacturer, and method of cavity preparation.
- Cementation and restoration: type of luting agent, restorative material, and surface treatment.
- Aging and loading protocol: thermocycling, mechanical load magnitude, and number of cycles.
- Outcome measures: parameters evaluated (e.g., fracture load, load bearing capacity, wear).
- Uncertainty assessment: identification and quantification of potential error sources (e.g., layer thickness variability, positioning deviation, or material property variation); report standard deviation, confidence intervals, or coefficient of variation (CV) for key parameters.
4.5. A Novel CAD/CAM Model for PDL Simulation
5. Conclusions
- The inclusion of a PDL analog is essential when evaluating mechanical properties such as the fracture resistance of fixed partial dentures, the strength of post-and-core restorations, and the wear resistance of prosthetic materials. Omission of this element may distort test outcomes, resulting in either overestimation or underestimation of the actual clinical performance of the material or the prosthetic structure.
- A comprehensive description of methodologies and materials used in in vitro studies is critical to ensure reproducibility, enable meaningful comparisons across studies, and support the development of reliable and clinically relevant conclusions.
- Experimental models incorporating PDL simulation should undergo functional validation in addition to geometric verification. This step is essential to confirm that the model accurately replicates the mechanical behavior of the natural PDL, thereby preventing biased or misleading results.
- The width of the simulated PDL space in experimental models should not be determined solely based on anatomical dimensions. Accurate reproduction requires calibration through functional testing, considering the mechanical properties of the material used to mimic PDL resilience and the anatomical configuration of the tooth replica.
- The CAD/CAM-based PDL simulation model proposed by the authors demonstrates high reproducibility, ease of fabrication, and accurate emulation of the elastic properties of the natural PDL. This model offers a viable alternative to conventional techniques and may be integrated into various experimental protocols.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAD/CAM | Computer-Aided Design/Computer-Aided Manufacturing |
| PDL | Periodontal Ligament |
| PMMA | Polymethyl methacrylate |
| PTV | Periotest Value |
| FPD | Fixed Partial Denture |
| FEA | Finite Element Analysis |
| TCML | Thermocycling and Mechanical Loading |
Appendix A
Appendix A.1. Literature Review Methodology
Appendix A.1.1. Search Strategy
Appendix A.1.2. Eligibility Criteria
- Published between 2005 and 2025
- Written in English
- Peer-reviewed original research
- Use of physical models simulating the PDL
Appendix A.1.3. Selection Process

Appendix A.1.4. Data Extraction
- Bibliographic details (authors, year of publication)
- Thickness of the simulated PDL layer
- Material used for PDL simulation
- Material of the abutment tooth
- Technique used for model fabrication
- Tested mechanical parameters
- Type of intervention tested
- Validation of the PDL model (if applicable)
- Assessment of the impact of PDL simulation on study outcomes
- Correlation of findings with in vivo data (if reported)
Appendix A.2
| Study (Authors, Year) | Layer Thickness | PDL Simulation Material | Tooth Material | Technique for Artificial PDL | Experimental Model Validation | Parameters Tested | Treatment Type | Influence of PDL on Results | In Vivo Measurements Correlation |
|---|---|---|---|---|---|---|---|---|---|
| Aboushelib (2013) [28] | 0.6 mm | Polyether impression material (Impregum; 3M ESPE, St. Paul, MN, USA) | Composite (Z250, 3M ESPE, St. Paul, MN, USA) | Polyether injection into the resin socket—technique not specified | Not specified | Cyclic loading resistance | Zirconia single crown | Lower peak stress in tested materials (lack of cone cracks typical for overloading in the testing environment) | Comparable survival rate |
| Alqarni et al. (2024) [48] | 0.2 mm | 3D printing resin—Flexible resin V2 (Formlabs Inc., Somerville, MA, USA) | Human teeth | PDL constructed using CAD/CAM process: root surface scanned and digitally offset 0.2 mm; PDL 3D-printed in flexible resin and fitted to the root, alveolar socket not mentioned | Not specified | Feasibility of digital workflow to fabricate PDL | None | Not specified | Not specified |
| AlZahrani & Richards (2018) [38] | 0.25 mm | Latex rubber die-spacer (Rubber-Sep, Kerr Dental, Brea, CA, USA)—direct technique; light-body silicone impression material (Imprint 4, 3M ESPE, St. Paul, MN, USA)—wax technique | Human teeth | 20 coats of paint-on latex rubber applied directly to the root (12 µm each) using a brush, tooth attached to a vertical rod with sticky wax to maintain position, embedded in acrylic resin (ProBase Cold, Ivoclar Vivadent, Schaan, Liechtenstein), comparison with the wax technique with teeth inserted with a silicone positioner | Geometric: Micro-CT—layer thickness measurement | Reproducibility and uniformity of PDL layer thickness (micro-CT analysis) | None | Not applicable | Not applicable |
| Berthold et al. (2011a) [61] | Not specified | Silicone (not specified) or silicone with rubber foam (not specified) | Stainless steel | Custom aluminum jaw model with alveolar sockets, silicone (not specified) or silicon with rubber foam (not specified) for increased mobility, apical screw for mobility adjustment | Functional: Universal testing machine—deflection measurement; Periotest | Tooth mobility: static (universal testing machine) and dynamic (Periotest) | Splint | Not applicable | Comparable damping capacity (Periotest) |
| Berthold et al. (2011b) [62] | Not specified | Silicone (not specified) or silicone with rubber foam (not specified) | Stainless steel | Custom aluminum jaw model with alveolar sockets, silicone (not specified) or silicon with rubber foam (not specified) for increased mobility, apical screw for mobility adjustment | Functional: Periotest | Different splint designs rigidity | Splint | Not applicable | Not specified |
| Boeckler et al. (2008) [49] | 0.25 mm | Silicone impression material (Flexistone, Detax, Ettlingen, Germany) | Human teeth | Teeth coated with a wax sheet and embedded in an epoxy resin (Technovit 5000, Heraeus Kulzer, Hanau, Germany), wax removed, socket filled with silicone (Flexistone, Detax, Ettlingen, Germany), specimens reinserted manually | Functional: Periotest | Crowns marginal gap | Tooth-implant supported fixed partial denture | Not applicable | Not specified |
| Bömicke et al. (2017) [59] | Not specified | Heat-shrink tubing (Protect, Bahag AG, Mannheim, Germany), Light body polyvinylsiloxane impression material (Flexitime Correct Flow, Heraeus Kulzer GmbH, Hanau, Germany) in apical area | Cobalt chromium alloy | Metal abutments with roots covered with heat-shrink tubing and apically sealed with polyvinylsiloxane impression material; embedded in acrylic resin (Technovit 4071; Heraeus Kulzer GmbH, Hanau, Germany) in aluminum blocks, teeth inserted using silicone positioners | Not specified | Load-bearing capacity, fracture patterns under dynamic and static loading | Zirconia and metal-ceramic inlay-retained and wing-retained resin bonded fixed partial dentures | Not specified | Similar failure modes as in clinical conditions |
| Bruschi-Alonso et al. (2010) [50] | 0.2–0.3 mm | Polyether impression material (Impregum; 3M ESPE St. Paul, MN, USA) | Bovine teeth | Roots dipped in melted wax, afterwards embedded in acrylic resin (Vipi Flash; Vipi Ind. Com., Pirassunununga, Brazil), wax removed, socket filled with polyether (Impregum), specimens reinserted manually | Not specified | Impact strength (fracture resistance at high speed impact) | Tooth fragment reattachment | Not specified | Not specified |
| Clausen et al. (2010) [40] | Not specified | Other elastomer/technical—gum resin (Anti-Rutsch-Lack, Wenko-Wenselaar GmbH, Hilden, Germany) | Human teeth | Roots coated directly with gum resin layer and embedded in acrylic resin (Technovit 4000, Heraeus Kulzer, Hanau,, Germany), roots secured in resin by 0.9 mm stainless steel bar in apical third of the root | Not specified | Survival under cyclic loading; fracture resistance | Ceramic overlay | Not specified | Not specified |
| Clavijo et al. (2009) [51] | 0.2–0.3 mm | Polyether impression material (Impregum; 3M ESPE St. Paul, MN, USA) | Bovine teeth | Roots dipped in melted wax, afterwards embedded in polystyrene resin (Cristal, Araquímica, Araçariguama, Brazil), wax removed, socket was filled with polyether (Impregum), specimens inserted manually | Not specified | Root fracture resistance | Intraradicular post | Not specified | Comparable fracture resistance |
| Dulaimi & Al-Hashimi (2005) [52] | Not specified | Silicone impression material (DoriDent, Dr Hirschberg GmbH, Vienna, Austria) | Human teeth | Roots wrapped in lead foil embedded in acrylic resin (Medicus Cold Cure, DMP Ltd., Attiki, Greece), lead foil removed and silicone material painted on the root, specimens inserted in the socket manually | Not specified | Endodontic spreader load and penetration depth | Endodontic treatment | Not specified | Not specified |
| Fulde et al. (2025) [41] | 0.2 mm | Other elastomer/technical—gum resin (Anti-Rutsch-Lack, Wenko-Wenselaar, Hilden, Germany) | Human teeth | Roots coated directly with 0.2 mm gum resin (dipping once and removing excess in apical portion), inserted into wax, 0.9 mm metal bar placed through the root to prevent rotation, specimens embedded in acrylic resin (Technovit 4000, Kulzer, Hanau, Germany), specimens reinserted using silicone positioner | Not specified | Survival after thermocycling and mechanical loading, fracture resistance under vertical load, failure mode, wear behaviour (chewing simulation) | Zirconia overlay | Not specified | Not specified |
| González-Lluch et al. (2016) [31] | 0.2 mm | Polyethyl methacrylate denture relining material (Visco-gel, Dentsply Detrey GmbH, Konstanz, Germany) | Human teeth | Roots coated with Visco-gel layer using a brush, inserted into acrylic resin blocks (Vertex Self-Curing Liquid, Zeist, The Netherlands) | Not specified | Fracture resistance and failure modes: teeth with vs. without PDL simulation | Fiber post retained restoration, single crown/no crown | No significant difference in fracture load between PDL vs. no-PDL (p = 0.185)—median (538 N vs. 395 N)—limited number of specimens | Not specified |
| Hasna et al. (2022) [29] | Not specified | Red wax (not specified) | Human teeth | Roots coated with red wax (not specified), embedded in acrylic resin blocks (JET, Artigos Odontológicos Clássico Ltda., São Paulo, Brazil)—technique not specified | Not specified | Deflection, fracture resistance after artificial aging | Endodontic access restoration | Not specified | Not specified |
| Hayashi et al. (2006) [13] | 0.2 mm | Polyvinylsiloxane impression material (Duplicone, Shofu, Kyoto, Japan) | Human teeth | Roots coated with polyvinylsiloxane, embedded in acrylic resin (Uni-Fast II, GC, Tokyo, Japan)—technique not specified | Not specified | Fracture resistance | Intraradicular post and crown | Without simulated PDL in the preliminary study, fracture resistance was approx. twice as high, acrylic resin in specimens without PDL acted as a ferrule | Not specified |
| Ille et al. (2023) [30] | Not specified | Polyvinylsiloxane impression material (Elite HD, Zhermack, Badia Polesine, Italy) | Human teeth | Teeth inserted in acrylic cylinder (not specified), apical part of the root embedded in polyvinylsiloxane low consistency impression material (not specified)—technique not specified | Not specified | Compressive strength | Ceramic/composite overlays | Not specified | Not specified |
| Jalalian & Mirzaei (2009) [33] | 0.2 mm | Polyether impression material (Impregum, 3M ESPE, St. Paul, MN, USA) | Human teeth | Roots wrapped in 0.2 mm aluminum foil, embedded in acrylic resin; foil removed, sockets filled with Impregum, specimens repositioned manually | Not specified | Compressive strength | Intraradicular fiber post | Not specified | Not specified |
| Kolbeck et al. (2008) [18] | Not specified | Polyether impression material (Impregum, 3M ESPE, Seefeld, Germany) | Human teeth | Roots coated with polyether, embedded in resin blocks (Palapress Vario: Heraeus Kulzer, Hanau, Germany)—technique not specified | Not specified | Fracture resistance after chewing simulation | Tooth-tooth and implant-tooth fixed partial dentures | Not applicable | Not specified |
| Kosewski et al. (2023) [15] | 0.85 mm | Polyether impression material (Impregum Penta; 3M ESPE, St. Paul, MN, USA) | Hybrid ceramic (Ambarino High Class, Creamed GmbH & Co., Marburg, Germany) | Teeth and bone constructed using CAD/CAM process: PMMA cubes with milled alveolar sockets digitally enlarged, teeth milled based on digital project, sockets filled with polyether material, specimens inserted in the socket with 3D-printed positioner | Functional: Periotest | Crown material wear | Single ceramic crown | Lower antagonist wear (0.172 mm vs. 0.220 mm), wear depth (0.098 mm vs. 0.186 mm), volume loss (0.107 mm3 vs. 0.322 mm3) | Not specified |
| Krummel et al. (2019) [42] | 0.25 mm | Other elastomer/technical—gum resin (Anti-Rutsch-Lack, Wenko-Wenselaar GmbH, Hilden, Germany) | Human teeth | Roots coated directly with 0.25 mm gum resin layer and embedded in acrylic resin (Technovit 4000, Heraeus Kulzer, Hanau, Germany), roots secured in resin by 0.9 mm stainless steel bar in apical third of the root | Not specified | Survival rate after dynamic loading; fracture resistance; failure mode analysis | Ceramic overlay | Not specified | Not specified |
| Marchionatti et al. (2014) [32] | 0.3 mm | Polyether impression material (Impregum Soft Medium Body, 3M ESPE, St. Paul, MN, USA) vs. silicone impression material (Express Medium Body, 3M ESPE, St. Paul, MN, USA) | Bovine teeth | Roots dipped in melted wax, afterwards embedded in acrylic resin (Dencrilay, Dencril, Caieiras, Brazil), wax removed, socket filled with simulation material, specimens reinserted manually | Not specified | Fiber posts bond strength, roots fracture resistance | Intraradicular fiber post | No effect | Not specified |
| Nawafleh et al. (2020) [17] | 0.3 mm | Light body elastomeric impression material (3M ESPE, St. Paul, MN, USA)—not specified | Epoxy resin (Exakto-Form, Bredent, Senden, Germany) | Wax layer added to the roots, embedded in in acrylic resin, wax substituted with elastomeric impression material, specimens reinserted manually | Not specified | Fatigue survival and fracture resistance | Single zirconia crown | No effect | Not specified |
| Oliveira et al. (2022) [53] | Not specified | Polyether impression material (Impregum; 3M ESPE, St. Paul, MN, USA) | Polystyrene resin (Cristal, Araquímica, Araçariguama, Brazil) | Alveolus in acrylic model adapted manually, polyether injected in the socket, specimens reinserted manually | Not specified | Proximal contact force | Direct restoration | Not applicable | Not specified |
| Pişkin et al. (2008) [54] | Not specified | Light body silicone impression material (Speedex light body, Coltene/Whaledent, Altstätten, Switzerland) | Human teeth | Tooth wrapped in a single layer of aluminium foil, inserted in polystyrene resin, after aluminium foil removal, silicone material injected in the sockets, specimens reinserted manually | Not specified | Fracture resistance | Endodontic treatment | Not specified | Not specified |
| Preis et al. (2015) [55] | 1 mm | Polyether impression material (Impregum, 3M Espe, Seefeld, Germany) | Human teeth | Roots dipped in melted wax, embedded in resin blocks (Palapress Vario, Kulzer, Hanau, Germany), wax removed, socket filled with polyether (Impregum), specimens reinserted manually | Not specified | Fracture resistance and marginal adaptation after thermocycling and mechanical loading under different cementation types | Monolithic zirconia-reinforced lithium silicate single crowns | Not specified | Not specified |
| Preis et al. (2018) [16] | 1 mm | Polyether impression material (Impregum, 3M, Seefeld, Germany) | Human teeth | Roots dipped in melted wax, embedded in resin blocks (Palapress Vario, Kulzer, Hanau, Germany), wax removed, socket filled with polyether (Impregum), specimens reinserted manually | Not specified | Survival after thermocycling and mechanical loading, fracture resistance, effect of restoration type (tooth/implant), polishing vs. glazing | Lithium aluminosilicate single crowns | No effect | Not specified |
| Puschmann et al. (2009) [43] | 0.2 mm | Latex rubber die-spacer (Erkoskin, Erkodent, Pfalzgrafenweiler, Germany) | Cobalt chromium alloy (Wironit; Bego, Bremen, Germany) | Roots of metal teeth coated directly with a 0.2 mm silicone layer; roots embedded in low-melting–point alloy (MCP 70) using a duplication form | Functional: Mobility checked with an electronical displacement transducer (W1T3; HBM, Darmstadt, Germany)—displacement of 50 µm under 20 N of horizontal load | Load-bearing capacity with and without fatigue loading; fracture mode analysis | Ceramic fixed partial denture | Not specified | Not specified |
| Rathke et al. (2022) [34] | 0.25 mm | Other elastomer/technical—latex rubber milk (Suter Kunststoffe, Fraubrunnen, Switzerland) | Human teeth | Roots coated directly with one layer of latex rubber milk (Suter Kunststoffe, Switzerland) and embedded in acrylic resin (Technovit 4071, Heraeus Kulzer, Hanau, Germany) | Not specified | Root fracture resistance and crack formation after chewing simulation and static loading | Intraradicular glass fiber and metal posts | Not specified | Not specified |
| Ribeiro et al. (2023) [12] | 0.3 mm | Polyether impression material (Impregum F, 3M Oral Care, St. Paul, MN, USA) | Bovine teeth | Roots coated with a 0.3 mm polyether layer and embedded in polystyrene resin (Cristal, Araquímica, Araçariguama, Brazil), technique not specified | Not specified | Survival rate and failure mode under cyclic loading | Intraradicular glass fiber post and direct restoration | Different results from studies without PDL simulation | Comparable differences between survival rates of teeth restored with fiber posts vs. no posts, and different fracture modes than in clinical studies |
| Rosentritt et al. (2006) [11] | 1 mm | Polyether impression material (Impregum, 3M ESPE, Seefeld, Germany) | Liquid crystal polymer (LCP) and human teeth | Roots dipped in melted wax, afterwards embedded in PMMA resin (not specified), wax removed, socket filled with polyether (Impregum), specimens reinserted manually | Not specified | Fracture resistance of all-ceramic fixed partial dentures after thermocycling and mechanical loading | Ceramic fixed partial denture | Fracture resistance reduced by up to 70%; omission of PDL simulation leads to overestimation of fracture resistance | Not specified |
| Rosentritt et al. (2011) [10] | 0.75 mm | Polyether impression matrial (Impregum, 3M ESPE, Seefeld, Germany) | Human teeth | Roots dipped in melted wax, embedded in PMMA (Palapress Vario, Kulzer, Hanau, Germany), wax removed, sockets filled with polyether (Impregum), specimens reinserted using repositioning cast | Functional/Geometric: Universal testing machine—deflection measurement; PDL thickness measured after slicing the specimens | Fracture resistance of three-unit fixed partial dentures after thermocycling and mechanical loading | Ceramic fixed partial denture | Fracture force reduced by 40–50% with PDL; no PDL led to overestimation; PDL presence seemed to be responsible for the ageing effect during TCML | Similar modes of failure as in clinical conditions |
| Rosentritt et al. (2020) [14] | 1 mm | Polyether impression material (Impregum, 3M ESPE, Seefeld, Germany) | Human teeth | Roots dipped in melted wax, embedded in resin blocks (Palapress Vario, Kulzer, Hanau, Germany), wax removed, socket filled with polyether (Impregum), specimens reinserted manually | Not specified | Number and depth of wear traces | Zirconia/ceramic single crown | Crown on implants displayed increased antagonistic wear compared to teeth with PDL | Wear patterns similar to clinical findings |
| Sarafidou et al. (2012) [44] | Not specified | Latex rubber die-spacer (Erkoskin, Erkodent, Pfalzgrafenweiler, Germany) | Polyurethane resin (PUR, Alpha-Die-Top, Schütz Dental, Rosbach vor der Höhe, Germany) | Roots coated directly with latex layer (Erkoskin); embedded manually in polyurethane base | Not specified | Load-bearing capacity after artificial ageing (thermocycling and mechanical loading); fracture mode analysis | Fixed partial denture | not specified | not specified |
| Sasse et al. (2015) [45] | Not specified directly, reference to previous studies (0.2 mm) | Other elastomer/technical—gum resin (Anti-Rutsch-Lack, Wenko-Wenselaar GmbH, Hilden, Germany) | Human teeth | Roots coated directly with gum resin layer and embedded in acrylic resin (Technovit 4000, Heraeus Kulzer, Hanau, Germany), roots secured in resin by 0.9 mm stainless steel bar in apical third of the root | Not specified | Survival rate after dynamic loading; fracture resistance; failure mode analysis | Ceramic overlay | Not specified | Not specified |
| Sivieri-Araujo et al. (2015) [35] | 0.25–0.3 mm | Polyether impression material (Impregum Soft, 3M ESPE AG, Seefeld, Germany) | Bovine teeth | Roots dipped in melted wax, embedded in polystyrene resin (not specified), wax removed, socket filled with polyether (Impregum), specimens reinserted manually | Not specified | Fracture resistance | Intraradicular post | Not specified | Not specified |
| Soares et al. (2005) [26] | 0.2–0.3 mm | Polyether impression material (Impregum F, 3M-ESPE, Seefeld, Germany)/Polysulfide (Permlastic, Kerr, Brea, CA, USA)/Polyurethane elastomer (Ultra Flex, Solplas, Santo André, Brazil) | Bovine teeth | Roots dipped in melted wax, embedded in self-cured acrylic resin (Jet Clássico, Artigos Odontológicos Clássico Ltda., São Paulo, Brazil)/polystyrene resin (Cristal, Araquímica, Araçariguama, Brazil), wax removed, sockets filled with simulation material, specimens reinserted manually | Not specified | Fracture resistance and fracture modes | None | Presence of PDL simulation significantly altered fracture modes (more root fractures); absence of PDL caused concentration of fractures at coronal region | Fracture modes with PDL simulation similar to clinical fracture patterns |
| Soares et al. (2011) [56] | 0.2–0.3 mm | Polyether impression material (Impregum F, 3M ESPE, St. Paul, MN, USA) | Human teeth | Roots dipped in melted wax, embedded in wax model of the mandible, wax from roots eliminated, wax mandible duplicated in polystyrene resin (Aerojet, Aerojet Fiberglass, São Paulo, Brazil), socket filled with polyether (Impregum), specimens reinserted manually | Not specified | Bone strain under different conditions of bone loss and periodontal splinting | Splint | Presence of artificial PDL allowed more realistic strain distribution | Strain values comparable to cadaveric bone measurements |
| Souza et al. (2014) [57] | 0.2–0.3 mm | Polyether impression material (Impregum Soft, 3M ESPE, Seefeld, Germany) | Bovine teeth | Roots dipped in melted wax, afterwards embedded in polystyrene resin (Cristal, Araquímica, Araçariguama, Brazil), wax removed, socket filled with polyether (Impregum), specimens reinserted manually | Not specified | Fracture resistance | Endodontic irrigation | Not specified | Not specified |
| Sterzenbach et al. (2011) [1] | 0.3 mm | Polyurethane (Anti-Rutschlack, Kaddi-Lack, Dortmund, Germany); Polyether (Impregum Penta, 3M ESPE, Seefeld, Germany); Polyvinylosiloxane (Mollosil, DETAX, Ettlingen, Germany) | Human teeth | Roots coated with 0.3 mm wax, embedded in acrylic resin (Technovit 4004), wax removed, sockets filled with simulation material; specimens reinserted manually | Functional: Periotest; universal testing machine—deflection measurement | Tooth deflection (mobility) under axial and perpendicular loading; damping capacity—Periotest values | None | Not applicable | Mobility similar to clinical conditions |
| Sterzenbach et al. (2012) [36] | 0.1 mm | Other elastomer/technical—gum resin (Anti-Rutsch-Lack, Wenko-Wenselaar GmbH, Hilden, Germany) | Human teeth | Roots coated directly with 0.1 mm silicone (Anti-Rutsch-Lack) and embedded in autopolymerizing acrylic resin (Technovit 4000, Heraeus Kulzer, Hanau, Germany); long axis angled at 135° probably with an index—technique not specified directly, reference to previous study | Not specified | Maximum load capability after thermocycling and mechanical loading; fracture patterns depending on restorative stage | Post-endodontic restorations: fiber post vs. post and core vs. post, core, ceramic crown | Not specified | Not specified |
| Tanomaru-Filho et al. (2014) [58] | 0.25 mm | Polyether impression material (Impregum Soft, 3M ESPE AG, Seefeld, Germany) | Bovine teeth | Roots dipped in melted wax, embedded in polystyrene resin (Cristal, Araquímica, Araçariguama, Brazil), wax removed, sockets filled with polyether (Impregum); specimens reinserted manually | Not specified | Fracture resistance | Intraradicular post/endodontic filling | not specified | Not specified |
| Waldecker et al. (2019) [9] | Not specified | Heat-shrink tubing (Protect, Bahag AG, Mannheim, Germany), low-viscosity polyvinylsiloxane impression material (Flexitime Correct Flow; Heraeus Kulzer GmbH, Hanau, Germany) in apical area | Cobalt-chromium alloy | Abutment roots covered with heat-shrink tubing sealed apically with polyvinyl siloxane; embedded in aluminum blocks with acrylic resin (Technovit 4071; Heraeus Kulzer GmbH, Hanau, Germany) | Functional: Model deflection measured under load of 100 N (technique not specified) | Validation of finite elements analysis model; stress distribution and fracture resistance under axial and oblique loading | Zirconia inlay-retained fixed partial denture | Absence of simulated resilience led to overestimation of fracture resistance by 50–95% | Good correlation with clinical behaviour |
| Weigl et al. (2018) [60] | 1 mm | Polyether impression material (Impregum, 3M Oral Care, Seefeld, Germany) | Hybrid ceramic (Ambarino High Class, Creamed GmbH & Co., Marburg, Germany) | Roots dipped in wax, embedded in resin blocks (Palapress Vario, Heraeus-Kulzer, Hanau, Germany), sockets filled with polyether—technique not specified in detail, reference to previous studies | Not specified | Survival under thermocycling and mechanical loading, fracture resistance, fracture mode, influence of crown thickness and cement type | Zirconia single crown | Not specified | Not specified |
| Wong & Botelho (2014) [8] | Not specified | Orthodontic elastic rings (Z-pak Elastics, Ormco Corp., Brea, CA, USA) | Stainless steel | Two or more elastic orthodontic rings placed apically and circumferentially around the tooth analog, rings placed in grooves in the root surfaces, specimens reinserted in metal base holders | Functional: Universal testing machine—deflection measurement | Fatigue bond strength under cyclic loading | Fixed-fixed/cantilever resin-bonded fixed partial denture | Not applicable | More mobility of the roots than in the clinical situation (550 µm vs. 25–100 µm) |
| Yeslam et al. (2023) [46] | 0.55 mm | Latex rubber die-spacer (Erkoskin, Erich Kopp GmbH, Pfalzgrafenweiler, Germany) | Die resin (Mirapont, Hager & Werken GmbH & Co. KG, Germany) | Roots painted directly with liquid latex (Erkoskin), embedded in die resin (Mirapont, Hager & Werken GmbH & Co. KG, Duisburg, Germany), manual insertion into the resin | Not specified | Fracture resistance | Hybrid ceramic single crown | Not specified | Fracture modes different than those in clinical conditions |
| Zhang et al. (2016) [69] | 0.3 mm | Polyvinylsiloxane impression material (Imprint II, 3M ESPE, St. Paul, MN, USA) | Composite resin (Filtek P60, 3M ESPE, St. Paul, MN, USA) | Roots coated with 0.3 mm polyvinylsiloxane and embedded in PMMA support base—technique not specified | Not specified | Validation of finite elements analysis model; crack initiation and propagation under loading | Zirconia inlay and onlay fixed partial denture | Not specified | Not specified |
| Zhu et al. (2015) [4] | 0.2–0.3 mm | Silicone impression material (Honigum-Mixstar Mono, DMG, Hamburg, Germany) | Human teeth | Roots dipped in melted wax, afterwards embedded in epoxy resin (Phoenix; Blue-star Manufacturing, Wuxi, China), wax removed, socket filled with silicone, specimens reinserted manually | Functional: Universal testing machine—compression testing—elastic modulus evaluation | Cyclic loading resistance | Vertical root fracture bonding | PDL simulation significantly increased fatigue resistance (PDL group: 150, 580 ± 21, 570 cycles vs. non-PDL group: 29, 2 [62] ±9, 473 cycles, p < 0.001) | Not specified |
| Zimmermann et al. (2020) [47] | 0.1 mm | Polyvinylsiloxane impression material (PRESIDENT light body, Coltène AG, Altstätten, Switzerland) | Composite resin (BRILLIANT Crios CAD/CAM, Coltène AG, Altstätten, Switzerland) | Teeth and bone constructed using CAD/CAM process: teeth and alveolar sockets milled from composite (Brilliant) and (PEEK BreCAM.bioHPP—bredent medical GmbH; Senden, Germany), elastomeric material injected into the socket, specimen insertion not specified | Not specified | Fracture resistance after thermocycling and mechanical loading | Ceramic/composite fixed partial denture | Not specified | Not specified |
Appendix A.3. Literature Review Results
References
- Sterzenbach, G.; Kalberlah, S.; Beuer, F.; Frankenberger, R.; Naumann, M. In-vitro simulation of tooth mobility for static and dynamic load tests: A pilot study. Acta Odontol. Scand. 2011, 69, 316–318. [Google Scholar] [CrossRef]
- Chang, H.-H.; Yeh, C.-L.; Wang, Y.-L.; Huang, Y.-C.; Tsai, S.-J.; Li, Y.-T.; Yang, J.-H.; Lin, C.-P. Differences in the biomechanical behaviors of natural teeth and dental implants. Dent. Mater. 2021, 37, 682–689. [Google Scholar] [CrossRef]
- Wen, X.; Pei, F.; Jin, Y.; Zhao, Z. Exploring the mechanical and biological interplay in the periodontal ligament. Int. J. Oral Sci. 2025, 17, 23. [Google Scholar] [CrossRef]
- Zhu, Y.N.; Yang, W.D.; Abbott, P.V.; Martin, N.; Wei, W.J.; Li, J.J.; Chen, Z.; Wang, W.M. The biomechanical role of periodontal ligament in bonded and replanted vertically fractured teeth under cyclic biting forces. Int. J. Oral Sci. 2015, 7, 125–130. [Google Scholar] [CrossRef]
- D’Arcangelo, C.; Vanini, L.; Rondoni, G.; Vadini, M.; De Angelis, F. Wear evaluation of prosthetic materials opposing themselves. Oper. Dent. 2018, 43, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Singer, L.; Fouda, A.; Bourauel, C. Biomimetic approaches and materials in restorative and regenerative dentistry: Review article. BMC Oral Health 2023, 23, 105. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.S.; Amin, F.; Fareed, M.A.; Ghabbani, H.; Riaz, S.; Khurshid, Z.; Kumar, N. Biomimetic aspects of restorative dentistry Biomaterials. Biomimetics 2020, 5, 34. [Google Scholar] [CrossRef]
- Wong, T.L.; Botelho, M.G. The fatigue bond strength of fixed-fixed versus cantilever resin-bonded partial fixed dental prostheses. J. Prosthet. Dent. 2014, 111, 136–141. [Google Scholar] [CrossRef]
- Waldecker, M.; Rues, S.; Rammelsberg, P.; Bömicke, W. Validation of in-vitro tests of zirconia-ceramic inlay-retained fixed partial dentures: A finite element analysis. Dent. Mater. 2019, 35, e53–e62. [Google Scholar] [CrossRef] [PubMed]
- Rosentritt, M.; Behr, M.; Scharnagl, P.; Handel, G.; Kolbeck, C. Influence of resilient support of abutment teeth on fracture resistance of all-ceramic fixed partial dentures: An in vitro study. Int. J. Prosthodont. 2011, 24, 465–468. [Google Scholar]
- Rosentritt, M.; Behr, M.; Gebhard, R.; Handel, G. Influence of stress simulation parameters on the fracture strength of all-ceramic fixed-partial dentures. Dent. Mater. 2006, 22, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.T.H.; Oliveira, G.; Oliveira, H.L.Q.; Mendoza, L.C.L.; Melo, C.; Silva Peres, T.; Soares, C.J. Survival of severely compromised endodontically treated teeth restored with or without a fiber glass post. J. Appl. Oral Sci. 2023, 31, e20230241. [Google Scholar] [CrossRef]
- Hayashi, M.; Takahashi, Y.; Imazato, S.; Ebisu, S. Fracture resistance of pulpless teeth restored with post-cores and crowns. Dent. Mater. 2006, 22, 477–485. [Google Scholar] [CrossRef]
- Rosentritt, M.; Schumann, F.; Krifka, S.; Preis, V. Influence of zirconia and lithium disilicate tooth- or implant-supported crowns on wear of antagonistic and adjacent teeth. J. Adv. Prosthodont. 2020, 12, 1–8. [Google Scholar] [CrossRef]
- Kosewski, P.; De Angelis, F.; Sorrentino, E.; Mielczarek, A.; Buonvivere, M.; D’Arcangelo, C. Effect of the abutment rigidity on the wear resistance of a lithium disilicate glass ceramic: An in vitro study. J. Funct. Biomater. 2023, 14, 395. [Google Scholar] [CrossRef]
- Preis, V.; Hahnel, S.; Behr, M.; Rosentritt, M. In vitro performance and fracture resistance of novel CAD/CAM ceramic molar crowns loaded on implants and human teeth. J. Adv. Prosthodont. 2018, 10, 300–307. [Google Scholar] [CrossRef]
- Nawafleh, N.; Bibars, A.R.; Elshiyab, S.; Janzeer, Y. In vitro simulation of periodontal ligament in fatigue testing of dental crowns. Eur. J. Dent. 2020, 14, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Kolbeck, C.; Behr, M.; Rosentritt, M.; Handel, G. Fracture force of tooth-tooth- and implant-tooth-supported all-ceramic fixed partial dentures using titanium vs. customised zirconia implant abutments. Clin. Oral Implant. Res. 2008, 19, 1049–1053. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, V.; Duruel, O.; Ataman-Duruel, E.T.; Tözüm, M.D.; Nares, S.; Tözüm, T.F. In-depth morphological evaluation of tooth anatomic lengths with root canal configurations using cone beam computed tomography in North American population. J. Appl. Oral Sci. 2020, 28, e20190103. [Google Scholar] [CrossRef]
- Kucher, M.; Dannemann, M.; Modler, N.; Bernhard, M.R.; Hannig, C.; Weber, M.T. Mapping of the micro-mechanical properties of human root dentin by means of microindentation. Materials 2021, 14, 505. [Google Scholar] [CrossRef]
- Kinney, J.; Marshall, S.; Marshall, G. The mechanical properties of human dentin: A critical review and re-evaluation of the dental literature. Crit. Rev. Oral Biol. Med. 2003, 14, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Chun, K.; Choi, H.; Lee, J. Comparison of mechanical property and role between enamel and dentin in the human teeth. J. Dent. Biomech. 2014, 5, 1758736014520809. [Google Scholar] [CrossRef]
- Andrejovská, J.; Petruš, O.; Medveď, D.; Vojtko, M.; Riznič, M.; Kizek, P.; Dusza, J. Hardness and indentation modulus of human enamel and dentin. Surf. Interface Anal. 2023, 55, 270–278. [Google Scholar] [CrossRef]
- Di Carlo, S.; De Angelis, F.; Brauner, E.; Pranno, N.; Tassi, G.; Senatore, M.; Bossù, M. Flexural strength and elastic modulus evaluation of structures made by conventional PMMA and PMMA reinforced with graphene. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5201–5208. [Google Scholar] [CrossRef] [PubMed]
- Odin, G.; Savoldelli, C.; Bouchard, P.O.; Tillier, Y. Determination of Young’s modulus of mandibular bone using inverse analysis. Med. Eng. Phys. 2010, 32, 630–637. [Google Scholar] [CrossRef]
- Soares, C.J.; Pizi, E.C.; Fonseca, R.B.; Martins, L.R. Influence of root embedment material and periodontal ligament simulation on fracture resistance tests. Braz. Oral Res. 2005, 19, 11–16. [Google Scholar] [CrossRef]
- Winkler, S.; Morris, H.F.; Spray, J.R. Stability of implants and natural teeth as determined by the Periotest over 60 months of function. J. Oral Implantol. 2001, 27, 198–203. [Google Scholar] [CrossRef]
- Aboushelib, M.N. Simulation of cumulative damage associated with long term cyclic loading using a multi-level strain accommodating loading protocol. Dent. Mater. 2013, 29, 252–258. [Google Scholar] [CrossRef]
- Hasna, A.A.; Pinto, A.B.A.; Coelho, M.S.; de Andrade, G.S.; Tribst, J.P.M.; de Castro Lopes, S.L.P.; Carvalho, C.A.T.; Borges, A.L.S. Fracture resistance and biomechanical behavior of different access cavities of maxillary central incisors restored with different composite resins. Clin. Oral Investig. 2022, 26, 6295–6303. [Google Scholar] [CrossRef]
- Ille, C.; Moacă, E.-A.; Pop, D.; Goguță, L.; Opriș, C.; Pîrvulescu, I.L.; Avram, L.; Faur, A.; Jivănescu, A. Compressive strength evaluation of thin occlusal veneers from different CAD/CAM materials, before and after acidic saliva exposure. Odontology 2023, 111, 360–374. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Lluch, C.; Rodriguez-Cervantes, P.J.; Forner, L.; Barjau, A. Inclusion of the periodontal ligament in studies on the biomechanical behavior of fiber post-retained restorations: An in vitro study and three-dimensional finite element analysis. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2016, 230, 230–238. [Google Scholar] [CrossRef]
- Marchionatti, A.M.; Wandscher, V.F.; Broch, J.; Bergoli, C.D.; Maier, J.; Valandro, L.F.; Kaizer, O.B. Influence of periodontal ligament simulation on bond strength and fracture resistance of roots restored with fiber posts. J. Appl. Oral Sci. 2014, 22, 450–458. [Google Scholar] [CrossRef]
- Jalalian, E.; Mirzaei, M. In vitro evaluation of different diameters of quartz fiber posts on root fracture resistance. J. Iran. Dent. Assoc. 2009, 21, 24–32. [Google Scholar]
- Rathke, A.; Frehse, H.; Hrusa, B. Vertical root fracture resistance and crack formation of root canal-treated teeth restored with different post-luting systems. Odontology 2022, 110, 719–725. [Google Scholar] [CrossRef]
- Sivieri-Araujo, G.; Tanomaru-Filho, M.; Guerreiro-Tanomaru, J.M.; Bortoluzzi, E.A.; Jorge, E.G.; Reis, J.M. Fracture resistance of simulated immature teeth after different intra-radicular treatments. Braz. Dent. J. 2015, 26, 211–215. [Google Scholar] [CrossRef]
- Sterzenbach, G.; Rosentritt, M.; Frankenberger, R.; Paris, S.; Naumann, M. Loading standardization of postendodontic restorations in vitro: Impact of restorative stage, static loading, and dynamic loading. Oper. Dent. 2012, 37, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Scharnagl, P.; Behr, M.; Rosentritt, M.; Leibrock, A.; Handel, G. Simulation of physiological tooth mobility in in-vitro stress examination of dental restorations in the masticator. J. Dent. Res. 1998, 77, 1260. [Google Scholar]
- AlZahrani, F.; Richards, L. Micro-CT evaluation of a novel periodontal ligament simulation technique for dental experimental models. Arch. Orofac. Sci. 2018, 13, 93–103. [Google Scholar]
- Heintze, S.D.; Monreal, D.; Reinhardt, M.; Eser, A.; Peschke, A.; Reinshagen, J.; Rousson, V. Fatigue resistance of all-ceramic fixed partial dentures—Fatigue tests and finite element analysis. Dent. Mater. 2018, 34, 494–507. [Google Scholar] [CrossRef]
- Clausen, J.O.; Abou Tara, M.; Kern, M. Dynamic fatigue and fracture resistance of non-retentive all-ceramic full-coverage molar restorations. Influence of ceramic material and preparation design. Dent. Mater. 2010, 26, 533–538. [Google Scholar] [CrossRef]
- Fulde, N.; Wille, S.; Kern, M. Fracture resistance and wear behavior of ultra-thin occlusal veneers made from translucent zirconia ceramics bonded to different tooth substrates. J. Esthet. Restor. Dent. 2025, 37, 1463–1473. [Google Scholar] [CrossRef]
- Krummel, A.; Garling, A.; Sasse, M.; Kern, M. Influence of bonding surface and bonding methods on the fracture resistance and survival rate of full-coverage occlusal veneers made from lithium disilicate ceramic after cyclic loading. Dent. Mater. 2019, 35, 1351–1359. [Google Scholar] [CrossRef]
- Puschmann, D.; Wolfart, S.; Ludwig, K.; Kern, M. Load-bearing capacity of all-ceramic posterior inlay-retained fixed dental prostheses. Eur. J. Oral Sci. 2009, 117, 312–318. [Google Scholar] [CrossRef]
- Sarafidou, K.; Stiesch, M.; Dittmer, M.P.; Jörn, D.; Borchers, L.; Kohorst, P. Load-bearing capacity of artificially aged zirconia fixed dental prostheses with heterogeneous abutment supports. Clin. Oral Investig. 2012, 16, 961–968. [Google Scholar] [CrossRef]
- Sasse, M.; Krummel, A.; Klosa, K.; Kern, M. Influence of restoration thickness and dental bonding surface on the fracture resistance of full-coverage occlusal veneers made from lithium disilicate ceramic. Dent. Mater. 2015, 31, 907–915. [Google Scholar] [CrossRef]
- Yeslam, H.E.; Aljadaani, A.K.; Almalky, A.M.; Zahran, M.M.; Hasanain, F.A. Effect of luting agent on the load-bearing capacity of milled hybrid ceramic single-tooth restoration. Ann. Dent. Spec. 2023, 11, 68–76. [Google Scholar] [CrossRef]
- Zimmermann, M.; Ender, A.; Attin, T.; Mehl, A. Fracture load of three-unit full-contour fixed dental prostheses fabricated with subtractive and additive CAD/CAM technology. Clin. Oral Investig. 2020, 24, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Alqarni, H.; Alfaifi, M.A.; Altoman, M.S.; AlHelal, A.A.; Magdy Ahmed, W.; Ahmed Azhari, A.; Kattadiyil, M.T. A novel digital workflow for fabricating artificial periodontal ligament using three-dimensional printing flexible resin: A dental technique. Saudi Dent. J. 2024, 36, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Boeckler, A.F.; Morton, D.; Kraemer, S.; Geiss-Gerstdorfer, J.; Setz, J.M. Marginal accuracy of combined tooth-implant-supported fixed dental prostheses after in vitro stress simulation. Clin. Oral Implant. Res. 2008, 19, 1261–1269. [Google Scholar] [CrossRef]
- Bruschi-Alonso, R.C.; Alonso, R.C.; Correr, G.M.; Alves, M.C.; Lewgoy, H.R.; Sinhoreti, M.A.; Puppin-Rontani, R.M.; Correr-Sobrinho, L. Reattachment of anterior fractured teeth: Effect of materials and techniques on impact strength. Dent. Traumatol. 2010, 26, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Clavijo, V.G.; Reis, J.M.; Kabbach, W.; Silva, A.L.; Oliveira Junior, O.B.; Andrade, M.F. Fracture strength of flared bovine roots restored with different intraradicular posts. J. Appl. Oral Sci. 2009, 17, 574–578. [Google Scholar] [CrossRef]
- Dulaimi, S.F.; Wali Al-Hashimi, M.K. A comparison of spreader penetration depth and load required during lateral condensation in teeth prepared using various root canal preparation techniques. Int. Endod. J. 2005, 38, 510–515. [Google Scholar] [CrossRef]
- Oliveira, L.; Melo, C.; Cavalcanti, K.; Soares, P.; Cardenas, A.; Soares, C.J. Effects of adjacent tooth type and occlusal fatigue on proximal contact force of posterior bulk fill and incremental resin composite restoration. Oper. Dent. 2022, 47, 64–75. [Google Scholar] [CrossRef]
- Piskin, B.; Aydin, B.; Sarikanat, M. The effect of spreader size on fracture resistance of maxillary incisor roots. Int. Endod. J. 2008, 41, 54–59. [Google Scholar] [CrossRef]
- Preis, V.; Behr, M.; Hahnel, S.; Rosentritt, M. Influence of cementation on in vitro performance, marginal adaptation and fracture resistance of CAD/CAM-fabricated ZLS molar crowns. Dent. Mater. 2015, 31, 1363–1369. [Google Scholar] [CrossRef]
- Soares, P.B.; Fernandes Neto, A.J.; Magalhaes, D.; Versluis, A.; Soares, C.J. Effect of bone loss simulation and periodontal splinting on bone strain: Periodontal splints and bone strain. Arch. Oral Biol. 2011, 56, 1373–1381. [Google Scholar] [CrossRef][Green Version]
- Souza, E.M.; Calixto, A.M.; Lima, C.N.; Pappen, F.G.; De-Deus, G. Similar influence of stabilized alkaline and neutral sodium hypochlorite solutions on the fracture resistance of root canal-treated bovine teeth. J. Endod. 2014, 40, 1600–1603. [Google Scholar] [CrossRef] [PubMed]
- Tanomaru-Filho, M.; Sivieri-Araujo, G.; Guerreiro-Tanomaru, J.M.; Bortoluzzi, E.A.; Jorge, E.G.; Abi-Rached, F.O.; Reis, J.M. Resistance of teeth with simulated incomplete rhizogenesis with intraradicular post or root canal filling. J. Contemp. Dent. Pract. 2014, 15, 413–416. [Google Scholar] [CrossRef]
- Bömicke, W.; Waldecker, M.; Krisam, J.; Rammelsberg, P.; Rues, S. In vitro comparison of the load-bearing capacity of ceramic and metal-ceramic resin-bonded fixed dental prostheses in the posterior region. J. Prosthet. Dent. 2018, 119, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Weigl, P.; Sander, A.; Wu, Y.; Felber, R.; Lauer, H.-C.; Rosentritt, M. In-vitro performance and fracture strength of thin monolithic zirconia crowns. J. Adv. Prosthodont. 2018, 10, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Berthold, C.; Auer, F.J.; Potapov, S.; Petschelt, A. In vitro splint rigidity evaluation—Comparison of a dynamic and a static measuring method. Dent. Traumatol. 2011, 27, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Berthold, C.; Auer, F.J.; Potapov, S.; Petschelt, A. Influence of wire extension and type on splint rigidity—Evaluation by a dynamic and a static measuring method. Dent. Traumatol. 2011, 27, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Re, D.; De Angelis, F.; Augusti, G.; Augusti, D.; Caputi, S.; D’Amario, M.; D’Arcangelo, C. Mechanical properties of elastomeric impression materials: An in vitro comparison. Int. J. Dent. 2015, 2015, 428286. [Google Scholar] [CrossRef]
- Natali, A.; Pavan, P.; Carniel, E.; Dorow, C. Viscoelastic response of the periodontal ligament: An experimental-numerical analysis. Connect. Tissue Res. 2004, 45, 222–230. [Google Scholar] [CrossRef]
- Rees, J.S. An investigation into the importance of the periodontal ligament and alveolar bone as supporting structures in finite element studies. J. Oral Rehabil. 2001, 28, 425–432. [Google Scholar] [CrossRef]
- Qian, L.; Todo, M.; Morita, Y.; Matsushita, Y.; Koyano, K. Deformation analysis of the periodontium considering the viscoelasticity of the periodontal ligament. Dent. Mater. 2009, 25, 1285–1292. [Google Scholar] [CrossRef]
- Berthold, C.; Holst, S.; Schmitt, J.; Goellner, M.; Petschelt, A. An evaluation of the Periotest method as a tool for monitoring tooth mobility in dental traumatology. Dent. Traumatol. 2010, 26, 120–128. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Zhang, Z.; Thompson, M.; Field, C.; Li, W.; Li, Q.; Swain, M.V. Fracture behavior of inlay and onlay fixed partial dentures—An in-vitro experimental and XFEM modeling study. J. Mech. Behav. Biomed. Mater. 2016, 59, 279–290. [Google Scholar] [CrossRef] [PubMed]





| Property | Human Dentin | Ambarino High Class |
|---|---|---|
| Elastic modulus (GPa) | 16–25 GPa [21,22,23] | 10 GPa |
| Hardness (GPa) | 0.4–0.7 GPa [20,23] | 0.71 GPa (≈710 MPa) |
| Compressive strength (MPa) | 190–300 MPa [21,22] | 500 MPa |
| Direction | Mean (PTV) | SD (PTV) | Standard Error (PTV) | 95% CI (PTV) | Minimum (PTV) | Maximum (PTV) |
|---|---|---|---|---|---|---|
| Buccal | 2.88 | 1.11 | 0.17 | [2.54, 3.21] | 1.00 | 4.90 |
| Palatal | 2.91 | 1.03 | 0.15 | [2.60, 3.22] | 1.10 | 4.90 |
| Mesial | 3.04 | 0.74 | 0.11 | [2.82, 3.26] | 1.70 | 4.50 |
| Distal | 3.14 | 0.75 | 0.11 | [2.91, 3.37] | 1.50 | 4.50 |
| Overall horizontal | 2.99 | 0.92 | 0.07 | [2.86, 3.13] | 1.00 | 4.90 |
| Vertical | −4.02 | 0.56 | 0.08 | [−4.19, −3.85] | −5.60 | −3.30 |
| Specimen | Mean (mm) | SD (mm) | Variance (mm2) | 95% CI (mm) | Minimum (mm) | Maximum (mm) |
|---|---|---|---|---|---|---|
| 1 | 0.86 | 0.05 | 0.003 | [0.83, 0.89] | 0.73 | 0.92 |
| 2 | 0.90 | 0.02 | 0.001 | [0.89, 0.92] | 0.86 | 0.95 |
| 3 | 0.87 | 0.08 | 0.006 | [0.83, 0.92] | 0.72 | 0.99 |
| 4 | 0.83 | 0.05 | 0.003 | [0.80, 0.86] | 0.77 | 0.94 |
| 5 | 0.82 | 0.04 | 0.002 | [0.79, 0.84] | 0.73 | 0.86 |
| 6 | 0.87 | 0.04 | 0.001 | [0.84, 0.89] | 0.81 | 0.92 |
| 7 | 0.84 | 0.07 | 0.005 | [0.80, 0.88] | 0.73 | 0.95 |
| 8 | 0.88 | 0.05 | 0.002 | [0.85, 0.90] | 0.81 | 0.94 |
| 9 | 0.87 | 0.05 | 0.003 | [0.84, 0.90] | 0.79 | 0.95 |
| 10 | 0.87 | 0.04 | 0.001 | [0.85, 0.89] | 0.81 | 0.92 |
| 11 | 0.87 | 0.04 | 0.002 | [0.84, 0.89] | 0.80 | 0.92 |
| 12 | 0.87 | 0.05 | 0.003 | [0.84, 0.90] | 0.80 | 0.93 |
| 13 | 0.86 | 0.05 | 0.002 | [0.83, 0.88] | 0.77 | 0.93 |
| 14 | 0.86 | 0.05 | 0.002 | [0.83, 0.89] | 0.80 | 0.94 |
| 15 | 0.85 | 0.05 | 0.002 | [0.82, 0.88] | 0.77 | 0.93 |
| Overall | 0.86 | 0.05 | 0.003 | [0.85, 0.87] | 0.72 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosewski, P.; Kosewski, J.; Mielczarek, A. Simulation of the Periodontal Ligament in Dental Materials Research: A CAD/CAM-Based Method for PDL Modeling. J. Funct. Biomater. 2025, 16, 429. https://doi.org/10.3390/jfb16120429
Kosewski P, Kosewski J, Mielczarek A. Simulation of the Periodontal Ligament in Dental Materials Research: A CAD/CAM-Based Method for PDL Modeling. Journal of Functional Biomaterials. 2025; 16(12):429. https://doi.org/10.3390/jfb16120429
Chicago/Turabian StyleKosewski, Przemysław, Juliusz Kosewski, and Agnieszka Mielczarek. 2025. "Simulation of the Periodontal Ligament in Dental Materials Research: A CAD/CAM-Based Method for PDL Modeling" Journal of Functional Biomaterials 16, no. 12: 429. https://doi.org/10.3390/jfb16120429
APA StyleKosewski, P., Kosewski, J., & Mielczarek, A. (2025). Simulation of the Periodontal Ligament in Dental Materials Research: A CAD/CAM-Based Method for PDL Modeling. Journal of Functional Biomaterials, 16(12), 429. https://doi.org/10.3390/jfb16120429








